Short abstract
Background
Lipoic acid, an antioxidant, has beneficial effects in experimental acute optic neuritis and autoimmune encephalomyelitis. Optical coherence tomography can detect retinal nerve fiber layer thinning, representing axonal degeneration, approximately 3–6 months after acute optic neuritis.
Objective
To determine whether lipoic acid is neuroprotective in acute optic neuritis.
Methods
A single-center, double-blind, randomized, placebo controlled, 24-week trial. Intervention included 6 weeks of once daily lipoic acid (1200 mg) or placebo within 14 days of acute optic neuritis diagnosis. The primary outcome was the mean difference in affected eye retinal nerve fiber layer (RNFL) thickness from baseline to 24 weeks.
Results
We enrolled 31 subjects (placebo n=16; lipoic acid n=15; average age 38.6 years (standard deviation (SD) 10.3)). Affected eye mean global RNFL thickness (µm) in the lipoic acid group decreased from 108.47 (SD 26.11) at baseline to 79.31 (SD 19.26) at 24 weeks. The affected eye RNFL in the placebo group decreased from 103.67 (SD 18.04) at baseline to 84.43 (SD 20.94) at 24 weeks. Unaffected eye RNFL thickness did not significantly change in either group over 24 weeks.
Conclusion
Six weeks of oral lipoic acid supplementation after acute optic neuritis is safe and well tolerated; however, because of insufficient recruitment, we could not conclude that lipoic acid treatment was neuroprotective in acute optic neuritis.
Keywords: Multiple sclerosis, lipoic acid, optic neuritis, intervention
Introduction
Acute optic neuritis (AON) is the most common optic neuropathy in young adults.1 AON affects up to 70% of people with multiple sclerosis (MS) over the course of their disease, either as the first demyelinating event or as a relapse.2 While corticosteroids accelerate AON recovery, neuroprotective therapies for AON are lacking.3–9 Approximately 3–6 months after AON, axonal degeneration of the retinal nerve ganglion cells can be seen as thinning of the retinal nerve fiber layer (RNFL) on optical coherence tomography (OCT).10
Lipoic acid (LA) is a neuroprotective antioxidant in an animal model of AON, experimental acute optic neuritis (EAON). The administration of oral LA in EAON reduces immune cell infiltration and axonal damage, and prevents motor disability when compared with mice treated with vehicle.11 In a recent pilot clinical trial, subjects with secondary progressive MS taking LA demonstrated a 68% reduction in annualized percentage brain volume change, suggesting a neuroprotective effect. LA was also noted to be safe and well tolerated over the 2-year trial period.12 We conducted a single center, double-blind, randomized, placebo controlled, pilot study to explore the tolerability and potential benefits of oral LA treatment for 6 weeks on visual outcomes in subjects with AON.
Methods
Design
This study was a single center, double-blind, randomized, placebo controlled, 24-week long pilot trial to determine the effects of oral LA on visual outcomes in AON. Within 14 days of AON diagnosis, we randomly assigned subjects to receive LA (1200 mg orally once a day) or placebo. Subjects participated in the 6-week treatment protocol, followed by an 18-week observation period. The primary outcome measure included the difference in affected eye RNFL thickness from baseline to 24 weeks post-enrollment. The secondary outcomes included the difference in affected eye RNFL thickness from baseline to 12 weeks post-enrollment, changes from baseline to 12 and 24 weeks in low and high-contrast visual acuity, contrast sensitivity, and visual fields. The Oregon Health and Science University (OHSU) institutional review board approved the study protocol. We obtained written informed consent from all study participants and registered the study at www.clinicaltrials.gov (NCT01294176).
Participants
We recruited subjects from the OHSU ophthalmology clinics and through national advertisements by the National MS Society. We included subjects between 18 and 65 years of age with a diagnosis of unilateral AON with visual symptoms (vision loss) for 14 days or less.2,13 Subjects had no previous history of optic neuritis or optic disc pallor at baseline in the affected eye. Subjects were determined to have a normal brain magnetic resonance imaging (MRI) scan or an abnormal brain MRI scan. We defined an abnormal MRI scan as having demyelinating brain lesions consistent with MS per the 2010 McDonald’s criteria. Subjects also had to be available for treatment initiation within 14 days of AON diagnosis,2,13 and were allowed to receive corticosteroids (oral or intravenously) prior to study drug initiation.
We excluded subjects with other causes of visual loss in the affected eye (e.g. amblyopia or glaucoma) and acute papillitis, as OCT was non-evaluable at the screening visit due to edema. In addition, we excluded subjects: (a) whose AON symptoms resolved prior to administration of the study medication; (b) who had fever or active infection at time of enrollment; (c) who were pregnant or breast feeding; or (d) who had diabetes mellitus or another significant health problem (e.g. active coronary heart disease, liver disease, significant pulmonary disease).
Procedure
Within 14 days of AON diagnosis, subjects returned to OHSU to receive their 6-week supply of LA or placebo. We collected data on the subjects’ clinical outcomes including visual acuity, contrast sensitivity, color vision, visual fields, and Expanded Disability Status Scale (EDSS). Subjects performed the timed 25-foot walk, the 9-hole peg test, and OCT imaging of both affected and unaffected eyes. At this visit, we provided detailed instructions to subjects regarding how to take the capsules. We called subjects one week after study drug initiation to find out if they had any questions. Two weeks after treatment initiation, subjects returned to determine their tolerability and monitor for side effects. At this visit, the study coordinator measured compliance by performing an accountability assessment of the remaining 4-week supply of the study drug. To ensure safety, we drew 20 mL of blood for a complete blood count with a differential and complete metabolic panel, including a liver function test. We also performed a urine dipstick for urinalysis and pregnancy test. At the end of the 6-week treatment period, visit 3, we performed the same blood safety labs and urine tests. At 12 weeks and 24 weeks after visit 1, subjects returned to OHSU for follow-up assessments as in visit 1.
Outcomes
Validated questionnaires or objective measures assessed study objectives. Descriptions of each outcome are listed below.
Optical coherence tomography
A commercially available optical coherence tomographer (Heidelberg Engineering Spectralis OCT, version 1.6.4.0, Heidelberg Engineering, Vista, CA, USA) obtained all quantitative measurements. The fast RNFL thickness scan acquisition performed RNFL analysis. This protocol acquires and compresses three 3.4 mm diameter circular scans centered on the optic disc into one scan. Each scan consists of 256 axial scans. We performed pharmacological pupillary dilation with 1% tropicamide in all subjects, and used an internal fixation. An expert operator took repeated measurements from the photography department at the OHSU Casey Eye Institute until three measurements were judged to be of good quality. We calculated the mean value. The average RNFL thickness (averaged for peripapillary retina 360° around the optic disc) and thickness values for each of the four quadrants (temporal, superior, nasal, inferior) were recorded from the OCT printouts.
Contrast sensitivity
We used the Pelli–Robson chart to test contrast sensitivity for each separate eye. This chart consists of 16 groups of three uppercase letters, and it captures the minimum contrast level at which patients can perceive letters of a single large size (1 m).
Visual acuity
We used the Snellen acuity chart to evaluate visual acuity for each eye, separately. We recorded the best-corrected visual acuity for each eye.
Visual fields
We performed the Humphrey field analyzer (Swedish Interactive Threshold Algorithm 24-2 threshold program) to assess visual fields. We then recorded the mean deviation in decibels for each eye.
Color vision
AO H–R–R (Hardy–Rand–Ritter) pseudo-isochromatic plates evaluated color vision by a common, comprehensive and rapid test of color vision.
Expanded Disability Status Scale
The EDSS14 is a standardized scale for assessing overall neurological impairment. It is based on seven functional status scales (mental status, vision, brainstem, pyramidal, cerebellar, sensory, and bladder and bowel) and on the patient’s ambulatory status.
Timed 25-foot walk (a measure of ambulation) and 9-hole peg test (a measure of upper extremity function)
These two tests15 are common objective measures of disability in MS studies to evaluate the treatment effect on disability.
Sample size and statistical analysis
Sample size
Power analysis indicated that 27 subjects in each arm (before 20% dropout) were needed to detect a 23.2 micrometer difference in the 24-week change in RNFL between the two groups with a power of 80% and an alpha of 0.05.
Statistical analysis
We used linear mixed models to determine whether subjects with AON who received LA have less permanent optic nerve injury than the placebo. Models accounted for the multiple measures over time with random intercepts. We performed the same analysis for all outcomes: global RNFL thickness, temporal superior quadrant, temporal quadrant, temporal inferior quadrant, nasal inferior quadrant, and nasal quadrant. Model covariates included group, time, MS diagnosis (yes/no), and MRI results (normal MRI vs. abnormal MRI). We used SAS version 9.4 (Cary, NC, USA) to perform all analysis.
Results
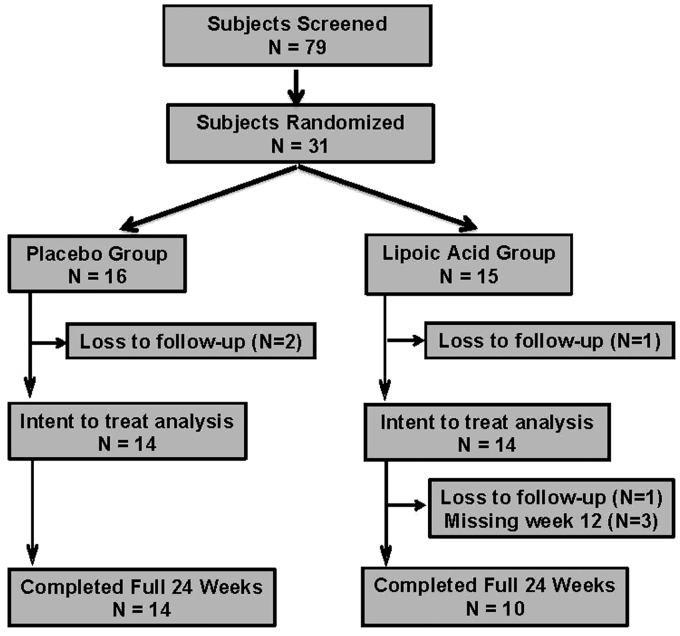
We enrolled subjects beginning in November 2011 and the last subject completed the study in November 2016. We did not achieve enrollment goals because of difficulty with subject recruitment. We recruited and randomly assiged 31 subjects, 16 to the placebo and 15 to the LA group, respectively. Four subjects were lost to follow-up (placebo n = 2; LA n = 2). The remaining subjects completed the study (although three subjects missed the week 12 assessment in the LA group). We describe the subject disposition details in Figure 1.
Figure 1.
Subject disposition.
Table 1 describes the comparable baseline characteristics of subjects in the two groups. An abnormal MRI scan was present in 69% of the placebo and 73% of the LA group subjects. At baseline, 50% of the placebo and 53% of the LA group subjects had confirmed MS diagnosis. Forty-four percent of the placebo group and 40% of the LA group subjects received steroid treatment (3 days of intravenous methylprednisolone at a dose of 1 g daily) after AON diagnosis.
Table 1.
Baseline demographics of study participants.
| Demographic characteristics (mean ± SD & N (%)) |
Placebo (n = 16) |
Lipoic acid (n = 15) |
|---|---|---|
| Age (years) | 36.1 ± 9.84 | 41.2 ± 10.51 |
| Gender | ||
| Female, % | 12 (75%) | 8 (53.3%) |
| Male, % | 4 (25%) | 7 (46.7%) |
| EDSS disability score | 2.59 ± 0.90 | 2.57 ± 1.02 |
| Abnormal MRI | 11 (68.75%) | 11 (73.33%) |
| MS diagnosis | 8 (50%) | 8 (53.33%) |
| Steroid treatment | 7 (44%) | 6 (40%) |
MS: multiple sclerosis; SD: standard deviation; EDSS: Expanded Disability Status Scale; MRI: magnetic resonance imaging.
Global thickness
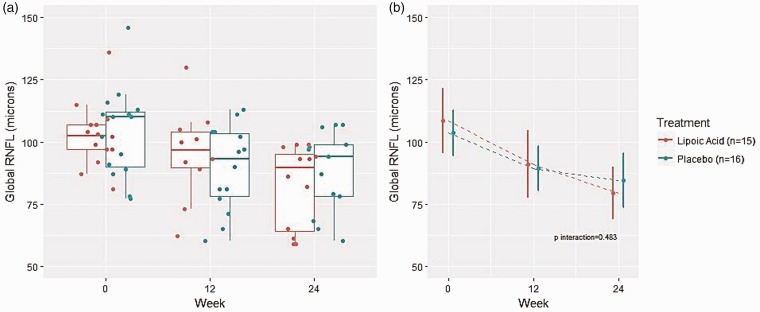
The mean global RNFL thickness (µm) in the placebo group affected eye was 103.67 at baseline (standard deviation (SD) 18.04), 89.43 at 12 weeks (SD 17.1), and 84.43 at 24 weeks (SD 20.94), respectively. The mean global RNFL thickness (µm) in the placebo group unaffected eye was 96.93 at baseline (SD 11.9), 96.00 at 12 weeks (SD 15.21), and 94.57 at 24 weeks (SD 16.37), respectively. The mean global RNFL thickness (µm) of the LA group affected eye was 108.47 at baseline (SD 26.11), 90.91 at 12 weeks (SD 23.02), and 79.31 at 24 weeks, respectively. The mean global RNFL thickness (µm) of the LA group unaffected eye was 96.40 (SD 8.3) at baseline, 96.27 at 12 weeks (SD 8.83), and 96.85 at 24 weeks (SD 9.30), respectively (Table 2, Figure 2).
Table 2.
Global RNFL thickness change between baseline, week 12, and week 24.
| Affected eye |
Unaffected eye |
||||
|---|---|---|---|---|---|
| Placebo | LA | Placebo | LA | ||
| Cross-sectional RNFL (µm) (mean ± SD) (95% CI) |
Baseline | 103.67 ± 18.04 | 108.47 ± 26.11 | 96.93 ± 11.90 | 96.40 ± 8.30 |
| (94.54, 112.80) | (95.25, 121.68) | (90.91, 102.95) | (92.20, 100.60) | ||
| 12 Weeks | 89.43 ± 17.10 | 90.91 ± 23.02 | 96.00 ± 15.21 | 96.27 ± 8.83 | |
| (80.47, 98.39) | (77.31, 104.51) | (88.03, 103.97) | (91.05, 101.49) | ||
| 24 Weeks | 84.43 ± 20.94 | 79.31 ± 19.26 | 94.57 ± 16.37 | 96.85 ± 9.30 | |
| (73.46, 95.40) | (68.84, 89.78) | (85.99, 103.15) | (91.79, 101.90) | ||
| Longitudinal change (µm) (mean ± SD) (95% CI) |
12 Week change (BL to W12) |
–13.77 ± 15.43 | –13.64 ± 29.20 | 1.00 ± 3.03 | 0.73 ± 3.44 |
| (–22.16, –5.38) | (–30.89, 3.62) | (–0.65, 2.65) | (–1.30, 2.76) | ||
| 12 Week change (W12 to W24) |
–5.00 ± 6.06 | –8.60 ± 10.78 | –1.43 ± 4.40 | –0.50 ± 1.27 | |
| (–8.18, –1.82) | (–15.28, –1.92) | (–3.73, 0.88) | (–1.29, 0.29) | ||
| 24 Week change (BL to W24) |
–18.77 ± 18.88 | –31.31 ± 38.30 | 0.00 ± 2.12 | 0.15 ± 2.48 | |
| (–29.03, –8.50) | (–52.13, –10.49) | (–1.15, 1.15) | (–1.19, 1.50) | ||
RNFL: retinal nerve fiber layer; LA: lipoic acid; SD: standard deviation; CI: confidence interval; BL: baseline.
Figure 2.
Changes in affected and unaffected eye global retinal nerve fiber layer thickness over time.
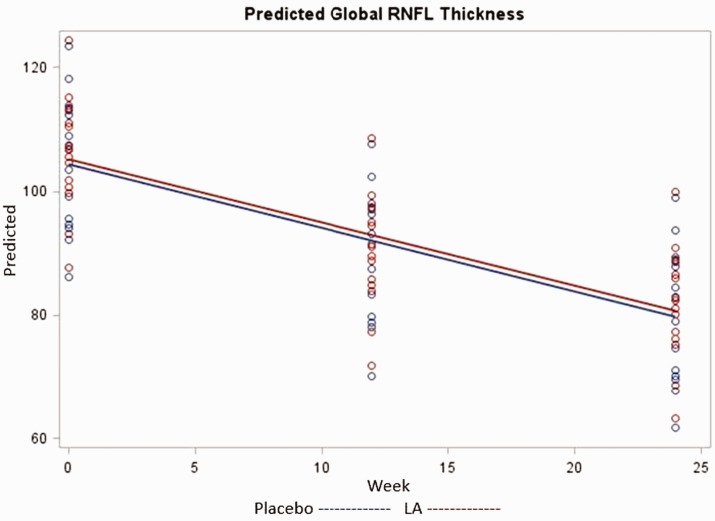
We first tested for a three-way interaction of group, week, and MS diagnosis, which was not significant (χ2 = 0.80, P = 0.67). In three subsequent models, we tested for interactions of week by group (χ2 = 1.42, P = 0.50), week by MS diagnosis (χ2 = 1.46, P = 0.49), and group by MS (χ2 = 0.36, P = 0.55). As none were significant, we retained only main effects in the final analytical model as shown in Table 3. Global RNFL thickness did not differ by group (χ2 = 0.06, P = 0.80), MS diagnosis (χ2 = 0.55, P = 0.46), or MRI result (χ2 = 1.88, P = 0.18). Global RNFL thickness decreased significantly over time (χ2 = 29.8, P < 0.001); the difference between thickness at baseline and 12 weeks was 15.88 µm (χ2 = 11.6, P = 0.001), and the difference between baseline and 24 weeks was 24.46 µm (χ2 = 28.9, P ≤ 0.0001). Post-hoc assessment found the thinning of global RNFL adequately powered at both 12 weeks and 24 weeks at greater than 99% achieved power. The remaining covariates were observed to have minimal power to detect significant effect with none reaching more than 45% power. Predicted values of global RNFL thickness over time by group are given in Figure 3. There was no change in visual acuity.
Table 3.
Results from linear mixed model for global RNFL thickness.
| Outcome global RNFL thickness | ||||
|---|---|---|---|---|
| Main effects model | ||||
| Variable | β | SE | P value | |
| Group | 1 | –1.47 | 5.87 | 0.80 |
| 2 | Ref | |||
| Time | 0 | Ref | ||
| 12 | –15.88 | 4.67 | 0.001 | |
| 24 | –24.46 | 4.55 | <0.0001 | |
| MS | MS diagnosis | 4.69 | 6.32 | 0.46 |
| No MS | Ref | |||
| MRI | Abnormal MRI | –9.56 | 6.97 | 0.18 |
| Normal MRI | Ref | |||
RNFL: retinal nerve fiber layer; MS: multiple sclerosis; MRI: magnetic resonance imaging.
Figure 3.
Predicted global retinal nerve fiber layer thickness.
Additional outcomes
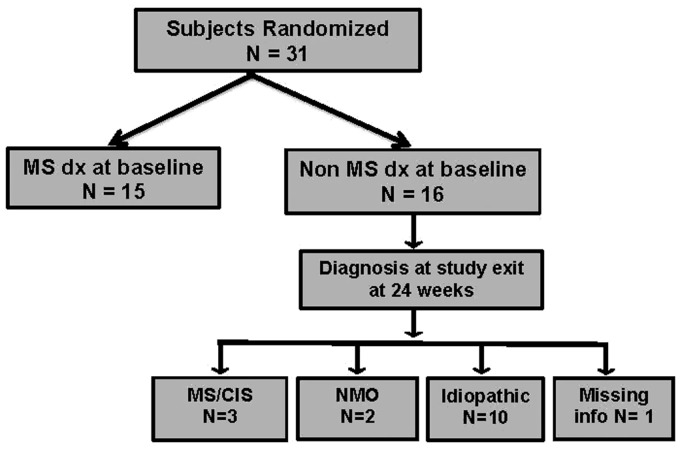
The same analysis was performed with all outcomes measured, including low and high-contrast visual acuity, contrast sensitivity, and visual fields. There were no interactions and no differences by group, MS diagnosis, or MRI result. All outcomes significantly decreased over time. At the 24-week visit, we reviewed the diagnosis of subjects determined to have non-MS AON at baseline (Figure 4). Out of the 16 subjects with non-MS AON, two subjects were diagnosed with neuromyeltitis optica (NMO) and 10 subjects were still categorized as idiopathic AON at week 24.
Figure 4.
Diagnosis: outcome of study subjects as of month 6 visit.
Safety
LA was safe and generally well tolerated. See Table 4 for a list of adverse events.
Table 4.
Adverse events.
| Placebo (n = 16) | Lipoic acid (n = 15) |
|---|---|
| Neurological (n = 7) | Neurological (n = 6) |
| Headache | Headache |
| Leg weakness | Foggy thinking |
| Tingling in toes and fingers | Fall, head injury |
| Intermittent bilateral toe numbness | Numbness/weakness bilateral hands |
| MS relapse (moderate) | Right hand numbness and tingling |
| Bilateral leg weakness (moderate) | Left leg periodic numbness and tingling |
| Recurrent AON due to NMO (moderate) | |
| Musculoskeletal (n = 4) | Musculoskeletal (n = 2) |
| Sciatica | Whiplash/ neck pain |
| Lower back pain | Back pain secondary to injury |
| Degenerative disc disease | |
| Significant lower back pain (moderate) | |
| Infection (n = 3) | Infection (n = 1) |
| Laryngitis | Pneumonia (moderate) |
| Yeast infection | |
| Possible pharyngitis (moderate) | |
| Skin (n = 2) | Skin (n = 1) |
| Basal cell carcinoma removal | Disseminated maculopapulary rash |
| Injection site reactions from copaxone | |
| Other (n = 2) | Other (n = 5) |
| Seasonal allergies | Odor in urine/feces |
| Vaginal bleeding | Upset stomach due to tecfidera |
| Bilateral eye pain | Left maxillary pain |
| Chest pain | |
| All mild severity unless stated | |
AON: acute optic neuritis; NMO: neuromyeltitis optica.
Discussion
In this prospective, randomized, placebo controlled clinical trial, 6 weeks of 1200 mg once daily oral LA, while safe, showed no effect on global RNFL thickness between baseline and 24 weeks in the affected eye after AON. The study, unfortunately, did not meet its enrollment goal and was therefore underpowered with no hint of effect. We, therefore, cannot conclude that 6 weeks of LA treatment is effective or ineffective as a neuroprotective agent in AON.
Pre-clinical studies in EAON show a significant reduction in axonal injury with LA treatment, but our study was unable to show similar outcomes in human subjects with AON.11 This observed dissonance between treatment efficacy in animal models and human subjects continues to be an intriguing challenge in neuroimmunology, and could be secondary to subtle differences in pathology. Moreover, neuroprotective agents in the brain may not have the same effect in the optic nerve. Limited clinical trial data suggest that LA may slow brain atrophy in progressive MS,12 but in this study RNFL did not correlate with whole-brain atrophy and did not change significantly with LA treatment.12,16 Many pharmacological agents including high-dose steroids,13 erythropoietin,17 phenytoin18 and opinicinumab19 failed to impact visual or clinical outcomes after AON in controlled clinical trials. These collective negative results in AON may suggest a differential capacity of the neuroprotective potential of the brain and optic nerve.
Our study has several limitations. Notably, we failed to reach our enrollment target, and the study was therefore underpowered with no hint of effect. There were several challenges in study recruitment including exclusion of subjects treated with steroids (earlier in the trial) and exclusion of patients with optic nerve edema. During the study design, we hypothesized that 6 weeks of LA treatment would reduce inflammation and promote neuroprotection in AON. This treatment duration was chosen to mirror the 4–6 week period of acute lesional inflammation in MS and AON. In addition, animal models of AON showed LA was neuroprotective as remotely as 26 days post-immunization.11,20,21 It is possible that a 6-week treatment duration was insufficient. The study results, unfortunately, were not sufficiently powered to prove or disprove our hypothesis. In addition, it is plausible that our treatment initiation window of 14 days after AON diagnosis missed the narrow therapeutic window to ameliorate optic nerve damage. Experts suggest that within 2 weeks of AON onset, the optic nerve axons undergo irreversible damage, which leads to poor visual outcomes.1 Out of the 16 subjects diagnosed with non-MS AON at baseline, two subjects were diagnosed with NMO and 10 subjects had idiopathic AON diagnosis. It is also plausible that heterogeneity of the study subjects impacted the treatment effect of LA for AON; however, the presence or absence of NMO subjects in the analysis did not change the study outcomes. The inclusion of AON subjects up to the age of 65 years is also a potential limitation. AON is less likely in patients over the age of 50 years, and other inflammatory and ischemic insults to the optic nerve are more common with advancing age. However, only five participants were over the age of 50 years, and four of these subjects had an abnormal MRI scan consistent with demyelinating brain lesions. Therefore, with our careful screening of AON subjects and supportive evidence of demyelination on imaging, potential mimics are unlikely. Finally, it is possible that a longer trial period and the inclusion of ganglion cell loss analysis could have reduced the potential confounding effects of edema.22 Future studies could consider adjusting the trial duration or including ganglion cell loss as an outcome measure to overcome this limitation.
In summary, this study shows that most of the RNFL loss after AON occurs within the first 12 weeks, and that 6 weeks of LA treatment did not change the course of AON. While LA did not appear to modify optic nerve atrophy in this underpowered trial, this study adds to our understanding of AON evolution over time. It is possible that a longer treatment duration is needed to achieve neuroprotection with LA in AON, or that the therapeutic window for LA is shorter than 14 days post-diagnosis. This study suggests that while 6 weeks of oral LA is safe, more research is needed to elucidate the efficacy of LA in AON.
Contributor Information
Julie Falardeau, Oregon Health and Science University, USA.
Allison Fryman, Department of Veterans Affairs, MS Center of Excellence – West, USA.
Rohan Wanchu, Oregon Health and Science University, USA.
Gail H Marracci, Oregon Health and Science University, USA; Department of Veterans Affairs, MS Center of Excellence – West, USA.
Michele Mass, Oregon Health and Science University, USA.
Charles F Murchison, Department of Biostatistics, University of Alabama at Birmingham, USA.
William L Hills, Oregon Health and Science University, USA.
Conflicts of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received funding from the National Multiple Sclerosis Society Foundation, Race to Erase MS Foundation, Study drug donation – Pure Encapsulations.
References
- 1.Toosy AT, Mason DF, Miller DH. Optic neuritis. Lancet Neurol 2014; 13: 83–99. [DOI] [PubMed] [Google Scholar]
- 2.Beck RW, Cleary PA. Optic neuritis treatment trial. One-year follow-up results. Arch Ophthalmol 1993; 111: 773–775. [DOI] [PubMed] [Google Scholar]
- 3.Kapoor R, Miller DH, Jones SJ, et al. Effects of intravenous methylprednisolone on outcome in MRI-based prognostic subgroups in acute optic neuritis. Neurology 1998; 50: 230–237. [DOI] [PubMed] [Google Scholar]
- 4.Sellebjerg F, Nielsen HS, Frederiksen JL, et al. A randomized, controlled trial of oral high-dose methylprednisolone in acute optic neuritis. Neurology 1999; 52: 1479–1484. [DOI] [PubMed] [Google Scholar]
- 5.Wakakura M, Mashimo K, Oono S, et al. Multicenter clinical trial for evaluating methylprednisolone pulse treatment of idiopathic optic neuritis in Japan. Optic Neuritis Treatment Trial Multicenter Cooperative Research Group (ONMRG). Jpn J Ophthalmol 1999; 43: 133–138. [DOI] [PubMed] [Google Scholar]
- 6.Katz B. The Tubingen Study on Optic Neuritis Treatment – a prospective, randomized and controlled trial. Surv Ophthalmol 1994; 39: 262–263. [DOI] [PubMed] [Google Scholar]
- 7.Rawson MD, Liversedge LA. Treatment of retrobulbar neuritis with corticotrophin. Lancet 1969; 2: 222. [DOI] [PubMed] [Google Scholar]
- 8.Rawson MD, Liversedge LA, Goldfarb G. Treatment of acute retrobulbar neuritis with corticotrophin. Lancet 1966; 2: 1044–1046. [DOI] [PubMed] [Google Scholar]
- 9.Bowden AN, Bowden PM, Friedmann AI, et al. A trial of corticotrophin gelatin injection in acute optic neuritis. J Neurol Neurosurg Psychiatry 1974; 37: 869–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costello F, Coupland S, Hodge W, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol 2006; 59: 963–969. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhary P, Marracci G, Yu X, et al. Lipoic acid decreases inflammation and confers neuroprotection in experimental autoimmune optic neuritis. J Neuroimmunol 2011; 233: 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spain R, Powers K, Murchison C, et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm 2017; 4: e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck RW, Cleary PA, Anderson MM, Jr, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med 1992; 326: 581–588. [DOI] [PubMed] [Google Scholar]
- 14.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 15.Fischer JS, Rudick RA, Cutter GR, et al. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler 1999; 5: 244–250. [DOI] [PubMed] [Google Scholar]
- 16.Winges KM, Murchison CF, Bourdette DN, et al. Longitudinal optical coherence tomography study of optic atrophy in secondary progressive multiple sclerosis: results from a clinical trial cohort. Mult Scler 2017; 25: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shayegannejad V, Shahzamani S, Dehghani A, et al. A double-blind, placebo-controlled trial of adding erythropoietin to intravenous methylprednisolone for the treatment of unilateral acute optic neuritis of unknown or demyelinative origin. Graefes Arch Clin Exp Ophthalmol 2015; 253: 797–801. [DOI] [PubMed] [Google Scholar]
- 18.Raftopoulos R, Hickman SJ, Toosy A, et al. Phenytoin for neuroprotection in patients with acute optic neuritis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol 2016; 15: 259–269. [DOI] [PubMed] [Google Scholar]
- 19.Cadavid D, Balcer L, Galetta S, et al. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol 2017; 16: 189–199. [DOI] [PubMed] [Google Scholar]
- 20.Hickman SJ, Toosy AT, Miszkiel KA, et al. Visual recovery following acute optic neuritis – a clinical, electrophysiological and magnetic resonance imaging study. J Neurol 2004; 251: 996–1005. [DOI] [PubMed] [Google Scholar]
- 21.Miller DH, Grossman RI, Reingold SC, et al. The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain 1998; 121 (Pt 1): 3–24. [DOI] [PubMed] [Google Scholar]
- 22.Huang-Link YM, Al-Hawasi A, Lindehammar H. Acute optic neuritis: retinal ganglion cell loss precedes retinal nerve fiber thinning. Neurol Sci 2015; 36: 617–620. [DOI] [PubMed] [Google Scholar]