Abstract
Odontogenic keratocysts make up 4%–12% of all odontogenic cysts. Most cysts are sporadic but sometimes they arise in the context of basal cell nevus syndrome (Gorlin syndrome). Most odontogenic keratocysts arise in the posterior region of the mandible, but they can occur anywhere in the jaw. In rare instances, they are located peripherally in the gingiva. Even more rare, they are found in the soft tissues of the mouth. There have been a few case reports and small case series of such peripheral odontogenic keratocysts. Some controversy exists as to whether these truly represent a peripheral counterpart of the intraosseous odontogenic keratocysts and if their origin is at all odontogenic. We hereby present two cases of peripheral odontogenic keratocysts, both being located in the soft tissue of the buccal mucosa, and review the literature on peripheral odontogenic keratocysts.
Keywords: Dentistry, pathology, keratocyst, peripheral keratocyst
Introduction
Odontogenic keratocysts (OKCs) are benign odontogenic cystic neoplasms occurring intraosseously, usually in the posterior part of the mandible. The mean age of occurrence is 40 years, and in most series there is a male preponderance.1 There have been case reports of OKCs in other, non-osseous locations. Most of these involve the gingiva, but mucosal, epidermal and even intramuscular sites have also been described (Table 1).2–9
Table 1.
Summary of reported cases of peripheral soft tissue odontogenic keratocysts in the literature.
| Reference | Year | Age | Sex | Location | Follow-up |
|---|---|---|---|---|---|
| Precheur and Krolls2 | 2009 | 59 | M | Right buccal space | Not stated |
| Ide et al.3 | 2010 | 60 | M | Left buccal mucosa | No recurrence |
| 16 | M | Right buccal mucosa | Not stated | ||
| Gröbe et al.4 | 2012 | 52 | M | Right buccal mucosa | No recurrence after 4 months |
| Yamamoto et al.5 | 2013 | 74 | M | Right buccal mucosa | No recurrence after 4 years |
| Kaminagakura et al.6 | 2013 | 37 | M | Left buccal mucosa | No recurrence after 12 months |
| Abé et al.7 | 2014 | 46 | M | Left temporalis muscle | No recurrence after 12 years |
| Zhu et al.8 | 2014 | 44 | F | Left soft palate and pharynx | Not stated |
| 69 | M | Right buccal mucosa | Not stated | ||
| Makarla et al.9 | 2015 | 62 | M | Right buccal space | No recurrence after 24 months |
| This article | 2018 | 63 | M | Right buccal space | Possible recurrence after 1 year. No recurrence after 4 years |
| 48 | F | Left buccal space | No recurrence after 1 year |
It is assumed that OKCs and other odontogenic cysts and tumours originate from dental lamina rests. The dental lamina is a primitive embryonal epithelial invaginated ridge that forms the tissues of the teeth. Despite the benign appearance of OKCs, they can behave aggressively and grow infiltratively. If not removed completely, they tend to recur in up to 62.5% of cases. Because OKCs harbour chromosomal abnormalities, the World Health Organization (WHO) reclassified them from a benign cyst to a neoplastic lesion in 2005. This was accompanied by a change in nomenclature from OKC to keratocystic odontogenic tumour. This decision was reversed in 2017.10 According to the authors and participants of the Consensus and Editorial Panel of the WHO, the evidence was not sufficient at the time to reclassify OKCs from benign to neoplastic. Although they harbour chromosomal abnormalities, only approximately 30% of spontaneous occurring OKCs do.10 This group is the largest of all OKCs. A smaller group of OKCs occur in the setting of basal cell nevus syndrome, of which approximately 85% harbour chromosomal abnormalities. Because basal cell nevus syndrome is the consequence of a germline PTCH1 gene mutation, this is not surprising. This germline mutation causes not only OKCs but also basal cell carcinomas and skeletal abnormalities, among other manifestations. The syndromic keratocysts also rarely occur in various skin locations. Because they are histologically similar in appearance to steatocystomas and appear to originate from the sebaceous duct, a unifying terminology of sebaceous duct cyst was proposed by Makhija11 in 2015.
The origin of OKCs or OKC-like lesions in sites other than intraosseously in the mandible or maxilla is still controversial.3,6
The purpose of this small case series is to present two cases of peripheral keratocysts and give a brief overview of the existing literature regarding soft tissue keratocysts and their presumed origin.
Case presentation
Case 1
A 63-year-old male patient presented with a painful swelling on the inside of the right cheek, increasing in size over the last month. He denied a history of jaw cysts. Clinical examination revealed a firm mobile nodule on the right buccal mucosa, just anterior to the ramus mandibulae, measuring 2.5 cm in diameter. The overlying mucosa was intact. A panoramic radiograph showed no bony lesions. Magnetic resonance imaging of the head and neck revealed an oval cystic structure in the retromolar trigone with a maximum diameter of 1.8 cm. An excision biopsy was performed. The differential diagnosis included mucus retention or mucous extravasation cyst, salivary gland tumour and a mesenchymal lesion.12 Microscopy showed a cyst wall, with a parakeratinized stratified squamous epithelium of 5–6 cell layers in thickness, a corrugated surface and basal palisading, consistent with the diagnosis of OKC. There was a possible recurrence after 1 year upon which the lesion was excised. Histology was not characteristic of OKC due to extensive reactive changes and inflammation. Follow-up after 4 years was uneventful.
Case 2
A 48-year-old female patient presented with a swelling on the inside of the left cheek. The medical history was unremarkable. A panoramic radiograph showed no bony lesions. An incisional biopsy was performed under the same differential diagnosis as case 1. The biopsy showed a histomorphology consistent with an OKC. The lesion was excised with a minimal margin. There was no recurrence after 1 year (Figures 1 and 2).
Figure 1.
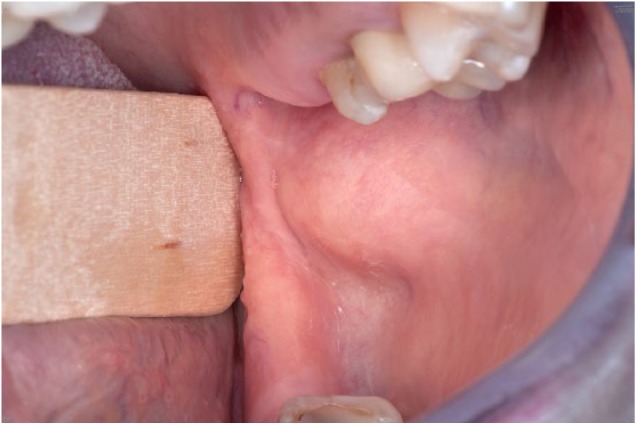
Case 2 – the swelling on the inside of the left cheek can be seen.
Figure 2.
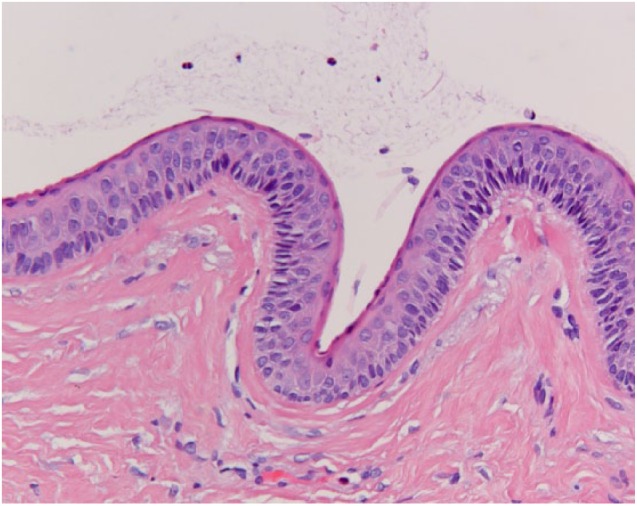
Histology of case 2 at 20× magnification. The squamous epithelium consists of 5–6 cell layers and there is basal palisading. A corrugated surface can be appreciated on the left side.
Review of the literature
A PubMed search of English literature was performed using the MeSH term ‘keratocyst’, combined with the word ‘peripheral’. The titles and abstracts were screened. The references of relevant articles were then checked for additional case reports that were not included in the primary search. This yielded 20 case reports and case series, in which a total of 29 cases of peripheral OKCs were presented. These consisted of 21 cases of gingival OKCs and 10 cases of soft tissue OKCs, 8 of which were located in the buccal mucosa. The cases located on the gingiva are outside the scope of this article and are not included in Table 1.
Discussion
To our knowledge, only 10 cases of OKC occurring in the soft tissue other than the gingiva have been described (Table 1). We are adding two.
There has been some controversy in the past as to whether peripheral OKCs truly represent a peripheral counterpart of the intraosseous OKC.3,6 This controversy is based on the fact that cutaneous OKCs are histomorphologically indistinguishable from (peripheral) OKCs, if only the surface epithelium is taken into consideration. Furthermore, sebaceous glands in the buccal mucosa are a variant of normal, so-called Fordyce spots. It would therefore not be surprising to encounter sebaceous duct neoplasms in the buccal mucosa. The epithelial lining of keratocysts, whether located intra- or extraosseously, shows a striking similarity to the epithelial lining of the sebaceous gland duct. This similarity led Makhija11 to propose a unifying terminology for cysts that appear to originate from the sebaceous duct epithelium: the cutaneous keratocyst and steatocystoma. He proposed the term ‘sebaceous duct cyst’. The immunohistochemical staining pattern for cytokeratins 10 and 17 is similar in OKCs and steatocystomas.13 Abé et al.7 confirmed this immunohistochemical staining pattern of both peripheral (intramuscular) and jawbone OKCs in 2013. The low prevalence of PTCH1 mutations in sporadic OKCs and the growing list of recently discovered mutations10 seem to make mutational analysis as evidence of their odontogenic origin untenable.
Another example of a presumably odontogenic cyst that is somewhat comparable to OKC is the orthokeratinized odontogenic cyst (OOC), although they show distinctive histological and clinical differences, mainly in their recurrence rate which is substantially lower than that in OKCs. There are no separate case reports regarding peripheral OOCs, probably because they were initially regarded as a variant of OKCs. However, an odontogenic origin is doubted altogether by some authors because their histologic features and immunohistochemical staining pattern are identical to those of epidermal inclusion cysts, which, surprisingly, also arise in the buccal mucosa.3,14
The recurrence rate of peripheral OKCs, excluding the gingival cases, is 12.5% (one out of eight cases in which follow-up was available). This is not in line with the higher recurrence rate of intraosseous OKCs (up to 62.5%). This could be due to better resectability in soft tissues or the fact that they are two separate entities with different biological behaviours.
The few cases of OKCs in the buccal mucosa that have been described were located either around the parotid papilla or in the posterior part of the buccal mucosa. Our two cases were also located around the parotid papilla. These findings make a sebaceous duct origin less likely, because Fordyce spots are not centred around the parotid papilla, but rather are randomly distributed. One would thus expect to find mucosal keratocysts more randomly distributed on the buccal mucosa. However, only eight cases have been described so far, so a coincidental clustering around the parotid papilla cannot be excluded.
Another argument against a sebaceous duct epithelium origin is that there are case reports of other tumours of odontogenic origin that arise in the buccal mucosa, for example, ameloblastomas.15 Interestingly, in these case reports, the odontogenic origin of the neoplasm is also put into question and a surface epithelial origin is discussed. There are very few and subtle histological features that distinguish peripheral ameloblastomas from (intraoral) basal cell carcinomas. For a definitive distinction, immunohistochemical staining for Ber-EP4 is recommended.16,17
Conclusion
We have added two cases to the list of peripheral, and specifically oral, soft tissue OKCs. A few authors have debated their origin, implying that an odontogenic origin might not hold true. At this moment, both hypotheses are plausible and there are insufficient data to confirm or reject these hypotheses.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: M Emma Witteveen  https://orcid.org/0000-0001-5619-8560
https://orcid.org/0000-0001-5619-8560
References
- 1. Zecha JAEM, Mendes RA, Lindeboom VB, et al. Recurrence rate of keratocystic odontogenic tumor after conservative surgical treatment without adjunctive therapies – a 35-year single institution experience. Oral Oncol 2010; 46(10): 740–742. [DOI] [PubMed] [Google Scholar]
- 2. Precheur HV, Krolls SO. An unusual presentation of an odontogenic keratocyst in the buccal space: case report. J Oral Maxillofac Surg 2009; 67(11): 2513–2515. [DOI] [PubMed] [Google Scholar]
- 3. Ide F, Kikuchi K, Miyazaki Y, et al. Keratocyst of the buccal mucosa: is it odontogenic? Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 110: 42–47. [DOI] [PubMed] [Google Scholar]
- 4. Gröbe A, Hanken H, Blessmann M, et al. An odontogenic keratocystic tumor in the buccal space: an unusual site of origin and a review of the literature. In Vivo 2012; 26(5): 847–851. [PubMed] [Google Scholar]
- 5. Yamamoto K, Matsusue Y, Kurihara M, et al. A keratocyst in the buccal mucosa with the features of keratocystic odontogenic tumor. Open Dent J 2013; 7: 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaminagakura E, Almeida JD, Carvalho YR, et al. Keratocyst of the buccal mucosa: case report and immunohistochemical comparative study with sporadic intraosseous keratocystic odontogenic tumor. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 116(5): e387–e392. [DOI] [PubMed] [Google Scholar]
- 7. Abé T, Maruyama S, Yamazaki M, et al. Intramuscular keratocyst as a soft tissue counterpart of keratocystic odontogenic tumor: differential diagnosis by immunohistochemistry. Hum Pathol 2014; 45(1): 110–118. [DOI] [PubMed] [Google Scholar]
- 8. Zhu L, Yang J, Zheng JW. Radiological and clinical features of peripheral keratocystic odontogenic tumor. Int J Clin Exp Med 2014; 7(1): 300–306. [PMC free article] [PubMed] [Google Scholar]
- 9. Makarla S, Bavle RM, Muniswamappa S, et al. A large extragnathic keratocystic odontogenic tumour. Case Rep Pathol 2015; 2015: 723010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wright JM, Vered M. Update from the 4th edition of the World Health Organization classification of head and neck tumours: odontogenic and maxillofacial bone tumors. Head Neck Pathol 2017; 11(1): 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Makhija M. Cutaneous keratocyst and steatocystoma unified as sebaceous duct cyst, a hamartoma resembling the sebaceous duct. Am J Dermatopathol 2015; 37(11): 871–873. [DOI] [PubMed] [Google Scholar]
- 12. Mortazavi H, Safi Y, Baharvand M, et al. Peripheral exophytic oral lesions: a clinical decision tree. Int J Dent 2017; 2017: 9193831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ide F, Mishima K, Saito I, et al. Rare peripheral odontogenic tumors: report of 5 cases and comprehensive review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 106(4): e22–e28. [DOI] [PubMed] [Google Scholar]
- 14. Tsuji K, Wato M, Hayashi T, et al. The expression of cytokeratin in keratocystic odontogenic tumor, orthokeratinized odontogenic cyst, dentigerous cyst, radicular cyst and dermoid cyst. Med Mol Morphol 2014; 47(3): 156–161. [DOI] [PubMed] [Google Scholar]
- 15. Goda H, Nakashiro K, Ogawa I, et al. Peripheral ameloblastoma with histologically low-grade malignant features of the buccal mucosa: a case report with immunohistochemical study and genetic analysis. Int J Clin Exp Pathol 2015; 8(2): 2085–2089. [PMC free article] [PubMed] [Google Scholar]
- 16. Upadhyaya JD, Bhattacharyya I, Fitzpatrick SG, et al. Peripheral ameloblastoma: a study of 18 cases and usage of Ber-EP4 immunohistochemistry to rule out a diagnosis of intraoral basal cell carcinoma. J Oral Maxillofac Surg 2018; 76(5): 996–1004. [DOI] [PubMed] [Google Scholar]
- 17. Woods TR, Cohen DM, Islam MN, et al. Intraoral basal cell carcinoma, a rare neoplasm: report of three new cases with literature review. Head Neck Pathol 2014; 8(3): 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]


