Short abstract
Endothelin-1 (ET-1) is a potent endogenous vasoconstrictor that has been widely known as a pain mediator involved in various pain states. Evidence indicates that ET-1 sensitizes transient receptor potential cation channel, subfamily A, member 1 (TRPA1) in vivo. But the molecular mechanisms still remain unknown. We aim to explore whether ET-1 sensitizes TRPA1 in primary sensory neurons and the molecular mechanisms. Ca2+ imaging, immunostaining, electrophysiology, animal behavioral assay combined with pharmacological experiments were performed. ET-1 sensitized TRPA1-mediated Ca2+ responses in human embryonic kidney (HEK)293 cells as well as in cultured native mouse dorsal root ganglion (DRG) neurons. ET-1 also sensitized TRPA1 channel currents. ET-1 sensitized TRPA1 activated by endogenous agonist H2O2. ETA receptor (ETAR) colocalized with TRPA1 in DRG neurons. ET-1-induced TRPA1 sensitization in vivo was mediated via ETAR and protein kinase A (PKA) pathway in HEK293 cells and DRG neurons. Pharmacological blocking of ETAR, PKA, and TRPA1 significantly attenuated ET-1-induced mechanical hyperalgesia in mice. Our results suggest that TRPA1 acts as a molecular target for ET-1, and sensitization of TRPA1 through ETAR–PKA pathway contributes to ET-1-induced mechanical hyperalgesia. Pharmacological targeting of TRPA1 and ETAR-PKA pathway may provide effective strategies to alleviate pain conditions associated with ET-1.
Keywords: TRPA1, endothelin, pain, protein kinase A, sensitization
Introduction
Endothelin-1 (ET-1) is a potent vasoconstrictor peptide that has been implicated in the pathogenesis of tissue inflammation and pain.1 Injection of ET-1 induced overt pain-like behavior and thermal and mechanical allodynia in animals.2–4 In humans, ET-1 injection produced severe pain and prolonged, touch-evoked allodynia.5 ET-1 exerts its effects mainly via acting on ETA and ETB receptors (ETAR and ETBR), both of which are G protein-coupled receptors.1 ETARs are abundantly expressed in primary sensory neurons, whereas ETBR are found exclusively in satellite glial cells.6,7 Extensive studies have been carried out to elucidate the mechanisms underlying ET-1-induced pain responses. ET-1 can induce hyperpolarizing shifts in voltage-dependent activation of TTX-R Na+ channels.8 ET-1 also potentiates TRPV1 channel via ETAR-mediated protein kinase C (PKC) signaling in both expression system and sensory neurons.6
Transient receptor potential cation channel, subfamily A, member 1 (TRPA1) is a nonselective cation ion channel mainly distributed in sensory neurons where it functions as a molecular detector to sense a variety of noxious stimuli. TRPA1 can be activated by a wide variety of endogenous and exogenous substances that elicit pain and irritation.9 Activation of TRPA1 depolarizes nociceptors and contributes to the perception of noxious stimuli.9 In addition, TRPA1 channel activity can be sensitized by inflammatory mediators, including bradykinin, trypsin, and nerve growth factor (NGF).10–12 TRPA1 can be sensitized via protein kinase A (PKA) and phospholipase C (PLC) pathways.10,13–15 ET-1 can potentiate cinnamaldehyde (a TRPA1 agonist)-induced nociception.16 ET-1-induced mechanical allodynia is inhibited by specific antagonists against TRPA1 and ETAR in vivo, suggesting a possible interaction between ETAR and TRPA1 in mediating pain responses.17 However, little is known about whether ET-1 sensitizes TRPA1 in primary sensory neurons and the detailed mechanisms.
Here, we investigated whether ET-1 sensitizes TRPA1 channel expressed in heterologous expression system and in cultured mouse primary sensory neurons. We further studied the molecular mechanisms underlying ET-1’s effect on TRPA1. Lastly, we examined the contribution of ETAR–PKA pathway and TRPA1 in ET-1-induced nocifensive response. Our results suggest that ET-1 sensitizes TRPA1 via ETAR and PKA signaling pathway both in vitro and in vivo, and this pathway contributes to ET-1-induced mechanical hyperalgesia.
Material and Methods
Animals
Male C57BL/6 mice (from Laboratory of Animal Research Center, Zhejiang Chinese Medical University, Hangzhou, China and Charles River Laboratories, Wilmington, MA, USA), six to eight weeks old, were used in this study. Trpa1–/– mice were a gift from David Julius (University of California, San Francisco, CA, USA). The mice were housed 5 per cage on a 12 h light/dark cycle with controlled temperature. Food and water were provided ad libitum. This study was carried out in accordance with the guidelines of National Institutes of Health guide for the care and use of laboratory animals and approved by the Animal Ethics Committee of Zhejiang Chinese Medical University.
Chemicals
Dimethyl sulfoxide (DMSO), HQ, ionomycin, capsaicin, and mustard oil (MO) were obtained from Sigma-Aldrich (St. Louis, MO, USA). HC-030031, ET-1, H89, BQ-123, BQ-788, edelfosine, forskolin, and bisindolylmaleimide (BIM) were purchased from Tocris (Minneapolis, MN, USA).
Cell culture
Human embryonic kidney (HEK)293 cells (ATCC, CRL-1573) were cultured in Dulbecco’s modified Eagle’s medium (Lonza, Belgium) supplemented with 10% fetal bovine serum (Lonza, Belgium), 2 mM L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were transfected by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instruction. Human TRPA1 (hTRPA1) is a gift from Professor David Julius (University of California, San Francisco), and ETAR is purchased from OriGene (Rockville, MD).
Adult mouse dorsal root ganglia (DRGs) were dissociated using 0.28 Wünsch units/ml Liberase Blendzyme 1 (Roche Diagnostics, Mannheim, Germany) as described previously.18 Neurons were cultured in Neurobasal-A medium (Invitrogen, Grand Island, NY) with B-27 supplement, 0.5 mM glutamine, and 50 ng/ml NGF (Calbiochem, La Jolla, CA) on an 8-well chambered coverglass coated with poly-D-lysine (Sigma, St. Louis, MO) and mouse laminin (Invitrogen, Carlsbad, CA, USA).
Immunofluorescence and confocal imaging
Mice were euthanized by CO2. Bilateral L3-5 DRGs were collected and immersed immediately in 4% paraformaldehyde overnight at 4°C. Then, DRGs were transferred to 15% and 30% sucrose for dehydration. Mouse DRGs were then frozen in frozen tissue matrix (OCT) and cut by cryostat in 8-μm sections. For immunostaining, the sections were first blocked with 1% BSA plus 10% donkey serum for 2 h at room temperature. The sections were then incubated overnight at 4°C with primary antibody against TRPA1 (1:200, Alomone Labs, Jerusalem, Israel) and ETAR (1:200, Abcam, Carlsbad, CA, USA). After washout, corresponding secondary antibodies (1:1000, Abcam, Carlsbad, CA, USA) were used for staining. Fluorescence signals were detected by Nikon A1R laser scanning confocal microscope (Nikon, Japan) and analyzed by ImageJ software. For quantification of immunofluorescent staining, two images were randomly selected per mouse tissue, and three mice were included in the present study.
Ca2+ imaging
For Ca2+ imaging of HEK293 cells, cells were used within 48 h after transfection. For DRG neurons, neurons were used 24 h after dissociation. Cells were loaded with Fura 2-AM (10 μM, Invitrogen) for 45 min in a loading buffer containing (mM) 140 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, and 10 HEPES (pH 7.4, adjusted with NaOH). Cells were subsequently washed three times and imaged in the loading buffer. Ratiometric Ca2+ imaging was performed on an Olympus IX51 microscope with a Polychrome V monochromator (Till Photonics, Hillsboro, Oregon, USA) and a PCO Cooke Sensicam QE CCD camera and Imaging Workbench 6 imaging software. Fura-2 emission images were obtained with exposures of 0.5 ms at 340 nm and 0.3 ms at 380 nm excitation wavelengths. Ratiometric images were generated using ImageJ software. A cell or neuron was considered responsive if the peak Ca2+ response is above 20% of the baseline.
Patch-clamp recordings
Recordings were carried out with borosilicate glass pipettes with initial series resistance of 2 to 4 MΩ after loading the pipette solution. Currents were filtered at 2.3 kHz and digitized at 100μs intervals using an EPC-10 amplifier and PatchMaster acquisition software (HEKA, Germany). Perforated whole-cell hTRPA1 currents in HEK293 cells were recorded by patch-clamp recordings with a pipette solution containing (in mM) 140 CsAsp, 2 MgCl2, 10 HEPES, and 10 Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) (pH 7.4, adjusted with CsOH), with ∼30 pM of amphotericin B added. The perfusion solution contained (in mM) 140 NaCl, 4 KCl, 2 EGTA, 2 MgCl2, 10 HEPES, and 8 glucose (pH 7.4, adjusted with NaOH).
Animal behavioral assay
Mechanical hyperalgesia was examined by von Frey hair test as described before.19,20 Briefly, mice were habituated for 30 min to the wire mesh surface before testing. Paw withdrawal thresholds (PWTs) were determined using a series of von Frey filaments (0.008–4.00 g) pressed against the plantar surface of the hind paw in ascending order beginning with the finest fiber following standard procedures. The minimum force (g) that caused the mouse to withdraw its hind paw away from the filament was considered as the withdrawal threshold. For each paw, a von Frey hair was applied 5 times at 10-s intervals. The threshold was determined when paw withdrawal was observed in more than three of five applications. ET-1 (20 ng/paw, dissolved in phosphate-buffered saline) was injected into the hind paw of mice using 1-ml syringe and 30-gauge needle in a volume of 20 μl. HC-030031 (10 µg/paw), H89 (5 µg/paw), and BQ-123 (1 µg/paw) was coinjected with ET-1, and the behavioral test was carried out thereafter at 0, 0.5, and 2.5 h after the injection. All behavioral tests were performed by an experimenter blinded to experimental conditions.
Statistics
Student’s t-test was used for comparison of data between two groups. One-way or two-way analysis of variance followed by Tukey post hoc test was used for comparison of ≥ 3 groups. Comparison is considered significantly different if the p value is less than 0.05. Data in bar graphs are expressed as means ± SE.
Results
ET-1 sensitizes hTRPA1 channel expressed in heterologous expression system
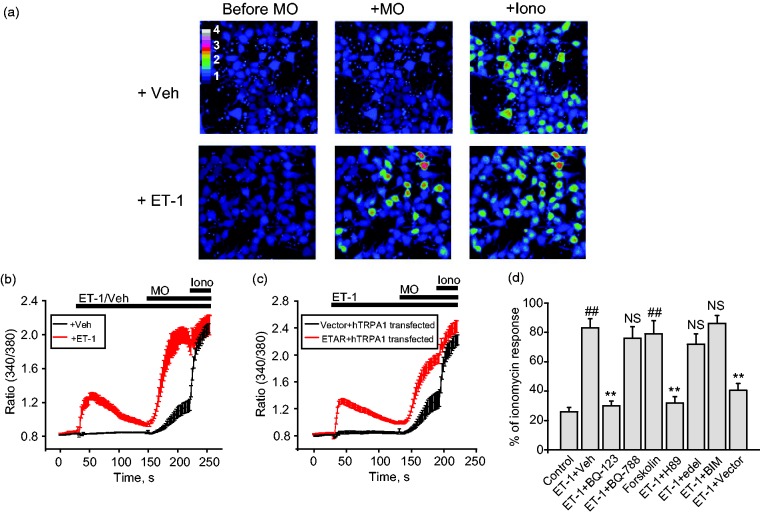
We transiently expressed hTRPA1 and ETAR together in HEK293 cells. To examine whether ET-1 sensitizes TRPA1, we measured its effects on Ca2+ responses to TRPA1 agonist MO in HEK293 cells. We tested the effects of 100 nM ET-1 in our in vitro experiments, which is a commonly used concentration in other studies and falls within the in vivo ET-1 concentration range.6,7,21 Pretreatment of HEK293 cells for 2 min with ET-1 (100 nM) significantly increased the magnitude of Ca2+ responses to MO (5 µM) compared to pretreatment with vehicle (0.1% DMSO), indicating sensitization effect of ET-1 (Figure 1(a) and (b)). ET-1 per se induced robust Ca2+ responses in HEK293 cells expressing both ETAR and TRPA1, which gradually returned to baseline level after 2 min (Figure 1(b)). Ionomycin (Iono) was applied at the end of Ca2+ imaging to identify all live cells (Figure 1(a) and (b)). We also tested HEK293 cells which are expressed with hTRPA1+empty vector pcDNA3.1 but with no ETAR. We found that ET-1 did not induce Ca2+ responses or potentiate MO’s response in cells expressing hTRPA1+pcDNA3.1 compared with cells expressing hTRPA1+ETAR (Figure 1(c) and (d)).
Figure 1.
ET-1 sensitizes TRPA1 channel exogenously expressed in HEK293 cells. (a) Pseudo color images from Fura-2 ratiometric imaging showing Ca2+ responses in HEK293 in response to TRPA1 agonist MO (5 µM) with or without pretreatment of ET-1 (100 nM). Ionomycin (1 µM) was applied at the end of the recording to determine all active cells. HEK293 cells were cotransfected with hTRPA1 and ETAR. (b) Averaged Ca2+ responses from experiments shown in panel (a). Red and black lines depict conditions with or without ET-1 pretreatment, respectively. n > 30 cells/group. (c) Averaged Ca2+ responses of HEK293 cells expressing hTRPA1 with ETAR or with empty vector (pcDNA3.1). n > 30 cells/group. (d) Pharmacological analysis of ET-1-induced TRPA1 sensitization in HEK293 cells. Cells were preincubated with ETAR antagonist BQ-123 (10 µM), ETBR antagonist BQ-788 (5 µM), PKA activator forskolin (15 µM), PKA inhibitor H89 (10 µM), PLC inhibitor edelfosine (10 µM), PKC inhibitor BIM (100 nM), or corresponding vehicle (0.1% DMSO) for 5 min, and then these reagents were coapplied with ET-1 (100 nM) during the imaging test. Ca2+ responses were normalized to ionomycin applied at the end of the tests for comparison (% response of ionomycin). n = 3–5 tests/group. Each test contains up to 30 cells. ##p < 0.01 versus control group. **p < 0.01 versus ET-1+Veh group (p > 0.05).
ET-1: endothelin-1; ETAR: ETA receptor; hTRPA1: human TRPA1; MO: mustard oil; NS: no significance; Iono: ionomycin; BIM: bisindolylmaleimide.
We proceeded to examine the mechanisms underlying ET-1-induced sensitization of TRPA1 in HEK293 cells. We used percent of ionomycin response, in which cell responses to MO were normalized to ionomycin, to compare Ca2+ responses among different groups. ETAR couples to PLC and PKA pathway, respectively.22,23 Since TRPA1 can be sensitized by PLC and PKA, we therefore examined the contributions of these two pathways to ET-1-induced sensitization of TRPA1 in HEK293 cells. ETAR-specific antagonist BQ-123 (10 µM), but not ETBR-specific antagonist BQ-788 (5 µM), at effective concentration, significantly reduced ET-1-induced sensitization of Ca2+ responses to MO in HEK293 cells.24–27 H89 (10 µM), a PKA antagonist, significantly reduced the sensitization, whereas BIM (100 nM), a PKC antagonist, had no effect (Figure 1(c)). In line with this observation, pretreating HEK293 cells with forskolin (15 µM), a PKA agonist, sensitizes the Ca2+ responses to MO in HEK293 cells, mimicking the effect of ET-1. On the contrary, the PLC-specific antagonist edelfosine, at effective concentration (10 µM), had no effect on ET-1-induced TRPA1 sensitization.28
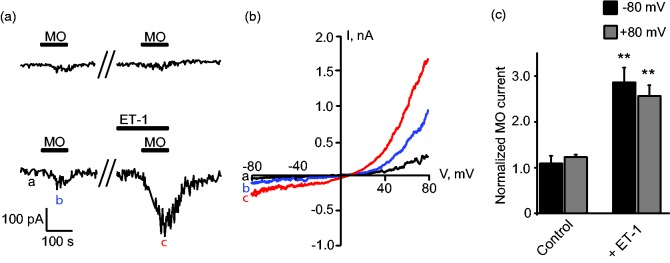
We further studied the effects of ET-1 on TRPA1/ETAR-expressing HEK293 cells via whole-cell patch-clamp recording. TRPA1 channel current was recorded under Ca2+-free extracellular solution to avoid channel inactivation as in our previous study.29 It was observed that 5 µM of MO induced small TRPA1 currents showing typical outward rectification property (Figure 2(a) and (b)). After 2 min pretreatment with 100 nM ET-1, reapplication of MO at the same dose (5 µM) elicited much larger current compared with control group (Figure 2(a) to (c)). The above results indicated that ET-1 sensitized TRPA1 via ETAR-mediated PKA signaling pathway in HEK293 cells.
Figure 2.
ET-1 sensitizes TRPA1 channel currents in HEK293 cells expressing TRPA1 and ETAR. (a) Representative inward current traces recorded at –80 mV in whole-cell configuration by patch clamp. MO (5 µM) and ET-1 (100 nM) were applied as indicated. (b) Current–voltage (I–V) curve recorded from HEK293 cell shown in the lower panel of (a). The letters a, b, c denote corresponding time point recorded in lower panel of (a). (c) Summarized data showing MO-activated TRPA1 currents were sensitized after ET-1 pretreatment. Currents were normalized to values first induced by MO application in the absence of ET-1. Inward and outward TRPA1 currents were recorded at –80 and +80 mV, respectively. n = 6 cells/group. **p < 0.01 versus control group.
ET-1: endothelin-1; MO: mustard oil.
ET-1 sensitizes TRPA1 channel in mouse primary sensory neurons
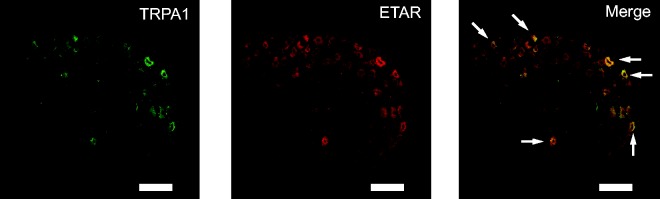
It is reported that ETARs are mainly expressed in DRG neurons.6,7 We therefore performed double immunostaining experiments using specific antibody against ETAR in conjunction with antibody against TRPA1. We found that a large population (over 80%) of TRPA1 positive neurons was labeled for ETAR (Figure 3). Out of 78 ETAR-positive neurons, 69 were stained positive for TRPA1 (6 sections obtained from 3 mice). This high percentage of coexpression suggests a possible functional interaction between TRPA1 and ETAR in mouse DRG neurons.
Figure 3.
TRPA1 showed coexpression with ETAR in mouse DRG neurons. Representative immunofluorescence images showing the expression of TRPA1 (in green, left panel), ETAR (in red, middle panel), and their coexpression (in yellow, right panel) in mouse DRG neurons. Scale bar indicates 100 µm.
TRPA1: transient receptor potential cation channel, subfamily A, member 1; ETAR: ETA receptor.
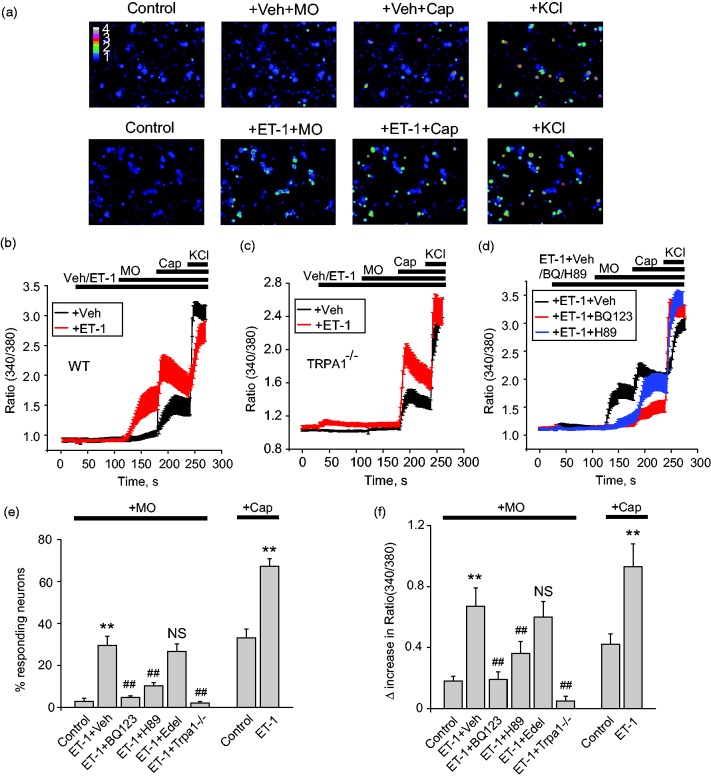
We began to examine the effects of ET-1 on primary sensory neurons. Cultured mouse dorsal root ganglion (DRG) neurons were loaded with Fura-2 for Ca2+ imaging. Application of ET-1 (100 nM) did not induce strong Ca2+ signals in mouse DRG neurons as in HEK293 cells (Figure 4(a) and (b)). The summarized percent of responding neurons to ET-1 application is not significantly different from vehicle-treated group (3. 3 ± 1.5% vs. 2.0 ± 0.9%, p > 0.05). We set to examine whether ET-1 was capable of sensitizing TRPA1 channel in DRG neurons. We used low concentrations of MO (5 µM) and capsaicin (10 nM) to activate TRPA1 and TRPV1 subsequently. In control group (vehicle-treated), 5 µM MO barely induced any Ca2+ signal, whereas subsequent application of 10 nM capsaicin induced only small Ca2+ signal in DRG neurons (Figure 4(a) and (b)). When DRG neurons were pretreated with ET-1(100 nM), however, larger Ca2+ responses were recorded with low concentration of MO (5 µM) (Figure 4(a) and (b)). Subsequent application of 10 nM capsaicin also induced higher Ca2+ responses (Figure 4(a) and (b)).
Figure 4.
ET-1 sensitizes TRPA1 in cultured mouse DRG neurons. (a) Pseudo color images from Fura-2 ratiometric imaging showing Ca2+ responses in mouse DRG neurons in response to MO (5 µM) with or without pretreatment of ET-1 (100 nM). Capsaicin (10 nM) was applied after MO application for comparison. KCl (40 mM) was applied at the end of recording to determine all live DRG neurons. (b) Averaged Ca2+ responses from experiments shown in panel (a). Red and black lines show conditions with or without ET-1 pretreatment, respectively. n > 20 cells/group. (c) Averaged Ca2+ responses of DRG neurons obtained from Trpa1–/– mice. n > 20 cells/group. (d) Pharmacological studies of ET-1-induced TRPA1 sensitization in mouse DRG neurons. Cells were preincubated with ETAR antagonist BQ-123 (10 µM), PKA antagonist H89 (10 µM), PLC antagonist edelfosine (10 µM), or corresponding vehicle (0.1% DMSO) for 5 min and then coapplied with ET-1. n > 20 cells/group. (e) Percentages of mouse DRG neurons responding to MO or Cap in control condition (no ET-1 added) and conditions of ET-1 with vehicle, BQ-123, H89, edelfosine, and TRPA1–/–. n = 5–6 tests/group, each group contains 150–200 neurons from 3 mice. (f) Summarized Δ increase in ratio of 340/380 of MO or Cap-induced Ca2+ responses in mouse DRG neurons as recorded in (e). **p < 0.01 versus control group, ##p < 0.01 versus ET-1+Veh group.
TRPA1: transient receptor potential cation channel, subfamily A, member 1; ET-1: endothelin-1; MO: mustard oil.
MO-induced Ca2+ signal sensitized by ET-1 was mediated via TRPA1, since it was completely eliminated in neurons derived from Trpa1 knockout (Trpa1–/–) mouse (Figure 4(c), (e), and (f)). In contrast, the sensitizing effect of ET-1 on capsaicin-induced Ca2+ signal remained unaltered in Trpa1–/– neurons (Figure 4(c), (e), and (f)). ETAR antagonist BQ-123 (10 µM) and PKA antagonist H89 (10 µM) largely eliminated ET-1-induced sensitization on TRPA1 (Figure 4(d), (e), and (f)). PLC antagonist edelfosine (10 µM) had no effect on ET-1-induced sensitization of TRPA (Figure 4(e) and (f)). Therefore, the above results demonstrated that ET-1 sensitizes TRPA1 in DRG neurons via ETAR and PKA pathway.
TRPA1 can be activated by a variety of endogenous agonists that activate TRPA1 to produce pain signals. We examined whether ET-1 sensitizes TRPA1 activated by hydrogen peroxide (H2O2), a well-established endogenous agonist for TRPA1.30 In HEK293 cells expressing ETAR and TRPA1, ET-1 (100 nM) treatment significant increased the magnitude of Ca2+ responses to H2O2 (1 mM) compared with vehicle-treated group (control) (Figure 5(a) and (b)). ET-1 (100 nM) treatment also significantly increased magnitude of Ca2+ responses to H2O2 in mouse DRG neurons (Figure 5(c) and (d)). These results suggest that ET-1 sensitizes TRPA1 channel activated by endogenous agonist H2O2.
Figure 5.
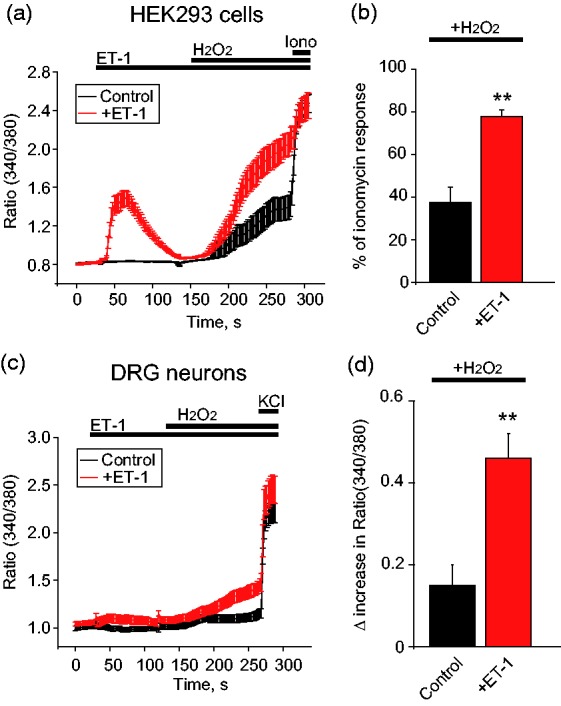
ET-1 sensitizes TRPA1 channel activated by endogenous agonist H2O2 in both HEK293 cells and mouse DRG neurons. (a) Averaged Ca2+ responses from HEK293 cells in control condition or treated with ET-1 (100 nM). HEK293 cells were expressed with TRPA1 and ETAR. H2O2 (1 mM) was applied as indicated to induce TRPA1 activation in HEK293 cells. n > 30 cells/group. (b) Summarized amplitude of H2O2-induced Ca2+ responses in control condition or treated with ET-1. Ca2+ responses were normalized to ionomycin (1 µM) applied at the end of the tests (% response of ionomycin). n = 3 tests/group. Each test contains up to 30 cells. (c) Averaged Ca2+ responses from mouse DRG neurons in control condition or treated with ET-1 (100 nM). H2O2 (1 mM) was applied as indicated to induce TRPA1 activation in DRG neurons. n > 20 cells/group. (d) Summarized Δ increase in ratio of 340/380 of H2O2-induced Ca2+ responses in DRG neurons as recorded in (c). n = 4 tests/group, each group contains 120–160 neurons from 3 mice. **p < 0.01 versus control group.
HEK293: human embryonic kidney 293 cells; ET-1: endothelin-1; DRG: dorsal root ganglion; Iono: ionomycin.
TRPA1 mediates ET-1-induced mechanical hyperalgesia in vivo
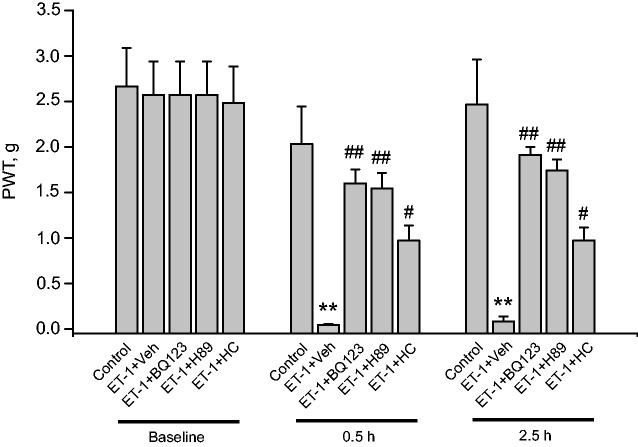
We examined the contribution of TRPA1 in ET-1-induced nocifensive behavior in vivo. Mice injected with ET-1 into hind paw (20 ng/paw) showed obvious signs of mechanical hyperalgesia compared with mice receiving vehicle injection only, measured 0.5 and 2.5 h after injection (Figure 6). HC-030031 (10 µg/paw), the TRPA1 antagonist, significantly reduced the mechanical hyperalgesia induced by ET-1. PKA antagonist H89 (5 µg/paw) and ETAR antagonist BQ-123 (1 µg/paw) both significantly attenuated the mechanical hyperalgesia induced by ET-1 (Figure 6). Injection of these antagonists alone in the same dosage as above did not produce any effects on PWTs compared with vehicle (control) group (Table 1). In all, these results demonstrated that ET-1 elicited mechanical hyperalgesia through TRPA1 via ETAR and PKA pathway.
Figure 6.
Pharmacological blocking of ETAR, PKA, and TRPA1 attenuates ET-1-induced mechanical hyperalgesia in mice. PWT of mice was measured by von Frey hair test. BQ-123 (1 µg/paw), H89 (5 µg/paw), or HC-030031 (10 µg/paw) was coinjected with ET-1 (20 ng/paw) into the hind paw of mice. Control group received vehicle (1% DMSO in PBS) injection only. PWT was measured before injection (baseline) and 0.5, 2.5 h after the injection. n = 7 mice/group. **p < 0.01 versus control group, ##p < 0.01, #p < 0.05 versus ET-1+Veh group.
ET-1: endothelin-1; PWT: paw withdraw threshold.
Table 1.
Effect of intraplantar injection of BQ-123, H89, or HC-030031 on the PWTs of mice.
| Group |
PWT (g) |
||
|---|---|---|---|
| 0 h | 0.5 h | 2.5 h | |
| Control | 2.7 ± 0.4 | 2.0 ± 0.4 | 2.5 ± 0.5 |
| +BQ-123 | 2.3 ± 0.3 (NS) | 2.1 ± 0.4 (NS) | 2.1 ± 0.4 (NS) |
| +H89 | 2.7 ± 0.4 (NS) | 2.0 ± 0.4 (NS) | 2.2 ± 0.4 (NS) |
| +HC-030031 | 2. 7± 0.4 (NS) | 2.2 ± 0.4 (NS) | 2.6 ± 0.5 (NS) |
PWT: paw withdrawal threshold; NS: no significance.
BQ-123 (1 µg/paw), H89 (5 µg/paw), or HC-030031 (10 µg/paw) and corresponding vehicle (1% dimethyl sulfoxide in phosphate-buffered saline, control) were administered intraplantarly into the hind paws of mice. PWTs were measured before and 0.5 and 2.5 h after drug/vehicle treatment.
Discussion
In the present study, we found that ET-1 sensitizes TRPA1 via ETAR and PKA-mediated signaling pathway both in vitro and in vivo. Our findings are based upon the following observations: First, ET-1 sensitizes TRPA1 channel in HEK293 cells via ETAR and PKA-mediated pathway. Second, ET-1 does not produce robust Ca2+ signals in DRG neurons but sensitizes MO-activated TRPA1 channel activity. Third, ET-1 sensitizes TRPA1 channel activated by endogenous agonist H2O2. Last, blocking TRPA1, ETAR, and PKA all significantly alleviated ET-1-induced mechanical hyperalgesia.
ETARs are widely expressed in small and medium-to-large diameter neurons and, in particular, in TRPV1-expressing small sensory neurons, whereas ETBRs are mainly found in satellite glial cells but not in sensory neurons.6,7 TRPA1 are distributed in sensory neurons that also express TRPV1.31 Previous studies revealed ETAR coexpressed largely with TRPV1 in mouse DRG neurons.6 This suggests that TRPA1 is likely to coexpress with ETAR in sensory neurons. Our immunostaining results demonstrated that a large population (over 80%) of TRPA1-positive DRG neurons also express ETAR. The high percentage of coexpression suggests a possible functional interaction between TRPA1 and ETAR in DRG neurons.
ETAR couples through Gαq/11 to PLCβ and the release of inositol trisphosphate (IP3) to induce intracellular Ca2+ release.23,32 In HEK293 cells which are overexpressed with ETAR, we found that ET-1 application elicited large Ca2+ responses, which gradually subsided in the continued presence of ET-1. This suggests that ET-1 binds with ETAR and initiates Ca2+ signals which is likely mediated via PLCβ-IP3 pathway in HEK293 cells. In contrast, ET-1 did not elicit obvious Ca2+ signals in native DRG neurons in our study. It is reported that ET-1 only induced quite small intracellular Ca2+ transients in mouse DRG neurons, but its effect on satellite nonneuronal cells is much larger.33 Recently, one study reported that only a small proportion (approximate 3%) of DRG neurons respond to ET-1 application in Ca2+ imaging.7 This responding rate to ET-1 is similar with our findings. But unfortunately, no comparisons between ET-1-responding and vehicle-responding rate were included in that study. In the present study and our previous publication, we found that even vehicle application produces small and random Ca2+ transients in DRG neurons during Ca2+ imaging.18 Therefore, it still remains to be investigated whether ET-1 can truly induce reliable and robust Ca2+ signals in mouse DRG neurons.
ETAR also couples to PKA signaling pathway.22,34 ET-1 induces intracellular cAMP level increase in HEK293 cells expressing ETAR but not ETBR, suggesting ETAR couples with PKA signaling in HEK293 cells.6 TRPA1 can be sensitized by inflammatory mediators, such as bradykinin and tryptase, via PKA and PLC pathways.10,13,15,35 Further study identified the amino acid residues involved in PKA-mediated phosphorylation and sensitization of TRPA1.14 We examined the contribution of these two pathways to ET-1-induced sensitization of TRPA1. Pharmacological blockage of ETAR by specific antagonist BQ-123 abolished ET-1-induced sensitization of TRPA1 Ca2+ signals in both HEK293 cell lines and DRG neurons. Furthermore, PKA antagonist H89 largely abolished ET-1-induced sensitization of TRPA1 Ca2+ signals in both HEK293 cell lines and DRG neurons. The PKA-specific agonist, forskolin, mimics the effect of ET-1 in sensitizing TRPA1 mediated Ca2+ signals. However, pretreating HEK293 cells and DRG neurons with PLC antagonist edelfosine did not affect ET-1-induced sensitization of TRPA1. These results demonstrate that ET-1-induced sensitization of TRPA1 in vitro requires ETAR-mediated PKA signaling pathway.
ET-1 potentiates TRPV1 channel, which underlies ET-1-induced nocifensive behavior.36 However, ET-1-induced nociceptive response is not completely inhibited in Trpv1–/– mice or by TRPV1 antagonist, suggesting other mechanisms are involved as well.3,37 In addition to TRPV1, TRPA1 is involved in ET-1-induced mechanical hypersensitivity.17 We found that ET-1 induced mechanical hyperalgesia is significantly reduced by TRPA1-specific antagonist HC-030031, as well as by PKA and ETAR antagonists. These findings suggest that TRPA1 is a molecular target of ET-1 in mediating nociceptive responses.
Oxidative stress occurs during many pathophysiological conditions including inflammation and tissue injury, which produces a variety of highly reactive oxygen species (ROS) including H2O2, lipid peroxidation products, like 4-hydroxy-2-nonenal and Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycerol-3-phosphatidylcholine (OxPAPC).38 These ROS products act as endogenous TRPA1 agonists and are involved in many inflammatory and neuropathic pain conditions.20,39 ET-1 is generated during tissue inflammation and damage and involved in pathogenesis of pain.1 The concentration of ET-1 (100 nM) we tested falls well within ET-1’s endogenous concentration range reported.21 Thus, our findings suggest that ET-1-induced TRPA1 sensitization is likely to occur in pathological conditions.
In addition to causing pain, ET-1 is also known to cause pruritus.7 This property is shared with many other stimulators of peripheral sensory neurons. Pain and itch sensations are activated by excitation of separate populations of peripheral sensory neurons, the nociceptors, and the pruriceptors, respectively.40 Recent studies demonstrated that nociceptors and pruriceptors engage spinal circuits that, depending on stimulus strength, duration, and lateral spread of inputs, control whether itch or pain is transduced, or whether itch is suppressed (by scratching, for example).40,41 It is possible that high local concentrations of ET-1 may favor pain since widespread nociceptor activation is known to suppress itch sensations, while lower local concentrations favor itch sensation. The role of TRPA1 in ET-1-induced pruritus remains controversial. While TRPA1 inhibitors were found to increase ET-1-induced scratching responses in mice immediately after injection, a study in Trpa1–/– mice observed that ET-1 induced scratching was attenuated.7,42 Additional studies, potentially using more selective inhibitors and longer observation time, may be necessary to clarify the role of TRPA1 in ET-1-induced pruritus.
It has been reported that ET-1 can potentiate TRPA1 agonist cinnamaldehyde-induced nociception in vivo.16 Further studies demonstrated that ET-1-induced mechanical allodynia is inhibited by specific antagonists against TRPA1 or ETAR in vivo, suggesting a possible interaction between ETAR and TRPA1 in mediating pain responses.17 However, little is known about whether ET-1 acts on TRPA1 in primary sensory neurons and the detailed molecular mechanisms. Our findings showed for the first time that ETAR couples with TRPA1 in primary sensory neurons, which provide another molecular mechanism for explaining ET-1-induced pain response. Our results demonstrate that TRPA1 acts as a novel molecular target for ET-1 and sensitization of TRPA1 through ETAR–PKA pathway contributes to ET-1-induced mechanical hyperalgesia. Targeting of TRPA1 and ETAR-PKA pathway may offer effective strategies to alleviate pain conditions related with ET-1.
Author Contributions
SEJ and BL conceived and designed the project. XZ, YT, DH, BL, CW and XS carried out the experiments and collected and analyzed the data. BL and SEJ prepared the manuscript.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Sven-Eric Jordt serves on the Scientific Advisory Board of Hydra Biosciences LLC (Cambridge, MA), a biopharmaceutical company developing TRP ion channel inhibitors for the treatment of pain and inflammation. Other authors state no conflict of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project is supported by funding from Zhejiang Provincial Natural Science Funds for Distinguished Young Scholars (LR17H270001), the National Natural Science Foundation of China (81873365, 81603676, 81770407, 81574056), Qianjiang Talent Program (QJD1702020), research fund from Zhejiang Chinese Medical University (2018ZY37, 2018ZY19), Scientific Research Foundation for the Returned Overseas Chinese Scholars of Hebei Province, Administration of Traditional Chinese Medicine of Hebei Province (2018139), and from the National Institute of Arthritis and Musculoskeletal and Skin Disease (R21AR070554 to S.E.J.).
References
- 1.Smith TP, Haymond T, Smith SN, Sweitzer SM. Evidence for the endothelin system as an emerging therapeutic target for the treatment of chronic pain. J Pain Res 2014; 7: 531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Souza RF, Oliveira LL, Nones CFM, Dos Reis RC, Araya EI, Kopruszinski CM, Rae GA, Chichorro JG. Mechanisms involved in facial heat hyperalgesia induced by endothelin-1 in female rats. Arch Oral Biol 2017; 83: 297–303. [DOI] [PubMed] [Google Scholar]
- 3.Motta EM, Chichorro JG, Rae GA. Role of ET(A) and ET(B) endothelin receptors on endothelin-1-induced potentiation of nociceptive and thermal hyperalgesic responses evoked by capsaicin in rats. Neurosci Lett 2009; 457: 146–150. [DOI] [PubMed] [Google Scholar]
- 4.Gomes LO, Chichorro JG, Araya EI, de Oliveira J, Rae GA. Facial hyperalgesia due to direct action of endothelin-1 in the trigeminal ganglion of mice. J Pharm Pharmacol 2018; 70: 893–900. [DOI] [PubMed] [Google Scholar]
- 5.Dahlof B, Gustafsson D, Hedner T, Jern S, Hansson L. Regional haemodynamic effects of endothelin-1 in rat and man: unexpected adverse reaction. J Hypertens 1990; 8: 811–817. [DOI] [PubMed] [Google Scholar]
- 6.Plant TD, Zollner C, Kepura F, Mousa SS, Eichhorst J, Schaefer M, Furkert J, Stein C, Oksche A. Endothelin potentiates TRPV1 via ETA receptor-mediated activation of protein kinase C. Mol Pain 2007; 3: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kido-Nakahara M, Buddenkotte J, Kempkes C, Ikoma A, Cevikbas F, Akiyama T, Nunes F, Seeliger S, Hasdemir B, Mess C, Buhl T, Sulk M, Muller FU, Metze D, Bunnett NW, Bhargava A, Carstens E, Furue M, Steinhoff M. Neural peptidase endothelin-converting enzyme 1 regulates endothelin 1-induced pruritus. J Clin Invest 2014; 124: 2683–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Z, Davar G, Strichartz G. Endothelin-1 (ET-1) selectively enhances the activation gating of slowly inactivating tetrodotoxin-resistant sodium currents in rat sensory neurons: a mechanism for the pain-inducing actions of ET-1. J Neurosci 2002; 22: 6325–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: a gatekeeper for inflammation. Annu Rev Physiol 2013; 75: 181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Dai Y, Fukuoka T, Yamanaka H, Kobayashi K, Obata K, Cui X, Tominaga M, Noguchi K. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain 2008; 131: 1241–1251. [DOI] [PubMed] [Google Scholar]
- 11.Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest 2007; 117: 1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malin S, Molliver D, Christianson JA, Schwartz ES, Cornuet P, Albers KM, Davis BM. TRPV1 and TRPA1 function and modulation are target tissue dependent. J Neurosci 2011; 31: 10516–10528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron 2009; 64: 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meents JE, Fischer MJ, McNaughton PA. Sensitization of TRPA1 by protein kinase A. PLoS One 2017; 12: e0170097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadkova A, Synytsya V, Krusek J, Zimova L, Vlachova V. Molecular basis of TRPA1 regulation in nociceptive neurons. A review. Physiol Res 2017; 66: 425–439. [DOI] [PubMed] [Google Scholar]
- 16.Liang J, Bi H, Ji W. Involvement of TRPA1 in ET-1-induced pain-like behavior in mice. Neuroreport 2010; 21: 201–205. [DOI] [PubMed] [Google Scholar]
- 17.Nodai T, Hitomi S, Ono K, Masaki C, Harano N, Morii A, Sago-Ito M, Ujihara I, Hibino T, Terawaki K, Omiya Y, Hosokawa R, Inenaga K. Endothelin-1 elicits TRP-mediated pain in an acid-induced oral ulcer model. J Dent Res 2018; 97: 901–908. [DOI] [PubMed] [Google Scholar]
- 18.Liu B, Tai Y, Achanta S, Kaelberer MM, Caceres AI, Shao X, Fang J, Jordt SE. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc Natl Acad Sci U S A. 2016; 113: E7572–E7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu B, Fan L, Balakrishna S, Sui A, Morris JB, Jordt SE. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 2013; 154: 2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu B, Tai Y, Caceres AI, Achanta S, Balakrishna S, Shao X, Fang J, Jordt SE. Oxidized phospholipid OxPAPC activates TRPA1 and contributes to chronic inflammatory pain in mice. PLoS One 2016; 11: e0165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Papendorp CL, Cameron IT, Davenport AP, King A, Barker PJ, Huskisson NS, Gilmour RS, Brown MJ, Smith SK, Research localization and endogenous concentration of endothelin-like immunoreactivity in human placenta. J Endocrinol 1991; 131: 507–511. [DOI] [PubMed] [Google Scholar]
- 22.Dulin NO, Niu J, Browning DD, Ye RD, Voyno-Yasenetskaya T. Cyclic AMP-independent activation of protein kinase A by vasoactive peptides. J Biol Chem 2001; 276: 20827–20830. [DOI] [PubMed] [Google Scholar]
- 23.Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS. ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol Lung Cell Mol Physiol 2003; 285: L680–L690. [DOI] [PubMed] [Google Scholar]
- 24.Hay DW, Luttmann MA. Nonpeptide endothelin receptor antagonists. IX. Characterization of endothelin receptors in guinea pig bronchus with SB 209670 and other endothelin receptor antagonists. J Pharmacol Exp Ther 1997; 280: 959–965. [PubMed] [Google Scholar]
- 25.Karaki H, Sudjarwo SA, Hori M. Novel antagonist of endothelin ETB1 and ETB2 receptors, BQ-788: effects on blood vessel and small intestine. Biochem Biophys Res Commun 1994; 205: 168–173. [DOI] [PubMed] [Google Scholar]
- 26.Yoon S, Zuccarello M, Rapoport RM. EndothelinA-endothelinB receptor cross-talk in rat basilar artery in situ. Naunyn-Schmiedeberg's Arch Pharmacol 2012; 385: 437–441. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto T, Ono K, Hitomi S, Harano N, Sago T, Yoshida M, Nunomaki M, Shiiba S, Watanabe S, Nakanishi O, Inenaga K. Endothelin receptor-mediated responses in trigeminal ganglion neurons. J Dent Res 2013; 92: 335–339. [DOI] [PubMed] [Google Scholar]
- 28.Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H, Gamper N. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl- channels. J Clin Invest 2010; 120: 1240–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tai Y, Wang C, Wang Z, Liang Y, Du J, He D, Fan X, Jordt SE, Liu B. Involvement of transient receptor potential cation channel member A1 activation in the irritation and pain response elicited by skin-lightening reagent hydroquinone. Sci Rep 2017; 7: 7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci 2008; 28: 2485–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004; 427: 260–265. [DOI] [PubMed] [Google Scholar]
- 32.Subedi KP, Son MJ, Chidipi B, Kim SW, Wang J, Kim KH, Woo SH, Kim JC. Signaling pathway for endothelin-1- and phenylephrine-induced cAMP response element binding protein activation in rat ventricular myocytes: role of inositol 1,4,5-trisphosphate receptors and CaMKII. Cell Physiol Biochem 2017; 41: 399–412. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto H, Kawamata T, Ninomiya T, Omote K, Namiki A. Endothelin-1 enhances capsaicin-evoked intracellular Ca2+ response via activation of endothelin a receptor in a protein kinase Cepsilon-dependent manner in dorsal root ganglion neurons. Neuroscience 2006; 137: 949–960. [DOI] [PubMed] [Google Scholar]
- 34.El-Mowafy AM, White RE. Evidence for a tyrosine kinase-dependent activation of the adenylyl cyclase/PKA cascade downstream from the G-protein-linked endothelin ETA receptor in vascular smooth muscle. Biochem Biophys Res Commun 1998; 251: 494–500. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Yang C, Wang ZJ. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience 2011; 193: 440–451. [DOI] [PubMed] [Google Scholar]
- 36.Kawamata T, Ji W, Yamamoto J, Niiyama Y, Furuse S, Omote K, Namiki A. Involvement of transient receptor potential vanilloid subfamily 1 in endothelin-1-induced pain-like behavior. Neuroreport 2009; 20: 233–237. [DOI] [PubMed] [Google Scholar]
- 37.Kawamata T, Ji W, Yamamoto J, Niiyama Y, Furuse S, Namiki A. Contribution of transient receptor potential vanilloid subfamily 1 to endothelin-1-induced thermal hyperalgesia. Neuroscience 2008; 154: 1067–1076. [DOI] [PubMed] [Google Scholar]
- 38.Fruhwirth GO, Loidl A, Hermetter A. Oxidized phospholipids: from molecular properties to disease. Biochim Biophys Acta 2007; 1772: 718–736. [DOI] [PubMed] [Google Scholar]
- 39.Trevisan G, Benemei S, Materazzi S, De Logu F, De Siena G, Fusi C, Fortes Rossato M, Coppi E, Marone IM, Ferreira J, Geppetti P, Nassini R. TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain 2016; 139: 1361–1377. [DOI] [PubMed] [Google Scholar]
- 40.Liu B, Jordt SE. Cooling the Itch via TRPM8. J Invest Dermatol 2018; 138: 1254–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross SE. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr Opin Neurobiol 2011; 21: 880–887. [DOI] [PubMed] [Google Scholar]
- 42.Liang J, Ji Q, Ji W. Role of transient receptor potential ankyrin subfamily member 1 in pruritus induced by endothelin-1. Neurosci Lett 2011; 492: 175–178. [DOI] [PubMed] [Google Scholar]