Abstract
Objectives:
Senility death is defined as natural death in the elderly who do not have a cause of death to be described otherwise and, if human life is finite, it may be one of the ultimate goals of medicine and healthcare. A recent survey in Japan reports that municipalities with a high senility death ratio have lower healthcare costs per late-elderly person. However, the causes of regional differences in senility death ratio and their biomedical determinants were unknown. In this study, we examined the relationships of the regional difference in senility death ratio with the regional differences in heart rate variability and physical activity.
Methods:
We compared the age-adjusted senility death ratio of all Japanese prefectures with the regional averages of heart rate variability and actigraphic physical activity obtained from a physiological big data of Allostatic State Mapping by Ambulatory ECG Repository (ALLSTAR).
Results:
The age-adjusted senility death ratio of 47 Japanese prefectures in 2015 ranged from 1.2% to 3.6% in men and from 3.5% to 7.8% in women. We compared these ratios with the age-adjusted indices of heart rate variability in 108,865 men and 136,536 women and of physical activity level in 16,661 men and 21,961 women. Heart rate variability indices and physical activity levels that are known to be associated with low mortality risk were higher in prefectures with higher senility death ratio.
Conclusion:
The regional senility death ratio in Japan may be associated with regional health status as reflected in heart rate variability and physical activity levels.
Keywords: Aging, ECG big data, heart rate variability, senility death, ALLSTAR
Introduction
The statistical information of the Japanese Ministry of Health, Labour and Welfare (Summary of the Age-adjusted Mortality Ratio by Prefecture in 2015)1 shows that senility is the fifth leading cause of death in Japan. The “Death Certificate Entry Manual (2018 edition)”2 published by the ministry defines senility death as “Elderly people who do not have a cause of death to be described otherwise, so-called natural death.” If human life is finite and the objectives of medicine and healthcare are in the eradication and prevention of all diseases, senility death may be one of their ultimate goals.
According to a recent survey by Nikkei newspaper (25 December 2017), Japan,3 the municipality with a high senility death ratio has lower healthcare costs per late-elderly person in Japan. Although they seem to place the high senility death ratio as a positive indicator for the qualities of regional healthcare and health policy, there is no convincing evidence that high senility death ratio is related to the high health status of the community. We have only limited knowledge even about the biomedical characteristics of individuals who are prone to senility death.
In this study, we addressed this issue through the analysis of big data. Using the regional differences in senility death ratio across Japan, we investigated whether regional differences in physiological parameters reflecting health status also have geographic patterns associated with it. We measured the heart rate variability (HRV) and physical activity (PA) levels, whose increases are known to associate with low mortality risk,4–8 from the Allostatic State Mapping by Ambulatory ECG Repository (ALLSTAR) database.9–11 We examined the relationships of the regional difference in senility death ratio with the regional differences in the indices of HRV and PA.
Methods
Death statistics
The statistical data of mortality were obtained from the home page of the Japanese Ministry of Health, Labour and Welfare (Summary of the Age-adjusted Mortality Ratio by Prefecture in 2015).1 We downloaded the figure and table data from the website, from which we used the age-adjusted senility death ratio by gender and prefecture.
ALLSTAR project
The ALLSTAR project was established with the cooperation of SUZUKEN Co., Ltd (Nagoya, Japan), the owner of the data, and researchers from seven universities across Japan. The ALLSTAR project is an inventory survey for a complete enumeration of Holter electrocardiogram (ECG) and triaxial accelerogram that were referred for analysis to an ECG analysis center of SUZUKEN Co., Ltd from medical institutions in Japan. Therefore, all of these Holter ECGs were recorded for certain medical purposes, including the screening and diagnosis of diseases and the evaluation of treatment effects. About 60,000 Holter ECG data per year, which are about 5% of the Holter ECG recorded in Japan, are being collected, and about 430,000 have already been registered so far.
This study was performed according to the protocol that has been approved by the Ethics Review Committee of Nagoya City University Graduate School of Medical Sciences and Nagoya City University Hospital (No. 709). Briefly, the data were anonymized by the ECG analysis centers and provided for this study with accompanying information, including age, sex, and recording date, time, and location (medical facilities’ postal code). Written informed consent was unable to obtain from each subject. Instead, according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects (by the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labour and Welfare, Japan, 22 December 2014), the purpose and information utilized in the ALLSTAR research projects have been public through the homepages of the ECG analysis centers (http://www.suzuken.co.jp/product/holter/detail/) and of the ALLSTAR project (http://www.med.nagoya-cu.ac.jp/mededu.dir/allstar/), in which opportunities to refuse the uses of information are ensured for the research subjects, except for the studies that have already been published.
Of the ALLSTAR data, those met the following inclusion criteria were used for this study: (1) Holter ECG data recorded between November 2007 and March 2014, (2) subjects’ age at the time of measurement was 20 years or older, and (3) basic cardiac rhythm was normal (defined as >80% of all recorded beats were in sinus rhythm).4 Data were excluded if ECG showed (1) atrial fibrillation, (2) artificial pacemaker rhythm, (3) pathological conduction block, or (4) ST-T changes suggesting transient myocardial ischemia. The reason why we chose ALLSTAR data for 2007–2014 to analyze the relationship with senility death ratio in 2015 was that we assumed that the senility death ratio is a result of long-term exposure to the biomedical status reflected in HRV and PA.
Data analysis
All of the 24-h ECG and actigraphic data were obtained with Holter recorders (Cardy series; SUZUKEN Co., Ltd), by which 24 h multi-channel ECG and triaxial acceleration data were measured, digitized at 125 and 31.25 Hz, respectively, and stored in memory. The digitized ECG and acceleration data were sent to ECG analysis center and analyzed with Holter ECG analyzers (Cardy Analyzer 05; SUZUKEN Co., Ltd) by skilled medical technologists. The temporal positions of all R-waves were detected, the rhythm annotations were given to all QRS complexes, and all errors in automated analysis were corrected manually by the technologists. Final ECG diagnosis of individual record was made by cardiologists who have contracted with each center.
Among HRV indices obtained from 24 h ECG, the standard deviation of 24 h normal-to-normal (N-N) R-R intervals (SDNN), deceleration capacity (DC),5 the power of the very-low-frequency (VLF),12 short-term (4–11 beats) scaling exponents α1,6 and amplitude (Acv) of cyclic variation of heart rate (CVHR)7 were used. These indices were calculated by the custom-made software. DC was computed as spontaneous increases within short (four beats) overlapping segments of N-N intervals averaged over 24 h by the method of phase-rectified signal averaging.13 The scaling exponent α1 was calculated as the exponent of linear-regression residual to the scale of observation windows by detrended fluctuation analysis (DFA).14,15 To calculate VLF and Acv, N-N interval time series were interpolated with a step function and resampled equidistantly. VLF was calculated as power between 0.0033 and 0.14 Hz by fast Fourier transformation of the resampled (131,072 points) 24-h time series. CVHR was detected from the resampled (at 2 Hz) time series during the night by the automated algorism of auto-correlated wave detection with adaptive threshold (ACAT).16 The changes in N-N intervals accompanying all episodes of CVHR were signal-averaged and Acv was obtained as the amplitude of signal-averaged response curve.7 In earlier studies, these HRV indices have been used for mortality risk stratification in post-myocardial infarction, end-stage renal failure, and chronic heart failure patients5–7 and in healthy elderly people.8 A decline in these HRV indices is an increased mortality risk, and their increase is associated with an increased probability of survival.4,17
From acceleration data, the body accelerations to left-to-right, caudocranial, and posteroanterior directions were obtained as x, y, and z values, respectively. Subjects were assumed to be in the lying position when the y-value was below a threshold and the ratio of time in lying position during the day was calculated as %lying time. Also, time series of x(t), y(t), and z(t) were resampled at 10 Hz and, after removing direct current component by a high-pass filter, combined into a composite vector value A(t) as
The average of A(t) over 24 h was used as the index of PA level.
Statistical analysis
We used the statistical program package of Statistical Analysis System (SAS Institute, Cary, NC, USA). The relationships between regional differences in senility death ratio and the regional differences in HRV and PA levels were examined in two ways. First, using the generalized linear model (GLM) procedure, the age-adjusted least-square means of HRV indices, PA, and %lying time were calculated for each prefecture and gender. Then, the relationships with age-adjusted senility death ratio of each prefecture were evaluated by scatter plots and linear-regression analyses in each sex. Second, the mean and SD of the age-adjusted senility death ratio among all prefectures were calculated for each gender. Next, the ratio in each prefecture was standardized as (ratio – mean)/SD. Using the standardized values, prefectures were classified into five prefecture groups (PGs), that is, PG1: < –1.5 SD, PG2: –1.5 to −0.5 SD, PG3: –0.5 to +0.5 SD, PG4: +0.5 to +1.5 SD, and PG5: > +1.5 SD. Then, the differences in HRV indices, PA, and %lying time among these PGs were evaluated by analysis of covariance (ANCOVA) using the GLM procedure for adjusting the effect of age. Post hoc multiple comparisons were performed for the differences of PG1, PG2, PG4, and PG5 from PG3 with the Bonferroni method.
For both analyses, the results are presented as least-square means and standard error of the mean adjusted for the effect of age. Statistical significance was determined at a type I error level α = 0.05.
Results
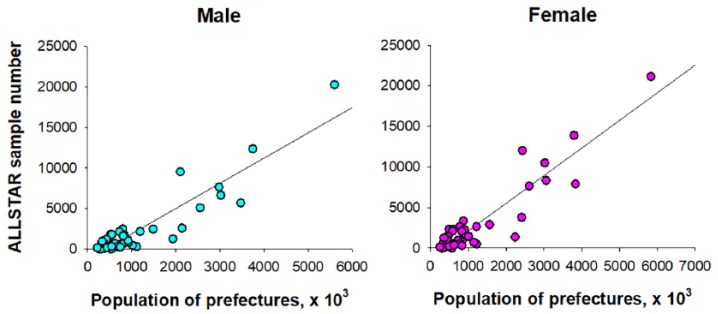
Among the ALLSTAR database, R-R interval data in 108,865 men and 136,536 women who fulfilled all of the inclusion criteria and had none of the exclusion criteria were selected. Among those, PA data were available in 16,661 men and 21,961 women. Figure 1 shows the relationship between the prefecture population (age: ⩾20 years) and the number of samples obtained from each prefecture, which shows that samples were selected in proportion to the prefecture population.
Figure 1.
Relationship between the prefecture population (age: ⩾ 20 years) and the number of samples obtained from each prefecture.
Gray line in each panel is the linear-regression line.
Figure 2 shows the relationships between the age-adjusted senility death ratio and age-adjusted heart rate and HRV indices obtained from each prefecture. For men, while heart rate showed no significant association with the senility death ratio, all HRV indices but DFA α1 showed positive correlations. Although similar trends were observed for women, the correlations reached a significant level only for DFA α1.
Figure 2.
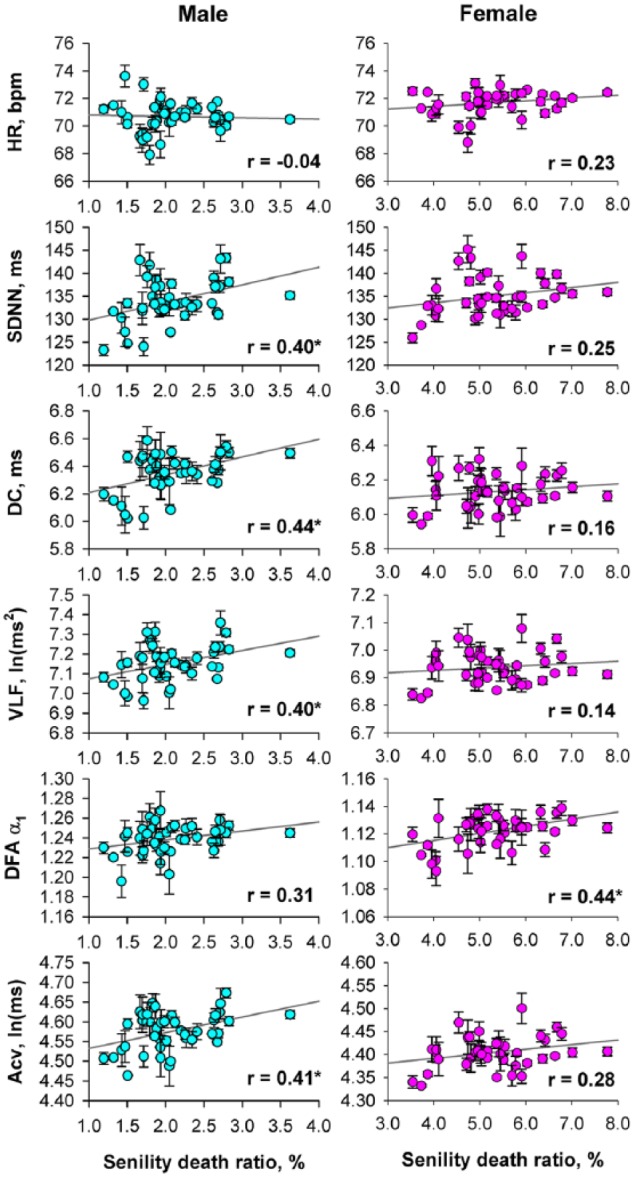
Relationships between age-adjusted senility death ratio and age-adjusted heart rate variability indices of prefectures in Japan.
Data and error bars indicate least-square means adjusted for the effect of age and the standard error of the mean. Gray line in each panel is the linear-regression line and r indicates the correlation coefficient
HR: heart rate; SDNN: 24 h standard deviation of normal-to-normal R-R interval; DC: deceleration capacity; VLF: power of very low frequency component; DFA: detrended fluctuation analysis; Acv: amplitude of cyclic variation of heart rate.
*Significant correlation coefficient.
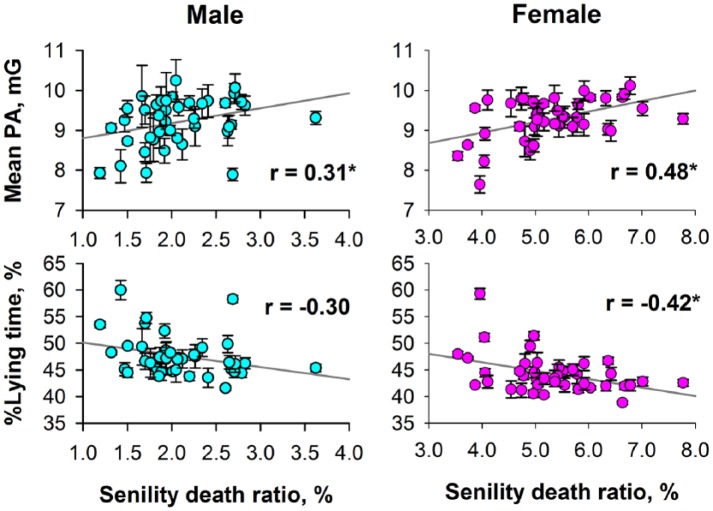
Figure 3 shows the relationships between the age-adjusted senility death ratio and age-adjusted mean PA and %lying time obtained from each prefecture. For women, 24 h mean of PA correlated positively and %lying time correlated negatively with the senility death ratio. Although similar trends were observed also for men, the positive correlation of only 24 h mean PA reached a significant level.
Figure 3.
Relationships between age-adjusted senility death ratio and age-adjusted PA measures of prefectures in Japan.
Data and error bars indicate least-square means adjusted for the effect of age and the standard error of the mean. Gray line in each panel is the linear-regression line and r indicates the correlation coefficient.
PA: physical activity.
*Significant correlation coefficient.
The age-adjusted senility death ratio of 47 prefectures in 2015 ranged from 1.2% to 3.6% (mean ± SD: 2.1% ± 0.5%) in men and from 3.5% to 7.8% (5.3% ± 1.0%) in women. These ratios were standardized, whereby the prefectures were categorized into five PGs. Table 1 shows the number of prefectures classified into each PG and the mean and SD of age-adjusted senility death ratio.
Table 1.
Prefecture groups by the level of age-adjusted senility death ratio.
| Variable | Prefecture group (PG) by age-adjusted senility death ratio |
||||
|---|---|---|---|---|---|
| PG1 |
PG2 |
PG3 |
PG4 |
PG5 |
|
| < –1.5 SD | –1.5 to −0.5 SD | –0.5 to +0.5 SD | +0.5 to +1.5 SD | > +1.5 SD | |
| Male | |||||
| No. of prefectures | 10 | 9 | 16 | 8 | 4 |
| Age-adjusted senility death ratio, % | 1.43 ± 0.17 | 1.79 ± 0.07 | 2.12 ± 0.17 | 2.65 ± 0.07 | 3.00 ± 0.42 |
| Female | |||||
| No. of prefectures | 7 | 9 | 17 | 10 | 4 |
| Age-adjusted senility death ratio, % | 3.86 ± 0.18 | 4.55 ± 0.32 | 5.27 ± 0.26 | 6.25 ± 0.31 | 7.06 ± 0.50 |
For both men and women, ANCOVA adjusted for the effect of age revealed that heart rate and all HRV indices significantly differed among five PGs (Table 2). Multiple comparisons revealed that, in the range from PG2 to PG5, the higher the senility death ratio, the higher the SDNN, DC, VLF, and Acv, for both men and women.
Table 2.
Indices of heart rate variability in prefectures grouped by the level of age-adjusted senility death ratio.
| Variable | Prefecture group (PG) by age-adjusted senility death ratio |
F-value | p-value | ||||
|---|---|---|---|---|---|---|---|
| PG1 |
PG2 |
PG3 |
PG4 |
PG5 |
|||
| < –1.5 SD | –1.5 to −0.5 SD | –0.5 to +0.5 SD | +0.5 to +1.5 SD | > +1.5 SD | |||
| Male | |||||||
| ALLSTAR subjects, N | 9972 | 22,183 | 45,980 | 24,379 | 6351 | ||
| Age (SD), years | 61 (20) | 67 (15) | 61 (19) | 61 (19) | 63 (18) | 456.48 | < 0.0001 |
| HR, bpm | 71.2 ± 0.11 | 70.7 ± 0.07* | 71.3 ± 0.05 | 71.4 ± 0.07 | 70.4 ± 0.13* | 23.74 | < 0.0001 |
| SDNN, ms | 131.6 ± 0.42 | 125.8 ± 0.29* | 130.8 ± 0.20 | 133.3 ± 0.29* | 138.4 ± 0.53* | 147.01 | < 0.0001 |
| DC, ms | 6.24 ± 0.018 | 6.07 ± 0.012* | 6.24 ± 0.008 | 6.31 ± 0.012* | 6.51 ± 0.022* | 98.28 | < 0.0001 |
| VLF, ln(ms)2 | 7.10 ± 0.008 | 7.00 ± 0.006* | 7.09 ± 0.004 | 7.13 ± 0.006* | 7.24 ± 0.011* | 118.93 | < 0.0001 |
| DFA α1 | 1.23 ± 0.002* | 1.23 ± 0.001* | 1.23 ± 0.001 | 1.24 ± 0.001 | 1.25 ± 0.003* | 16.21 | < 0.0001 |
| Acv, ln(ms) | 4.54 ± 0.005 | 4.48 ± 0.004* | 4.54 ± 0.002 | 4.57 ± 0.004* | 4.63 ± 0.007* | 129.01 | < 0.0001 |
| Female | |||||||
| ALLSTAR subjects, N | 5472 | 33,271 | 51,991 | 37,994 | 7808 | ||
| Age (SD), years | 66 (17) | 68 (16) | 64 (18) | 64 (18) | 65 (17) | 301.91 | < 0.0001 |
| HR, bpm | 71.8 ± 0.13 | 71.5 ± 0.05* | 72.1 ± 0.04 | 72.3 ± 0.055* | 72.0 ± 0.11 | 26.61 | < 0.0001 |
| SDNN, ms | 131.5 ± 0.53* | 130.0 ± 0.21* | 133.6 ± 0.17 | 134.4 ± 0.20* | 136.9 ± 0.44* | 85.44 | < 0.0001 |
| DC, ms | 6.13 ± 0.021 | 5.97 ± 0.009* | 6.08 ± 0.007 | 6.12 ± 0.008* | 6.16 ± 0.018* | 54.30 | < 0.0001 |
| VLF, ln(ms)2 | 6.92 ± 0.01 | 6.84 ± 0.004* | 6.9 ± 0.003 | 6.91 ± 0.004* | 6.95 ± 0.009* | 67.65 | < 0.0001 |
| DFA α1 | 1.12 ± 0.003 | 1.11 ± 0.001* | 1.12 ± 0.001 | 1.12 ± 0.001 | 1.13 ± 0.002* | 48.71 | < 0.0001 |
| Acv, ln(ms) | 4.4 ± 0.007 | 4.34 ± 0.003* | 4.38 ± 0.002 | 4.4 ± 0.003* | 4.42 ± 0.006* | 72.69 | < 0.0001 |
ALLSTAR: Allostatic State Mapping by Ambulatory ECG Repository project; HR: heart rate; SDNN: 24 h standard deviation of normal-to-normal R-R interval; DC: deceleration capacity; VLF: power of very low frequency component; DFA: detrended fluctuation analysis; Acv: amplitude of cyclic variation of heart rate.
Age is presented as mean (SD) and other data as least-square means ± standard error of the mean adjusted for the effect of age.
Significantly different from the value for PG3.
Table 3 shows the results of ANCOVA for 24 h mean PA and %lying time. For both men and women, 24 h mean PA and %lying time differed among PGs. Multiple comparisons revealed that 24 h mean PA increases and %lying time decreases with advancing senility death ratio from PG1 to PG5, indicating that the higher the prefectural senility death ratio, the higher the level of PA.
Table 3.
Physical activities in prefectures grouped by the level of age-adjusted senility death ratio.
| Variable | Prefecture group (PG) by age-adjusted senility death ratio |
F-value | p-value | ||||
|---|---|---|---|---|---|---|---|
| PG1 |
PG2 |
PG3 |
PG4 |
PG5 |
|||
| < –1.5 SD | –1.5 to −0.5 SD | –0.5 to +0.5 SD | +0.5 to +1.5 SD | > +1.5 SD | |||
| Male | |||||||
| ALLSTAR subjects, N | 2220 | 2737 | 7711 | 2986 | 1007 | ||
| Mean PA, mG | 8.68 ± 0.073* | 8.78 ± 0.066* | 9.13 ± 0.039 | 9.46 ± 0.069* | 9.50 ± 0.108* | 24.74 | < 0.0001 |
| %Lying time, % | 50.1 ± 0.32* | 49.3 ± 0.29* | 46.3 ± 0.17 | 44.06 ± 0.30* | 45.2 ± 0.48 | 71.9 | < 0.0001 |
| Female | |||||||
| ALLSTAR subjects, N | 1538 | 4653 | 9826 | 4708 | 1236 | ||
| Mean PA, mG | 8.7 ± 0.074* | 8.96 ± 0.042* | 9.31 ± 0.029 | 9.7 ± 0.042* | 9.59 ± 0.082* | 58.77 | < 0.0001 |
| %Lying time, % | 47.2 ± 0.35* | 45.7 ± 0.20* | 42.9 ± 0.14 | 41.3 ± 0.20* | 42.4 ± 0.39 | 93.49 | < 0.0001 |
ALLSTAR: Allostatic State Mapping by Ambulatory ECG Repository project; PA: physical activity.
Significantly different from the value for PG3.
Discussions
Using the ALLSTAR database, we examined the relationship between the regional difference in age-adjusted senility death ratio and the regional difference in HRV indices and PA in Japan. We observed that the HRV indices and PA whose increases are known to associate with decreased mortality risk are higher for the areas with higher senility death ratio. These observations support the hypothesis that the level of regional senility death ratio is associated with good regional health status reflected in HRV and PA.
To our knowledge, this is the first study to investigate the relationships between regional senility death ratio and physiological indicator. In a recent survey conducted by Nikkei newspaper, Japan,3 the relationships between senility death ratio and regional medical expenses were analyzed for ~130 municipalities with a population more than 200,000 in Japan. Their results showed that the medical expenses per capita of late-elderly people were lower as the ratio of late-elderly people who die from increased senility. Although they speculated that the higher proportion of healthy elderly people and the responsiveness of the surrounding medical facilities might be associated with a higher ratio of senility death, they failed to provide convincing evidence for their speculations. In response to this problem, this study has shown that HRV and PA levels are high in geographic regions with high senility death ratio, suggesting a possible association between regional senility death ratio and health status of the region.
This study, however, may have an important limitation. The ALLSTAR database comprised data obtained from people who have visited a healthcare facility and have undergone Holter ECG testing. Thus, all subjects in the database had the health problem or suspicion that triggered Holter ECG examination. Although we included only data with normal sinus rhythm without atrial fibrillation, artificial pacemaker, pathological conduction block, or ST-T changes suggesting transient myocardial ischemia, it was not possible to control the influences of potential health problems on HRV indices and PA. Therefore, it is not appropriate to consider HRV and PA levels obtained from each prefecture as reflecting the health status of the general population of the prefecture.
Nevertheless, we observed the association in the geographic patterns between senility death ratio and HRV and PA levels. In previous studies, we also observed that the HRV indices such as SDNN and PA level are associated with regional healthy life expectancy.9,10 Although the data were sampled from subjects who required Holter ECG examination, the conditions of data sampling may be assumed to be the same for all prefectures and thus, there seemed no apparent factors that could cause sampling biases among prefectures. Although the relevant mechanism of the association between senility death ration and regional HRV and PA levels is unknown from this study, one may speculate that regional differences in genetic background and/or environmental factors may affect both the senility death ratio and the physiological markers, creating an indirect link between them. Also, such genetic and/or environmental factors, if any, may have affected not only general population but also the subjects of ALLSTAR database. HRV and PA levels observed in the ALLSTAR database may be correlated with the regional factors affecting health status and thereby associated with the senility death ratio.
Conclusion
To investigate the factors related to regional differences in senility death ratio in Japan, we examined the relationships with HRV and PA levels obtained from the ALLSTAR big data. The results suggested that the regional senility death ratio in Japan is associated with regional health status as reflected in HRV and PA levels.
Acknowledgments
The authors deeply appreciate the support of SUZUKEN Co., Ltd, who provided the database for this research.
Footnotes
Authors’ note: The short abstract (<200 words) of this work has been published in the program of 2018 3rd International Conference on Biomedical Signal and Image Processing, 22–24 August 2018, Seoul, South Korea.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from the Ethics Review Committee of Nagoya City University Graduate School of Medical Sciences and Nagoya City University Hospital (Approval No. 709).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The ALLSTAR project was supported by the grant of the Knowledge Hub of Aichi, Japan (the Priority Research Project, P3-G1 S1-2b to J. Hayano) and the Japan Society for the Promotion of Science, Japan (Grant-in-Aid for Scientific Research (C) 23591055 to J. Hayano, Grant-in-Aid for Scientific Research (C) 25461062 to H. Fukuta, Grant-in-Aid for Scientific Research (B) 15H03095 to T. Nakamura, Grant-in-Aid for Scientific Research (C) 16K09097 to K. Ueda, and Grant-in-Aid for Scientific Research (A) 17H00878 to Y. Yamamoto).
Informed consent: Informed consent was not sought for the present study because we used the existing clinical data obtained for clinical purposes. To utilize the existing clinical data for this study, we followed the Ethical Guidelines for Medical and Health Research Involving Human Subjects (by the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labour and Welfare, Japan, 22 December 2014). According to the guideline, the purposes of the study and information used for the study have been public through the homepages of the ALLSTAR research project (http://www.med.nagoya-cu.ac.jp/mededu.dir/allstar/) and of SUZUKEN Co., Ltd (http://www.suzuken.co.jp/product/holter/detail/), in which opportunities to refuse the uses of information are ensured for the research subjects. These procedures have been approved by the Ethics Review Committee of Nagoya City University Graduate School of Medical Sciences and Nagoya City University Hospital (Approval No. 709)
Trial registration: Not applicable because this is a observational study.
ORCID iDs: Junichiro Hayano  https://orcid.org/0000-0002-5340-6325
https://orcid.org/0000-0002-5340-6325
Hiroyuki Sakano  https://orcid.org/0000-0002-5473-2202
https://orcid.org/0000-0002-5473-2202
References
- 1. Japanese Ministry of Health Law. Summary of the Age-adjusted Mortality Ratio by Prefecture in 2015. Tokyo, Japan: Japanese Ministry of Health Law, 2017. [Google Scholar]
- 2. Japanese Ministry of Health Law. Death Certificate Entry Manual (2018 Edition). Tokyo, Japan: Japanese Ministry of Health Law, 2018. [Google Scholar]
- 3. Inc. N. Regional difference in senility death (authors’ translation). Health expenditures topics 6 2017. [Google Scholar]
- 4. Camm AJ, Malik M, Bigger JT, et al. Task force of the European society of cardiology and the North American society of pacing and electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996; 93: 1043–1065. [PubMed] [Google Scholar]
- 5. Bauer A, Kantelhardt JW, Barthel P, et al. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: cohort study. Lancet 2006; 367(9523): 1674–1681. [DOI] [PubMed] [Google Scholar]
- 6. Huikuri HV, Makikallio TH, Peng CK, et al. Fractal correlation properties of R-R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation 2000; 101(1): 47–53. [DOI] [PubMed] [Google Scholar]
- 7. Hayano J, Yasuma F, Watanabe E, et al. Blunted cyclic variation of heart rate predicts mortality risk in post-myocardial infarction, end-stage renal disease, and chronic heart failure patients. Europace 2017; 19(8): 1392–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsuji H, Venditti FJ, Jr, Manders ES, et al. Reduced heart rate variability and mortality risk in an elderly cohort: the Framingham heart study. Circulation 1994; 90: 878–883. [DOI] [PubMed] [Google Scholar]
- 9. Yuda E, Furukawa Y, Yoshida Y, et al. Association between regional difference in heart rate variability and inter-prefecture ranking of healthy life expectancy: ALLSTAR big data project in Japan. In: Jung JJ, Kim P. (eds) Big data technologies and applications: Proceedings of the 7th EAI international conference, BDTA 2016 Seoul: Springer, 2017, pp. 23–28. [Google Scholar]
- 10. Hayano J, Yuda E, Furukawa Y, et al. Association of 24-hour heart rate variability and daytime physical activity: ALLSTAR big data analysis. Int J Biosci Biochem Bioinform 2018; 8: 61–67. [Google Scholar]
- 11. Hayano J, Kiyono K, Yuda E, et al. Holter ECG big data project: Allostatic State Mapping by Ambulatory ECG Repository (ALLSTAR). Int J Inform Res Review 2018; 5: 5617–5624. [Google Scholar]
- 12. Bigger JT, Jr, Fleiss JL, Steinman RC, et al. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 1992; 85: 164–171. [DOI] [PubMed] [Google Scholar]
- 13. Kantelhardt JW, Bauer A, Schumann AY, et al. Phase-rectified signal averaging for the detection of quasi-periodicities and the prediction of cardiovascular risk. Chaos 2007; 17(1): 015112. [DOI] [PubMed] [Google Scholar]
- 14. Peng CK, Buldyrev SV, Havlin S, et al. Mosaic organization of DNA nucleotides. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics 1994; 49(2): 1685–1689. [DOI] [PubMed] [Google Scholar]
- 15. Iyengar N, Peng CK, Morin R, et al. Age-related alterations in the fractal scaling of cardiac interbeat interval dynamics. Am J Physiol 1996; 271(4 Pt.2): R1078–R1184. [DOI] [PubMed] [Google Scholar]
- 16. Hayano J, Watanabe E, Saito Y, et al. Screening for obstructive sleep apnea by cyclic variation of heart rate. Circ Arrhythm Electrophysiol 2011; 4(1): 64–72. [DOI] [PubMed] [Google Scholar]
- 17. Hayano J, Yuda E. Pitfalls of assessment of autonomic function by heart rate variability. J Physiol Anthropol 2019; 38(1): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]