Abstract
Human papillomavirus (HPV) is the most common sexually transmitted disease in the world. The aim of our study is to describe the differences in HPV‐vaccination coverage and screening programs in WHO European Countries notably according to income levels. Multiple correspondence analysis was applied to examine the association among the following variables: Gross National Income (GNI) levels (Lower‐Middle Income, LMI; Upper‐Middle Income, UMI; and High Income, HI); type of CC screening program (coverage; opportunistic/organized); vaccination payment policies (free or partial or total charge); mortality rates/100 000 (≤3; >3‐6; >6‐9; >9); incidence rates/100 000 (≤7; >7‐15; >15‐21; >21). Data HPV‐vaccination start (years) (2006‐2008; 2009‐2011; 2012‐2014; >2014; no program); coverage HPV‐vaccination percentage (≤25; 26‐50; 51‐75; >75); data screening start (years) (<1960; 1960‐1980; 1981‐2000; >2000); primary screening test (HPV, cytology), and screening coverage percentage (≤25; >25‐50; >50‐75; >75). A high income is associated with: start of screening before 1960, medium‐high screening coverage, organized screening, start of vaccination in the periods 2009‐2011 and 2012‐2014 and high immunization coverage. On the other hand, lower‐middle income is associated with: late start of vaccination and screening programs with cytology as primary test, high mortality and incidence rates and lower‐medium vaccination coverage. Our results show a useful scenario for crucial support to public health decision‐makers. Public health authorities should monitor the HPV‐vaccinated population in order to determine more precisely the effects on short‐ and long‐term incidence and mortality rates. In fact, the greater the vaccination coverage, the greater will be the efficacy of the program for the prevention of CC and other HPV‐related diseases.
Keywords: cervical cancer, coverage, HPV vaccination, income level, screening programs, surveillance
1. INTRODUCTION
Human papillomavirus (HPV) is the most common sexually transmitted disease in the world.1 The persistent infection with high‐risk HPV causes Cervical Cancer (CC).2 In female population it is the fourth cancer and the second most common from 25 to 40 years of age.3 Strategies against HPV infection are vaccination and safe sex education.4 Countries that have performed HPV‐vaccination programs have showed a decrease in the prevalence in the population of the HPV 16, 18 genotypes.5 HPV‐related disease incidence and mortality are the most common measures used to evaluate the impact of vaccination in European Countries.6 In Europe, HPV‐vaccination coverage rates vary from 30% to 80% with school‐based programs.7 Information campaigns of health interventions are closely linked to the success of a vaccination program. In fact, the greater the vaccination coverage, the greater will be the efficacy of the program for the prevention of CC and other HPV‐related diseases.8 In 2006, the European Medicines Agency (EMA) endorsed the quadrivalent HPV vaccine, in 2007 the bivalent, while in June 2015 a 9‐valent vaccine was recommended.9
It is important to underline that the two primary (HPV vaccination) and secondary strategies (screening, early diagnosis) will lead to the reduction of incidence and mortality for CC.10 Relatively to Europe, with regard to CC, vaccination and screening programs show differences among Countries; indeed, relatively to screening, there are organized and nonorganized (opportunistic) programs.11 Knowledge of the onset of CC, new technologies, HPV test as primary screening test11 along with home self‐sampling12, 13, 14 modified screening programs in many European Countries.15
Cervical cancer screening programs together with primary prevention could contribute to reducing social inequalities between central and eastern European Countries.16
The aim of the study was to describe the differences in HPV‐vaccination coverage and screening programs in WHO European Countries notably according to income levels.
2. MATERIALS AND METHODS
2.1. Gross national income (GNI)
According to the World Bank, economies can be divided into low income (LI), lower‐middle income (LMI), upper‐middle income (UMI), and high income (HI) in relation to GNI per capita17 (Figure 1). In this study, the 53 WHO ER Countries were thus divided into: LMI, $1026‐4035 (Armenia, Georgia, Kyrgyzstan, Moldova, Tajikistan, Ukraine, and Uzbekistan); UMI, $4036‐12 475 (Albania, Azerbaijan, Belarus, Bosnia and Herzegovina, Bulgaria, Kazakhstan, FYR of Macedonia [FM], Hungary, Montenegro, Romania, Serbia, Turkey, and Turkmenistan); and HI, $12 476 (Austria, Belgium, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Norway, Poland, Portugal, Slovakia Republic, Slovenia, Spain, Sweden, Switzerland, the Netherlands, and the United Kingdom, Andorra, Croatia, Cyprus, Malta, Monaco, Latvia, Lithuania, Russian Federation, and San Marino) (World Bank and Lending Groups 2016) (Table 1).18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37
Figure 1.
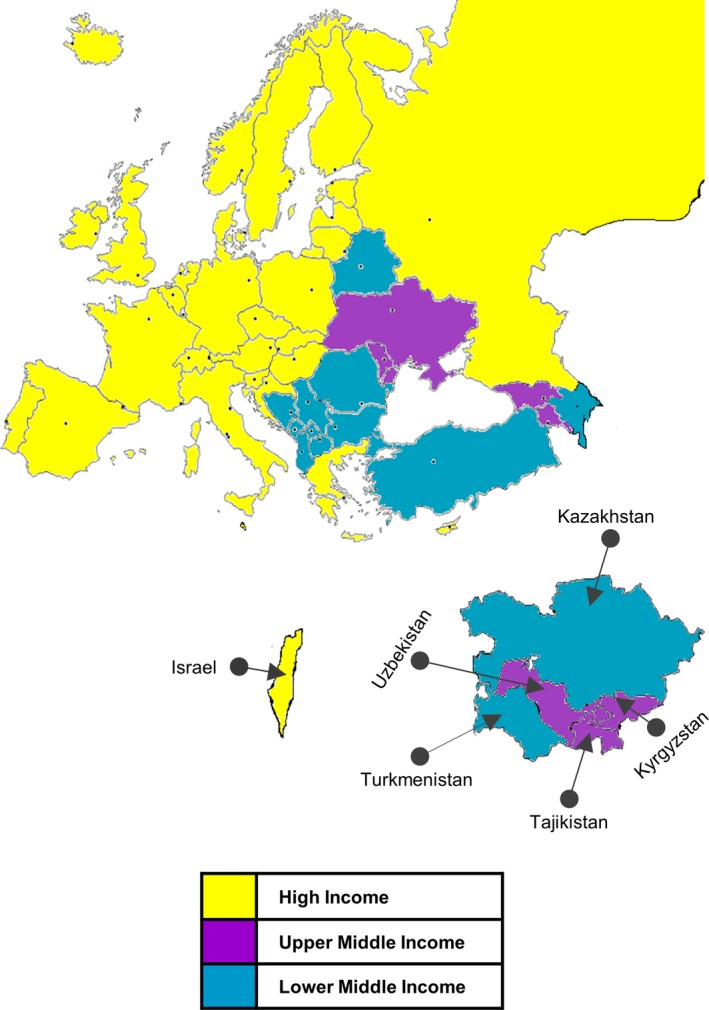
Map of Countries grouped according to income levels
Table 1.
Differences of CC burden, primary and secondary prevention programs in 53 Countries of the WHO Region
| Country | National immunization | Cancer screening | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Incidence 100.000 |
Mortality 100.000 |
Date start Age at beginning |
Policy payment Coverage% (year) |
Organization Start date |
Regions Coverage % (year) |
Primary Test |
Age target Age |
Screening Interval Years |
Payment Policy |
|
|
2008 2012 |
2008 2012 |
|||||||||
| Austria |
5.7 5.8 |
2.2 2.6 |
2014 9‐12 |
Fully covered by patient NR |
Opportunistic 1970 |
National 86.6 (2014) |
Cytology PAP | >18 | 1 | Free of Charge |
| Andorra |
NR NR |
NR NR |
2014 12 |
NR NR |
Opportunistic NR |
NR 61.4 (2011) |
Cytology PAP | >18 | 1 | NR |
| Belgium |
8.4 8.6 |
2.5 2.3 |
2007 12 |
75% supported by national health authorities NR |
Opportunistic 1965 Organized 1994 |
Regional 68.7 (2013) |
Cytology PAP | 25‐64 | 3 | Free of Charge |
| Croatia |
11.8 10.0 |
4.2 3.4 |
2016 NR |
Fully covered by national health authorities23
NR |
Opportunistic 1960 Organized 2012 |
National 65.3 (2003) |
Cytology PAPa | 25‐64 | 3 | Free of Charge |
| Cyprus |
4.5 4.1 |
2.6 1.3 |
2016 11‐12 |
NR NR |
Opportunistic NR |
NR 67.4 (2012) |
Cytology PAP | 24‐65 | NR | NR |
| Czech Republic |
14.0 14.1 |
4.6 4.8 |
2012 13 |
Covered by general health insurance for routine 81.0 (2011) |
Opportunistic 1947 Organized 2008 |
National 87.2 (2014) |
Cytology PAP | 25‐60 | 1 | Free of Charge |
| Denmark |
12.1 10.6 |
3.1 2.6 |
2009 12 |
Fully covered by national health authorities 79.0 (2011) |
Opportunistic 1962 Organized 2006 |
National policy, local implementation 64.1 (2014) |
HPV Cytology PAP12 |
60‐64 HPVb
23‐59 Cytol. |
5 (60‐64) HPV 3 (23‐59) Cytology |
Free of Charge |
| Estonia |
15.8 19.9 |
7.3 7.5 |
No program | ‐ | Organized 2006 |
National 57.7 (2014) |
Cytology PAP12 | 30‐59 | 5 | Free of Charge |
| Finland |
4.5 4.3 |
1.5 1.3 |
2013 11‐12 |
Fully covered by national health authorities 29.3 (2012) |
Organized 1963 |
National 69.0 (2015) |
HPV Cytology PAP12 |
30‐64 | 5c | Free of Charge |
| France |
7.1 6.8 |
1.9 1.8 |
2007 11‐14 |
65% supported by national health authorities 24.0 (2008) |
Organized 1991 |
Regional12
75.4 (2014) |
Cytology PAP | 25‐65 | 3 |
Insurance Copayment |
| Germany |
6.9 8.2 |
2.6 2.6 |
2007 9‐14 |
Fully covered by national health authorities NR |
Organized 1971 (west) 1991 (expanded to the eastern country)17 |
National 80.4 (2014) |
Cytology PAP | ≥20 | 1 | Free of Charge |
| Greece |
4.1 5.2 |
1.4 1.9 |
2008 11‐15 |
Fully covered by national health authorities NR |
Opportunistic 1991 |
National 75.5 (2014) |
Cytology PAP | ≥20 | 1 | NR |
| Hungary |
16.6 18.0 |
6.6 6.2 |
2014 NR |
NR 88.0 (2014) |
Opportunistic 1950 Organized 2003 |
National 40.1 (2015) |
Cytology PAP | 25‐65 | 3 | Free of Charge |
| Iceland |
8.4 7.9 |
0.8 1.9 |
2011 12 |
Fully covered by national health authorities 84.0 (2013) |
Organized 196420 |
National 71.0 (2015) |
Cytology PAP12 | 20‐69 |
2 (20‐39) 4 (40‐69) |
NR |
| Ireland |
10.9 13.6 |
3.6 4.0 |
2010 12‐13 |
Fully covered by national health authorities 84.9 (2014) |
Organized 2008 |
National 78.7 (2015) |
Cytology PAP | 25‐60 |
3 (25‐44) 5 (45‐60) |
Free of Charge |
|
Israel Outside European institutions |
NR 4.6 |
2.4 2.0 |
2013 13 |
NR 80.0 (2011) |
Opportunistic NR |
Regional 32.0 (2008) |
Cytology PAP | 25‐6521 | 3 | Free of Charge31 |
| Italy |
6.7 6.7 |
0.8 0.9 |
2007 12 |
Fully covered by national health authorities 65.0 (2011) |
Organized 1996 |
National policy, local implementation14
79.0 (2015) |
HPV Cytology PAP |
25‐64 |
5 HPV 3 Cytology |
Free of Charge |
| Latvia |
12.4 17.3 |
6.9 8.5 |
2010 12 |
Fully covered by national health authorities NR |
Opportunistic 1960 Organized 2009 |
National 25.2 (2016) |
Cytology PAP18, d | 25‐70 | 3 | Free of Charge |
| Lithuania |
21.0 26.1 |
10.6 9.0 |
2016 NR |
NR 29.0 (2009) |
Organized 2004 |
National 74.0 (2014) |
Cytology PAP | 25‐60 | 3 | Free of Charge |
| Luxembourg |
6.3 4.9 |
5.6 1.7 |
2008 12‐18 |
Fully covered by national health authorities 17.0 (2009) |
Opportunistic 1962 Organized 1990 |
National 83.6 (2014) |
Cytology PAP | >15 | 1 | NR |
| Malta |
2.1 3.8 |
2.1 2.4 |
2012 12 |
Fully covered by national health authorities NR |
Opportunistic NR |
National 49.3 (2008) |
HPV Cytology PAP |
>30 HPV 25‐50 Cytol. |
5 HPV 3 Cytologye |
Free of Charge |
| Monaco |
NR NR |
NR NR |
2011 14 |
NR NR |
Opportunistic NR |
NR NR |
Cytology PAP | 21‐65 | 1f | NR |
| Norway |
9.4 9.8 |
3.0 2.1 |
2009 12 |
Fully covered by national health authorities 63.0 (2011) |
Opportunistic 197012
Organized 199512 |
National 74.1 (2015) |
Cytology PAP12, g | 25‐69 | 3 | NR |
| Poland |
11.6 12.2 |
7.3 6.7 |
No Program |
‐ ‐ |
Opportunistic 1970 Organized 2006 |
National 21.2 (2013) |
Cytology PAP | 25‐59 | 3 | Free of Charge |
| Portugal |
12.2 9.0 |
3.4 2.8 |
2008 13 |
Fully covered by national health authorities 84.0 (2011) |
Organized Central Region 1990 Alentejo Region 2008 |
Regional 70.7 (2014) |
Cytology PAP | 25‐64 | 3 | Free of Charge |
|
Russian Federation Outside European institutions |
13.3 15.3 |
6.6 6.9 |
Partial program 2009 12‐13 |
NR NR |
Organized NR |
NR 72.0 (2012) |
Cytology PAP | >18 | 1 | NR |
| San Marino |
NR NR |
NR NR |
2008 11‐14 |
Fully covered by national health authorities NR |
Opportunistic 1968 Organized 200633 |
National 82.0 (2017) 19 |
HPV Cytology PAP |
30‐65 HPV 25‐30 Cytol. |
5 HPV 3 Cytology |
NR |
| Slovakia Republic |
15.8 16.1 |
6.5 6.9 |
2014 12 |
NR 55.0 (2012) |
Opportunistic 1980 Organized 2008 |
National 69.0 (2014) |
Cytology PAP | 23‐64 | 1 | Free of Charge |
| Slovenia |
11.1 10.5 |
3.1 2.9 |
2009 11 |
Fully covered by national health authorities 70.8 (2012) |
Opportunistic 1960 Organized 2003 |
National 71.9 (2016) |
Cytology PAP | 20‐64 | 3 | Free of Charge |
| Spain |
6.3 7.8 |
2.1 2.1 |
2007 11‐14 |
Fully covered by national health authorities 78.5 (2010) |
Organized 1993 |
National18
72.7 (2014) |
HPV Cytology PAP |
30‐65 HPV 25‐65 Cytol. |
5 HPV 3 Cytology |
Free of Charge |
|
Sweden 4,100,000 |
7.8 7.4 |
2.2 2.8 |
2010 10‐12 |
Fully covered by national health authorities 82.0 (2012)24 |
Opportunistic 1950 Organized 1967 |
National 81.7 (2015) |
HPV Cytol. PAP12, h |
30‐64 HPV 23‐29 Cytol.12 |
3 (30‐50) 7 (51‐64) HPV. 3 (23‐29) Cytology |
Free of Charge |
| Switzerland |
4.0 3.6 |
1.4 1.5 |
2008 11‐14 |
NR NR |
Opportunistic NR |
NR 74.5 (2012) |
Cytology PAP | >20 | 3 |
Insurance Copayment |
| Netherlands |
6.8 6.8 |
2.3 1.9 |
2010 12 |
Fully covered by national health authorities 79.5 (2014) |
Opportunistic 1970 Organized 1980 |
National 64.4 (2015) |
HPVi
Cytology PAP |
30‐60 |
5 HPV 5 Cytology |
Free of Charge |
| United Kingdom |
7.2 7.1 |
2.4 2.2 |
2008 12‐13 |
Fully covered by national health authorities 91.4 (2013) |
Opportunistic 1964 Organized 1988 |
National 77.5 (2016) |
Cytology PAPj | 25‐64 |
3 (25‐49); 5 (50‐64) |
Free of Charge |
| Albania |
7.1 5.0 |
1.5 1.83 |
No Program |
NR NR |
Opportunistic NR |
NR 2.7 (2002) |
Cytology PAP | >20 | 2‐3 | NR |
|
Azerbaijan Outside European institutions |
NR 9.8 |
NR 3.93 |
No Program |
NR NR |
No Program | 1.1 (2001) | Acetic acid visualization VIA | NR | ‐ | NR |
|
Belarus Outside European institutions |
13.2 13.2 |
6.2 4.73 |
No Program |
NR NR |
Opportunistic NR |
NR 75 (2015) |
Cytology PAP | >18 | 1 | NR |
| Bosnia and Herzegovina |
9.1 13.7 |
NR 2.7 |
No Program |
NR NR |
Organized NR |
National 39.8 (2003) |
Cytology PAP | 21‐70 | 1 | NR |
| Bulgaria |
21.9 24.5 |
7.0 7.8 |
2012 12 |
Covered by general health; catch‐up is opportunistic and not free of charge NR |
Opportunistic NR |
NR 46.8 (2008) |
Cytology PAP | 30‐59 | 3 | NR |
|
Kazakhstan Outside European institutions |
NR 29.4 |
8.8 9.1 |
Partial program 2013 11 |
NR NR |
Organized NR |
National 80.3 (2003) |
Cytology PAP | 30‐60 | 5 | NR |
| FRY of Macedonia |
22.0 12.4 |
4.1 3.5 |
2009 12 |
NR NR |
Organized 2015 |
National 60.0 (2015) |
Cytology PAP | 30‐55 | 3 | NR |
| Montenegro |
13.0 20.2 |
5.2 5.83 |
No Program |
NR NR |
Opportunistic NR |
NR NR |
Cytology PAP | 25‐64 | 3 | NR |
| Romania |
23.9 28.6 |
13.7 12.0 |
2008 12‐14 |
Fully covered by national health authorities NR |
Opportunistic 1965 Organized 2012 |
National 8.1 (2014) |
Cytology PAP | 25‐64 | 5 | Free of Charge |
| Serbia |
20.9 23.8 |
10.3 9.3 |
201721
12 |
NR NR |
Opportunistic 1960 Organized 201122 |
National 57.1 (2013) |
Cytology PAP | 25‐65 | 3 | Free of Charge |
| Turkey |
NR 4.3 |
NR 1.7 |
No Program |
NR NR |
Opportunistic 1985 Organized 200423 |
National 46.5 (2015) |
HPV23, k | 30‐65 | 5 | NR |
|
Turkmenistan Outside European institutions |
NR 13.1 |
5.9 10.1 |
2016 9 |
NR NR |
Opportunistic NR |
National NR |
Cytology PAP | >20 | 1 | NR |
| Armenia |
NR 13.8 |
3.7 5.5 |
No Program |
NR NR |
Opportunistic NR |
NR 9.3 (2010) |
Cytology PAP | 30‐60 | 3 | NR |
|
Georgia EU19 |
NR 14.2 |
NR 5.7 |
NR |
NR 36.2 (2012) |
Opportunistic NR |
NR 9.0 (2011) |
Cytology PAPl | 25‐60 | 3 | Free of Charge |
|
Kyrgyzstan Outside European institutions |
NR 23.7 |
12.6 11.4 |
No Program |
NR 53.4 |
Opportunistic NR |
NR 10‐50 (2015) |
Cytology PAP | NR | 5 | NR |
| Republic of Moldova |
17.1 19.6 |
8.6 7.5 |
NR |
NR NR |
Organized NR |
National 70.0 (2015) |
Cytology PAP | >20 | 2 | NR |
|
Tajikistan Outside European institutions |
NR 9.9 |
NR 4.9 |
NR |
NR 65.0 (2012) |
Opportunistic NR |
NR 10‐50 (2015) |
Cytology PAP | >20 | NR | NR |
| Ukraine |
NR 16.6 |
7.4 7.5 |
No Program |
NR 86.7 (2014) |
Opportunistic NR |
NR 73.7 (2003) |
Cytology PAP | 18‐65 | 1 | NR |
|
Uzbekistan Outside European institutions |
NR 13.5 |
NR 6.4 |
Announced 12 |
NR NR |
Opportunistic NR |
NR NR |
Cytology PAP | 25‐49 | NR | NR |
R: Not Reported.
Acetic acid visualization VIA HPV secondary test as a triage to borderline cytology and as a follow‐up after treatment of severe cervical lesions.
Interval between negative screens is three years for women aged 23‐49 and five years for women aged 50‐64. The primary screening test is cytology for women aged 23‐59 with HPV as a triage test. HPV DNA test is primary screening for women aged 60‐64 years.
Primary screening test is predominantly cytology but can also be HPV. The sample is examined for cell changes (the traditional Pap test) or the Human Papillomavirus. If there is cancer‐related HPV, the screening sample is checked for possible cervical cell changes (Pap test).
HPV testing is not reimbursed.
Screening ages: Above 25 (cytology), Above 30 (HPV test). Screening interval: Cytology every 3 years (ages 25‐50), VIA every 5 years (above 50). HPV test every 5 years.
1, 3 after 2 consecutive annual negative Cytology test.
HPV as primary screening test is underway in part of the country for women between 34 and 69 years of age.
Reflex testing with HPV is done for cytology positive test (ASCUS/LSIL or worse) below the age of 30 and reflex testing with cytology for HR HPV positive test above the age of 30. A double test (cytology and HPV) is recommended for women at age 41. Women with HPV positive/cytology negative tests should repeat screening after 3 years. Women with ASCUS/LSIL (regardless of HPV status) below the age of 28 are not referred to colposcopy, but repeat cytology.
Replace Pap‐test with hrHPV DNA test as primary screening test (since 2016).
If slightly abnormal cells are present, the human papillomavirus (HPV) will be tested.
HPV test since 2015.
HPV test undergoing project.
2.2. Sources of WHO European epidemiological data
The main data source, the GLOBOCAN 2012 website of the International Agency for Research on Cancer (IARC), provides access to several databases that allow assessing the impact of CC in 184 Countries or territories.38
These data were supplemented using the literature, ministerial web site of WHO ER Countries, World Bank Open Data Web site and the World Cancer Registry (X edition).17
2.3. Statistical analysis
Multiple correspondence analysis (MCA) was applied to examine the association among the following variables: GNI levels (LMI, UMI, and HI); type of CC screening program in each country (coverage; opportunistic/organized); vaccination payment policies (free or partial or total charge); mortality rates/100 000 (≤3; >3‐6; >6‐9; >9); incidence rates/100 000 (≤7; >7‐15; >15‐21; >21). Data HPV‐vaccination start (years) (2006‐2008; 2009‐2011; 2012‐2014; >2014; no program); HPV‐vaccination coverage percentage (≤25; 26‐50; 51‐75; >75); data screening start (years) (<1960; 1960‐1980; 1981‐2000; >2000); primary screening test (HPV, cytology); screening coverage percentage (≤25; >25‐50; >50‐75; >75).
These variables were coded as ordinal, nominal or dummy, as appropriate, and incorporated into the model.
3. RESULTS
3.1. Multiple correspondence analysis
The results of MCA are shown in Figures 2 and 3. We identified two dimensions that explain 82% of the variance: the first is 49% and the second being 33%.
Figure 2.
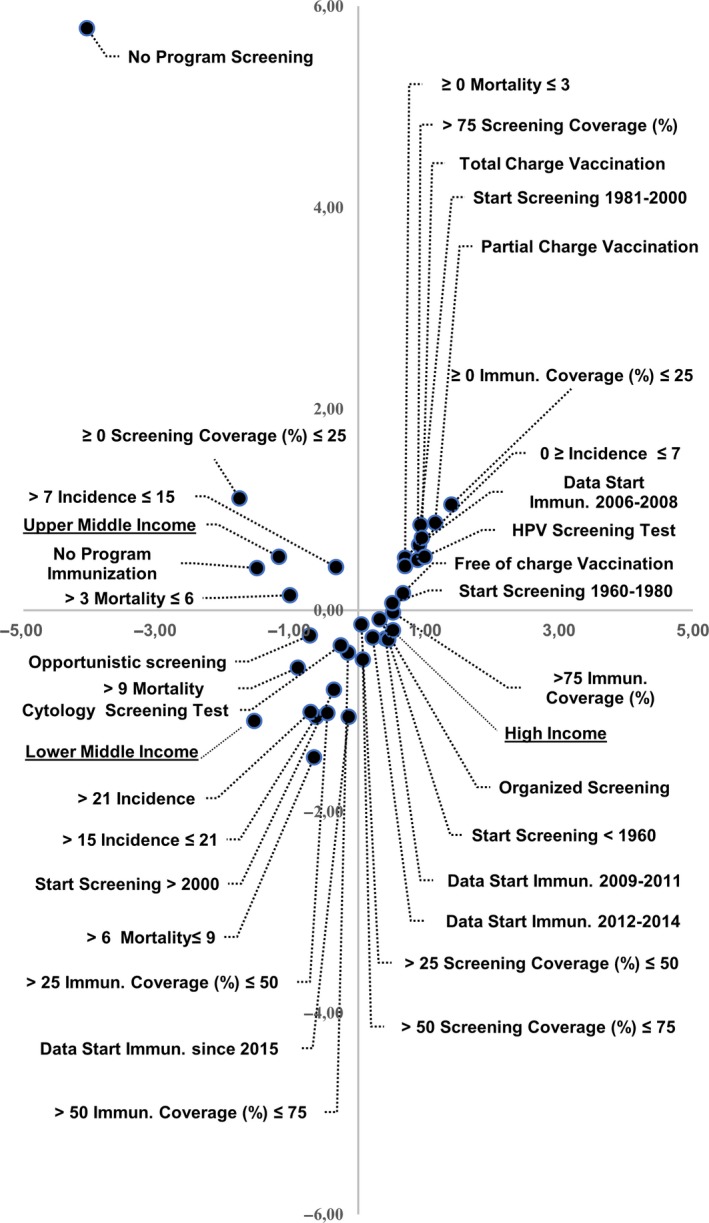
Association among variables included in model of multiple correspondence analysis
Figure 3.
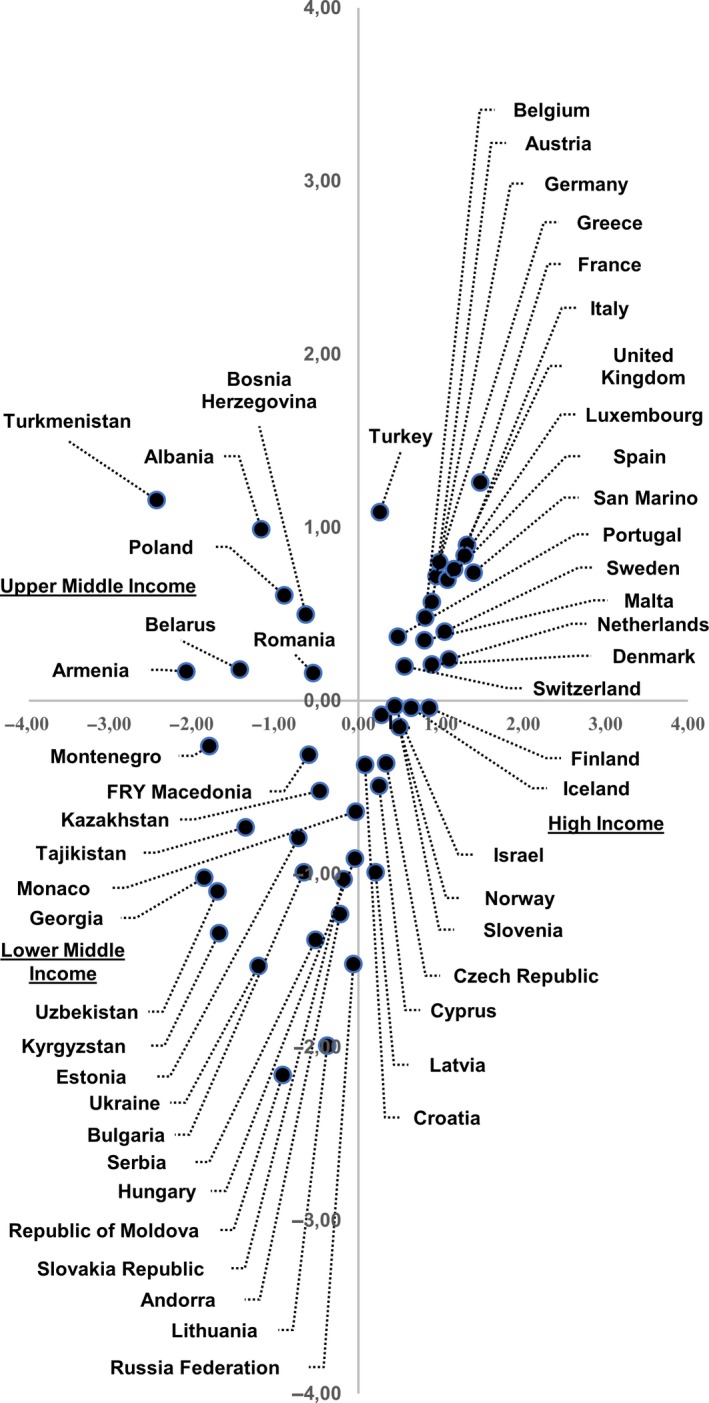
Distribution of 53 European Countries according to multiple correspondence analysis
The first quadrant (top right) identified the following variables: an early initiation of vaccination programs based on HPV screening as primary test; a high‐screening coverage and low incidence and mortality rates. In addition, low‐vaccination coverage and different payment policies (free, partial or total charge) for vaccination programs are located in this quadrant. High income, screening before 1960, medium‐high screening coverage, start of vaccination in the periods 2009‐2011 and 2012‐2014, and high‐immunization coverage are in the fourth quadrant (bottom‐right). On the left side, we can see medium‐low and medium‐high income, low attention to primary and secondary prevention with high rates of occurrence. In the second quadrant (top left), instead, we can observe upper‐middle income, total absence of screening and vaccination programs, medium‐low incidence and mortality rates. The third quadrant (bottom left) stands out with lower‐middle income, late start of vaccination programs and screening with cytology as primary test, medium‐high mortality and incidence rates, and medium vaccination coverage (Figure 2). It is important to highlight that most EU‐28 Countries are mainly located between the first and fourth quadrants with high income. On the contrary, the Countries outside of the EU‐28 are located between the second and third quadrant with upper‐middle income and lower‐middle income (Figure 3).
4. DISCUSSION
In 2015, 526.000 women developed CC worldwide and caused 239.000 deaths.39 The pap‐test screening programs, allowing an early diagnosis of precancerous lesions and a timely treatment of the same, have allowed to reduce the incidence of cervical cancer. Vaccination prevents precancerous lesions, reduces cancer and related treatments to eliminate precancerous lesions. Vaccination, acting much earlier in the history of disease development, prevents chronic infection resulting in pre‐cancerous lesions. Vaccination and screening programs are fundamental because they are potentially cost‐effective and allow decreasing incidence and mortality rates of CC.40 Screening, however, will remain fundamental for prevention of CC despite HPV vaccines.41 In fact, a factor that determines the differences in the incidence of CC among Countries is the screening coverage of the population.7
Monitoring HPV‐vaccination coverage is important to evaluate the performance of vaccination programs and the potential impact of HPV vaccine on cervical cancer. In fact, cervical cancer screening programs will need to be adjusted to the number of vaccinated people eligible for screening. However, despite the documented effectiveness of HPV vaccine, there is still an incomplete availability to this prevention action in the world population. Bruni et al42 showed high differences in number of women vaccinated according to gross income level countries; in fact, high‐quality primary and secondary cancer prevention is nearly always available in wealthy countries with gross national income (GNI) level.42 Moreover, higher income allows access to better resources and living standards and can increase the ability to maintain healthy behaviors.43 Syse and Lyngstand showed that high income is also related to higher survival rate.44
Our study shows that European Countries with higher income have higher screening and immunization coverage probably due to organized screenings starting before 1960 that determined low incidence and mortality rates, respect to those with lower‐middle income. High‐income countries have HPV screening test as the primary test and total or free partial charge HPV vaccination.
Eastern European and Asian Countries have lower‐middle income and show high incidence and mortality rates. These countries have an opportunistic screening with lower‐screening coverage and lower‐immunization coverage probably because HPV vaccine was introduced later. Globally, the coverage of vaccination is higher in countries with high income; by 2016, 71% of HI countries, 35% UMI countries, 8% of LMI countries, and 6% of LI countries had introduced the HPV vaccine.45
Only eight of the 70 countries who reported HPV vaccine introduction by the end of 2016, made the vaccine available to boys in addiction to girls (Australia, Austria, Barbados, Brazil, Canada, Italy, Switzerland, and the United States).46 According to Brisson et al,47 greater benefits can be acquired for both female and male by increasing HPV‐vaccination coverage among girls. In addition, vaccination of both sexes would be more equitable.48
In light of this, we would like to point out that: first, the strategy of including males in vaccination campaigns has, without a doubt, the function of reducing the circulation of the virus (herd‐effect) and the transmission of infection between the two sexes. It has also the advantage of countering the occurrence of HPV‐related diseases affecting male anatomic sites, such as the penis. Second, it is important to stress that both sexes have the same right to benefit from the advantages of anti‐HPV vaccination. In fact, according to European regulations, it is a right of every citizen to take advantage of disease prevention programs, where there is an effective means of prevention like the anti‐HPV vaccine. Third, a universal anti‐HPV vaccination program reduces the prejudices created around a female‐only vaccination, helping to reduce sociocultural barriers and thereby increasing acceptability and vaccination coverage.
Public health authorities should monitor the HPV‐vaccinated population in order to determine more precisely the effects on short‐ and long‐term incidence and mortality rates.
A useful scenario for crucial support to public health decision‐makers is the strength of our paper. On the other hand, a limitation could be that the data that came from low‐income countries must be considered with caution, both because they come from local registries (rather than the population‐based cancer registries used for the other countries) and because the International Classification Disease, 9th revision, codes are not always accurate.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Altobelli E, Rapacchietta L, Profeta VF, Fagnano R. HPV‐vaccination and cancer cervical screening in 53 WHO European Countries: An update on prevention programs according to income level. Cancer Med. 2019;8:2524‐2534. 10.1002/cam4.2048
REFERENCES
- 1. Harper DM, DeMars LR. HPV vaccines – a review of the first decade. Gynecol Oncol. 2017;146:196‐204. [DOI] [PubMed] [Google Scholar]
- 2. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12‐19. [DOI] [PubMed] [Google Scholar]
- 3. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer.2015;136(5):E359-386. [DOI] [PubMed] [Google Scholar]
- 4. Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342‐350. [DOI] [PubMed] [Google Scholar]
- 5. Castle PE, Maza M. Prophylactic HPV vaccination: past, present, and future. Epidemiol Infect. 2016;44(3):449‐468. [DOI] [PubMed] [Google Scholar]
- 6. Elfström KM, Dillner J, Arnheim‐Dahlström L. Organization and quality of HPV vaccination programs in Europe. Vaccine. 2015;33(14):1673‐1681. [DOI] [PubMed] [Google Scholar]
- 7. Hillemanns P, Soergel P, Hertel H, Jentschke M. Epidemiology and early detection of cervical cancer. Oncol Res Treat. 2016;39:501‐506. [DOI] [PubMed] [Google Scholar]
- 8. Bray F, Loos AH, McCarron P, et al. Trends in cervical squamous cell carcinoma incidence in 13 European countries: changing risk and the effects of screening. Cancer Epidemiol Biomarkers Prev. 2005. Mar;14(3):677‐686. [DOI] [PubMed] [Google Scholar]
- 9. Joura EA, Giuliano AR, Iversen OE, et al. A 9‐valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711‐723. [DOI] [PubMed] [Google Scholar]
- 10. Arbyn M, Castellsaguè X, de Sanjosè S. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675‐2686. [DOI] [PubMed] [Google Scholar]
- 11. Altobelli E, Scarselli G, Lattanzi A, Fortunato C, Profeta VF. A comparison between Pap and HPV screening tests and screening methods. Mol Clin Oncol. 2016;5(2):348‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Del Mistro A, Frayle H, Ferro A, Fantin G, Altobelli E, Giorgi Rossi P. Efficacy of self‐sampling in promoting participation to cervical cancer screening also in subsequent round. Prev Med Rep. 2016;5:166‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Del Mistro A, Frayle H, Rizzi M, et al. Methylation analysis and HPV genotyping of self‐collected cervical samples from women not responding to screening invitation and review of the literature. PLoS ONE. 2017;12(3):e0172226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giorgi Rossi P, Fortunato C, Barbarino P, et al. HPV Self‐sampling Italian Working Group Self‐sampling to increase participation in cervical cancer screening: an RCT comparing home mailing, distribution in pharmacies, and recall letter. Br J Cancer. 2015;112(4):667‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Altobelli E, Lattanzi A. Cervical carcinoma in the European Union. An update on disease burden, screening program state of activation, and coverage as of March 20014. Int J Gynecol Cancer. 2015;25:474‐483. [DOI] [PubMed] [Google Scholar]
- 16. Altobelli E. Improving cervical cancer screening in Baltic, central, and eastern European Countries. Lancet Oncol. 2016;17:1349‐1350. [DOI] [PubMed] [Google Scholar]
- 17. World Bank Country and Lending Groups for July 2016. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519.
- 18. Kesic V, Poljak M, Rogovskaya S. Cervical cancer burden and prevention activities in Europe. Cancer Epidemiol Biomarkers Prevention. 2012;21(9):1423-1433. [DOI] [PubMed] [Google Scholar]
- 19. https://dw.euro.who.int/api/v3/export?code=HFA_144
- 20. GLOBOCAN . 2012. http://globocan.iarc.fr/Default.aspx; https://nccd.cdc.gov/uscs/cancersrankedbystate.aspx
- 21. WHO cancer country profile. http://www.who.int/cancer/country-profiles/en/. Last accessed March 23, 2018.
- 22. Against Cancer . Cancer Screening in the European Union. Report on the Implementation of the Council Recommendation on Cancer Screening. 2017. Reprint May 2017. https://ec.europa.eu/health/sites/health/files/major_chronic_diseases/docs/2017_cancerscreening_2ndreportimplementation_en.pdf
- 23. http://www.hpvcentre.net/statistics/reports/XWX.pdf
- 24. OECD.STAT . http://stats.oecd.org/index.aspx?queryxml:id=30159.
- 25. https://gateway.euro.who.int/en/indicators/cah_63-hpv-vaccine-coverage/
- 26. Bruni L, Diaz M, Barrionuevo‐Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453‐e463. [DOI] [PubMed] [Google Scholar]
- 27. ECDC . Introduction of HPV Vaccines in European Countries – An Update. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/20120905_GUI_HPV_vaccine_update.pdf
- 28. http://nordscreen.org. Last accessed March 23, 2018. http://nordscreen.org/wp-content/uploads/2017/07/cervix-fact-sheet-iceland-2017.pdf; http://nordscreen.org/wp-content/uploads/2017/08/cervix-fact-sheet-denmark-2017.pdf; http://nordscreen.org/wp-content/uploads/2017/07/cervix-fact-sheet-estonia-2017.pdf; http://nordscreen.org/wp-content/uploads/2017/07/cervix-fact-sheet-finland-2017.pdf; http://nordscreen.org/wp-content/uploads/2017/07/cervix-fact-sheet-norway-2017.pdf; http://nordscreen.org/wp-content/uploads/2017/07/cervix-fact-sheet-sweden-2017.pdf
- 29. Hamers FF, Duport N, Beltzer N. Population‐based organized cervical cancer screening pilot program in France. Eur J Cancer Prev. 2018;27(5):486‐492. [DOI] [PubMed] [Google Scholar]
- 30. National Cancer Institute . International Screening Cancer Network. Cervical Cancer Screening Programs in 19 ICSN Countries. Organization, Policies, and Program Reach; 2012. https://www.cancer.gov/about-nci/organization/cgh/research/icsn [Google Scholar]
- 31. http://www.hpvcentre.net. Last accessed March 23, 2018 http://www.hpvcentre.net/statistics/reports/AUT.pdf http://www.hpvcentre.net/statistics/reports/AND.pdf http://www.hpvcentre.net/statistics/reports/BEL.pdf http://www.hpvcentre.net/statistics/reports/HRV.pdf; http://www.hpvcentre.net/statistics/reports/CYP.pdf http://www.hpvcentre.net/statistics/reports/CZE.pdf http://www.hpvcentre.net/statistics/reports/DNK.pdf http://www.hpvcentre.net/statistics/reports/EST.pdf http://www.hpvcentre.net/statistics/reports/FIN.pdf http://www.hpvcentre.net/statistics/reports/FRA.pdf http://www.hpvcentre.net/statistics/reports/DEU.pdf http://www.hpvcentre.net/statistics/reports/GRC.pdf http://www.hpvcentre.net/statistics/reports/HUN.pdf http://www.hpvcentre.net/statistics/reports/ISL.pdf http://www.hpvcentre.net/statistics/reports/IRL.pdf http://www.hpvcentre.net/statistics/reports/ISR.pdf http://www.hpvcentre.net/statistics/reports/ITA.pdf http://www.hpvcentre.net/statistics/reports/LVA.pdf http://www.hpvcentre.net/statistics/reports/LTU.pdf http://www.hpvcentre.net/statistics/reports/LUX.pdf http://www.hpvcentre.net/statistics/reports/MLT.pdf http://www.hpvcentre.net/statistics/reports/MCO.pdf http://www.hpvcentre.net/statistics/reports/NOR.pdf http://www.hpvcentre.net/statistics/reports/POL.pdf http://www.hpvcentre.net/statistics/reports/PRT.pdf http://www.hpvcentre.net/statistics/reports/RUS.pdf http://www.hpvcentre.net/statistics/reports/SMR.pdf http://www.hpvcentre.net/statistics/reports/SVK.pdf http://www.hpvcentre.net/statistics/reports/SVN.pdf http://www.hpvcentre.net/statistics/reports/ESP.pdf http://www.hpvcentre.net/statistics/reports/SWE.pdf http://www.hpvcentre.net/statistics/reports/CHE.pdf http://www.hpvcentre.net/statistics/reports/NLD.pdf http://www.hpvcentre.net/statistics/reports/GBR.pdf http://www.hpvcentre.net/statistics/reports/ALB.pdf http://www.hpvcentre.net/statistics/reports/AZE.pdf http://www.hpvcentre.net/statistics/reports/BLR.pdf http://www.hpvcentre.net/statistics/reports/BIH.pdf http://www.hpvcentre.net/statistics/reports/BGR.pdf http://www.hpvcentre.net/statistics/reports/KAZ.pdf http://www.hpvcentre.net/statistics/reports/MKD.pdf http://www.hpvcentre.net/statistics/reports/MNE.pdf http://www.hpvcentre.net/statistics/reports/ROU.pdf http://www.hpvcentre.net/statistics/reports/SRB.pdf http://www.hpvcentre.net/statistics/reports/TUR.pdf http://www.hpvcentre.net/statistics/reports/TKM.pdf http://www.hpvcentre.net/statistics/reports/ARM.pdf http://www.hpvcentre.net/statistics/reports/GEO.pdf http://www.hpvcentre.net/statistics/reports/KGZ.pdf http://www.hpvcentre.net/statistics/reports/MDA.pdf http://www.hpvcentre.net/statistics/reports/TJK.pdf http://www.hpvcentre.net/statistics/reports/UKR.pdf http://www.hpvcentre.net/statistics/reports/UZB.pdf
- 32. European Guidelines for Quality Assurance in Cervical Cancer Screening, Second edition. Supplements.pdf.https://www.gisci.it/documenti/news/EW0115451ENN_002.pdf [DOI] [PMC free article] [PubMed]
- 33. Schenck U, von Karsa L. Cervical cancer screening in Germany. Eur J Cancer. 2000;36(17):2221‐2226. [DOI] [PubMed] [Google Scholar]
- 34. Vīberga I, Poljak M. Cervical cancer screening in Latvia: a brief history and recent improvements (2009‐2011). Acta Dermatovenerol Alp Pannonica Adriat. 2013;22(1):27‐30. [PubMed] [Google Scholar]
- 35. Seme K, Maver PJ, Korać T, et al. Current status of human papillomavirus vaccination implementation in central and eastern Europe. Acta Dermatovenerol Alp Pannonica Adriat. 2013;22(1):21‐25. [PubMed] [Google Scholar]
- 36. http://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/news/news/2016/12/public-debate-on-hpv-immunization-in-serbian-parliament. Last accessed March 23, 2018.
- 37. Periši Z, Plešinac‐Karapandži V, Džini M, Zamurov M, Periš N. Cervical cancer screening in Serbia. Vojnosanit Pregl. 2013;70(1):86‐89. [DOI] [PubMed] [Google Scholar]
- 38. Gultekin M, Zayifoglu Karaca M, Kucukyildiz I, et al. Initial results of population based cervical cancer screening program using HPV testing in one million Turkish women. Int J Cancer. 2018;142(9):1952‐1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferlay J, Soerjomataran I, Ervik M, et al. GLOBOCAN 2012 Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. Lyon, France: International Agency for Research on Cancer; 2013. https://globocan.iarc.fr. [Google Scholar]
- 40. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Klemp Gjertsen M, Neilson AR, de Freiesleben Blasio B. Cost‐Effectiveness of Human Papillomavirus (HPV) Vaccination in Norway [Internet]. Oslo, Norway: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH); 2007. http://www.ncbi.nlm.nih.gov/books/NBK464826/. [PubMed] [Google Scholar]
- 42. Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first‐line screening test. Gynecol Oncol. 2015;136:189‐197. [DOI] [PubMed] [Google Scholar]
- 43. Bruni L, Diaz M, Barrionuevo‐Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Global Health. 2016;4:e453‐e463. [DOI] [PubMed] [Google Scholar]
- 44. Kawachi I, Adler NE, Dow WH. Money, schooling, and health: mechanisms and causal evidence. Annals of New York Academy of Sciences. 2010;1186(1):56‐58. [DOI] [PubMed] [Google Scholar]
- 45. Syse A, Lyngstad TH. In sickness and in health: the role of marital partners in cancer survival. SSM Popul Health. 2016;3:99‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bloem P, Ogbuanu O. vaccination to prevent human papillomavirus infections: from promise to practice. PLoS Med. 2017;14(6):e1002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brotherton J, Zuber P, Bloem P. Primary prevention of HPV through vaccination: update on the current global status. Curr Obst Gynecol Reports. 2016;5:210‐224. [Google Scholar]
- 48. Brisson M, Bénard É, Drolet M, et al. Population‐level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta‐analysis of predictions from transmission‐dynamic models. Lancet Public Health. 2016;1:e8‐e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brisson M, Van de Velde N, Boily MC. Economic evaluation of human papillomavirus vaccination in developed countries. Public Health Genomics. 2009;12:343‐351. [DOI] [PubMed] [Google Scholar]


