Abstract
Aim
To assess the efficacy of oral NEPA (netupitant‐palonosetron 300/0.50 mg) over multiple chemotherapy cycles.
Methods
Two randomized phase III studies evaluated a single dose of oral NEPA given on day 1 in chemotherapy‐naive patients receiving anthracycline‐cyclophosphamide (AC)–based (Study 1) or highly (HEC)/moderately (MEC) emetogenic chemotherapy (safety Study 2). Oral NEPA was compared with oral palonosetron 0.50 mg (Study 1) or oral aprepitant 125 mg day 1, 80 mg days 2‐3/palonosetron 0.50 mg (Study 2; no formal statistical comparisons). Oral dexamethasone was administered in all treatment groups. Complete response (CR; no emesis/no rescue medication), no emesis, and no significant nausea (NSN) rates during acute (0‐24 h) and delayed (>24‐120 h) phases of chemotherapy cycles 1‐4 in each study were evaluated.
Results
In Study 1, 1450 patients received 5969 chemotherapy cycles; in Study 2, 412 patients received 1961 chemotherapy cycles. In each study, ≥75% of patients completed 4 or more cycles. In Study 1, oral NEPA was superior to palonosetron in preventing chemotherapy‐induced nausea and vomiting (CINV) in the acute and delayed phases of cycle 1, with higher rates of CR (all P < 0.05), no emesis (all P < 0.05), and NSN (delayed phase P < 0.05 cycles 1, 2, and 4) reported across 4 cycles. In Study 2, oral NEPA had numerically higher CR and NSN rates in the acute and delayed phases than aprepitant‐palonosetron in MEC/HEC patients.
Conclusion
Oral NEPA was highly effective in preventing both acute and delayed CINV over multiple chemotherapy cycles of HEC, AC, and MEC regimens.
Clinical trial registration numbers
Study 1, NCT01339260; Study 2, NCT01376297.
Keywords: CINV, delayed phase, efficacy, multiple cycles, NEPA, netupitant
1. INTRODUCTION
Chemotherapy‐induced nausea and vomiting (CINV) is a debilitating complication and one of multiple adverse events frequently reported in patients receiving routine chemotherapy. If poorly controlled, CINV impairs quality of life1 and may compromise anticancer treatment adherence (reviewed in Hesketh2). Nausea and vomiting belong to a cluster of symptoms that if correctly managed, may lead to longer survival.3, 4 CINV is primarily mediated through neurotransmitters, such as serotonin, substance P, and dopamine,5 and can be categorized into acute (0‐24 h) and delayed phases (>24‐ 120 h).2 Acute CINV results mainly from serotonin's action on the 5‐hydroxytryptamine‐3 (5‐HT3) receptor, while delayed CINV is mainly mediated by substance P acting on the neurokinin‐1 (NK1) receptor.6
Delayed CINV occurs more frequently than acute CINV,1, 7 and is experienced by over 50% of patients receiving chemotherapy, despite antiemetic prophylaxis use.1, 7 It tends to be underreported by patients, and the incidence is underestimated by most oncology physicians and nurses.7, 8, 9
For effective antiemetic prophylaxis, control throughout the entire period of emetic risk in the first and subsequent chemotherapy cycles is necessary.10 If CINV is inadequately controlled in the first cycle, it may become more difficult to manage,11 with a 14% increased risk of CINV in subsequent cycles.12 Controlling CINV over multiple cycles with an NK1 receptor antagonist (RA) has been shown to reduce resource utilization, particularly by preventing delayed CINV.13, 14 Prevention of CINV from the first cycle therefore remains the main goal for successful CINV control.
Current antiemetic guidelines of the National Comprehensive Cancer Network (NCCN)15 and the Multinational Association of Supportive Care in Cancer (MASCC)/European Society for Medical Oncology (ESMO)16 recommend the triplet combination of an NK1 RA, a 5‐HT3 RA, and dexamethasone (DEX) to prevent CINV in patients receiving highly emetogenic chemotherapy (HEC).15, 16, 17 The American Society of Clinical Oncology (ASCO) and NCCN endorse the addition of olanzapine to this triplet in patients receiving HEC and before the start of chemotherapy, with additional prophylaxis (DEX and olanzapine) on days 2‐4 of chemotherapy; NK1 RA aprepitant (APR) is part of the prophylaxis on days 2‐3 if given on day 1.15, 17 For patients receiving moderately emetogenic chemotherapy (MEC), a 5‐HT3 RA in combination with DEX on day 1 is generally advised,15, 16, 17 with the addition of an NK1 RA for certain patients with additional risk factors or previous treatment failure when receiving a steroid plus 5‐HT3 RA alone15; additional prophylaxis may be offered on days 2 and 3 of chemotherapy if necessary.15, 17 MASCC/ESMO first introduced a new recommendation for adding an NK1 RA to the 5‐HT3 RA plus DEX regimen for patients receiving carboplatin, regardless of dose.16 The NCCN and ASCO guidelines now also make the same recommendation for carboplatin area under the curve (AUC) ≥4 mg mL−1 minied carboplatin.15, 17 Only the NCCN guidelines have reclassified carboplatin AUC ≥4 mg mL−1 min from a MEC to a HEC agent.15
Oral NEPA is the first and only antiemetic combination agent; it is composed of netupitant (300 mg), a highly selective NK1 RA, and the pharmacologically18 and clinically19 distinct 5‐HT3 RA palonosetron (PALO, 0.50 mg). NEPA thereby antagonizes 2 key neurotransmitters involved in the pathophysiology of CINV, and provides acute and delayed CINV control with a single dose. Real‐world evidence suggests complicated antiemetic schedules are often not followed by patients, leading to mistakes/missed doses of prophylactic agents prescribed to be taken over multiple days.5 The convenient administration schedule of oral NEPA may therefore enable improved adherence to the antiemetic regimen and guidelines. Oral NEPA has been shown to be superior to oral PALO in preventing CINV during the acute, delayed, and overall phases following the first cycle of cisplatin‐based20 or anthracycline‐cyclophosphamide (AC)‐based chemotherapy.21 Oral NEPA is well tolerated, with a safety profile consistent with the NK1 RA and 5‐HT3 RA classes.20, 21 An intravenous (IV) formulation of the NEPA fixed combination has been developed to offer clinicians and patients further convenience, and IV NEPA plus DEX was recently approved by the US Food and Drug Administration for the prevention of CINV in patients receiving HEC, with a limitation of use in AC‐based chemotherapy.22 A phase IIIb study evaluating the safety of IV NEPA in patients receiving AC‐based chemotherapy is ongoing.
Two phase III studies, evaluating the efficacy and safety of oral NEPA over multiple cycles of chemotherapy, form the basis of this report.21, 23, 24 In both studies, previously published data demonstrated that oral NEPA maintained antiemetic control during the 5‐day period following chemotherapy (overall phase) over at least 4 cycles of chemotherapy.23, 24 Oral NEPA also showed superior complete response (CR) rates compared with oral PALO during the overall phase in cycle 1, and the difference was statistically significant over repeated cycles.23 At the time both trials were conducted, MEC included AC‐based regimens in patients with breast cancer (referred to herein as AC MEC). However, AC‐based chemotherapy has since been reclassified as HEC.15, 16, 17 For consistency with the original publications of each trial, patients are referred to as receiving AC MEC in Study 121 and HEC or non‐AC MEC in Study 2 (breast cancer patients scheduled to receive AC‐based chemotherapy in Study 2 were not eligible).24 This report focuses on the efficacy of oral NEPA during the acute and delayed phases over 4 cycles of chemotherapy in these studies.
2. METHODS
2.1. Studies
Two international, randomized, double‐blind phase III trials, Study 1 (NCT01339260)21 and Study 2 (NCT01376297),24 were analyzed. Both evaluated the efficacy of oral NEPA in patients with solid tumors, including patients diagnosed with any malignant tumor in Study 2. Detailed study designs, methods, and eligibility criteria have been reported previously and are summarized in Figure 1.21, 24 Oral DEX was open‐label and the dosing schedule was based on the emetogenicity of the chemotherapy according to the antiemetic guidelines valid at the time the studies were performed.25 Both study protocols were approved by the relevant ethical review committees, all patients provided written informed consent, and all investigators and site personnel followed International Conference on Harmonization E6 Good Clinical Practice guidelines, Declaration of Helsinki (2008) ethical principles, and local laws and regulations.
Figure 1.
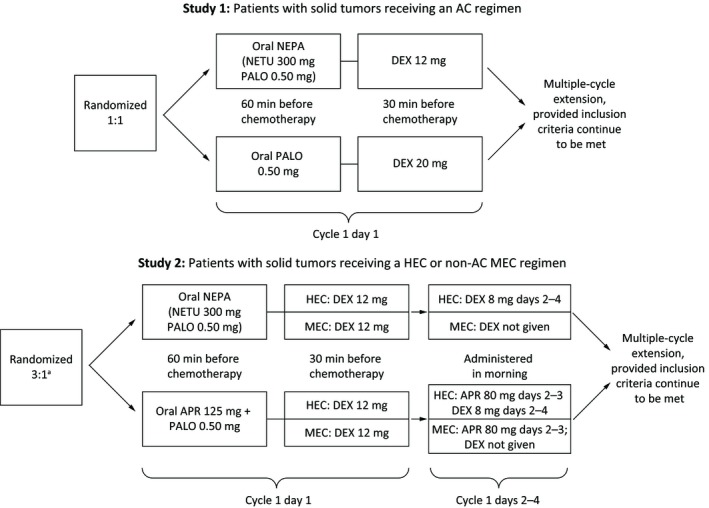
Schemas of 2 international, randomized, double‐blind phase III trials: Study 1 (NCT01339260)21 and Study 2 (NCT01376297).24 a3:1 randomization NEPA: APR + PALO; the protocol also specified that 75% of patients would receive MEC and 25% HEC. AC, anthracycline‐ cyclophosphamide; APR, aprepitant; DEX, dexamethasone; HEC, highly emetogenic chemotherapy; MEC, moderately emetogenic chemotherapy; NEPA, netupitant‐palonosetron; NETU, netupitant; PALO, palonosetron
2.2. Assessments
CR (defined as no emesis and no rescue medication) and no significant nausea (NSN; defined as a score of <25 mm on a visual analog scale of 100 mm) were assessed during the acute and delayed phases after chemotherapy initiation, for the first 4 cycles; “no emesis” was also collected in Study 1.
2.3. Statistical considerations
Efficacy data were not pooled across the 2 studies because of different study objectives, designs, chemotherapy regimens, and populations. All efficacy analyses were undertaken on the full analysis set (defined as all randomized patients who received chemotherapy and study drug). In Study 1, CR, no emesis, and NSN rates for the oral NEPA and oral PALO arms were compared using a 2‐sided Cochran‐Mantel‐Haenszel test stratified by age class (<55 years, ≥55 years) and region (US, Latin America, Europe, Commonwealth of Independent States, and Asia) for all 4 cycles; this test was the same used at cycle 1 for the primary and key secondary efficacy analyses as per prespecified study plan. For cycles 2‐4, a formal statistical comparison was not prespecified; no method to adjust for multiplicity was applied. No formal comparisons of efficacy were made between the oral NEPA and oral APR‐PALO arms in Study 2, as its primary endpoint was safety. CR and NSN rates are also reported separately for the subsets of NEPA patients receiving either MEC or HEC; APR‐PALO data are not included for these chemotherapy subsets since the small sample size (considering the 3:1 randomization ratio) hindered interpretation, especially for repeated cycles. As most patients completed their planned therapy after 4 treatment cycles, efficacy data are presented over only cycles 1‐4 (safety data have been previously published21).
3. RESULTS
3.1. Analyzed patient population
In total, 1455 patients were randomized in Study 1: 726 to oral NEPA and 729 to oral PALO (Table 1). Of these, 1450 patients (99.7%) were treated for a total of 5969 chemotherapy cycles; 1438 patients (98.8%) completed cycle 1, and 1286 patients (88.4%) entered the multiple‐cycle extension 23; 1107 patients (76.1%) completed 4 or more cycles. Most patients completed their planned chemotherapy after 4 treatment cycles; 35.7% of patients received a fifth cycle, and 26.7% received a sixth cycle (data not shown).
Table 1.
Summary of patient disposition from Study 1 and Study 2—all randomized patients
| Study 1a | Study 2b | |||||
|---|---|---|---|---|---|---|
|
NEPA (N = 726) (C = 2983) |
PALO (N = 729) (C = 2986) |
Overall (N = 1455) (C = 5969) |
NEPA (N = 309) (C = 1446) |
APR‐PALO (N = 104) (C = 515) |
Overall (N = 413) (C = 1961) |
|
| Treated, n (%) | 724 (99.7) | 726 (99.6) | 1450 (99.7) | 309 (100.0) | 103 (99.0) | 412 (99.8) |
| Completed cycle 1, n (%) | 719 (99.0) | 719 (98.6) | 1438 (98.8) | 303 (98.1) | 102 (98.1) | 405 (98.1) |
| Completed cycle 2, n (%) | 630 (86.8) | 645 (88.5) | 1275 (87.6) | 278 (90.0) | 94 (90.4) | 372 (90.1) |
| Completed cycle 3, n (%) | 596 (82.1) | 603 (82.7) | 1199 (82.4) | 255 (82.5) | 88 (84.6) | 343 (83.1) |
| Completed cycle 4, n (%) | 548 (75.5) | 559 (76.7) | 1107 (76.1) | 230 (74.4) | 81 (77.9) | 311 (75.3) |
C = Total number of cycles started for all treated patients.
APR, aprepitant; NEPA, netupitant‐palonosetron; PALO, palonosetron.
Data corresponding to cycles 5‐8 not shown.
Data corresponding to cycles 5‐14 not shown.
In Study 2, 413 patients were randomized: 309 to oral NEPA and 104 to oral APR‐PALO (Table 1). Of these, 412 patients (99.8%) were treated for a total of 1961 chemotherapy cycles; 405 patients (98.1%) completed cycle 1, 311 patients (75.3%) completed 4 or more cycles; 51.6% of patients received a fifth cycle, and 40.0% received a sixth cycle (data not shown).
Baseline and disease characteristics from both studies are reported in Table 2. These characteristics remained consistent across cycles, and were similar between treatment arms. In Study 1, the median age was 54 years, 98.1% of patients were female, and 97.4% had breast cancer. In Study 2, the median age was 58 years, 50% of patients were female, and the most prevalent cancer was lung/respiratory cancer (37.4%). Per protocol, most patients (75.7%) received MEC (Table 3).
Table 2.
Baseline and disease characteristics of patients from Study 1 and Study 2—safety population (cycle 1)
| Study 1 | Study 2 | |||||
|---|---|---|---|---|---|---|
|
NEPA (N = 725) |
PALO (N = 725) |
Overall (N = 1450) |
NEPA (N = 308) |
APR‐PALO (N = 104) |
Overall (N = 412) |
|
| Gender, % | ||||||
| Male | 1.9 | 1.9 | 1.9 | 49.7 | 51.0 | 50.0 |
| Female | 98.1 | 98.1 | 98.1 | 50.3 | 49.0 | 50.0 |
| Median age, years | 54.0 | 54.0 | 54.0 | 57.0 | 58.5 | 58.0 |
| Cancer type, % | ||||||
| Breast | 97.7 | 97.2 | 97.4 | 12.7 | 8.7 | 11.7 |
| Lung/respiratory | — | — | — | 39.6 | 30.8 | 37.4 |
| Ovarian | — | — | — | 10.7 | 17.3 | 12.4 |
| Head and neck | — | — | — | 6.5 | 10.6 | 7.5 |
| Colorectal | — | — | — | 16.2 | 22.1 | 17.7 |
| Gastric | — | — | — | 2.3 | 1.0 | 1.9 |
| Bladder | — | — | — | 1.3 | 2.9 | 1.7 |
| Othera | 2.3 | 2.8 | 2.6 | 16.7 | 6.7 | 9.7 |
| Extent of cancer at entry, % | ||||||
| Primary | 81.8 | 82.9 | 82.3 | 43.8 | 51.9 | 45.9 |
| Metastatic | 16.3 | 15.6 | 15.9 | 51.9 | 43.3 | 49.8 |
| Local recurrence | 1.9 | 1.5 | 1.7 | 4.2 | 4.8 | 4.4 |
| Site of metastasis, % | ||||||
| Lymph nodes | 10.8 | 11.7 | 11.2 | 33.1 | 21.2 | 30.1 |
| Other | 5.5 | 3.2 | 4.3 | 15.6 | 19.2 | 16.5 |
| Liver | 2.9 | 2.1 | 2.5 | 12.0 | 12.5 | 12.1 |
| Bone | 3.7 | 3.6 | 3.7 | 5.8 | 4.8 | 5.6 |
| Brain | 0.3 | 0 | 0.1 | 1.6 | 2.9 | 1.9 |
| ECOG performance status, % | ||||||
| 1 | 69.5 | 69.2 | 69.4 | 47.4 | 48.1 | 47.6 |
| 2 | 29.7 | 30.6 | 30.1 | 51.0 | 50.0 | 50.7 |
| 3 | 0.8 | 0.1 | 0.5 | 1.6 | 1.9 | 1.7 |
APR, aprepitant; ECOG, Eastern Cooperative Oncology Group; NEPA, netupitant‐palonosetron; PALO, palonosetron.
The category “other” included any other type of cancer not listed in the prespecified categories, including, but not limited to, those of the uterus, larynx, and endometrium.
Table 3.
Chemotherapy received in patients from Study 1 and Study 2—safety population (cycle 1)
| Study 1 chemotherapya, % | NEPA (N = 725) | PALO (N = 725) | Overall (N = 1450) |
|---|---|---|---|
| ACb | |||
| Doxorubicin | 68.0 | 63.6 | 65.8 |
| Cyclophosphamide | 99.9 | 99.9 | 99.9 |
| Epirubicin | 32.0 | 36.3 | 34.2 |
| Study 2 chemotherapya, % | NEPA (N = 308) | APR‐PALO (N = 104) | Overall (N = 412) |
|---|---|---|---|
| MECc | 75.7 | 75.7 | 75.7 |
| Carboplatin | 60.3 | 61.5 | 60.6 |
| Oxaliplatin | 20.1 | 24.4 | 21.2 |
| Doxorubicind | 11.1 | 6.4 | 9.9 |
| Cyclophosphamided | 3.4 | 2.6 | 3.2 |
| Irinotecan | 3.0 | 3.8 | 3.2 |
| Epirubicind | 1.7 | 1.3 | 1.6 |
| Daunorubicin | 0.4 | 0 | 0.3 |
| HECc | 24.3 | 24.3 | 24.3 |
| Cisplatin | 96.0 | 92.0 | 95.0 |
| Dacarbazine | 4.0 | 4.0 | 4.0 |
| Carmustine | 0 | 4.0 | 1.0 |
AC, anthracycline‐cyclophosphamide; APR, aprepitant; HEC, highly emetogenic chemotherapy; MEC, moderately emetogenic chemotherapy; NEPA, netupitant‐palonosetron, PALO: palonosetron.
Percentages are based on efficacy (full analysis) population, while all others are based on safety population (cycle 1).
Breast cancer patients scheduled to receive AC‐based chemotherapy in Study 2 were not eligible.
Cycle 1 chemotherapy.
Cyclophosphamide and doxorubicin or epirubicin were administered together as “AC” in Study 1.
3.2. Efficacy
3.2.1. Study 1
Oral NEPA was superior to oral PALO in preventing CINV in the acute and delayed phases in cycle 1 (Table 3, Figure 2A), with high rates of control maintained in subsequent cycles. In the oral NEPA group, CR rates ranged from 88.4% to 91.6%, and 76.9% to 85.5% for the acute and delayed phases, respectively, across 4 cycles. These rates were higher and differences were statistically significant compared with the PALO group (between‐group comparisons for individual cycles, all P < 0.05 not adjusted for multiplicity). Likewise, higher rates of NSN and no emesis were observed for oral NEPA versus oral PALO (Table 4). In the oral NEPA group, the rates of no emesis across 4 cycles ranged from 90.9% to 93.1%, and 81.8% to 89.5% for the acute and delayed phases, respectively, and differences were statistically significant compared with the PALO‐treatment group (between‐group comparisons for individual cycles, all P < 0.05 not adjusted for multiplicity). In the oral NEPA group, the rates of NSN across 4 cycles ranged from 87.3% to 91.3%, and 76.9% to 81.7% for the acute and delayed phases, respectively; differences were statistically significant in the delayed‐phase NSN rates compared with the PALO group (between‐group comparisons for individual cycles, P < 0.05 for cycles 1, 2, and 4 not adjusted for multiplicity).
Figure 2.
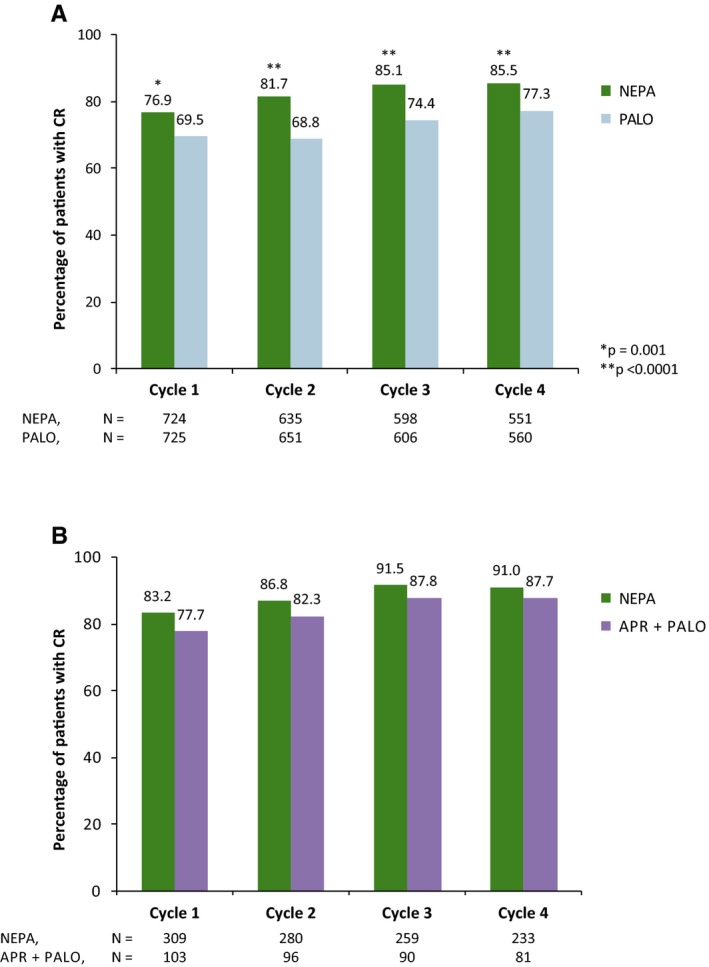
CR (no emesis, no rescue medication) rates in the delayed phase (>24‐120 h) of cycles 1‐4 in: A) Study 1 (patients receiving AC‐based chemotherapy), and B) Study 2 (patients receiving HEC or MECa). Full analysis set. aBreast cancer patients were not allowed AC‐based regimens. AC, anthracycline‐cyclophosphamide; APR, aprepitant; CR, complete response; HEC, highly emetogenic chemotherapy; MEC, moderately emetogenic chemotherapy; NEPA, netupitant‐palonosetron; PALO, palonosetron
Table 4.
CR, no emesis, and NSN rates in the acute (0‐24 h) and delayed (>24‐120 h) phase of cycles 1‐4—full analysis set
| Study 1 | Cycle (N = NEPA/PALO) | Cycle 1 (N = 724/725) | Cycle 2 (N = 635/651) | Cycle 3 (N = 598/606) | Cycle 4 (N = 551/560) | |
|---|---|---|---|---|---|---|
| CR | Acute | NEPA, % | 88.4 | 89.9 | 91.6 | 91.5 |
| PALO, % | 85.0 | 83.7 | 83.8 | 86.8 | ||
| P valuea | 0.047 | 0.001 | <0.001 | 0.011 | ||
| Delayed | NEPA, % | 76.9 | 81.7 | 85.1 | 85.5 | |
| PALO, % | 69.5 | 68.8 | 74.4 | 77.3 | ||
| P valuea | 0.001 | <0.001 | <0.001 | <0.001 | ||
| Overall | NEPA, % | 74.3 | 80.3 | 83.8 | 83.8 | |
| PALO, % | 66.6 | 66.7 | 70.3 | 74.6 | ||
| P valuea | 0.001 | <0.001 | <0.001 | <0.001 | ||
| No emesis | Acute | NEPA, % | 90.9 | 92.6 | 93.0 | 93.1 |
| PALO, % | 87.3 | 86.8 | 87.5 | 88.4 | ||
| P valuea | 0.025 | <0.001 | 0.002 | 0.006 | ||
| Delayed | NEPA, % | 81.8 | 86.3 | 89.5 | 88.8 | |
| PALO, % | 75.6 | 76.5 | 81.0 | 82.3 | ||
| P valuea | 0.004 | <0.001 | <0.001 | 0.002 | ||
| Overall | NEPA, % | 79.8 | 85.5 | 88.3 | 87.3 | |
| PALO, % | 72.1 | 73.7 | 77.2 | 79.5 | ||
| P valuea | <0.001 | <0.001 | <0.001 | <0.001 | ||
| NSNb | Acute | NEPA, % | 87.3 | 88.8 | 89.1 | 91.3 |
| PALO, % | 87.9 | 87.3 | 87.1 | 88.9 | ||
| P value | 0.747 | 0.431 | 0.297 | 0.181 | ||
| Delayed | NEPA, % | 76.9 | 79.5 | 79.8 | 81.7 | |
| PALO, % | 71.3 | 74.0 | 75.4 | 76.4 | ||
| P valuea | 0.014 | 0.017 | 0.062 | 0.025 | ||
| Overall | NEPA, % | 74.6 | 77.3 | 78.4 | 80.2 | |
| PALO, % | 69.1 | 71.6 | 73.3 | 75.2 | ||
| P valuea | 0.020 | 0.016 | 0.033 | 0.035 | ||
| Study 2 | Cycle (N = NEPA/APR + PALO) | Cycle 1 (N = 309/103) | Cycle 2 (N = 280/96) | Cycle 3 (N = 259/90) | Cycle 4 (N = 233/81) | |
|---|---|---|---|---|---|---|
| CR | Acute | NEPA, % | 92.9 | 96.4 | 96.1 | 96.6 |
| APR + PALO, % | 94.2 | 91.7 | 95.6 | 96.3 | ||
| Delayed | NEPA, % | 83.2 | 86.8 | 91.5 | 91.0 | |
| APR + PALO, % | 77.7 | 82.3 | 87.8 | 87.7 | ||
| Overall | NEPA, % | 80.6 | 86.1 | 90.7 | 90.1 | |
| APR + PALO, % | 75.7 | 81.3 | 86.7 | 87.7 | ||
| NSNb | Acute | NEPA, % | 90.6 | 95.4 | 96.1 | 97.0 |
| APR + PALO, % | 93.2 | 92.7 | 93.3 | 95.1 | ||
| Delayed | NEPA, % | 85.1 | 87.5 | 90.0 | 91.8 | |
| APR + PALO, % | 81.6 | 86.5 | 84.4 | 86.4 | ||
| Overall | NEPA, % | 84.1 | 86.8 | 89.6 | 91.8 | |
| APR + PALO, % | 80.6 | 86.5 | 83.3 | 86.4 | ||
APR, aprepitant; CR, complete response; NEPA, netupitant‐palonosetron; NSN, no significant nausea; PALO, palonosetron; VAS, visual analog scale.
Test prespecified and adjusted for multiplicity for CR at cycle 1 only; post‐hoc for cycles 2‐4 (not adjusted for multiplicity).
Defined as maximum daily nausea score <25 mm on 100‐mm VAS.
3.2.2. Study 2
In Study 2, the CR rates were high across cycles 1‐4 for both treatment groups. In patients treated with oral NEPA, CR rates ranged from 92.9% to 96.6%, and 83.2% to 91.5% for the acute and delayed phases, respectively. These rates were similar but numerically higher compared with oral APR‐PALO, except for acute CR and acute NSN in cycle 1 (Table 4, Figure 2B). Similar results were seen in the subsets of NEPA patients receiving MEC (n = 235 at cycle 1). Across cycles 1‐4, CR rates of 93.2%, 97.2%, 96.4%, and 97.2% were observed in the acute phase, and 81.7%, 88.7%, 90.8%, and 91.7% in the delayed phase. For APR‐PALO patients receiving MEC (n = 77 at cycle 1), CR rates of 93.5%, 94.6%, 97.3%, and 97.0% were observed across cycles 1‐4 in the acute phase, and 84.4%, 85.1%, 87.8%, and 88.1% in the delayed phase (data not shown). For the subset of NEPA patients receiving HEC (n = 74 at cycle 1), CR rates of 91.9%, 94.1%, 95.2%, and 94.2% were reported across cycles 1‐4 in the acute phase, and 87.8%, 80.9%, 93.7%, and 88.5% in the delayed phase. For APR‐PALO patients receiving HEC (n = 26 at cycle 1), CR rates of 96.2%, 81.8%, 87.5%, and 92.9% were reported across cycles 1‐4 in the acute phase, and 57.7%, 72.7%, 87.5%, and 85.7% in the delayed phase (data not shown).
NSN rates were also high in both treatment groups, with numerically higher rates for NEPA compared with APR‐PALO. Across cycles 1‐4, NEPA‐treated patients had NSN rates ranging from 90.6% to 97.0%, and 85.1% to 91.8% in the acute and delayed phases, respectively (Table 4).
4. DISCUSSION
This report presents the efficacy results of 2 pivotal trials evaluating the safety and efficacy of oral NEPA in the acute and delayed phases over multiple chemotherapy cycles in patients with solid tumors (in Study 2, patients with any malignant tumor were eligible). In Study 1, superiority of NEPA to PALO in preventing CINV in the acute, delayed, and overall phases during cycle 1 of AC‐based chemotherapy was clearly demonstrated,21 with overall CR sustained across multiple cycles23 (overall CR for oral NEPA was 74.3%–83.8% across 4 cycles; overall no emesis and NSN rates for oral NEPA were 79.8%–88.3% and 74.6%–80.2%, respectively, all across 4 cycles [Table 3]). The current analysis now reports higher response rates with NEPA, compared with PALO, for all 3 efficacy measures (CR, NSN, and no emesis) during the acute and delayed phases across all 4 chemotherapy cycles (P < 0.05 not adjusted for multiplicity). In the previously reported results of Study 2, the safety and efficacy with NEPA were maintained, and NEPA resulted in numerically higher overall CR rates than APR‐PALO in cycle 1 and subsequent cycles24 (overall CR for oral NEPA was 80.6%–90.7% across 4 cycles; overall NSN for oral NEPA was 84.1%–91.8% across 4 cycles [Table 3]). This current analysis also found CR and NSN rates were numerically higher for NEPA in the acute and delayed phases for both the overall population and the MEC and HEC subgroups.
Collectively, these data show the efficacy of oral NEPA in preventing CINV, particularly in the delayed phase in cycle 1; this was maintained over multiple cycles. NK1 RA regimens have historically been studied primarily in cisplatin‐based HEC and AC settings, so these data expand the known efficacy of NEPA, ergo NK1 RA regimens, to MEC regimens as well. These findings are thus relevant to a broad range of chemotherapeutic settings, which is important considering the recent reclassification in the guidelines of carboplatin AUC ≥ 415 and AC combination regimens to HEC.15, 16, 17
The high NSN rates are notable given the unmet clinical need for CINV prevention in the delayed setting, particularly for nausea control.26 Evidence from phase III studies of APR showed “no nausea” rates between 44% and 71%, and NSN rates between 57% and 78% in the delayed phase.27, 28, 29, 30, 31 A study of the NK1 RA rolapitant added to a granisetron‐DEX regimen reported no statistically significant improvement to nausea control in patients receiving AC and non‐AC HEC.32 Another pooled analysis of 2 studies in patients receiving cisplatin‐based HEC reported statistically higher rates of NSN and “no nausea” in patients receiving rolapitant‐granisetron‐DEX, compared with those receiving granisetron‐DEX alone. However, when analyzed separately, 1 of the 2 trials showed no statistically significant difference in NSN rates.33 Another study reported statistically significantly higher NSN but not “no nausea” rates in patients receiving rolapitant‐ondansetron‐DEX compared with ondansetron‐DEX with cisplatin‐based HEC.34
Each of the 2 studies in this analysis reported more than 3‐quarters of patients evaluable at the end of cycle 4. This is in contrast to other studies investigating antiemetic usage across multiple cycles. A recent post‐hoc analysis of pooled efficacy data from 4 trials of rolapitant presenting data from 2637 patients undergoing up to 6 cycles of chemotherapy reported that only ~50% completed cycle 4.35 Other multiple‐cycle trials of antiemetics also reported high dropout rates, which has hampered the interpretation of results in these studies.36, 37, 38, 39, 40 Despite NEPA's sustained efficacy over multiple cycles reported in the present analysis, there are limitations to our methodology. The 2 studies were sufficiently heterogeneous, preventing pooled analysis, and the main focus of Study 2 was on safety, hence not designed to formally compare the efficacy of oral NEPA with an APR triplet (the APR‐PALO arm was included only as a safety reference). Furthermore, the increased percentage of patients in both studies with CR and NSN in subsequent cycles may reflect a selection bias in which responders, but not nonresponders, preferentially continued to receive further cycles. In the subset of patients treated with MEC, 60% of patients received carboplatin. It is noteworthy that carboplatin was considered as MEC by all guidelines at the time the study was conducted; however, all guidelines currently recommend antiemetics consistent with those administered for HEC in patients receiving carboplatin AUC ≥ 4 mg mL−1 min.
In conclusion, oral NEPA, the first antiemetic combination agent targeting 2 critical emetic pathways, demonstrated superiority over PALO in terms of CR in all 3 phases of cycle 1 (Study 1); also, NEPA resulted in high CR and NSN rates during the acute and delayed phases, as well as in the overall phase across the 4 cycles (Studies 1 and 2), regardless of whether patients were receiving an AC MEC, non‐AC MEC, or HEC regimen. Preservation of the antiemetic effect of oral NEPA over multiple cycles suggests the utility of this agent in providing sustained CINV control beyond the first cycle. The convenient fixed single‐dose, once‐per‐cycle administration of oral NEPA may improve adherence to antiemetic guidelines and increase treatment compliance, hence improve CINV prevention; this will need to be verified in prospective studies.
CONFLICT OF INTEREST
The authors have the following conflicts of interest to disclose: LS: consultant for Helsinn Healthcare, Tesaro, Merck, and Heron. MK: consultant for Helsinn Healthcare, MSD, and Tesaro. GRo, GRi, and MEB: employees of Helsinn Healthcare. HSR: research funding from UCSF and Eisai. KJ: consultant or received honoraria from Helsinn Healthcare, Tesaro, and Merck/MSD. VH: none declared.
ACKNOWLEDGMENTS
The authors thank the clinical investigators, patients, and site personnel who participated in the studies. Funding: The trials described in this paper were sponsored by Helsinn Healthcare SA, Lugano, Switzerland. Editorial and medical writing assistance was provided by Joanne Franklin, PhD, CMPP, and Shilu Amin, PhD, of Aptitude Health, The Hague, The Netherlands, and funded by Helsinn Healthcare SA, Lugano, Switzerland.
Schwartzberg L, Karthaus M, Rossi G, et al. Fixed combination of oral NEPA (netupitant‐palonosetron) for the prevention of acute and delayed chemotherapy‐induced nausea and vomiting in patients receiving multiple cycles of chemotherapy: Efficacy data from 2 randomized, double‐blind phase III studies. Cancer Med. 2019;8:2064–2073. 10.1002/cam4.2091
Funding information
Helsinn Healthcare SA provided financial support for the study. Helsinn participated in the design, collection, analysis and interpretation of data, as well as the review, and approval of the manuscript before the submission.
REFERENCES
- 1. Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H. Chemotherapy‐induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer. 2007;15(5):497‐503. [DOI] [PubMed] [Google Scholar]
- 2. Hesketh PJ. Chemotherapy‐induced nausea and vomiting. N Engl J Med. 2008;358(23):2482‐2494. [DOI] [PubMed] [Google Scholar]
- 3. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient‐reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient‐reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aapro M, Ruffo P, Panteri R, Costa S, Piovesana V. Oncologist perspectives on chemotherapy‐ induced nausea and vomiting (CINV) management and outcomes: A quantitative market research‐based survey. Cancer Rep. 2018;1:e1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hesketh PJ, Van Belle S, Aapro M, et al. Differential involvement of neurotransmitters through the time course of cisplatin‐ induced emesis as revealed by therapy with specific receptor antagonists. Eur J Cancer. 2003;39(8):1074‐1080. [DOI] [PubMed] [Google Scholar]
- 7. Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy‐induced nausea and emesis after modern antiemetics. Cancer. 2004;100(10):2261‐2268. [DOI] [PubMed] [Google Scholar]
- 8. Salsman JM, Grunberg SM, Beaumont JL, et al. Communicating about chemotherapy‐induced nausea and vomiting: a comparison of patient and provider perspectives. J Natl Compr Canc Netw. 2012;10(2):149‐157. [DOI] [PubMed] [Google Scholar]
- 9. Majem M, Moreno ME, Calvo N, et al. Perception of healthcare providers versus patient reported incidence of chemotherapy‐induced nausea and vomiting after the addition of NK‐1 receptor antagonists. Support Care Cancer. 2011;19(12):1983‐1990. [DOI] [PubMed] [Google Scholar]
- 10. Schnell FM. Chemotherapy‐induced nausea and vomiting: the importance of acute antiemetic control. Oncologist. 2003;8(2):187‐198. [DOI] [PubMed] [Google Scholar]
- 11. Dranitsaris G, Molassiotis A, Clemons M, et al. The development of a prediction tool to identify cancer patients at high risk for chemotherapy‐induced nausea and vomiting. Ann Oncol. 2017;28(6):1260‐1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Molassiotis A, Lee PH, Burke TA, et al. Anticipatory nausea, risk factors, and its impact on chemotherapy‐induced nausea and vomiting: results from the Pan European Emesis Registry study. J Pain Symptom Manage. 2016;51(6):987‐993. [DOI] [PubMed] [Google Scholar]
- 13. Schwartzberg L, Harrow B, Lal LS, Radtchenko J, Lyman GH. Resource utilization for chemotherapy‐induced nausea and vomiting events in patients with solid tumors treated with antiemetic regimens. Am Health Drug Benefits. 2015;8(5):273‐282. [PMC free article] [PubMed] [Google Scholar]
- 14. Ihbe‐Heffinger A, Ehlken B, Bernard R, et al. The impact of delayed chemotherapy‐induced nausea and vomiting on patients, health resource utilization and costs in German cancer centers. Ann Oncol. 2004;15(3):526‐536. [DOI] [PubMed] [Google Scholar]
- 15. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Antiemesis, Version3. 2018. http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed October 2018.
- 16. Roila F, Molassiotis A, Herrstedt J, et al; participants of the MASCC/ESMO Consensus Conference Copenhagen 2015 . 2016 MASCC and ESMO guideline update for the prevention of chemotherapy‐ and radiotherapy‐induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119–v133. [DOI] [PubMed] [Google Scholar]
- 17. Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(28):3240‐3261. [DOI] [PubMed] [Google Scholar]
- 18. Rojas C, Raje M, Tsukamoto T, Slusher BS. Molecular mechanisms of 5‐HT(3) and NK(1) receptor antagonists in prevention of emesis. Eur J Pharmacol. 2014;722:26‐37. [DOI] [PubMed] [Google Scholar]
- 19. Jordan K, Jahn F, Aapro M. Recent developments in the prevention of chemotherapy‐ induced nausea and vomiting (CINV): a comprehensive review. Ann Oncol. 2015;26(6):1081‐1090. [DOI] [PubMed] [Google Scholar]
- 20. Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy‐induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose‐ranging pivotal study. Ann Oncol. 2014;25(7):1340‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aapro M, Rugo H, Rossi G, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed‐dose combination of netupitant and palonosetron, for prevention of chemotherapy‐induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. 2014;25(7):1328‐1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akynzeo® (netupitant and palonosetron) [prescribing information] Dublin, Ireland: Helsinn Birex Pharmaceuticals Ltd. 2018. https://www.akynzeo.com/hcp/assets/pdf/Prescribing_Information.pdf. Accessed March 4, 2019. [Google Scholar]
- 23. Aapro M, Karthaus M, Schwartzberg L, et al. NEPA, a fixed oral combination of netupitant and palonosetron, improves control of chemotherapy‐induced nausea and vomiting (CINV) over multiple cycles of chemotherapy: results of a randomized, double‐bind, phase 3 trial versus oral palonosetron. Support Care Cancer. 2017;25(4):1127‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gralla RJ, Bosnjak SM, Hontsa A, et al. A phase III study evaluating the safety and efficacy of NEPA, a fixed‐dose combination of netupitant and palonosetron, for prevention of chemotherapy‐induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol. 2014;25(7):1333‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roila F, Herrstedt J, Aapro M, et al; ESMO/MASCC Guidelines Working Group . Guideline update for MASCC and ESMO in the prevention of chemotherapy‐ and radiotherapy‐induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(suppl 5):v232‐v243. [DOI] [PubMed] [Google Scholar]
- 26. Bosnjak SM, Dimitrijevic J, Djordjevic F. Cancer and chemotherapy‐induced nausea and vomiting: a focus on olanzapine. Curr Opin Support Palliat Care. 2016;10(2):180‐188. [DOI] [PubMed] [Google Scholar]
- 27. Hesketh PJ, Grunberg SM, Gralla RJ, et al; Aprepitant Protocol 052 Study Group . The oral neurokinin‐1 antagonist aprepitant for the prevention of chemotherapy‐induced nausea and vomiting: a multinational, randomized, double‐blind, placebo‐controlled trial in patients receiving high‐dose cisplatin–the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21(22):4112‐4119. [DOI] [PubMed] [Google Scholar]
- 28. Poli‐Bigelli S, Rodrigues‐Pereira J, Carides AD, et al. Aprepitant Protocol 054 Study Group. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy‐induced nausea and vomiting. Results from a randomized, double‐blind, placebo‐controlled trial in Latin America. Cancer. 2003;97(12):3090‐3098. [DOI] [PubMed] [Google Scholar]
- 29. Warr DG, Grunberg SM, Gralla RJ, et al. The oral NK1 antagonist aprepitant for the prevention of acute and delayed chemotherapy‐induced nausea and vomiting: pooled data from 2 randomised, double‐blind, placebo controlled trials. Eur J Cancer. 2005;41(9):1278‐1285. [DOI] [PubMed] [Google Scholar]
- 30. Roila F, Ruggeri B, Ballatori E, del Favero A, Tonato M. Aprepitant versus dexamethasone for preventing chemotherapy‐induced delayed emesis in patients with breast cancer: a randomized double‐blind study. J Clin Oncol. 2014;32(2):101‐106. [DOI] [PubMed] [Google Scholar]
- 31. Roila F, Ruggeri B, Ballatori E, et al; Italian Group for Antiemetic Research . Aprepitant versus metoclopramide, both combined with dexamethasone, for the prevention of cisplatin‐induced delayed emesis: a randomized, double‐blind study. Ann Oncol. 2015;26(6):1248‐1253. [DOI] [PubMed] [Google Scholar]
- 32. Schwartzberg LS, Modiano MR, Rapoport BL, et al. Safety and efficacy of rolapitant for prevention of chemotherapy‐induced nausea and vomiting after administration of moderately emetogenic chemotherapy or anthracycline and cyclophosphamide regimens in patients with cancer: a randomised, active‐controlled, double‐blind, phase 3 trial. Lancet Oncol. 2015;16(9):1071‐1078. [DOI] [PubMed] [Google Scholar]
- 33. Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy‐induced nausea and vomiting after administration of cisplatin‐based highly emetogenic chemotherapy in patients with cancer: two randomised, active‐controlled, double‐blind, phase 3 trials. Lancet Oncol. 2015;16(9):1079‐1089. [DOI] [PubMed] [Google Scholar]
- 34. Rapoport B, Chua D, Poma A, Arora S, Wang Y, Fein LE. Study of rolapitant, a novel, long‐acting, NK‐1 receptor antagonist, for the prevention of chemotherapy‐induced nausea and vomiting (CINV) due to highly emetogenic chemotherapy (HEC). Support Care Cancer. 2015;23(11):3281‐3288. [DOI] [PubMed] [Google Scholar]
- 35. Rapoport B, Schwartzberg L, Chasen M, et al. Efficacy and safety of rolapitant for prevention of chemotherapy‐induced nausea and vomiting over multiple cycles of moderately or highly emetogenic chemotherapy. Eur J Cancer. 2016;57:23‐30. [DOI] [PubMed] [Google Scholar]
- 36. de Wit R, Schmitz PI, Verweij J, et al. Analysis of cumulative probabilities shows that the efficacy of 5HT3 antagonist prophylaxis is not maintained. J Clin Oncol. 1996;14(2):644‐651. [DOI] [PubMed] [Google Scholar]
- 37. de Wit R, Herrstedt J, Rapoport B, et al. Addition of the oral NK1 antagonist aprepitant to standard antiemetics provides protection against nausea and vomiting during multiple cycles of cisplatin‐based chemotherapy. J Clin Oncol. 2003;21(22):4105‐4511. [DOI] [PubMed] [Google Scholar]
- 38. de Wit R, Herrstedt J, Rapoport B, et al. The oral NK1 antagonist, aprepitant, given with standard antiemetics provides protection against nausea and vomiting over multiple cycles of cisplatin‐based chemotherapy: a combined analysis of two randomised, placebo‐controlled phase III clinical trials. Eur J Cancer. 2004;40(3):403‐410. [PubMed] [Google Scholar]
- 39. Herrstedt J, Muss HB, Warr DG, et al; Aprepitant Moderately Emetogenic Chemotherapy Study Group . Efficacy and tolerability of aprepitant for the prevention of chemotherapy‐induced nausea and emesis over multiple cycles of moderately emetogenic chemotherapy. Cancer. 2005;104(7):1548‐1555. [DOI] [PubMed] [Google Scholar]
- 40. Nathan PC, Tomlinson G, Dupuis LL, et al. A pilot study of ondansetron plus metopimazine vs. ondansetron monotherapy in children receiving highly emetogenic chemotherapy: a Bayesian randomized serial N‐of‐1 trials design. Support Care Cancer. 2006;14(3):268‐276. [DOI] [PubMed] [Google Scholar]


