Abstract
Background:
The chronobiotic antidepressant, agomelatine, acts via re-entrainment of circadian rhythms. Earlier work has demonstrated late-life anxiety and reduced corticosterone in post-weaning social isolation reared (SIR) rats. Agomelatine was anxiolytic in this model but did not reverse hypocortisolemia. Reduced corticosterone or cortisol (in humans) is well-described in anxiety states, although the anxiolytic-like actions of agomelatine may involve targeting another mechanism. Central oxytocin and vasopressin exert anxiolytic and anxiogenic effects, respectively, and are subject to circadian fluctuation, while also showing sex-dependent differences in response to various challenges.
Aims and methods:
If corticosterone is less involved in the anxiolytic-like actions of agomelatine in SIR rats, we wondered whether effects on vasopressin and oxytocin may mediate these actions, and whether sex-dependent effects are evident. Anxiety as assessed in the elevated plus maze, as well as plasma vasopressin, oxytocin, and corticosterone were analyzed in social vs SIR animals receiving sub-chronic treatment with vehicle or agomelatine (40 mg/kg/day intraperitoneally at 16:00) for 16 days.
Results:
Social isolation rearing induced significant anxiety together with increased plasma vasopressin levels, but decreased corticosterone and oxytocin. While corticosterone displayed sex-dependent changes, vasopressin, and oxytocin changes were independent of sex. Agomelatine suppressed anxiety as well as reversed elevated vasopressin in both male and female rats and partially reversed reduced oxytocin in female but not male rats.
Conclusion:
SIR-associated anxiety later in life involves reduced corticosterone and oxytocin, and elevated vasopressin. The anxiolytic-like effects of agomelatine in SIR rats predominantly involve targeting of elevated vasopressin.
Keywords: Neurodevelopmental model, neuropeptide, circadian rhythm, anxiety, hypothalamic-pituitary axis, sex differences
Introduction
Agomelatine is a first in class antidepressant that acts via the stimulation of melatonin (MT1/MT2) receptors and antagonism of serotonin (5-HT)2C receptors, most notably in the suprachiasmatic nucleus of the hypothalamus (Guardiola-Lemaitre et al., 2014). By virtue of this action, it acts predominantly to re-entrain altered circadian rhythms, thereby exerting a chronobiotic action (Quera Salva and Hartley, 2012). Early-life adversity is a causal factor in the development of an anxiety disorder later in life (Carr et al., 2013; Heim and Nemeroff, 2001). Recently we confirmed this construct in post-weaning social isolation reared (SIR) rats, while furthermore describing the anxiolytic-like effects of agomelatine in this neurodevelopmental model of anxiety (Regenass et al., 2018). We found this model to be associated with significant late-life anxiety-related behavior in both sexes, while at the same time demonstrating a significant reduction in plasma corticosterone, also in both sexes. Reduced corticosterone (in rodents) or cortisol (in humans) is a pathological feature of anxiety-like behavior, e.g. in posttraumatic stress disorder (PTSD) (Lisieski et al., 2018), possibly related to overt suppression of the hypothalamic-pituitary-adrenal (HPA) axis (Lisieki et al., 2018). However, a rather unexpected finding in our study was that, despite the anxiolytic-like properties of agomelatine in this model, it failed to reverse reduced corticosterone (Regenass et al., 2018). This observation suggests that reduced corticosterone is not a major contributing factor to SIR-related anxiety, and/or that agomelatine’s anxiolytic-like actions involve another mechanism, at least in this model.
Vasopressin and oxytocin are gaining increasing importance with respect to anxiety, stress-coping and sociality (Neumann and Landgraf, 2012). Importantly, central oxytocin exerts anxiolytic and antidepressant effects, whereas vasopressin tends to show anxiogenic and depressive actions (Neumann and Landgraf, 2012). Altered circadian fluctuation of these neuropeptides has been described in chronic anxiety states, especially vasopressin (Kalsbeek et al., 2012; McClung, 2013). Oxytocin and vasopressin have shown sex-specific effects in animal models (Bisagno and Cadet, 2014). However, there is a paucity of data in female animals (Janeček and Dabrowska, 2019; Voskuhl, 2016), which is problematic considering that stress-induced mental disorders, including PTSD, are two to three times more prevalent in females than in males (Olff et al., 2007). In anxiety-related conditions specifically, oxytocin exerts contrasting sex-dependent effects on amygdala activity (Domes et al., 2010) while for vasopressin prenatal stress-induced social memory deficits are more prominent in females than males, with vasopressin-1a receptor mRNA expression in the lateral septum and bed nucleus of stria terminalis unaltered in males but significantly lower in females (Grundwald et al., 2016). In a stress-induced depression model, Karisetty et al. (2017) also showed that stress-regulated transcriptional changes in the hypothalamus are sex-specific and ovarian hormone-dependent.
Considering agomelatine’s chronobiotic actions to re-entrain disordered circadian hormones, such as cortisol and melatonin (De Berardis et al., 2015; Leproult et al., 2005), and that it presents with clinically relevant anxiolytic activity (Buoli et al., 2017), we pondered the question whether vasopressin and oxytocin may underlie the earlier observed anxiolytic-like actions of agomelatine in SIR rats, perhaps more so than corticosterone, and whether any sex specific effects are evident.
Subjects and methods
A total of 72 Sprague–Dawley rats (36 males and 36 females) were provided by the Vivarium of the North-West University (NWU). Animals were bred and housed at the Vivarium (South African Veterinary Council reg. number FR15/13458; South African National Accreditation System good laboratory practice compliance number G0019) of the Pre-Clinical Drug Development Platform of the NWU. All experiments were ethically approved (National Health Research Ethics Council reg. number AREC-130913-015) to comply with the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines (Kilkenny et al., 2010) as well as the code of ethics in research, training and testing of drugs in South Africa (ethics approval number NWU-00347-15-S5).
Using the social isolation rearing protocol described previously (Regenass et al., 2018), animals were randomized post weaning (post-natal day (PND) 21) to either social isolation rearing (one animal per cage) or social rearing (three animals per cage) for eight weeks (PND 77) (Möller et al., 2012, 2013a). Groups consisted of 12 rats per group, with male and female rats housed separately but in the same room. Housing conditions were as described by Regenass et al. (2018). Since regular handling reduces the effects of social isolation rearing (Fone and Porkess, 2008; Wall et al., 2012), vaginal smears for documenting the specific estrous cycle of females was not performed.
Social and isolation reared animals received sub-chronic treatment with vehicle (1% hydroxyethylcellulose (HEC)) or agomelatine (Servier, Suresnes, France; 40 mg/kg/day), the latter freshly prepared in 1% HEC suspension (pH=6.4). Agomelatine or vehicle were administered intraperitoneally (i.p.) at 16:00 each day (Banasr et al., 2006) in a total injection volume of 1 mL/kg for 16 days from PND 61 (Möller et al., 2012, 2013a). The dosage regimen for agomelatine was derived from earlier studies suggesting its antidepressant-like and anxiolytic-like actions are best captured in rodents when administered in the late afternoon and at the above-noted dose and duration of treatment (Banasr et al., 2006; Norman et al., 2012; Papp et al., 2006; Regenass et al., 2018). Since agomelatine failed to alter behavior in socially reared control rats (Coutts, 2015) and to conserve animals, this group was not included in the study (see also Regenass et al., 2018).
On the last day of drug treatment (PND 78), rats were euthanized (at 08:00–09:00) with no prior anesthesia. Trunk blood was collected in pre-chilled, 4 mL vacutainer tubes (SGVac) containing dipotassium ethylenediaminetetraacetic acid (K2EDTA) solution as anti-coagulant. The blood was centrifuged at 20,000× g at 4°C for 10 min and the plasma stored at −80°C. On the day of analysis, plasma samples were thawed on ice, centrifuged again as described above, and the plasma used for the analysis of corticosterone, vasopressin, and oxytocin. Plasma corticosterone was quantified using a solid-phase extraction (SPE) high-performance liquid chromatography (HPLC) method, as described previously (Regenass et al., 2018). Plasma vasopressin and oxytocin were assayed using enzyme-linked immunosorbent assay (ELISA) (Arg8-Vasopressin ELISA Kit Catalog No: ab205928, Abcam; Oxytocin ELISA Kit Catalog No: E-EL-0029, Elabscience)
Selected behavioral data for correlation analysis
The relationship between anxiety-like behavior as reported previously (Regenass et al., 2018) and plasma oxytocin, vasopressin, and corticosterone levels was determined. Although we had previously performed a diverse range of anxiety tests in SIR animals receiving agomelatine or vehicle (see Regenass et al., 2018), the correlation analysis undertaken here used only percentage time in open arms (TOA) as reference anxiety parameter. Briefly, anxiety-like behavior was assessed in the elevated plus maze (EPM; n=12/group) within the first two hours of the dark cycle (18:00–20:00) on PND 76, three hours after the last dose of agomelatine (Regenass et al. (2018). Behavior was scored by Noldus EthoVision XT 12 under dim white light (10 lux) conditions (Hogg, 1996; Mora et al., 1996) and expressed as percentage TOA. Arm entries were scored only when all four paws entered the arm.
Statistical analysis
Graphpad Prism version 6 for Windows (Graphpad Software, San Diego, USA) was used for all statistical analysis and graphical presentations. Normality of the data was determined using the Shapiro-Wilk test. Data were analyzed by two-way analysis of variance (ANOVA) with respect to sex and treatment to determine the anxiolytic-like effects of agomelatine. Where an interaction and/or simple main effect were found in the ANOVA analysis, this was followed by a Bonferroni post-hoc analysis or an unpaired student’s t-test. In all cases, data are expressed as the mean±standard error of the mean, with a p value of <0.05 deemed statistically significant. The correlation between corticosterone, vasopressin, oxytocin, and percentage TOA was determined using Pearson’s rank correlation in all treatment groups. Pearson’s correlation coefficient (r) was defined as 0.1⩽r<0.3 indicating a small effect, 0.3⩽r<0.5 a medium effect, and r⩾0.5 a large effect. A probability level of 95% was used to determine statistical significance (p<0.05).
Results
Effect of social isolation rearing with/without agomelatine treatment on plasma corticosterone and neuropeptide levels
Regenass et al. (2018) described a significant anxiogenic effect for social isolation rearing in female and male rats, evinced as decreased percentage TOA (amongst other anxiety-related behaviors) with an associated hypocortisolemia. Agomelatine treatment reversed anxiety in both female and male SIR rats but not hypocortisolemia (Regenass et al., 2018). We have now re-analyzed the percentage TOA and corticosterone data presented in Regenass et al. (2018) (Table 1 and Figure 1(a) respectively), while in addition introducing novel analysis of plasma oxytocin and vasopressin.
Table 1.
Pearson correlation matrix depicting associations between oxytocin, vasopressin, corticosterone, and percentage time in open arms (%TOA) across all treatment groups (male and female) (n=72; 12 rats/group).
| Vasopressin | Oxytocin | Corticosterone | %TOA | |
|---|---|---|---|---|
|
r (95% CI) |
r (95% CI) |
r (95% CI) |
r (95% CI) |
|
| Vasopressin | - | −0.45 (−0.24 – −0.62)
p<0.0001 |
−0.55 (−0.37 – −0.69)
p<0.0001 |
−0.26 (−0.034 – −0.47)
p=0.025 |
| Oxytocin | −0.45 (−0.24 – −0.62)
p<0.0001 |
- | 0.31 (0.51–0.008)
p=0.007 |
NS |
| Corticosterone a | −0.55 (−0.37 – −0.69)
p<0.0001 |
0.31 (0.51–0.008)
p=0.007 |
- | NS |
| %TOA | −0.26 (−0.034 – −0.47)
p=0.025 |
NS | NS | - |
CI: confidence interval; NS: non-significant.
Data from Regenass et al., 2018 re-presented and re-analyzed.
Figure 1.
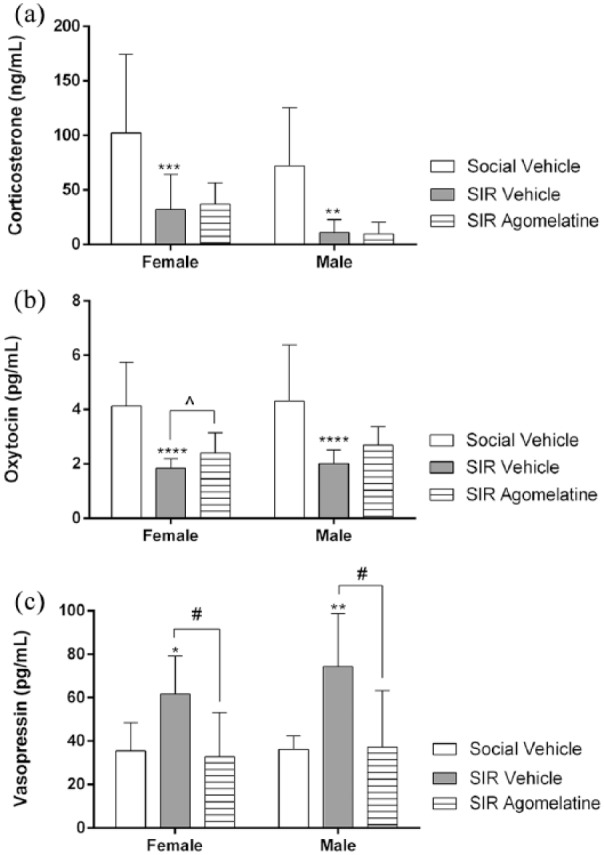
Plasma levels of (a) corticosterone, (b) oxytocin, and (c) vasopressin respectively in socially reared animals receiving vehicle treatment and social isolation reared (SIR) animals receiving vehicle and agomelatine treatment, presented as mean±standard error of the mean (SEM) (n=12 rats/group). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 vs social vehicle; #p<0.05 vs SIR vehicle (two-way analysis of variance (ANOVA), Bonferroni’s post-hoc analysis); ^p<0.05 vs SIR vehicle (unpaired student’s t-test).
A two-way ANOVA revealed no significant gender×treatment interaction on corticosterone (F(2,65)=0.07, p=0.9). However, a significant main effect regarding gender on corticosterone levels was observed (F(1,65)=7.46, p=0.008) and a significant treatment effect (F(2,65)=20.13, p<0.0001). Two-way ANOVA indicated no significant gender×treatment interaction with respect to oxytocin (F(2,66)=0.02, p=0.97) and vasopressin (F(2,66)=0.5, p=0.59). Unlike with corticosterone, gender had no significant main effect on oxytocin (F(1,66)=0.59, p=0.44) or vasopressin (F(1,66)=2.01, p=0.16). However, treatment had a significant main effect on oxytocin (F(2,66)=24.59, p<0.0001) and vasopressin (F(2,66)=8.35, p=0.0006).
Social isolation rearing significantly decreased plasma corticosterone in both females (p=0.0002) and males (p=0.001), while agomelatine did not reverse this affect (Regenass et al., 2018) (Figure 1(a)). Social isolation rearing significantly decreased plasma oxytocin in both females and males (p<0.0001) (Figure 1(b)) and significantly increased plasma vasopressin in females (p=0.01) and males (p=0.003) (Figure 1(c)). Agomelatine significantly reversed elevated vasopressin in both females (p=0.03) and males (p=0.01) (Figure 1(c)). Although not significant in the two-way ANOVA, agomelatine partially reversed reduced oxytocin levels in females (p=0.03) but not in males (p=0.07) (Figure 1(b); unpaired student’s t-test).
Correlation analyses were performed in the combined dataset of all the female and male drug-treated groups (n=72) (Table 1). No noteworthy correlations were evident in the separate groups. Considering the cross-correlation neuropeptide analysis, significant negative correlations were observed between vasopressin and oxytocin (Pearson’s r=−0.45, p<0.0001) and between vasopressin and corticosterone (Pearson’s r=−0.55, p<0.0001), while a significant positive correlation was evident with respect to oxytocin and corticosterone (Pearson’s r=0.31, p=0.007). Considering the neuropeptide-anxiety correlation, vasopressin levels and percentage TOA showed a significant negative correlation (Pearson’s r=−0.26, p=0.025) (Table 1), and depicted specifically in Figure 2. Oxytocin (Pearson’s r=0.22, p=0.08) and corticosterone (Pearson’s r=0.23, p=0.07) narrowly missed a significant correlation with percentage TOA.
Figure 2.
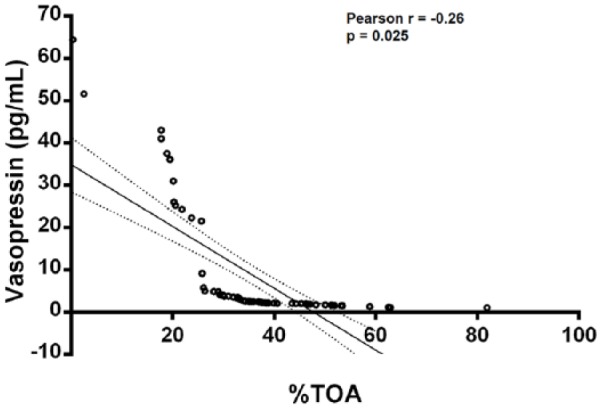
Pearson correlation analysis between percentage time in open arms (%TOA) and plasma vasopressin in all treatment groups (male and female) (n=72).
Discussion
Early life adversity (e.g. social isolation rearing) exerts profound neurodevelopmental effects that alter 5-HT as well as other neurotransmitters, culminating in late-life presentation of anxiety, depression and antisocial behavior (Carr et al., 2013; Fone and Porkess, 2008; Heim and Nemeroff, 2001; Möller et al., 2015). 5-HT1A and 5-HT2A receptors are expressed in the rat brain from gestational day (GD) 16 (Patel and Zhou, 2005) and PND 3 (Li et al., 2003), respectively, and may be compromised by an early-life neurodevelopmental challenge (Maniam and Morris, 2010; Matsuzaki et al., 2011). Indeed, earlier work has noted that social isolation rearing significantly alters regional brain 5-HT levels (Möller et al., 2013b; Trabace et al., 2012). Although 5-HT reuptake inhibitors (SSRIs) may offer effective treatment, full remission in anxiety (Taylor et al., 2012) and mood (Fava, 2003) disorders is seldom attained, emphasizing the need to identify new biological targets as well as drug treatments. In this regard, disturbed circadian rhythm is a recognized contributor to these disorders (Quera Salva and Hartley, 2012), with chronobiotic agents like agomelatine offering hope for more effective pharmacotherapy.
Both animal and human studies suggest that plasma vasopressin levels correlate with anxiety in adulthood (Varga et al., 2015). To the best of our knowledge, this is the first study to investigate concomitant changes in corticosterone, vasopressin and oxytocin following social isolation rearing in rats and their association with anxiety-like behavior. Here we report that rearing condition, i.e. be it group- or isolation-housed, engenders a significant and negative correlation between anxiety and plasma vasopressin. Thus, social isolation rearing is associated with reduced %TOA and elevated plasma vasopressin. Although social isolation rearing significantly decreased plasma oxytocin levels, the association between anxiety and plasma oxytocin was insignificant in all the treatment groups. Given the anxious state of these animals (Regenass et al., 2018), these findings support the anxiogenic role of vasopressin in mediating anxious states, less so the anxiolytic actions of oxytocin (Neumann and Landgraf, 2012). Similarly, although significant hypocortisolemia was evident in SIR animals (Regenass et al., 2018), the correlation between anxiety and plasma corticosterone was insignificant in all the treatment groups.
This and our earlier work (Regenass et al., 2018) provides unequivocal evidence that post-weaning social isolation rearing induces anxiety-like behavior later in life, as well as reduces plasma corticosterone and oxytocin but elevates vasopressin. Moreover, we corroborate the probable causal association of SIR-induced anxiety with increased vasopressin rather than with reduced corticosterone or oxytocin. The interactive nature of these neuropeptides is important to consider here. Indeed, a medium effect size negative correlation was observed between vasopressin and oxytocin, as well as a large effect size negative correlation between vasopressin and corticosterone. On the other hand, we observed a medium effect size positive correlation between oxytocin and corticosterone. The significant negative correlation between vasopressin and oxytocin confirms earlier findings within the context of anxiety (Neumann and Landgraff, 2012). However, corticotrophin-releasing hormone (CRH) (and its subsequent activation of glucocorticoid release) and vasopressin are the central drivers of the stress hormone system (Holsboer and Ising, 2010). The negative correlation between vasopressin and corticosterone thus appear counter-intuitive with respect to the genesis of anxiety. Oxytocin on the other hand plays a moderating role on bio-behavioral responses to stress (Windle et al., 2006). Since we observed a positive correlation between oxytocin and corticosterone, it is interesting that elevated oxytocin reduces stress-induced corticosterone release (Windle et al., 1997). Given the juxtaposed findings noted with regard to vasopressin and oxytocin vs. corticosterone, we posit that under extended stressful conditions (e.g. social isolation rearing) certain inherent protective mechanisms are compromised resulting in an anxiogenic response.
5-HT plays an important role in regulating the release of the above hormones (Jørgensen et al., 2003; Oosthuizen et al., 2005), which as noted earlier may be compromised by social isolation rearing (Möller et al., 2013b; Trabace et al., 2012). Thus, the serotonergic system plays a key role in the anxiolytic effects of oxytocin (Yoshida et al., 2009), with 5-HT-induced oxytocin secretion mediated by 5-HT1A (inhibitory) and 5-HT2A/C (excitatory) receptors (Jørgensen et al., 2003) in the paraventricular nucleus (PVN) (Zhang et al., 2004). 5-HT also stimulates vasopressin release at the neurohypophyseal level (Lemay et al., 1979) via 5-HT2C, 5-HT4, and 5-HT7 receptor activation (Jørgensen et al., 2003) while, conversely, vasopressin can promote the synthesis and release of 5-HT in the hippocampus (Auerbach and Lipton, 1982). With agomelatine significantly reversing SIR-induced elevations in vasopressin in both male and female rats, our data supports a definite role for vasopressin in the anxiolytic actions of agomelatine. On the other hand, agomelatine treatment only reversed SIR-induced oxytocin deficits in female rats (t-test), thereby suggesting a lesser role for oxytocin in this regard, at least in the social isolation rearing model. Taken together, 5-HT2C receptor antagonism by agomelatine may curb 5-HT-mediated vasopressin release, and in this way abrogate anxiety-like behavior (Guardiola-Lemaitre et al., 2014), a conclusion corroborated by a significant negative correlation between percentage TOA and vasopressin plasma levels. That said, it is possible that agomelatine may exert different dose-related effects on these neuropeptides with effects on corticosterone and oxytocin revealed at another dose. However, although 5-HT2C antagonism is generally regarded as evoking an anxiolytic response (De Berardis et al., 2015), agomelatine’s short duration of receptor occupancy argues for another mechanism (Harvey and Slabbert, 2014).
Social isolation rearing is known to destabilize circadian rhythms (Gambardella et al., 1994), while altered circadian fluctuation of the above neuropeptides has been described in chronic anxiety states, especially vasopressin (Kalsbeek et al., 2012; McClung, 2013). Considering the chronobiotic effect of agomelatine via the stimulation and block of MT1/MT2 and 5-HT2C receptors in the suprachiasmatic nucleus respectively, agomelatine may modulate oxytocin and vasopressin release indirectly via re-synchronization of disordered neuropeptide rhythms following chronic stress (Koresh et al., 2012), or in this instance following social isolation rearing. The anxiolytic actions of agomelatine can thus be attributed to it reducing vasopressin and to a lesser degree increasing oxytocin. Since social isolation rearing emphasizes early life social distress as a central construct, and wherein oxytocin and vasopressin are noted to have a prominent role (Neumann and Landgraf, 2012), these actions offer a reasonable explanation for how agomelatine may exert anxiolytic-like effects in SIR rats.
Finally, our findings reveal a significant effect of sex on corticosterone in response to social isolation rearing but not with respect to oxytocin or vasopressin. Such sexual dimorphic responses have been suggested to explain the higher incidence of anxiety disorders and depression in women or antisocial behavior in males (Neumann and Landgraf, 2012). Agomelatine involves pronounced reversal of SIR-associated elevations in vasopressin in both females and males together with partial reversal of reduced oxytocin observed in females, but not males. Although both oxytocin and vasopressin have specific functions in males and females (Neumann and Landgraf, 2012), these effects may differ between the sexes (Bisagno and Cadet, 2014).
Conclusion
Our combined data suggests that social isolation rearing is anxiogenic, with co-presentation of reduced plasma corticosterone and oxytocin and increased vasopressin, with a gender effect noted with respect to corticosterone. However, both sexes demonstrated significant association between anxiety-like behavior and elevated vasopressin. Furthermore, the anxiolytic-like effects of agomelatine involves a sex-independent reversal of SIR-associated elevations in vasopressin. This response was noted to a lesser extent with regard to oxytocin and only in female rats. As noted earlier (Regenass et al., 2018), corticosterone is not involved in this response. The anxiolytic response to agomelatine is likely to involve modulation of processes involved in vasopressin and oxytocin release, either via direct serotonergic and/or indirect chronobiotic mechanisms. This unique profile involving modulation of anxiety neuropeptides is of particular interest and warrants deeper study. Further study in other neurodevelopmental or genetic models is necessary to verify these findings.
Acknowledgments
The following author contributions were made: WR treated the animals and collected the samples, with WD performing the ELISA assays. MM advised on the setting up of the social isolation rearing model, undertook the statistical analysis, and co-prepared the table. BHH initiated and designed the study, co-supervised WR, contributed towards the preparation of the table, interpreted the results and prepared the manuscript for submission. All the authors read and approved the final version of the manuscript for submission.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare that over the past three years, BHH has participated in advisory boards and received honoraria from Servier, and has received research funding from Servier and Lundbeck. The authors declare that, except for income from the primary employer and research funding to BHH from the below-mentioned organizations and agencies, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional services, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The authors declare that this work has been partially funded by the National Research Foundation (NRF) (BHH; grant number CPRR14080485951). BHH acknowledges that opinions, findings and conclusions or recommendations expressed in any publication generated by NRF supported research are those of the authors, and that the NRF accepts no liability whatsoever in this regard. This work was also partially funded through an unrestricted grant by Servier Laboratories (SA), although Servier (SA) did not provide any input into the study design, data collection or interpretation, reporting or publication. Opinions, findings, and conclusions or recommendations expressed in this paper are those of the authors and not those of Servier (SA).
References
- Auerbach S, Lipton P. (1982) Vasopressin augments depolarization-induced release and synthesis of serotonin in hippocampal slices. J Neurosci 2: 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Soumier A, Hery M, et al. (2006) Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry 59: 1087–1096. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Cadet JL. (2014) Stress, sex, and addiction: Potential roles of corticotropin-releasing factor, oxytocin, and arginine-vasopressin. Behav Pharmacol 25: 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buoli M, Grassi S, Serati M, et al. (2017) Agomelatine for the treatment of generalized anxiety disorder. Expert Opin Pharmacother 18: 1373–1379. [DOI] [PubMed] [Google Scholar]
- Carr CP, Martins CM, Stingel AM, et al. (2013) The role of early life stress in adult psychiatric disorders: A systematic review according to childhood trauma subtypes. J Nerv Ment Dis 201: 1007–1020. [DOI] [PubMed] [Google Scholar]
- Coutts DE. (2015) Behavioural, neuroendocrine and neurochemical studies on agomelatine in social isolation reared rats. MSc dissertation, North-West University, Potchefstroom, South Africa. [Google Scholar]
- De Berardis D, Fornaro M, Serroni N, et al. (2015) Agomelatine beyond borders: Current evidences of its efficacy in disorders other than major depression. Int J Mol Sci 16: 1111–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, et al. (2010) Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology 35: 83–93. [DOI] [PubMed] [Google Scholar]
- Fava M. (2003) Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 53: 649–659. [DOI] [PubMed] [Google Scholar]
- Fone KC, Porkess MV. (2008) Behavioural and neurochemical effects of post-weaning social isolation in rodents—relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev 32: 1087–1102. [DOI] [PubMed] [Google Scholar]
- Gambardella P, Greco AM, Sticchi R, et al. (1994) Individual housing modulates daily rhythms of hypothalamic catecholaminergic system and circulating hormones in adult Male rats. Chronobiol Int 11: 213–221. [DOI] [PubMed] [Google Scholar]
- Grundwald NJ, Benítez DP, Brunton PJ. (2016) Sex-dependent effects of prenatal stress on social memory in rats: A role for differential expression of central vasopressin-1a receptors. J Neuroendocrinol 28(4): Published online 25 April 2016. doi: 10.1111/jne.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola-Lemaitre B, De Bodinat C, Delagrange P, et al. (2014) Agomelatine: Mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol 171: 3604–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BH, Slabbert FN. (2014) New insights on the antidepressant discontinuation syndrome. Hum Psychopharmacol 29: 503–516. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. (2001) The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol Psychiatry 49: 1023–1039. [DOI] [PubMed] [Google Scholar]
- Hogg S. (1996) A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav 54: 21–30. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. (2010) Stress hormone regulation: Biological role and translation into therapy. Annu Rev Psychol 61: 81–109, C1–C11. [DOI] [PubMed] [Google Scholar]
- Janeček M, Dabrowska J. (2019) Oxytocin facilitates adaptive fear and attenuates anxiety responses in animal models and human studies—potential interaction with the corticotropin-releasing factor (CRF) system in the bed nucleus of the stria terminalis (BNST). Cell Tissue Res 375: 143–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen H, Riis M, Knigge U, et al. (2003) Serotonin receptors involved in vasopressin and oxytocin secretion. J Neuroendocrinol 15: 242–249. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van der Spek R, Lei J, et al. (2012) Circadian rhythms in the hypothalamo–pituitary–adrenal (HPA) axis. Mol Cell Endocrinol 349: 20–29. [DOI] [PubMed] [Google Scholar]
- Karisetty BC, Khandelwal N, Kumar A, et al. (2017) Sex difference in mouse hypothalamic transcriptome profile in stress-induced depression model. Biochem Biophys Res Commun 486: 1122–1128. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, et al. (2010) Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koresh O, Kozlovsky N, Kaplan Z, et al. (2012) The long-term abnormalities in circadian expression of Period 1 and Period 2 genes in response to stress is normalized by agomelatine administered immediately after exposure. Eur Neuropsychopharmacol 22: 205–221. [DOI] [PubMed] [Google Scholar]
- Lemay A, Brouillette A, Denizeau F, et al. (1979) Melatonin-and serotonin-stimulated release of vasopressin from rat neurohypophysis in vitro. Mol Cell Endocrinol 14: 157–166. [DOI] [PubMed] [Google Scholar]
- Leproult R, Van Onderbergen A, L’hermite-Balériaux M, et al. (2005) Phase-shifts of 24-h rhythms of hormonal release and body temperature following early evening administration of the melatonin agonist agomelatine in healthy older men. Clin Endocrinol (Oxf) 63: 298–304. [DOI] [PubMed] [Google Scholar]
- Li Q, Nakadate K, Tanaka-Nakadate S, et al. (2003) Unique expression patterns of 5-HT2A and 5-HT2C receptors in the rat brain during postnatal development: Western blot and immunohistochemical analyses. J Comparat Neurol 496: 128–140. [DOI] [PubMed] [Google Scholar]
- Lisieski MJ, Eagle AL, Conti AC, et al. (2018) Single-prolonged stress: A review of two decades of progress in a rodent model of post-traumatic stress disorder. Front Psychiatry 9: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniam J, Morris MJ. (2010) Voluntary exercise and palatable high-fat diet both improve behavioural profile and stress responses in male rats exposed to early life stress: Role of hippocampus. Psychoneuroendocrinology 35:1553–1564. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Izumi T, Horinouchi T, et al. (2011) Juvenile stress attenuates the dorsal hippocampal postsynaptic 5-HT1A receptor function in adult rats. Psychopharmacology (Berl) 214: 329–337. [DOI] [PubMed] [Google Scholar]
- McClung CA. (2013) How might circadian rhythms control mood? Let me count the ways. Biol Psychiatry 74: 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller M, Du Preez JL, Emsley R, et al. (2012) Social isolation rearing in rats alters plasma tryptophan metabolism and is reversed by subchronic clozapine treatment. Neuropharmacology 62: 2499–2506. [DOI] [PubMed] [Google Scholar]
- Möller M, Du Preez JL, Viljoen FP, et al. (2013. a) Social isolation rearing induces mitochondrial, immunological, neurochemical and behavioural deficits in rats, and is reversed by clozapine or N-acetyl cysteine. Brain Behav Immun 30: 156–167. [DOI] [PubMed] [Google Scholar]
- Möller M, Du Preez JL, Viljoen FP, et al. (2013. b) N-Acetyl cysteine reverses social isolation rearing induced changes in cortico-striatal monoamines in rats. Metab Brain Dis 28: 687–696. [DOI] [PubMed] [Google Scholar]
- Möller M, Swanepoel T, Harvey BH. (2015) Neurodevelopmental animal models reveal the convergent role of neurotransmitter systems, inflammation, and oxidative stress as biomarkers of schizophrenia: Implications for novel drug development. ACS Chem Neurosci 6: 987–1016. [DOI] [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Diaz-Veliz G. (1996) Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology 21: 609–620. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. (2012) Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviours. Trends Neurosci 35: 649–659. [DOI] [PubMed] [Google Scholar]
- Norman TR, Cranston I, Irons JA, et al. (2012) Agomelatine suppresses locomotor hyperactivity in olfactory bulbectomised rats: A comparison to melatonin and to the 5-HT 2C antagonist, S32006. Eur J Pharmacol 674: 27–32. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Draijer N, et al. (2007) Gender differences in posttraumatic stress disorder. Psychol Bull 133:183–204. [DOI] [PubMed] [Google Scholar]
- Oosthuizen F, Wegener G, Harvey BH. (2005) Nitric oxide as inflammatory mediator in post-traumatic stress disorder (PTSD): Evidence from an animal model. Neuropsychiatr Dis Treat 1: 109–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M, Litwa E, Gruca P, et al. (2006) Anxiolytic-like activity of agomelatine and melatonin in three animal models of anxiety. Behav Pharmacol 17: 9–18. [DOI] [PubMed] [Google Scholar]
- Patel D, Zhou FC. (2005) Ontogeny of 5-HT1A receptor expression in the developing hippocampus. Dev Brain Res 157: 42–57. [DOI] [PubMed] [Google Scholar]
- Quera Salva MA, Hartley S. (2012) Mood disorders, circadian rhythms, melatonin and melatonin agonists. J Cent Nerv Syst Dis 4: 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenass W, Möller M, Harvey BH. (2018) Studies into the anxiolytic actions of agomelatine in social isolation reared rats: Role of corticosterone and sex. J Psychopharmacology 32: 134–145. [DOI] [PubMed] [Google Scholar]
- Taylor S, Abramowitz JS, Mckay D. (2012) Non-adherence and non-response in the treatment of anxiety disorders. J Anxiety Disord 26: 583–589. [DOI] [PubMed] [Google Scholar]
- Trabace L, Zotti M, Colaianna M, et al. (2012). Neurochemical differences in two rat strains exposed to social isolation rearing. Acta Neuropsychiatrica 24: 286–295. [DOI] [PubMed] [Google Scholar]
- Varga J, Fodor A, Klausz B, et al. (2015) Anxiogenic role of vasopressin during the early postnatal period: Maternal separation-induced ultrasound vocalization in vasopressin-deficient Brattleboro rats. Amino Acids 47: 2409–2418. [DOI] [PubMed] [Google Scholar]
- Voskuhl R. (2016) Preclinical studies of sex differences: A clinical perspective. Biol Sex Differ 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall VL, Fischer EK, Bland ST. (2012) Isolation rearing attenuates social interaction-induced expression of immediate early gene protein products in the medial prefrontal cortex of male and female rats. Physiol Behav 107: 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Gamble LE, Kershaw YM, et al. (2006) Gonadal steroid modulation of stress-induced hypothalamo-pituitary-adrenal activity and anxiety behavior: Role of central oxytocin. Endocrinology 147: 2423–2431. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, et al. (1997) Central oxytocin administration reduces stress-induced corticosterone release and anxiety behaviour in rats. Endocrinology 138: 2829–2834. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, et al. (2009) Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci 29: 2259–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Grey TS, D’Souza DN, et al. (2004) Desensitization of 5-HT1A receptors by 5-HT2A receptors in neuroendocrine neurons in vivo. J Pharmacol Exp Ther 310: 59–66. [DOI] [PubMed] [Google Scholar]


