Abstract
Background
Previous studies have suggested an association between the use of direct‐acting antiviral agents (DAAs) for treating hepatitis C virus (HCV) infection and the resulting decrease in the incidence of hepatocellular carcinoma (HCC); however, it is unclear whether DAAs prevent the recurrence of HCC after curative treatment for HCC. This study aimed to prospectively investigate HCC recurrence and its predictors after curative treatment for HCC.
Methods
A total of 3012 patients with chronic HCV infection, with or without cirrhosis, who were treated with DAAs were enrolled between January 1, 2015 and January 31, 2017 as per the institutional review board approved study protocol at 15 institutions, including 10 university hospitals and five high‐volume centers in the Kyusyu area of Japan. Of the 3012 patients, 459 patients who had HCC but were cured with surgery or ablation therapy (curative treatment) before the use of DAAs were included in the analysis.
Results
During a mean follow‐up period of 29.4 months, 217 (47.2%) patients developed HCC recurrence. The median time to recurrence was 34.0 months, and the 1‐, 2‐, and 3‐year cumulative HCC recurrence rates were 27.1%, 43.4%, and 50.8%, respectively. The risk factors for HCC recurrence were the α‐fetoprotein (AFP) level before DAA therapy (P = 0.0047) and the number of curative treatments for HCC before DAA therapy (P < 0.0001).
Conclusions
A high AFP level and multiple occurrences of HCC before DAA therapy are associated with a high risk for HCC recurrence after curative treatment. Follow‐up after DAA therapy should include special attention to the abovementioned risk factors.
Keywords: DAA, HCV, hepatocarcinogenesis, liver cancer, SVR
1. INTRODUCTION
Chronic hepatitis C virus (HCV) infection is a major cause of liver cirrhosis and hepatocellular carcinoma (HCC).1, 2 HCC is a frequent consequence of HCV‐related cirrhosis, with an annual incidence of 1%‐8%.3 In addition, HCV infection may promote carcinogenesis; hence, its eradication will directly decrease the risk of developing HCC.4, 5 With the recent development of interferon (IFN)‐free direct‐acting antiviral agents (DAAs), high rates of sustained virological response (SVR) have been achieved in patients with chronic HCV infection.6, 7, 8
For many decades, IFN‐based regimens had been the standard care for treating HCV infection and reducing the risk of HCC development.9, 10, 11 Risk factors for HCC are old age, advanced liver fibrosis, male sex, post‐IFN α‐fetoprotein (AFP) levels, glucose metabolism disorders, lipid metabolism disorders, and alcohol intake.12
Although the SVR with DAAs reduces the incidence of HCC, an increase in unexpected early occurrence or recurrence of HCC after HCV elimination has been reported with DAA therapies.13, 14, 15, 16, 17 Recent studies have reported that HCV‐infected patients with HCC who had an initial complete response to hepatic resection or local ablation and subsequently had DAA‐related SVR experienced an increased risk of HCC recurrence.16, 17 The annual incidence of de novo HCC after inducing SVR with DAAs has been variably reported to be 3%‐5%, which is higher than that observed with IFN‐based therapy.18, 19, 20
Although previous studies have suggested an association between the use of DAAs for treating HCV infection and resulting decrease in the incidence of HCC, it is unclear whether DAAs used for treating HCV infection can prevent the recurrence of HCC after curative treatment for HCC. This study aimed to prospectively investigate the HCC recurrence and predictors of HCC recurrence after curative treatment for HCC.
2. PATIENTS AND METHODS
2.1. Patients
In this cohort study, we recruited 3012 patients with chronic HCV infection, with or without cirrhosis, who were treated with DAAs between January 1, 2015 and January 31, 2017. Of the 3012 patients, 459 patients who had HCC but were cured with surgery or ablation therapy (curative treatment) prior to the DAA therapy were included in the analysis. We specifically focused on the identification of predictors for HCC recurrence after DAA therapy in the 459 patients with precured HCC, while the remaining 2553 patients were subjected to an independent analysis (Figure 1), because the characteristics of the two populations, and thus the risk factors for HCC development were shown to be significantly different in previous studies.11 The SVR was confirmed by the absence of serum HCV‐RNA at 12 weeks after the discontinuation of DAAs. After the SVR was obtained, the patients were followed‐up prospectively. Patients were excluded if they had hepatitis B virus surface antigen or other forms of liver disease.
Figure 1.

Flow chart detailing the criteria of patient selection
2.2. DAA therapy
The DAA therapy was provided at 15 institutions, including 10 universities and five high‐volume centers in the Kyusyu area of Japan. The therapeutic regimen included asunaprevir plus daclatasvir for 24 weeks, sofosbuvir (SOF)/ledipasvir for 12 weeks, SOF plus ribavirin for 12 weeks, and paritaprevir/ombitasvir/ritonavir for 12 weeks. The main outcome was HCC recurrence. The endpoint was the date on which HCC was diagnosed in the patients who had developed HCC or the date on which the absence of HCC was confirmed at the last follow‐up on January 31, 2018. The period of observation was defined as time from the initiation of DAA therapy to the endpoint.
2.3. Laboratory results
The results of routine tests for blood cell count, liver biochemical parameters, and AFP levels before starting the DAA therapy were collected. Aspartate aminotransferase to platelet count ratio index (APRI) was calculated as AST (/ULN)/platelet count [109/L] × 100.21 Fibrosis‐4 (FIB‐4) index was calculated as age × AST/platelet count [109/L] × ALT1/2.22 If patients had idiopathic thrombocytopenic purpura or other forms of hematological disorder, platelet count, APRI, and FIB‐4 index data were excluded. All patients had chronic HCV genotype (serotype) 1 or 2 infection. The attending physician clinically diagnosed the presence of cirrhosis. Alcoholism was defined as an average daily consumption of >75 g of ethanol. Patients with a bright liver, deep attenuation, and hepatorenal contrast on abdominal ultrasonography were diagnosed with fatty liver. A diagnosis of diabetes mellitus was made in accordance with the diagnostic criteria established by the Japan Diabetes Society.23 The protocol was approved by the institutional review boards of all hospitals, and the study was conducted in compliance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all participating patients.
2.4. HCC diagnosis
HCC was confirmed either histologically or diagnosed using noninvasive criteria according to the European Association for the Study of the Liver.24 Intrahepatic lesions and vascular invasion were diagnosed using a combination of contrast‐enhanced computed tomography, magnetic resonance imaging, and ultrasonography.
2.5. Registration of the study
This study has been registered in the UMIN Clinical Trials Registry (http://www.umin.ac.jp/ctr/) (Trial ID: UMIN000027988).
2.6. Statistical analysis
The Cox proportional‐hazards regression model was used to estimate hazards ratios for risk factors. The cumulative incidence curve was determined using the Kaplan‐Meier method, and differences between groups were assessed using the log‐rank test. For all methods, the level of significance was set at P < 0.05. All statistical analyses were performed using the JMP pro 13 software (SAS Institute, Cary, NC).
3. RESULTS
3.1. Patient characteristics
Table 1 shows the characteristics of the enrolled patients (n = 459). Table 2 shows the characteristics of patients with or without HCC recurrence after the DAA therapy. There were significant differences in sex, platelet count, AFP level, the presence of cirrhosis, and number of curative treatments for HCC before the DAA therapy between the patients with and without HCC recurrence after the DAA therapy. Mean follow‐up period was 29.4 ± 6.8 months.
Table 1.
Patient characteristics
| Patients (n = 459) | |
|---|---|
| Age before DAA therapy (y) | 74.9 ± 7.6 |
| Sex (male/female) | 269/190 |
| AST level before DAA therapy (IU/L) | 58.6 ± 31.1 |
| ALT level before DAA therapy (IU/L) | 48.5 ± 32.2 |
| GGTP level before DAA therapy (IU/L) | 48.8 ± 54.8 |
| Platelet count before DAA therapy (×104/μL) | 10.7 ± 4.9 |
| AFP level before DAA therapy (ng/mL) | 28.5 ± 67.3 |
| APRI before DAA therapy | 2.32 ± 1.81 |
| FIB‐4 index before DAA therapy | 7.10 ± 4.16 |
| Geno/serotype (1/2/1+2/unknown) | 407/49/1/2 |
| Habitual alcohol intake (presence/absence/unknown) | 55/333/71 |
| Diabetes mellitus (presence/absence/unknown) | 128/315/16 |
| Fatty liver (presence/absence/unknown) | 33/321/105 |
| Cirrhosis (presence/absence/unknown) | 323/135/1 |
| Number of curative treatments for HCC before DAA therapy (1/2/3 or more/unknown) | 262/88/105/4 |
Results are expressed as mean ± SD.
AFP, alpha‐fetoprotein; ALT, alanine aminotransferase; APRI, aspartate aminotransferase to platelet count ratio index; AST, aspartate aminotransferase; DAA, direct‐acting antiviral agents.; FIB‐4 index, Fibrosis‐4 index; GGTP, gamma‐glutamyl transpeptidase; HCC, hepatocellular carcinoma
Table 2.
Patient characteristics with or without HCC recurrence after DAA therapy
|
Patients with HCC recurrence (n = 217) |
Patients without HCC recurrence (n = 242) |
P‐value | |
|---|---|---|---|
| Age before DAA therapy (y) | 74.9 ± 7.7 | 75.0 ± 7.5 | 0.9327 |
| Sex (male/female) | 138/79 | 131/111 | 0.0399 |
| AST level before DAA therapy (IU/L) | 61.6 ± 34.7 | 55.9 ± 27.2 | 0.0518 |
| ALT level before DAA therapy (IU/L) | 50.2 ± 35.8 | 47.0 ± 28.6 | 0.2883 |
| GGTP level before DAA therapy (IU/L) | 47.4 ± 39.0 | 50.0 ± 65.8 | 0.6204 |
| Platelet count before DAA therapy (×104/μL) | 10.0 ± 4.8 | 11.4 ± 5.0 | 0.0021 |
| AFP level before DAA therapy (ng/mL) | 36.3 ± 88.3 | 21.6 ± 39.0 | 0.0196 |
| APRI before DAA therapy | 2.54 ± 1.85 | 2.12 ± 1.74 | 0.0117 |
| FIB‐4 index before DAA therapy | 7.70 ± 4.00 | 6.56 ± 4.23 | 0.0032 |
| Geno/serotype (1/2/1+2/unknown) | 190/26/0/1 | 217/23/1/1 | 0.3982 |
| Habitual alcohol intake (presence/absence/unknown) | 32/153/32 | 23/180/39 | 0.0923 |
| Diabetes mellitus (presence/absence/unknown) | 59/150/8 | 69/165/8 | 0.7707 |
| Fatty liver (presence/absence/unknown) | 16/154/47 | 17/167/58 | 0.9555 |
| Cirrhosis (presence/absence/unknown) | 169/48/0 | 154/87/1 | 0.0011 |
| Number of curative treatments for HCC before DAA therapy (1/2/3 or more/unknown) | 98/46/71/2 | 164/42/34/2 | <0.0001 |
Results are expressed as mean ± SD.
AFP, alpha‐fetoprotein; ALT, alanine aminotransferase; APRI, aspartate aminotransferase to platelet count ratio index; AST, aspartate aminotransferase; DAA, direct‐acting antiviral agents.; FIB‐4 index, Fibrosis‐4 index; GGTP, gamma‐glutamyl transpeptidase; HCC, hepatocellular carcinoma
3.2. Incidence of HCC
Of the 459 patients, 217 (47.2%) had developed HCC. Figure 2 shows time‐to‐recurrence in the enrolled patients. The median time‐to‐recurrence was 34.0 months, and the 1‐, 2‐, and 3‐year cumulative HCC recurrence rates were 27.1%, 43.4%, and 50.8%, respectively. Twelve patients (2.6%) died during the observation period. Of these, nine patients had HCC recurrence before they died. When the remaining three patients were excluded from the analysis, the 1‐, 2‐, and 3‐year cumulative HCC recurrence rates were 27.1%, 43.6%, and 51.1%, respectively.
Figure 2.
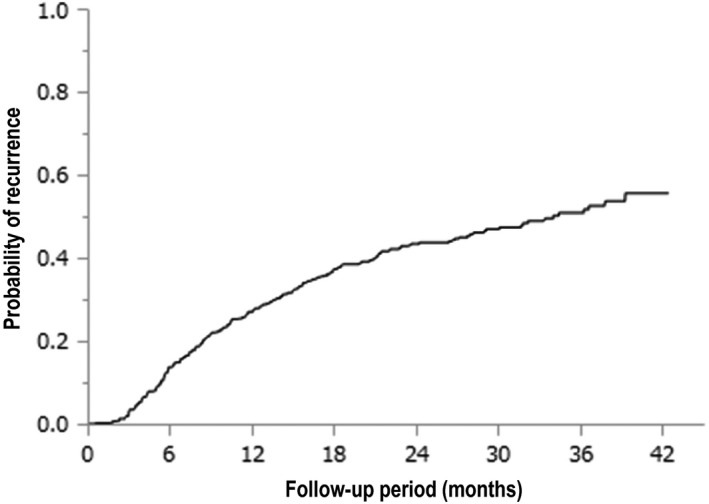
Kaplan‐Meier analysis of the time‐to‐recurrence in the enrolled patients
3.3. Risk factors associated with HCC recurrence
Table 3 shows the risk factors associated with HCC recurrence after the DAA therapy. Based on the univariate analysis, sex, platelet count, AFP level, APRI, FIB‐4 index, the presence of cirrhosis, and number of curative treatments for HCC before the DAA therapy were identified as factors that were significantly associated with HCC recurrence after the DAA therapy. On the basis of multivariate analysis, the AFP level before the DAA therapy and number of curative treatments for HCC before the DAA therapy were identified as independent factors that were significantly associated with HCC recurrence after the DAA therapy.
Table 3.
Univariate and multivariate analyses of HCC recurrence after DAA therapy in all patients
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Age before DAA therapy (by every 10 y) | 0.986 (0.827‐1.179) | 0.8841 | 0.982 (0.747‐1.291) | 0.8992 |
| Sex (female) | 1 | 1 | ||
| (male) | 1.340 (1.019‐1.774) | 0.0357 | 1.355 (0.958‐1.926) | 0.0854 |
| AST level before DAA therapy (by every 10 IU/L) | 1.033 (0.991‐1.073) | 0.1055 | 1.066 (0.937‐1.212) | 0.3290 |
| ALT level before DAA therapy (by every 10 IU/L) | 1.018 (0.976‐1.058) | 0.3802 | 0.996 (0.877‐1.131) | 0.9535 |
| GGTP level before DAA therapy (by every 10 IU/L) | 0.995 (0.965‐1.018) | 0.7610 | 0.996 (0.950‐1.044) | 0.8872 |
| Platelet count before DAA therapy (by every 104/μL) | 0.952 (0.922‐0.981) | 0.0019 | 0.995 (0.941‐1.053) | 0.8840 |
| AFP level before DAA therapy (by every 10 ng/mL) | 1.019 (1.003‐1.031) | 0.0046 | 1.021 (1.006‐1.036) | 0.0062 |
| APRI before DAA therapy (by every 1 unit) | 1.078 (1.009‐1.147) | 0.0195 | 0.835 (0.561‐1.243) | 0.3756 |
| FIB‐4 index before DAA therapy (by every 1 unit) | 1.040 (1.011‐1.067) | 0.0044 | 1.054 (0.912‐1.218) | 0.4718 |
| Geno/serotype (1) | 1 | 1 | ||
| (2) | 1.396 (0.904‐2.066) | 0.1270 | 1.128 (0.664‐1.917) | 0.6543 |
| Habitual alcohol intake (absence) | 1 | 1 | ||
| (presence) | 1.384 (0.929‐1.999) | 0.1067 | 1.315 (0.799‐2.165) | 0.2808 |
| Diabetes mellitus (absence) | 1 | 1 | ||
| (presence) | 0.975 (0.716‐1.311) | 0.8738 | 0.939 (0.637‐1.358) | 0.7440 |
| Fatty liver (absence) | 1 | 1 | ||
| (presence) | 1.019 (0.585‐1.652) | 0.9407 | 1.078 (0.569‐1.897) | 0.8048 |
| Cirrhosis (absence) | 1 | 1 | ||
| (presence) | 1.696 (1.240‐2.363) | 0.0007 | 1.332 (0.871‐2.086) | 0.1883 |
| Number of curative treatments for HCC before DAA therapy (1) | 1 | 1 | ||
| (2) | 1.539 (1.074‐2.170) | 0.0191 | 1.608 (1.054‐2.407) | 0.0277 |
| (3 or more) | 2.561 (1.878‐3.476) | <0.0001 | 2.610 (1.762‐3.843) | <0.0001 |
AFP, alpha‐fetoprotein; ALT, alanine aminotransferase; APRI, aspartate aminotransferase to platelet count ratio index; AST, aspartate aminotransferase; CI, confidence interval; DAA, direct‐acting antiviral agents; FIB‐4 index, Fibrosis‐4 index; GGTP, gamma‐glutamyl transpeptidase; HCC, hepatocellular carcinoma; HR, hazards ratio.
3.4. AFP level before the DAA therapy
In addition, we determined the cutoff value for the AFP level before the DAA therapy via the receiver operating characteristic (ROC) curve analysis. According to the ROC analysis, AFP = 5.4 ng/ml was identified as the cutoff value. Figure 3 shows the cumulative HCC recurrence stratified with AFP level before the DAA therapy. The 1‐, 2‐, and 3‐year cumulative HCC recurrence rates of the group with AFP level <5.4 ng/mL (solid line; n = 132) were 18.9%, 31.6%, and 45.4%, respectively. The median time‐to‐recurrence in the group with AFP level ≥5.4 ng/mL (dotted line; n = 327) was 29.0 months, and the 1‐, 2‐, and 3‐year cumulative HCC recurrence rates were 30.0%, 48.1%, and 53.2%, respectively. These were found to be statistically significant (P = 0.0047).
Figure 3.
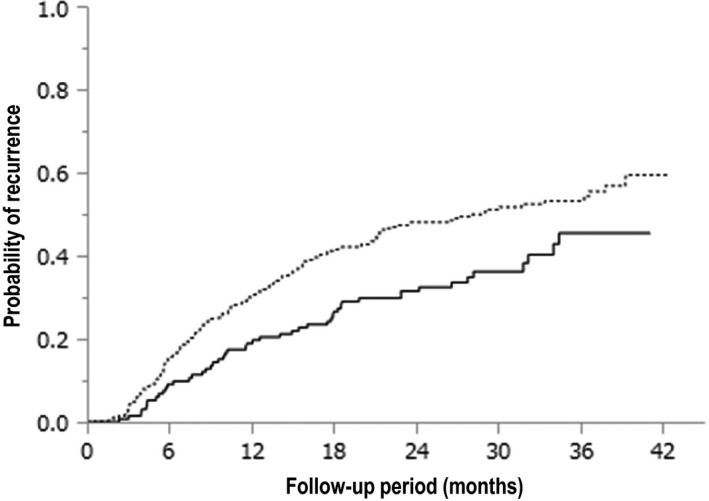
Kaplan‐Meier analysis of the cumulative hepatocellular carcinoma (HCC) recurrence in patients stratified based on serum alpha‐fetoprotein (AFP) levels (P = 0.0047). The probability of HCC recurrence in the group with AFP <5.4 ng/mL is indicated by the solid line (n = 132), and that in the group with AFP ≥5.4 ng/mL is indicated by the dotted line (n = 327)
3.5. Number of curative treatments for HCC before the DAA therapy
Figure 4 shows the cumulative HCC recurrence stratified with number of curative treatments for HCC before the DAA therapy. The 1‐, 2‐, and 3‐year cumulative HCC recurrence rates in the group with once curative treatment for HCC before the DAA therapy (the solid line; n = 262) were 19.4%, 33.0%, and 42.0%, respectively. The median time‐to‐recurrence in the group with twice curative treatments for HCC before the DAA therapy (the dotted line; n = 88) was 31.7 months, and the 1‐, 2‐, and 3‐year cumulative HCC recurrence rates were 30.7%, 45.6%, and 56.4%, respectively. The median time‐to‐recurrence in the group with thrice or more curative treatments for HCC before the DAA therapy (the solid and dotted line; n = 105) was 14.0 months, and the 1‐, 2‐, and 3‐year cumulative HCC recurrence rates were 43.8%, 67.1%, and 68.6%, respectively. These were also statistically significant (P < 0.0001). Moreover, the number of curative treatments for HCC before the DAA therapy was unknown in four patients.
Figure 4.
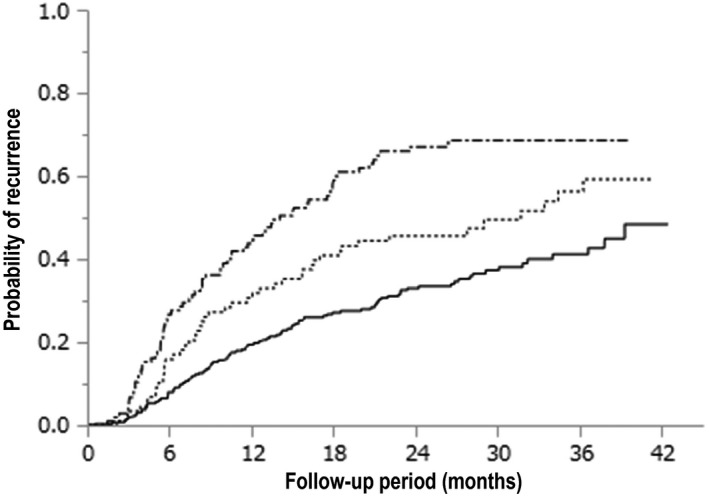
Kaplan‐Meier analysis of the cumulative hepatocellular carcinoma (HCC) recurrence in patients stratified based on the number of curative treatments for HCC before the direct‐acting antiviral agent (DAA) therapy (P < 0.0001). The probability of HCC recurrence for once curative treatment for HCC before the DAA therapy is indicated by the solid line (n = 262), that for twice curative treatments for HCC before the DAA therapy is indicated by the dotted line (n = 88), and that for thrice or more curative treatments for HCC before the DAA therapy is indicated by the solid and dotted line (n = 105). The number of curative treatments for HCC before the DAA therapy was unknown in four patients
3.6. Correlation between the duration of HCC recurrence and maximum tumor size
Figure 5 shows the lack of correlation between the duration of HCC recurrence and maximum tumor size (r = −0.0130, P = 0.8517).
Figure 5.
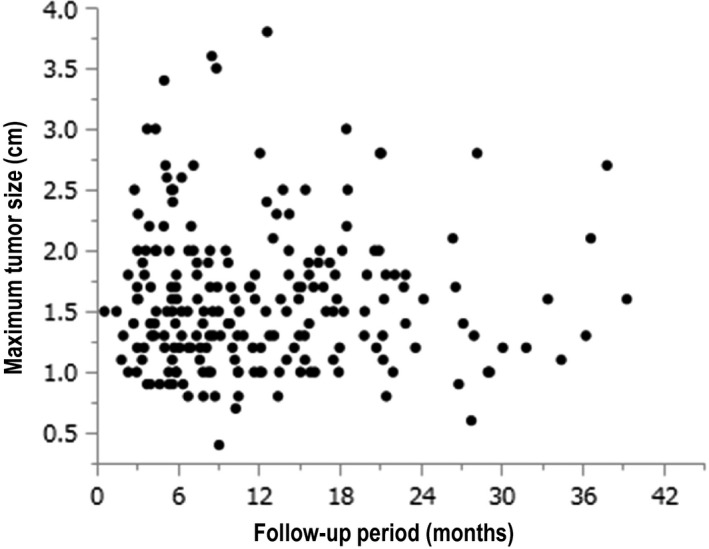
Correlation between the duration of hepatocellular carcinoma (HCC) recurrence and maximum tumor size (r = −0.0130, P = 0.8517)
4. DISCUSSION
The IFN‐based treatment is often difficult to perform in patients with HCV infection because of the associated adverse events, such as high fever, general fatigue, loss of appetite, and platelet count reduction, especially in the elderly patients and patients with cirrhosis, with a low platelet count. Recently, DAAs have been introduced as an easy and safe antiviral oral therapy for HCV infection. The development of DAAs has rendered the treatment of HCV infection easier in the elderly patients and patients with cirrhosis, with a reduced platelet count, at not only specialized high‐volume centers but also general physician clinics. The eradication of HCV infection has been reported to reduce the risk of HCC.25 It is reported that after achieving SVR with DAAs, the risk of developing HCC was the same as that with the IFN‐based therapy.9, 10, 11, 12 According to these studies, DAAs do not increase the risk of de novo HCC after achieving an SVR in many patients with the other risk factors of HCC.10 Recently, SVR with DAAs has reduced the incidence of HCC in a large prospective study of patients with cirrhosis.13
In the present study, the factors associated with HCC recurrence after the DAA therapy were the AFP level and number of curative treatments for HCC before the DAA therapy. In general, the AFP level and frequency of HCC recurrence are suggested to be closely related to malignancy of HCC. An elevated serum AFP level has been reported as a risk factor for HCC in HCV‐infected patients with or without attaining SVR following the IFN therapy.26, 27, 28 An elevated serum AFP level before the DAA therapy has also been recognized as one of the common risk factors for HCC.26, 27, 28 The post‐IFN therapy AFP level was reported as one of the risk factors for developing HCC in patients with old age, advanced liver fibrosis, male sex, glucose metabolism disorders, lipid metabolism disorders, and alcohol intake.12 These risk factors have already been reported in patients treated with the IFN‐based therapy.29 Especially, the old age, male sex, and advanced liver fibrosis have been reported to be the important risk factors for HCC.30, 31 However, in our study, age, sex, advanced liver fibrosis, and alcohol intake were not associated with HCC recurrence after the DAA therapy. On the contrary, it suggested that HCC recurrence after the DAA therapy was associated with tumor‐related factors, such as high AFP level and multiple occurrences of HCC before the DAA therapy.
Moreover, in our study, there was no correlation between the duration of HCC recurrence and the maximum tumor size. This suggested the possibility that a relatively large‐sized hepatic tumor will recur relatively early after the DAA therapy. Thus, it is necessary to carefully follow up for recurrence after curative treatment for HCC using image inspection and laboratory data, especially in patients with high AFP level and multiple occurrences of HCC before the DAA therapy.
Our study had several limitations. First, the observation period was short; thus, another study with a longer observation period is needed to confirm our results. Second, the sample size of our study cohort was small; therefore, additional investigations with a higher number of patients and participating institutions are needed.
In conclusion, high AFP level and multiple occurrences of HCC before the DAA therapy indicate a high risk for HCC recurrence after the curative treatment. The DAA‐induced SVR does not always warrant a long HCC‐free status. Thus, follow up after the DAA therapy should include special attention to the abovementioned risk factors.
CONFLICTS OF INTEREST
Akio Ido has COI as follows: (1) Speaking fees or honoraria; AbbVie GK, Bristol Myers Squibb Co. Ltd., Gilead Sciences Inc, and Eisai Co. Ltd. (2) Research grants; AbbVie GK, Otsuka Pharmaceutical Co. Ltd., MSD KK, Chugai Pharmaceutical Co. Ltd., Eisai Co. Ltd., Astellas Pharmaceutical Inc, and Takeda Pharmaceutical Co. Ltd. The other authors disclose no conflicts.
ACKNOWLEDGEMENTS
The authors thank the staffs of the 15 institutions, including 10 university hospitals and five high‐volume centers in the Kyusyu area of Japan, and Editage (www.editage.jp) for English language editing.
Nakano M, Koga H, Ide T, et al. Predictors of hepatocellular carcinoma recurrence associated with the use of direct‐acting antiviral agent therapy for hepatitis C virus after curative treatment: A prospective multicenter cohort study. Cancer Med. 2019;8:2646–2653. 10.1002/cam4.2061
REFERENCES
- 1. El‐Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118‐1127. [DOI] [PubMed] [Google Scholar]
- 2. Gower E, Estes C, Blach S, Razavi‐Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 Suppl):S45‐57. [DOI] [PubMed] [Google Scholar]
- 3. El‐Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60:1767‐1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ioannou GN, Green PK, Berry K. HCV eradication induced by direct‐acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2018;68:25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lemon SM, McGivern DR. Is hepatitis C virus carcinogenic? Gastroenterology. 2012;142:1274‐1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alqahtani SA, Afdhal N, Zeuzem S, et al. Safety and tolerability of ledipasvir/sofosbuvir with and without ribavirin in patients with chronic hepatitis C virus genotype 1 infection: Analysis of phase III ION trials. Hepatology. 2015;62:25‐30. [DOI] [PubMed] [Google Scholar]
- 7. Kwo P, Gitlin N, Nahass R, et al. Simeprevir plus sofosbuvir (12 and 8 weeks) in hepatitis C virus genotype 1‐infected patients without cirrhosis: OPTIMIST‐1, a phase 3, randomized study. Hepatology. 2016;64:370‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pol S, Bourliere M, Lucier S, et al. Safety and efficacy of daclatasvir‐sofosbuvir in HCV genotype 1‐mono‐infected patients. J Hepatol. 2017;66:39‐47. [DOI] [PubMed] [Google Scholar]
- 9. Hiramatsu N, Oze T, Takehara T. Suppression of hepatocellular carcinoma development in hepatitis C patients given interferon‐based antiviral therapy. Hepatol Res. 2015;45:152‐161. [DOI] [PubMed] [Google Scholar]
- 10. Roche B, Coilly A, Duclos‐Vallee JC, Samuel D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018;38(Suppl 1):139‐145. [DOI] [PubMed] [Google Scholar]
- 11. Waziry R, Hajarizadeh B, Grebely J, et al. Hepatocellular carcinoma risk following direct‐acting antiviral HCV therapy: A systematic review, meta‐analyses, and meta‐regression. J Hepatol. 2017;67:1204‐1212. [DOI] [PubMed] [Google Scholar]
- 12. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El‐Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct‐acting antiviral agents. Gastroenterology. 2017;153:996‐1005.e1. [DOI] [PubMed] [Google Scholar]
- 13. Calvaruso V, Cabibbo G, Cacciola I, et al. Incidence of hepatocellular carcinoma in patients with HCV‐associated cirrhosis treated with direct‐acting antiviral agents. Gastroenterology. 2018;155:411‐421. [DOI] [PubMed] [Google Scholar]
- 14. Kozbial K, Moser S, Schwarzer R, et al. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon‐free direct‐acting antiviral treatment. J Hepatol. 2016;65:856‐858. [DOI] [PubMed] [Google Scholar]
- 15. Nakao Y, Hashimoto S, Abiru S, et al. Rapidly growing, moderately differentiated HCC: A clinicopathological characteristic of HCC occurrence after IFN‐free DAA therapy? J Hepatol. 2017;68:854‐855. [DOI] [PubMed] [Google Scholar]
- 16. Reig M, Mariño Z, Perelló C, et al. Unexpected high rate of early tumor recurrence in patients with HCV‐related HCC undergoing interferon‐free therapy. J Hepatol. 2016;65:719‐726. [DOI] [PubMed] [Google Scholar]
- 17. Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV‐related cirrhosis treated with direct‐acting antivirals. J Hepatol. 2016;65:727‐733. [DOI] [PubMed] [Google Scholar]
- 18. Llovet JM, Villanueva A. Liver cancer: effect of HCV clearance with direct‐acting antiviral agents on HCC. Nat Rev Gastroenterol Hepatol. 2016;13:561‐562. [DOI] [PubMed] [Google Scholar]
- 19. Cheung M, Walker AJ, Hudson BE, et al. Outcomes after successful direct‐acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65:741‐747. [DOI] [PubMed] [Google Scholar]
- 20. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck‐Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta‐analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329‐337. [DOI] [PubMed] [Google Scholar]
- 21. Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518‐526. [DOI] [PubMed] [Google Scholar]
- 22. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317‐1325. [DOI] [PubMed] [Google Scholar]
- 23. Kuzuya T, Nakagawa S, Satoh Jo, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract. 2002;55:65‐85. [DOI] [PubMed] [Google Scholar]
- 24. Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona‐2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421‐430. [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi M, Suzuki F, Fujiyama S, et al. Sustained virologic response by direct antiviral agents reduces the incidence of hepatocellular carcinoma in patients with HCV infection. J Med Virol. 2017;89:476‐483. [DOI] [PubMed] [Google Scholar]
- 26. Tada T, Kumada T, Toyoda H, et al. Post‐treatment levels of alpha‐fetoprotein predict long‐term hepatocellular carcinoma development after sustained virological response in patients with hepatitis C. Hepatol Res. 2017;47:1021‐1031. [DOI] [PubMed] [Google Scholar]
- 27. Huang CM, Hu TH, Chang KC, et al. Dynamic noninvasive markers predict hepatocellular carcinoma in chronic hepatitis C patients without sustained virological response after interferon‐based therapy: Prioritize who needs urgent direct‐acting antiviral agents. Medicine (Baltimore). 2017;96:e8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oze T, Hiramatsu N, Yakushijin T, et al. Post‐treatment levels of alpha‐fetoprotein predict incidence of hepatocellular carcinoma after interferon therapy. Clin Gastroenterol Hepatol. 2014;12:1186‐1195. [DOI] [PubMed] [Google Scholar]
- 29. Ikeda K, Saitoh S, Arase Y, et al. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: A long‐term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124‐1130. [DOI] [PubMed] [Google Scholar]
- 30. Kasahara A, Hayashi N, Mochizuki K, et al. Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Osaka Liver Disease Study Group. Hepatology. 1998;27:1394‐1402. [DOI] [PubMed] [Google Scholar]
- 31. Yoshida H, Shiratori Y, Moriyama M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174‐181. [DOI] [PubMed] [Google Scholar]


