Abstract
Combination regimens are a standard of care for many cancers. However, components of such regimens are typically first developed individually and subsequently combined using strategies to minimize toxicity. Little or no consideration is given to strategies that potentially maximize efficacy. In contrast, CPX-351 (Vyxeos®) is a dual-drug liposomal encapsulation of cytarabine and daunorubicin that was rationally designed to improve efficacy over the traditional 7+3 cytarabine/daunorubicin chemotherapy regimen for patients with acute myeloid leukemia (AML). The notable clinical efficacy of CPX-351 is achieved through maintenance of a synergistic 5:1 molar ratio of cytarabine and daunorubicin within the liposome after intravenous injection. The CPX-351 liposome, which is formulated to contain bilayers of distearoylphosphatidylcholine, distearoylphosphatidylglycerol, and cholesterol at a 7:2:1 molar ratio and remains in a gel phase at body temperature, provides stability without polyethylene glycol, controlled release of cytarabine and daunorubicin, limited systemic drug distribution, and preferential internalization within malignant myeloblasts in the bone marrow via active uptake of liposomes into cytoplasmic vacuoles. Thus, the CPX-351 liposome protects cytarabine and daunorubicin from metabolism and elimination, while overcoming pharmacokinetic differences between the two agents. In clinical studies, these liposome properties markedly increased the elimination half-life of CPX-351 versus free cytarabine and daunorubicin and maintained a synergistic drug ratio for over 24 hrs after administration. Preferential uptake of liposomes by leukemia cells suggests that relatively large amounts of cytarabine and daunorubicin enter malignant cells via liposomes, potentially bypassing P-glycoprotein-based efflux pumps, which are important mediators of chemotherapy resistance, and contribute to the rapid clearance of leukemia cells from the circulation and bone marrow. These pharmacologic advantages, a direct consequence of properties of the encapsulating liposome, may explain the efficacy of CPX-351 in patients with newly diagnosed high-risk/secondary AML and the reduced drug exposure in off-target tissues that contribute to a manageable safety profile.
Keywords: acute myeloid leukemia, CPX-351, cytarabine, daunorubicin, molar ratio, nanoscale liposomes
Introduction
Historically, liposomal delivery of individual anticancer agents has focused on improving targeted drug delivery to tumor tissues and, consequently, reducing toxicity. However, liposomal encapsulation or nanoparticle capture of multiple drugs within a single formulation provides an alternative approach for administration of drug combinations. This novel approach enables a fixed additive or synergistic ratio of drugs to be administered and maintained within a single delivery vehicle.
Combination treatment with multiple drugs exhibiting different modes of action is common in the management of diseases such as infection and cancer.1–3 The theoretical rationale for such approaches includes increasing the therapeutic effect, decreasing dosage to reduce toxicity, and delaying the development of drug resistance.4 However, typically, the components of such regimens are first developed individually, without consideration of the many issues that may arise when they are used in combination, such as on-target antagonism and potentiation of adverse events.4 A more efficient approach to identifying synergistic drug combinations would involve a system of dual-drug screening and nanoscale formulation at the preclinical stage.5 Due to their in vivo stability, liposomes allow for a greater regulation of drug release and prolonged drug exposure. Together, these properties overcome issues with differing pharmacokinetic and pharmacodynamic profiles and fluctuating molar ratios in vivo.6,7 In addition, liposomal encapsulation may allow preferential targeting of specific tissues, thereby enhancing drug concentrations at the site of disease while reducing exposure to healthy tissues.7 This review summarizes this approach and explains how a novel drug carrier delivering a fixed molar ratio of cytarabine and daunorubicin (referred to as CPX-351 [Vyxeos®; Jazz Pharmaceuticals, Inc.]) to malignant myeloid target cells has specific pharmacologic advantages that enhance efficacy.
Acute myeloid leukemia (AML) is a good example of an indication that is commonly treated with combination therapy, where progress may require improved understanding of the therapeutic interactions between drugs and their ability to delay or prevent drug resistance. Annually in the United States, more than 19,000 new cases of AML are diagnosed and almost 11,000 people die of AML.8 The overwhelming majority of cases (>80%) occur in adults aged 45 years and over.9,10 Five-year survival is approximately 20% overall and decreases sharply in older patients.10 Multiple disease and demographic variables, including age, the presence of certain genetic or chromosomal abnormalities, white cell count at diagnosis, and specific comorbidities, can influence prognosis.11,12 Secondary AML (sAML), an AML subtype that develops after a previous hematologic disorder or as a consequence of prior cytotoxic chemotherapy or radiation (ie, therapy-related AML) has an especially poor prognosis.13
Combination chemotherapy remains the standard of care for intensive AML induction therapy; treatment regimens typically consist of multiple-day infusion of cytarabine in combination with an anthracycline. In the United States and Europe, the “7+3” regimen of cytarabine and daunorubicin, ie, continuous intravenous (IV) cytarabine administration for 7 days with IV doses of daunorubicin for the first 3 days, is broadly accepted as a standard of care treatment.14,15 This regimen has remained largely unchanged for more than 40 years but is limited by low rates of complete remission (CR) in older patients who have an increased prevalence of AML-specific genomic and cytogenetic risk factors associated with a poor prognosis. Thus, outcomes remain dismal in older (>70 years) adults and individuals with sAML.16,17
The 7+3 regimen was devised by Yates et al in 1973 after trial and error evaluations of other dosing approaches in AML patients.18 Subsequent clinical studies have assessed variations on the 7+3 regimen—namely, intensified doses of cytarabine or daunorubicin, or the addition of other therapeutic agents—with inconsistent or limited benefits to remission rates or survival. While rational, the traditional combination chemotherapy approach does not consider drug interactions that may potentially impact efficacy. In some cases, drug interactions affecting efficacy depend on the relative concentrations of each drug in the regimen, with synergy apparent at specific molar ratios and additivity or antagonism evident at other ratios. Varying the molar ratio of each drug can be used to exploit synergistic interactions, and avoiding antagonistic ratios could further improve combination regimens.
In vitro studies have shown that cytarabine and daunorubicin achieve maximal synergy when malignant cells are exposed to five times as much cytarabine as daunorubicin. Achievement and maintenance of this 5:1 molar ratio maximizes efficacy and minimizes antagonism across multiple tumor cell lines,19 but the synergistic ratio must be delivered to the malignant cells to be effective. However, traditional drug administration methods may be insufficient to achieve and maintain a particularly efficacious molar ratio within a malignant cell. For example, the administration of cytarabine by 7-day continuous infusion and daunorubicin by rapid IV infusion on days 1, 2, and 3, while appropriate for each individual agent, does not maintain a synergistic cytarabine:daunorubicin molar ratio. After achieving equilibrium, the plasma concentration of cytarabine stays relatively constant during the 7-day infusion. However, the plasma concentration of daunorubicin changes after each rapid infusion, as the drug is distributed and eliminated, resulting in a constantly changing cytarabine:daunorubicin ratio within the plasma and, presumably, within individual malignant cells. The traditional method of cytarabine administration by 7-day continuous infusion also presents logistical challenges because 24-hr infusions commonly require inpatient support, which adds to treatment complexity, costs, and patient discomfort.20
Early high-cholesterol liposomal products, such as those used for liposomal daunorubicin (DaunoXome®) or doxorubicin HCl liposome injection (Doxil®) only encapsulated a single agent, not a combination of drugs. Since these and other single-agent liposomal agents still had their own pharmacokinetic profiles, combined administration of these individual agents was unable to retain combinations at a fixed synergistic ratio for more than a few hours. Therefore, manipulation of liposomal composition was required to maintain a synergistic molar ratio for extended periods of time in vivo. To achieve this goal, CPX-351, which is a dual-drug liposomal encapsulation of daunorubicin and cytarabine for IV injection, was developed as a low-cholesterol liposome with distearoylphosphatidylcholine (DSPC), distearoylphosphatidylglycerol (DSPG), and cholesterol at a 7:2:1 molar ratio.21 Importantly, these innovative biophysical properties underlie the pharmacologic advantages of CPX-351 and, consequently, improved efficacy compared with conventional chemotherapy and other liposomal formulations. CPX-351 was approved by the US Food and Drug Administration in August 2017 and by the European Medicines Agency in August 2018 for the treatment of adults with newly diagnosed, therapy-related AML (tAML) or AML with myelodysplasia-related changes (MRC). National Comprehensive Cancer Network guidelines recommend the use of induction or consolidation with CPX-351 for newly diagnosed patients with tAML or AML-MRC.14 Here, we describe the pharmacologic properties of the CPX-351 dual-drug liposomal formulation and discuss how these properties relate to pharmacokinetic and pharmacodynamic characteristics that contribute to the observed clinical efficacy and tolerability of this treatment for AML.
Clinical efficacy and safety of CPX-351 in AML
The recommended induction dose of CPX-351 was established in a first-in-human, Phase I dose-escalation study of CPX-351 in patients with relapsed/refractory AML.22 For induction therapy, CPX-351 is administered as a 90-min infusion of 100 units/m2, corresponding to cytarabine 100 mg/m2 plus daunorubicin 44 mg/m2, on days 1, 3, and 5 (days 1 and 3 for the second induction cycle). CPX-351 consolidation was initially administered at the induction dose on days 1 and 3 in the Phase II studies but was reduced to a dose of 65 units/m2, corresponding to cytarabine 65 mg/m2 plus daunorubicin 29 mg/m2, in the Phase III study to reduce the duration of myelosuppression.23
The efficacy and safety of CPX-351 were subsequently assessed in a Phase II, randomized, open-label study conducted in 121 patients who had experienced a first relapse of their AML after previous successful treatment.24 Patients received induction and consolidation therapy with CPX-351 or the investigators’ choice of salvage therapy. A greater proportion of patients in the CPX-351 group achieved CR than in the control group (49.4% vs 40.9%).24 Although median overall survival (OS) was longer in the CPX-351 group, this difference did not reach statistical significance compared with the control group (8.5 vs 6.3 months; P=0.19). However, among patients classified as poor-risk per the European Prognostic Index,25 median OS was significantly longer with CPX-351 (6.6 months) than with control therapy (4.2 months; P=0.02).
A separate Phase II randomized, open-label, parallel arm clinical study compared CPX-351 with 7+3 in 126 older patients (aged 60–75 years) with newly diagnosed AML.26 Remission (CR or CR with incomplete platelet or neutrophil recovery [CRi]) was achieved by 66.7% of patients in the CPX-351 arm and by 51.2% of patients in the 7+3 arm (P=0.07). In the overall study population, the median OS was not significantly longer in patients receiving CPX-351 compared with those receiving 7+3 (14.7 months vs 12.9 months; P=0.61, with significance prospectively defined as P<0.1); notably, this analysis was confounded by inclusion of patients who had crossed over to receive CPX-351 after failure of 7+3 study therapy). However, in a pre-specified subgroup analysis of patients with sAML (n=52), median OS was significantly longer with CPX-351 versus 7+3 (12.1 vs 6.1 months; P=0.01).26 These results provided the rationale for a Phase III study that compared CPX-351 with 7+3 in patients with high-risk/sAML.
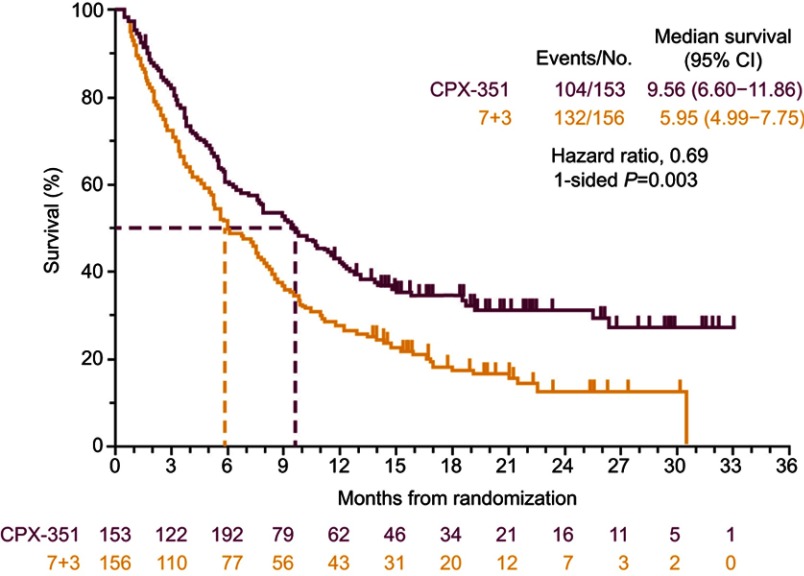
The subsequent Phase III, randomized, controlled study enrolled 309 patients aged 60–75 years with newly diagnosed high-risk/sAML.23 As in the Phase II studies, CPX-351 was compared with the 7+3 regimen. Median OS in the CPX-351 group was significantly longer than in the 7+3 group (9.56 vs 5.95 months; 1-sided P=0.003; Figure 1).23 A greater proportion of patients achieved remission (CR+CRi) with CPX-351 than with 7+3 (47.7% vs 33.3%, respectively; 2-sided P=0.016) and were able to proceed to hematopoietic cell transplantation (HCT; 34.0% vs 25.0%). For those patients who underwent HCT, median OS landmarked from the time of transplantation was not reached in the CPX-351 arm and was 10.25 months in the 7+3 arm (1-sided P=0.009).23 Early mortality occurred within 30 days for 5.9% and 10.6% of patients randomized to CPX-351 and 7+3, respectively, while death within 60 days was reported for 13.7% and 21.2% of patients, respectively.23 Among the 43 patients who harbored a FLT3 mutation, which is associated with a poor prognosis,27 remission (CR+CRi) was achieved by 68% of patients randomized to CPX-351 and 24% randomized to 7+3; median OS in these patients appeared to be longer in the CPX-351 arm (10.25 vs 4.60 months), but the difference was not statistically significant (hazard ratio=0.76 [95% CI: 0.34–1.66]).23
Figure 1.
Overall survival in the Phase III clinical study comparing CPX-351 and cytarabine:daunorubicin 7+3 in patients with newly diagnosed high-risk/sAML.
Notes: Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (Cytarabine: daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684–2692. Reprinted with permission. ©(2018) American Society of Clinical Oncology. All rights reserved.23
Abbreviations: CI, confidence interval; sAML, secondary acute myeloid leukemia.
Across studies, the safety profile of CPX-351 was generally consistent with the known safety profile of 7+3, except that delayed recovery of platelets and neutrophils was observed with CPX-351 treatment (Table 1).23,24,26 In both the CPX-351 and 7+3 treatment arms, the most commonly reported grade ≥3 adverse events included febrile neutropenia, infections, respiratory events, and fatigue.23,24,26 Of note, while the proportions of patients experiencing adverse events in the Phase III study were similar between cohorts, patients treated with CPX-351 were more likely to receive consolidation, resulting in a longer median treatment phase and, thus, a longer adverse event reporting period. To normalize to the length of the treatment phase, the median rate of adverse events per patient year was calculated: 75.68 with CPX-351 and 87.22 with 7+3.23
Table 1.
Overview of clinical safety for CPX-351 in AML
| Study | Phase (N) | Patient population | Common adverse events | Early mortality | Median time to neutrophil recovery |
|---|---|---|---|---|---|
| Study 101 NCT0038942822 |
I (48) |
Relapsed/refractory AML or ALL or high-risk MDS | DLTs: congestive heart failure, hypertensive crisis, persistent cytopenias beyond 56 days (in 1 patient each) | ||
| Study 204 NCT0078889224 |
II (126) |
Newly diagnosed AML in patients aged ≥60 and ≤75 years |
CPX-351 vs 7+3, respectively: Common (>15%) grade ≥3 adverse events: febrile neutropenia, 63.5% vs 51.2%; bacteremia, 35.3% vs 19.5%; pneumonia, 15.3% vs 19.5%; hypokalemia, 15.3% vs 12.2% |
CPX-351 vs 7+3, respectively: 30-day: all patients, 3.5% vs 7.3% (P=0.053); high-risk, 5.8% vs 12.0%; sAML, 3.0% vs 15.8% 60-day: all patients, 4.7% vs 14.6%; high-risk; 7.7% vs 24.0%; sAML, 6.1% vs 31.6% |
CPX-351 vs 7+3, respectively: To neutrophil recovery to ≥1000/µL: 36 vs 32 days; to platelet recovery to ≥100,000/µL: 37 vs 28 days; infection-related deaths: 3.5% vs 7.3% |
| Study 205 NCT0082209426 |
II (125) |
First relapse AML in patients aged ≥18 and ≤65 years |
CPX-351 vs 7+3, respectively: Common (>15%) grade ≥3 adverse events: febrile neutropenia, 54% vs 34%; bacteremia, 30% vs 43%; pneumonia, 22% vs 9% |
CPX-351 vs 7+3, respectively: 30-day: all patients, 7.4% vs 4.5%; poor-risk, 8.9% vs 6.9% 60-day: all patients, 14.8% vs 15.9%; poor-risk, 16.1% vs 24.1% 90-day: all patients, 18.5% vs 29.5%; poor-risk, 21.4% vs 37.9% |
CPX-351 vs 7+3, respectively: To neutrophil recovery to >1000/µL, 42 vs 34 days; to platelet recovery to >100,000/µL, 45 vs 35 days; infection-related deaths: 9.9% vs 9.1% |
| Study 301 NCT0169608423 |
III (309) |
Newly diagnosed, high-risk/sAML in patients aged 60–75 years |
CPX-351 vs 7+3, respectively: Common (>15%) grade ≥3 adverse events: febrile neutropenia, 68% vs 71%; pneumonia, 20% vs 15%; and hypoxia, 13% vs 15% |
CPX-351 vs 7+3, respectively: 30-day: all patients, 5.9% vs 10.6% 60-day: all patients, 13.7% vs 21.2% |
CPX-351 vs 7+3, respectively: Patients with CR + CRi, to neutrophil recovery to >500/µL, 35 vs 29 days (first induction), 35 vs 28 days (second induction); to platelet recovery to >50,000/µL, 36.5 vs 29 days (first induction), 35 vs 24 days (second induction) |
Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; DLT, dose-limiting toxicity; sAML, secondary AML; CR, complete remission; CRi, CR with incomplete platelet or neutrophil recovery.
As CPX-351 is administered as three 90-min infusions on alternate days, rather than the 7-day continuous infusion required for administration of 7+3, an exploratory post hoc analysis of the Phase III study evaluated outpatient administration of CPX-351.28 In the Phase III study, CPX-351 was administered in the outpatient setting for 51.0% (n=25/49) of patients who received consolidation cycle 1 and 60.9% (n=14/23) of patients who received consolidation cycle 2; in contrast, 6.3% (n=2/32) and 0% (n=0/12) of patients treated with conventional chemotherapy received consolidation cycles 1 and 2 in the outpatient setting, respectively. Administration of CPX-351 in the outpatient setting was associated with a reduction in the number of hospitalizations during the treatment phase of the study compared with 7+3. However, among responding patients, the total number of days in the hospital for induction plus consolidation was similar between treatment arms (a median of 42 days with CPX-351 and 43 with 7+3). Importantly, median OS was not diminished for patients receiving CPX-351 consolidation in the outpatient versus inpatient setting.28
Key formulation design features responsible for the improved efficacy of CPX-351
CPX-351 was developed using a drug design methodology (CombiPlex®), with the primary aim of achieving a controlled release of both cytarabine and daunorubicin from nanoscale liposomes, permitting the maintenance of a stable, synergistic molar ratio in vivo between the two drugs, following IV administration.29 Synergy between the two drugs was measured in vitro using the Chou–Talalay Combination Index method (where a Combination Index <0.9 is synergistic, a Combination Index 0.9–1.1 is additive, and a Combination Index >1.1 is antagonistic).30 When evaluated against the P388 leukemia cell line, it was established that cytarabine:daunorubicin molar ratios of 1:1, 5:1, and 10:1 were synergistic in terms of cytotoxic activity in vitro (death of 90% of all cells; CI values of 0.65, 0.72, and 0.75, respectively). Conversely, ratios of 1:5 and 1:10 were antagonistic (CI values of approximately 1.5 and 1.2).29 Subsequent studies found that the 5:1 molar ratio was the most synergistic in terms of growth inhibition in a panel of 15 tumor cell lines in vitro.19 Given the differential distribution and metabolism of these two drugs in vivo,31 maintaining a fixed, synergistic molar ratio for optimal use in AML patients was critical.32
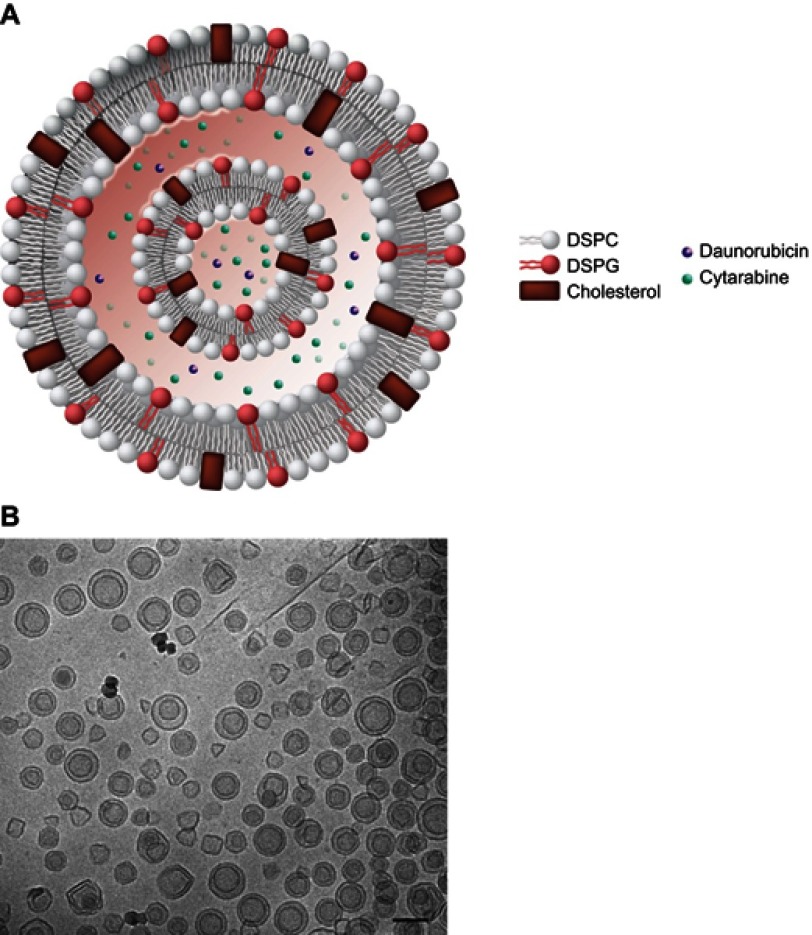
Many single-agent liposomal formulations employ polyethylene glycol (PEG)–modified lipids to increase the duration of circulation within the blood.6,7 However, the rate of drug clearance from these carriers may be increased due to immune system recognition of the PEG component.33 CPX-351 contains anionic phosphatidylglycerol, which is an alternative stabilizing phospholipid.19 The liposome bilayers of CPX-351 comprise a 7:2:1 molar ratio of DSPC, DSPG, and cholesterol (Figure 2). This composition conferred a high melting point to the liposome, keeping it in the gel phase at body temperature,21 such that it could maintain the synergistic drug ratio in vivo for >24 hrs post-injection.19,34 The incorporation of a low level of cholesterol into the membrane acts to decrease the loss of hydrophilic substances and stabilize the lipid bilayer.35 Optimization of the lipid ratio and cholesterol content to coordinate the release of two water soluble drugs, such as cytarabine and daunorubicin, was previously investigated.36 The diameter of the liposomes was designed to be approximately 100 nm and have a net negative surface zeta potential of approximately –30 mV.21
Figure 2.
Schematic representation of the CPX-351 liposome (A). CPX-351 contains bilayers of DSPC, DSPG, and cholesterol at a 7:2:1 molar ratio. This structure provides stability without polyethylene glycol, controlled release of cytarabine and daunorubicin, limited systemic drug distribution, and preferential internalization within malignant myeloblasts in the bone marrow. Cryogenic transmission electron microscopy images of CPX-351 (B). The formulation shows a regular spherical morphology that is primarily bilamellar. The scale bar represents 100 nm.
Notes: Image (A) Copyright©(2018). Future Medicine Ltd. Reproduced from Tolcher AW, Mayer LD. Improving combination cancer therapy: the CombiPlex development platform. Future Oncol. 2018;14(13):1317–1332.5
Abbreviations: DSPC, distearoylphosphatidylcholine; DSPG, distearoylphosphatidylglycerol.
Cytarabine and daunorubicin are encapsulated in the aqueous environment within the liposome at the predetermined molar ratio of 5:1. Cytarabine is passively encapsulated into the preformed liposomes in the presence of copper gluconate buffer, which is used during liposome extrusion. Daunorubicin is subsequently actively encapsulated with high efficiency through complexation with intra-liposomal copper.19,21 The copper gluconate buffer forms a complex with daunorubicin at a ratio of 1:1 to 1:2; and these interactions between drug and metal provide adequate retention of both cytarabine and daunorubicin within the liposome.21 As a consequence, the disparity between the pharmacokinetic profiles of cytarabine and daunorubicin is overcome because the liposome determines the distribution and half-life of both drugs within the body and the preferential delivery of both drugs to the malignant myeloblasts.
The inclusion of copper gluconate within the liposome raises concerns about potential toxicity. The tolerable upper intake level for copper is 10 mg/day for adults.37 A CPX-351 dose of cytarabine 100 mg/m2 plus daunorubicin 44 mg/m2 is equivalent to the administration of 36 mg of elemental copper with each dose administered for a patient with a 2.0 m2 body surface area;38,39 however, in a Phase I study of CPX-351, total serum copper levels in patients with normal copper metabolism who received three doses of CPX-351 were elevated on day 7 (2 days after the last dose) but returned to normal levels in most patients by day 14.39 All patients had serum copper levels in the normal range by day 42 after induction. No acute toxicities attributable to copper exposure were observed.
CPX-351 pharmacology
In a CCRF-CEM leukemia model in Rag2-M mice treated with CPX-351, approximately twice as much liposomal lipid was taken up by leukemia cells relative to healthy cells, and the concentrations of cytarabine and daunorubicin were several times higher.40 Additionally, leukemia cells were undetectable in femurs from these mice at day 14 following treatment with CPX-351; in contrast, residual leukemia cells rapidly repopulated the bone marrow following free drug cocktail administration. Thus, relative to free drugs, CPX-351 enabled rapid clearance of malignant myeloblasts from the bone marrow in this preclinical model, a phenomenon that may be associated with the improved response rate in AML patients.40,41
The preferential internalization of CPX-351 liposomes within malignant myeloblasts relative to healthy cells within the bone marrow is thought to occur via active uptake of liposomes into cytoplasmic vacuoles. Using spinning disc confocal microscopy, CPX-351 liposomes were visible first along the plasma membrane of malignant myeloblasts and then, over 24 hrs, the plasma membrane-associated liposomes decreased concurrently with an increase in the accumulation of daunorubicin within the nuclei.40 In cells taken from patients with AML versus healthy control subjects, CPX-351 demonstrated significantly greater killing of leukemia precursors while normal hematopoietic precursors were more resistant.42 Notably, this difference in cell killing between leukemia precursors versus normal hematopoietic precursors was most notable for leukemia precursor cells from patients who achieved a CR to CPX-351 treatment. Furthermore, the cytotoxicity of CPX-351 is especially enhanced in AML cells harboring the FLT3 mutation.43 This finding correlates with the favorable clinical activity of CPX-351 in this subgroup of patients.41
Several studies have assessed the antitumor efficacy of CPX-351 in mouse leukemia models. A notable finding from these studies was that matched separate liposomal doses of cytarabine (10 mg/kg) and daunorubicin (4 mg/kg) demonstrated significantly less antitumor activity than CPX-351 administered at 10:4 mg/kg on days 1, 3, and 5.19 Furthermore, tumor cell killing was approximately 1,000-fold greater with CPX-351 than the additive contribution of the individual drug-containing liposomes, confirming the synergistic rather than additive nature of the formulation. Using an induction regimen with CPX-351 in the CCRF-CEM tumor model, the median OS of mice with leukemic tumors was 78 days versus 35 days in mice receiving placebo (P<0.001).44 To mimic a consolidation regimen, CPX-351 was administered on days 1, 3, and 5 at 3 weeks after the initiation of induction therapy. The effect of this treatment was to extend the median OS to 134 days (P<0.001 vs induction regimen alone). In contrast, similar induction and consolidation regimens using the free drug cocktail of cytarabine and daunorubicin at their maximum tolerated doses increased median OS more modestly, from 58 to 76 days.44
Multidrug resistance is a concern when using combination drug therapy. There are multiple mechanisms of cellular resistance to chemotherapeutic agents, such as cytarabine and daunorubicin.45 Resistance may be conferred by removal of drugs from the cytoplasm into intracellular compartments, which serves to limit their access to the nucleus.46 Additionally, increased expression of transporters responsible for pumping active cytarabine metabolites out of leukemic cells is associated with treatment resistance and poor clinical outcomes.47 Multidrug resistance protein-1 (MDR1) is responsible for the export of various substances from cells, including anthracyclines such as daunorubicin. Its activity is increased in some tumor cell types, including AML. Notably, elevated expression of MDR1 is associated with poor response and reduced OS to 7+3 induction therapy for AML.48,49 Other transporters that mediate drug efflux from cells may also be modified in leukemia cells resistant to anthracyclines.45 Conversely, leukemic cells, including those harboring the FLT3 mutation, demonstrate decreased expression of ENT1, a carrier that is responsible for the cellular uptake of cytarabine and therefore confers resistance to free (unencapsulated) cytarabine.50
Although there is preferential uptake of CPX-351 liposomes by malignant myeloblasts, uptake by normal hematopoietic precursors does occur. In preclinical studies, CPX-351 administration induced marked reductions in healthy cells within the bone marrow.40 When compared with 7+3 in mice, CPX-351 demonstrated a lower nadir of all blood cells and their precursors. The consolidation regimen of CPX-351 administered on days 1, 3, and 5 was associated with prolonged cytopenias; however, modifying the consolidation regimen to be administered on days 1 and 3, days 1 and 5, or days 1 and 7 resulted in substantially lower reductions in bone marrow cell counts than the 1, 3, 5 regimen.44 Consistent with preclinical models, clinical data have indicated a prolonged period of thrombocytopenia and neutropenia following CPX-351 administration. This observed delayed recovery of platelets and neutrophils is thought to be due to the prolonged drug exposure in the bone marrow that is achieved with CPX-351. In the Phase III clinical study, the consolidation regimen of CPX-351 was further reduced to cytarabine 65 mg/m2 plus daunorubicin 29 mg/m2 (vs cytarabine 100 mg/m2 plus daunorubicin 44 mg/m2 in the Phase II studies).23
CPX-351 pharmacokinetics
The combined pharmacokinetic properties of the CPX-351 liposome and its drug cargo determine the accumulation and distribution of the encapsulated drugs. In mice, at 15 mins after an injection of free cytarabine and daunorubicin (in the saline vehicle), these two compounds are largely eliminated from the blood (96% and 99% of the injected dose, respectively). In contrast, 15 mins after a single IV injection of CPX-351, plasma concentrations of cytarabine and daunorubicin were approximately 90% of the injected dose.19 Elimination half-lives for cytarabine and daunorubicin were 11.6 and 8.5 hrs following injection of CPX-351 compared with 0.26 and 0.27 hrs following injection of the free drug cocktail in this mouse model. Additionally, during the first 24 hrs after injection of CPX-351, the molar ratio of cytarabine to daunorubicin remained between 5:1 and 9:1 (within a synergistic range), whereas within 15 mins following administration of free drugs, the ratio increased to 1,923:1.19
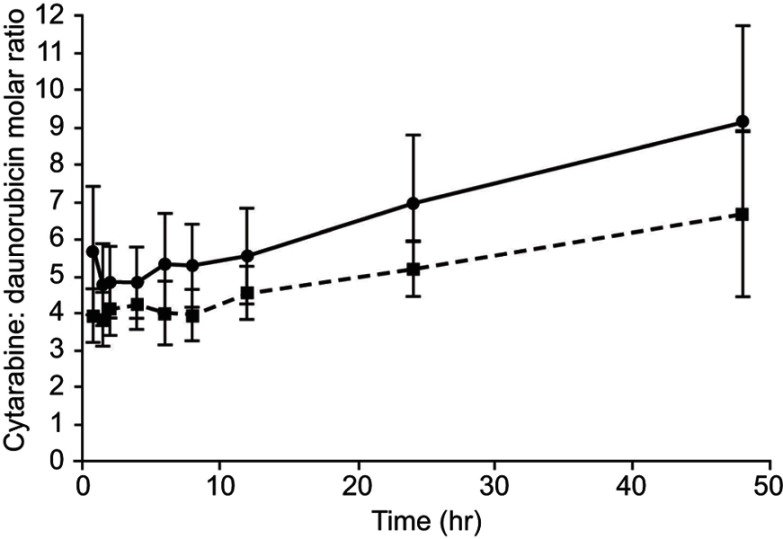
In patients with advanced AML, the molar ratio of cytarabine:daunorubicin after a 90-min infusion of CPX-351 at doses of cytarabine 24–134 mg/m2 plus daunorubicin 10.56–58.96 mg/m2 was maintained at approximately 5:1 from 45 mins to 48 hrs after day 1 and day 5.34 The molar ratio following the administration of CPX-351 at a dose of cytarabine 101 mg/m2 plus daunorubicin 44 mg/m2 is shown in Figure 3. The median time to achieve maximum plasma concentrations for both cytarabine and daunorubicin following infusion of CPX-351 at a dose of cytarabine 101 mg/m2 plus daunorubicin 44 mg/m2 was 2 hrs, and the elimination half-lives were 42.5 and 22.1 hrs, respectively (Table 2). As intended, exposure to cytarabine was substantially higher than to daunorubicin.34 It has been shown that plasma clearance of cytarabine and daunorubicin following administration of CPX-351 is minimally affected by renal impairment.51 Taken together, preclinical and clinical data indicate that the pharmacokinetic properties of CPX-351 prolong exposure to a synergistic drug ratio compared with administration of free drugs. It is possible that the persistence of drug in the circulation and within the bone marrow contributes to the improved clinical efficacy of CPX-351 compared with 7+3 by prolonging the period of time when slowly dividing malignant cells can be killed by chemotherapy.
Figure 3.
Molar ratio of cytarabine to daunorubicin following infusion of CPX-351 at a dose of cytarabine 101 mg/m2 plus daunorubicin 44 mg/m2 over 90 mins. Following infusion administered on day 1: dashed line; following infusion administered on Day 5: solid line (n=13). Error bars represent standard deviation.
Notes: Reprinted from Leuk Res, 36(10), Feldman EJ, Kolitz JE, Trang JM, et al, Pharmacokinetics of CPX-351; a nano-scale liposomal fixed molar ratio formulation of cytarabine: daunorubicin, in patients with advanced leukemia, 1283–1289, Copyright (2012), with permission from Elsevier.34
Table 2.
Pharmacokinetics of cytarabine and daunorubicin after day 1 90-min infusion of CPX-351 at a dose of cytarabine 101 mg/m2 plus daunorubicin 44 mg/m2 in 13 patients
| Cytarabine | Daunorubicin | |
|---|---|---|
| Mean Cmax, μg/mL | 42.6 (19) | 24.8 (18) |
| Median tmax, hr | 2 | 2 |
| Mean AUC∞, μg·mL/hr | 2280 (60) | 725 (36) |
| Mean VZ, L/m2 | 2.75 (31) | 2.00 (21) |
| Mean VSS, L/m2 | 2.74 (27) | 1.91 (21) |
| Mean CL, mL/hr/m2 | 62.5 (67) | 68.4 (36) |
Notes: Values are shown as mean (CV%) unless otherwise noted. Reprinted from Leuk Res, 36(10), Feldman EJ, Kolitz JE, Trang JM, et al, Pharmacokinetics of CPX-351; a nano-scale liposomal fixed molar ratio formulation of cytarabine: daunorubicin, in patients with advanced leukemia, 1283–1289, Copyright (2012), with permission from Elsevier.34
Abbreviations: Cmax, peak concentration; tmax, time to peak concentration; AUC, area under the curve; VZ, volume of distribution based on terminal phase; VSS, volume of distribution at steady state; CL, total body clearance; CV%, coefficient of variation.
Similar pharmacokinetic behavior was observed within the bone marrow. Following single IV injections of CPX-351 or saline-based free drug cocktail in mice, there were stark differences in bone marrow residency.19 Due to the differing pharmacokinetic profiles of free cytarabine and daunorubicin, bone marrow concentrations of cytarabine decreased by 92% between 15 mins and 2 hrs after administration, while those of daunorubicin were 147% times higher than plasma concentrations at 1 hr after administration. As a result, the cytarabine:daunorubicin molar ratio in the bone marrow fluctuated from 90:1 at 15 mins to 0.7:1 at 8 hrs after injection of the free drugs. In contrast, bone marrow levels of both drugs peaked shortly after injection of CPX-351 and remained relatively constant. At 16 hrs post-injection, the levels of cytarabine and daunorubicin had decreased by only 29% and 6% of the values observed at 15 mins. Importantly, the molar ratio of drugs was maintained near 5:1 for over 48 hrs.19 The ability to maintain a synergistic drug ratio at the tumor site for a prolonged period of time may contribute to the enhanced efficacy observed with CPX-351.
Quantitative whole-body autoradiography of rats that were administered CPX-351 has demonstrated that both daunorubicin and cytarabine remained localized to the bone marrow at 24 hrs following exposure. Although high concentrations of both drugs were also apparent in the spleen and, to a lesser extent, the pancreas, in general, there was less systemic distribution of daunorubicin and cytarabine to tissues compared with rats that received the individual free drugs, at both 1 hr and 24 hrs after exposure.52 Reduced drug exposure of off-target tissues may contribute to the manageable safety profile observed with CPX-351.
Conclusions
The development of CPX-351 demonstrates the benefits of rational combination therapy design. The composition of the CPX-351 liposome, which is unique from prior single-drug liposomal formulations, enables prolonged retention of cytarabine and daunorubicin within the liposome at the intended 5:1 molar ratio, thereby protecting both drugs from metabolism and elimination, overcoming the differences in pharmacokinetic profiles between the two compounds and reducing exposure of normal tissues to cytarabine and daunorubicin. The long plasma half-lives of cytarabine and daunorubicin following CPX-351 administration indicate prolonged circulation of liposomes within the vascular space, which likely maintains and prolongs drug exposure within the bone marrow environment. Leukemic cells exhibit preferential uptake of CPX-351 liposomes when compared with healthy cells. Preferential uptake of CPX-351 liposomes by leukemia cells suggests that relatively large amounts of cytarabine and daunorubicin enter malignant cells via liposomes, potentially bypassing P-glycoprotein-based efflux pumps, which are important mediators of resistance to chemotherapy. This may contribute to the rapid clearance of leukemia cells from the circulation and bone marrow, enabling the higher rate of CR observed with CPX-351 following the initial course of induction treatment. The pharmacologic advantages described above, which are due to the unique properties of the CPX-351 liposome, may help explain the observed efficacy of CPX-351 in patients with newly diagnosed sAML.
This rational method for developing drug combinations may shorten the time required for clinical development of new combinations by reducing or eliminating the requirement that each component be developed to the point of approval before combination studies are initiated. Moreover, this methodology may help to maximize the efficacy of new combinations where favorable drug interactions exist. Combination therapy is the standard of care for numerous malignancies, and the rational design of combination therapy using nanoscale liposomes and particles has the potential to transform the process by which these therapies are developed.
Acknowledgments
Medical writing and editorial assistance were provided by Kimberly Brooks, PhD, CMPP™, of SciFluent Communications and were financially supported by Jazz Pharmaceuticals, Inc.
Disclosure
AC Louie, P Tardi, and LD Mayer are employees of Jazz Pharmaceuticals, Inc. LD Mayer reports personal fees from Jazz Pharmaceuticals, outside the submitted work; in addition, LD Mayer has patents issued for CombiPlex drug combinations, low cholesterol liposomes, clinical use of CPX-351, copper-mediated drug encapsulation, and synergistic ratio of cytarabine and daunorubicin. P Tardi reports personal fees from Celator and Jazz Pharmaceuticals during the conduct of the study; personal fees from Celator and Jazz Pharmaceuticals outside the submitted work; and several patents issued to Celator Pharmaceuticals (patent nos: 7238367, 8518437, 8022279, 7850990, and 10028912). AC Louie has a patent for CPX-351. The authors report no other conflicts of interest in this work.
References
- 1.Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev. 2012;25(3):450–470. doi: 10.1128/CMR.05041-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson LB, Saravolatz LD. The quad pill, a once-daily combination therapy for HIV infection. Clin Infect Dis. 2014;58(1):93–98. doi: 10.1093/cid/cit637 [DOI] [PubMed] [Google Scholar]
- 3.Mokhtari RB, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022–38043. doi: 10.18632/oncotarget.16723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–681. doi: 10.1124/pr.58.3.10 [DOI] [PubMed] [Google Scholar]
- 5.Tolcher AW, Mayer LD. Improving combination cancer therapy: the CombiPlex development platform. Future Oncol. 2018;14(13):1317–1332. doi: 10.2217/fon-2017-0607 [DOI] [PubMed] [Google Scholar]
- 6.Ashley JD, Quinlan CJ, Schroeder VA, et al. Dual carfilzomib and doxorubicin-loaded liposomal nanoparticles for synergistic efficacy in multiple myeloma. Mol Cancer Ther. 2016;15(7):1452–1459. doi: 10.1158/1535-7163.MCT-15-0867 [DOI] [PubMed] [Google Scholar]
- 7.Liu GX, Fang GQ, Xu W. Dual targeting biomimetic liposomes for paclitaxel/DNA combination cancer treatment. Int J Mol Sci. 2014;15(9):15287–15303. doi: 10.3390/ijms150915287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Cancer Society. What are the key statistics about acute myeloid leukemia?; 2017. Available from: https://www.cancer.org/cancer/acute-myeloid-leukemia/about/key-statistics.html. Accessed December7, 2018.
- 9.Nagel G, Weber D, Fromm E, et al. Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO). Ann Hematol. 2017;96(12):1993–2003. doi: 10.1007/s00277-017-3150-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulte D, Jansen L, Castro FA, et al. Survival in patients with acute myeloblastic leukemia in Germany and the United States: major differences in survival in young adults. Int J Cancer. 2016;139(6):1289–1296. doi: 10.1002/ijc.30186 [DOI] [PubMed] [Google Scholar]
- 11.Wheatley K, Brookes CL, Howman AJ, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009;145(5):598–605. doi: 10.1111/j.1365-2141.2009.07663.x [DOI] [PubMed] [Google Scholar]
- 12.Mohammadi M, Cao Y, Glimelius I, Bottai M, Eloranta S, Smedby KE. The impact of comorbid disease history on all-cause and cancer-specific mortality in myeloid leukemia and myeloma - a Swedish population-based study. BMC Cancer. 2015;15:850. doi: 10.1186/s12885-015-1584-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granfeldt Østgård LS, Medeiros BC, Sengeløv H. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: A national population-based cohort study. J Clin Oncol. 2015;33(31):3641–3649. doi: 10.1200/JCO.2014.60.0890 [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Acute Myeloid Leukemia. Version 3.2018. 2018; NCCN.org.
- 15.Fey MF, Buske C, ESMO Guidelines Working Group. Acute myeloblastic leukaemias in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi138–vi143. doi: 10.1093/annonc/mdt320 [DOI] [PubMed] [Google Scholar]
- 16.Rai RK, Holland JF, Glidewell OJ, et al. Treatment of acute myelocytic leukemia: a study by cancer and leukemia group B. Blood. 1981;58(6):1203–1212. [PubMed] [Google Scholar]
- 17.Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184 [DOI] [PubMed] [Google Scholar]
- 18.Yates JW, Wallace HJ Jr, Ellison RR, Holland JF. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep. 1973;57(4):485–488. [PubMed] [Google Scholar]
- 19.Tardi P, Johnstone S, Harasym N, et al. In vivo maintenance of synergistic cytarabine: daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33(1):129–139. doi: 10.1016/j.leukres.2008.06.028 [DOI] [PubMed] [Google Scholar]
- 20.Vaughn JE, Othus M, Powell MA, et al. Comparative analysis of resource utilization and safety of outpatient management following intensive induction or salvage chemotherapy. JAMA Oncol. 2015;1(8):1120–1127. doi: 10.1001/jamaoncol.2015.2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dicko A, Kwak S, Frazier AA, Mayer LD, Liboiron BD. Biophysical characterization of a liposomal formulation of cytarabine and daunorubicin. Int J Pharm. 2010;391(1–2):248–259. doi: 10.1016/j.ijpharm.2010.02.014 [DOI] [PubMed] [Google Scholar]
- 22.Feldman EJ, Lancet JE, Kolitz JE, et al. First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol. 2011;29(8):979–985. doi: 10.1200/JCO.2010.30.5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (Cytarabine: daunorubicin)liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684–2692. doi: 10.1200/JCO.2017.77.6112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortes JE, Goldberg SL, Feldman EJ, et al. Phase II, multicenter, randomized trial of CPX-351 (cytarabine: daunorubicin) liposome injection versus intensive salvage therapy in adults with first relapse AML. Cancer. 2015;121(2):234–242. doi: 10.1002/cncr.28974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23(9):1969–1978. doi: 10.1200/JCO.2005.06.027 [DOI] [PubMed] [Google Scholar]
- 26.Lancet JE, Cortes JE, Hogge DE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123(21):3239–3246. doi: 10.1182/blood-2013-12-540971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar CC. Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes Cancer. 2011;2(2):95–107. doi: 10.1177/1947601911408076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolitz JE, Strickland SA, Cortes JE, et al. Efficacy by consolidation administration site: subgroup analysis of a phase 3 study of CPX-351 versus 7+3 in older adults with newly diagnosed, high-risk acute myeloid leukemia (AML). Presented at: Annual Meeting of the American Society of Clinical Oncology (ASCO); June1–5, 2017; Chicago, IL Abstract 7035. [Google Scholar]
- 29.Mayer LD, Harasym TO, Tardi PG, et al. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5(7):1854–1863. doi: 10.1158/1535-7163.MCT-06-0118 [DOI] [PubMed] [Google Scholar]
- 30.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. [DOI] [PubMed] [Google Scholar]
- 31.Mayer LD, Janoff AS. Optimizing combination chemotherapy by controlling drug ratios. Mol Interv. 2007;7(4):216–223. doi: 10.1124/mi.7.4.8 [DOI] [PubMed] [Google Scholar]
- 32.Harasym TO, Liboiron BD, Mayer LD. Drug ratio-dependent antagonism: a new category of multidrug resistance and strategies for its circumvention. Methods Mol Biol. 2010;596:291–323. doi: 10.1007/978-1-60761-416-6_13 [DOI] [PubMed] [Google Scholar]
- 33.Koide H, Asai T, Hatanaka K, et al. T cell-independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. Int J Pharm. 2010;392(1–2):218–223. doi: 10.1016/j.ijpharm.2010.03.022 [DOI] [PubMed] [Google Scholar]
- 34.Feldman EJ, Kolitz JE, Trang JM, et al. Pharmacokinetics of CPX-351; a nano-scale liposomal fixed molar ratio formulation of cytarabine: daunorubicin, in patients with advanced leukemia. Leuk Res. 2012;36(10):1283–1289. doi: 10.1016/j.leukres.2012.07.006 [DOI] [PubMed] [Google Scholar]
- 35.Eloy JO, Claro de Souza M, Petrilli R, et al. Liposomes as carriers of hydrophilic small molecule drugs: strategies to enhance encapsulation and delivery. Colloids Surf B Biointerfaces. 2014;123:345–363. doi: 10.1016/j.colsurfb.2014.09.029 [DOI] [PubMed] [Google Scholar]
- 36.Tardi PG, Gallagher RC, Johnstone S, et al. Coencapsulation of irinotecan and floxuridine into low cholesterol-containing liposomes that coordinate drug release in vivo. Biochim Biophys Acta. 2007;1768(3):678–687. doi: 10.1016/j.bbamem.2006.11.014 [DOI] [PubMed] [Google Scholar]
- 37.Agency for Toxic Substances and Disease Registry. Copper. 2017. Available from: https://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=37. Accessed November20, 2017.
- 38.Lancet JE, Uy GL, Cortes JE, et al. Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML. J Clin Oncol. 2016;34(18 Suppl):7000. doi: 10.1200/JCO.2016.34.15_suppl.7000 [DOI] [Google Scholar]
- 39.Data on file. Jazz Pharmaceuticals, Inc., Palo Alto, CA. [Google Scholar]
- 40.Lim WS, Tardi PG, Dos Santos N, et al. Leukemia-selective uptake and cytotoxicity of CPX-351, a synergistic fixed-ratio cytarabine: daunorubicin formulation, in bone marrow xenografts. Leuk Res. 2010;34(9):1214–1223. doi: 10.1016/j.leukres.2010.01.015 [DOI] [PubMed] [Google Scholar]
- 41.Medeiros BC, Hogge D, Newell LF, et al. Overall survival and transplantation in patients with FLT3 mutations: subgroup analysis of a Phase 3 study of CPX-351 versus 7+3 in older adults with newly diagnosed, high-risk acute myeloid leukemia. Presented at: 22nd Annual Congress of the European Hematology Association (EHA); June22–25, 2017; Madrid, Spain Abstract P210. [Google Scholar]
- 42.Kim HP, Gerhard B, Harasym TO, Mayer LD, Hogge DE. Liposomal encapsulation of a synergistic molar ratio of cytarabine and daunorubicin enhances selective toxicity for acute myeloid leukemia progenitors as compared to analogous normal hematopoietic cells. Exp Hematol. 2011;39(7):741–750. doi: 10.1016/j.exphem.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 43.Gordon MJ, Tardi P, Loriaux MM, et al. CPX-351 exhibits potent and direct ex vivo cytotoxicity against AML blasts with enhanced efficacy for cells harboring the FLT3-ITD mutation. Leuk Res. 2017;53:39–49. doi: 10.1016/j.leukres.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 44.Lim WS, Tardi PG, Xie X, et al. Schedule- and dose-dependency of CPX-351, a synergistic fixed ratio cytarabine: daunorubicin formulation, in consolidation treatment against human leukemia xenografts. Leuk Lymphoma. 2010;51(8):1536–1542. doi: 10.3109/10428194.2010.490312 [DOI] [PubMed] [Google Scholar]
- 45.Marin JJ, Briz O, Rodriguez-Macias G, Diez-Martin JL, Macias RI. Role of drug transport and metabolism in the chemoresistance of acute myeloid leukemia. Blood Rev. 2016;30(1):55–64. doi: 10.1016/j.blre.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 46.Chapuy B, Koch R, Radunski U, et al. Intracellular ABC transporter A3 confers multidrug resistance in leukemia cells by lysosomal drug sequestration. Leukemia. 2008;22(8):1576–1586. doi: 10.1038/leu.2008.103 [DOI] [PubMed] [Google Scholar]
- 47.Guo Y, Kock K, Ritter CA, et al. Expression of ABCC-type nucleotide exporters in blasts of adult acute myeloid leukemia: relation to long-term survival. Clin Cancer Res. 2009;15(5):1762–1769. doi: 10.1158/1078-0432.CCR-08-0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Den Heuvel-Eibrink MM, van der Holt B, Burnett AK, et al. CD34-related coexpression of MDR1 and BCRP indicates a clinically resistant phenotype in patients with acute myeloid leukemia (AML) of older age. Ann Hematol. 2007;86(5):329–337. doi: 10.1007/s00277-007-0269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chauhan PS, Bhushan B, Singh LC, et al. Expression of genes related to multiple drug resistance and apoptosis in acute leukemia: response to induction chemotherapy. Exp Mol Pathol. 2012;92(1):44–49. doi: 10.1016/j.yexmp.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 50.Jin G, Matsushita H, Asai S, et al. FLT3-ITD induces ara-C resistance in myeloid leukemic cells through the repression of the ENT1 expression. Biochem Biophys Res Commun. 2009;390(3):1001–1006. doi: 10.1016/j.bbrc.2009.10.094 [DOI] [PubMed] [Google Scholar]
- 51.Lin TL, Newell LF, Stuart RK, et al. CPX-351 (cytarabine: daunorubicin) liposome injection, (Vyxeos) does not prolong QTcF intervals, requires no dose adjustment for impaired renal function and induces high rates of complete remission in acute myeloid leukemia. Presented at: 57th Annual Meeting and Exposition of the American Society of Hematology (ASH); December5–8, 2015; Orlando, FL Abstract 2510. [Google Scholar]
- 52.Liboiron BD, Louie AC, Mayer LD. Nanoscale complexes. A novel nanotechnology-based platform to optimize combination cancer therapies: rational development & improved delivery using CombiPlex®. Drug Dev Delivery. 2016;16(1):34–39. [Google Scholar]