Short abstract
Electroacupuncture has been shown to effectively reduce chronic pain in patients with nerve injury. The underlying mechanisms are not well understood. Accumulated evidence suggests that purinergic P2X3 receptors (P2X3Rs) in dorsal root ganglion neurons play a major role in mediating chronic pain associated with nerve injury. The aim of this study is to determine if electroacupuncture stimulation alters P2X3R activity in dorsal root ganglia to produce analgesia under neuropathic pain condition. Peripheral neuropathy was produced by ligation of the left lumbar 5 (L5) spinal nerve in rats. Low-frequency (2 Hz) electrical stimulation was applied to ipsilateral ST36 and BL60 acupoints in rats. The P2X3R agonist (α,β-meATP)-induced flinch responses were reduced after electroacupuncture treatment. Western analyses showed that P2X3R expression was upregulated in nerve-uninjured lumbar 4 (L4) dorsal root ganglion neurons ipsilateral to the spinal nerve ligation. Electroacupuncture-stimulation reversed the upregulation. In nerve-injured L5 dorsal root ganglia, P2X3R expression was substantially reduced. Electroacupuncture had no effect on the reduction. We also determined the injury state of P2X3R expressing dorsal root ganglion neurons using the neuronal injury marker, activating transcription factor 3 (ATF3). Immunohistochemical assay showed that in L4 dorsal root ganglia, almost all P2X3Rs were expressed in uninjured (ATF3−) neurons. Spinal nerve ligation increased the expression of P2X3Rs. Electroacupuncture reduced the increase in P2X3R expression without affecting the percentage of ATF + neurons. In ipsilateral L5 dorsal root ganglion neurons, spinal nerve ligation reduced the percentage of P2X3R + neurons and markedly increased the percentage of ATF3 + cells. Almost all of P2X3Rs were expressed in damaged (ATF3+) neurons. Electroacupuncture had no effect on spinal nerve ligation-induced changes in the percentage of P2X3R or percentage of ATF3 + cells in L5 dorsal root ganglia. These observations led us to conclude that electroacupuncture effectively reduces injury-induced chronic pain by selectively reducing the expression of P2X3Rs in nerve-uninjured L4 dorsal root ganglion neurons.
Keywords: Electroacupuncture, P2X3R, dorsal root ganglion, spinal nerve ligation, activating transcription factor 3
Introduction
Chronic pain resulting from tissue or nerve injuries is difficult to treat and often leads to a great deal of patient suffering.1–3 Electroacupuncture (EA), a method combining acupuncture and electric stimulation, has been increasingly used in treating pain patients.4 Better understanding of underlying mechanisms of EA analgesia is important to improve its effectiveness and usage.
It has been shown that EA produces analgesia by modulating the activity of receptors that are involved in pain transmission.5,6 Ligand-gated purinergic P2XRs were found to express in dorsal root ganglia (DRGs) and the spinal cord. Among them, P2X3, P2X4, and P2X7 receptor subtypes are particularly important in mediating pain signaling. P2X3R is the most abundant P2XR subtype expressed in DRG neurons.7 The P2X3R directly participate in signal transmission from peripheral to central terminals.8,9 P2X4Rs are expressed in injury surveillance glial cells, i.e., microglia in the spinal cord and macrophages in DRGs.10 They modulate the P2X3R activity under injurious conditions.10,11 P2X7R are expressed mostly in satellite glial cells. They modulate the activity of P2X3Rs in neurons under both normal and injurious conditions.12 In this study, we concentrated our study on the effect of EA stimulation on the P2X3R activity in DRGs.
The P2X3R is abundantly expressed in small and medium (20–32 µm) diameter DRGs.13 There is good evidence that ATP-P2X3R plays an essential role in transmitting and processing nociceptive information.7,14,15 It was found that damaged skin cells can induce an increase in cytosolic ATP release to evoke large P2X3R-mediated current responses in DRG neurons.16 P2X3Rs in central processes of DRGs facilitate transmission of nociceptive signals from peripheral to spinal dorsal horns.8,9 Increasing ATP release after tissue injury was shown to enhance pain behaviors.17 Tissue injury potentiates P2X3R expression, increases current responses, and promotes the excitability of P2X3Rs in DRG neurons.18 These changes can be blocked by selective P2X3R antagonists19,20 or by antisense oligonucleotides.21,22
Several nerve injury models have been used to determine changes in P2X3R properties following chronic nerve injury. They are (1) chronic constriction injury (CCI) model in which the sciatic nerve proximal to trifurcation23 or the trigeminal mandibular inferior alveolar nerve is loosely ligated,24 (2) spared nerve injury (SNI) model in which the tibial and common peroneal terminal nerve branches of the sciatic nerve are transected,25 and (3) spinal nerve ligation (SNL) model in which the spinal nerves in one lumbar 5 (L5) or L5 and L6 are tightly ligated or transected.26,27 In CCI, and SNI models, P2X3R protein and mRNA expression in DRGs are increased after nerve injury.24,28,29 Membrane expression of P2X3Rs was found to increase three to seven days after SNI injury.30 In contrast, in L5-6 SNL model, P2X3 expression in both L5 and L6 ganglia is downregulated.31,32
EA was found to reduce P2X3R activation and expression to produce analgesia in CCI-induced injury,33–36 to reduce chronic visceral hypersensitivity37,38 or to downregulate SNI-induced hyperalgesia.39 It is unclear if EA analgesia is associated with P2X3Rs in DRGs of SNL rats. We therefore determined if changes of P2X3R activity in L4 and L5 DRG neurons can contribute to EA-induced analgesia. A unique feature of SNL injury model is that damaged (axotomized) L5 DRG neurons are separated from neighboring undamaged L4 and L6 DRG neurons. Using this model allowed us to distinguish EA effects on P2X3R activity in injured or uninjured DRGs thus contributing to EA-induced reduction of neuropathic pain. In addition, we also used activating transcription factor 3 (ATF3),40 a neuronal-injury marker, to monitor the damaged state of P2X3R containing DRG neurons in L4 and L5 DRGs.
Materials and methods
Animals
Adult male Sprague-Dawley rats weighing 180 to 200 g were obtained from the Animal Resource Center of University of Texas Medical Branch. All rats were acclimatized for five days before the experiments. The animals were subjected to light/dark cycles of 12 h at a constant temperature (25 ± 1°C). Food and water were available ad libitum. The animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and performed in accordance with the guidelines of the National Institutes of Health and the International Association for the Study of Pain.
SNL model
SNL model was established using previously described method.26,27 Briefly, the left L5 spinal nerve was exposed and tightly ligated with a 5–0 silk thread under isoflurane anesthesia (5% for anesthesia induction, then adjusted to 2% for continuous anesthesia). The wound was sutured and maintained with postoperative care. Sham surgery consisted of exposing the nerve in the same manner but without nerve ligation.
Forty-seven rats were used in our studies. They were randomly divided into three groups: The first group received only sham surgery nerve, the second group received SNL and sham EA stimulation, and the third group received SNL and EA stimulation. The experiment timeline is described in Results section.
EA application
To minimize the stress of immobilization, rats were acclimated under a black cotton head retainer for 30 min/day for seven days (Patent No. ZL 2014 2 0473579.9, State Intellectual Property Office of the P.R.C), with both hind legs and the tail exposed. After the acclimation period, rats would stay clam during EA stimulation. The fur on the left hind leg of rats was shaved. Stainless steel acupuncture needles (0.18 mm in diameter, 13 mm in length, Wuji USA Co., Austin, USA) were inserted to a depth of 5 mm at ipsilateral ST36 (Zusanli) (5 mm lateral to and below the anterior tubercule of the tibia) and BL60 (Kunlun) (at the level of the ankle joint between the tip of the lateral malleolus and the Achilles tendon). The needles were connected to a HANS Acupuncture point Nerve Stimulator (HANS-200E, Huawei Co., Ltd., Beijing, China). Three consecutive square waves of 0.6 ms duration and amplitude of 0.5, 1.0, 1.5 mA for 10 min each (a total 30 min) were applied at 2 Hz frequency.41 The EA stimulation was given once per day for seven days. Sham EA group rats received oblique needle-insertion into subcutaneous layer of the same point (approximately 2 mm in depth) but without electrical stimulation.
Paw withdrawal thresholds
Mechanical allodynia was determined by measuring paw withdrawal thresholds (PWTs) in response to von Frey filament stimulation on days 0, 7, and 13 after SNL. Using up and down method, a series of von Frey filaments (ranging from 0.4 to 15.0 g) were used to the lateral plantar surface of the ipsilateral paw. To avoid injury during tests, the cutoff strength of the von Frey filaments was set at 15 g. PWTs were calculated using the method described by Chaplan et al.42
α,β-meATP induced nociceptive flinch responses
At day 14 post-SNL, the nocifensive behavior elicited by activation of P2X3Rs was analyzed using a previously described procedure.30,43 Briefly, rats were placed under individual cages and acclimated for 1 h before EA stimulation. Immediately after the completion of EA or s-EA stimulation, a 50 μl of α,β-meATP (1 mM) was injected intradermally into the plantar surface of rat’s left hindpaw. The rat was returned to the cage immediately and flinching behavioral tests were performed. Paw flinch durations (PFDs), an accumulative duration that the hindpaw was lifted in the air in a 1-min time bin, were used to assess flinching behavior. By taking into account both paw lift frequency and duration, PFD gives a more accurate measure of nociceptive behavior than measuring paw lift frequency alone. All behavioral studies including PWTs and PFD measurements tests were obtained under blind conditions.
Western blotting
Protein extraction was prepared using described procedures.44,45 Ipsilateral L4 or L5 DRGs were homogenized in radio-immunoprecipitation assay lysis buffer (Beyotime Biotechnology). The homogenate was centrifuged at 14,000 r/min at 4°C for 5 min and the supernatant was collected. The protein samples were loaded into wells of 8% sodium dodecyl sulfate/polyacrylamide gel electrophoresis gels (15μg/well) and underwent electrophoresis. The samples were then electro-transferred to a polyvinylidene difluoride membrane, which was subsequently incubated in a Tris-buffered saline blocking buffer containing 5% w/v fat-free dry milk at room temperature (RT) for 1 h. The membrane was then immunoblotted with rabbit anti-P2X3R primary antibody (1:1000, Alomone Labs) at 4°C, overnight followed by horse radish peroxidase-conjugated goat anti-rabbit IgG (1:10000, Abcam) at RT for 1 h. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), probed with rabbit anti-GAPDH antibody (1:1000, Cell Signaling Technilogy), was used as loading control. The immunoreactive proteins were detected by enhanced chemiluminescence kit (Beyotime Biotechnology) and analyzed by using Image Quant TL 7.0 analysis software (General Electric).
Immunohistochemistry
For immunohistochemical staining DRG neurons in slices, rats were deeply anesthetized with 1% pentobarbital sodium (50 mg/kg i.p.) and perfused with 4°C saline followed by 4% paraformaldehyde in 0.1 mol/L phosphate-buffered saline (PBS) (pH 7.2–7.4). The ipsilateral L4 and L5 DRGs were removed, post-fixed for 2 h and cryoprotected in 30% sucrose in 0.1 M PBS overnight at 4°C and then cut into 10 µm sections in a cryostat. Sample sections were blocked with 0.2% H2O2 for 20 min, then with 10% normal goat serum and 0.3% Triton X-100 for 1 h at room temperature. Tissue sections were incubated with guinea pig anti-P2X3R (1:5000, Millipore) for 36 h, and then with rabbit anti-rat ATF-3 (1:500, Santa Cruz) overnight at RT. All antibodies were diluted in PBS containing 1% Normal Goat Serum and 0.3% Triton X-100. Sections were then incubated with Alexa Fluor 594 labeled goat anti-guinea pig IgG (1:500; Molecular Probes) or Alexa 488 labeled goat anti-rabbit IgG (1:500; Molecular Probes) for 1 h at RT. Tissue sections were imaged with a confocal microscope (A1RMP, Nikon). Positive cells were counted by Image Pro Plus 6.0 software. To avoid the possibility of double counting the same cell, every fifth DRG sections were analyzed. Positive ratio is representative as (number of positive neurons/total number of neurons)× 100%. A minimum of five DRG sections per rat were analyzed to evaluate the percentage of immunoreactivity (ir) cells. To determine the intensities of P2X3R labels, integrated optical densities (IODs) of P2X3R-labeled neurons in L4 DRG neurons were also determined.
Statistical analysis
All data were expressed as mean ± standard error. Statistical analyses were performed using SPSS 20.0 software (Serial CN 2010096, SPSS China). Comparisons between multiple means were done with one-way analysis of variance followed by Student–Newman–Keuls post hoc test. P < 0.05 was considered statistically significant.
Results
Experiment timeline
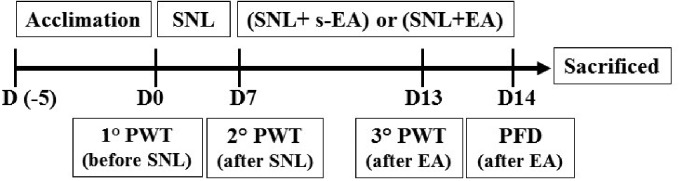
Rats were divided into three groups: (1) rats received only sham surgery (Control), (2) SNL rats received sham EA (s-EA) stimulation (SNL + s-EA), and (3) SNL rats received EA stimulation (SNL+EA). Following five days acclimation period, on Day 0 (D0) before SNL surgery, PWTs (1° PWT) in response to von Frey filament stimulation were measured on the left paw of all three rat groups (Figure 1). Afterward, SNL procedure, i.e., tight ligation of the left lumbar 5 (L5) spinal nerve, was performed on groups 2 and 3 rats. Seven days (D7) after the SNL operation, PWTs were measured the second time (2° PWT). Sham-EA and EA stimulations were subsequently applied to rat groups 2 and 3, respectively, for 30 min once/day for seven consecutive days. On D13, PWT measurements were performed the third time (3° PWT). On day 14, PFD in response to intraplantar injection of α,β-meATP on the left paw was obtained. Rats were subsequently sacrificed and L4 and L5 DRGs were removed for Western or immunohistochemical analyses.
Figure 1.
Experiment protocol. Detailed under results. SNL: spinal nerve ligation; EA: electroacupuncture; PWT: paw withdrawal threshold; PFD: Paw Flinch Duration.
SNL-induced tactile allodynia was reduced by EA stimulation
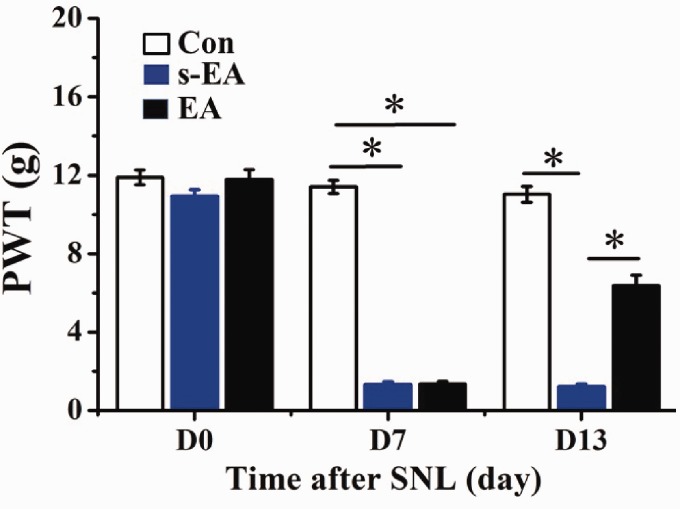
Prior to SNL, no significant difference was found in ipsilateral PWT measurements obtained from three rat groups (Figure 2). Seven days (D7) after SNL, PWTs of both SNL + s-EA and SNL + EA rats were significantly reduced, a sign of tactile allodynia. On D13, seven days after s-EA or EA stimulation, PWTs in SNL + s-EA rats remained at same low level. In contrast, PWTs in SNL + EA rats were significantly larger than those in SNL + s-EA rats, although they were still lower than control rats. Thus, EA stimulation partially reversed the SNL-induced reduction in PWTs.
Figure 2.
EA stimulation increased paw withdrawal threshold in SNL rats. On D0, under control condition, PWTs of control (Con), SNL+s-EA (s-EA) and SNL+EA (EA) rats were similar in magnitude (Con: 11.9 ± 0.4 g, s-EA: 10.9 ± 0.3 g, EA: 11.8 ± 0.5 g). Seven days after SNL prior to EA application (D7), PWTs of s-EA and EA rats were significantly lowered to a similar extent (Con: 11.4 ± 0.3 g, s-EA: 1.3 ± 0.2g, EA: 1.4 ± 0.11g). On D13, seven days after s-EA stimulation, PWTs remained at the same low level (1.2 ± 0.1 g). In contrast, the PWTs of EA rats were significantly higher than that of s-EA group. Rat number (n), Con: n = 13, s-EA: n = 17, EA: n = 17. *P < 0.01. SNL: spinal nerve ligation; EA: electroacupuncture; PWT: paw withdrawal threshold.
α,β-meATP-induced flinch responses were decreased following EA stimulation
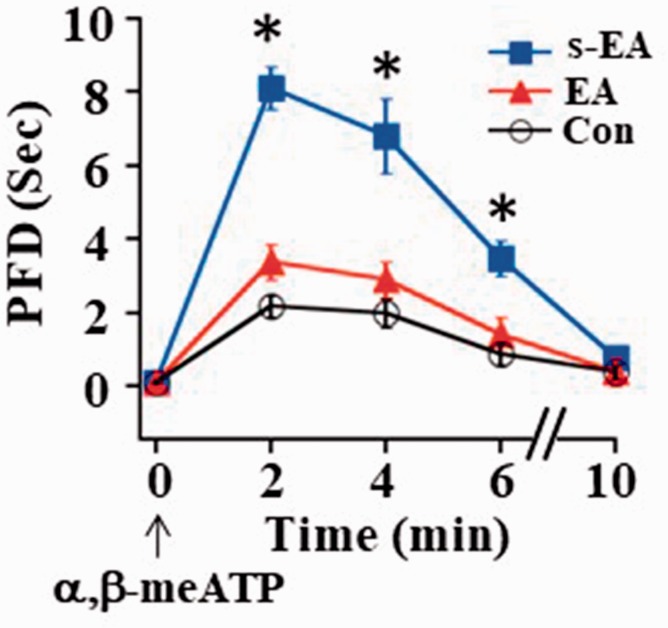
We then studied EA effects on P2X3R-mediated activity. On D14, the P2X3R agonist, α,β-meATP, was injected into the plantar surface of rat left paw to activate P2X3Rs to elicit nociceptive responses. This induced rapid flinching and prolonged paw withdrawal, thus resulting in an increase in PFD. PFDs were peaked ∼2 min after α,β-meATP injection and returned to the control level 10 min later (Figure 3). The peak PFD in SNL + s-EA rats (∼8 s) was significantly longer than that in control rats (∼2 s). The peak PFD measured in SNL+EA rats (∼3 s) approached to the control level (Figure 3). These results suggest that P2X3R-mediated responses were enhanced after SNL and EA stimulation inhibited P2X3R-mediated hyperalgesia in SNL rats.
Figure 3.
EA stimulation reduced α,β-meATP-evoked prolongation of paw flinch duration. Following injection of α,β-meATP (50 nmole in 50 µl) into the plantar surface of rat left hindpaw, the peak PFD increased from 2.2 ± 0.2 s in control rats to 8.1 ± 0.6 s in SNL rats. Following EA stimulation, the peak PFD reduced to 3.5 s, which was not significantly different from that in control rats (Con: n = 6, s-EA: n = 11, EA: n = 11). *P < 0.01 compared to the control group. EA: electroacupuncture; PFD: Paw Flinch Duration.
P2X3R protein expression in ipsilateral uninjured L4 DRG neurons was lowered after EA stimulation
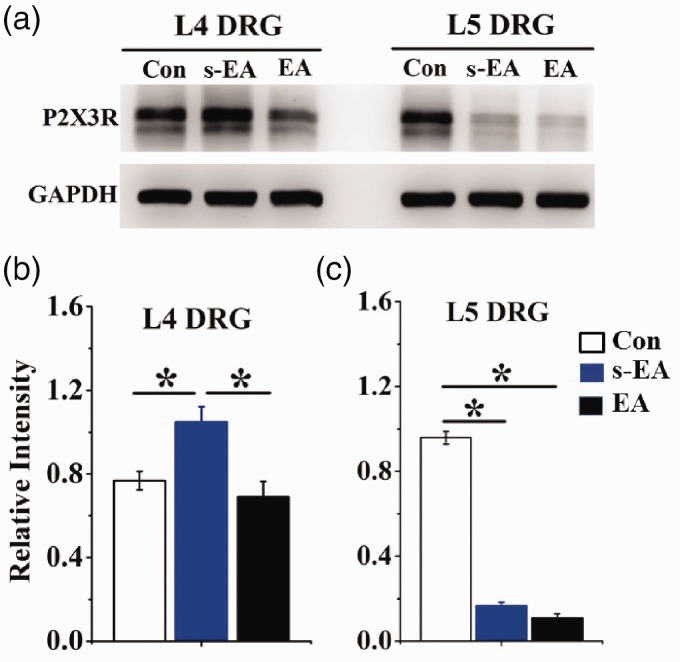
P2X3R protein expression in ipsilateral L4 and L5 DRGs was determined using Western analyses (Figure 4). The level of P2X3R expression in ipsilateral L4 DRGs obtained from SNL + s-EA rats was 1.4 times higher than that in control rats (Figure 4(a) and (b)). P2X3R expression in SNL + EA rats was close to the level of control rats. Thus, EA treatment significantly lowered the increased P2X3R expression induced by SNL injury. Studying P2X3R expression in ipsilateral L5 DRGs isolated from SNL + s-EA rats, we found that P2X3R expression was much lower than that of control rats (Figure 4(a) and (c)). EA stimulation did not change the reduced P2X3R expression in SNL rats.
Figure 4.
SNL-induced changes in P2X3R expression in ipsilateral L4 and L5 DRG were altered by EA stimulation. (a) Examples of P2X3R expressions in different rat groups. (b) EA-induced changes in P2X3R protein expression in L4 DRGs. Following SNL+ s-EA stimulation, P2X3R expression was 1.4 times (= 1.1/0.77) higher than that in control rats. The expression was retuned close to control level (0.7/0.77 = 0.9) after EA stimulation. (c) EA-induced changes P2X3R protein expressions in L5 DRGs. The expression of P2X3R was much reduced after SNL+s-EA treatment (0.2/0.97 = 0.21) or after SNL+EA treatment (0.16/0.97 = 0.17). (n = 6 for each rat group, *P < 0.01). DRG: dorsal root ganglion; EA: electroacupuncture; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
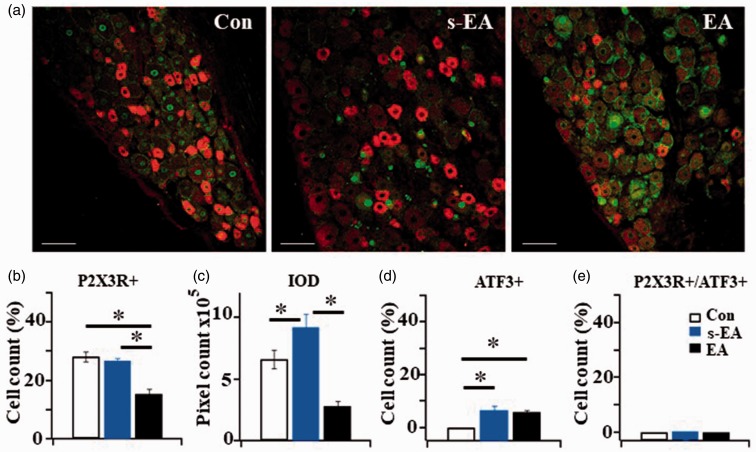
EA stimulation reduced P2X3R-containing neurons in uninjured L4 DRGs
Immunohistochemical studies were conducted to further determine changes in P2X3R-immunoreactivity (P2X3R-ir) in neurons of nerve-uninjured L4 DRGs. ATF3, which is expressed only in nuclei of injured cells, was used to identify damaged (axotomized) neurons in DRGs. We found no significant difference in the percentage of P2X3R-ir positive neurons between control and SNL + s-EA rats. The percentage of P2X3R + neurons in SNL + EA rats was significantly reduced (Figure 5(a) and (b)). This was in contrast to the Western analyses in which P2X3R expression in SNL+s-EA rats was higher than that in control rats (Figure 4). We then determined if the difference between the two analyses was a result of changes in the intensity of P2X3R fluorescent labels after SNL treatment. IOD of P2X3R labels in DRG neurons was studied in the three rat groups. The IOD level in SNL + s-EA neurons was higher than that in control neurons suggesting an SNL-induced increase in P2X3R expression (Figure 5(c)). In SNL+EA neurons, the IOD level of P2X3R-containing neurons was much reduced. To investigate the state of neuronal injury in ipsilateral L4 DRGs, the number of ATF3 + neurons was determined. Following SNL, the percentage of ATF3 + neurons in DRGs of SNL + s-EA and of SNL+EA rats was increased moderately. No significant difference was found between the two rat groups (Figure 5(d)), suggesting that EA stimulation had no effect on SNL-induced damaged neurons. We then determined the percentage of P2X3R + expressed in injured neurons by double labeling P2X3R and ATF3 in L4 DRGs. There was no P2X3Rs expressed in damaged neurons, i.e., (P2X3R+/ATF+) = 0 (Figure 5(b)). All P2X3Rs were expressed in uninjured neurons. Thus, EA stimulation reduced P2X3R expression in L4 DRG neurons to produce analgesia.
Figure 5.
The effect of immunohistochemical study of P2X3R-ir and ATF3-ir expression in L4 DRG neurons. (a) P2X3R-ir (red) + ATF3-ir (green) double labeled DRG neurons in Control (Con), SNL+ s-EA (s-EA) and SNL+EA (EA) rats. Bar =100 µm. (b) Percentages of P2X3R+ expressing neurons in Control and SNL+ s-EA rats were similar (28.24 ± 1.69% in Con vs. 26.83 ± 0.9% in s-EA rats), The percentage of P2X3R+ neurons in SNL+ EA rats was significantly reduced (15.37 ± 1.75%). (c) Integrated optical density measured in SNL+s-EA rats ((9.21 ± 1.08) × 105) was higher than that in control rats ((6.64 ± 0.75) ×105) and much reduced ((2.81 ± 0.39) × 105) in SNL+EA rats. (d) The percentage of ATF+ neurons in SNL+ s-EA and in SNL+EA rats were not significantly different (6.7 ± 1.7 vs. 6.1 ± 0.6%, P > 0.05). Both were higher than control rats (0%). (e) Almost no P2X3+/ATF3+ neurons were expressed in the three rat groups. Thus, all of the P2X3R+ were expressed in un-injured neurons (Con: n = 7, SNL+ s-EA: n = 3, SNL+EA: n = 3). *P < 0.01. EA: electroacupuncture; ATF3: activating transcription factor 3; IOD: integrated optical density.
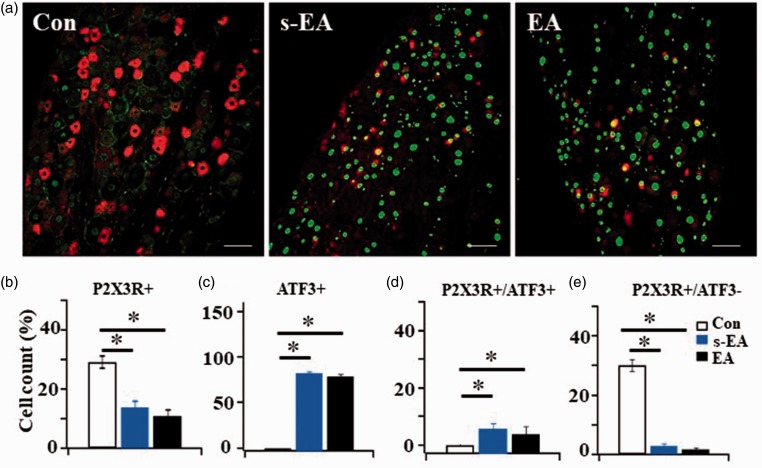
EA stimulation had no effect on SNL-induced P2X3R and ATF3 + expression changes in nerve-injured L5 DRGs
When we did the same analyses on ipsilateral nerve-injured L5 DRGs, different observations were obtained. After SNL, the percentage of P2X3R + neurons in SNL + s-EA (∼14%) and SNL+EA rats (11%) was substantially lower than that in control rats (∼29%) (Figure 6(a) and (b)). The level of reduction was similar in these two rat groups. Thus, EA stimulation could not reverse the SNL-induced decrease in P2X3R expression.
Figure 6.
EA stimulation did not affect P2X3R or ATF3 expression in injured neurons ipsilateral to L5 DRGs. (a) Examples of P2X3R-ir + ATF3-ir double labeled neurons in L5 DRGs of three rat groups, Bar = 100 µm. (b) Compared to control rats, L5 nerve ligation induced a substantial reduction in P2X3R+ expression in DRGs of SNL+ s-EA or SNL+EA rats (from 29.0 ± 2.1% to 13.7 ± 4.9% and to 11.0 ± 3.3% respectively). (c) Unlike the minimal expression of ATF3+ cells (0.4 ± 0.2%) in L5 DRG of control rats, a majority of L5 DRG neurons in SNL+ s-EA rat group (85.7 ± 1.6%) and in SNL+EA group (81.7 ± 3.1%) were ATF3+. (d) Most P2X3Rs in injured L5 DRGs were expressed in ATF3+ neurons (P2X3R/ATF3+ =10.6% in SNL+ s-EA rats and =8.8% in SNL+EA rats). (e) The cell count of P2X3R+/ATF3− cells was reduced from 30.0 ± 2.0% in control L5 DRGs to 2.7 ± 0.8% in SNL+ s-EA and 1.8 ± 0.5% in SNL+EA rats. EA stimulation did not reverse the reduction in P2X3R expression induced by SNL (Con: n = 7, SNL+ s-EA: n = 3, SNL+EA: n = 3). *P < 0.01. EA: electroacupuncture; ATF3: activating transcription factor 3.
To determine the degree of injury in L5 DRGs induced by SNL, the expression of ATF3 + in L5 DRG neurons was studied. In contrast to minimal ATF3 + cells found in control rats, a majority (>80%) of L5 DRG neurons in SNL + s-EA groups were ATF3+ (Figure 6(c)). We also studied the co-expression of P2X3R and ATF3 in ipsilateral L5 DRGs in SNL + s-EA rats. Almost all of the P2X3Rs expressing cells were ATF3 + (Figure 6(d)). In addition, the percentage of cells expressing in undamaged neurons, i.e., P2X3R+/ATF3− cells, was reduced to lower than 3% in SNL + s-EA rats (Figure 6(e)). Thus, very few P2X3R + were expressed in undamaged, i.e., ATF3−, neurons. EA treatment did not reverse the damage incurred in nerve-injured L5 DRGs.
Discussion
We showed in this study that following L5 SNL, P2X3R expression was upregulated in ipsilateral uninjured L4 DRGs. This resulted in reduced PWTs in response to von Frey filament stimulation (Figure 2) and a prolongation of in α,β-meATP-induced flinch responses, i.e., an increase in PFD (Figure 3). EA stimulation reduced P2X3R upregulation in L4 DRGs (Figures 4 and 5), thus increasing PWTs and reducing α,β-meATP induced PFDs to produce analgesia. In contrast, L5 nerve ligation induced severe injury in L5 DRGs. This resulted in a reduction in the percentage of P2X3R + neurons and large increase in percentage of ATF3 + neurons. EA had no effect on P2X3R expression and did not alter the severe nerve injury in L5 DRG (Figure 6).
There is a great deal of evidence that P2X3Rs play a crucial role in processing information in various pain states. Different nerve injury models, including CCI, SNI, and SNL models,23,25,27 have been used by researchers to study the involvement of P2X3Rs in chronic pain. Because of differences in the injury states produced by these models, changes in P2X3R activities were different. In those partial nerve-ligated CCI and SNI models, each ganglion contained a mixture of injured and uninjured neurons. It is difficult to determine the effect of EA on P2X3R activity in different injury state neurons. We therefore chose SNL model to study EA effects on uninjured L4 DRG and injured L5 DRGs separately. In addition, DRG neurons were doubled labeled with P2X3R and ATF3 antibodies. These strategies allowed us to differentiate EA effects on P2X3R activity in different injury state DRG neurons. We found that EA stimulation reduced the P2X3R expression in un-damaged DRG neurons (Figure 5).
In our immunohistochemical studies, we found that SNL did not alter the percentage of P2X3R-containing neurons in L4 DRGs (Figure 5(b)). Similar results were obtained by Fukuoka et al.46 who showed that SNL did not change P2X3R mRNA expression in non-injured L4 DRG neurons. The results had led the authors to conclude that SNL induced an increase in TRPV1 rather than P2X3R expression to produce the thermal hyperalgesia in their SNL-induced pain model. Nevertheless, we conclude that P2X3Rs mediated EA-induced analgesia in our studies for several reasons. First, in our behavioral studies, we studied mechanical-induced, not thermal hyperalgesia as done by Fukuoka et al.46 Second, we studied not only PWTs induced by SNL-induced injury (Figure 2), but also the effect of P2XR-agonist, α,β-meATP on PFDs (Figure 3). Although α,β-meATP is known to bind to both P2X1R and P2X3R, the effect of the agonist observed in our study is likely to be mediated by P2X3Rs because P2X1Rs are minimally expressed in DRG neurons.47 Our PFD experiments clearly showed that activation of P2X3Rs produced a large change in flinch behaviors after SNL and EA stimulation reversed the enhanced PFD (Figure 3). Third, our Western analyses showed that P2X3R expression in L4 DRGs was upregulated after SNL injury and EA stimulation reduced the P2X3R expression in L4 DRG neurons (Figure 4(a) and (b)).
Using immunohistochemical technique, we determined P2X3R expression in both damaged and undamaged DRG neurons through changes in cell counts. This method of analyses did not provide quantitative information on the magnitude changes of protein expression of P2X3Rs. We therefore performed additional analyses of our immunohistochemical data by determining IOD of P2X3R labels. These analyses showed that P2X3R expression was increased after SNL + s-EA stimulation (Figure 5(c)). It is also interesting to find that after SNL, P2X3Rs were expressed in larger neurons (data not shown). The IOD data were consistent with our studies of P2X3R expression using Western analyses in which SNL injury upregulated P2X3R expression in L4 DRGs. (Figure 4(b)). In addition, the IOD analysis showed that EA reduced P2X3R expression to a level lower than that in control rats (Figure 5(b)). The reason for this observation needs to be explored in the future. In the study of EA effect on nerve-injured L5 DRG, we found that EA had no effect on SNL-induced changes in P2X3R expression (Figure 6). The underlying mechanism of this EA effects has yet to be defined.
EA was also found to reduce upregulation of TRPV1 and calcitonin gene-related peptide expression in uninjured L4 and L6 DRG neurons in SNL rats48 suggesting EA can induce analgesia through multiple targets. Furthermore, since ATP is not the only substance released in response to injury, the release of other substances and/or activation of other proteins in response to tissue injury is also important.49,50 It is of interest to determine the relationship among different transmitters, neuropeptides, and protein molecules in response to EA stimulation to produce analgesia. From our studies, we conclude that EA-induced analgesic effect in SNL induced chronic pain through downregulation of P2X3Rs in L4 DRG adjacent to the spinal nerve-ligated L5 DRGs.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NIDCR R01 DE017813 and NINDS RO1 NS30045.
References
- 1.Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, Portenoy RK, Rice ASC, Stacey BR, Treede R-D, Turk DC, Wallace MS. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007; 132: 237–251. [DOI] [PubMed] [Google Scholar]
- 2.Bouhassira D, Lanteri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008; 136: 380–387. [DOI] [PubMed] [Google Scholar]
- 3.Harifi G, Amine M, Ait Ouazar M, Boujemaoui A, Ouilki I, Rekkab I, Belkhou A, El Bouchti I, Niamane R, El Hassani S. Prevalence of chronic pain with neuropathic characteristics in the Moroccan general population: a national survey. Pain Med 2013; 14: 287–292. [DOI] [PubMed] [Google Scholar]
- 4.Estores I, Chen K, Jackson B, Lao L, Gorman PH. Auricular acupuncture for spinal cord injury related neuropathic pain: a pilot controlled clinical trial. J Spinal Cord Med 2017; 40: 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayor D. An exploratory review of the electroacupuncture literature: clinical applications and endorphin mechanisms. Acupunct Med 2013; 31: 409–415. [DOI] [PubMed] [Google Scholar]
- 6.Zhang R, Lao L, Ren K, Berman BM. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology 2014; 120: 482–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnstock G. Purinergic mechanisms and pain–an update. Eur J Pharmacol 2013; 716: 24–40. [DOI] [PubMed] [Google Scholar]
- 8.Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature 1997; 389: 749–753. [DOI] [PubMed] [Google Scholar]
- 9.Gu JG. P2X receptor-mediated modulation of sensory transmission to the spinal cord dorsal horn. Neuroscientist 2003; 9: 370–378. [DOI] [PubMed] [Google Scholar]
- 10.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003; 424: 778–783. [DOI] [PubMed] [Google Scholar]
- 11.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005; 438: 1017–1021. [DOI] [PubMed] [Google Scholar]
- 12.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 2005; 114: 386–396. [DOI] [PubMed] [Google Scholar]
- 13.Gu Y, Li G, Huang L. Inflammation induces Epac-protein kinase C alpha and epsilon signaling in TRPV1-mediated hyperalgesia. Pain 2018; 159: 2383–2393. [DOI] [PubMed] [Google Scholar]
- 14.Wirkner K, Sperlagh B, Illes P. P2X3 receptor involvement in pain states. Mol Neurobiol 2007; 36: 165–183. [DOI] [PubMed] [Google Scholar]
- 15.Huang LY, Gu Y, Chen Y. Communication between neuronal somata and satellite glial cells in sensory ganglia. Glia 2013; 61: 1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook SP, McCleskey EW. Cell damage excites nociceptors through release of cytosolic ATP. Pain 2002; 95: 41–47. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton SG, Wade A, McMahon SB. The effects of inflammation and inflammatory mediators on nociceptive behaviour induced by ATP analogues in the rat. Br J Pharmacol 1999; 126: 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu GY, Huang LY. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J Neurosci 2002; 22: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanfa LC, Kontinen VK, Dickenson AH. Effects of spinally administered P2X receptor agonists and antagonists on the responses of dorsal horn neurones recorded in normal, carrageenan-inflamed and neuropathic rats. Br J Pharmacol 2000; 129: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballini E, Virginio C, Medhurst SJ, Summerfield SG, Aldegheri L, Buson A, Carignani C, Chen YH, Giacometti A, Lago I, Powell AJ, Jarolimek W. Characterization of three diaminopyrimidines as potent and selective antagonists of P2X3 and P2X2/3 receptors with in vivo efficacy in a pain model. Br J Pharmacol 2011; 163: 1315–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barclay J, Patel S, Dorn G, Wotherspoon G, Moffatt S, Eunson L, Abdel'al S, Natt F, Hall J, Winter J, Bevan S, Wishart W, Fox A, Ganju P. Functional downregulation of P2X3 receptor subunit in rat sensory neurons reveals a significant role in chronic neuropathic and inflammatory pain. J Neurosci 2002; 22: 8139–8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honore P, Kage K, Mikusa J, Watt AT, Johnston JF, Wyatt JR, Faltynek CR, Jarvis MF, Lynch K. Analgesic profile of intrathecal P2X(3) antisense oligonucleotide treatment in chronic inflammatory and neuropathic pain states in rats. Pain 2002; 99: 11–19. [DOI] [PubMed] [Google Scholar]
- 23.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988; 33: 87–107. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson J, Bongenhielm U, Kidd E, Matthews B, Fried K. Distribution of P2X3 receptors in the rat trigeminal ganglion after inferior alveolar nerve injury. Neurosci Lett 1998; 254: 37–40. [DOI] [PubMed] [Google Scholar]
- 25.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 2000; 87: 149–158. [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992; 50: 355–363. [DOI] [PubMed] [Google Scholar]
- 27.Chung JM, Kim HK, Chung K. Segmental spinal nerve ligation model of neuropathic pain. Methods Mol Med 2004; 99: 35–45. [DOI] [PubMed] [Google Scholar]
- 28.Novakovic SD, Kassotakis LC, Oglesby IB, Smith JA, Eglen RM, Ford AP, Hunter JC. Immunocytochemical localization of P2X3 purinoceptors in sensory neurons in naive rats and following neuropathic injury. Pain 1999; 80: 273–282. [DOI] [PubMed] [Google Scholar]
- 29.Tsuzuki K, Kondo E, Fukuoka T, Yi D, Tsujino H, Sakagami M, Noguchi K. Differential regulation of P2X(3) mRNA expression by peripheral nerve injury in intact and injured neurons in the rat sensory ganglia. Pain 2001; 91: 351–360. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Li GW, Wang C, Gu Y, Huang LY. Mechanisms underlying enhanced P2X receptor-mediated responses in the neuropathic pain state. Pain 2005; 119: 38–48. [DOI] [PubMed] [Google Scholar]
- 31.Kage K, Niforatos W, Zhu CZ, Lynch KJ, Honore P, Jarvis MF. Alteration of dorsal root ganglion P2X3 receptor expression and function following spinal nerve ligation in the rat. Exp Brain Res 2002; 147: 511–519. [DOI] [PubMed] [Google Scholar]
- 32.Kim C, Chung JM, Chung K. Changes in the gene expression of six subtypes of P2X receptors in rat dorsal root ganglion after spinal nerve ligation. Neurosci Lett 2003; 337: 81–84. [DOI] [PubMed] [Google Scholar]
- 33.Tu WZ, Cheng RD, Cheng B, Lu J, Cao F, Lin HY, Jiang YX, Wang JZ, Chen H, Jiang SH. Analgesic effect of electroacupuncture on chronic neuropathic pain mediated by P2X3 receptors in rat dorsal root ganglion neurons. Neurochem Int 2012; 60: 379–386. [DOI] [PubMed] [Google Scholar]
- 34.Cheng RD, Tu WZ, Wang WS, Zou EM, Cao F, Cheng B, Wang JZ, Jiang YX, Jiang SH. Effect of electroacupuncture on the pathomorphology of the sciatic nerve and the sensitization of P2X(3) receptors in the dorsal root ganglion in rats with chronic constrictive injury. Chin J Integr Med 2013; 19: 374–379. [DOI] [PubMed] [Google Scholar]
- 35.Yu J, Zhao C, Luo X. The effects of electroacupuncture on the extracellular signal-regulated kinase 1/2/P2X3 signal pathway in the spinal cord of rats with chronic constriction injury. Anesth Analg 2013; 116: 239–246. [DOI] [PubMed] [Google Scholar]
- 36.Wang WS, Tu WZ, Cheng RD, He R, Ruan LH, Zhang L, Gong YS, Fan XF, Hu J, Cheng B, Lai YP, Zou EM, Jiang SH. Electroacupuncture and A-317491 depress the transmission of pain on primary afferent mediated by the P2X3 receptor in rats with chronic neuropathic pain states. J Neurosci Res 2014; 92: 1703–1713. [DOI] [PubMed] [Google Scholar]
- 37.Weng Z, Wu L, Lu Y, Wang L, Tan L, Dong M, Xin Y. Electroacupuncture diminishes P2X2 and P2X3 purinergic receptor expression in dorsal root ganglia of rats with visceral hypersensitivity. Neural Regen Res 2013; 8: 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weng ZJ, Wu LY, Zhou CL, Dou CZ, Shi Y, Liu HR, Wu HG. Effect of electroacupuncture on P2X3 receptor regulation in the peripheral and central nervous systems of rats with visceral pain caused by irritable bowel syndrome. Purinergic Signal 2015; 11: 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng J, Cui LY, Feng Y, Ding MX. Electroacupuncture relieves neuropathic pain via upregulation of glutamate transporters in the spinal cord of rats. Neurosci Lett 2016; 620: 38–42. [DOI] [PubMed] [Google Scholar]
- 40.Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci 2000; 15: 170–182. [DOI] [PubMed] [Google Scholar]
- 41.Han JS. Acupuncture and endorphins. Neurosci Lett 2004; 361: 258–261. [DOI] [PubMed] [Google Scholar]
- 42.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 43.Wang C, Gw L, Huang LY. Prostaglandin E2 potentiation of P2X3 receptor mediated currents in dorsal root ganglion neurons. Mol Pain 2007; 3: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu Y, Li G, Chen Y, Huang LY. Epac-protein kinase C alpha signaling in purinergic P2X3R-mediated hyperalgesia after inflammation. Pain 2016; 157: 1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gu Y, Wang C, Li G, Huang LY. EXPRESS: F-actin links Epac-PKC signaling to purinergic P2X3 receptors sensitization in dorsal root ganglia following inflammation. Mol Pain. Epub ahead of print 5 July 2016. DOI: 10.1177/1744806916660557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukuoka T, Tokunaga A, Tachibana T, Dai Y, Yamanaka H, Noguchi K. VR1, but not P2X(3), increases in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain 2002; 99: 111–120. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Differential expression patterns of mRNAs for P2X receptor subunits in neurochemically characterized dorsal root ganglion neurons in the rat. J Comp Neurol 2005; 481: 377–390. [DOI] [PubMed] [Google Scholar]
- 48.Jiang YL, Yin XH, Shen YF, He XF, Fang JQ. Low frequency electroacupuncture alleviated spinal nerve ligation induced mechanical allodynia by inhibiting TRPV1 upregulation in ipsilateral undamaged dorsal root ganglia in rats. Evid Based Complement Alternat Med 2013; 2013: 170910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xue C, Xie L, Li X, Cai J, Gu Z, Wang K. Analgesic mechanism of electroacupuncture in a rat L5 spinal nerve ligation model. Exp Ther Med 2015; 9: 987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang Y, Du JY, Qiu YJ, Fang JF, Liu J, Fang JQ. Electroacupuncture attenuates spinal nerve ligation-induced microglial activation mediated by p38 mitogen-activated protein kinase. Chin J Integr Med 2016; 22: 704–713. [DOI] [PubMed] [Google Scholar]