Abstract
Objectives:
Fatigue is a frequent and often disabling phenomenon that occurs in patients with chronic inflammatory and immunological diseases, and the underlying biological mechanisms are largely unknown. Because fatigue is generated in the brain, we aimed to investigate cerebrospinal fluid and search for molecules that participate in the pathophysiology of fatigue processes.
Methods:
A label-free shotgun proteomics approach was applied to analyze the cerebrospinal fluid proteome of 20 patients with primary Sjögren’s syndrome. Fatigue was measured with the fatigue visual analog scale.
Results:
A total of 828 proteins were identified and the 15 top discriminatory proteins between patients with high and low fatigue were selected. Among these were apolipoprotein A4, hemopexin, pigment epithelium-derived factor, secretogranin-1, secretogranin-3, selenium-binding protein 1, and complement factor B.
Conclusion:
Most of the discriminatory proteins have important roles in regulation of innate immunity, cellular stress defense, and/or functions in the central nervous system. These proteins and their interacting protein networks may therefore have central roles in the generation and regulation of fatigue, and the findings contribute with evidence to the concept of fatigue as a biological phenomenon signaled through specific molecular pathways.
Keywords: Brain, cerebrospinal fluid, fatigue, proteomics, Sjögren’s syndrome, sickness behavior
Introduction
Fatigue can be defined as an overwhelming sense of tiredness, lack of energy, and a feeling of exhaustion.1 It is a frequent and often disabling phenomenon that occurs in patients with chronic immunological diseases, cancer, neurological diseases, and several other conditions in which inflammation and/or cellular stress occurs.2 Nevertheless, chronic fatigue has a substantial impact on the patient’s quality of life and is a major cause of sick leave and disability.
It is a common view that inflammation and disease activity directly influence the severity of fatigue, and several studies have reported associations between fatigue and inflammatory markers such as C-reactive protein, pro-inflammatory cytokines, and other variables.3,4 However, some authors question these observations and argue that studies using fatigue instruments that do not capture elements of disease activity (generic fatigue instruments) do not confirm these associations.5,6 Another seemingly contradictory observation is that instead of experiencing relief, fatigue worsens during chemotherapy or radiation treatment in cancer patients.7 In chronic fatigue syndrome (CFS)—a much-debated condition in which no specific underlying disease can be revealed—several inconsistent disturbances in genetic, immunological, and molecular markers have been described over the years, so far with no definite and uniform overarching theory and conclusion reached.8,9
The biological mechanisms that cause fatigue are largely unknown, the hypotheses are conflicting, and it is important to uncover the pathophysiology and identify signaling pathways that generate and regulate this important phenomenon.2,10,11
Primary Sjögren’s syndrome
Primary Sjögren’s syndrome (pSS) is an autoimmune disease clinically characterized by inflammation of the exocrine glands, leading to dry eyes and dry mouth, and with fatigue as a prevalent feature.12,13 As no effective treatment is available today, pSS is an optimal disease to investigate fatigue mechanisms as molecular pathways can be considered “pure and undisturbed” in most patients, in contrast to other relevant diseases in which cytostatics and immunomodulating drugs are widely used.
Present study
In this study, we examined the cerebrospinal fluid proteome of 20 pSS patients—10 with high and 10 with low fatigue—using shotgun proteomics with the aim to identify proteins potentially involved in the pathogenesis of fatigue. The protein content of the cerebrospinal fluid comprises approximately 80% plasma-derived and 20% brain-derived proteins and contains several high abundant proteins, which are important to remove prior to proteomic analysis to minimize abundant proteins masking the detection of less abundant proteins.14,15
Identification of proteins expressed in cerebrospinal fluid that differentiate patients with high and low fatigue was the feasibility objective of the present pilot study. Furthermore, the clinical relevance of these proteins in relation to sickness behavior/fatigue was a criterion for the success of the study. Usually, a difference in fatigue of 20 or more in fatigue visual analog scale (fVAS) scores is considered clinically meaningful. In this study, the high fatigue group had a median fVAS score five times higher than the median of the low fatigue group, well above this limit (Table 1).
Table 1.
Selected clinical data for 20 patients with primary Sjögren’s syndrome.
| Variables | High fatigue (n = 10) | Low fatigue (n = 10) | p |
|---|---|---|---|
| Age, yearsa | 61 (42–78) | 56 (36–68) | 0.16 |
| Gender (male/female) | 1/9 | 2/8 | 0.54 |
| Disease duration, yearsa | 10 (1–15) | 4 (2–11) | 0.09 |
| Hgb, g/dLa | 13 (12–14) | 13 (12–15) | 0.51 |
| ESR, mm/ha | 17 (6–31) | 7 (2–28) | 0.04 |
| CRP (mg/L)a | 3 (0–9) | 0 (0–4) | 0.05 |
| Anti-SSA/La abs | |||
| (Pos/neg) | 8/2 | 6/4 | 0.34 |
| Anti-SSB/Ro abs | |||
| (Pos/neg) | 4/6 | 4/6 | 1 |
| BDI scorea | 12 (8–27) | 7 (0–18) | 0.01 |
| Fatigue VAS scorea | 79 (76–91) | 13 (3–29) | < 0.001 |
| Medication | |||
| Prednisolone (%) | 3 (30) | 0 | |
| Antimalarials (%) | 2 (20) | 3 (30) | |
| Prednisolone and antimalarial (%) | 0 | 1 | |
BDI: Beck depression inventory; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; Hgb: hemoglobin; SSA/La abs: Sjögren’s syndrome A antibodies; SSB/Ro abs: Sjögren’s syndrome B antibodies; VAS: visual analog scale.
Results presented as median and range (Mann–Whitney U test).
Methods
Patients and fatigue measures
Seventy-two Caucasian patients fulfilling the American-European Consensus Group (AECG) criteria for pSS16and followed at the Department of Internal Medicine at Stavanger University Hospital participated in a clinical study in which patients were investigated during a two-day stay in the hospital for research purposes only.12 Fatigue was measured with the fVAS, a widely used and accepted generic and uni-dimensional fatigue instrument.17 Fifty-five (76%) of the 72 pSS patients underwent lumbar puncture. To increase the probability of finding discriminatory proteins, the 10 patients with the lowest fVAS scores (between 3 and 29) and the 10 patients with the highest fVAS scores (between 76 and 91) were selected for study, regardless of other clinical characteristics. Selected demographic and clinical data are summarized in Table 1, and fVAS data for all 55 patients are present in Supplementary Table 1.
Sample collection
All lumbar punctures were performed at a fixed time (between 01:00 and 02:00 p.m.). A specialist in internal medicine using aseptic technique performed all punctures, and all patients tolerated the procedure well without serious adverse events. Results of routine cerebrospinal fluid (CSF) analyses such as cells and glucose were not recorded except albumin and IgG levels for assessment of blood-brain barrier function (Supplementary Table 2). Tests for autoimmune encephalitis or pathogens were not performed. CSF was collected in cooled glass tubes and immediately placed on ice. Samples were centrifuged at 2500 g for 10 min at 4°C and the supernatant distributed in 200 µL aliquots. Samples were stored at −80°C until analysis.
Sample preparation
All CSF samples were subjected to a high abundant protein depletion system consisting of the following components: an Agilent Human 14 Multiple Affinity Removal Column (MARS-human 14, Agilent Technologies, USA), which is designed to remove 14 high abundant proteins from human body fluids (albumin, IgG, antitrypsin, IgA, transferrin, haptoglobin, fibrinogen, alpha-2-macroglobulin, alpha-1-acid glycoprotein, IgM, apolipoprotein Al, apolipoprotein AII, complement C3, and transthyretin); a liquid chromatography instrument (Waters 2795, USA); a switch valve (IDEX ® Health & Science LLC, USA) connected downstream of the column; and a fraction collector (Kromatek CHF100SA, UK).
Two hundred microliters of CSF from each patient was loaded onto the MARS column. The non-retained proteins were eluted with a flow rate of 0.2 mL/min for 6 min in MARS Buffer A (Agilent Technologies, USA), resulting in a 1.2 mL fraction per sample injected.
The column was cleaned using MARS Buffer B (Agilent Technologies, Santa Clara, USA) and re-equilibrated with MARS Buffer A before the next sample was injected.
The 1.2 mL fractions were transferred to a 3 kDa spin filter (Merck KGaA, Germany) for up-concentration and buffer exchange, according to the manufacturer’s protocol. The retentate was dissolved in 200 µL of 20% ammonium carbonate and 80% acetonitrile (ACN).
Protein digestion
Two hundred nanograms of trypsin (Promega, USA) was added to each sample, incubated for 1 h at 37°C and dried in a vacuum centrifuge (Concentrator 5301, Eppendorf, Germany) for 1 h at 60°C. One hundred microliters of 0.1% formic acid (FA, mobile phase A) was then added to each sample.
LC-MS/MS analysis
Liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis was performed using an UltiMate3000 dual-pump nanoflow HPLC system (Dionex USA) connected to a linear ion trap-orbitrap mass spectrometer (LTQ-Orbitrap XL, Thermo Fischer Scientific, USA). Two columns were used in series: a Nanoviper monolithic PS-DVB precolumn (PepSwift 200 µm × 5 mm) and a monolithic PS-DVB PepSwift® analytical column (100 µm ID, 25 cm length; Nano Viper, Thermo Fischer Scientific, USA). The separation mobile phase A was 2.5% ACN in 0.1% FA, and separation mobile phase B was 80% ACN in 0.1% FA. The injection volume was 5 µL and the analytical flow was set at 300 nL/min. An optimized multistep linear gradient was used: 0–10 min, 100% A; 10–175 min, 100%–70% A; 175–185 min, 70%–0% A; 185–215 min, 0%–0% A; 215–220 min, 0%–100% A; and 220–250 min, 100%–100% A. The MS method was data dependent, using dynamic exclusion-based MS/MS analysis on peptides with two or more charges.
Samples were randomized during sample preparation and instrumental analysis in order to eliminate the potential of systematic error, such as drift in instrument response.
Data analysis
All patients’ raw data files were analyzed using Proteome Discoverer 2.0 (Thermo Fischer Scientific, USA) to search against the Homo sapiens database (UNIPROT, downloaded on 07 September 2016, 70367 sequences). Peptide and fragment ion mass tolerance was set at 10 ppm and 0.6 Da. Trypsin was set as the digestion enzyme, allowing for up to two missed cleavages. Oxidation of methionine was set as the dynamic modification. Peptide identification was performed by correlating the experimental MS/MS spectra with theoretical spectra predicted for each peptide in the protein sequence database. The best scoring peptide-spectrum match (PSM) was considered to be the peptide identification, and PSMs were filtered using Percolator18 at a false discovery rate (FDR) of 1% (strict) and 5% (relaxed). Only peptides identified with high confidence (X correlation 1.9 (z = 2), 2.3 (z = 3), and 2.6 (z ⩾ 4)) were considered in this study.19
Relative protein quantitation was performed using a label-free approach based on spectral counts. This method counts the total number of PSMs for a protein, including those redundantly identified.20 The PSMs were normalized by multiplying each variable by the ratio of total spectral counts for a sample and the average spectral counts for the corresponding group. The output value was then divided by the number of amino acids of the corresponding protein.
To examine potential differences in protein profiles derived from patients with low fatigue versus patients with high fatigue, a multivariate approach using two pattern recognition methods was performed on the normalized data set using SIRIUS 8.1 software (Pattern Recognition System AS, Norway). Compared to the classical univariate approaches, where each variable (i.e. protein) is considered as independent from each other, multivariate methods take into consideration the correlation structure and the potential interactions between the proteins and should thus be the preferred statistical approach for proteomic studies.21 Principal components analysis (PCA) is an unsupervised statistical procedure that reduces the dimensionality of a data set by finding a new set of variables that is smaller than the original set of variables but nonetheless retains most of the sample’s information.22 Partial least square–discriminant analysis (PLS-DA) is a supervised statistical classification analysis method that uses the information from the group membership of objects to find the maximal variation between the groups.23 Most proteomic studies (including this one) are characterized by a large number of variables characterizing each sample, accompanied by a small set of samples available, increasing the risk of identification of false positive protein candidates. However, as both PCA- and PLS-based methods can provide a dimensionality reduction by considering few latent variables or principal components rather than a large number of original variables, both of these approaches have become quite widespread in proteomics in recent years, to reduce the risk of identification of false positive protein candidates.21 Residual standard deviation versus Hotelling’s T-square resulted in three outliers for the PLS-DA and the PCA, respectively. To determine which proteins contributed the most to the separation of the two groups, a target projection–based selectivity ratio was used for discriminatory protein selection. A discriminatory variable plot was used to obtain probability boundaries.24,25 Selecting a mean correct classification rate of 80% for a non-parametric Mann–Whitney test resulted in a discriminatory selectivity ratio of 0.23.
QIAGEN’s Ingenuity Pathway Analysis (IPA, Ingenuity Systems, Inc., USA, September 2016) was used for functional interpretation of the differentially regulated proteins based on the Ingenuity Knowledge Base. The differentially expressed proteins with their PSM ratios were uploaded for pathway and network analysis to find relevant biological interactions between the molecules.
All analyses were performed in 2013 and 2014.
Ethics
This study was carried out in compliance with the Helsinki Declaration and approved by the Regional Committee for Medical and Health Research, West (2010/1455). All subjects gave informed written consent to participate in the study.
Results
The patient cohort consisted of 17 (85%) women and three (15%) men. The median (range) age of the cohort was 59 (36–78) years, disease duration 6 (0–24) years, Beck depression inventory (BDI) score 9 (0–27), and fVAS score 53 (3–91). Hemoglobin was 13 (12–15) g/dL, C-reactive protein (CRP) 1 (0–9) mg/L, and erythrocyte sedimentation rate (ESR) 12 (2–31) mm/h. Sixteen patients (80%) had a positive anti-nuclear antibody (ANA) test; 14 of these (70%) had anti-SSA (Sjögren’s syndrome A) antibodies, and eight (40%) anti-SSB (Sjögren’s syndrome B) antibodies. Seven (35%) out of these had both anti-SSA/Ro and anti-SSB/La antibodies. Three (15%) patients were on prednisolone, five (25%) on antimalarials (hydroxychloroquine), and one on combined prednisolone and antimalarials. Two patients had a well-regulated hypothyroidism and one a substituted vitamin-B12 deficiency. Selected demographic and laboratory variables for the individual groups with high and low fatigue are shown in Table 1.
There was no association between fatigue and any conventional disease-associated variable (e.g. hemoglobin, ESR, CRP, ANA test), age, or gender.
A total of 828 proteins were identified in one or more of the 20 CSF samples analyzed (Supplementary Table 3). No single protein had a statistically significant influence on fatigue using univariate analysis. However, because multiple variables often work together, changes may occur in the interactions between variables that cannot be detected by analyzing one variable at a time. A multivariate approach was therefore applied, and to reveal trends and outliers, a PCA was performed for the normalized data set. Based on the plot and outlier testing, three samples were removed from the analysis, one from the high fatigue group and two from the low fatigue group. A supervised partial least square discriminant model was then calculated using the 828 proteins as the explanatory variables and group classification as the response variable. The aim was to identify a protein pattern that was differentially expressed by the two groups and yielded optimal classification accuracy. Since PLS-DA can often lead to chance classifications, that is, models that by chance give a good classification of two groups in data sets where the number of samples are much smaller than the number of variables, double cross validation was used to validate the model and only statistically significant components were included. A two-dimensional partial least square discriminant scoring plot is reported in Figure 1, showing a separation between the two groups along component 1. To determine which proteins contribute the most to the separation of the two groups, a target projection–based selectivity ratio was used for discriminatory protein selection. Of the 828 proteins identified in total, only 15 proteins had a selectivity ratio of 0.23 or higher (Table 2) and these proteins were selected as the most important variables involved in the class discrimination observed. A new PCA, including only information from the 15 selected proteins, was then performed to confirm that the set of selected protein candidates could separate most of the patients into two groups based on their level of fatigue (Figure 2).
Figure 1.

Supervised partial least square–discriminant analysis (PLS-DA) based on peptide-spectrum matches (PSMs) shows a clear separation of primary Sjögren’s Syndrome (pSS) patients with high (square) and low (circle) fatigue. Three patients (one in the high fatigue group and two in the low fatigue group) were categorized as outliers and were therefore removed from the statistical analysis.
Table 2.
Fold change, peptide-spectrum match selectivity ratio, and biological function of the 15 proteins in cerebrospinal fluid highly contributing to separation of primary Sjogren’s Syndrome patients with high versus low fatigue.
| Accession | Gene names | Protein names | Fold change | Total peptide-spectrum match |
Biological function | ||
|---|---|---|---|---|---|---|---|
| High | Low | ||||||
| H7C5I0 | ITIH1 | Inter-alpha-trypsin inhibitor heavy chain H1 | 4.25 | ↑ | 17 | 4 | Protease inhibitor, mood |
| H0YJW9 | Uncharacterized protein | 1.77 | ↑ | 39 | 22 | ||
| H7C5H1 | CFB | Complement factor B | 1.7 | ↑ | 104 | 61 | Innate immunity |
| P02790 | HPX | Hemopexin | 1.54 | ↑ | 188 | 122 | Depression, cellular stress defense |
| P06727 | APOA4 | Apolipoprotein A-IV | 1.37 | ↑ | 183 | 134 | Depression, loss of appetite |
| P36955 | SERPINF1 | Pigment epithelium-derived factor | 1.2 | ↑ | 415 | 346 | Cellular stress defense, innate immunity, depression |
| E5RJZ5 | CLU | Clusterin | 1.13 | ↑ | 61 | 54 | Cellular stress defense |
| P10451 | OPN | Osteopontin | 0.98 | ↓ | 352 | 360 | Cell adhesion, wound healing |
| A0A0A0MT66 | CHGB | Secretogranin-1 | 0.91 | ↓ | 307 | 338 | Depression |
| C9JP35 | FAM3C | Protein FAM3C | 0.59 | ↓ | 17 | 29 | Expressed in neurons, unknown function |
| H3BQ34 | PKM | Pyruvate kinase | 0.58 | ↓ | 49 | 85 | Cell metabolism |
| C9J8J8 | CDH2 | Cadherin-2 | 0.5 | ↓ | 26 | 52 | Neuronal signaling |
| F8WCR4 | SELENBP1 | Selenium-binding protein 1 | 0.16 | ↓ | 3 | 19 | Cellular stress defense, loss of appetite |
| P40925 | MDH1 | Malate dehydrogenase, cytoplasmic | 0.14 | ↓ | 4 | 29 | Cellular metabolism |
| H0YKC2 | SCG3 | Secretogranin-3 | 0.14 | ↓ | 1 | 7 | Loss of appetite, depression |
Figure 2.

Unsupervised principal components analysis (PCA) based on normalized peptide-spectrum matches (PSMs) from the 15 top discriminatory proteins shows a separation along principal component 1 between most of patients in the two groups with high (square) and low fatigue (circle). Three patients were categorized as outliers (one in the high fatigue group and two in the low fatigue group) and were therefore removed from the PCA analysis.
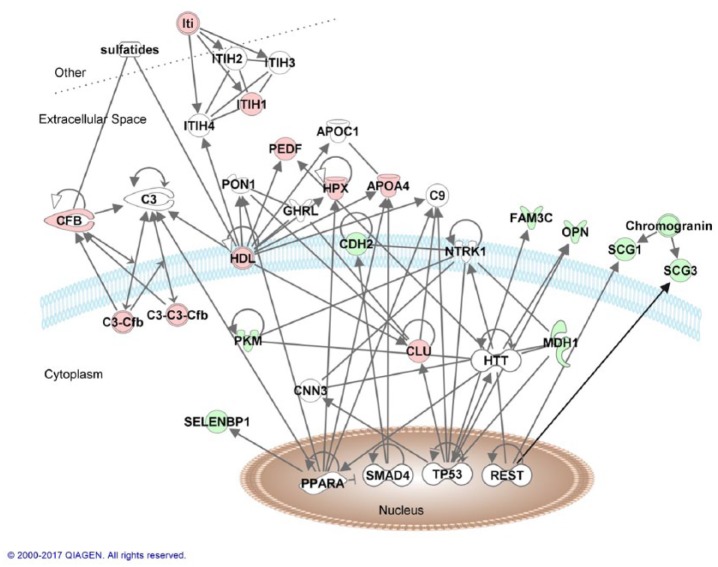
To understand the potential biological relevance of the observed protein pattern, the possible interactions between the 15 selected proteins were examined by use of ingenuity pathway analysis. All of the selected proteins were included in the analysis, except for the uncharacterized protein H0YJW9. The top network generated included all 14 proteins and proteins that are directly connected to several of the proteins in our analysis (Figure 3).
Figure 3.
Top network from ingenuity pathway analysis with 14 of the differentially expressed proteins (red = upregulated, green = downregulated) and proteins that are directly associated with them (white molecules). The different lines correspond as follows: only line = binding, filled closed arrow = acts on, closed arrow = translocation, open arrow = reaction, and butt line = inhibition.
Discussion
In this study, a CSF protein pattern associated with the level of fatigue in pSS patients was identified, with many novel protein candidates detected. Several of these proteins have important functions in the central nervous system and add evidence to the concept of fatigue as a cerebral phenomenon. Of special interest is that some of the proteins are associated with severe depression (hemopexin, apolipoprotein A4, pigment epithelium-derived factor, and secretogranin-3) and/or loss of appetite (apolipoprotein A4, selenium-binding protein 1, and secretogranin-3), which are cardinal findings of sickness behavior, in which fatigue constitutes a major element. In addition, three of the 15 proteins have previously been identified in the CSF of patients with CFS (hemopexin, pigment epithelium-derived factor, and secretogranin-3).
Proteins with important functions in the innate immune system were also revealed as discriminatory proteins. Several of these play roles in downregulation of inflammation and in cellular stress defense (hemopexin, pigment epithelium-derived factor, clusterin, osteopontin, and selenium-binding protein 1). These observations strengthen our hypothesis that some fatigue signaling pathways are associated with “cellular protection and defense” and are not directly related to pro-inflammatory factors.26 This is in line with several well-designed studies in various inflammatory diseases that were not able to verify an association between disease activity or the degree of inflammation and the severity of fatigue.5,6 The origin of the proteins are multiple with some obviously originating from activated neurons or different types of glial cells. Others involved in innate immunity and cellular stress responses may have been produced by brain-resident microglia, astroglia, or even been produced in the periphery and transported across the blood-brain barrier.
In general, our findings correspond to an increasing understanding of fatigue as part of the “sickness behavior response” observed during damage, cellular stress, infection, or chronic inflammation.2,12 Sickness behavior is characterized by fatigue, sleepiness, depression, social withdrawal and loss of appetite, thirst and grooming, and is hypothesized to be an automated and non-conscious survival enhancing strategy that is deeply conserved during evolution.27 In the individual, the protective molecular processes of cellular life are complemented by an element of survival behavior. In states of chronic inflammation and autoimmunity, these mechanisms are constantly active and fatigue is chronic.
The significance of our findings is strengthened by the associations with other highly relevant proteins in the pathway analysis (Figure 3), which illustrates the interplay between pro-inflammatory signals, downregulation of inflammation, and cellular protection against oxidative stress and other cellular stressors. In line with this, we recently showed that heat shock protein 90 in blood is strongly associated with increased fatigue in patients with pSS.26 Only a few previous studies have investigated the CSF proteome in states with chronic fatigue, such as post-treatment Lyme disease,28 multiple sclerosis,29 Persian Gulf War syndrome, fibromyalgia, and CFS.30
In detail, the following proteins collectively separated pSS patients with high and low fatigue:
Inter-alpha-trypsin inhibitor heavy chain H1 (ITIH1), also known as inter-alpha-trypsin inhibitor complex component III or serum-derived hyaluronan-associated protein, belongs to a family of protease inhibitors induced during the acute phase response. One genome-wide association study found an association between bipolar disorders and a variant of the ITIH1 gene.31
Complement factor B (CFB), also known as C3/C5 convertase, glycine-rich beta glycoprotein or properdin factor B, is a component in the alternative pathway of complement activation by innate immunity and acts as a downstream effector of Toll-like receptor signaling pathways. A number of other components in the complement cascade, such as Complement subcomponent 1, Complement C4A/B, and Complement subcomponent C1q were identified in the CSF of patients with CFS and Persian Gulf War Illness,28,30 indicating that the innate immune system has a role in fatigue generation.
Hemopexin (HPX), or beta-1B-glycoprotein, is an acute phase protein that binds heme with high affinity. HPX provides neuroprotection in mouse models of stroke and intracerebral hemorrhage and protects neurons in vitro against heme or reactive oxygen species toxicity via heme oxygenase-1 activity. Several studies have observed that HPX is associated with depression, with higher levels found in depressed than in non-depressed subjects.32 HPX was also identified as one of the 19 CFS-related proteins discovered by Baraniuk et al.30
Apolipoprotein A4 (ApoA-IV), in the brain, strongly suppresses food intake by inhibiting specific neurons in the hypothalamus.33 Loss of appetite and thirst is one of the fundamental characteristics of chronic fatigue, and thus the differential expression of ApoA-IV represents a highly relevant finding. A polymorphism of the APOA4 gene is associated with depression,34 and upregulation of APOA4 gene expression was significantly associated with post-stroke depression.35 In addition, in a recent plasma proteomic study of late-life depression, ApoA-IV protein was one of a panel of three proteins that could discriminate depressive from non-depressive elderly subjects.36 Depression and loss of desire to eat are hallmarks of fatigue, and these observations indicate that ApoA-IV may be of importance for generation of chronic fatigue.
Pigment epithelium-derived factor (PEDF), also known as cell-proliferation-inducing gene 35 protein, EPC-1, serpin F1, or serpin-1, is a multifunctional protein that inhibits lipopolysaccharide-driven macrophage activation and induces peroxisome proliferator-activated receptor gamma.37 Thus, this protein suppresses inflammation and contributes to the regulation of wound healing.38 It protects neurons in vitro against oxidant injury and has been postulated to represent a biomarker for major depression.39,40 Of special interest is a CSF proteomic study in CFS, in which PEDF was one of the five proteins that separated subjects with CFS or Persian Gulf War syndrome from healthy control subjects.30
Clusterin (CLU), also known as apolipoprotein J, aging-associated gene 4 protein, complement cytolysis inhibitor, complement-associated protein SP-40, KU70-binding protein 1, NA1/NA2 or testosterone-repressed prostate message 2, is a secreted small heat shock protein that can, under stress conditions, be found in the cell cytosol. CLU is involved in many diseases related to oxidative stress, including neurodegenerative diseases, cancers, inflammatory diseases, and aging. Plasma levels of CLU correlate with the severity of Alzheimer’s disease.41
Secretogranin-1 (SCG1), or chromogranin B, is a calcium-binding neuroendocrine secretory granule protein that may be a precursor for other biologically active peptides. Its functions are largely unknown, but one recent proteomic study of CSF found an increase of SCG1 in the early phase of multiple sclerosis.42 We observed a low fold change in our study, and interestingly an animal study reported that mice lacking SCG1 exhibited increased depressive-like behavior.43 SCG1 was also identified as one of the 19 CFS-related proteins discovered by Baraniuk et al.30
Osteopontin (OPN), also known as bone sialoprotein 1, nephropontin, secreted phosphoprotein 1, urinary stone protein, or uropontin, is an organic component of bone but is also synthesized in a variety of tissues and cells. It is produced after stimulation with tumor necrosis factor alpha and interleukin 1 beta, and it influences autoimmune demyelinating disease.44 OPN also functions as an adhesion protein and is involved in cell attachment and wound healing.
Protein FAM3C (FAM3C), or interleukin-like EMT inducer, shows widespread expression in neurons, especially in the presynaptic terminals, but its function is not completely understood.45
Pyruvate kinase (PKM), also known as cytosolic thyroid hormone-binding protein, Opa-interacting protein 3, pyruvate kinase 2/3, thyroid-hormone binding protein or p58, catalyzes the final step of glycolysis. This enzyme is inhibited by reactive oxygen species.46
Cadherin-2 (CHD2), also known as neural cadherin or CdW325, is a transmembrane protein primarily found in neurons. It is of importance for signaling across neuronal synapses, thus playing roles in learning and memory consolidation.47
Selenium-binding protein 1 (SELENBP1), also known as 56-kDa selenium binding protein, has an inverse relationship with the ubiquitously expressed glutathione peroxidase (GPX1) enzyme such that low levels of SELENBP1 are accompanied by high levels of GPX1. GPX1 detoxifies lipid and hydrogen peroxides during oxidative stress conditions and is a cellular protector.48 SELENBP1 is upregulated in the brain and blood of patients with schizophrenia.49 However, even more interestingly, in mice with cancer, the lack of desire to eat has been associated with changes in the hypothalamic proteome, in which SELENBP1 has been identified.50
Malate dehydrogenase (MDH1), also known as cytosolic malate dehydrogenase or diiodophenylpyruvate reductase, is a ubiquitous enzyme that catalyzes the NAD/NADH-dependent conversion of oxaloacetate and malate. This reaction is important for mitochondrial function and thus for cellular metabolism in general.
Secretogranin-3 (SCG3) belongs to a family of neuroendocrine secretory proteins that may serve as precursors of biologically active peptides. Studies have shown that SCG3 mRNA is expressed in areas of hypothalamus with important functions for appetite regulation, and the protein may interact with appetite-regulating neuropeptides such as orexin (hypocretin), indicating a role for the involvement of SCG3 in appetite regulation.51 In addition, increased gene expression of SCG3 has been described in patients with major depressive disorder.52
There are important weaknesses of this study: First, it needs to be replicated in another pSS cohort, as well as in a non-pSS cohort. The sample size is small and a healthy control cohort is lacking. It was not possible to stratify completely for all clinical variables in the two groups. These shortcomings are partly due to the difficulties of obtaining relevant biological material (CSF) in a high enough number of subjects. Many countries do not permit spinal tap for research purposes only, and few studies have been performed of the CSF proteome that can enlighten fatigue signaling mechanisms. Our study is therefore a pilot study in this context. It should be noted that the high abundance protein depletion step also most likely has removed proteins bound to these proteins, which could have been relevant for this study. However, removal of high abundance proteins is a necessary step to minimize abundant proteins masking the detection of less abundant proteins in the CSF.
It is necessary to validate the present findings in CSF of other diseases characterized by fatigue, as well as in blood, a much more easily accessible fluid than CSF. Whether some of the proteins may represent consistent biomarkers of fatigue for future research and routine diagnostics, therefore, remains to be seen.
In conclusion, the present findings give further support to the concept of fatigue as a sickness behavior phenomenon that has been strongly conserved during evolution, generated and regulated through a redundancy of molecular signaling pathways to the brain. The findings also support the hypothesis that fatigue signaling is in part associated with cellular stress defense mechanisms, wound healing, and both up- and downregulation of innate immunity. This can explain the frequent finding of lack of association between severity of fatigue and disease activity in several studies.
Supplemental Material
Supplemental material, Supplementary_table_1_fVAS_09.11-2018_(1) for Fatigue in primary Sjögren’s syndrome: A proteomic pilot study of cerebrospinal fluid by Eivind Larssen, Cato Brede, Anne Hjelle, Anne Bolette Tjensvoll, Katrine Brække Norheim, Kjetil Bårdsen, Kristin Jonsdottir, Peter Ruoff, Roald Omdal and Mari Mæland Nilsen in SAGE Open Medicine
Supplemental Material
Supplemental material, Supplementary_Table_3_09.12.2018 for Fatigue in primary Sjögren’s syndrome: A proteomic pilot study of cerebrospinal fluid by Eivind Larssen, Cato Brede, Anne Hjelle, Anne Bolette Tjensvoll, Katrine Brække Norheim, Kjetil Bårdsen, Kristin Jonsdottir, Peter Ruoff, Roald Omdal and Mari Mæland Nilsen in SAGE Open Medicine
Supplemental Material
Supplemental material, Suppl_Table_2_17.04.2019 for Fatigue in primary Sjögren’s syndrome: A proteomic pilot study of cerebrospinal fluid by Eivind Larssen, Cato Brede, Anne Hjelle, Anne Bolette Tjensvoll, Katrine Brække Norheim, Kjetil Bårdsen, Kristin Jonsdottir, Peter Ruoff, Roald Omdal and Mari Mæland Nilsen in SAGE Open Medicine
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from the Regional Committee for Medical and Health Research, REK Vest (2010/1455).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: All subjects gave informed written consent to participate in the study.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs: Cato Brede  https://orcid.org/0000-0003-2691-6052
https://orcid.org/0000-0003-2691-6052
Roald Omdal  https://orcid.org/0000-0002-6051-4658
https://orcid.org/0000-0002-6051-4658
References
- 1. Krupp LB, Pollina DA. Mechanisms and management of fatigue in progressive neurological disorders. Curr Opin Neurol 1996; 9(6): 456–460. [DOI] [PubMed] [Google Scholar]
- 2. Norheim KB, Jonsson G, Omdal R. Biological mechanisms of chronic fatigue. Rheumatology 2011; 50: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 3. de Raaf PJ, Sleijfer S, Lamers CH, et al. Inflammation and fatigue dimensions in advanced cancer patients and cancer survivors: an explorative study. Cancer 2012; 118(23): 6005–6011. [DOI] [PubMed] [Google Scholar]
- 4. Kappelman MD, Long MD, Martin C, et al. Evaluation of the patient-reported outcomes measurement information system in a large cohort of patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2014; 12(8): 1315–1323. e1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Hoogmoed D, Fransen J, Bleijenberg G, et al. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology 2010; 49(7): 1294–1302. [DOI] [PubMed] [Google Scholar]
- 6. Wang B, Gladman DD, Urowitz MB. Fatigue in lupus is not correlated with disease activity. J Rheumatol 1998; 25: 892–895. [PubMed] [Google Scholar]
- 7. Prue G, Rankin J, Allen J, et al. Cancer-related fatigue: a critical appraisal. Eur J Cancer 2006; 42(7): 846–863. [DOI] [PubMed] [Google Scholar]
- 8. Peterson D, Brenu EW, Gottschalk G, et al. Cytokines in the cerebrospinal fluids of patients with chronic fatigue syndrome/myalgic encephalomyelitis. Mediators Inflamm 2015; 2015: 929720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smylie AL, Broderick G, Fernandes H, et al. A comparison of sex-specific immune signatures in Gulf War illness and chronic fatigue syndrome. BMC Immunol 2013; 14: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bower JE. Fatigue, brain, behavior, and immunity: summary of the 2012 Named Series on fatigue. Brain Behav Immun 2012; 26(8): 1220–1223. [DOI] [PubMed] [Google Scholar]
- 11. Klimas NG, Broderick G, Fletcher MA. Biomarkers for chronic fatigue. Brain Behav Immun 2012; 26: 1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harboe E, Tjensvoll AB, Vefring HK, et al. Fatigue in primary Sjögren’s syndrome–a link to sickness behaviour in animals? Brain Behav Immun 2009; 23: 1104–1108. [DOI] [PubMed] [Google Scholar]
- 13. Norheim KB, Le Hellard S, Nordmark G, et al. A possible genetic association with chronic fatigue in primary Sjögren’s syndrome: a candidate gene study. Rheumatol Int 2014; 34: 191–197. [DOI] [PubMed] [Google Scholar]
- 14. Kroksveen AC, Opsahl JA, Guldbrandsen A, et al. Cerebrospinal fluid proteomics in multiple sclerosis. Biochim Biophys Acta 2015; 1854: 746–756. [DOI] [PubMed] [Google Scholar]
- 15. Kroksveen AC, Opsahl JA, Aye TT, et al. Proteomics of human cerebrospinal fluid: discovery and verification of biomarker candidates in neurodegenerative diseases using quantitative proteomics. J Proteomics 2011; 74(4): 371–388. [DOI] [PubMed] [Google Scholar]
- 16. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002; 61: 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hewlett S, Dures E, Almeida C. Measures of fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for severity, effect, and coping, Chalder Fatigue Questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), Fatigue Severity Scale (FSS), Functional Assessment Chronic Illness Therapy (Fatigue) (FACIT-F), Multi-Dimensional Assessment of Fatigue (MAF), Multi-Dimensional Fatigue Inventory (MFI), Pediatric Quality Of Life (PedsQL) Multi-Dimensional Fatigue Scale, Profile of Fatigue (ProF), Short Form 36 Vitality Subscale (SF-36 VT), and Visual Analog Scales (VAS). Arthritis Care Res 2011; 63(Suppl. 11): S263–S286. [DOI] [PubMed] [Google Scholar]
- 18. Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods 2007; 4(3): 207–214. [DOI] [PubMed] [Google Scholar]
- 19. Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom 1994; 5: 976–989. [DOI] [PubMed] [Google Scholar]
- 20. Borg J, Campos A, Diema C, et al. Spectral counting assessment of protein dynamic range in cerebrospinal fluid following depletion with plasma-designed immunoaffinity columns. Clin Proteomics 2011; 8(1): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robotti E, Manfredi M, Marengo E. Biomarkers discovery through multivariate statistical methods: a review of recently developed methods and applications in proteomics. J Proteom Bioinform 2014, https://www.omicsonline.org/open-access/biomarkers-discovery-through-multivariate-statistical-methods-jpb.S3-003.php?aid=33716
- 22. Joliffe IT, Morgan BJ. Principal component analysis and exploratory factor analysis. Stat Methods Med Res 1992; 1: 69–95. [DOI] [PubMed] [Google Scholar]
- 23. Wold S, Sjöström M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemomet Intell Lab Syst 2001; 58: 109–130. [Google Scholar]
- 24. Rajalahti T, Arneberg R, Berven FS, et al. Biomarker discovery in mass spectral profiles by means of selectivity ratio plot. Chemomet Intell Lab Syst 2009; 95: 35–48. [Google Scholar]
- 25. Rajalahti T, Arneberg R, Kroksveen AC, et al. Discriminating variable test and selectivity ratio plot: quantitative tools for interpretation and variable (biomarker) selection in complex spectral or chromatographic profiles. Anal Chem 2009; 81: 2581–2590. [DOI] [PubMed] [Google Scholar]
- 26. Bårdsen K, Nilsen MM, Kvaløy JT, et al. Heat shock proteins and chronic fatigue in primary Sjögren’s syndrome. Innate Immun 2016; 22: 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9(1): 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schutzer SE, Angel TE, Liu T, et al. Distinct cerebrospinal fluid proteomes differentiate post-treatment lyme disease from chronic fatigue syndrome. PLoS ONE 2011; 6(2): e17287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Opsahl JA, Vaudel M, Guldbrandsen A, et al. Label-free analysis of human cerebrospinal fluid addressing various normalization strategies and revealing protein groups affected by multiple sclerosis. Proteomics 2016; 16(7): 1154–1165. [DOI] [PubMed] [Google Scholar]
- 30. Baraniuk JN, Casado B, Maibach H, et al. A chronic fatigue syndrome—related proteome in human cerebrospinal fluid. BMC Neurol 2005; 5: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scott LJ, Muglia P, Kong XQ, et al. Genome-wide association and meta-analysis of bipolar disorder in individuals of European ancestry. Proc Natl Acad Sci U S A 2009; 106(18): 7501–7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frye MA, Nassan M, Jenkins GD, et al. Feasibility of investigating differential proteomic expression in depression: implications for biomarker development in mood disorders. Transl Psychiatry 2015; 5: e689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yan C, He Y, Xu Y, et al. Apolipoprotein A-IV inhibits AgRP/NPY neurons and activates pro-opiomelanocortin neurons in the arcuate nucleus. Neuroendocrinology 2016; 103(5): 476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ota VK, Chen ES, Ejchel TF, et al. APOA4 polymorphism as a risk factor for unfavorable lipid serum profile and depression: a cross-sectional study. J Investig Med 2011; 59(6): 966–970. [DOI] [PubMed] [Google Scholar]
- 35. Nguyen VA, Carey LM, Giummarra L, et al. A pathway proteomic profile of ischemic stroke survivors reveals innate immune dysfunction in association with mild symptoms of depression—a pilot study. Front Neurol 2016; 7: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Diniz BS, Lin CW, Sibille E, et al. Circulating biosignatures of late-life depression (LLD): towards a comprehensive, data-driven approach to understanding LLD pathophysiology. J Psychiatr Res 2016; 82: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zamiri P, Masli S, Streilein JW, et al. Pigment epithelial growth factor suppresses inflammation by modulating macrophage activation. Invest Ophthalmol Vis Sci 2006; 47(9): 3912–3918. [DOI] [PubMed] [Google Scholar]
- 38. Phang WM, Tan AA, Gopinath SC, et al. Secretion of N- and O-linked glycoproteins from 4T1 murine mammary carcinoma cells. Int J Med Sci 2016; 13(5): 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ditzen C, Tang N, Jastorff AM, et al. Cerebrospinal fluid biomarkers for major depression confirm relevance of associated pathophysiology. Neuropsychopharmacology 2012; 37(4): 1013–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanchez A, Tripathy D, Yin X, et al. Pigment epithelium-derived factor (PEDF) protects cortical neurons in vitro from oxidant injury by activation of extracellular signal-regulated kinase (ERK) 1/2 and induction of Bcl-2. Neurosci Res 2012; 72(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schrijvers EM, Koudstaal PJ, Hofman A, et al. Plasma clusterin and the risk of Alzheimer disease. JAMA 2011; 305(13): 1322–1326. [DOI] [PubMed] [Google Scholar]
- 42. Kroksveen AC, Jaffe JD, Aasebo E, et al. Quantitative proteomics suggests decrease in the secretogranin-1 cerebrospinal fluid levels during the disease course of multiple sclerosis. Proteomics 2015; 15(19): 3361–3369. [DOI] [PubMed] [Google Scholar]
- 43. Pereda D, Pardo MR, Morales Y, et al. Mice lacking chromogranins exhibit increased aggressive and depression-like behaviour. Behav Brain Res 2015; 278: 98–106. [DOI] [PubMed] [Google Scholar]
- 44. Chabas D, Baranzini SE, Mitchell D, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 2001; 294: 1731–1735. [DOI] [PubMed] [Google Scholar]
- 45. Liu L, Watanabe N, Akatsu H, et al. Neuronal expression of ILEI/FAM3C and its reduction in Alzheimer’s disease. Neuroscience 2016; 330: 236–246. [DOI] [PubMed] [Google Scholar]
- 46. Anastasiou D, Poulogiannis G, Asara JM, et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 2011; 334(6060): 1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schrick C, Fischer A, Srivastava DP, et al. N-cadherin regulates cytoskeletally associated IQGAP1/ERK signaling and memory formation. Neuron 2007; 55(5): 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ansong E, Yang W, Diamond AM. Molecular cross-talk between members of distinct families of selenium containing proteins. Mol Nutr Food Res 2014; 58(1): 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Udawela M, Money TT, Neo J, et al. SELENBP1 expression in the prefrontal cortex of subjects with schizophrenia. Transl Psychiatry 2015; 5: e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ihnatko R, Post C, Blomqvist A. Proteomic profiling of the hypothalamus in a mouse model of cancer-induced anorexia-cachexia. Br J Cancer 2013; 109(7): 1867–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tanabe A, Yanagiya T, Iida A, et al. Functional single-nucleotide polymorphisms in the secretogranin III (SCG3) gene that form secretory granules with appetite-related neuropeptides are associated with obesity. J Clin Endocrinol Metab 2007; 92(3): 1145–1154. [DOI] [PubMed] [Google Scholar]
- 52. Teyssier JR, Ragot S, Chauvet-Gelinier JC, et al. Activation of a DeltaFOSB dependent gene expression pattern in the dorsolateral prefrontal cortex of patients with major depressive disorder. J Affect Disord 2011; 133(1–2): 174–178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_table_1_fVAS_09.11-2018_(1) for Fatigue in primary Sjögren’s syndrome: A proteomic pilot study of cerebrospinal fluid by Eivind Larssen, Cato Brede, Anne Hjelle, Anne Bolette Tjensvoll, Katrine Brække Norheim, Kjetil Bårdsen, Kristin Jonsdottir, Peter Ruoff, Roald Omdal and Mari Mæland Nilsen in SAGE Open Medicine
Supplemental material, Supplementary_Table_3_09.12.2018 for Fatigue in primary Sjögren’s syndrome: A proteomic pilot study of cerebrospinal fluid by Eivind Larssen, Cato Brede, Anne Hjelle, Anne Bolette Tjensvoll, Katrine Brække Norheim, Kjetil Bårdsen, Kristin Jonsdottir, Peter Ruoff, Roald Omdal and Mari Mæland Nilsen in SAGE Open Medicine
Supplemental material, Suppl_Table_2_17.04.2019 for Fatigue in primary Sjögren’s syndrome: A proteomic pilot study of cerebrospinal fluid by Eivind Larssen, Cato Brede, Anne Hjelle, Anne Bolette Tjensvoll, Katrine Brække Norheim, Kjetil Bårdsen, Kristin Jonsdottir, Peter Ruoff, Roald Omdal and Mari Mæland Nilsen in SAGE Open Medicine