Abstract
Objective
Cisplatin is the first‐line chemotherapy for ovarian cancer. However, cisplatin resistance is severely affecting the treatment efficacy. FOXO3a has been reported to be involved in reversing chemotherapy resistance. However, whether low‐dose fraction radiation therapy (LDFRT) can reverse cisplatin resistance remains unclear. This study aimed to explore the effect of LDFRT on cisplatin resistance and its relation with FOXO3a expression in vitro.
Methods
The toxicity of cisplatin on SKOV3/DDP cells was evaluated by CCK8 assay and cell apoptosis was measured by Annexin V‐FITC staining as well as Hoechst33342 staining. The expression of FOXO3a and other relative proteins was measured by western blot.
Results
Our study found that LDFRT enhanced cisplatin‐induced apoptosis of SKOV3/DDP cells and promoted the expression of FOXO3a and pro‐apoptotic protein PUMA. In addition, overexpression of FOXO3a promoted PUMA activity and toxicity of cisplatin on SKOV3/DDP cells.
Conclusion
LDFRT reverses cisplatin resistance of SKOV3/DDP cells possibly by upregulating the expression of FOXO3a and its downstream target PUMA, suggesting that LDFRT might be a potent chemosensitizer for the treatment of ovarian cancer.
Keywords: cisplatin resistance, FOXO3a, LDFRT, ovarian cancer
1. INTRODUCTION
Ovarian cancer is the second leading diagnosed tumor in the female reproductive tract and metastasis has been observed in 80% of patients in the first diagnosis and more than 60% of metastasis occurs in the abdominopelvic cavity with a 5‐year survival rate less than 50%.1, 2, 3 Although cisplatin is beneficial in the initial treatment, after a period of time, cisplatin resistance occurs, which causes ovarian cancer recurrence and relapse in the abdominal cavity.4 Therefore, identifying a new useful way to reverse cisplatin resistance during the treatment of ovarian cancer is urgently required.
Radiation was discovered in the late 19th century and is widely used in the clinic including imaging diagnosis and cancers therapy. Ovarian cancer is sensitive to radiation and abdominal radiotherapy is an effective adjuvant radiotherapy for ovarian cancer, but the low tolerance of the up‐abdominal organs limits the application of convention radiation.3, 5
Low‐dose fraction radiation therapy (LDFRT), the total dose of low‐dose radiation divided into smaller doses called fractions, can improve the activity of the immune system and promote normal cells growth but inhibit cancer cells growth.6, 7, 8, 9 Clinical trials showed that LDFRT enhances the chemotherapeutic effect in human prostate cancer cells 10 and low‐dose abdominal radiation (60 cGy X 4 fractions) can act as a docetaxel chemosensitizer for recurrent ovarian cancer.11 However, whether LDFRT can reverse cisplatin resistance remains unclear.
FOXO3a, also known as forkhead boxO3, belongs to the family of forkhead transcription factors and plays a regulatory role in cells growth, differentiation, and apoptosis.12 Recently, some studies found that FOXO3a plays an important role in reversing chemoresistance and inhibiting tumor proliferation and development.13, 14, 15 Importantly, the accumulation of FOXO3 at laser‐induced damage was observed in cultured tumor cells treated by focused laser micro‐irradiation.16 PUMA, a downstream target of FOXO3a, was also implicated in the radiosensitivity of tumor cells.17, 18 In this study, we aimed to explore the effect of LDFRT on cisplatin resistance and its relation with FOXO3a and PUMA expression in vitro.
2. MATERIALS AND METHODS
2.1. Materials
The drug‐resistant ovarian cancer cell line, SKOV3/DDP, was bought from the Institute of Cancer Research, Chinese Academy of Medical Sciences (Beijing, China).19, 20 Cell counting kit‐8 (CCK‐8) and Annexin V‐FITC were purchased from Jiamay Biotech (The catalog numbers are AP1008 and LHK601‐100, respectively; China). Primary antibodies against FOXO3a or PUMA were purchased from Abcam (The catalog numbers are ab12162 and ab9643, respectively; USA). β‐actin antibody was purchased from Bioss (Catalog number: bs‐0061R; China). Plasmids GFP‐Foxo3a and GFP‐vector were purchased from Gene (Shanghai, China). Lipofectamine 2000 was purchased from Invitrogen (Catalog number: 11668019; USA).
2.2. Radiation treatment
Cells were cultivated in 1640 (HyClone ltd, China) medium supplemented with 10% fetal bovine serum (BI ltd, USA) and 1.25 µg/mL cisplatin to sustain drug resistance at 37℃ incubator with 5% CO2 and 95% O2. Cells were randomly divided into control group, low‐dose radiation (LDR) group, low‐dose fraction radiation (LDFRT) group and conventional group (CR). After cells reached a confluence of 40%‐50%, radiation treatment was applied. LDFRT group received two fractions of 0.5 gy per day (10:00 AM and 4:00 PM) for two days of continuous treatment.21 At the last radiation, the LDR group received 0.5 gy, CR group received 2 gy and the control group received no radiation. Twenty‐four hours after last radiation, all groups received cisplatin for another 24 hours followed by further analysis.
2.3. Cell proliferation/cytotoxicity assay
CCK‐8 is a more sensitive WST‐8‐based colorimetric assay than other tetrazolium salts such as MTT or MTS‐based assays in determining the cell viability regarding the cell proliferation and cytotoxicity. In cells, the tetrazolium salt, WST‐8, is reduced by dehydrogenases to generate a yellow color formazan dye, which is water soluble and directly proportional to the number of living cells.22 In our study, cells were digested by trypsin and replanted into 96‐well plates at a concentration of 5 × 103 cells/100 µL followed by 24‐hour culture. Then, different concentrations of cisplatin (0, 1.25, 2.5, 5, 10, 20 µg/mL) were added into each well and incubated for 24 hours followed by addition of CCK8 reagent and incubation at 37°C. At the end time point, the optical density value at a wavelength of 490 nm was measured by a microplate reader (iMarkTM, Bio‐Rad, USA). The viability in the cells without cisplatin treatment was set as 1.0, and this value was used to calculate the relative viability in cells treated with different concentrations of cisplatin. The GraphPad Prism software can easily fit a dose‐response curve to determine the IC50 (GraphPad Software, USA).
2.4. Apoptosis analysis
A total of 1 × 106 cells were cultured overnight and collected by trypsin digestion. The cells were then washed with PBS followed by addition of Annexin V‐FITC and propidium iodide (PI) double staining and subsequent incubation at room temperature under dark for 15 minutes according to the manufacturer's protocol. Then, cell apoptosis was detected by a flow cytometer (BD Biosciences, USA).23
2.5. Western blot
Cells were washed with pre‐ice PBS and lysed with RIPA buffer for 30 minutes followed by centrifugation to collect the supernatant. The concentration of proteins was measured using the BCA protein assay kit. Proteins were separated by SDS‐PAGE at 100 V for 2 hours and wet transferred to the PVDF membranes at 350 mA for 1.5 hours. Then, the membrane was blocked with 5% skimmed milk at room temperature for 1 hour followed by incubation with primary antibodies (anti‐FOXO3a antibody, 1:2500; anti‐PUMA antibody, 1 µg/mL, and anti‐β‐actin antibody, 1:200) overnight at 4°C. After that, the membrane was washed with TBST three times and incubated with HRP‐conjugated second antibody (bs‐0295G‐HRP, 1:3000; Bioss, China) at room temperature for 2 hours. At last, the ECL reagent was added to visualize the proteins and band densities were determined by the ImageQuant TL software (GE Healthcare, USA).24 Every experiment was performed in triplicate.
2.6. Cell transfection
The cell transfection was conducted as previously described.25 Briefly, SKOV3/DPP cells were seeded into 24‐well plates. After 24‐h culture, the CMV‐MCS‐EGFP‐SV40‐FOXO3a plasmid or CMV‐MCS‐EGFP‐SV40 vector was transfected into cells using lipofectamine 2000 based on the manufacturer's protocol. Two days after transfection, the cells were examined under a fluorescence microscope (Leica, USA) and the overexpressed FOXO3a was confirmed using western blot.
2.7. Hoechst staining
We performed the Hoechst staining as previously stated.26 The SKOV3/DPP cells were seeded on glass coverslips (0.5 × 106 cells/well) for 24 hours. Cells were then treated with cisplatin at the concentration of 5 µg/mL for 24 hours. Cells were fixed by 4% paraformaldehyde at room temperature for 10 minutes. Cells were then incubated in 1 μg/mL of Hoechst 33342 (Thermo Fisher Scientific, USA) for 1 hour, followed by washing twice with PBS. The coverslips were mounted by Fluoromount media (SouthernBiotech, USA) and examined using a fluorescent microscope (BX60; Olympus Optical Co., Tokyo, Japan).
2.8. Statistics analysis
All the experiments were repeated three times and data were processed by SPSS 20.0 software. The data were expressed as the mean ± standard deviation (SD).27 Student’s t test was performed for comparison of the differences between two independent groups and one‐way ANOVA was for the comparison of the differences between multiple groups. P < 0.05 was considered statistically significant.
3. RESULTS
3.1. The effects of cisplatin on SKOV3/DDP cells
To evaluate the toxicity of cisplatin on SKOV3/DDP cells, the CCK8 assay was performed. We found that, in all groups, the cisplatin affected the cell viability in a concentration‐dependent manner, with the higher concentration of cisplatin, the greater toxicity on SKOV3/DDP cells (Figure 1A). Furthermore, compared with the control group and the LDR group, the cell viability of SKOV3/DDP cells was much lower in the LDFRT group. Besides, the IC50 of cisplatin was much lower in the LDFRT group compared with the control group and LDR group (Figure 1B). Consistently, the cell percentage of cisplatin‐induced apoptotic SKOV3/DDP cells was much higher in the LDFRT group when comparing to the control group and LDR group (Figure 1C,D). Notably, similar results were obtained in the LDFRT and CR groups regarding the effects of cisplatin on SKOV3/DDP cells.
Figure 1.
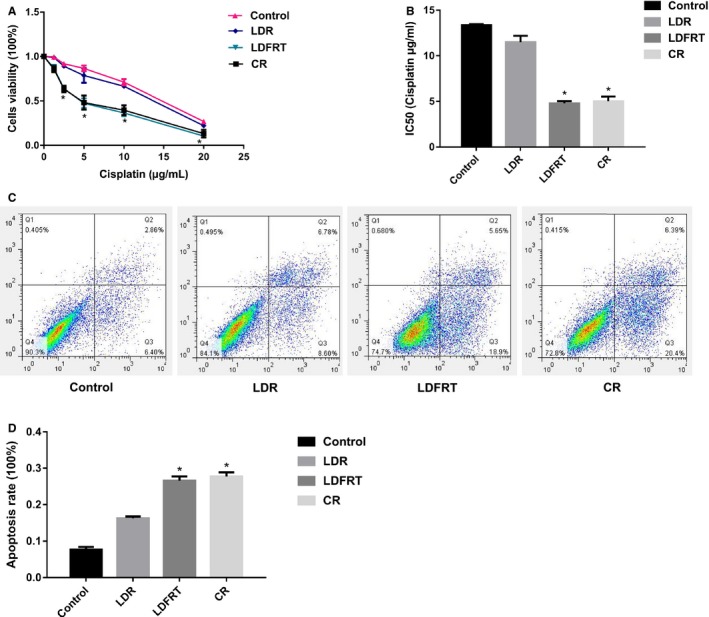
The effects of cisplatin on SKOV3/DDP cells. A, The toxicity of cisplatin on SKOV3/DDP cells. B, IC50 of cisplatin. C, Representative image showing the effects of cisplatin on the cell apoptosis of SKOV3/DDP cells. D, Quantification of the effects of cisplatin on the cell apoptosis of SKOV3/DDP cells. LDR, low‐dose radiation group; LDFRT, low‐dose fraction radiation group; CR, conventional group. Data were expressed as the mean ± SD. One‐way ANOVA was used for comparison of the differences between multiple groups. *P < 0.05 (n = 3) vs control group
3.2. The expression of FOXO3a and relative proteins
After 24‐h pre‐radiation, the cells in the control, LDR, and CR groups had a lower expression of FOXO3a (Figure 2). However, LDFRT upregulated the expression of FOXO3a and its downstream target PUMA.
Figure 2.
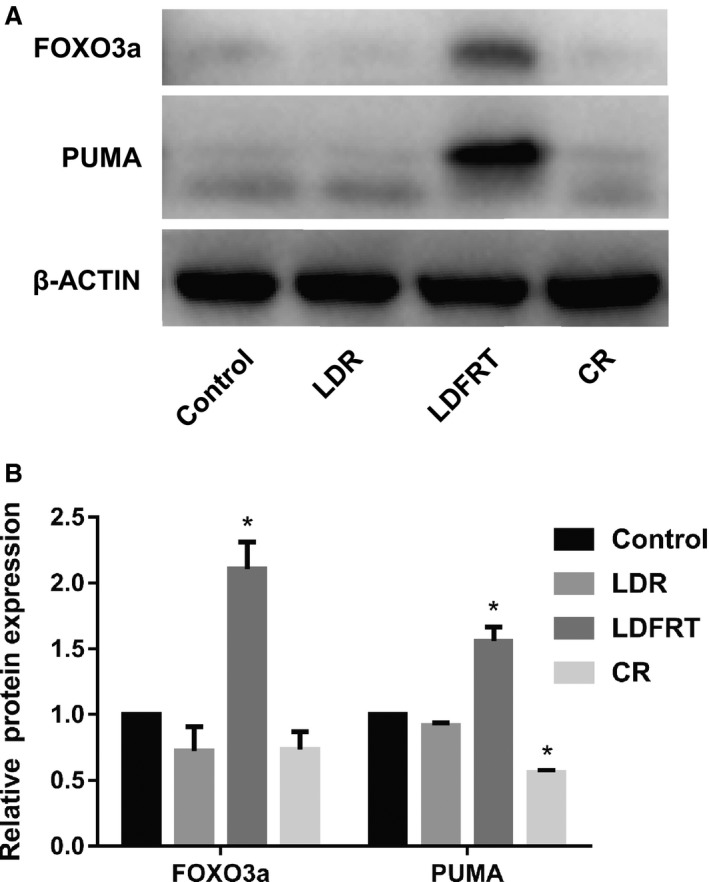
Western blot analysis of the expression of FOXO3a and PUMA. A, Representative western blot. B, Quantification of FOXO3a and PUMA protein levels. After 24‐h pre‐radiation, the expression of FOXO3a and PUMA was examined. Data were expressed as the mean ± SD. Student’s t test was performed for comparison of the differences between two independent groups. *P < 0.05 (n = 3) vs control group
3.3. Overexpression of FOXO3a enhances the toxicity of cisplatin on SKOV3/DDP cells
To evaluate the role of FOXO3a in reversing cisplatin resistance, we transfected the SKOV3/DDP cells with the CMV‐MCS‐EGFP‐SV40‐FOXO3a plasmid to overexpress FOXO3a. As shown in Figure 3A,B, a significantly increased expression of FOXO3a and its downstream target PUMA were observed in SKOV3/DDP cells after transfection compared with the control group, indicating the successful transfection. We next assessed the effect of FOXO3a overexpression on cisplatin toxicity and apoptosis of SKOV3/DDP cells and found that overexpression FOXO3a increased the toxic effect of cisplatin (Figure 3C, D) and cisplatin‐induced cell apoptosis (Figure 3E) on SKOV3/DDP cells.
Figure 3.
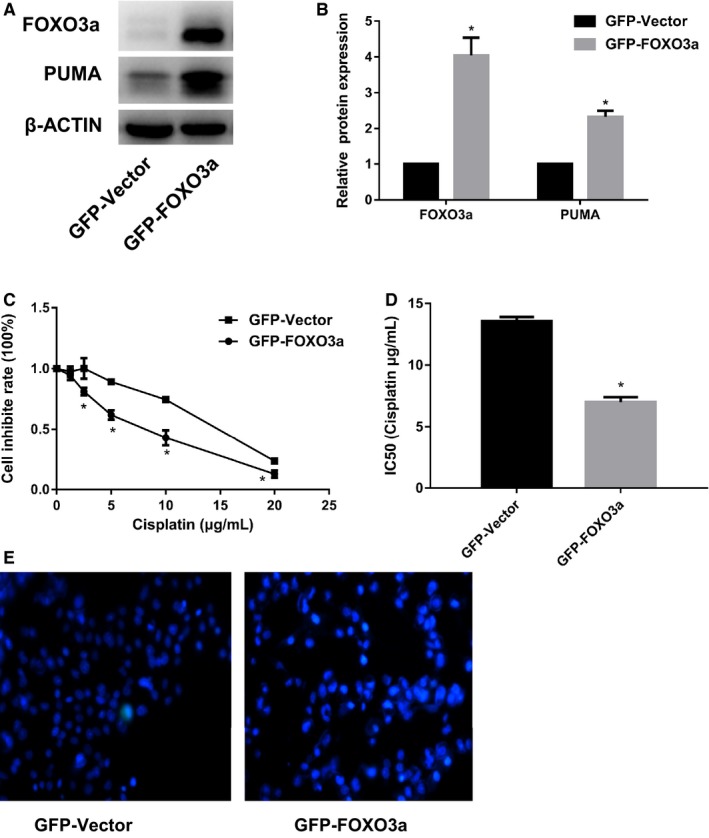
Effects of overexpression of FOXO3a on cisplatin‐induced toxicity and apoptosis of SKOV3/DDP cells. A, Representative western blot. The CMV‐MCS‐EGFP‐SV40‐FOXO3a plasmid was transfected into SKOV3/DDP cell followed by western blot analysis of the expression of FOXO3a and PUMA. B, Quantification of FOXO3a and PUMA protein levels. C, The toxicity of cisplatin on SKOV3/DDP cells. D, IC50 of cisplatin. E, Hoechst 33342 showing the cell apoptosis. After FOXO3a was overexpressed, cisplatin‐induced cell toxicity and cell apoptosis were measured by CCK8 assay and Hoechst33342 staining, respectively. Data were expressed as the mean ± SD. Student’s t test was performed for comparison of the differences between two independent groups. *P < 0.05 (n = 3) vs control group
4. DISCUSSION
According to the USA cancer statistics, ovarian cancer is a high‐malignant female reproductive system tumor.28 There are no signs and symptoms in the early stages of ovarian cancer patients and some patients are already in the advanced stage at the time of being diagnosed.1 The standard therapeutic approach is cisplatin combined with surgical debulking for the advanced stage cancer.28 However, after initial treatment, cisplatin resistance is observed in some patients, which severely affects the treatment efficacy and outcome, leading to a lower 5‐year survival rate (<30%).29, 30 Therefore, discovering alternative approaches to overcome cisplatin resistance is extremely urgent.
Studies have shown that LDFRT can act as a chemosensitizer to reverse chemotherapy resistance,11, 31 as LDR can enhance immunity capacity, promote normal cells growth but inhibit cancer cells growth and induce radiation hypersensitivity.6, 7, 8, 32, 33, 34 Consistently, our study found that LDFRT enhanced the toxicity of cisplatin on SKOV3/DDP cells as well as cisplatin‐induced apoptosis of SKOV3/DDP cells.
As a tumor suppressor, FOXO3a in the nucleus is a conservative transcription factor that belongs to forkhead transcription factors and plays important roles in the regulation of cell differentiation, apoptosis, longevity, and metabolism.35, 36 The activity of FOXO3a depends on two patterns, posttranscription modification and subcellular localization. Phosphorylation is not only one of the posttranscriptions but also modifies the cellular localization of FOXO3a; the inactivity of FOXO3a was affected by phosphorylation of different sites.39 Although originally identified as a p53 downstream target,40, 41 PUMA expression was also regulated by FOXO3a in response to cytokine or growth factor deprivation. PUMA deficiency is known to protect cells from genotoxic stress that causes activation of p53. Additionally, cells lacking PUMA are also resistant to several p53‐independent death stimuli.42 Over the past three decades, studies have proved that many drug resistance cancer cells have a low expression of FOXO3a, which is a poor predictive factor. However, upregulation of FOXO3a can reverse cisplatin resistance.38, 43, 44 Consistent with these, in the present study, we found significantly lower expression of FOXO3a as well as its downstream target PUMA in SKOV3/DDP cells. However, LDFRT treatment significantly increased FOXO3a expression in SKOV3/DDP cells and reversed cisplatin resistance. In addition, overexpression of FOXO3a through transfection of the plasmid into SKOV3/DDP cells could also reverse cisplatin resistance together with increased expression of PUMA (Figure 4). Notably, PUMA expression is visible in the cells transfected with control vector, possibly due to the high basal level of PUMA in SKOV3/DDP cells.
Figure 4.
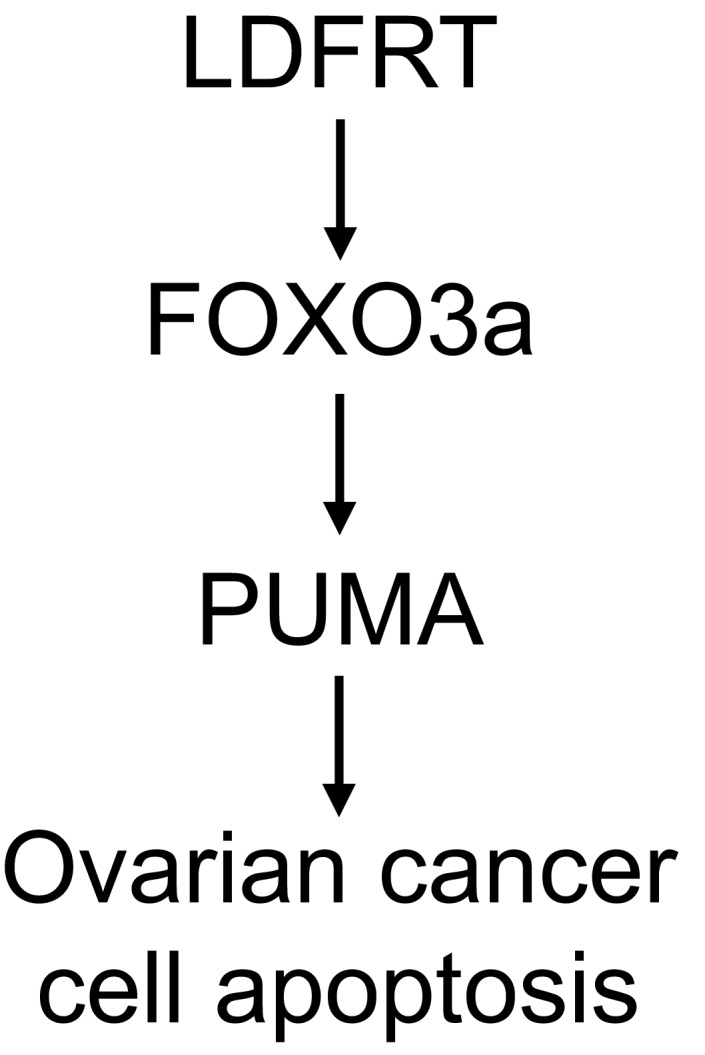
Diagram depicting the main players
In conclusion, our study shows that LDFRT promotes the expression of FOXO3a and its downstream target PUMA, as well as reverses cisplatin resistance, suggesting that low‐dose fraction radiation may be served as an effective complementary adjuvant radiotherapy in the treatment of ovarian cancer.
CONFLICT OF INTEREST
All the authors have no conflict of interests to declare.
ACKNOWLEDGMENTS
This work was supported by Natural Science Foundation (No. ZR2015HM025), China and Technology Development Program of Shandong Province (No. 2011YD18003), China.
Zhao L, Liu S, Liang D, et al. Resensitization of cisplatin resistance ovarian cancer cells to cisplatin through pretreatment with low‐dose fraction radiation. Cancer Med. 2019;8:2442–2448. 10.1002/cam4.2116
REFERENCES
- 1. Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376‐1388. [DOI] [PubMed] [Google Scholar]
- 2. Piver MS, Barlow JJ, Lele SB. Incidence of subclinical metastasis in stage I and II ovarian carcinoma. Obstet Gynecol. 1978;52(1):100‐104. [PubMed] [Google Scholar]
- 3. Fields EC, McGuire WP, Lin L, Temkin SM. Radiation treatment in women with ovarian cancer: past, present, and future. Front Oncol. 2017;7:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dembo AJ. Epithelial ovarian cancer: the role of radiotherapy. Int J Radiat Oncol Biol Phys. 1992;22(5):835‐845. [DOI] [PubMed] [Google Scholar]
- 5. Rai B, Bansal A, Patel FD, Sharma SC. Radiotherapy for ovarian cancers ‐ redefining the role. Asian Pac J Cancer Prev. 2014;15(12):4759‐4763. [DOI] [PubMed] [Google Scholar]
- 6. Jiang H, Li W, Li X, Cai L, Wang G. Low‐dose radiation induces adaptive response in normal cells, but not in tumor cells: in vitro and in vivo studies. J Radiat Res. 2008;49(3):219‐230. [DOI] [PubMed] [Google Scholar]
- 7. Jiang H, Xu Y, Li W, Ma K, Cai L, Wang G. Low‐dose radiation does not induce proliferation in tumor cellsin vitroand in vivo. Radiat Res. 2008;170(4):477‐487. [DOI] [PubMed] [Google Scholar]
- 8. Farooque A, Mathur R, Verma A, et al. Low‐dose radiation therapy of cancer: role of immune enhancement. Expert Rev Anticancer Ther. 2011;11(5):791‐802. [DOI] [PubMed] [Google Scholar]
- 9. Larson SM, Carrasquillo JA, Cheung NK, Press OW. Radioimmunotherapy of human tumours. Nat Rev Cancer. 2015;15(6):347‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oseni S, Kumi‐Diaka J, Branly R, Jebelli J, Warrick J, Goldsmith H. Pyroelectrically generated very low dose ionizing radiation enhances chemopreventive and chemotherapeutic effects of genistein isoflavone in human prostate cancer cells. J Cancer Prev Curr Res. 2014;1(2):00010. [Google Scholar]
- 11. Kunos CA, Sill MW, Buekers TE, et al. Low‐dose abdominal radiation as a docetaxel chemosensitizer for recurrent epithelial ovarian cancer: a phase I study of the Gynecologic Oncology Group. Gynecol Oncol. 2011;120(2):224‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Gan B, Liu D, Paik J‐H. FoxO family members in cancer. Cancer Biol Ther.. 2014;12(4):253‐259. [DOI] [PubMed] [Google Scholar]
- 13. Shiota M, Yokomizo A, Kashiwagi E, et al. Foxo3a expression and acetylation regulate cancer cell growth and sensitivity to cisplatin. Cancer Sci. 2010;101(5):1177‐1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fei M, Zhao Y, Wang Y, et al. Low expression of Foxo3a is associated with poor prognosis in ovarian cancer patients. Cancer Invest. 2009;27(1):52‐59. [DOI] [PubMed] [Google Scholar]
- 15. Hornsveld M, Dansen TB, Derksen PW, Burgering B. Re‐evaluating the role of FOXOs in cancer. Semin Cancer Biol. 2018;50:90‐100. [DOI] [PubMed] [Google Scholar]
- 16. Chung YM, Park S‐H, Tsai W‐B, et al. FOXO3 signalling links ATM to the p53 apoptotic pathway following DNA damage. Nat Commun. 2012;3:1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qiu W, Carson‐Walter EB, Liu H, et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell. 2008;2(6):576‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu H, Shen H, Yuan Y, et al. Deletion of Puma protects hematopoietic stem cells and confers long‐term survival in response to high‐dose gamma‐irradiation. Blood. 2010;115(17):3472‐3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu J, Wu X, Liu H, et al. Expression of microRNA‐30a‐5p in drug‐resistant and drug‐sensitive ovarian cancer cell lines. Oncol Lett. 2016;12(3):2065‐2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen L, Sun B, Sheng J, et al. PGC1alpha promotes cisplatin resistance in human ovarian carcinoma cells through upregulation of mitochondrial biogenesis. Int J Oncol. 2018;53(1):404‐416. [DOI] [PubMed] [Google Scholar]
- 21. Prasanna A, Ahmed MM, Mohiuddin M, Coleman CN. Exploiting sensitization windows of opportunity in hyper and hypo‐fractionated radiation therapy. J Thorac Dis. 2014;6(4):287‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo J, Chen Li, Luo N, et al. LPS/TLR4‐mediated stromal cells acquire an invasive phenotype and are implicated in the pathogenesis of adenomyosis. Sci Rep. 2016;6:21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ju X, Yu H, Liang D, et al. LDR reverses DDP resistance in ovarian cancer cells by affecting ERCC‐1, Bcl‐2, Survivin and Caspase‐3 expressions. Biomed Pharmacother. 2018;102:549‐554. [DOI] [PubMed] [Google Scholar]
- 24. Li S, Wang J, Wei Y, et al. Crucial role of TRPC6 in maintaining the stability of HIF‐1alpha in glioma cells under hypoxia. J Cell Sci. 2015;128(17):3317‐3329. [DOI] [PubMed] [Google Scholar]
- 25. Yang L, Ma L, Lai D. Over‐expression of fibroblast activation protein alpha increases tumor growth in xenografts of ovarian cancer cells. Acta Biochim Biophys Sin (Shanghai). 2013;45(11):928‐937. [DOI] [PubMed] [Google Scholar]
- 26. Paramee S, Sookkhee S, Sakonwasun C, et al. Anti‐cancer effects of Kaempferia parviflora on ovarian cancer SKOV3 cells. BMC Complement Altern Med. 2018;18(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barde MP, Barde PJ. What to use to express the variability of data: Standard deviation or standard error of mean? Perspect Clin Res. 2012;3(3):113‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 29. Coleman RL, Monk BJ, Sood AK, Herzog TJ. Latest research and treatment of advanced‐stage epithelial ovarian cancer. Nat Rev Clin Oncol. 2013;10(4):211‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cornelison R, Llaneza DC, Landen CN. Emerging therapeutics to overcome chemoresistance in epithelial ovarian cancer: a mini‐review. Int J Mol Sci. 2017;18(10):2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gleason JF Jr, Kudrimoti M, Van Meter EM, et al. Low‐dose fractionated radiation with induction chemotherapy for locally advanced head and neck cancer: 5 year results of a prospective phase II trial. J Radiat Oncol. 2013;2(1):35‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pollycove M, Feinendegen LE. Low‐dose radioimmuno‐therapy of cancer. Hum Exp Toxicol. 2008;27(2):169‐175. [DOI] [PubMed] [Google Scholar]
- 33. Dai X, Tao D, Wu H, Cheng J. Low dose hyper‐radiosensitivity in human lung cancer cell line A549 and its possible mechanisms. J Huazhong Univ Sci Technolog Med Sci. 2009;29(1):101‐106. [DOI] [PubMed] [Google Scholar]
- 34. Harney J, Short SC, Shah N, Joiner M, Saunders MI. Low dose hyper‐radiosensitivity in metastatic tumors. Int J Radiat Oncol Biol Phys. 2004;59(4):1190‐1195. [DOI] [PubMed] [Google Scholar]
- 35. Martins R, Lithgow GJ, Link W. Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell. 2016;15(2):196‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murtaza G, Khan AK, Rashid R, Muneer S, Hasan S, Chen J. Transcriptional factors and long‐term. Living. Oxid Med Cell Longev. 2017;2017:3494289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Farhan M, Wang HT, Gaur U, Little PJ, Xu JP, Zheng WH. FOXO signaling pathways as therapeutic targets in cancer. Int J Biol Sci. 2017;13(7):815‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ning Y, Luo C, Ren K, Quan M, Cao J. FOXO3a‐mediated suppression of the self‐renewal capacity of sphere‐forming cells derived from the ovarian cancer SKOV3 cell line by 7‐difluoromethoxyl‐5,4'‐di‐n‐octyl genistein. Mol Med Rep. 2014;9(5):1982‐1988. [DOI] [PubMed] [Google Scholar]
- 39. Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479‐2487. [DOI] [PubMed] [Google Scholar]
- 40. Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7(3):673‐682. [DOI] [PubMed] [Google Scholar]
- 41. Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7(3):683‐694. [DOI] [PubMed] [Google Scholar]
- 42. You H, Pellegrini M, Tsuchihara K, et al. FOXO3a‐dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203(7):1657‐1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang J‐Y, Chang C‐J, Xia W, et al. Activation of FOXO3a is sufficient to reverse mitogen‐activated protein/extracellular signal‐regulated kinase kinase inhibitor chemoresistance in human cancer. Cancer Res. 2010;70(11):4709‐4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sunters A, Fernández de Mattos S, Stahl M, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel‐treated breast cancer cell lines. J Biol Chem. 2003;278(50):49795‐49805. [DOI] [PubMed] [Google Scholar]
- 45. Arimoto‐Ishida E, Ohmichi M, Mabuchi S, et al. Inhibition of phosphorylation of a forkhead transcription factor sensitizes human ovarian cancer cells to cisplatin. Endocrinology. 2004;145(4):2014‐2022. [DOI] [PubMed] [Google Scholar]
- 46. Lu M, Chen X, Xiao J, Xiang J, Yang L, Chen D. FOXO3a reverses the cisplatin resistance in ovarian cancer. Arch Med Res. 2018;49(2):84‐88. [DOI] [PubMed] [Google Scholar]


