Short abstract
A well-recognized relationship exists between aging and increased susceptibility to chronic pain conditions, underpinning the view that pain signaling pathways differ in aged individuals. Yet despite the higher prevalence of altered pain states among the elderly, the majority of preclinical work studying mechanisms of aberrant sensory processing are conducted in juvenile or young adult animals. This mismatch is especially true for electrophysiological studies where patch clamp recordings from aged tissue are generally viewed as particularly challenging. In this study, we have undertaken an electrophysiological characterization of spinal dorsal horn neurons in young adult (3–4 months) and aged (28–32 months) mice. We show that patch clamp data can be routinely acquired in spinal cord slices prepared from aged animals and that the excitability properties of aged dorsal horn neurons differ from recordings in tissue prepared from young animals. Specifically, aged dorsal horn neurons more readily exhibit repetitive action potential discharge, indicative of a more excitable phenotype. This observation was accompanied by a decrease in the amplitude and charge of spontaneous excitatory synaptic input to dorsal horn neurons and an increase in the contribution of GABAergic signaling to spontaneous inhibitory synaptic input in aged recordings. While the functional significance of these altered circuit properties remains to be determined, future work should seek to assess whether such features may render the aged dorsal horn more susceptible to aberrant injury or disease-induced signaling and contribute to increased pain in the elderly.
Keywords: Pain, spinal cord, dorsal horn, aging, patch clamp, synaptic transmission, action potential
Introduction
The incidence of pain increases with advancing age. Epidemiological evidence suggests that while approximately 20% of young adults report persistent pain, 40%–80% of people aged 55–65 report pain that interferes with daily activities.1 The clinical literature shows that various indices of pain perception during repetitive noxious stimulation are enhanced by age.2 Likewise, capsaicin-induced pain lasts longer in aged individuals and is more resistant to local anaesthesia.3 The production of endogenous analgesic substances and the expression of opioid receptors also decline with age,4,5 and functional magnetic resonance imaging studies have shown that striatal inhibitory pain modulatory responses are reduced in aged individuals.6 Finally, electroencephalography (EEG) work has demonstrated that both peripheral and central nociceptive pathways are altered in the elderly.6,7 These data suggest that the detection and processing of pain-related information are altered with age.
Data from preclinical pain studies in aged animals are more varied than the clinical literature, most likely reflecting differences in experimental design and an over reliance on reflexive testing.8 Nevertheless, this body of work suggests that pain following inflammatory and neuropathic insults is greater in severity and duration in aged animals.9 At the cellular level, a number of baseline differences have been identified in the aged nociceptive system. For example, myelinated unmyelinated fibers are reduced in peripheral nerves,10,11 as well as the expression of key pain transducers and signaling receptors such as TRPV1 and NaV1.8.12,13 Together, these changes would reduce nociceptive signaling. Peripheral nerves also show signs of Wallerian degeneration with age,10,14 and the presence of increased numbers of macrophages and mast cells15 indicate increased inflammatory damage. At a functional level, the inflammatory response of nociceptive afferents also differs markedly in young versus aged animals. Young nociceptive afferents strongly sensitize to acute but desensitize to chronic inflammation.16 In contrast, aged nociceptive afferents do not exhibit robust sensitization or desensitization under either acute or chronic inflammatory conditions. These observations for inflammatory conditions imply the mechanisms underlying increased pain in aged animals lies in central pain pathways.
Some groups have provided clear evidence of altered excitability in central nociceptive pathways in aged animals. For example, extracellular in vivo recordings show that dorsal horn (DH) neurons in aged rats exhibit significantly higher background activity and higher discharge rates during noxious stimulation. After discharge is also enhanced following noxious stimulation and the receptive field of putative nociceptors is larger in aged versus young adult animals.17–19 In contrast, in inflammatory and neuropathic pain models, DH neuron background activity, discharge during nociceptive stimulation, receptive field size, and degree of sensory-evoked activation do not change dramatically in aged animals but are substantially increased in young adults.17,18 Together, these findings have been interpreted as evidence that aged spinal nociceptive circuits are sensitized in naive animals and thus have limited capacity for further injury-induced plasticity.
To date, detailed information on the excitatory and inhibitory synaptic mechanisms, as well as the intrinsic excitability in DH circuits, required to better understand how DH circuits differ in aged animals, has not been reported. This is in part because of a widely held view that high-resolution patch clamp recordings are not possible in spinal cord slices prepared from aged animals. Thus, much of the work on these properties has only been undertaken in young animals. Notwithstanding these challenges, in this study, we compare the intrinsic and synaptic properties of DH neurons in aged and young adult mice, highlighting a number of differences under baseline conditions. This information suggests that a variety of properties are altered with aging, and these may change DH circuit signaling under pathological conditions.
Experimental procedures
All experiments were approved by the University of Newcastle (UoN) Animal Care and Ethics Committee. Male C57BL/6 mice were used for all experiments and housed in a temperature- and humidity-controlled environment under a 12-h light and/or dark cycle. All animals had unlimited access to standard rodent chow and water. Two age groups were used for all experiments: mice that could be considered young adults (aged 3–5 months) and aged animals (24–32 months).
Acute spinal slice preparation
An identical spinal cord slicing procedure was used for young adult and aged mice, as previously described.20,21 Briefly, mice were anaesthetized with ketamine (100 mg/kg i.p.) and decapitated. Using a ventral approach, the lumbosacral enlargement of the spinal cord was rapidly removed and placed in ice-cold sucrose substituted artificial cerebrospinal fluid (ACSF) containing (in mM): 250 sucrose, 25 NaHCO2, 10 glucose, 2.5 KCl, 1 NaH2PO4, 1 MgCl2, and 2.5 CaCl2. Parasagittal slices (from L3 to L5 segments cut at 300 µm thickness) were obtained using a vibrating microtome (Leica VT-1000S, Heidelberg, Germany). Slices were then transferred to an interface incubation chamber containing oxygenated ACSF (118 mM NaCl substituted for sucrose) and allowed to equilibrate for 1 h (at 22°C–24°C) prior to recording.
Electrophysiology
Slices were transferred to a recording chamber and continually superperfused (bath volume 0.4 ml; exchange rate 4–6 bath volumes/min) with ACSF bubbled with Carbonox (95% O2 and 5% CO2) to achieve a final pH of 7.3–7.4. Neurons were visualized using near-infrared differential interference contrast optics. All recordings were limited to neurons within or dorsal to the substantia gelatinosa, which were easily identified by its translucent appearance. This meant neurons were sampled in laminae I and II. Recordings were obtained at room temperature (21°C–24°C). Patch pipettes (4–8 MΩ) were filled with one of the two internal solutions. A potassium gluconate-based internal solution was used to record action potential (AP) discharge and excitatory postsynaptic currents (EPSCs), containing (in mM): 135 C6H11KO7, 6 NaCl, 2 MgCl2, 10 HEPES, 0.1 EGTA, 2 MgATP, and 0.3 NaGTP (pH adjusted to 7.3 with KOH). A cesium chloride-based internal was used for recording inhibitory postsynaptic currents (IPSCs), containing (in mM): 130 CsCl, 10 HEPES, 10 EGTA, 1 MgCl2, 2 ATP, and 0.3 GTP (pH adjusted to 7.35 with 1 M CsOH). Neurobiotin (0.2%, Vector Laboratories, Peterborough, UK) was included in the internal solutions to access neuronal location and morphology in a subset of experiments. Initially, all recordings were made in the voltage-clamp mode (holding potential −70 mV). Data were acquired using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA) and digitized online (sampled at 10–20 kHz and filtered at 5–10 kHz), via an ITC-18 computer interface (Instrutech, Long Island, NY, USA), and stored on a Macintosh computer using Axograph X software (Kagi, Berkley, CA, USA). Series resistance, neuron input resistance, and membrane capacitance were calculated at the beginning of each recording, based on the response to a 5 mV hyperpolarizing voltage step (10 ms duration, holding potential −70 mV). These values were reassessed at the end of each recording session, and data were rejected if they changed by more than 30%.
AP discharge was assessed by switching to current-clamp mode with bridge balance monitored throughout recordings. Neurons were left for 60 s following this switch to allow membrane potential to stabilize and this value was taken as resting membrane potential (RMP). Neuronal excitability and AP discharge were studied by injecting a series of depolarizing step currents from RMP (800 ms duration, 20 pA increments, delivered every 8 s) into the recorded neuron. During this protocol, maximum voltage deflections were limited to −20 mV, in parts of the voltage trace not containing APs. This avoided cell damage.
Spontaneous EPSCs (sEPSCs), which represent the postsynaptic response to AP-dependent and independent neurotransmitter release,22–24 were pharmacologically isolated by including the GABAA receptor and glycine receptor antagonists bicuculline (10 μM) and strychnine (1 μM) in the bath perfusate. The identity of these currents as excitatory was subsequently confirmed by bath application of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-kainate receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μM). Spontaneous IPSCs (sIPSCs) were recorded in a similar manner except CNQX was included initially, to isolate mixed sIPSCs (i.e., those mediated by GABA and glycine), before the GABAA receptor antagonist bicuculline (10 μM) was added to isolate glycinergic currents. At least 3 min of data were acquired under each pharmacological condition for subsequent analysis.
Data analysis
Criteria for inclusion of recordings for analysis were an RMP more negative than −50 mV and a series resistance < 30 MΩ (filtered at 5 KHz). All data were analyzed offline using Axograph software. Analysis of sEPSCs and sIPSCs used a sliding template method (semiautomated procedure within Axograph X) to capture individual events.25 Captured currents were inspected and excluded from further analysis if multiple events overlapped or had an unstable baseline before their rise or during their decay phase. Data were also rejected if a significant time-dependent trend in either the amplitude or the interval between currents was observed during the analysis period. The peak current amplitude and rise time (measured over 10%–90% of the maximum current amplitude) were measured for all accepted events (via semiautomated procedures in Axograph). Mean frequency was calculated as the number of accepted events detected divided by the duration of analysis period (in seconds). Analysis of decay time constant (calculated over 20%–80% of the current’s decay phase) was undertaken on averaged currents, generated by aligning the rising phase of all accepted events in a recording. Averaged currents were also used to calculate charge transfer, defined as the area under the trace.
For the analysis of AP discharge, responses were classified according to previously published criteria.26,27 Tonic firing was characterized by continuous AP discharge throughout the depolarizing step, initial bursting (IB) was characterized by AP discharge limited to the beginning of the depolarizing step, delayed firing featured a prominent delay between the onset of the depolarizing step and AP discharge, single spiking was characterized by discharge limited to one or two APs at depolarizing step onset. Individual APs elicited by step-current injection were also captured using a derivative threshold method with the inflection point during spike initiation (dV/dt ≥ 15 V/s) defined as AP threshold. The difference between AP threshold and its maximum positive peak was defined as AP amplitude. AP base width was measured at AP threshold. AP afterhyperpolarization (AHP) amplitude was taken as the difference between AP threshold and the maximum negative peak following the AP. Rheobase current was defined as the smallest step-current that elicited at least one AP. Three parameters were used to further classify each neuron’s AP discharge pattern during step-currents: discharge latency which reflects time from the onset of step-current injection to the first evoked AP, discharge duration which reflects the time between the onset of the first and last AP during step-current injection, and number of spikes discharged per step. These properties were also measured and compared for young adult and aged neurons that exhibited tonic firing, IB, and delayed firing. This allowed us to assess whether the profile of AP discharge was altered in each category.
Statistical analysis was carried out using SPSS v10 (SPSS Inc., Chicago, IL, USA). Student t tests were used to compare variables between young adult and aged data sets, with nonparametric data compared using Kruskal–Wallis test. G tests, with Williams’ correction, were used to determine whether the proportions of AP discharge patterns and voltage-activated currents differed between groups. Statistical significance was set at p < 0.05. All values are presented as means ± standard error of the mean.
Histochemistry
At the conclusion of some experiments the slice was stored in 4% paraformaldehyde at 4°C for subsequent analysis of neurobiotin-filled neurons (one neuron per slice). Slices were washed in 0.1 M phosphate-buffered saline (PBS; 3×15 min) and incubated in Rhodamine Red Streptavidin (1:50, overnight) using antibody diluent (0.3% Trident). Slices were then washed in PBS (3×15 min), cover-slipped, and imaged on an epifluorescent microscope. The distance of recovered neurons from the dorsal white–gray border was recorded to allow between-group comparison across similar recording sites between the young adult and aged DH. Some recovered neurons from aged tissue were imaged on a confocal microscope and classified morphologically if neurons soma and dendritic processes were clearly visible.
Drugs
All drugs were prepared and stored at 1000× final concentration and then diluted in bath perfusate. All drugs were purchased from Sigma-Aldrich (Sydney, Australia).
Results
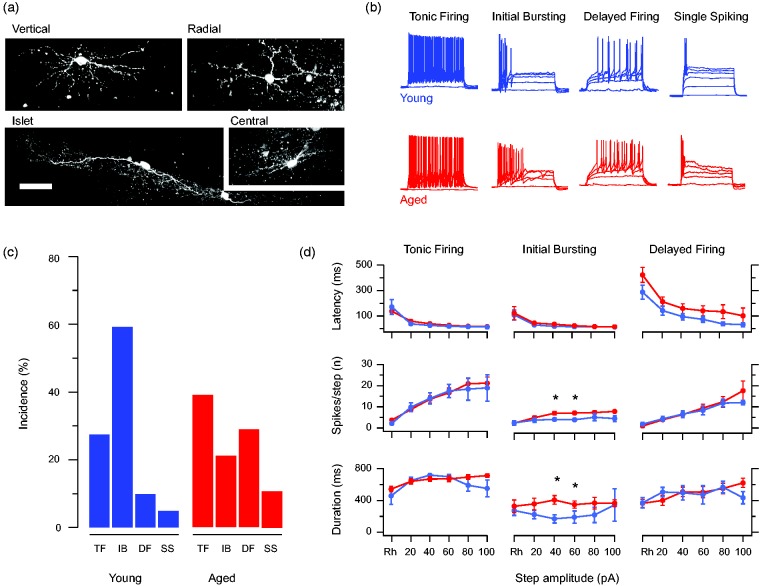
Whole-cell patch clamp recordings were obtained from 112 neurons (40 from 15 young adult mice and 72 from 24 aged mice). Our recordings characterized the active and passive membrane properties as well as fast excitatory and inhibitory synaptic transmission. The mean distance of neurons from the dorsal border of the gray and white matter (80.5 ± 8.9 μm vs. 78.5 ± 6.5 μm), input resistance (471.4 ± 54.5 MΩ vs. 478.8 ± 40.1 MΩ), and membrane potential (−54.3. ± 2.1 mV vs. −53.6 ± 3.1 mV) of neurons were similar in both age groups. A subset of aged DH neurons were also filled and recovered by including neurobiotin in the recording pipette. Although recovery from aged spinal cord tissue was limited, the range of morphologies typically reported for lamina II neurons in young animals were observed, including vertical, radial, islet, and central morphologies (Figure 1(a)). Together, these comparisons suggest any differences we observed were not due to location, cell type, or recording bias. Most importantly, they show that the functional properties of DH neurons can be studied in spinal cord slices from very old mice.
Figure 1.
Altered intrinsic excitability of aged DH neurons. (a) Representative morphology of neurobiotin recovered DH neurons recorded from aged mice. Recovered neurons exhibited all the morphological classes typically identified in studies from younger animals including vertical, radial, islet, and central cells. (b) Overlaid traces show AP discharge responses recorded during step current injections (800 ms duration, 20 pA increments) from young (blue) and aged (red) DH neurons. These responses included the range of responses typically reported in the literature including TF, IB, DF, and SS were observed in both young and aged recordings. (c) Bar plots compare the relative incidence of each discharge category (shown in panel b) between young and aged recordings. IB dominated in the young recording’s but was less common in the aged sample where TF and DF responses were more prominent. (d) Line plots compare spiking characteristics (duration of spiking, number of spikes, and latency to spiking) in young and aged recordings from DH neurons that exhibit repetitive AP discharge (TF, IB, and DF). Data are normalized to compare the spiking at rheobase and then subsequent 20 pA step responses up to 100 pA. All spiking characteristics assessed were similar in young and aged TF (left) and DF (right) DH neurons, whereas aged IB DH neurons exhibited a greater number of spikes per step and longer spiking duration (at 40 and 60 pA above rheobase) than young IB DH neurons. Thus, across these findings, the aged DH neurons sampled exhibited greater excitability than those recorded from young animals. TF: tonic firing; IB: initial bursting; DF: delayed firing; SS: single spiking.
Intrinsic excitability is enhanced in aged DH neurons
DH neurons exhibit well-established heterogeneity in their AP discharge patterns. Accordingly, we compared neuronal responses to depolarizing current step injections (20 pA increments, 800 ms duration) from young adult and aged animals (Figure 1). Comparison of the rheobase APs from young and aged animals showed no difference in AP threshold (−29.90 ± 1.32 vs. −30.18 ± 1.05 mV, p = 0.87), AP amplitude (55.12 ± 1.85 vs. 53.91 ± 1.72 mV, p = 0.65), AP half-width (2.11 ± 0.14 vs. 2.16 ± 0.13 ms, p = 0.77), and AHP amplitude (−19.55 ± 1.53 vs. −20.47 ± 1.25 mV, p = 0.65). Four types of AP discharge were observed in both young and aged neurons. These included tonic firing, IB, delayed firing, and single spiking (Figure 1(b)). In contrast, reluctant firing responses that have been reported in some work were not observed in our study.24,25 Comparison of the distribution of these discharge responses showed that IB responses dominated the young sample but became less common in the aged animals (Figure 1(c)). Despite this observation, the high variability in both samples meant that the distribution in both groups was statistically similar. However, when neurons were collapsed into a group that showed adaptive, limited AP discharge (IB and single spiking); or extended discharge and a linear relationship between current step amplitude and spike number (tonic firing and delayed firing), there was a significant rise in responses that supported repetitive discharge in the aged recordings (tonic firing and delayed firing, Chi squared p < 0.05).
To further assess the impact of advanced age on intrinsic excitability recordings, Young and aged DH neurons that exhibited repetitive discharge at multiple steps (tonic firing, IB, and delayed firing) were separated into discharge patterns, and the features of AP discharge (delay, duration, and frequency) were compared across multiple currents step injections (Figure 1(d)). This analysis showed that tonic firing neurons exhibited latency to discharge, number of AP spikes per step, and duration of discharge responses that was similar across both ages. IB neurons exhibited a similar latency to AP discharge for both ages; however, aged neurons produced more AP spikes per step and longer discharge durations in response to the 40 and 60 pA steps above rheobase. Finally, delayed firing neurons exhibited similar latency to discharge, number of AP spikes per step, and duration of discharge responses in young and aged recordings. Thus, in addition to an overall shift to responses that featured sustained AP discharge in the aged DH (tonic firing and delayed firing), IB neurons in the aged DH also discharged more APs producing longer responses. Together, these analyses indicate certain populations of neurons in the aged DH become more excitable. This could disrupt normal spinal sensory processing mechanisms and alter pain signaling in this region.
The strength of excitatory input is reduced in aged DH neurons
We next examined the effect of age on spontaneous excitatory synaptic input, which is critical for driving activity in DH circuits under both normal and pathological conditions. sEPSCs were recorded in young adult and aged DH neurons from a holding potential −70 mV (Figure 2). sEPSC amplitude was reduced in aged DH neurons (15.83 ± 0.65 pA vs. 20.33 ± 1.03 pA, p < 0.05). The time course of these currents was unaffected by age, with sEPSC rise time (1.27 ± 0.06 ms vs. 1.23 ± 0.08 ms, p = 0.654) and decay time constants being similar in both groups (5.24 ± 0.27 ms vs. 4.78 ± 0.52 ms, p = 0.424). sEPSCs frequency was also similar in neurons from young adult and aged mice (2.78 ± 0.63 Hz vs. 2.02 ± 0.63 Hz, p = 0.436). We also calculated the average charge that would be transferred by excitatory synaptic events in the two groups. Charge was lower in the aged sample (107.1 ± 5.9 pA.ms vs. 165.6 ± 33.9 pA.ms, p < 0.05). Thus, excitatory synaptic drive is reduced in the DH of aged animals.
Figure 2.
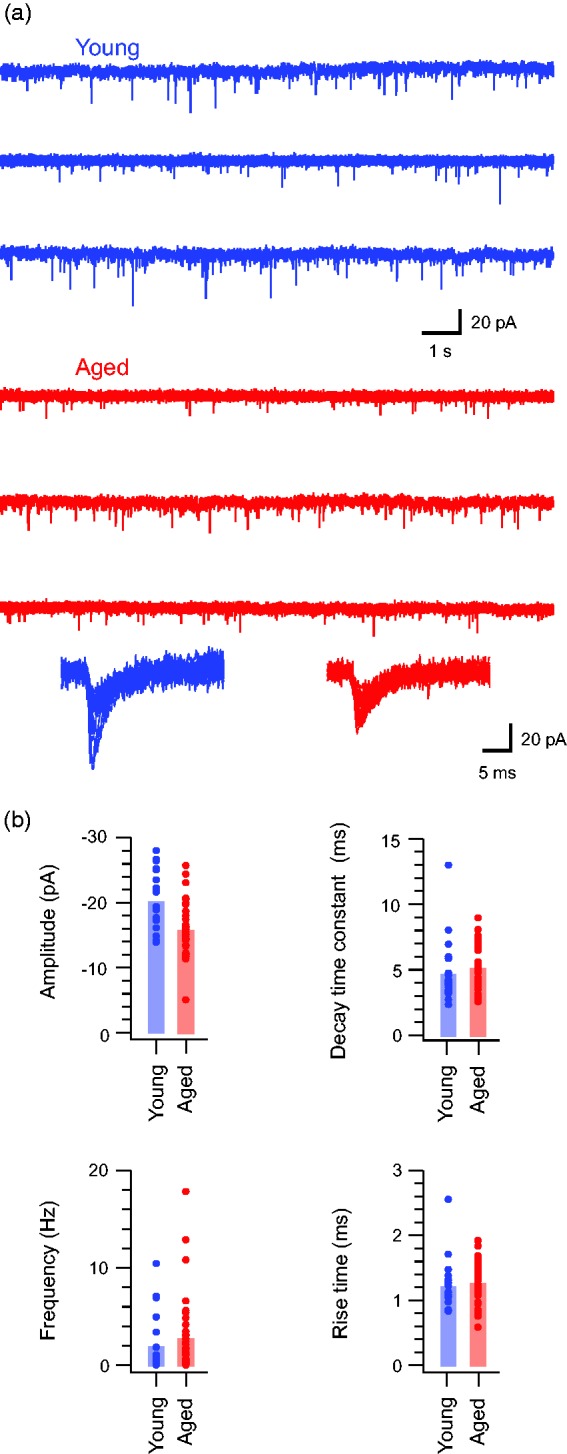
Excitatory drive is decreased in aged DH neurons. (a) Traces show continuous recordings of spontaneous excitatory postsynaptic currents (sEPSC) from DH neurons in young (upper blue) and aged (lower red) animals. Lower overlaid traces are captured sEPSCs from young and aged recordings highlighting the amplitude range and time course of events recorded from young and aged animals. (b) Group data plots compare sEPSC properties in young and aged recordings. sEPSC amplitude was significantly decreased in aged recordings, whereas rise time decay time constant and frequency were similar in both samples.
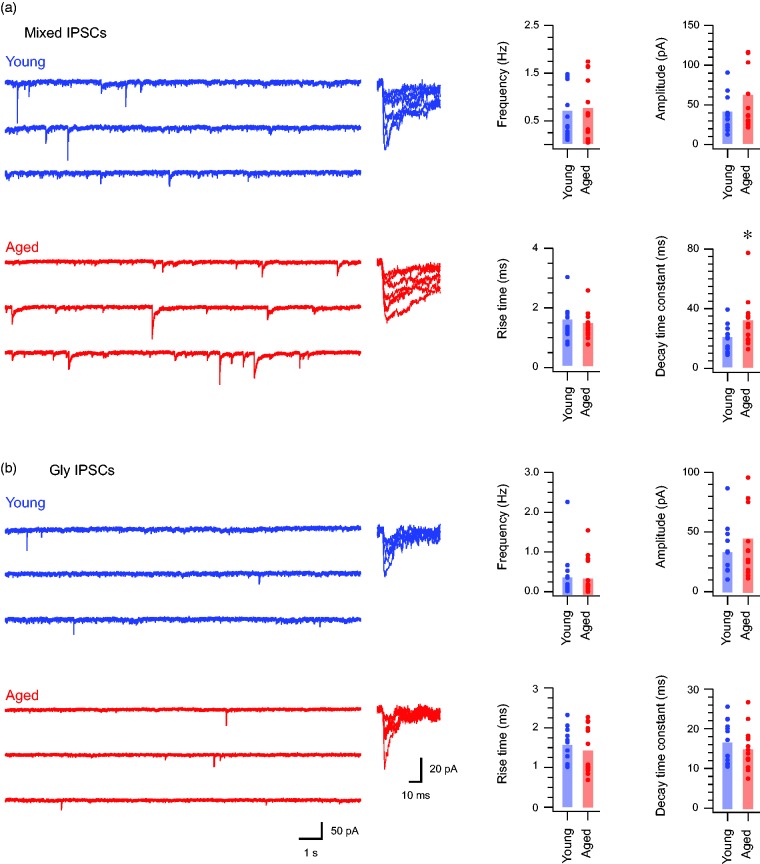
The contribution of GABAergic inhibition increases in aged DH neurons
Activity and processing within DH circuits is also under fast synaptic inhibitory control mediated by GABAergic and glycinergic inhibition. To assess the effect of age on inhibitory signaling in the DH, we recorded sIPSCs in young and aged DH neurons (Figure 3). Mixed sIPSCs, which represent the combined actions of GABAergic and glycinergic input, were first examined. Our analysis showed mixed sIPSC amplitude (54.47 ± 11.36 pA vs. 36.22 ± 5.83 pA, p = 0.146), rise time (1.41 ± 0.12 ms vs. 1.53 ± 0.16 ms, p = 0.524), and frequency (0.73 ± 0.16 Hz vs. 0.67 ± 0.15 Hz, p = 0.801) were similar in aged and young adult recordings. In contrast, the decay time constant of these currents was markedly slower in aged recordings (30.70 ± 4.35 ms vs. 19.65 ± 2.47 ms, p < 0.05). This slower decay time course meant mixed sIPSC charge was larger in aged recordings (1161.71 ± 203.51 pA.ms vs. 637.49 ± 94.95 pA.ms, p < 0.05).
Figure 3.
GABAergic inhibition is more prominent in aged DH neurons. (a) Traces show continuous recordings of mixed (GABA and glycine) sIPSCs recorded from DH neurons in young (upper blue) and aged (lower) animals. The right overlaid traces are captured sEPSCs from young and aged recordings highlighting the amplitude range and time course of events recorded from young and aged animals. Note that currents exhibit similar amplitudes, but time course is extended in the aged recordings. Group data plots (right) compare mixed sIPSC properties, showing similar eIPSC amplitude, rise time, and frequency between samples by slower decay time constants in the aged recordings. (b) Traces show continuous recordings of glycinergic sIPSCs in young and aged DH neurons, isolated by bath addition of bicuculline to block GABAergic components. The right overlaid traces are captured glycinergic sEPSCs highlighting the amplitude range and time course of events. Note that currents from young and aged recordings have a similar appearance. Group data plots (right) compare glycinergic sIPSC properties, showing DH neurons from young and aged animals exhibit similar values for amplitude, rise time decay time constant, and frequency values. sIPSCs: spontaneous inhibitory postsynaptic currents.
Given GABAergic synaptic currents are known to produce slow decaying inhibitory synaptic currents whereas glycinergic synaptic currents decay more rapidly,21 we next isolated the glycinergic component of sIPSCs in a subset of neurons by bath application of bicuculline (10 μM). As with mixed sIPSCs, glycinergic sIPSCs exhibited similar amplitudes (48.35 ± 10.49 pA vs. 35.73 ± 5.99 pA, p = 0.317), frequencies (0.38 ± 0.13 Hz vs. 0.42 ± 0.16 Hz, p = 0.854), and rise times (1.40 ± 0.16 ms vs. 1.54 ± 0.11 ms, p = 0.474). After removal of GABAergic input, the remaining glycinergic sIPSCs exhibited similar decay time constants (14.59 ± 1.38 ms vs. 16.35 ± 1.44 ms, p = 0.386) and synaptic charge (673.26 ± 163.19 pA.ms vs. 554.91 ± 82.79 pA.ms, p = 0.386). These data suggest that the difference we observed in mixed sIPSC decay (Figure 3(a)) in aged versus young adult recordings was mediated by GABAergic input. This interpretation was further supported by the relative change in sIPSC properties between mixed and glycinergic-only conditions in aged and young adult samples. Specifically, sIPSC frequency, decay time constant, and charge all fell after removal of GABA-mediated currents in the aged but not young adult sample. Together, these findings suggest that the relative balance of GABAergic and glycinergic shifts to a greater dominance of GABAergic sources in the aged DH.
Discussion
The well-documented shift in susceptibility to, and experience of, pathological pain conditions with advancing age has been seen as evidence for a change in the way nociceptive input is haracte and processed by the aged central nervous system (CNS). Data presented here provide a systematic appraisal of the key factors that shape nociceptive processing in the spinal DH by characterizing intrinsic excitability, excitatory synaptic signaling, and inhibitory synaptic signaling. Together, our results suggest that several alterations occur in aged spinal DH circuits that would impact sensory processing. Specifically, with age, there is a shift in the intrinsic excitability of DH neurons with an increased capacity to support repetitive spiking. In parallel, there is an overall decrease in excitatory synaptic drive. Most notably, there is a shift in the balance of inhibitory input contribution from GABAergic sources which increases with age.
While the relationship between the above changes is unclear, our findings are consistent with the concept of homeostatic plasticity, where dynamic interplay between the factors that govern excitability is adjusted to maintain neuronal output within functional limits.28 For example, layer 2/3 pyramidal neurons in the rat visual cortex exhibit enhanced intrinsic excitability following monocular deprivation that blocks visual input and reduces excitatory drive to these neurons.29 Conversely, reduced inhibitory input in the naturally occurring glycine receptor-mutant mouse, spastic, lead to reduced intrinsic excitability in hypoglossal motor neurons, presumably to limit neuronal output in the face of compromised inhibitory regulation.30 Importantly, similar adjustments have also been identified in the DH of the same glycine receptor-mutant mouse, where intrinsic excitability is downregulated via enhanced A-type potassium currents that limit/delay AP discharge.26 In the case of aged DH neurons, we observed an increase in repetitive AP discharge—that is, enhanced neuronal excitability.
Regarding potential mechanisms for a shift in excitability, a substantial body of work has shown that features of AP discharge in the DH are not static. Rather many neurons can exhibit a range of discharge responses depending on factors such as membrane potential, neuromodulator signaling, and voltage-gated ion channel expression patterns.31–35 For example, a shift to tonic firing could result if there were a reduction in A-type potassium currents either due to reduced expression of the underlying potassium channel or if channel function were modulated.36,37 Such conditions would diminish the capacity of the A-type potassium current to delay AP discharge and produce tonic firing. Alternatively, a change in the relative expression or activation state of voltage-gated sodium and potassium currents could allow switching from adaptive AP discharge to a tonic firing.33 While our data set does not allow us to differentiate these mechanisms, or others, the resting membrane potentials were similar in young and aged recordings. Thus, future studies will be required to directly assess the effect of aging on the range of voltage activated currents that influence tonic AP discharge.
We observed an overall reduction in excitatory synaptic strength in aged DH circuits (Figure 2(a)). This is similar to recordings in acute slices from rat parietal cortex where sEPSC amplitude decreases with advanced age.38 Likewise, reports in rat CA3 hippocampal neurons have shown an age-associated decrease in synaptic strength in the form of reduced sEPSC amplitude.39 Interestingly, this reduced excitatory drive was accompanied by enhanced intrinsic excitability, an observation our DH data set mirrors. Recordings in prefrontal cortex from nonhuman primates have also shown decreased excitatory synaptic transmission, in this case, however, the cause was a reduction in sEPSC frequency with little change in amplitude.40 Finally, others have reported that excitatory inputs do not differ in the primary somatosensory cortex of young and aged rats.41 Thus, age-related changes to excitatory synaptic function maybe region-specific.
The ultimate impact of reduced excitatory signaling in the DH would be reduced signaling through DH circuits. Given, excitatory (glutamatergic) drive in the DH comes predominantly from primary afferents and local excitatory interneurons populations in this region,42,43 differentiating the source of specific types of excitatory input in the aged DH will also be important. Our recordings were of spontaneous excitatory events so the source of input cannot be distinguished. Thus, the reduction to sEPSC amplitude in aged DH recordings may be conserved across both afferent and interneuron inputs or restricted to one type. This will be important to resolve in the future as a selective reduction to interneuron or primary afferent signals in aged DH neurons may have differential effects on spinal sensory processing. Likewise, the impact of these changes will depend on whether they occur across the DH population, or selectively on excitatory versus inhibitory cell types. Previous work has suggested DH neurons can be functionally identify differentiated by AP discharge patterns, with tonic firing common for inhibitory interneurons and delayed firing common for excitatory populations.44–46 Unfortunately, the relative incidence of each in our data set meant that statistically meaningful comparisons could not be made to test if reduced excitatory signaling was confined to excitatory or inhibitory DH neurons. Future work using aged transgenic mice with genetically labeled DH neuron subpopulations such as the Gad67::eGFP or vGluT2::eGFP lines could directly address this issue.47,48
Our assessment of inhibitory synaptic input in the aged DH showed a marked shift to GABAergic inhibition with age. The effect of advanced age on inhibition has also been studied in other CNS regions with a range of findings, though generally advanced age is accompanied by diminished inhibitory function.49 For example, inhibition to CA3 pyramidal neurons in rat hippocampus is reduced through reductions in both the amplitude and frequency of sIPSCs in aged animals.39 Similarly, sIPSC amplitude and frequency are reduced in rat medial geniculate neurons.50 Pyramidal neurons in rat parietal cortex also exhibit reduced sIPSC frequency, though other properties such as amplitude and time course are unaffected.51 In contrast, inhibition of pyramidal neurons in somatosensory cortex is unchanged in aged rats.41 Findings in nonhuman primates also vary, with increased sIPSC frequency in layer II/III pyramidal cells in prefrontal cortex, but sIPSC amplitude and frequency unchanged in hippocampal dentate gyrus granule cells in aged animals. Of note, however, the decay time course of inhibitory GABAergic currents in the dentate was slower in recordings from aged animals.40 This work concluded that presynaptic release mechanisms were unaltered, but age had changed postsynaptic GABAA receptor kinetics. Our findings mirror this observation, with decay time the only property of sIPSCs that was altered in aged mouse DH neurons. Unlike dentate sIPSCs that are solely mediated by GABA, sIPSCs in DH neurons are mediated by both GABA and glycine. Importantly, GABA receptor-mediated currents decay with slow kinetics (>20 ms), whereas glycine receptors decay with fast kinetics (5–10 ms). Thus, the slower decay kinetics in our sIPSC recordings appear to reflect a shift to greater GABAergic inhibition in the aged DH. This is supported by a more pronounced shift to faster decay kinetics when GABAergic signaling is blocked in aged compared to young sIPSC recordings, as well as a greater decrease in sIPSC frequency under these conditions.
Many studies, including our own, have highlighted the importance of inhibition from both GABAergic and glycinergic sources in the mouse DH, and the impact that altering this inhibition has for nociceptive processing.21,52–57 In addition, previous work in rat has suggested a developmental shift in the relative balance of GABA and glycine. Specifically, glycinergic inputs were not detectable at birth but emerged approximately two weeks later.58 In contrast, GABAergic input was present at birth and remained relatively strong even after the establishment of glycinergic input. Other rat data also indicate that once glycinergic input is established, a period follows where the majority of DH neurons receive mixed inhibition from both glycinergic and GABAergic sources, with up to a quarter of these recordings also displaying evidence of GABA/glycine cotransmission at individual synapses.59 This transient period of mixed inhibition then declines, and most DH neurons receive either predominantly glycine or GABA dominant inhibition by three weeks postnatal and into adulthood. Work in mouse broadly agrees with this segregation of glycine or GABA-dominant inhibition in DH subpopulations and has shown subsets of excitatory and inhibitory interneurons exist that preferentially receive strong inhibition from one transmitter system in adulthood.56,60 This literature has also shown that neurons located more superficially tend to receive GABA dominant inhibition, whereas synaptic inhibition in more ventrally located populations has a stronger glycinergic component.57,61 Our experiments in sagittal slices will have included recordings from both populations described in this work, but we did not specifically differentiate the more superficial and ventral populations. Regardless, the strong dominance of GABAergic input in our data highlights that advanced age causes a relative reduction in glycinergic inhibition that has the potential to alter the profile of synaptic inhibition in aged DH circuits. The functional significance of this shift remains to be determined; however, given glycinergic currents decay more rapidly than GABAergic currents, the precision, temporal qualities, and potential for summation will be altered. It follows that these altered properties will affect normal and pathological signaling within aged DH circuits. In addition, a substantial literature suggests that altered chloride transporter function under neuropathic conditions can diminish synaptic inhibition and even convert inhibitory synapses to excite their postsynaptic targets.62–65 If these observations hold true in the aged DH, they may be further exacerbated by the greater dominance of slower decaying GABAergic currents and produce sustained excitation within the DH.
The mechanisms that drive the sequence of changes we have observed in DH neurons remains to be determined; however, as noted, these differences may be interrelated with both the consequences of aging and other compensatory adjustments to maintain normal signaling. Regardless, the aging literature contains a growing body of evidence, suggesting that the aged nervous system is maintained in a chronic neuro-inflammatory state, in part due to dysfunction of glial cell populations. For example, microglia isolated from aged mice express greater quantities of pro-inflammatory cytokines tumor necrosis factor α (TNFα), interleukin-1 β (IL-1β), transforming growth factor β, and IL-6 mRNA compared to young microglia.66,67 In aged brain slices, microglia become less dynamic and ramified. Moreover, they exhibit slower acute responses to laser-evoked focal tissue injury; however, the duration of their responses is extended.68 Together, these data support widespread disturbances to microglial structure, activation, and pro-inflammatory cytokine release with advancing age. Astrocytes have also been implicated in the heightened inflammatory status of the aged CNS. Astroglial morphology is considerably affected by age, with hypertrophic, reactive astrocytes predominating in the aged brain.69 Astrocytes isolated from aged cortex produce larger quantities of IL-6 than those from young cortex.70 Further, the increased TNFα, IL-1β, and IL-6 in aged cortex and striatum are more extensively colocalized with astrocytes than microglia or neurons.70
This literature is particularly relevant in the DH as neuro-inflammation has emerged as a key driver of altered sensory signaling following nerve injury and neuropathic pain. Specifically, a neuro-inflammatory cascade has been shown to provoke significant plasticity in excitatory and inhibitory DH signaling, starting with the release of a number of pro-inflammatory cytokines. Damage to primary sensory neurons results in their rapid de novo synthesis of C-C motif chemokine ligand 2.71 Oligodendrocyte-derived IL-33 induces the production and release of TNFα and IL-1β from astrocytes and microglia within the spinal DH.72 Activation of microglia by these pro-inflammatory cytokines results in upregulation of their synthesis and surface expression of the ionotropic purine receptor 4 (P2X4). P2X4 receptor activation by ATP in turn causes brain-derived neurotrophic factor release from microglia,73 facilitating neuropathic pain by two mechanisms. Activation of TrkB (tropomyosin receptor kinase B) can induce phosphorylation of the obligatory N-methyl-D-aspartate (NMDA) receptor subunit GRIN1 (glutamate ionotropic receptor NMDA type subunit 1), increasing the amplitude of evoked excitatory synaptic inputs.74 TrkB can also inhibit KCC2 (potassium/chloride cotransporter 2), resulting in reduced chloride reversal potential, rendering GABAergic and glycinergic inhibition less effective (as noted earlier).63–65
Given these established links between neuro-inflammation and altered synaptic function under neuropathic conditions, chronic neuro-inflammatory conditions appear a likely contributor to the altered synaptic and intrinsic excitability we see in the naive aged DH may also be driven by. Adding another dimension to these data, work on young animals has indicated that neuro-inflammatory mechanisms are developmentally sensitive, suppressing neuropathic dysfunction in the spinal cord.75,76 Our data suggest that a shift in the relationship between neuro-inflammation and neuropathic injury may also exist with advanced age. Differences between our data and the neuropathic literature may reflect the immediate, transient inflammatory state produced by nerve injury versus age-related neuro-inflammation that is likely to develop more slowly and chronically. The question of whether treating this chronic neuro-inflammation might restore aged DH circuits to resemble that of younger animals may have implications for future therapies. Regardless, it will be of particular importance, to establish if injury and pathological signaling, such as that under neuropathic conditions, is altered when superimposed upon an aged DH circuitry. The outcome of both phenomena converging in the DH will provide important insights into our understanding and treatment of pain in aged populations.
Author Contributions
BAG, DGS, and RJC conceived the study. BAG and RJC designed the experiments. JAM and BAG performed experiments and analyzed data; BAG, DGS, JAM, FRW, and RJC drafted the manuscript and finished the final version of the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the National Health and Medical Research Council of Australia (grants 631000 and 1043933 to BAG and 1067146 to RJC) and the Hunter Medical Research Institute (grant to BAG and RJC).
References
- 1.Melding PS. Is there such a thing as geriatric pain? Pain 1991; 46: 119–121. [DOI] [PubMed] [Google Scholar]
- 2.Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: clinical relevance in healthy older and younger adults. J Pain 2001; 2: 307–317. [DOI] [PubMed] [Google Scholar]
- 3.Gagliese L. Pain and aging: the emergence of a new subfield of pain research. J Pain 2009; 10: 343–353. [DOI] [PubMed] [Google Scholar]
- 4.Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain 2005; 115: 410–418. [DOI] [PubMed] [Google Scholar]
- 5.Hess GD, Joseph JA, Roth GS. Effect of age on sensitivity to pain and brain opiate receptors. Neurobiol Aging 1981; 2: 49–55. [DOI] [PubMed] [Google Scholar]
- 6.Cole LJ, Farrell MJ, Gibson SJ, Egan GF. Age-related differences in pain sensitivity and regional brain activity evoked by noxious pressure. Neurobiol Aging 2010; 31: 494–503. [DOI] [PubMed] [Google Scholar]
- 7.Creac HC, Bertholon A, Convers P, Garcia-Larrea L, Peyron R. Effects of aging on laser evoked potentials. Muscle Nerve 2015; 51: 736–742. [DOI] [PubMed] [Google Scholar]
- 8.Yezierski RP. The effects of age on pain sensitivity: preclinical studies. Pain Med 2012; 13: S27–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novak JC, Lovell JA, Stuesse SL, Cruce WL, McBurney DL, Crisp T. Aging and neuropathic pain. Brain Res 1999; 833: 308–310. [DOI] [PubMed] [Google Scholar]
- 10.O'Sullivan DJ, Swallow M. The fibre size and content of the radial and sural nerves. J Neurol Neurosurg Psychiatry 1968; 31: 464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochoa J, Mair WG. The normal sural nerve in man. I. Ultrastructure and numbers of fibres and cells. Acta Neuropathol 1969; 13: 197–216. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Davis BM, Zwick M, Waxman SG, Albers KM. Reduced thermal sensitivity and Nav1.8 and TRPV1 channel expression in sensory neurons of aged mice. Neurobiol Aging 2006; 27: 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Albers KM. Behavioral and cellular level changes in the aging somatosensory system. Ann N Y Acad Sci 2009; 1170: 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drac H, Babiuch M, Wiśniewska W. Morphological and biochemical changes in peripheral nerves with aging. Neuropatol Pol 1991; 29: 49–67. [PubMed] [Google Scholar]
- 15.Ceballos D, Cuadras J, Verdu E, Navarro X. Morphometric and ultrastructural changes with ageing in mouse peripheral nerve. J Anatomy 1999; 195: 563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weyer AD, Zappia KJ, Garrison SR, O'Hara CL, Dodge AK, Stucky CL. Nociceptor sensitization depends on age and pain chronicity(1,2,3. ). eNeuro 2016; 3: pii: ENEURO.0115-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitagawa J, Kanda K, Sugiura M, Tsuboi Y, Ogawa A, Shimizu K, Koyama N, Kamo H, Watanabe T, Ren K, Iwata K. Effect of chronic inflammation on dorsal horn nociceptive neurons in aged rats. J Neurophysiol 2005; 93: 3594–3604. [DOI] [PubMed] [Google Scholar]
- 18.Iwata K, Tsuboi Y, Shima A, Harada T, Ren K, Kanda K, Kitagawa J. Central neuronal changes after nerve injury: neuroplastic influences of injury and aging. J Orofac Pain 2004; 18: 293–298. [PubMed] [Google Scholar]
- 19.Iwata K, Fukuoka T, Kondo E, Tsuboi Y, Tashiro A, Noguchi K, Masuda Y, Morimoto T, Kanda K. Plastic changes in nociceptive transmission of the rat spinal cord with advancing age. J Neurophysiol 2002; 87: 1086–1093. [DOI] [PubMed] [Google Scholar]
- 20.Smith KM, Boyle KA, Madden JF, Dickinson SA, Jobling P, Callister RJ, Hughes DI, Graham BA. Functional heterogeneity of calretinin-expressing neurons in the mouse superficial dorsal horn: implications for spinal pain processing. J Physiol 2015; 593: 4319–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham BA, Schofield PR, Sah P, Callister RJ. Altered inhibitory synaptic transmission in superficial dorsal horn neurones in spastic and oscillator mice. J Physiol 2003; 551: 905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bekkers JM, Stevens CF. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature 1989; 341: 230–233. [DOI] [PubMed] [Google Scholar]
- 23.Callister RJ, Walmsley B. Amplitude and time course of evoked and spontaneous synaptic currents in rat submandibular ganglion cells. J Physiol 1996; 490: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz B, Miledi R. Spontaneous and evoked activity of motor nerve endings in calcium Ringer. J Physiol (Lond) 1969; 203: 689–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clements JD, Bekkers JM. Detection of spontaneous synaptic events with an optimally scaled template. Biophys J 1997; 73: 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham BA, Brichta AM, Schofield PR, Callister RJ. Altered potassium channel function in the superficial dorsal horn of the spastic mouse. J Physiol 2007; 584: 121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham BA, Brichta AM, Callister RJ. In vivo responses of mouse superficial dorsal horn neurones to both current injection and peripheral cutaneous stimulation. J Physiol 2004; 561: 749–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis CG. Mechanisms of chronic pain from whiplash injury. J Forensic Leg Med 2013; 20: 74–85. [DOI] [PubMed] [Google Scholar]
- 29.Lambo ME, Turrigiano GG. Synaptic and intrinsic homeostatic mechanisms cooperate to increase L2/3 pyramidal neuron excitability during a late phase of critical period plasticity. J Neurosci 2013; 33: 8810–8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tadros MA, Farrell KE, Schofield PR, Brichta AM, Graham BA, Fuglevand AJ, Callister RJ. Intrinsic and synaptic homeostatic plasticity in motoneurons from mice with glycine receptor mutations. J Neurophysiol 2014; 111: 1487–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu H-J, Carrasquillo Y, Karim F, Jung WE, Nerbonne JM, Schwarz TL, Gereau RW. The kv4.2 potassium channel subunit is required for pain plasticity. Neuron 2006; 50: 89–100. [DOI] [PubMed] [Google Scholar]
- 32.Melnick IV. A-type K+ current dominates somatic excitability of delayed firing neurons in rat substantia gelatinosa. Synapse 2011; 65: 601–607. [DOI] [PubMed] [Google Scholar]
- 33.Melnick IV, Santos SF, Szokol K, Szucs P, Safronov BV. Ionic basis of tonic firing in spinal substantia gelatinosa neurons of rat. J Neurophysiol 2004; 91: 646–655. [DOI] [PubMed] [Google Scholar]
- 34.Ruscheweyh R, Sandkuhler J. Lamina-specific membrane and discharge properties of rat spinal dorsal horn neurones in vitro. J Physiol 2002; 541: 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham BA, Brichta AM, Callister RJ. Recording temperature affects the excitability of mouse superficial dorsal horn neurons, in vitro. J Neurophysiol 2008; 99: 2048–2059. [DOI] [PubMed] [Google Scholar]
- 36.Hu H-J, Alter BJ, Carrasquillo Y, Qiu C-S, Gereau RW. Metabotropic glutamate receptor 5 modulates nociceptive plasticity via extracellular signal-regulated kinase-Kv4.2 signaling in spinal cord dorsal horn neurons. J Neurosci 2007; 27: 13181–13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu HJ, Glauner KS, Gereau R. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. I. Modulation of A-type K+ currents. J Neurophysiol 2003; 90: 1671–1679. [DOI] [PubMed] [Google Scholar]
- 38.Wong TP, Marchese G, Casu MA, Ribeiro-da-Silva A, Cuello AC, De Koninck Y. Loss of presynaptic and postsynaptic structures is accompanied by compensatory increase in action potential-dependent synaptic input to layer V neocortical pyramidal neurons in aged rats. J Neurosci 2000; 20: 8596–8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villanueva-Castillo C, Tecuatl C, Herrera-López G, Galván E J. Aging-related impairments of hippocampal mossy fibers synapses on CA3 pyramidal cells. Neurobiol Aging 2017; 49: 119–137. [DOI] [PubMed] [Google Scholar]
- 40.Luebke JI, Chang YM, Moore TL, Rosene DL. Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience 2004; 125: 277–288. [DOI] [PubMed] [Google Scholar]
- 41.Hickmott P, Dinse H. Effects of aging on properties of the local circuit in rat primary somatosensory cortex (S1) in vitro. Cereb Cortex 2013; 23: 2500–2513. [DOI] [PubMed] [Google Scholar]
- 42.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 2010; 11: 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kato G, Kawasaki Y, Koga K, Uta D, Kosugi M, Yasaka T, Yoshimura M, Ji RR, Strassman AM. Organization of intralaminar and translaminar neuronal connectivity in the superficial spinal dorsal horn. J Neurosci 2009; 29: 5088–5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain 2010; 151: 475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heinke B, Ruscheweyh R, Forsthuber L, Wunderbaldinger G, Sandkuhler J. Physiological, neurochemical and morphological properties of a subgroup of GABAergic spinal lamina II neurones identified by expression of green fluorescent protein in mice. J Physiol 2004; 560: 249–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boakye PA, Schmidt EKA, Rancic V, Kerr B, Ballanyi K, Smith PA. Characterization of superficial dorsal horn neurons from “tamamaki” mice and stability of their GAD67-EGFP phenotype in defined-medium organotypic culture. Neuroscience 2018; 372: 126–140. [DOI] [PubMed] [Google Scholar]
- 47.Punnakkal P, von Schoultz C, Haenraets K, Wildner H, Zeilhofer HU. Morphological, biophysical and synaptic properties of glutamatergic neurons of the mouse spinal dorsal horn. J Physiol 2014; 592: 759–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schoffnegger D, Heinke B, Sommer C, Sandkuhler J. Physiological properties of spinal lamina II GABAergic neurons in mice following peripheral nerve injury. J Physiol 2006; 577: 869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rozycka A, Liguz-Lecznar M. The space where aging acts: focus on the GABAergic synapse. Aging Cell 2017; 16: 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richardson BD, Ling LL, Uteshev VV, Caspary DM. Reduced GABA(A) receptor-mediated tonic inhibition in aged rat auditory thalamus. J Neurosci 2013; 33: 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong TP, Marchese G, Casu MA, Ribeiro-da-Silva A, Cuello AC, De Koninck Y. Imbalance towards inhibition as a substrate of aging-associated cognitive impairment. Neurosci Lett 2006; 397: 64–68. [DOI] [PubMed] [Google Scholar]
- 52.Polgar E, Durrieux C, Hughes DI, Todd AJ. A quantitative study of inhibitory interneurons in laminae I-III of the mouse spinal dorsal horn. PloS One 2013; 8: e78309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Labrakakis C, Rudolph U, De Koninck Y. The heterogeneity in GABAA receptor-mediated IPSC kinetics reflects heterogeneity of subunit composition among inhibitory and excitatory interneurons in spinal lamina II. Front Cell Neurosci 2014; 8: 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foster E, Wildner H, Tudeau L, Haueter S, Ralvenius WT, Jegen M, Johannssen H, Hosli L, Haenraets K, Ghanem A, Conzelmann KK, Bosl M, Zeilhofer HU. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron 2015; 85: 1289–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gradwell MA, Boyle KA, Callister RJ, Hughes DI, Graham BA. Heteromeric alpha/beta glycine receptors regulate excitability in parvalbumin-expressing dorsal horn neurons through phasic and tonic glycinergic inhibition. J Physiol 2017; 595: 7185–7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takazawa T, MacDermott AB. Glycinergic and GABAergic tonic inhibition fine tune inhibitory control in regionally distinct subpopulations of dorsal horn neurons. J Physiol 2010; 588: 2571–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson WB, Graham BA, Beveridge NJ, Tooney PA, Brichta AM, Callister RJ. Different forms of glycine- and GABA(A)-receptor mediated inhibitory synaptic transmission in mouse superficial and deep dorsal horn neurons. Mol Pain 2009; 5: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baccei ML, Fitzgerald M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J Neurosci 2004; 24: 4749–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keller AF, Coull JA, Chery N, Poisbeau P, De Koninck Y. Region-specific developmental specialization of GABA-glycine cosynapses in laminas I-II of the rat spinal dorsal horn. J Neurosci 2001; 21: 7871–7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takazawa T, Choudhury P, Tong CK, Conway CM, Scherrer G, Flood PD, Mukai J, MacDermott AB. Inhibition mediated by glycinergic and GABAergic receptors on excitatory neurons in mouse superficial dorsal horn is location-specific but modified by inflammation. J Neurosci 2017; 37: 2336–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cronin JN, Bradbury EJ, Lidierth M. Laminar distribution of GABAA- and glycine-receptor mediated tonic inhibition in the dorsal horn of the rat lumbar spinal cord: effects of picrotoxin and strychnine on expression of Fos-like immunoreactivity. Pain 2004; 112: 156–163. [DOI] [PubMed] [Google Scholar]
- 62.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 2003; 424: 938–942. [DOI] [PubMed] [Google Scholar]
- 63.Ferrini F, Trang T, Mattioli TA, Laffray S, Del'Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu JM, Cahill CM, Salter MW, De Koninck Y. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(-) homeostasis. Nat Neurosci 2013; 16: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005; 438: 1017–1021. [DOI] [PubMed] [Google Scholar]
- 65.Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, Saarma M. BDNF-induced TrkB activation down-regulates the K+-Cl- cotransporter KCC2 and impairs neuronal Cl- extrusion. J Cell Biol 2002; 159: 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ritzel RM, Patel AR, Pan S, Crapser J, Hammond M, Jellison E, McCullough LD. Age- and location-related changes in microglial function. Neurobiol Aging 2015; 36: 2153–2163. [DOI] [PubMed] [Google Scholar]
- 67.Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 2007; 55: 412–424. [DOI] [PubMed] [Google Scholar]
- 68.Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell 2011; 10: 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev 2007; 65: S173–S176. [DOI] [PubMed] [Google Scholar]
- 70.Campuzano O, Castillo-Ruiz MM, Acarin L, Castellano B, Gonzalez B. Increased levels of proinflammatory cytokines in the aged rat brain attenuate injury-induced cytokine response after excitotoxic damage. J Neurosci Res 2009; 87: 2484–2497. [DOI] [PubMed] [Google Scholar]
- 71.Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain 2009; 13: 263–272. [DOI] [PubMed] [Google Scholar]
- 72.Zarpelon AC, Rodrigues FC, Lopes AH, Souza GR, Carvalho TT, Pinto LG, Xu D, Ferreira SH, Alves-Filho JC, McInnes IB, Ryffel B, Quesniaux VF, Reverchon F, Mortaud S, Menuet A, Liew FY, Cunha FQ, Cunha TM, Verri WA., Jr. Spinal cord oligodendrocyte-derived alarmin IL-33 mediates neuropathic pain. FASEB J 2016; 30: 54–65. [DOI] [PubMed] [Google Scholar]
- 73.Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 2008; 28: 11263–11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Slack SE, Pezet S, McMahon SB, Thompson SW, Malcangio M. Brain-derived neurotrophic factor induces NMDA receptor subunit one phosphorylation via ERK and PKC in the rat spinal cord. Eur J Neurosci 2004; 20: 1769–1778. [DOI] [PubMed] [Google Scholar]
- 75.Fitzgerald M, McKelvey R. Nerve injury and neuropathic pain – a question of age. Exp Neurol 2016; 275: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McKelvey R, Berta T, Old E, Ji RR, Fitzgerald M. Neuropathic pain is constitutively suppressed in early life by anti-inflammatory neuroimmune regulation. J Neurosci 2015; 35: 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]