Abstract
The Nrf2/Keap1 pathway is an important signaling cascade responsible for the resistance of oxidative damage induced by exogenous chemicals. It maintains the redox homeostasis, exerts anti‐inflammation and anticancer activity by regulating its multiple downstream cytoprotective genes, thereby plays a vital role in cell survival. Interestingly, in recent years, accumulating evidence suggests that Nrf2 has a contradictory role in cancers. Aberrant activation of Nrf2 is associated with poor prognosis. The constitutive activation of Nrf2 in various cancers induces pro‐survival genes and promotes cancer cell proliferation by metabolic reprogramming, repression of cancer cell apoptosis, and enhancement of self‐renewal capacity of cancer stem cells. More importantly, Nrf2 is proved to contribute to the chemoresistance and radioresistance of cancer cells as well as inflammation‐induced carcinogenesis. A number of Nrf2 inhibitors discovered for cancer treatment were reviewed in this report. These provide a new strategy that targeting Nrf2 could be a promising therapeutic approach against cancer. This review aims to summarize the dual effects of Nrf2 in cancer, revealing its function both in cancer prevention and inhibition, to further discover novel anticancer treatment.
Keywords: cancer, chemoresistance, inflammation, Keap1, Nrf2
1. INTRODUCTION
Nuclear factor (erythroid‐derived 2)‐like 2 (Nrf2) encoded by NFE2L2 gene belongs to a transcription factor subfamily with a Cap “n” Collar (CNC) structure and contains a basic leucine zipper DNA binding domain (b‐Zip) at the C terminus. Nrf2 possesses six highly conserved domains named Nrf2‐ECH homology (Neh) domains. The bZip in Neh1 domain allows Nrf2 to heterodimerize with small musculoaponeurotic fibrosarcoma proteins (sMafs).1 The Neh2 domain allows Nrf2 to bind and regulate its cytoplasmic chaperone molecule Kelch‐like‐ECH‐associated protein 1 (Keap1) in physiological manner.2 The two motifs of Neh2 domain, ETGE and DLG, bind to similar sites on the bottom surface of the Keap1 Kelch motif.3 Neh3 domain is necessary for protein stability and transcriptional activation.4 Neh4 and Neh5 function as two transactivation domains by interacting with CREB‐binding protein (CBP).2 Neh6 domain is rich in serine residues and contains a degron that is involved in the degradation of Nrf2 in oxidatively stressed cells.5 The activity of this degron could be increased by glycogen synthase kinase‐3 (GSK‐3) activity, suggesting that the stimulation of the degron of Neh6 domain could be an effective method to overcome the constitutive upregulation of Nrf2.6
2. NRf2 ACTIVATION
2.1. Keap1/Nrf2/ARE signaling pathway
The Keap1‐Nrf2 system is of primary importance in maintaining cellular homeostasis in order to respond adaptively to xenobiotic and oxidative stress. Under basal condition, Nrf2 interacts with two molecules of Keap1 through its Neh2 ETGE and DLG motifs to activate Cullin 3 (Cul3)‐based E3 ligase complex‐mediated Nrf2 ubiquitination reaction.7 Once Nrf2 is ubiquitinated, it will be rapidly degraded by 26S proteasome and maintained at a very low level in the cytoplasm.8 On exposure of cells to oxidative stress or chemopreventive compounds, the cysteine residues of Keap1 are modified and the conformation changes, resulting in the detachment of Nrf2 DLG motifs from Keap1, disrupting the ubiquitination and degradation of Nrf2. The binding affinity between Nrf2 and Keap1 is reduced and the ubiquitination system of Nrf2‐Cul3 is disrupted. This allows the de novo‐synthesized Nrf2 to translocate into nucleus, forms a heterodimer with one of sMafs, and binds to antioxidant element (ARE) in the upstream promoter region of multiple genes. On recovery of the redox balance, Nrf2 is dissociated from the ARE sequence. Keap1 enters into the nucleus and escorts Nrf2 out of the nucleus to the cytoplasmic Cul3‐E3 ubiquitin ligase machinery for degradation. Ultimately, a low level of Nrf2 is reattained; the Nrf2/Keap1 signaling pathway is switched off (Figure 1).9, 10
Figure 1.
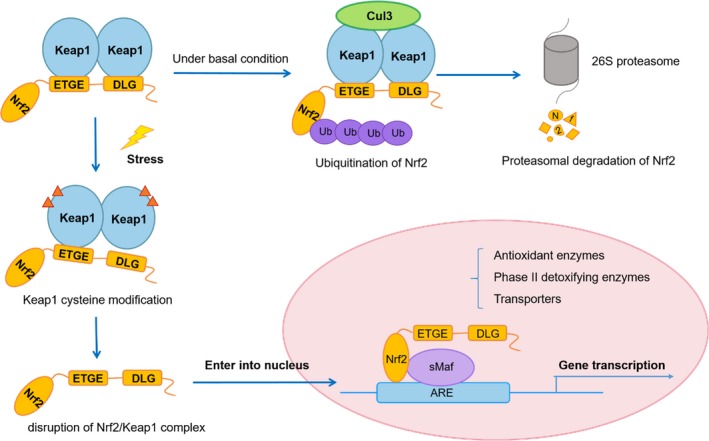
Nrf2/Keap1 signaling pathway. Under basal conditions, Nrf2 binds to Keap1 by its two motifs (ETGE and DLG) and activates Cul3‐mediated ubiquitination followed by proteasomal degradation. Under stress conditions, due to the modification of Keap1 cysteine residues, Nrf2 dissociates from Keap1 and translocates into the nucleus. Nrf2 then forms a heterodimer with sMaf protein and binds to ARE to initiate the transcription of various downstream genes
2.2. Downstream targets of Nrf2
The Nrf2 downstream targets are classified into three major groups: phase I and phase II drug metabolizing enzymes as well as phase III drug transporters (Table 1). Briefly, phase I enzymes oxidize drugs or xenobiotics; while phase II enzymes conjugate products of phase I reactions, phase III enzymes transport the final metabolites out of cells, cooperating to exert a cytoprotective function. Phase I‐metabolizing enzymes oxidize, reduce, or hydrolyze xenobiotics and drugs, such as aldo‐keto reductases (AKR) and cytochrome P450s (CYPs) encoded by genes regulated by Nrf2.
Table 1.
Downstream targets regulated by Nrf2
| Abbreviation | Name | General biochemical function | Ref. |
|---|---|---|---|
| Xenobiotic detoxification | |||
| NQO1 | NAD(P)H:quinone oxidoreductase 1 | Degradation of reactive quinone and scavenge of superoxide | 11, 12 |
| AKR | Aldo‐keto reductases | Reduce aldehydes and ketones | 13 |
| CBR | carbonyl reductase | prostaglandin metabolism | 14 |
| ADH | Alcohol dehydrogenase | Alcohol metabolism | 15 |
| ALDH | Aldehyde dehydrogenase | Catalyzes the oxidation of aldehydes | 16 |
| CYPs | cytochrome P450s | Catalyze the oxidation, reduction, and dehalogenation of various xenobiotics | 17 |
| CES | Carboxylesterases | Catalyze the hydrolysis of ester‐ and amide‐containing chemicals | 18 |
| SOD | Superoxide dismutase | Catalyzes the dismutation of the superoxide | 19 |
| EPHX1 | Epoxide hydrolase | Catalyzes the hydrolysis of arene and aliphatic epoxides | 20 |
| Conjugation | |||
| UGT | UDP‐glucuronosyltransferase | Conjugates glycosyl group with xenobiotics for detoxification | 21 |
| GST | Glutathione S‐transferases | Conjugate reduced GSH with xenobiotics for detoxification | 22 |
| SULT | sulfotransferases | Conjugate sulfate group with xenobiotics for detoxification | 23 |
| NAT | N‐acetyltransferase | Conjugates acetyl group with xenobiotics for detoxification | 24 |
| GSH metabolism | |||
| xCT | the subunit of system xc– | Imports cysteine into the cell | 25 |
| GCLC | Glutamate‐cysteine ligase | Catalytic subunit in rate‐limiting step of GSH synthesis | 26 |
| GCLM | Glutamate‐cysteine ligase | Modifier subunit in rate‐limiting step of GSH synthesis | 26 |
| GPX | Glutathione peroxidase | Catalyzes the oxidation of GSH | 27 |
| GSR | Glutathione reductase | Catalyzes the NADPH‐dependent reduction of GSSG | 22 |
| Thioredoxin enzyme system | |||
| Prxs | Peroxiredoxins | Catalyze the reduction of peroxides | 28 |
| Txn1 | Thioredoxin‐1 | Catalyzes the reduction of oxidized proteins | 29 |
| TrxR1 | Thioredoxin reductases‐1 | Catalyze the NADPH‐dependent reduction of oxidized Trx | 30 |
| Srxn1 | Sulfiredoxin‐1 | Reactivates Prxs | 31 |
| Heme metabolism | |||
| HO‐1 (HMOX1) | Heme oxygenase 1 | Cleaves heme to form biliverdin for the degradation of heme | 32 |
| BLVR | Biliverdin reductase | Reduction of biliverdin to bilirubin for the degradation of heme | 33 |
| FECH | Ferrochelatase | Converts Fe2+ to heme for the degradation of heme | 34 |
| FTH | Ferritin heavy chain | Storage of Fe for degradation of heme | 35 |
| FTL | Ferritin light chain | Storage of Fe for degradation of heme | 35 |
| NADPH generation and PPP pathway | |||
| G6PD | Glucose‐6‐phosphate 1‐dehydrogenase | NADPH production in oxidative phase of PPP | 36 |
| PGD | 6‐phosphogluconate dehydrogenase | NADPH production in oxidative phase of PPP | 36 |
| IDH1 | Isocitrate dehydrogenase 1 | NADPH production in oxidative phase of PPP | 36 |
| ME1 | Malic enzyme 1 | NADPH production, pyruvate regeneration for TCA cycle | 36 |
| TKT | Transketolase | directing carbon flux in nonoxidative phase of PPP | 36 |
| TALDO1 | Transaldolase1 | directing carbon flux in nonoxidative phase of PPP | 36 |
| Xenobiotic transporters | |||
| MRPs | Multidrug resistance‐associated proteins | Transport or excrete drug metabolites out of cells | 37 |
| OATP2 | Organic anion‐transporting polypeptide | Mediates the Na+‐independent uptake of organic anions | 38 |
| P‐gp | P‐glycoprotein | an ATP‐dependent efflux pump of wide range of xenobiotics | 39 |
| Fatty acid synthesis and oxidation | |||
| ACL | ATP‐citrate lyase | Synthesis of cytosolic acetyl‐CoA for fatty acid synthesis | 40 |
| ACC | Acetyl‐CoA carboxylase | Synthesis of malonyl‐CoA for fatty acid synthesis | 41 |
| FASN | Fatty acid synthase | Synthesis of long‐chain saturated fatty acid for fatty acid synthesis | 40 |
| SCD | Stearoyl CoA desaturase | Introduction of a double bond for unsaturated fatty acid synthesis | 40 |
| Purine biosynthesis | |||
| PPAT | Phosphoribosyl pyrophosphate amidotransferase | Catalyzes the rate‐limiting step in the de novo purine biosynthetic pathway | 36 |
| MTHFD2 | Methylenetetrahydrofolate dehydrogenase 2 | Provides one‐carbon units for purine biosynthesis | 36 |
| Transcription factors | |||
| AhR | Aromatic hydrocarbon receptor | Promotes the expression of cytochrome P450s (CYPs) for inhibition of adipogenesis | 42 |
| PPARγ | Peroxisome proliferators activator receptors gamma | Promotes the expression of CYP4A gene for adipocyte differentiation and reduction in inflammation | 43 |
| CEBPα | CCAAT/enhancer‐binding protein alpha | Binds to the CCAAT box motif in various gene for adipocyte differentiation and macrophage function | 44 |
| RXRα | Retinoid X receptor alpha | Interacts with Neh7 domain of Nrf2 for the inhibition of Nrf‐Keap1 pathway | 45 |
Phase II drug‐metabolizing enzymes regulated by Nrf2 are mostly engaged in metabolic pathways through metabolizing xenobiotics via glucuronidation, glutathione conjugation, and sulfation. The primary function of intracellular redox‐balancing proteins is to maintain the cellular levels of glutathione (GSH) and thioredoxin (Trx), which scavenge reactive oxygen species (ROS) and reactive nitrogen species (RNS) in cells.46 Nrf2/Keap1 signaling governs the expression of the xCT (aka SLC7a11 or system xc–),25 which imports cysteine into the cell, along with glutamate‐cysteine ligase (GCL) that catalyzes the rate‐limiting step in glutathione (GSH) biosynthesis.26 Nrf2 regulates glutathione peroxidase (GPx) to maintain peroxides level in order to produce oxidized glutathione (GSSG),27 and glutathione reductase‐1 (GSR1), which reduces GSSG to maintain intracellular levels of reduced GSH.22 The induction of thioredoxin‐1 (Txn1),29 thioredoxin reductases‐1 (TrxR1),30 peroxiredoxins (Prxs),28 and sulfiredoxin‐1 (Srxn1)31 is also subject to Nrf2 regulation for the reduction of oxidized protein thiols and the removal of peroxides. Importantly, NADPH is required as a coenzyme for many xenobiotic metabolism and antioxidant enzymes, including AKR, NAD(P)H: quinone oxidoreductase‐1 (NQO1), and GSR1. Additionally, Nrf2 regulates four NADPH‐generating enzymes, namely glucose‐6‐phosphate dehydrogenase (G6PD), 6‐phosphogluconate dehydrogenase (PGD), isocitrate dehydrogenase‐1 (IDH1), and malic enzyme‐1 (ME1). Heme and quinone both transfer electrons and are therefore direct sources of free radicals and ROS. Moreover, Nrf2 regulates heme oxygenase‐1 (HO‐1) and NQO1 to catalyze heme and quinone degradation.11
Phase III drug transporters extrude endogenous xenobiotics and conjugated metabolites out of cells, including multidrug resistance‐associated proteins (MRPs), P‐glycoprotein (P‐pg), and organic anion‐transporting polypeptide (OATP).37
2.3. General function of Nrf2 pathway
Nrf2 downstream genes, illustrated in Figure 1, are involved in intracellular redox balancing, xenobiotic response, metabolism, and cell survival. Living cells require cellular homeostasis for metabolic process and the ability to rapidly respond to various stresses imposed by toxic exposure. ROS and toxic metabolites generated by ROS‐mediated cell damage give rise to oxidative stress that is apparently adverse to cell survival and further contribute to the induction of tumorigenesis.47 The Nrf2‐mediated antioxidant response is one of the major cellular defense mechanisms that protect against oxidative stress. The activation of Nrf2 signaling pathway scavenges ROS and RNS by upregulating the expression of multiple drug‐metabolizing enzymes, such as GCL, AKR, UGT, and MRPs. Likewise, Nrf2‐regulated metabolic pathways including pentose phosphate pathway (PPP) and fatty acid pathway, which are essential contributors to the maintenance of cellular redox and normal cell proliferation.
2.4. Nrf2 in cancer prevention
There are abundant evidences that the activation of Nrf2 is able to suppress carcinogenesis, especially in its early stage. Under the physiological condition, Nrf2 maintains the cellular redox homeostasis and exerts anti‐inflammatory functions and further anticancer activities, hence supports cell survival.
2.5. Nrf2 maintain cellular redox homeostasis
Under the physiological condition, Nrf2 implements its general function of maintaining cellular redox homeostasis and regulating cell growth, which is molecular basic of Nrf2 to prevent the tumorigenesis.
The physiological relevance between Nrf2, cellular redox homeostasis, and tumorigenesis has been shown by numerous studies. Nrf2‐deficient mice are generally more susceptible to redox disturbances and easily developing drug toxicity.48, 49 For instance, loss of Nrf2 initiates a detrimental cascade of reduced GST expression and elevates ROS level, ultimately leading to DNA damage and tumorigenesis.50 NFE2L2 gene knockout mice are more sensitive to exogenous chemicals, leading to the formation of bleomycin‐induced pulmonary fibrosis and hyperoxic lung injury51 as well as acetylhydrolase‐induced liver cancer.52 Oxidative tissue damage after ischemia and reperfusion, including that resulting in noise‐induced hearing loss, is effectively suppressed by Nrf2 activation through its antioxidant function.53
2.6. Nrf2 exerts an anti‐inflammatory activity
Inflammation induces the generation of ROS and other reactive species, causing DNA damage, activating oncogenes or inactivating tumor suppressor genes, and stimulating proliferation of initiated cells, metastasis, and angiogenesis. Inflammation‐induced carcinogenesis is attributed as part of cytokines that retaining the pro‐inflammatory properties. Nrf2 regulates the expression of antioxidant and cytoprotective enzymes to guard against oxidative electrophilic insults and inhibit excessive production of pro‐inflammatory mediators, thereby constitute to the fundamental line for the chemoprevention of inflammation‐associated cancer. The Nrf2‐regulated Gpx and Trx are verified to suppress inflammatory response.54, 55 Cyclooxygenase‐2 (COX‐2), inducible nitric oxide synthase (iNOS), and tumor necrosis factor (TNF‐α) are significantly elevated in the Nrf2‐deficient mice, indicating an inhibitory function of Nrf2 toward pro‐inflammatory mediators.56 Additionally, the overexpression of HO‐1, a Nrf2 downstream target, attenuates TNF‐α‐induced oxidative stress and interleukin (IL)‐3 via the suppression of the DNA‐binding activity of activator protein‐1 (AP‐1).57 Similarly, Nrf2‐dependent induction of NQO1 downregulates lipopolysaccharide (LPS)‐induced expression of TNF‐α and IL‐1β, thereby impairs the inflammatory response.58 To conclude, another significant role of Nrf2 is to suppress inflammatory response and protect cells against inflammatory injury and inflammation‐induced carcinogenesis.
2.7. Nrf2 inhibits tumorigenesis
The activation of Nrf2/Keap1 pathway is one of the most important mechanisms in anti‐tumorigenesis. In tumor microenvironment, Nrf2 is activated by tumor suppressor genes BRCA1 and protein p21 via the inhibition of Keap1/Nrf2 complex formation59, 60 and is blocked by oncogene Fyn‐mediated degradation.61 The expression of antioxidant and phase II enzymes was found to be abrogated in the Nrf2‐deficient mice. The aggravated oxidative stress caused by quinone rendered the Nrf2‐deficient mice to be more prone to skin cancer, while the expression of NQO1 and GST regulated by Nrf2 decreased when compared to wild‐type mice.62 Similarly, Nrf2‐deficient mice treated with carcinogens develop the much larger number of tumors in the forestomach,63 liver,64 and urinary bladder65 compared with that of the wild‐type mice, suggesting that Nrf2 protects against inflammation‐induced carcinogenesis.
Many compounds from plants such as sulforaphane (an isothiocyanates in broccoli), curcumin, carnosol, and resveratrol, certain synthetic chemicals such as oltipraz (an antischistosomal drug and a 1,2‐dithiole‐3‐thione derivative) as well as synthetic oleanane triterpenoids have been discovered to exert chemopreventive activities through the induction of Nrf2/ARE‐regulated genes. Most of these Nrf2 activators affect Nrf2 activity by modifying intermolecular disulfide bonds between two Keap1 molecules at Cys273 and Cys288, to stabilize and enhance nuclear accumulation of Nrf2.66 For instance, sulforaphane induces the transcription of phase II enzymes, while inhibits phase I enzymes expression and facilitates cancer cell apoptosis via p53 mechanisms.67 Additionally, sulforaphane targets NF‐κB68 as well as both JUN and FOS of the AP‐1 complex to perform an anti‐inflammatory effect.69 Another well‐studied chemopreventive drug, synthetic oleanane triterpenoids, inhibits carcinogenesis through the suppression of oncogenes transcription as diverse as K‐Ras, TP53, Brca1, and Erbb2 in many organs such as pancreas, breast, or lung.70, 71 Indeed, many drugs that function by enhancing the Nrf2 activity are now in clinical trials for numerous indications. These studies show that Nrf2 plays an essential role in tumorigenesis inhibition and can be further strengthened by chemopreventive compounds.
3. NRf2 IN CANCER TREATMENT
The constitutive activation of Nrf2 promotes the development of various cancers and notably increases the cancer resistance. Clinically, the excessive expression of Nrf2 is always observed with poor prognosis.72 Several mechanisms by which Nrf2 signaling pathway is constitutively activated in various cancers have been described (Figure 2): (a) facilitative Nrf2 transcription by oncogenic Myc, K‐Ras, and B‐Raf mutation via mitogen‐activated protein kinases (MAPKs)47; (b) somatic mutations in Keap1, Nrf2, or Cul3 disrupting Nrf2/Keap1 interaction73, 74, 75; (c) loss of exons in Nrf2 mRNA resulting in Nrf2 mutants that do not interact with Keap176; (d) epigenetic DNA methylation of Keap1 reducing Keap1 expression level77; (e) mutations of tumor suppressor gene PTEN and epigenetic changes amplifying Nrf2 level36; (f) Keap1‐competing proteins such as partner and localizer of BRCA2(PALB2), dipeptidyl peptidase 3 (DPP3), Wilms tumor gene on X chromosome (WTX), p21 and p62 disrupt Nrf2/Keap1 interaction60, 78, 79, 80; (g) succination of Keap1 cysteine due to the loss‐of‐function mutations in fumarate hydratase resulting in the reduction of Nrf2‐Keap1‐binding affinity and blockage of Nrf2 ubiquitination.81 Cancer cells acquire new characteristics through Nrf2 hyperactivity including infinite cell growth and proliferation, avoidance of apoptosis, enhanced chemoresistance and radioresistance, and induction of angiogenesis and metastasis. The mechanisms of persistent activation of Nrf2 in cancer promotion are discussed in many respects.
Figure 2.
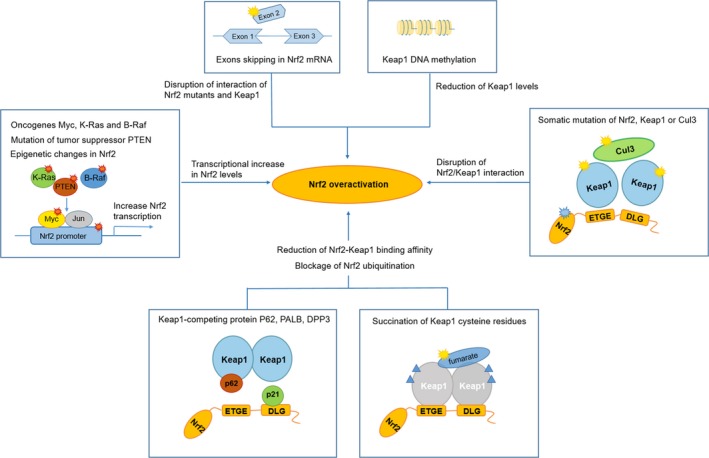
Mechanisms for Nrf2 overactivation in cancers. Oncogenes (Myc, K‐Ras and B‐Raf), mutations of tumor suppressor PTEN, and epigenetic changes in Nrf2 lead to the transcriptional increase in Nrf2 levels. Keap1 methylation transcriptionally reduces Keap1 levels. Exon skipping of Nrf2 and somatic mutations of Nrf2, Keap1, or Cul3 disrupt Nrf2/Keap1 interaction. Succination of Keap1 cysteine and Keap1‐competing protein such as p62 results in the reduction of Nrf2‐Keap1‐binding affinity and blockage of Nrf2 ubiquitination
3.1. Nrf2 promotes cancer cell growth and proliferation
Cancer cells differ from normal cells by its enormous growth and proliferative capacity, which is often observed with Nrf2 overactivation. The reduced state of GSH is indispensable for cell proliferation due to its detoxification, antioxidant defense function, etc. The excessive activation of Nrf2 greatly facilitates transcriptions of several genes involved in the formation of NADPH, the main cofactor in GSH synthesis.82 Nrf2 overactivation in cancer cells results in the significantly elevated expression of G6PD, TKT, PGD, and other metabolic enzymes. These highly expressed metabolic enzymes promote glucose and glutamine metabolism in the PPP and augment the synthesis of purine and amino acids, all of which contribute to metabolic reprogramming for cell proliferation.36 Nrf2 also regulates genes involved in metabolism of fatty acids and other lipids.83 Moreover, microRNA miR‐1 and miR‐206 are under control of Nrf2 to direct carbon flux toward PPP and tricarboxylic acid (TCA) cycle.84
Nrf2 promotes cell proliferation not only by metabolic reprogramming. The cell cycle regulation is closely related to Nrf2 activity by its target proliferation‐associated genes, including Bmpr1a, Igf1, Itgb2, Jag1, and Pdgf‐c.85 G2/M‐phase arrest is observed with Nrf2 deficiency, suggesting that Nrf2 is necessary for cell cycle regulation by controlling the inhibitory cell‐cycle regulators.86 The activation of the phosphoinositide 3‐kinase (PI3K)/protein kinase B (AKT) signaling in hepatocytes was attenuated in Nrf2‐deficient mice,87 revealing that persistently elevated Nrf2 promotes the phosphorylation of AKT, GSK3, and the PPP, establishing a crosstalk with the PI3K/AKT pathway to enhance anabolic efficiency.36 Besides, Nrf2 participates in healthy mitochondrial maintenance by controlling substrate availability for mitochondrial respiration, inducing mitochondrial biogenesis, and removing damaged mitochondria.88 A recent study found that loss of Nrf2 led to impaired mRNA translation in pancreatic cancer cells due to the defects in epidermal growth factor receptor (EGFR) signaling pathway and oxidation of specific translational regulatory proteins, demonstrating that Nrf2 is necessary for cancer cell maintenance by modulating mRNA translation.89
3.2. Nrf2 suppresses cancer cell apoptosis
In addition to the ability of unlimited proliferation, cancer cells are also characterized to escape from cell apoptosis. Cancer cells frequently express high levels of ROS‐scavenging enzymes, which confer resistance to ROS‐mediated cell death. The excessive activation of Nrf2 counterbalances the accumulated ROS by upregulating antioxidant enzymes, for instance, GCL and GSR that required for the synthesis of GSH.90 Nrf2 contributes to the avoidance of cancer cell death also by interacting with other pathways within the cells. The tumor suppressor p53 triggers cell growth arrest and apoptosis partly dependent on decreased Nrf2 activity via counteracting the expression of x‐CT, NQO1, and GST.91 Additionally, Nrf2 downstream target glutathione‐S‐transferase pi 1 (GSTP1) suppresses the activation of proapoptotic c‐Jun N‐terminal kinases (JNKs).92 Another downstream target p62 creates a positive feedback loop via initiation of selective autophagy of Keap1, to further block apoptosis.93 Moreover, Nrf2 mediates the upregulation of antiapoptotic protein B‐cell lymphoma 2 (Bcl‐2) which leads to the decrease in etoposide‐mediated cell apoptosis and increases cell survival.94 TNF‐induced cell death is also suppressed through the upregulation of HO‐1.95 In conclusion, the continuous activation of Nrf2 in cancer cells inhibits cell apoptosis, enhances the survival benefits of oxidized damaged cells, thereby promoting cancer development and progression.
3.3. Nrf2 promotes self‐renewal of cancer stem cells
Cancer steam cells (CSCs), characterized by their self‐renewal and differentiation properties, are thought to be partly responsible for anticancer drug resistance and tumor relapse after therapy. The self‐renewal capacity of CSCs is largely due to elevated expression of antioxidant enzymes, drug transporters, cell cycle quiescence, and enhanced DNA repair capacity.96 Nrf2 expression has been proved to regulate cell cycle quiescence and cell fate determination as well as reconstitution capacity of hematopoietic stem cells (HSCs).97, 98 These findings are consistent with the results in Drosophila intestinal stem cells; the cell quiescence was obtained through the reduction in ROS level due to Nrf2 upregulation.99 The persistent activation of Nrf2 also drives the myeloid differentiation and represses erythroid and lymphoid differentiation.97 Numerous studies have demonstrated that Nrf2 plays a vital role in various CSCs survival and self‐renewal so far. Nrf2 level was higher in glioma stem cells (GSCs) than non‐GSCs fraction,100 and Nrf2 overactivation induced its transcriptional network to perform self‐renewal capacity in GSCs with the treatment of anticancer drugs.101 Similarly, in neural stem cells (NSCs), amyloid‐β (Aβ1)‐induced toxicity was alleviated by Nrf2 overexpression along with increased expression of GCL, NQO1, and HO‐1, while Nrf2 deficiency enhanced the Aβ1‐42‐induced reduction of neuronal differentiation.102 In mesenchymal stem cells (MSCs), the continuous activation of Nrf2 reduced oxidative stress‐induced apoptosis and cytotoxicity.103
3.4. Nrf2 promotes anti‐inflammation activities
The activation of Nrf2/ARE signaling pathway plays a critical role in alleviation of chronic inflammation, which is associated with cancers. Since Nrf2 positively regulates a large number of cytoprotective proteins, elimination of ROS has been widely accepted as the molecular basis of Nrf2‐mediated anti‐inflammation. This has been proved by several reports that Nrf2‐deficient mice showed greater LPS‐induced pulmonary inflammation, which could be attenuated by Nrf2 inducers.104, 105 Nrf2 represses the activation of pro‐inflammatory genes and potentiates the anti‐inflammatory signaling. Nrf2 interferes with LPS‐induced transcriptional upregulation of pro‐inflammatory cytokines in macrophages (IL‐6 and IL‐1β), by binding to proximity of corresponding genes and inhibiting RNA polymerase II recruitment.109 Similarly, Nrf2 upregulates pro‐inflammatory chemokine IL‐8 to promote anti‐inflammation activities. Nrf2 activation is directly associated with the induction of IL‐8 expression by increasing the half‐life of IL‐8 mRNA.110 Indirectly, hypoxia‐inducible factor (HIF‐1) induction attenuated Nrf2‐dependent IL‐8 expression in human endothelial cells, suggesting a crosstalk between Nrf2 and HIF‐1 to promote IL‐8 expression.111 Likewise, Nrf2 activation protects cells against sepsis and H2O2‐mediated injury via p38/MAPK pathway to modulate pro‐inflammatory cytokine including monocyte chemotactic protein 1 (MCP1), vascular cell adhesion molecule 1 (VCAM1), TNF, and macrophage inflammatory protein 2 (MIP2).112, 113 The recent studies revealed that Nrf2 crosstalks with other important inflammatory pathway to bring coordinated innate immune response in form of inflammation. The toll‐like receptors (TLRs) signaling pathway plays a key role in modulating immune responses by eliciting inflammatory reactions via the production of inflammatory cytokines (TNF‐α, IL‐6), chemokines (IL‐8, MIP2), and interferons (type‐I). Several cell‐specific kinases (protein kinase C, MAPK, Bruton's tyrosine kinase, and PI3Ks) are known to mediate crosstalk between TLR and Nrf2 either by regulation of p62‐mediated autophagy,93 expression of anti‐inflammatory proteins (eg, HO‐1, NQO1, SOD),114, 115 or suppression of pro‐inflammatory cytokines (eg, IL‐6, IL‐1β).117 Nrf2 inducers attenuate TLR‐driven inflammation as well as pro‐inflammatory cytokines (IL‐6, IL‐1β, TNF) in sepsis or inflammatory disorders, while TLRs agonists may also act as activator of Nrf2 pathway, augment the expression of antioxidant proteins, and contribute to cell survival.108, 114 Reportedly, Nrf2 crosstalks with TLR also result from the interactions of former with nuclear factor kappa‐B (NF‐κB) pathway. Nrf2 target gene HMOX1 possesses prominent anti‐inflammatory despite of its cytoprotection role. Nrf2 activation inhibits NF‐ĸB pathway mainly by elevating HO‐1 expression and antioxidant defenses which neutralize ROS and detoxifying chemicals.118 It also controls NF‐κB pathway through Keap1, which stabilizes the NF‐ĸB inhibitor (IKB)‐α and represses degradation of NF‐ĸB kinase inhibitor (IKK)‐β, leading negatively to the regulation of NF‐κB pathway.119 NF‐ĸB exerts its negative effect on Nrf2‐driven gene expression through p65, along with MafK and histone deacetylase3 (HDAC3). Moreover, NF‐ĸB‐mediated transcription reduces Nrf2 activation by competing with Nrf2 for CBP.120 Since Nrf2 contributes to anti‐inflammatory effects and cell survival, Nrf2 inducers may be further explored for novel therapeutic treatment in inflammatory diseases and inflammation‐associated cancers.
3.5. Nrf2 promotes angiogenesis
The induction of angiogenesis is one of the hallmarks of inflammation‐induced carcinogenesis. Angiogenesis is under control in normal physiological processes, whereas it is continuously activated in cancers. HO‐1 overexpression augmented vascular endothelial growth factor (VEGF) production and VEGF‐mediated angiogenic activities by increasing proliferation, migration, the formation of tubes on Matrigel, and the outgrowth of capillaries from endothelial spheroids.121 The proangiogenic role of VEGF/Nrf2‐dependent pathway induced by Nrf2 activation was proved in rat gastric epithelial cells122 as well as glioma cells123 and pancreatic cancer cells.124 Nrf2 blockade inhibited hypoxia‐induced activation of HIF‐1α/VEGF signaling to suppress tumor angiogenesis, indicating a crosstalk mechanism between Nrf2 and HIF‐1α in angiogenesis.125
3.6. Nrf2 enhances chemoresistance of cancer cells
Multiple factors contribute to the buildup of the chemoresistance of cancer cells. Several mechanisms of chemoresistance have been proposed to be associated with the pharmacokinetics and pharmacodynamics. Chemical activation of Nrf2 by pretreatment with tBHQ increases the survival of neuroblastoma cell in response to three chemotherapeutic drugs, namely cisplatin, doxorubicin, and etoposide.12 Drug resistance toward tamoxifen in MCF‐7 cell lines driven by Nrf2 activation is attributed to antioxidant enzymes, including Trx, Prx, and GCL.11 Several phase II enzymes regulated by Nrf2 have also proved to be chemoresistance. The downregulation of Nrf2 by siRNA rendered cancer cells more susceptible to cisplatin due to the suppression of NQO1.126 The transient transfection of GSTP1 enhances the drug resistance to adriamycin, cisplatin, melphalan, and etoposide in colon cancer cells.127 The chemoresistance role of Nrf2 is consistent by repressing HO‐1 in lung carcinoma A549 cells.32 Notably, the depletion of GSH elevated the drug sensitivity to cisplatin, and the amplification of cellular GSH level protects against toxic effects of cytotoxic drug both in vivo and in vitro, indicating a role for GSH in chemoresistance.126, 128 Moreover, Nrf2 overactivation drives the expression of MRPs, thus reduces anticancer drug accumulation in cancer cells.129, 130 These efflux transport proteins have been an attractive target for many researchers to prevent chemoresistance, such as identifying peptides that hindering MRPs, finding the way to downregulate MRP genes, or designing new drugs that are not substrate of MRPs. Peroxisome proliferators activator receptors gamma (PPARγ) has been reported to effectively suppress cancer progression.132 When treated with Keap1 shRNA, PPARγ of the non‐small cell lung cancer (NSCLC) cells increase, and cells were more susceptible to arsenic trioxide, etoposide, and doxorubicin, suggesting that Nrf2 contributes to drug resistance by the downregulation of PPARγ.133 To summarize, when Nrf2 is overactivated in cancer cells, specific antioxidant enzymes, phase II enzymes, and transporters are excessively expressed. These downstream proteins hinder the entry of drug into the cells and block the drugs decomposition rate and efflux in cells to decrease the efficacy of drugs.
3.7. Nrf2 enhances radioresistance of cancer cells
Radiation therapy leads to cancer cell death through the generation of ROS with the combination of chemotherapeutic agents.134 However, persistent activation of Nrf2 significantly enhances the cancer cell resistance to ROS by upregulating antioxidant enzymes and attenuating the sensitivity to cytotoxic chemotherapeutic agents.135 The upregulated Nrf2 in radiation therapy is found to be associated with higher expression of HO‐1, NQO1, Prx, and other Nrf2 downstream targets that promote GSH synthesis. Nrf2 also establishes the crosstalk with other radioresistance‐related signaling pathways such as HIF‐1136 and NF‐kB137 against radiotherapy. Reduction of Nrf2 levels in NSCLC cells led to a dramatic increase in endogenous ROS levels. Similarly, significantly higher γ‐irradiation‐induced protein carbonyl levels were observed in Nrf2‐depleted lung cancer cells. These findings conclude that Nrf2 confers to radioresistance against ionizing radiation toxicity. Targeting Nrf2 activity in tumors may be an effective method to avoid radioresistance.138
3.8. Nrf2 inhibitors
As the pro‐tumorigenic role of Nrf2 in cancer cells has been clearly shown, pharmacological modulation of the Nrf2 pathway offers novel therapeutic opportunities for oxidative stress‐related diseases, such as cancer, diabetes, Alzheimer's disease, arteriosclerosis, inflammation, and myocarditis. Most of currently known Nrf2 activators (eg, curcumin, sulforaphane, and oltipraz) lack specificity, leading to the rising risk of “off‐target” toxic effects due to their ability to react with the cysteine residues of other enzymes and proteins. Another problem is the metabolic instability, poor membrane permeability, and low bioavailability of some Nrf2 modulators, such as curcumin and its analogs. Demanding the modulators of Nrf2‐Keap1 pathway, therefore, requires not only potent efficacy but also good bioavailability and specificity.
A number of natural compounds and synthetics have been identified as Nrf2 inhibitors, but currently none has yielded strong and practicable results. Ascorbic acid (Vitamin C) prevents Nrf2‐mediated redox imbalance caused by hydrogen peroxide and UV irradiation, however, cannot efficiently protect cells from apoptosis by metabolic dysregulation.139 Retinoic acid is a metabolite of dietary vitamin A, which binds to RARα and inhibits Nrf2 binding to the ARE.140 These vitamins and their derivatives do not exert potent inhibitory of Nrf2, and the high concentrations may result in opposite effects.
A large number of substances extracted from natural compounds were proved to exert Nrf2 inhibitory effect. EGCG (Epigallocatechin 3‐gallate), the major polyphenol found in green tea, shows the capability of suppressing Nrf2 activity and reducing HO‐1 expression, although the concentrations of EGCG required for its inhibitory effect were high (>200 μmol/L).141 Luteolin, a polyphenolic flavonoid, elicited a dramatic reduction in Nrf2 at both the mRNA and the protein levels in A549 cells142 and cholangiocarcinoma cells,143 resulting in downregulation of Nrf2 target genes. However, Luteolin shows an opposite effect of activating Nrf2 in several other cell lines (eg, PC12, HepG2)144, 145 and rat models (eg, colorectal cancer).146 Hence, luteolin function as an Nrf2 modulator still needs to be considered. Other flavonoids activate Nrf2 pathway includes procyanidin,147 apigenin,148 and chrysin.149 Overall, the effects of flavonoids on the Nrf2/ARE pathway appear to be cell type‐specific, concentration dependent, and stage dependent and may vary depending on cancer properties.
Brusatol, a component of Brucea javanica seeds, strongly reduces the protein level of Nrf2 in A549 cells, sensitizing them to cisplatin and other chemotherapeutic drugs.150 Nevertheless, Brusatol was proved lately to be a nonspecific inhibitor as it rapidly and significantly reduces the level of a large amount of proteins, demonstrating its potential role as a protein translation machinery inhibitor.151 Ochratoxin A (OTA), produced by Aspergillusand Penicilliumspecies, is both a nephrotoxin and a renal carcinogen. OTA dramatically alleviates the mRNA level of Nrf2 as well as GCL and GST levels in LLC‐PK1 cells.152 There are several potential mechanisms for OTA‐induced Nrf2 inhibition: (a) blocking nuclear import of Nrf2; (b) reducing Nrf2‐DNA binding; (c) epigenetic modification of Nrf2 through upregulation of miR‐132.153 In another study, reduction by OTA in Nrf2‐dependent gene expression was observed in the kidney, but not liver.154 Coupled with the facts that OTA possess severe toxicity, this may not be an ideal agent for clinical purpose.
A few substances isolated from traditional herbs are also identified to be Nrf2 inhibitors. Cryptotanshinone, extracted from Salvia miltiorrhiza, inhibits Nrf2 protein expression and sensitizes A549 cells to cisplatin. However, its poor bioactivity is a major obstacle to utilize cryptotanshinone in clinical therapy.155 Wogonin (5,7‐dihydroxy‐8‐methoxyflavone), isolated form Scutellariae radix, is known to have anti‐inflammatory, antiviral, and anticancer effects. It reduces Nrf2 activity by suppressing the PI3K/Akt and Stat3/NF‐κB signaling and reverses chemoresistance.156, 157 Inversely, wogonin has also been reported to activate Nrf2 expression to exerts its antioxidant and anti‐inflammatory effects. The roles of wogonin in Nrf2 modulatory are contradictory though wogonin has low toxicity and good pharmacokinetic properties.157
Some approved medicines for the treatment of various diseases are found to block Nrf2 activity. All‐trans‐retinoic acid (ATRA), approved for medical use for the treatment of acne and acute promyelocytic leukemia, has been proposed as a specific Nrf2 inhibitor, which enables Nrf2 forms a complex with retinoid X receptor alpha (RARα), blocking activation of the Nrf2 pathway to suppress chemoresistance.140 ATRA at different concentrations elicits distinct Nrf2 modulation. Micromolar concentration of ATRA suppresses Nrf2 activation; in contrast, highly toxic concentration (10−7‐10−5 mol/L) of ATRA activates Nrf2 and induces Nrf2 target genes.158 Halofuginone is the derivative of febrifugine which is a bioactive component of the traditional Chinese medical herb Dichroa febrifuga. It has now been tested in phase II clinical trials for cancer and fibrotic diseases.159, 160 Recently, halofuginone was found to decrease Nrf2 protein synthesis by inhibiting prolyl‐tRNA synthetase, although halofuginone is not a specific inhibitor of Nrf2. However, cotreatment with halofuginone strengths the effects of conventional anticancer drugs in a xenograft tumor model, such as cisplatin or doxorubicin, illustrating a novel therapeutic cotreatment that alleviates Nrf2‐mediated chemoresistance.161 Metformin, a drug widely used in the treatment of type 2 diabetes, reduces mRNA and protein levels of Nrf2 through the suppression of Raf/ERK/Nrf2 signaling.162 Another postulated mechanism is that metformin may induce miR‐34a that decreases the protein expression of Nrf2 through the Sirt1/PGC‐1α/Nrf2 signaling.163 Conversely, metformin represses cancer cell growth and induces autophagy through the AMPK/mTOR pathway, which could also potentially activates Nrf2 in a p62‐dependent manner.164 Another therapeutic drug for type II diabetes, trigonelline inhibits Nrf2‐dependent proteasomal activity and interferes with Nrf2 nuclear import at low concentrations (0.0001‐1 μmol/L).165 The research of metformin and trigonelline in combination therapy with cancer chemotherapeutic drugs is currently ongoing. Clobetasol propionate, a glucocorticoid for various skin disorders, has been demonstrated as a potent Nrf2 inhibitor due to its capability of preventing nuclear accumulation and promoted β‐TrCP‐dependent degradation of Nrf2 in a glucocorticoid receptor‐ and a GSK3‐dependent manner. The combination of clobetasol propionate and rapamycin is proposed as a potential therapeutic strategy for tumors harboring both Keap1 and LKB1 mutations. On the basis of screening, the study also proposed that three classes of drugs: glucocorticoids, cardiac glycosides, and antimetabolites have the potential to be potent Nrf2 inhibitors.166
Some studies have discovered small molecule inhibitors of Nrf2 by high‐throughput screening of compound libraries. ML385, a thiazole‐indoline compound that specifically binds to Neh1 domain of Nrf2 and interferes with the binding of the MafG‐Nrf2 interaction to inhibit Nrf2 pathway. In preclinical models of Nrf2‐mediated NSCLC, ML385 shows significant antitumor activity in combination with carboplatin.167 ARE expression modulator 1 (AEM1) contains a thienopyrimidine structure and inhibits mRNA and protein expression of Nrf2. Interestingly, AEM1 exerts its inhibitory role by an unknown mechanism other than altering the protein levels of Nrf2 or Keap1. A cyclin‐dependent kinases (CDK) inhibitor, PHA‐767491, was identified to be a potent inhibitor of Nrf2 transcriptional activity through a quantitative high‐throughput screening.168 The mechanism of its inhibitory effect needs further investigation. IM3829 (4‐[2‐Cyclohexylethoxy] aniline) decreases Nrf2 mRNA and protein levels, and combined treatment with radiation is able to significantly inhibit cancer cell survival, suggesting a promising radiosensitizer in lung cancer treatment.135 By screening a siRNA library that targets the majority of the druggable genome, a study found that Grassypeptolide A, an active peptide isolated from marine cyanobacteria contains actin, is able to attenuate Nrf2 activity.169 However, the cytotoxicity and bioactivity of these compounds are still unclear.
Moreover, miRNAs that negatively regulate Nrf2 expression offer additional putative targets to manipulate Nrf2 pathway. miR‐144 was the first identified miRNA for the decreasing of Nrf2 protein level via targeting two distinct sites in the Nrf2 untranslated region.170 Similarly, miR‐28 targets the 3'UTR of Nrf2 mRNA and decreases Nrf2 protein expression.171
Identified proteins that involved in the regulation of Nrf2 signaling pathway, such as protein kinases, provide more opportunities to modulate Nrf2 pathway. For instance, GSK‐3β has been observed to indirectly modulate Nrf2 via tyrosine‐protein kinase Fyn phosphorylation. Fyn translocates to the nucleus resultant to GSK‐3β phosphorylation, and in turn phosphorylates Nrf2, which stimulates its activation. GSK‐3β inhibitors for the blockage of Nrf2 have been studied for neurodegenerative diseases including Alzheimer's disease (AD) and brain ischemia.172 This allows pre‐existing drug, such as kinase inhibitors, to inhibit Nrf2 activation and potentially represses cancer development.
4. CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Overall, the role of Nrf2 activation in cancer is paradoxical and requires further exploration. For the prevention of chronic diseases and cancer in which oxidative and inflammatory stress contributes to the pathogenesis, enhancing Nrf2 activity is still a traditional and effective approach. However, studies in the past few decades have proposed that the overactivation of Nrf2 promotes cancer cell growth and proliferation, blocks cell apoptosis, strengthens CSCs self‐renewal capacity, most importantly, enhances the chemoresistance and radioresistance of cancer cells. Hence, it is reasonable to consider that blocking the Nrf2 activity in fully malignant cells may be a considerable way for cancer prevention. Previous works have provided a large number of Nrf2 inhibitors that regulate Nrf2 at different levels and proved the anticancer effect of Nrf2 inhibition. However, currently, none has yielded strong and practicable results. A few small molecules discovered currently display promising availabilities in Nrf2 inhibition, which still needs to be further investigated and optimized. An ideal inhibitor for clinical application requires not only potent efficiency and specificity but also less toxicity, good bioactivity, and pharmacokinetics. A better strategy is probably not only to focus on directly targeting Nrf2 but also to explore indirect methods such as the inhibition of upstream miRNAs or protein kinases.
CONFLICT OF INTEREST
There are no conflicts of interest to this work.
AUTHOR CONTRIBUTIONS
Shijia Wu wrote the original draft. Hong Lu and Yongheng Bai reviewed and edited the manuscript.
ACKNOWLEDGMENTS
This study was sponsored by Wenzhou Science and Technology Plan Project, China (Grant No. Y20180100).
Wu S, Lu H, Bai Y. Nrf2 in cancers: A double‐edged sword. Cancer Med. 2019;8:2252–2267. 10.1002/cam4.2101
REFERENCES
- 1. Motohashi H, Katsuoka F, Engel JD, Yamamoto M. Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1‐Nrf2 regulatory pathway. Proc Natl Acad Sci U S A. 2004;101(17):6379‐6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Motohashi H, Yamamoto M. Nrf2‐Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10(11):549‐557. [DOI] [PubMed] [Google Scholar]
- 3. Tong Ki, Padmanabhan B, Kobayashi A, et al. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol Cell Biol. 2007;27(21):7511‐7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nioi P, Nguyen T, Sherratt PJ, Pickett CB. The carboxy‐terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol Cell Biol. 2005;25(24):10895‐10906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Redox‐regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox‐sensitive Neh2 degron and the redox‐insensitive Neh6 degron. J Biol Chem. 2004;279(30):31556‐31567. [DOI] [PubMed] [Google Scholar]
- 6. Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD. Nrf2 is controlled by two distinct beta‐TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK‐3 activity. Oncogene. 2013;32(32):3765‐3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two‐site molecular recognition model. Mol Cell Biol. 2006;26(8):2887‐2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kobayashi A, Kang M‐i, Okawa H, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3‐based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24(16):7130‐7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Itoh K, Chiba T, Takahashi S, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313‐322. [DOI] [PubMed] [Google Scholar]
- 10. Kobayashi A, Kang M‐i, Watai Y, et al. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26(1):221‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim SK, Yang JW, Kim MR, et al. Increased expression of Nrf2/ARE‐dependent anti‐oxidant proteins in tamoxifen‐resistant breast cancer cells. Free Radic Biol Med. 2008;45(4):537‐546. [DOI] [PubMed] [Google Scholar]
- 12. Wang X‐j, Sun Z, Villeneuve Nf, et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29(6):1235‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. MacLeod AK, Acosta‐Jimenez L, Coates PJ, et al. Aldo‐keto reductases are biomarkers of NRF2 activity and are co‐ordinately overexpressed in non‐small cell lung cancer. Br J Cancer. 2016;115(12):1530‐1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miura T, Taketomi A, Nishinaka T, Terada T. Regulation of human carbonyl reductase 1 (CBR1, SDR21C1) gene by transcription factor Nrf2. Chem Biol Interact. 2013;202(1–3):126‐135. [DOI] [PubMed] [Google Scholar]
- 15. Goto M, Kitamura H, Alam MM, et al. Alcohol dehydrogenase 3 contributes to the protection of liver from nonalcoholic steatohepatitis. Genes Cells. 2015;20(6):464‐480. [DOI] [PubMed] [Google Scholar]
- 16. Duong HQ, You KS, Oh S, Kwak SJ, Seong YS. Silencing of NRF2 reduces the expression of ALDH1A1 and ALDH3A1 and sensitizes to 5‐FU in pancreatic cancer cells. Antioxidants (Basel). 2017;6(3):E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ashino T, Ohkubo‐Morita H, Yamamoto M, Yoshida T, Numazawa S. Possible involvement of nuclear factor erythroid 2‐related factor 2 in the gene expression of Cyp2b10 and Cyp2a5. Redox Biol. 2014;2:284‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen YT, Shi D, Yang D, Yan B. Antioxidant sulforaphane and sensitizer trinitrobenzene sulfonate induce carboxylesterase‐1 through a novel element transactivated by nuclear factor‐E2 related factor‐2. Biochem Pharmacol. 2012;84(6):864‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yama K, Sato K, Murao Y, Tatsunami R, Tampo Y. Epalrestat upregulates heme oxygenase‐1, superoxide dismutase, and catalase in cells of the nervous system. Biol Pharm Bull. 2016;39(9):1523‐1530. [DOI] [PubMed] [Google Scholar]
- 20. Su S, Yang X, Omiecinski CJ. Intronic DNA elements regulate Nrf2 chemical responsiveness of the human microsomal epoxide hydrolase gene (EPHX1) through a far upstream alternative promoter. Biochim Biophys Acta. 2014;1839(6):493‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yueh MF, Tukey RH. Nrf2‐Keap1 signaling pathway regulates human UGT1A1 expression in vitro and in transgenic UGT1 mice. J Biol Chem. 2007;282(12):8749‐8758. [DOI] [PubMed] [Google Scholar]
- 22. Shih AY, Johnson DA, Wong G, et al. Coordinate regulation of glutathione biosynthesis and release by Nrf2‐expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23(8):3394‐3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alnouti Y, Klaassen CD. Regulation of sulfotransferase enzymes by prototypical microsomal enzyme inducers in mice. J Pharmacol Exp Ther. 2008;324(2):612‐621. [DOI] [PubMed] [Google Scholar]
- 24. Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol. 2013;100:30‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Habib E, Linher‐Melville K, Lin HX, Singh G. Expression of xCT and activity of system xc(‐) are regulated by NRF2 in human breast cancer cells in response to oxidative stress. Redox Biol. 2015;5:33‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Solis WA, Dalton TP, Dieter MZ, et al. Glutamate‐cysteine ligase modifier subunit: mouse Gclm gene structure and regulation by agents that cause oxidative stress. Biochem Pharmacol. 2002;63(9):1739‐1754. [DOI] [PubMed] [Google Scholar]
- 27. Singh A, Rangasamy T, Thimmulappa RK, et al. Glutathione peroxidase 2, the major cigarette smoke‐inducible isoform of GPX in lungs, is regulated by Nrf2. Am J Respir Cell Mol Biol. 2006;35(6):639‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim YJ, Ahn JY, Liang P, Ip C, Zhang Y, Park YM. Human prx1 gene is a target of Nrf2 and is up‐regulated by hypoxia/reoxygenation: implication to tumor biology. Cancer Res. 2007;67(2):546‐554. [DOI] [PubMed] [Google Scholar]
- 29. Kim YC, Masutani H, Yamaguchi Y, Itoh K, Yamamoto M, Yodoi J. Hemin‐induced activation of the thioredoxin gene by Nrf2. A differential regulation of the antioxidant responsive element by a switch of its binding factors. J Biol Chem. 2001;276(21):18399‐18406. [DOI] [PubMed] [Google Scholar]
- 30. Sakurai A, Nishimoto M, Himeno S, et al. Transcriptional regulation of thioredoxin reductase 1 expression by cadmium in vascular endothelial cells: role of NF‐E2‐related factor‐2. J Cell Physiol. 2005;203(3):529‐537. [DOI] [PubMed] [Google Scholar]
- 31. Soriano FX, Léveillé F, Papadia S, et al. Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H–1,2‐dithiole‐3‐thione. J Neurochem. 2008;107(2):533‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim H‐R, Kim S, Kim E‐J, et al. Suppression of Nrf2‐driven heme oxygenase‐1 enhances the chemosensitivity of lung cancer A549 cells toward cisplatin. Lung Cancer. 2008;60(1):47‐56. [DOI] [PubMed] [Google Scholar]
- 33. Foresti R, Bains SK, Pitchumony TS, et al. Small molecule activators of the Nrf2‐HO‐1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharmacol Res. 2013;76:132‐148. [DOI] [PubMed] [Google Scholar]
- 34. Han L, Batistel F, Ma Y, Alharthi A, Parys C, Loor JJ. Methionine supply alters mammary gland antioxidant gene networks via phosphorylation of nuclear factor erythroid 2‐like 2 (NFE2L2) protein in dairy cows during the periparturient period. J Dairy Sci. 2018;101(9):8505‐8512. [DOI] [PubMed] [Google Scholar]
- 35. Pietsch EC, Chan JY, Torti FM, Torti SV. Nrf2 mediates the induction of ferritin H in response to xenobiotics and cancer chemopreventive dithiolethiones. J Biol Chem. 2003;278(4):2361‐2369. [DOI] [PubMed] [Google Scholar]
- 36. Mitsuishi Y, Taguchi K, Kawatani Y, et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22(1):66‐79. [DOI] [PubMed] [Google Scholar]
- 37. Maher JM, Dieter MZ, Aleksunes LM, et al. Oxidative and electrophilic stress induces multidrug resistance‐associated protein transporters via the nuclear factor‐E2‐related factor‐2 transcriptional pathway. Hepatology. 2007;46(5):1597‐1610. [DOI] [PubMed] [Google Scholar]
- 38. Cheng X, Maher J, Dieter MZ, Klaassen CD. Regulation of mouse organic anion‐transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos. 2005;33(9):1276‐1282. [DOI] [PubMed] [Google Scholar]
- 39. Jeddi F, Soozangar N, Sadeghi MR, et al. Nrf2 overexpression is associated with P‐glycoprotein upregulation in gastric cancer. Biomed Pharmacother. 2018;97:286‐292. [DOI] [PubMed] [Google Scholar]
- 40. Ludtmann MH, Angelova PR, Zhang Y, Abramov AY, Dinkova‐Kostova AT. Nrf2 affects the efficiency of mitochondrial fatty acid oxidation. Biochem J. 2014;457(3):415‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shin S, Wakabayashi J, Yates MS, et al. Role of Nrf2 in prevention of high‐fat diet‐induced obesity by synthetic triterpenoid CDDO‐imidazolide. Eur J Pharmacol. 2009;620(1–3):138‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsuji G, Takahara M, Uchi H, et al. Identification of ketoconazole as an AhR‐Nrf2 activator in cultured human keratinocytes: the basis of its anti‐inflammatory effect. J Invest Dermatol. 2012;132(1):59‐68. [DOI] [PubMed] [Google Scholar]
- 43. Lee C. Collaborative Power of Nrf2 and PPARgamma activators against metabolic and drug‐induced oxidative injury. Oxid Med Cell Longev. 2017;2017:1378175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hou Y, Xue P, Bai Y, et al. Nuclear factor erythroid‐derived factor 2‐related factor 2 regulates transcription of CCAAT/enhancer‐binding protein beta during adipogenesis. Free Radic Biol Med. 2012;52(2):462‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang H, Liu K, Geng M, et al. RXRalpha inhibits the NRF2‐ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013;73(10):3097‐3108. [DOI] [PubMed] [Google Scholar]
- 46. Hayes JD, McLellan LI. Glutathione and glutathione‐dependent enzymes represent a co‐ordinately regulated defence against oxidative stress. Free Radic Res. 1999;31(4):273‐300. [DOI] [PubMed] [Google Scholar]
- 47. DeNicola GM, Karreth FA, Humpton TJ, et al. Oncogene‐induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475(7354):106‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Enomoto A, Itoh K, Nagayoshi E, et al. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE‐regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59(1):169‐177. [DOI] [PubMed] [Google Scholar]
- 49. Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte‐specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. 2006;339(1):79‐88. [DOI] [PubMed] [Google Scholar]
- 50. Frohlich DA, McCabe MT, Arnold RS, Day ML. The role of Nrf2 in increased reactive oxygen species and DNA damage in prostate tumorigenesis. Oncogene. 2008;27(31):4353‐4362. [DOI] [PubMed] [Google Scholar]
- 51. Xu C, Huang MT, Shen G, et al. Inhibition of 7,12‐dimethylbenz(a)anthracene‐induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2‐related factor 2. Cancer Res. 2006;66(16):8293‐8296. [DOI] [PubMed] [Google Scholar]
- 52. Marhenke S, Lamlé J, Buitrago‐Molina LE, et al. Activation of nuclear factor E2‐related factor 2 in hereditary tyrosinemia type 1 and its role in survival and tumor development. Hepatology. 2008;48(2):487‐496. [DOI] [PubMed] [Google Scholar]
- 53. Honkura Y, Matsuo H, Murakami S, et al. NRF2 is a key target for prevention of noise‐induced hearing loss by reducing oxidative damage of cochlea. Sci Rep. 2016;6:19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chu FF, Esworthy RS, Doroshow JH. Role of Se‐dependent glutathione peroxidases in gastrointestinal inflammation and cancer. Free Radic Biol Med. 2004;36(12):1481‐1495. [DOI] [PubMed] [Google Scholar]
- 55. Ohashi S, Nishio A, Nakamura H, et al. Overexpression of redox‐active protein thioredoxin‐1 prevents development of chronic pancreatitis in mice. Antioxid Redox Signal. 2006;8(9–10):1835‐1845. [DOI] [PubMed] [Google Scholar]
- 56. Chowdhry S, Nazmy MH, Meakin PJ, et al. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radic Biol Med. 2010;48(2):357‐371. [DOI] [PubMed] [Google Scholar]
- 57. Lee IT, Luo SF, Lee CW, et al. Overexpression of HO‐1 protects against TNF‐alpha‐mediated airway inflammation by down‐regulation of TNFR1‐dependent oxidative stress. Am J Pathol. 2009;175(2):519‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rushworth SA, MacEwan DJ, O'Connell MA. Lipopolysaccharide‐induced expression of NAD(P)H:quinone oxidoreductase 1 and heme oxygenase‐1 protects against excessive inflammatory responses in human monocytes. J Immunol. 2008;181(10):6730‐6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gorrini C, Baniasadi PS, Harris IS, et al. BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J Exp Med. 2013;210(8):1529‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen W, Sun Z, Wang X‐J, et al. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2‐mediated antioxidant response. Mol Cell. 2009;34(6):663‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Niture SK, Khatri R, Jaiswal AK. Regulation of Nrf2‐an update. Free Radic Biol Med. 2014;66:36‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Long DJ 2nd, Waikel RL, Wang XJ, Perlaky L, Roop DR, Jaiswal AK. NAD(P)H:quinone oxidoreductase 1 deficiency increases susceptibility to benzo(a)pyrene‐induced mouse skin carcinogenesis. Cancer Res. 2000;60(21):5913‐5915. [PubMed] [Google Scholar]
- 63. Ramos‐Gomez M, Kwak M‐k, Dolan Pm, et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor‐deficient mice. Proc Natl Acad Sci U S A. 2001;98(6):3410‐3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kitamura Y, Umemura T, Kanki K, et al. Increased susceptibility to hepatocarcinogenicity of Nrf2‐deficient mice exposed to 2‐amino‐3‐methylimidazo[4,5‐f]quinoline. Cancer Sci. 2007;98(1):19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Iida K, Itoh K, Maher Jm, et al. Nrf2 and p53 cooperatively protect against BBN‐induced urinary bladder carcinogenesis. Carcinogenesis. 2007;28(11):2398‐2403. [DOI] [PubMed] [Google Scholar]
- 66. Wakabayashi N, Dinkova‐Kostova AT, Holtzclaw WD, et al. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci U S A. 2004;101(7):2040‐2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gamet‐Payrastre L, Li P, Lumeau S, et al. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60(5):1426‐1433. [PubMed] [Google Scholar]
- 68. Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor kappa B is a molecular target for sulforaphane‐mediated anti‐inflammatory mechanisms. J Biol Chem. 2001;276(34):32008‐32015. [DOI] [PubMed] [Google Scholar]
- 69. Dickinson SE, Melton TF, Olson ER, Zhang J, Saboda K, Bowden GT. Inhibition of activator protein‐1 by sulforaphane involves interaction with cysteine in the cFos DNA‐binding domain: implications for chemoprevention of UVB‐induced skin cancer. Cancer Res. 2009;69(17):7103‐7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liby Kt, Royce Db, Risingsong R, et al. Synthetic triterpenoids prolong survival in a transgenic mouse model of pancreatic cancer. Cancer Prev Res (Phila). 2010;3(11):1427‐1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kim E‐h, Deng C, Sporn Mb, et al. CDDO‐methyl ester delays breast cancer development in BRCA1‐mutated mice. Cancer Prev Res (Phila). 2012;5(1):89‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Solis Lm, Behrens C, Dong W, et al. Nrf2 and Keap1 abnormalities in non‐small cell lung carcinoma and association with clinicopathologic features. Clin Cancer Res. 2010;16(14):3743‐3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yoo NJ, Kim HR, Kim YR, An CH, Lee SH. Somatic mutations of the KEAP1 gene in common solid cancers. Histopathology. 2012;60(6):943‐952. [DOI] [PubMed] [Google Scholar]
- 74. Kim YR, Oh JE, Kim MS, et al. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J Pathol. 2010;220(4):446‐451. [DOI] [PubMed] [Google Scholar]
- 75. Ooi A, Dykema K, Ansari A, et al. CUL3 and NRF2 mutations confer an NRF2 activation phenotype in a sporadic form of papillary renal cell carcinoma. Cancer Res. 2013;73(7):2044‐2051. [DOI] [PubMed] [Google Scholar]
- 76. Goldstein L, Lee J, Gnad F, et al. Recurrent loss of NFE2L2 Exon 2 is a mechanism for Nrf2 pathway activation in human cancers. Cell Rep. 2016;16(10):2605‐2617. [DOI] [PubMed] [Google Scholar]
- 77. Hanada N, Takahata T, Zhou Q, et al. Methylation of the KEAP1 gene promoter region in human colorectal cancer. BMC Cancer. 2012;12:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ma J, Cai H, Wu T, et al. PALB2 interacts with KEAP1 to promote NRF2 nuclear accumulation and function. Mol Cell Biol. 2012;32(8):1506‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Komatsu M, Kurokawa H, Waguri S, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12(3):213‐223. [DOI] [PubMed] [Google Scholar]
- 80. Hast Be, Goldfarb D, Mulvaney Km, et al. Proteomic analysis of ubiquitin ligase KEAP1 reveals associated proteins that inhibit NRF2 ubiquitination. Cancer Res. 2013;73(7):2199‐2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Adam J, Hatipoglu E, O'Flaherty L, et al. Renal cyst formation in Fh1‐deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell. 2011;20(4):524‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wu KC, Cui JY, Klaassen CD. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol Sci. 2011;123(2):590‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kitteringham NR, Abdullah A, Walsh J, et al. Proteomic analysis of Nrf2 deficient transgenic mice reveals cellular defence and lipid metabolism as primary Nrf2‐dependent pathways in the liver. J Proteomics. 2010;73(8):1612‐1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Singh A, Happel C, Manna SK, et al. Transcription factor NRF2 regulates miR‐1 and miR‐206 to drive tumorigenesis. J Clin Invest. 2013;123(7):2921‐2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Malhotra D, Portales‐Casamar E, Singh A, et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP‐Seq profiling and network analysis. Nucleic Acids Res. 2010;38(17):5718‐5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Reddy Nm, Kleeberger Sr, Bream Jh, et al. Genetic disruption of the Nrf2 compromises cell‐cycle progression by impairing GSH‐induced redox signaling. Oncogene. 2008;27(44):5821‐5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Beyer TA, Xu W, Teupser D, et al. Impaired liver regeneration in Nrf2 knockout mice: role of ROS‐mediated insulin/IGF‐1 resistance. EMBO J. 2008;27(1):212‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Holmstrom Km, Baird L, Zhang Y, et al. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open. 2013;2(8):761‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chio I, Jafarnejad SM, Ponz‐Sarvise M, et al. NRF2 Promotes Tumor Maintenance by Modulating mRNA Translation in Pancreatic Cancer. Cell. 2016;166(4):963‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma‐glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem. 1999;274(47):33627‐33636. [DOI] [PubMed] [Google Scholar]
- 91. Faraonio R, Vergara P, Di Marzo D, et al. p53 suppresses the Nrf2‐dependent transcription of antioxidant response genes. J Biol Chem. 2006;281(52):39776‐39784. [DOI] [PubMed] [Google Scholar]
- 92. Elsby R, Kitteringham NR, Goldring CE, et al. Increased constitutive c‐Jun N‐terminal kinase signaling in mice lacking glutathione S‐transferase Pi. J Biol Chem. 2003;278(25):22243‐22249. [DOI] [PubMed] [Google Scholar]
- 93. Jain A, Lamark T, Sjottem E, et al. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element‐driven gene transcription. J Biol Chem. 2010;285(29):22576‐22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Niture SK, Jaiswal AK. Nrf2 protein up‐regulates antiapoptotic protein Bcl‐2 and prevents cellular apoptosis. J Biol Chem. 2012;287(13):9873‐9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rushworth SA, MacEwan DJ. HO‐1 underlies resistance of AML cells to TNF‐induced apoptosis. Blood. 2008;111(7):3793‐3801. [DOI] [PubMed] [Google Scholar]
- 96. Blanpain C, Mohrin M, Sotiropoulou PA, Passegue E. DNA‐damage response in tissue‐specific and cancer stem cells. Cell Stem Cell. 2011;8(1):16‐29. [DOI] [PubMed] [Google Scholar]
- 97. Murakami S, Shimizu R, Romeo PH, Yamamoto M, Motohashi H. Keap1‐Nrf2 system regulates cell fate determination of hematopoietic stem cells. Genes Cells. 2014;19(3):239‐253. [DOI] [PubMed] [Google Scholar]
- 98. Murakami S, Suzuki T, Harigae H, Romeo PH, Yamamoto M, Motohashi H. NRF2 activation impairs quiescence and bone marrow reconstitution capacity of hematopoietic stem cells. Mol Cell Biol. 2017;37(19):e00086‐e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Owusu‐Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461(7263):537‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhu J, Wang H, Ji X, et al. Differential Nrf2 expression between glioma stem cells and non‐stem‐like cells in glioblastoma. Oncol Lett. 2014;7(3):693‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Singer E, Judkins J, Salomonis N, et al. Reactive oxygen species‐mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015;6:e1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Karkkainen V, Pomeshchik Y, Savchenko E, et al. Nrf2 regulates neurogenesis and protects neural progenitor cells against Abeta toxicity. Stem Cells. 2014;32(7):1904‐1916. [DOI] [PubMed] [Google Scholar]
- 103. Mohammadzadeh M, Halabian R, Gharehbaghian A, et al. Nrf‐2 overexpression in mesenchymal stem cells reduces oxidative stress‐induced apoptosis and cytotoxicity. Cell Stress Chaperones. 2012;17(5):553‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Thimmulappa RK, Lee H, Rangasamy T, et al. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116(4):984‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Iizuka T, Ishii Y, Itoh K, et al. Nrf2‐deficient mice are highly susceptible to cigarette smoke‐induced emphysema. Genes Cells. 2005;10(12):1113‐1125. [DOI] [PubMed] [Google Scholar]
- 106. Taguchi K, Takaku M, Egner PA, et al. Generation of a new model rat: Nrf2 knockout rats are sensitive to aflatoxin B1 toxicity. Toxicol Sci. 2016;152(1):40‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hayashi M, Takai J, Yu L, Motohashi H, Moriguchi T, Yamamoto M. Whole‐body in vivo monitoring of inflammatory diseases exploiting human interleukin 6‐luciferase transgenic mice. Mol Cell Biol. 2015;35(20):3590‐3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chen J‐Y, Zhu G‐Y, Su X‐H, et al. 7‐deacetylgedunin suppresses inflammatory responses through activation of Keap1/Nrf2/HO‐1 signaling. Oncotarget. 2017;8(33):55051‐55063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kobayashi EH, Suzuki T, Funayama R, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhang X, Chen X, Song H, Chen HZ, Rovin BH. Activation of the Nrf2/antioxidant response pathway increases IL‐8 expression. Eur J Immunol. 2005;35(11):3258‐3267. [DOI] [PubMed] [Google Scholar]
- 111. Loboda A, Stachurska A, Florczyk U, et al. HIF‐1 induction attenuates Nrf2‐dependent IL‐8 expression in human endothelial cells. Antioxid Redox Signal. 2009;11(7):1501‐1517. [DOI] [PubMed] [Google Scholar]
- 112. Kong X, Thimmulappa R, Craciun F, et al. Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. Am J Respir Crit Care Med. 2011;184(8):928‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chen XL, Dodd G, Thomas S, et al. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol. 2006;290(5):H1862‐1870. [DOI] [PubMed] [Google Scholar]
- 114. Nadeem A, Siddiqui N, Al‐Harbi NO, Al‐Harbi MM, Ahmad SF. TLR‐7 agonist attenuates airway reactivity and inflammation through Nrf2‐mediated antioxidant protection in a murine model of allergic asthma. Int J Biochem Cell Biol. 2016;73:53‐62. [DOI] [PubMed] [Google Scholar]
- 115. Vijayan V, Baumgart‐Vogt E, Naidu S, Qian G, Immenschuh S. Bruton's tyrosine kinase is required for TLR‐dependent heme oxygenase‐1 gene activation via Nrf2 in macrophages. J Immunol. 2011;187(2):817‐827. [DOI] [PubMed] [Google Scholar]
- 116. Lee I‐t, Wang S‐w, Lee C‐w, et al. Lipoteichoic acid induces HO‐1 expression via the TLR2/MyD88/c‐Src/NADPH oxidase pathway and Nrf2 in human tracheal smooth muscle cells. J Immunol. 2008;181(7):5098‐5110. [DOI] [PubMed] [Google Scholar]
- 117. Brandenburg LO, Kipp M, Lucius R, Pufe T, Wruck CJ. Sulforaphane suppresses LPS‐induced inflammation in primary rat microglia. Inflamm Res. 2010;59(6):443‐450. [DOI] [PubMed] [Google Scholar]
- 118. Soares Mp, Seldon Mp, Gregoire Ip, et al. Heme oxygenase‐1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172(6):3553‐3563. [DOI] [PubMed] [Google Scholar]
- 119. Kim JE, You DJ, Lee C, Ahn C, Seong JY, Hwang JI. Suppression of NF‐kappaB signaling by KEAP1 regulation of IKKbeta activity through autophagic degradation and inhibition of phosphorylation. Cell Signal. 2010;22(11):1645‐1654. [DOI] [PubMed] [Google Scholar]
- 120. Liu GH, Qu J, Shen X. NF‐kappaB/p65 antagonizes Nrf2‐ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta. 2008;1783(5):713‐727. [DOI] [PubMed] [Google Scholar]
- 121. Meng D, Wang X, Chang Q, et al. Arsenic promotes angiogenesis in vitro via a heme oxygenase‐1‐dependent mechanism. Toxicol Appl Pharmacol. 2010;244(3):291‐299. [DOI] [PubMed] [Google Scholar]
- 122. Shibuya A, Onda K, Kawahara H, et al. Sofalcone, a gastric mucosa protective agent, increases vascular endothelial growth factor via the Nrf2‐heme‐oxygenase‐1 dependent pathway in gastric epithelial cells. Biochem Biophys Res Commun. 2010;398(3):581‐584. [DOI] [PubMed] [Google Scholar]
- 123. Morita K, Lee MS, Her S. Possible relation of hemin‐induced HO‐1 expression to the upregulation of VEGF and BDNF mRNA levels in rat C6 glioma cells. J Mol Neurosci. 2009;38(1):31‐40. [DOI] [PubMed] [Google Scholar]
- 124. Sunamura M, Duda DG, Ghattas MH, et al. Heme oxygenase‐1 accelerates tumor angiogenesis of human pancreatic cancer. Angiogenesis. 2003;6(1):15‐24. [DOI] [PubMed] [Google Scholar]
- 125. Kim T‐h, Hur E‐g, Kang S‐j, et al. NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia‐induced activation of HIF‐1alpha. Cancer Res. 2011;71(6):2260‐2275. [DOI] [PubMed] [Google Scholar]
- 126. Cho JM, Manandhar S, Lee HR, Park HM, Kwak MK. Role of the Nrf2‐antioxidant system in cytotoxicity mediated by anticancer cisplatin: implication to cancer cell resistance. Cancer Lett. 2008;260(1–2):96‐108. [DOI] [PubMed] [Google Scholar]
- 127. Ban N, Takahashi Y, Takayama T, et al. Transfection of glutathione S‐transferase (GST)‐pi antisense complementary DNA increases the sensitivity of a colon cancer cell line to adriamycin, cisplatin, melphalan, and etoposide. Cancer Res. 1996;56(15):3577‐3582. [PubMed] [Google Scholar]
- 128. Kaur T, Khanduja KL, Gupta R, Gupta NM, Vaiphei K. Changes in antioxidant defense status in response to cisplatin and 5‐FU in esophageal carcinoma. Dis Esophagus. 2008;21(2):103‐107. [DOI] [PubMed] [Google Scholar]
- 129. Aleksunes LM, Slitt AL, Maher JM, et al. Induction of Mrp3 and Mrp4 transporters during acetaminophen hepatotoxicity is dependent on Nrf2. Toxicol Appl Pharmacol. 2008;226(1):74‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD. Induction of the multidrug resistance‐associated protein family of transporters by chemical activators of receptor‐mediated pathways in mouse liver. Drug Metab Dispos. 2005;33(7):956‐962. [DOI] [PubMed] [Google Scholar]
- 131. Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance‐associated protein 1 in mouse embryo fibroblasts. Biochem Biophys Res Commun. 2003;310(3):824‐829. [DOI] [PubMed] [Google Scholar]
- 132. Reka AK, Goswami MT, Krishnapuram R, Standiford TJ, Keshamouni VG. Molecular cross‐regulation between PPAR‐gamma and other signaling pathways: Implications for lung cancer therapy. Lung Cancer. 2011;72(2):154‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zhan L, Zhang H, Zhang Q, et al. Regulatory role of KEAP1 and NRF2 in PPARgamma expression and chemoresistance in human non‐small‐cell lung carcinoma cells. Free Radic Biol Med. 2012;53(4):758‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jayakumar S, Kunwar A, Sandur SK, Pandey BN, Chaubey RC. Differential response of DU145 and PC3 prostate cancer cells to ionizing radiation: role of reactive oxygen species, GSH and Nrf2 in radiosensitivity. Biochim Biophys Acta. 2014;1840(1):485‐494. [DOI] [PubMed] [Google Scholar]
- 135. Lee S, Lim M‐J, Kim M‐H, et al. An effective strategy for increasing the radiosensitivity of Human lung Cancer cells by blocking Nrf2‐dependent antioxidant responses. Free Radic Biol Med. 2012;53(4):807‐816. [DOI] [PubMed] [Google Scholar]
- 136. Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF‐1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5(5):429‐441. [DOI] [PubMed] [Google Scholar]
- 137. Nair S, Doh ST, Chan JY, Kong AN, Cai L. Regulatory potential for concerted modulation of Nrf2‐ and Nfkb1‐mediated gene expression in inflammation and carcinogenesis. Br J Cancer. 2008;99(12):2070‐2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Singh A, Bodas M, Wakabayashi N, Bunz F, Biswal S. Gain of Nrf2 function in non‐small‐cell lung cancer cells confers radioresistance. Antioxid Redox Signal. 2010;13(11):1627‐1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gegotek A, Bielawska K, Biernacki M, Zareba I, Surazynski A, Skrzydlewska E. Comparison of protective effect of ascorbic acid on redox and endocannabinoid systems interactions in in vitro cultured human skin fibroblasts exposed to UV radiation and hydrogen peroxide. Arch Dermatol Res. 2017;309(4):285‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wang XJ, Hayes JD, Henderson CJ, Wolf CR. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proc Natl Acad Sci U S A. 2007;104(49):19589‐19594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kweon MH, Adhami VM, Lee JS, Mukhtar H. Constitutive overexpression of Nrf2‐dependent heme oxygenase‐1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3‐gallate. J Biol Chem. 2006;281(44):33761‐33772. [DOI] [PubMed] [Google Scholar]
- 142. Chian S, Thapa R, Chi Z, Wang XJ, Tang X. Luteolin inhibits the Nrf2 signaling pathway and tumor growth in vivo. Biochem Biophys Res Commun. 2014;447(4):602‐608. [DOI] [PubMed] [Google Scholar]
- 143. Kittiratphatthana N, Kukongviriyapan V, Prawan A, Senggunprai L. Luteolin induces cholangiocarcinoma cell apoptosis through the mitochondrial‐dependent pathway mediated by reactive oxygen species. J Pharm Pharmacol. 2016;68(9):1184‐1192. [DOI] [PubMed] [Google Scholar]
- 144. Lin CW, Wu MJ, Liu IY, Su JD, Yen JH. Neurotrophic and cytoprotective action of luteolin in PC12 cells through ERK‐dependent induction of Nrf2‐driven HO‐1 expression. J Agric Food Chem. 2010;58(7):4477‐4486. [DOI] [PubMed] [Google Scholar]
- 145. Paredes‐Gonzalez X, Fuentes F, Jeffery S, et al. Induction of NRF2‐mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm Drug Dispos. 2015;36(7):440‐451. [DOI] [PubMed] [Google Scholar]
- 146. Pandurangan AK, Ananda Sadagopan SK, Dharmalingam P, Ganapasam S. Luteolin, a bioflavonoid inhibits Azoxymethane‐induced colorectal cancer through activation of Nrf2 signaling. Toxicol Mech Methods. 2014;24(1):13‐20. [DOI] [PubMed] [Google Scholar]
- 147. Ohnuma T, Matsumoto T, Itoi A, et al. Enhanced sensitivity of A549 cells to the cytotoxic action of anticancer drugs via suppression of Nrf2 by procyanidins from Cinnamomi Cortex extract. Biochem Biophys Res Commun. 2011;413(4):623‐629. [DOI] [PubMed] [Google Scholar]
- 148. Gao AM, Zhang XY, Ke ZP. Apigenin sensitizes BEL‐7402/ADM cells to doxorubicin through inhibiting miR‐101/Nrf2 pathway. Oncotarget. 2017;8(47):82085‐82091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wang J, Wang H, Sun K, et al. Chrysin suppresses proliferation, migration, and invasion in glioblastoma cell lines via mediating the ERK/Nrf2 signaling pathway. Drug Des Devel Ther. 2018;12:721‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ren D, Villeneuve Nf, Jiang T, et al. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2‐mediated defense mechanism. Proc Natl Acad Sci U S A. 2011;108(4):1433‐1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Vartanian S, Ma TP, Lee J, et al. Application of mass spectrometry profiling to establish brusatol as an inhibitor of global protein synthesis. Mol Cell Proteomics. 2016;15(4):1220‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Boesch‐Saadatmandi C, Wagner AE, Graeser AC, Hundhausen C, Wolffram S, Rimbach G. Ochratoxin A impairs Nrf2‐dependent gene expression in porcine kidney tubulus cells. J Anim Physiol Anim Nutr (Berl). 2009;93(5):547‐554. [DOI] [PubMed] [Google Scholar]
- 153. Limonciel A, Jennings P. A review of the evidence that ochratoxin A is an Nrf2 inhibitor: implications for nephrotoxicity and renal carcinogenicity. Toxins (Basel). 2014;6(1):371‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Marin‐Kuan M, Nestler S, Verguet C, et al. A toxicogenomics approach to identify new plausible epigenetic mechanisms of ochratoxin a carcinogenicity in rat. Toxicol Sci. 2006;89(1):120‐134. [DOI] [PubMed] [Google Scholar]
- 155. Xia C, Bai X, Hou X, et al. Cryptotanshinone reverses cisplatin resistance of human lung carcinoma A549 cells through down‐regulating Nrf2 pathway. Cell Physiol Biochem. 2015;37(2):816‐824. [DOI] [PubMed] [Google Scholar]
- 156. Xu X, Zhang X, Zhang Y, et al. Wogonin reversed resistant human myelogenous leukemia cells via inhibiting Nrf2 signaling by Stat3/NF‐kappaB inactivation. Sci Rep. 2017;7:39950. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 157. Yao J, Zhao L, Zhao Q, et al. NF‐kappaB and Nrf2 signaling pathways contribute to wogonin‐mediated inhibition of inflammation‐associated colorectal carcinogenesis. Cell Death Dis. 2014;5:e1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Tan KP, Kosuge K, Yang M, Ito S. NRF2 as a determinant of cellular resistance in retinoic acid cytotoxicity. Free Radic Biol Med. 2008;45(12):1663‐1673. [DOI] [PubMed] [Google Scholar]
- 159. Elkin M, Ariel I, Miao HQ, et al. Inhibition of bladder carcinoma angiogenesis, stromal support, and tumor growth by halofuginone. Cancer Res. 1999;59(16):4111‐4118. [PubMed] [Google Scholar]
- 160. Pines M, Snyder D, Yarkoni S, Nagler A. Halofuginone to treat fibrosis in chronic graft‐versus‐host disease and scleroderma. Biol Blood Marrow Transplant. 2003;9(7):417‐425. [DOI] [PubMed] [Google Scholar]
- 161. Tsuchida K, Tsujita T, Hayashi M, et al. Halofuginone enhances the chemo‐sensitivity of cancer cells by suppressing NRF2 accumulation. Free Radic Biol Med. 2017;103:236‐247. [DOI] [PubMed] [Google Scholar]
- 162. Do MT, Kim HG, Khanal T, et al. Metformin inhibits heme oxygenase‐1 expression in cancer cells through inactivation of Raf‐ERK‐Nrf2 signaling and AMPK‐independent pathways. Toxicol Appl Pharmacol. 2013;271(2):229‐238. [DOI] [PubMed] [Google Scholar]
- 163. Do MT, Kim HG, Choi JH, Jeong HG. Metformin induces microRNA‐34a to downregulate the Sirt1/Pgc‐1alpha/Nrf2 pathway, leading to increased susceptibility of wild‐type p53 cancer cells to oxidative stress and therapeutic agents. Free Radic Biol Med. 2014;74:21‐34. [DOI] [PubMed] [Google Scholar]
- 164. Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A. Pre‐treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis. 2015;30(3):747‐754. [DOI] [PubMed] [Google Scholar]
- 165. Arlt A, Sebens S, Krebs S, et al. Inhibition of the Nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity. Oncogene. 2013;32(40):4825‐4835. [DOI] [PubMed] [Google Scholar]
- 166. Choi E‐j, Jung B‐j, Lee S‐h, et al. A clinical drug library screen identifies clobetasol propionate as an NRF2 inhibitor with potential therapeutic efficacy in KEAP1 mutant lung cancer. Oncogene. 2017;36(37):5285‐5295. [DOI] [PubMed] [Google Scholar]
- 167. Singh A, Venkannagari S, Oh KH, et al. Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1‐Deficient NSCLC Tumors. ACS Chem Biol. 2016;11(11):3214‐3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Liu HY, Tuckett AZ, Fennell M, Garippa R, Zakrzewski JL. Repurposing of the CDK inhibitor PHA‐767491 as a NRF2 inhibitor drug candidate for cancer therapy via redox modulation. Invest New Drugs. 2018;36(4):590‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Matthews JH, Liang X, Paul VJ, Luesch H. A Complementary chemical and genomic screening approach for druggable targets in the Nrf2 pathway and small molecule inhibitors to overcome cancer cell drug resistance. ACS Chem Biol. 2018;13(5):1189‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Sangokoya C, Telen MJ, Chi JT. microRNA miR‐144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood. 2010;116(20):4338‐4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Yang M, Yao Y, Eades G, Zhang Y, Zhou Q. MiR‐28 regulates Nrf2 expression through a Keap1‐independent mechanism. Breast Cancer Res Treat. 2011;129(3):983‐991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Gameiro I, Michalska P, Tenti G, et al. Discovery of the first dual GSK3beta inhibitor/Nrf2 inducer. A new multitarget therapeutic strategy for Alzheimer's disease. Sci Rep. 2017;7:45701. [DOI] [PMC free article] [PubMed] [Google Scholar]


