Abstract
A 41-year-old Caucasian woman with a history of infertility dating from 2011 was identified as wild-type (no mutations) for methylenetetrahydrofolate reductase single nucleotide polymorphisms (MTHFR-SNPs). Previous treatment included three failed in vitro fertilization/intracytoplasmic sperm injection cycles as well as one failed cycle of in vitro fertilization/intracytoplasmic sperm injection with donated oocytes. Counseling for a further oocyte donation cycle included advice to take high doses of folic acid (5 mG per day). Prior to initiation of this cycle, in October 2017 she attended our unit for general gynecological assessment and was found to have a slightly increased level of homocysteine, 12.2 µmol/L. A further test in February 2018 showed an increase to 17.2 µmol/L. Folic acid was stopped, and she was treated with 5-MTHF (500 µG daily), which supports the one-carbon cycle. After 5 days of treatment, her homocysteine level dropped to a baseline level of 8.2 µmol/L. As previously described in mice, high doses of folic acid can induce a “pseudo MTHFR” syndrome in wild-type patients, leading to an elevated unmetabolized folic acid syndrome which results in increased serum levels of homocysteine.
Keywords: Folic acid, pseudo-MTHFR, homocysteine, 5-MTHF (methyltetrahydrofolate), UMFA (unmetabolized folic acid)
Introduction
Folic acid (FA, Pteroyl glutamic acid) supplementation has for many years been considered a dogma, based on the fact that FA intake during the periconception period decreases the risk of neural tube defects (NTDs) in the babies conceived.1 FA is a synthetic compound that must undergo a 2-step transformation by dihydrofolate reductase (DHFR) before it can enter the FA cycle (Figure 1). The folate cycle is an obligatory component of all methylation processes that are ubiquitous and of major importance in cell physiology. The FA cycle is linked to the one-carbon cycle (1-CC), which recycles homocysteine (Hcy) to methionine (Met). Hcy is a toxic inhibitor of methylation,2 competes with Met for the same amino acid transporter, and is known to induce numerous pathologies.3 During reproduction, methylation processes are involved in oogenesis and spermatogenesis: methylation of DNA and histones regulates epigenesis and imprinting. Anomalies of methylation, especially those linked to polymorphism of enzymes involved in the 1-CC will also affect early embryo trophoblast growth and implantation.4 Methylenetetrahydrofolate reductase (MTHFR) is the most common single nucleotide polymorphism (SNP), affecting up to 50% of the population in some geographical areas.5 Women who carry MTHFR have up to 75% reduction in the capacity to form active folate (5-MTHF: 5-methyltetrahydrofolate). Liver DHFR activity is slow and weak, so that the capacity for synthetic FA to enter the FA cycle is reduced.6 High doses of FA (5 mG/day) are usually recommended prior to conception because the neural tube closes at around 28 days post fertilization. FA at these doses can decrease circulating Hcy to some extent, during advancing in pregnancy, but has no effect on Lipoprotein(a) in pregnant patients, irrespective of their genetic MTHFR SNP background.7 However, at this time, the placenta has also a regulatory role in Hcy metabolism, depending upon the paternal genetic background.8 Poor FA metabolism may lead to its accumulation in high concentrations, an unmetabolized FA (UMFA) syndrome (Figure 1).9,10 Non-metabolized FA competes with natural folate (5-MTHF) for binding and transport into the cells, leading to a pseudo-MTHFR deficiency11 with altered lipid metabolism: this can lead to fetal losses and other harmful effects. UMFA is strongly suspected to be involved in the flare-up of some tumors (colorectal and prostate).10 This case report describes a wild-type (WT) patient who developed a pseudo-MTHFR syndrome with continuous elevation of Hcy after taking high doses of FA prior to an oocyte donation cycle.
Figure 1.
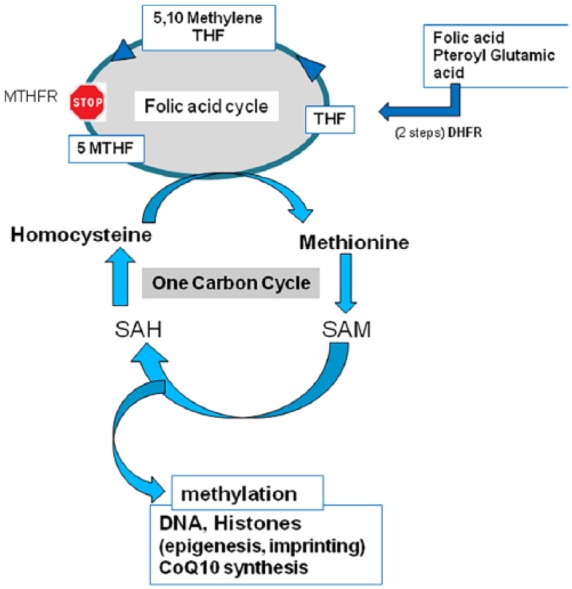
The one-carbon cycle (1-CC) and the folic acid (FA) cycle: The poor capacity to metabolize high doses of FA by the liver (6) induces an accumulation of homocysteine and unmetabolized FA and may induce a reversal of the 1-CC.
SAM: S-adenosyl methionine; SAH: S-adenosyl homocysteine; DHFR: dihydrofolate reductase; MTHFR, methylenetetrahydrofolate reductase; THF: tetrahydrofolate; MTHF: methyltetrahydrofolate.
Case report
A 41-year-old French Caucasian woman presented with infertility dating from 2011. Her husband (53 years old) had oligoasthenospermia (3.8 million sperm/mL, 1% motility). The couple had experienced 3 failed assisted reproductive technology (ART)/intracytoplasmic sperm injection (ICSI) cycles, with 10 metaphase II oocytes injected, 5 oocytes fertilized, and a total of 3 embryos transferred. A subsequent oocyte donation cycle (carried out in Spain due to restrictive laws in France) also failed to achieve a pregnancy. At the beginning of October 2017, the Spanish center prescribed a dose of 5 mG/day FA prior to starting a second oocyte donation cycle. She attended our center for full gynecological assessment, and as per our routine was tested for MTHFR SNPs C677T and A1298C, and for serum Hcy levels. She was found to be WT for both SNPs; her Hcy was 12.2 µmol/L on 26 October. This level is higher than the expected baseline (7.8 µmol/L) usually observed in WT patients. The oocyte donation cycle was delayed for unknown reasons, and she returned on 13 February 2018 for further assessment. Her Hcy level was 17.2 µmol/L, which is greater than the level we observe in C677T patients (14.2 µmol/L). Our policy is to treat patients with elevated Hcy levels with a supplement containing 5-MTHF, 500 µG daily, which supports the 1-CC. (Tetrafolic®, Nurilia, France, or Impryl®, Parthenogen CH). 5-MTHF is known to reduce circulating Hcy,12,13 without contributing to UMFA syndrome. It bypasses most of the mutations that may affect the FA cycle and is directly metabolized by Met synthase. Pre-conceptional support of the 1-CC has a positive effect in hypofertile patients.14–17 This patient stopped FA and started Tetrafolic® on 17 February. Monitoring on 21 February revealed that her Hcy level had fallen to 8.2 µmol/L, an appropriate baseline level for WT patients.
Discussion
General nutritional supplementation, especially in the United States and Canada, has led to the disturbing observation that UMFA can be found in a large number of patients. Of even greater concern is the fact that high levels of UMFA, at least five times greater than is generally considered safe, are found in the blood and the umbilical cord of pregnant patients9 who have been prescribed very high doses of FA.10 In men, high doses of FA induce sperm DNA methylation anomalies that are known to affect sperm fertility and methylation patterns/epigenesis in particular.18,19 This case report demonstrates that high doses of FA can reverse the FA cycle in WT patients, resulting in increased circulating Hcy, a situation that is observed in patients who carry MTHFR SNPs. This can be explained in two ways: (1) circulating UMFA competes with the “natural” folate 5-MTHF and may increase “folate deficiency” and the ability to recycle Hcy and (2) accumulated UMFA can lead to “excess-substrate inhibition,” a common deviation from Michaelis–Menten enzyme kinetics: this inhibits DHFR activity, which is already slow and variable.6 DHFR activity is required to allow synthetic FA to enter the folate cycle (see Figure 1).
A second similar case also attended our clinic: a female patient awaiting oocyte donation was found to have elevated circulating Hcy, up to 15.2 µmol/L. Interrupting FA treatment and prescribing 5-MTHF allowed the Hcy level to rapidly return to baseline, confirming that 5-MTHF is bio-available as a substrate for Met synthase. In our experience with MTHFR SNP carriers, return to a correct physiological level of around 10 µmol/L is observed after 10 days of treatment. The slow and low level of liver DHFR activity results in a weak ability to provide the correct input to the 1-CC: this should to be taken into account when FA is prescribed, especially in consideration of the fact that MTHF minimizes the risk of birth defects.20 Although nutritional supplementation with FA has reduced the incidence of NTDs by 20%–30% in the United States,21 the policy is now due for re-evaluation.
Conclusion
Although the prevalence of MTHFR SNPs is high, and these patients have a higher risk of Hcy elevation, the majority of pregnant patients are not tested for MTHFR SNPs. It is now clear that the practice of prescribing high doses of synthetic FA should be at least a matter for debate. 5-MTHF, the “active” folate that is immediately available for conversion of Hcy to Met, should be proposed instead of FA for periconceptional support and even for nutritional supplementation in general.
Acknowledgments
The authors want to thank Dr Kay Elder, MD, PhD for her help in the translation corrections.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article. In France, by law, the consent for the testing is mandatory. It has to be signed by the doctor, the laboratory making the tests, and the patients. Specific form has to be filled and signed. The consent for anonymous publication is oral, not written. During consultation, the doctor cannot propose a form for written consent for anonymous publication. As soon as the patient agrees for the test, the test can be used anonymously, for publication or for statistics.
ORCID iD: Yves Menezo  https://orcid.org/0000-0001-6861-8905
https://orcid.org/0000-0001-6861-8905
References
- 1. Hibbard BM, Hibbard E, Jeffcoate TN. Folic acid and reproduction. Acta Obstet Gynecol Scand 1965; 44: 375–400. [DOI] [PubMed] [Google Scholar]
- 2. Menezo Y, Khatchadourian C, Gharib A, et al. Regulation of S-adenosyl methionine synthesis in the mouse embryo. Life Sci 1989; 44(21): 1601–1609. [DOI] [PubMed] [Google Scholar]
- 3. Skovierova H, Vidomanová E, Mahmood S, et al. The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int J Mol Sci 2016; 17(10): E173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Enciso M, Sarasa J, Xanthopoulou L, et al. Polymorphisms in the MTHFR gene influence embryo viability and the incidence of aneuploidy. Hum Genet 2016; 135(5): 555–568. [DOI] [PubMed] [Google Scholar]
- 5. Zappacosta B, Graziano M, Persichilli S, et al. 5,10-Methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C polymorphisms: genotype frequency and association with homocysteine and folate levels in middle-southern Italian adults. Cell Biochem Funct 2014; 32(1): 1–4. [DOI] [PubMed] [Google Scholar]
- 6. Bailey SW, Ayling JE. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc Natl Acad Sci U S A 2009; 106(36): 15424–15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hekmati Azar Mehrabani Z, Ghorbanihaghjo A, Sayyah Melli M, et al. Effects of folic acid supplementation on serum homocysteine and lipoprotein (a) levels during pregnancy. Bioimpacts 2015; 5(4): 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mohanraj PS, Rahat B, Mahajan A, et al. Temporal expression of genes involved in folate metabolism and transport during placental development, preeclampsia and neural tube defects. Mol Biol Rep. Epub ahead of print 2 April 2019. DOI: 10.1007/s11033-019-04776-w. [DOI] [PubMed] [Google Scholar]
- 9. Plumptre L, Masih SP, Ly A, et al. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am J Clin Nutr 2015; 102(4): 848–857. [DOI] [PubMed] [Google Scholar]
- 10. Crider KS, Bailey LB, Berry RJ. Folic acid food fortification—its history, effect, concerns, and future directions. Nutrients 2011; 3(3): 370–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christensen KE, Mikael LG, Leung KY, et al. High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. Am J Clin Nutr 2015; 101(3): 646–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Venn BJ, Green TJ, Moser R, et al. Comparison of the effect of low-dose supplementation with L-5-methyltetrahydrofolate or folic acid on plasma homocysteine: a randomized placebo-controlled study. Am J Clin Nutr 2003; 77: 658–662. [DOI] [PubMed] [Google Scholar]
- 13. Lamers Y, Prinz-Langenohl R, Moser R, et al. Supplementation with [6S]-5-methyltetrahydrofolate or folic acid equally reduces plasma total homocysteine concentrations in healthy women. Am J Clin Nutr 2004; 79(3): 473–478. [DOI] [PubMed] [Google Scholar]
- 14. Servy EJ, Jacquesson-Fournols L, Cohen M, et al. MTHFR isoform carriers. 5-MTHF (5-methyl tetrahydrofolate) vs folic acid: a key to pregnancy outcome: a case series. J Assist Reprod Genet 2018; 35(8): 1431–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goyco Ortiz LE, Servy EJ, Menezo YJR. A successful treatment with 5 methyltetrahydrofolate of a 677 TT MTHFR woman suffering premature ovarian insufficiency post a NHL (non-Hodgkin’s lymphoma) and RPL (repeat pregnancy losses). J Assist Reprod Genet 2019; 36: 65–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dattilo M, D’Amato G, Caroppo E, et al. Improvement of gamete quality by stimulating and feeding the endogenous antioxidant system: mechanisms, clinical results, insights on gene-environment interactions and the role of diet. J Assist Reprod Genet 2016; 33: 1633–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cornet D, Amar E, Cohen M, et al. Clinical evidence for the importance of 1-carbon cycle support in subfertile couples. Austin J Reprod Med Infertil 2015; 2: 1011. [Google Scholar]
- 18. Aarabi M, SanGabriel MC, Chan D, et al. High-dose folic acid supplementation alters the human sperm methylome and is influenced by the MTHFR C677T polymorphism. Hum Mol Genet 2015; 24(22): 6301–6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aarabi M, Christensen KE, Chan D, et al. Testicular MTHFR deficiency may explain sperm DNA hypomethylation associated with high dose folic acid supplementation. Hum Mol Genet 2018; 27(7): 1123–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bailey SW, Ayling JE. The pharmacokinetic advantage of 5-methyltetrahydrofolate for minimization of the risk for birth defects. Sci Rep 2018; 8(1): 4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Honein MA, Paulozzi LJ, Mathews TJ, et al. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 2001; 285(23): 2981–2986. [DOI] [PubMed] [Google Scholar]


