Abstract
Vertical sleeve gastrectomy (VSG) and Roux-en-Y gastric bypass (RYGB) are the most common surgical options for the treatment of obesity and metabolic disorder. Whereas RYGB may result in greater and more durable weight loss, recent clinical and pre-clinical studies in rats have raised concerns that RYGB surgery may increase risk for alcohol use disorder (AUD). In contrast, recent clinical reports suggest a lesser risk for AUD following VSG, although no preclinical studies have been done to confirm that. Therefore, the present study sought to determine the effects of VSG on ethanol intake and preferences in rodent models using protocols similar to those previously used in animal studies for RYGB. Male Sprague Dawley rats and male C57B6 mice were made obese on a high fat diet (60% kcal from fat) and received VSG or no surgery (controls). All animals then were given access to increasing concentrations of ethanol (2%, 4%, 6%, and 8%), presented for few days each. Compared to controls, VSG rats consumed significantly less of 2, 6 and 8% ethanol and showed significantly reduced preferences to 6 and 8% ethanol over water. VSG mice also displayed reduced intake and preference for 6 and 8% ethanol solutions. After a two-week period of forced abstinence, 8% ethanol was reintroduced and the VSG rats and mice continued to exhibit reduced consumption and less preference for ethanol. Regarding the underlying mechanism, we hypothesized that the removal of the ghrelin producing part of the stomach in the VSG surgery is a possible contributor to the observed reduced ethanol preference. To test for functional changes at the ghrelin receptors, the VSG and control rats were given IP injections of acyl-ghrelin (2.5 nmol and 5 nmol) prior to ethanol access. Neither concentration of ghrelin resulted in a significant increase in 8% ethanol consumption of VSG or control subjects. Next, the rats were given IP injections of the ghrelin receptor antagonist, JMV (2.5 mg/kg body weight). This dose induced a significant reduction in 8% ethanol consumption in the VSG group, but no effect on ethanol intake in the controls. While ghrelin injection was uninformative, increased sensitivity to subthreshold doses of the ghrelin receptor antagonist may indicate reduced ghrelin signaling following VSG. Overall, these findings suggest that bariatric patients with increased susceptibility to AUD may benefit from receiving VSG instead of RYGB surgery, and that changes in ghrelin signaling, at least in part, may play a role in the differential AUD risks between the two most commonly performed bariatric surgical procedures.
Keywords: dietary obesity, bariatric surgery, rodent models, gut-brain signaling, ghrelin, alcohol use disorder
1. Introduction
Obesity and its associated health consequences are among the main causes of preventable morbidity and mortality (Flegal, Carroll et al. 2012). Finding a cure for obesity has proven remarkably challenging given its multifactorial etiology and the complexity of systems involved. Currently, the only effective treatment for obesity and sustained weight loss is surgery, with the two most common bariatric operations used as a treatment for obesity and associated metabolic disorder being the vertical sleeve gastrectomy (VSG) and the Roux-en-Y gastric bypass (RYGB) surgery. A recent worldwide survey (Angrisani, Santonicola et al. 2017) found the most commonly performed procedure in the world was VSG that reached 45.9%, followed by Roux-en-Y gastric bypass (RYGB) (39.6%). The older RYGB procedure involves creating a stomach pouch out of a small portion of the stomach and attaching it directly to the small intestine, bypassing a large part of the stomach and the entire duodenum. With the VSG, more than half of the stomach is removed, leaving a thin vertical sleeve. It is estimated that over 200,000 bariatric procedures are performed annually in the U.S. (Prachand 2011), and patients following RYGB typically lose approximately 30% of total body weight or 60–70% of excess body weight (Padwal, Klarenbach et al. 2011). Weight loss and metabolic effects of VSG are similar, although some studies suggested that VSG results in less durable effects (Garb, Welch et al. 2009). These changes can be life-improving, if not life-saving. That said, clinical observations also suggest an increased risk among RYGB patients for use of alcohol (Hsu, Benotti et al. 1998, Ertelt, Mitchell et al. 2008, King, Chen et al. 2012, Suzuki, Haimovici et al. 2012, Blackburn, Hajnal et al. 2016, King, Chen et al. 2017) or other substances (Dutta, Morton et al. 2006, Fogger and McGuinness 2012, Conason, Teixeira et al. 2013), raising concerns about the development of alcohol use disorder (AUD) or addiction in this population. Additionally, Suzuki et al. (Suzuki, Haimovici et al. 2012) found that ~10% of 51 bariatric surgery patients who had either RYGB or gastric banding met criteria for alcohol abuse or dependence 2–5 years post-surgery; none of them had met criteria before surgery.
Different surgical techniques result in different changes in the anatomy of the gastrointestinal tract, and this difference in procedure has posed the question if these anatomical changes can result in technique-specific changes in alcohol use. For example, three prospective studies (King, Chen et al. 2012, Suzuki, Haimovici et al. 2012, Conason, Teixeira et al. 2013, King, Chen et al. 2017) have shown an effect of RYGB, but not gastric banding, a fully restrictive procedure, in increasing alcohol use after 2+ years follow-up. Despite contradictory findings on whether RYGB or restrictive procedures, including VSG, have a similar effect on the pharmacokinetic and metabolism of ethanol (Maluenda, Csendes et al. 2010, Holt 2011, Gallo, Berducci et al. 2015, Pepino, Okunade et al. 2015), larger studies suggest that AUD occurs in significantly fewer patients following restrictive procedures than after RYGB (King, Chen et al. 2012, Ostlund, Backman et al. 2013, Svensson, Anveden et al. 2013, King, Chen et al. 2017).
Rat studies (Hajnal, Zharikov et al. 2012, Thanos, Subrize et al. 2012, Davis, Tracy et al. 2013, Polston, Pritchett et al. 2013) also support that RYGB increases alcohol drinking and reward pointing to a biological mechanism. In contrast, no preclinical studies have yet investigated the effect of VSG on alcohol intake or preference. Therefore, the present study replicated our previous study (Thanos, Subrize et al. 2012) using high fat diet-induced obese rats tested for alcohol intake and preferences in an identical alcohol regimen, but instead of RYGB surgery, the rats and mice in the current study received VSG surgeries.
Regarding a plausible underlying mechanism, recent studies have suggested changes in brain dopamine functions following bariatric surgery (Dunn, Cowan et al. 2010, Steele, Prokopowicz et al. 2010, de Weijer, van de Giessen et al. 2014, Reddy, Wasserman et al. 2014, Hankir, Ashrafian et al. 2015, Blackburn, Hajnal et al. 2016, Han, Tellez et al. 2016, van der Zwaal, de Weijer et al. 2016). In fact, dopamine and related brain areas have been shown to play a critical role in ethanol consumption and addiction (Tabakoff and Hoffman 2013, Vanderlinden, Saba et al. 2013). Additionally, hormones that change after bariatric surgery, such as leptin and ghrelin (Korner, Inabnet et al. 2009, Shin, Zheng et al. 2010, Beckman, Beckman et al. 2011), are also known to modulate the dopamine reward system (Abizaid, Liu et al. 2006, Abizaid 2009, Figlewicz and Benoit 2009, Dunn, Kessler et al. 2012), as well as ethanol consumption (Wurst, Rasmussen et al. 2007, Jerlhag, Egecioglu et al. 2009, Dunn, Kessler et al. 2012). Consistent with the above preclinical work, clinical studies have reported changes in blood ghrelin levels in alcoholic patients versus controls and a positive correlation between blood levels of ghrelin and alcohol craving (Addolorato, Capristo et al. 2006, Badaoui, De Saeger et al. 2008, Koopmann, von der Goltz et al. 2012, Leggio, Ferrulli et al. 2012). Notably, a recent human laboratory study with heavy drinking alcohol-dependent subjects demonstrated a causal link by showing that IV ghrelin infusion, compared to placebo, resulted in an acute increase in cue-induced craving for alcohol (Leggio, Zywiak et al. 2014).
Ghrelin is a 28-amino acid peptide mainly produced by the stomach and acts as the endogenous ligand for the growth hormone secretagogue receptor (GHS-R1a) (Kojima, Hosoda et al. 1999). After acetylation in position 3, acyl-ghrelin acts via its ghrelin 1A receptor (GHS-R1A), which is expressed in the brain. Ghrelin is able to cross the blood-brain-barrier via a saturable transporter (Banks, Tschöp et al. 2002) where it activates hypothalamic orexigenic neurons and inhibits anorectic neurons to induce hunger and stimulate feeding (Tschop, Smiley et al. 2000, Druce, Wren et al. 2005). GHS-R1a’s are highly co-expressed with dopamine receptors in the midbrain, raphe nuclei, and ventral tegmental area (VTA) (Katayama, Nogami et al. 2000, Jiang, Betancourt et al. 2006, Zigman, Jones et al. 2006), suggesting that ghrelin modulates reward processing.
Relevant to the present study, ghrelin increases ethanol consumption and GHS-R1A antagonism blocks the rewarding effects of ethanol in rodents (Jerlhag, Egecioglu et al. 2009, Landgren, Simms et al. 2011). Ghrelin also stimulates dopamine neurons in the VTA (Tessari, Catalano et al. 2007, Disse, Bussier et al. 2010, Egecioglu, Jerlhag et al. 2010, Perello, Sakata et al. 2010, Skibicka, Hansson et al. 2011, Skibicka, Shirazi et al. 2012) and increases dopamine release in terminal areas, including the nucleus accumbens (Jerlhag, Egecioglu et al. 2006, Jerlhag, Egecioglu et al. 2007, Abizaid, Mineur et al. 2011). Remarkably, ethanol-induced dopamine release is absent in ghrelin knock-out mice (Jerlhag, Landgren et al. 2011). Because most studies in obese subjects find lower plasma ghrelin levels (Tschop, Weyer et al. 2001), it may be assumed that chronically reduced ghrelin levels may also contribute to obesity-related dopamine deficits (Wang, Volkow et al. 2001, Stice, Spoor et al. 2008, Geiger, Haburcak et al. 2009, Stice, Yokum et al. 2011). As a case in point, a recent PET study reported an association between blood ghrelin levels and dopamine D2 receptor availability in obese subjects’ limbic brain areas (Dunn, Kessler et al. 2012). A recent study (Hajnal, Zharikov et al. 2012) showed that RYGB rats were more sensitive to a ghrelin receptor antagonist in reducing their ethanol intake compared to obese controls, thus suggesting a functional relationship between improved ghrelin signaling and increased ethanol reward. Based on these and other considerations, we tested involvement of ghrelin in ethanol intake and preferences in rats that received VSG or control surgery. Specifically, rats were treated with ghrelin or the ghrelin receptor antagonist, JMV 2959 (EMD Millipore), and ethanol intake was measured to determine whether VSG alters ghrelin receptor sensitivity in controlling ethanol intake and preferences.
2. Material and Methods
2.1. Animals
2.1.1. Rats
Sixteen adult, male Sprague-Dawley rats (Charles River Laboratories, Wilmington, Massachusetts) were maintained on high fat diet (60% kcal from fat) for the entirety of the study. These diet induced obese rats were then separated in two groups. One group of eight rats received VSG surgeries (VSG rats) and the other group of eight rats received no surgery and served as high fat diet controls (Control rats). All rats were housed in single cages on a 12hr light-dark cycle, and maintained on ad libitum water.
2.1.2. Mice
Nine adult, C57B6 male mice (Jackson Laboratory) were placed on a high fat diet (60% kcal from fat). Four received vertical sleeve gastrectomy surgeries (VSG mice), while the remaining five received no surgery (Control mice). All mice were housed in single cages on a 12-hr light dark cycle, and maintained on ad libitum water.
2.2. Two Bottle Choice Tests
Two bottle choice tests were used to determine ethanol preference between groups in both rats and mice. Animals were habituated to ethanol via access to ethanol solution increasing in percentage over time following previously published protocols (Thanos, Subrize et al. 2012). Each ethanol solution was made using 190 proof ethyl alcohol (Pharmco-Aaper) and dH2O. Rats had 24 hour access to inverted graduated cylinders with sipper tubes, one containing tap water and the other containing a designated percentage of ethanol solution. Water and ethanol bottle positions were swapped left and right each day. Food consumption and body weights of each animal were measured at gram accuracy once a week. The order and period of time exposed to each ethanol concentration followed the protocols used previously to test RYGB rats (Thanos, Subrize et al. 2012), and is as follows. Animals were given access to 2% ethanol solution for the first four days of the study. The ethanol percentage was increased to 4% for days 5–8, which was then increased to 6% for the following two weeks. Then animals were given 8% ethanol solution for 10 days. After this period of habituation, all participants had a two week period of forced abstinence, where the two bottle choice was still present, however both bottles contained water. After this period of force abstinence 8% ethanol solution was reinstated for four days.
2.3. Ghrelin treatment
Acylated rat ghrelin (TOCRIS) was dissolved in 0.9% sodium chloride to the concentrations of 2.5 nmol/0.5 ml and 5 nmol/0.5 ml. All injections, (regardless of concentration) were intraperitoneal at a volume of 0.5 ml at 9am (two hours into day cycle) and 24 hour ethanol intake was measured.
On the first day, each animal was given an intraperitoneal (IP) injection of a vehicle consisting of 0.5 ml 0.9% sodium chloride. On day two, a ghrelin injection was given as a single dosage of 2.5 nmol/0.5 ml, which has been established as an effective dosage for ethanol intake (Cepko, Selva et al. 2014). A vehicle injection of 0.5 ml 0.9% sodium chloride was administered on day three. On day four, each animal was again given a single injection of the ghrelin solution, this time at a concentration of 5 nmol/0.5 ml saline. On all days, food (high fat diet - of 60% kcal from fat), water, and an 8% ethanol solution were available ad libitum. Body weight was taken daily and food, water, and ethanol intake was measured every 2, 4, 6, and 24 hours post injection.
2.4. Ghrelin receptor antagonist treatment
After a washout period following ghrelin administration, ghrelin receptor antagonist, JMV 2959 (EMD Millipore), was dissolved in 0.9% sodium chloride and given IP at a dosages of 2.5 mg/kg and 5 mg/kg body weight an hour before the beginning of the dark cycle and overnight ethanol intake was measured.
In the first two days, both groups of rats were injected with 1 ml 0.9% sodium chloride (vehicle). On the third day, an injection of JMV at a dosage of 2.5 mg/kg body weight was administered, as this was a minimum dosage found to be effective in changing ethanol consumption in rats (Landgren, Simms et al. 2012). On all days, food (high fat diet - of 60% kcal from fat), water, and an 8% ethanol solution were available ad libitum and intake of each was measured every 24 hours. Daily measurements were continued for another two days following JMV injections. This protocol was then repeated with a dosage of 5 mg/kg body weight solely for the control group.
2.5. Statistical analyses
2.5.1. Two Bottle Choice Tests
Ethanol consumption was analyzed using 2-Way ANOVA comparing the daily intakes of VSG and control groups at individual concentrations of ethanol (2, 4, 6, and 8%). Daily intake of ethanol was converted to grams and then adjusted for kg body weight of each animal. This body weight adjustment was conducted for all percentages and 2-Way ANOVA was used to compare intake of each percentage between VSG and control groups.
Percent preference was determined by dividing ethanol intake by the cumulative water and ethanol intake for each day. The percent preference for VSG and control groups was compared for each percentage using 2-Way ANOVA.
For the 8% ethanol reinstatement portion of the experiment the overall intake of ethanol was averaged over the four days of the experiment and a t-test was used to compare VSG to control groups. Daily intake was corrected for body weight and averaged over the 4 day exposure period for both groups and then compared using an unpaired t-test.
Percent preference was calculated by dividing ethanol intake by the cumulative water and ethanol intake for each day. These preferences were then averaged over the four days for individual animals and the VSG and control groups were compared using an unpaired t-test.
2.5.2. Ghrelin Treatment
Two days of saline injections were averaged and only animals with an intake above 0.0 ml were used for comparison purposes. Consumption of 8% ethanol was measured daily, adjusted to individual body weight, and preference for ethanol over water was calculated. The daily means for the saline, 2.5 nmol, and 5 nmol treatments were compared between the VSG and control groups using 2-Way ANOVA (repeated measures) and post-hoc Sidak’s multiple comparisons tests. The percent change from the baseline ethanol intake (i.e., following saline injection) was calculated for 2.5 nmol and 5 nmol injections and the surgical groups (VSG and control) were compared using 2-Way ANOVA (repeated measures).
2.5.3. Ghrelin receptor antagonist treatment
Two days of saline injections were averaged and only animals with an intake above 0.0 ml were used for comparison purposes. An intake below zero demonstrates an animal that did not drink ethanol, therefore attempting to reduce the intake would result in confounding data. Consumption of 8% ethanol was measured daily, adjusted to individual body weight, and preference for ethanol over water was calculated. The means for JMV and saline treatments were compared between the VSG and control groups using 2- Way ANOVA and post-hoc Sidak’s multiple comparisons tests.
3. Results
3.1. Two Bottle Choice Tests
The results of the two bottle choice tests over the 2, 4, 6, and 8% ethanol access periods for the rat and mice groups are summarized in Figure 1. VSG rats displayed an overall reduced ethanol intake compared to controls (Fig 1A). During the 2% ethanol concentration period, the VSG rats consumed significantly less ethanol than the control group (F1,56=10.53, P= 0.0020). The VSG rats continued to consume less ethanol at the 4% concentration; however statistical tests showed no significant difference between the groups. During the 6% ethanol concentration period, the VSG rats once again consumed significantly less ethanol than the control group (F1,196=33.47, P<0.0001). At the 8% ethanol concentration, VSG rats drank significantly less ethanol solution than controls (F1,140=36.47, P<0.0001).
Figure 1.
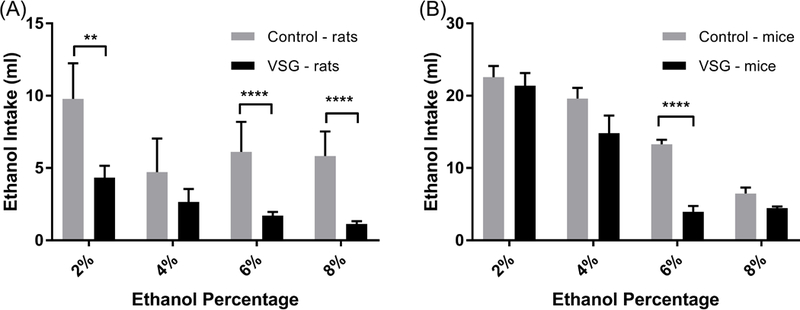
Mean ethanol intake for increasing concentrations of ethanol by VSG and Control groups. (A) Rats: 2-Way ANOVA showed significant difference between VSG and control groups for 2% (**P ≤ 0.01), 6%, and 8% (****P ≤ 0.0001) ethanol concentrations. (B) Mice: 2-Way ANOVA shows significant difference between VSG and Control groups for the 6% (****P ≤ 0.0001) ethanol concentration.
VSG mice showed similar results in the two bottle tests (Fig. 1B) in that they consistently drank less ethanol than controls at 2, 4, 6 and 8% solutions. However this reduced intake did not reach statistical significance for 2%, 4%, or 8%. VSG mice did consume significantly less ethanol than controls for the 6% ethanol solution (F1,91=22.03, P<0.0001).
When ethanol intake was adjusted to rat body weight (Fig. 2A), only the 6% and 8% ethanol solutions showed significant differences between the VSG and control rats. Specifically, the VSG group consumed significantly less of the 6% (F1,196=28.69, P<0.0001) and 8% (F1,140=33.15, P<0.0001)ethanol solution compared to controls.
Figure 2.
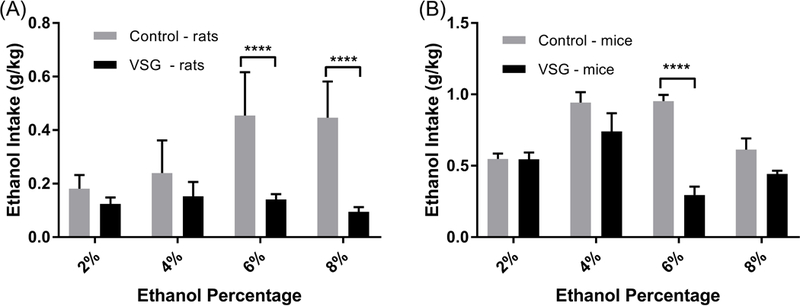
Mean consumption of 2, 4, 6, and 8% ethanol solution normalized to g/kg body weight. (A) Rats: 2-Way ANOVA revealed significant difference between VSG and Control groups for 6% and 8% (****P ≤ 0.0001) ethanol concentrations. (B) Mice: 2-Way ANOVA revealed significant difference between VSG and Control groups for the 6% (****P≤ 0.0001) ethanol concentration.
Mean ethanol intake adjusted for mouse body weight is summarized in Figure 2B. Once again reduced ethanol intake in VSG mice compared to controls did not reach significance with the 2%, 4%, and 8% solutions. However, VSG mice consumed significantly less 6 % ethanol solution than controls (F1,91=21.02, P<0.0001).
A similar pattern appeared for reduced intake by the VSG rats when computing percent preference for ethanol over water, as shown in Figure 3A. Overall, control rats had a higher percent preference for ethanol over water than VSG rats for all percentages. However, the 2% and 4% ethanol solutions did not show statistical difference between the two groups. During the 6% ethanol exposure, control rats had a higher percent preference for ethanol over water (F1,196=28.65, P<0.0001). The 8% ethanol concentration showed a similar result, with control rats having a higher percent preference for ethanol over water (F1,140=35.89, P<0.0001).
Figure 3.
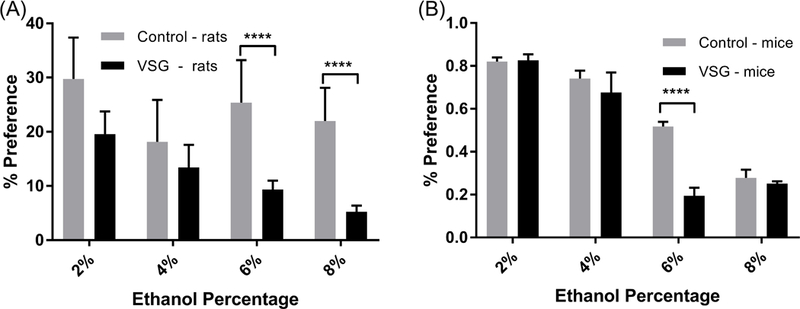
Mean percent preference for 2, 4, 6, and 8% ethanol solutions over water. (A) Rats: 2-Way ANOVA revealed significant differences between VSG and control groups at 6% and 8% (****P ≤ 0.0001) ethanol concentrations. (B) Mice: 2- Way ANOVA revealed significant difference between VSG and Control groups for the 6% (****P ≤ 0.0001) ethanol concentrations.
Figure 3B summarizes mouse preference for ethanol over water at 2, 4, 6, and 8% ethanol concentrations. This data follows the same pattern of the previous mouse data, in that no significant difference between VSG and control mice is seen for 2%, 4%, and 8% ethanol solutions. VSG mice show significant reduction in preference for ethanol over water for 6% ethanol (F1,91=18.18, P<0.0001).
The period of reinstatement of intake of 8% ethanol concentration for rats and mice is summarized in Figure 4. The VSG rats showed a significantly lower intake of ethanol averaged across a four day period (t(14)=2.287, P=0.0383) (Fig. 4A). When ethanol consumption was adjusted for body weight, VSG rats consistently drank less ethanol overall (Fig. 5A). However, statistical analysis of ethanol consumption in g/kg BW revealed a p-value just outside the significant range (t(14)=2.135, P=0.0509). VSG mice showed significantly reduced ethanol consumption compared to controls overall (t(10)=3.19, P=0.0096)and when adjusted for body weight(t(10)=3.044, P=0.0124)(Figs. 4B, 5B). Percent preference was then calculated for rats and mice over the four day period and compared using a t-test. Overall similar results were achieved for rats in that the control rats tended to have a higher percent preference for ethanol over water, but the t-test missed statistical significance (t(14)=1.986, P = 0.0670) (Fig. 6A). VSG mice displayed a significantly reduced preference for ethanol over water compared to controls (t(10)=2.459, P=0.0337) (Fig. 6B).
Figure 4.
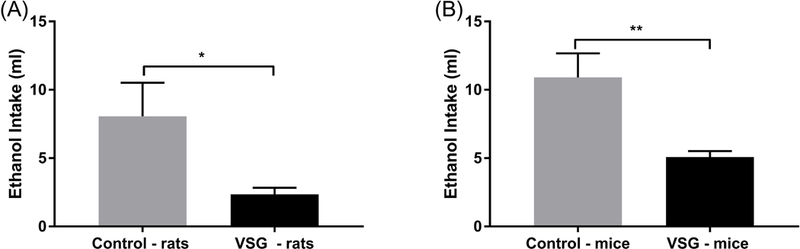
Mean ethanol intake averaged over 4 days of 8% ethanol re-exposure after a 2-week period of forced abstinence. (A) Rats: an unpaired t-test comparing the VSG to control intake revealed a significant difference (*P ≤ 0.05). (B) Mice: an unpaired t-test comparing VSG to control intake revealed a significant difference between VSG and Control ethanol intake (**P ≤ 0.01).
Figure 5.
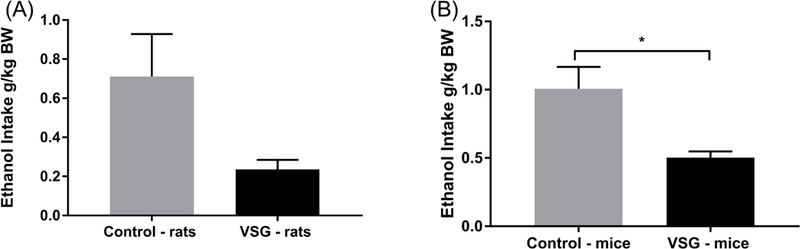
Mean consumption following reinstatement of 8% ethanol adjusted for body weight. (A) Rats: an unpaired t-test did not show statistically significant difference between the two groups. (B) Mice: an unpaired t-test showed significant difference between VSG and Control groups (*P ≤ 0.05)
Figure 6.
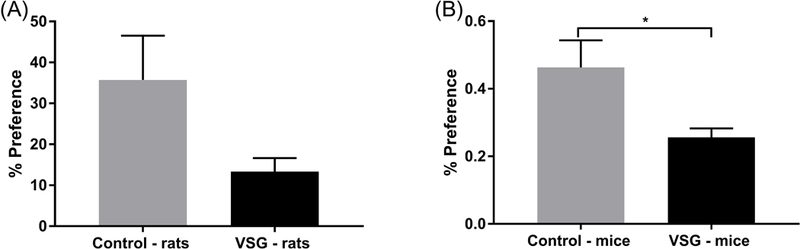
Mean percent preference for ethanol following reinstatement of 8% ethanol averaged across a 4 day experimental period. (A) Rats: an unpaired t-test did not show significant difference between the VSG and control groups. (B) Mice: an unpaired t-test showed a significant difference between VSG and Control groups (*P ≤ 0.05).
3.2. Ghrelin administration
Acylated rat ghrelin was given intraperitoneally to VSG and control groups at 2.5 nmol and 5 nmol concentrations and 8% ethanol intake was monitored daily. No change in overall ethanol consumption or ethanol consumption adjusted for body weight is apparent for the VSG and control groups after the 2.5 or 5 nmol injections (Figs. 7A and 8A). 2-Way ANOVAS were performed on 24 hour ethanol consumption and consumption adjusted for body weight and did not result in a significant difference.
Figure 7.
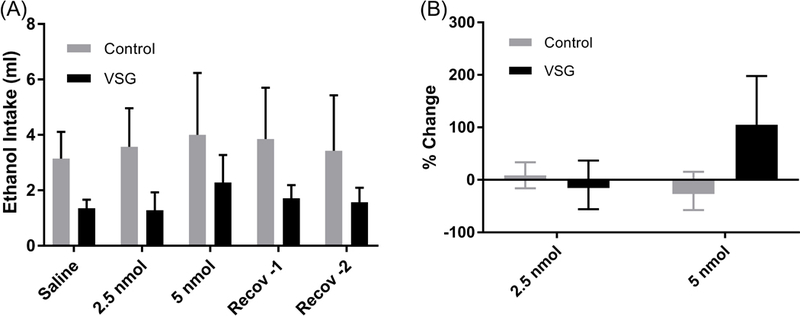
Mean 8% ethanol intake and percent change in consumption from saline. (A) Mean intake of 8% ethanol post IP injection of 2.5 and 5 nmol ghrelin. 2-Way ANOVA resulted in no significant difference between VSG and control groups. (B) % Change: One Way ANOVA showed no significant difference among the three treatments (saline, 2.5 nmol ghrelin, 5 nmol ghrelin).
Figure 8.
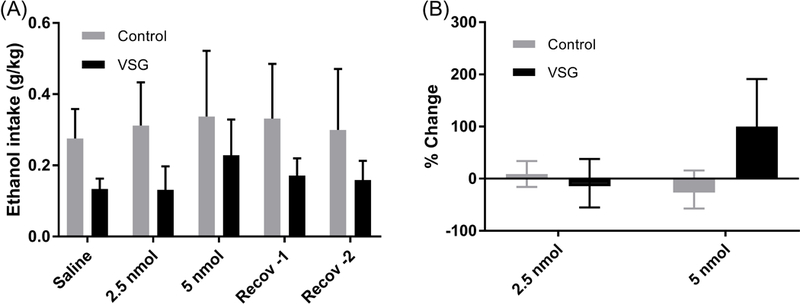
Mean 8% ethanol intake by body weight and percent change in consumption from saline. (A) Mean intake of 8% ethanol adjusted for body weight post IP injection of 2.5 and 5 nmol ghrelin. 2-Way ANOVA resulted in no significant difference between VSG and control groups. (B) % Change: One Way ANOVA showed no significant difference among the three treatments (saline, 2.5 nmol ghrelin, 5 nmol ghrelin).
A percent difference was calculated for both injection concentrations, using saline injections as baseline consumption and is summarized in Figures 7B and 8B. For the 2.5nmol injections, neither the VSG nor the control groups showed a change from the baseline. A mean increase in ml ethanol consumption of 104% was calculated for the 5 nmol injection in the VSG group, while the controls showed little to no change (Fig. 7B). 2-Way ANOVA for percent change in ml ethanol consumption was not significant. Percent change in ethanol consumption adjusted for body weight after IP injections is summarized in Figure 8B. When adjusted for body weight (g/kg), there remained little difference in percent change from the saline baseline for the 2.5 nmol injections. However, 5 nmol injections resulted in a 99% increase in percent change in the VSG group. No such increase was observed for the control group at the 5 nmol ghrelin concentration. However, 2-Way ANOVA resulted in no significant difference in percent change between VSG and control groups.
Percent preference for the 8% ethanol over water was calculated for VSG and Control groups and summarized in Figure 9A. VSG and control groups maintained their earlier pattern with no change in either the 2.5 or 5 nmol injections. Two-way ANOVA of ethanol preference resulted in no significant difference between VSG and control groups.
Figure 9.
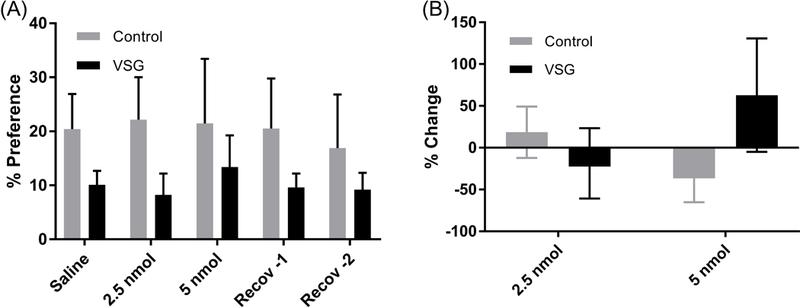
Mean percent preference for 8% ethanol and percent change in consumption from saline. (A) Mean percent preference for 8% ethanol over water post IP injection of 2.5 and 5 nmol ghrelin. % Preference: 2-Way ANOVA resulted in no significant difference between VSG and control groups. (B) % Change: One Way ANOVA showed no significant difference among the three treatments (saline, 2.5 nmol ghrelin, 5 nmol ghrelin).
Percent change in VSG and control preference for ethanol from a saline baseline was calculated for the 2.5 and 5 nmol injection days (Fig. 9B). The 2.5 nmol injections showed little to no change in preference for the control and VSG groups. The 5 nmol injections showed a slight decrease in preference for the control group but a mean increase in preference of 62% for the VSG rats. Two-way ANOVA of percent change in preference for ethanol over water however, resulted in no significant difference between VSG and control groups.
3.3. Ghrelin receptor antagonist treatment
Ghrelin receptor antagonist (JMV) was given as IP injections to both groups of rats at a dose of 2.5 mg/kg and 8% ethanol intake was measured for the VSG and control groups every 24 hours for three days following the injection. Overall ethanol intake was reduced for the VSG group the day of and two days following JMV injection (Figure 10A). Two-way ANOVA showed statistical difference between VSG and control groups (F1,7=6.539, P=0.0377) and Sidak’s multiple comparison’s test confirmed statistical differences between the two groups on recovery days 1 and 2 (P=0.0358, P=0.0480). When ethanol intake is adjusted for body weight, the decrease in ethanol intake for VSG animals is still apparent and the difference between the two groups remains significant (Figure 10B). Two-way ANOVA resulted in significant difference between the two groups (F1, 7=6.466, P=0.0385) and Sidak’s multiple comparisons tests confirm a significant difference between the two groups on the second day of recovery (P=0.0364). IP injection of JMV also resulted in a decreased preference for ethanol over water within the VSG group but not the control group (Figure 10C). Two-way ANOVA resulted in a significant difference between the two groups with (F1, 7=7.011, P=0.0330) and Sidak’s multiple comparisons test confirmed a significant difference between the two groups on recovery days 1 and 2 (P=0.0181, P=0.0323). Following a similar pattern as ethanol intake, ethanol preference returns to pre-injection levels by the third day of recovery. A second dose of JMV was then given to the control rat groups at 5 mg/kg in an attempt to induce a reduction in ethanol consumption as was seen in the VSG rats at 2.5 mg/kg and is summarized in Figures 10A–C. This dosage was found to reduce the overall consumption of ethanol and preference for ethanol over water in the control group on the day of injection with a gradual return to normal intake and preference over the next 2 days.
Figure 10.
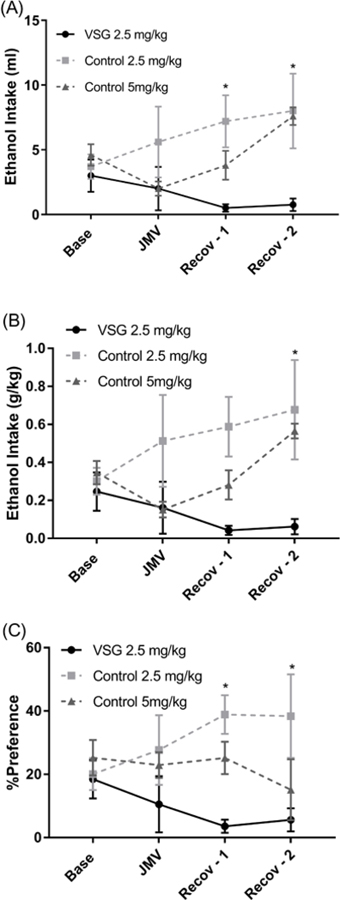
Mean ethanol intake and preference post JMV injection. (A)Mean ethanol (8%) intake in ml of VSG and control rats post injection of JMV. Two-way ANOVA showed significant difference between VSG and control groups for 2.5 mg/kg injections (*P ≤ 0.05). (B) Mean ethanol (8%) intake of VSG and control rats adjusted for body weight post injection of JMV. Two-way ANOVA showed significant difference between VSG and Control groups for 2.5 mg/kg injections (*P ≤ 0.05). (C) Mean percent preference for ethanol (8%) over water of VSG and control rats post injection of JMV. 2 – Way ANOVA showed a significant difference between VSG and Control groups for 2.5mg/kg injections (*P ≤ 0.05).
4. Discussion
4.1. Summary of findings
The far reaching goal of this study was to establish VSG surgery as a viable option for weight loss by demonstrating a lack of increased ethanol intake after surgery. What we showed here is that both rats and mice, having undergone VSG surgery, reduce, rather than increase, their overall ethanol consumption and reduced, rather than increase, their preference for ethanol over water in comparison to non-surgical controls.
In the case of the mouse studies, there was a tendency for reduced intake and preference of VSG mice for the 4, 6 and 8% concentrations, with the intake and preference for the 6% solution reaching significance. It should be noted that, despite the lack of statistically significant effect, VSG mice consistently demonstrated lower ethanol consumption and preference for ethanol throughout the acclimation period. The most telling observation, however, was made for mice when evaluated following a 2 week abstinence period. At this juncture, overall ethanol intake, ethanol intake adjusted for body weight, and preference for ethanol over water were all significantly lower for the VSG mice than for the control mice. Based on this finding, we can confidently conclude that mice having undergone VSG surgery will consume less ethanol and have a lower hedonic motivation (preference) to drink ethanol than mice that have not had surgery at all.
In the rat studies, the VSG rats showed an overall reduced consumption of ethanol solutions during the acclimation period. Most notable is that the ethanol intakes of the higher concentrations (6 and 8% EtOH) were significantly lower in the VSG rats than in controls. Therefore, these VSG rats not only consumed less ethanol overall but also displayed a greater lack of preference for higher ethanol percentages than their non-surgical counterparts. This decreased preference for high concentrations of ethanol is carried over after the abstinence period and into the 8% re-exposure period, where the VSG rats continued to consume significantly less ethanol and showed a lower preference for ethanol over water than control rats. This part of the study demonstrates, in both mice and rats, that not only does VSG surgery not increase ethanol intake, as it does in RYGB rats (Hajnal, Zharikov et al. 2012, Thanos, Subrize et al. 2012, Polston, Pritchett et al. 2014), it actually decreases the intake and preference for ethanol. These findings point to VSG as a possible alternative to RYGB weight loss surgery, particularly for those at risk for alcohol use disorder.
Based on evidence for the role of ghrelin on ethanol intake (see Introduction) and a previous study conducted in RYGB rats (Hajnal, Zharikov et al. 2012), we hypothesized that altered ghrelin signaling may also play a role in the differential effects of RYGB and VSG surgeries on ethanol intake and preference. In order to test this hypothesis, we gave IP injections of acylated ghrelin at 2.5 and 5 nmol concentrations to both VSG and control rat groups. It was assumed that ghrelin signaling is reduced after VSG because of the removal of much of the body of the stomach, whose lining produces most of the body’s circulating ghrelin. Therefore, one may assume that due to lower ghrelin production, and in turn to upregulation of the ghrelin receptor, over time an individual with VSG would become more sensitive to increases in ghrelin. If this was indeed the case, then the injection of subthreshold amounts of ghrelin should have an effect of increased ethanol consumption in a more sensitive animal (VSG) and produce little to no effect in an animal that does not have increased sensitivity to such signaling (control). However, both the 2.5 and 5 nmol injections of acyl-ghrelin resulted in no significant change in overall consumption of ethanol or in preference for ethanol over water. This lack of change in ethanol consumptive behavior after injection of exogenous ghrelin could be due in part to the varying levels of endogenous ghrelin in both the VSG and control groups and therefore may not be the most appropriate indicator of altered ghrelin signaling. Furthermore, the subthreshold levels used were found to be effective in normal diet, non-obese rats (Cepko, Selva et al. 2014) therefore it may be more appropriate, for studies such as the one performed here, to establish a dose dependent curve for HFD animals based on body weight for future studies. It should be noted that ghrelin also mediates consumptive behaviors via the GHS-R1α type receptors on the vagus nerve by suppressing vagal afferent firing (Inui, Asakawa et al. 2004, Grabauskas, Wu et al. 2015) but is blocked after surgical procedures involving vagotomy (Date, Murakami et al. 2002, Inui, Asakawa et al. 2004, le Roux, Neary et al. 2005).However, retrograde labeling using the fast blur tracer showed that afferent and efferent neurons at the nodose ganglion and dorsal motor nucleus of the vagus remain unchanged after VSG surgery but are significantly diminished after RYGB surgery (Ballsmider, Vaughn et al. 2015). This observation suggest that a change in gastric vagal afferent sensing of ghrelin following the VSG surgery, at least in our model, is not a major contributing factor.
After the ghrelin was shown to not have differential effects on VSG and control groups, we then checked to see whether the antagonist would also be successful in causing a differential change in ethanol consumption in VSG rats occurring at lower doses than in controls. The ghrelin receptor (GHS-R1a) antagonist was given as IP injections of 2.5 mg/kg body weight and was expected to result in a decrease in ethanol intake and preference. The VSG group displayed the expected decrease while the control groups did not. It is interesting to note that the injection of controls with 5 mg/kg JMV resulted in a successful reduction in ethanol consumption and preference.
Collectively, these results demonstrate an increased sensitivity to ghrelin antagonism at subthreshold doses in the VSG animals interfering with ethanol intake compared to controls, hence further supporting a role for altered ghrelin signaling in postoperative ethanol intake.
4.2. Clinical and preclinical studies on alcohol use after bariatric surgery
Although not without some conflicting results, most of the published literature suggests that bariatric surgery represents a risk for increased ethanol use in humans (Hsu, Benotti et al. 1998, Ertelt, Mitchell et al. 2008, King, Chen et al. 2012, Suzuki, Haimovici et al. 2012, Conason, Teixeira et al. 2013, King, Chen et al. 2017), and as discussed in a recent systematic review (Blackburn, Hajnal et al. 2016). Longitudinal studies have shown new onset ethanol use with a significant increase in consumption after RYGB. Specifically, three prospective studies have shown an effect of RYGB, but not gastric banding, in increasing alcohol use after 2+ years follow-up (King, Chen et al. 2012, Conason, Teixeira et al. 2013, Ivezaj, Saules et al. 2014). These results point to a concern that, as the number RYGB surgeries increases, alcohol treatment centers are seeing more and more preventable cases of AUD. For example, a recent retrospective study looking at electronic medical records reported that out of 823 patients seeking treatment for AUD, 4.9% had a RYGB procedure (Cuellar-Barboza, Frye et al. 2015). Given that up to 200,000 procedures are done every year this puts a large part of the population at potential risk. Furthermore, in a 22-year long study comparing gastric bypass to other weight loss surgeries (i.e. banding surgeries), as well as non-surgical controls, gastric bypass patients were consistently shown to be at higher risk for AUD diagnosis (Svensson, Romeo et al. 2012).
To date there are only a few animal studies that investigated the effects of bariatric surgery on ethanol intake, and all were limited to the RYGB procedure. To our best knowledge, the present study represents the first preclinical study on the effect of VSG surgery on alcohol use. Davis and colleagues reported a decreased risk for ethanol abuse following RYGB surgery (Davis, Schurdak et al. 2012). This study, however, used ethanol preferring rats. In contrast, in outbred high fat diet-induced obese rats that maybe viewed as a better model for the human RYGB population (i.e., with a multigenic and non-alcoholic background), RYGB rats showed increased ethanol preference and consumed twice as much ethanol as sham-operated obese controls and 50% more than normal-diet lean controls (Thanos, Subrize et al. 2012, Fonseca, Schuster et al. 2013). Of special importance to separating gastrointestinal factors from more direct effects of ethanol on the brain, recent studies (Hajnal, Zharikov et al. 2012, Polston, Pritchett et al. 2013) found increased ethanol self-administration in RYGB rats with both oral and intravenous routes of administration. These findings provide strong evidence that the facilitative effect of RYGB on ethanol intake is also present when the pharmacokinetic effects from the changes in absorption of ethanol from the gut is controlled for.
4.3. Potential underlying mechanisms of altered alcohol intake following bariatric surgery
The increase in ethanol intake and preference in rats, found in previous experiments (Hajnal, Zharikov et al. 2012, Thanos, Subrize et al. 2012, Fonseca, Schuster et al. 2013), supports the notion that biological changes following RYGB surgery contribute to increased risk of AUD in surgical patients. Multiple factors likely contribute to increased chronic ethanol use after RYGB. For example, RYGB patients have higher and longer-lasting blood ethanol concentrations, and a shorter period of onset than non-surgical controls when consuming similar amounts of ethanol (Klockhoff, Naslund et al. 2002, Hagedorn, Encarnacion et al. 2007, Holt 2011, Woodard, Downey et al. 2011, Pepino, Okunade et al. 2015). Changes in ethanol’s pharmacokinetics may alter not only ethanol bioavailability and stimulating properties, but may also influence the neuronal and hormonal signals upstream of the reward system. A few studies have investigated the effects of VSG on alcohol metabolism and have generated contradictory findings. For example, two studies (Changchien, Woodard et al. 2012, Gallo, Berducci et al. 2015) found no change in alcohol metabolism following VSG, whereas another alcohol challenge study (Maluenda, Csendes et al. 2010) reported increased blood alcohol levels and prolonged time to return to zero after VSG surgery compared to the patients preoperative baseline data. Recent studies have also found that bariatric surgery candidates with Binge Eating Disorder (10–27% of RYGB patients) (Mitchell, King et al. 2014) demonstrated some addictive personalities (Gossop and Eysenck 1980, Lent and Swencionis 2012). The ‘symptom substitution’ theory (Kazdin 1982) posits that the elimination of a particular symptom without treating the underlying cause will result in the appearance of a substitute symptom (Niego, Kofman et al. 2007). Similar to the ‘symptom substitution theory’ is the concept of ‘reward-transfer’ (Blum, Bailey et al. 2011). Brain imaging studies suggest that following RYGB, food-cues may elicit reduced activation in brain reward areas (Ochner, Kwok et al. 2011, Ochner, Stice et al. 2012, Scholtz, Miras et al. 2014). RYGB results in blunted activation in the prefrontal cortex (Ochner, Stice et al. 2012), an area involved in inhibition of impulsive behaviors. Collectively, these data suggest a greater risk for some patients to engage in alternative excessive behavior (Wang, Volkow et al. 2004, Kalivas and Volkow 2005, Goldstein, Alia-Klein et al. 2007, Volkow and Baler 2013). However, the extent to which RYGB compared to VSG may alter the motivation to consume ethanol has remained largely unexplored.
As mentioned previously, both RYGB and VSG may disrupt the natural ghrelin response to fasting and food. Given the role for ghrelin in stimulating ethanol reward and intake in both rodents and humans (see the Introduction), therefore, ghrelin is a strong candidate for influencing consumption and preference for ethanol after VSG or RYGB procedures. Ghrelin release from the stomach to the systemic circulation is increased during periods of fasting and subsequently decreases once feeding recommences (Toshinai, Mondal et al. 2001). This occurs partly because ghrelin producing cells in the stomach are closed type cells that are activated by distension, releasing ghrelin when the stomach is relaxed and empty and ceasing ghrelin release when the stomach is full and distended (Sakata and Sakai 2010). After the VSG surgery, this release of ghrelin is greatly reduced (Ramón, Salvans et al. 2012, Chambers, Kirchner et al. 2013) as a likely result of the excision of a large portion of the stomach. On the other hand, RYGB surgeries maintain the entire stomach and the continuity between the stomach and the rest of the GI tract, while the stomach itself is bypassed by the connection of the duodenum to the esophagus. Findings regarding the actual plasma ghrelin levels after RYGB are controversial, with some studies reporting a significant reduction in ghrelin levels right after RYGB both in obese patients and in a rat model of RYGB (Korner, Inabnet et al. 2009, Shin, Zheng et al. 2010) and others reporting increased or unaltered ghrelin levels at various time points after RYGB in humans (Garcia-Fuentes, Garrido-Sanchez et al. 2008, Matzko, Argyropoulos et al. 2012, Barazzoni, Zanetti et al. 2013). Notably, a recent study comparing RYGB and VSG surgical patients at 6 and 18 months post-surgery, found significantly increased plasma ghrelin levels in the RYGB group and significantly decreased plasma ghrelin levels in the VSG group (Alamuddin, Vetter et al. 2017). One may speculate that, at least under certain conditions, the bypassed stomach may continue releasing ghrelin to the circulation, possibly at an even higher amount because the stomach no longer experiences the post meal distension that attenuates ghrelin release. Long term differential release of ghrelin could potentially effect receptor expression, such that increased exposure to ghrelin after RYGB may result in downregulation GHS- R1α, while reduced exposure to ghrelin after VSG may result in upregulation of GHS- R1α. Such changes in postoperative ghrelin signaling and consequently receptor expression and activity may explain the decreased consumption and preference for ethanol observed in VSG rats. Furthermore, the differential effects of ghrelin antagonist on the VSG versus non-surgical group merits further study of this peptide and its role post bariatric surgery.
4.4. Clinical relevance
Worldwide bariatric procedures are increasing in number with RYGB and VSG surgeries as the two most common types of bariatric surgery, each exceeding 200,000 a year (Angrisani, Santonicola et al. 2017). RYGB surgeries in particular are shown to have greater risk for increased ethanol consumption in comparison to other weight loss surgeries, which show no such increase (Conason, Teixeira et al. 2013). Because of its popularity and the increasing data supporting risk of AUD in association with RYGB surgery it is important to consider alternatives for patients that may be at risk. Those individuals with a history of AUD have been shown to be at increased risk for relapse after RYGB surgery specifically (Suzuki, Haimovici et al. 2012). In this paper, we have successfully demonstrated that VSG surgeries are not associated with increased ethanol consumption or preference in rat and mouse models. Therefore, it is a promising option for further study and consideration in the context of AUD and bariatric surgery.
The role that ghrelin plays in the possible mechanism underlying differential ethanol consumption and preference between VSG and RYGB groups helps to shed light on the long term effects of these surgeries, and possibly on unrecognized factors affecting alcohol and substance use disorders as well. As such, further study into this mechanism could also aid in understanding the motivation and mechanisms underlying addictive and drug seeking behaviors.
5. Conclusion
Here we show that as opposed to RYGB, VSG does not increase, and in fact decreases, ethanol intake in rats or mice when maintained on a diet with identical dietary fat content and tested on the same schedule of ethanol access (Thanos, Subrize et al. 2012). These findings are consistent with clinical studies showing that RYGB patients have a significantly increased risk for alcohol abuse diagnosis, self-reported alcohol problems, and have more than double the risk of inpatient treatment for AUD, compared to patients who underwent procedures limited to reduction of the stomach either by vertical banding or VSG (for a review, see (Blackburn, Hajnal et al. 2016)).
Regarding one plausible underlying mechanism, we found differential sensitivity to exogenous manipulation of ghrelin signaling on influencing ethanol intake between RYGB and VSG rats. Specifically, as opposed to RYGB (Hajnal, Zharikov et al. 2012), VSG rats’ increased sensitivity to the ghrelin antagonist may suggest reduced ghrelin signaling contributes to reduced ethanol intake and preferences.
Nevertheless, further clinical and preclinical studies are warranted to identify underlying mechanisms responsible for the differences in alcohol effects following different surgical protocols, including metabolic, pharmacokinetic, neural and hormonal factors. Investigating the potential mechanisms through which bariatric surgery may result in increased alcohol use is important not only to identify patients that may be potentially at increased risk for AUD after surgery (and therefore could be advised on choosing alternative therapies), but also to identify plausible pathways that may represent novel pharmacological targets for the treatment of AUD in general.
Acknowledgements
This research is supported by NIH grant AA024490 (to A.H.).
References
- Abizaid A (2009). “Ghrelin and dopamine: new insights on the peripheral regulation of appetite.” Journal of Neuroendocrinology 21(9): 787–793. [DOI] [PubMed] [Google Scholar]
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB and Horvath TL (2006). “Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite.” Journal of Clinical Investigation 116(12): 3229–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abizaid A, Mineur YS, Roth RH, Elsworth JD, Sleeman MW, Picciotto MR and Horvath TL (2011). “Reduced locomotor responses to cocaine in ghrelin-deficient mice.” Neuroscience 192: 500–506. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Capristo E, Leggio L, Ferrulli A, Abenavoli L, Malandrino N, Farnetti S, Domenicali M, D’Angelo C, Vonghia L, Mirijello A, Cardone S and Gasbarrini G (2006). “Relationship between ghrelin levels, alcohol craving, and nutritional status in current alcoholic patients.” Alcohol Clin Exp Res 30(11): 1933–1937. [DOI] [PubMed] [Google Scholar]
- Alamuddin N, Vetter ML, Ahima RS, Hesson L, Ritter S, Minnick A, Faulconbridge LF, Allison KC, Sarwer DB, Chittams J, Williams NN, Hayes MR, Loughead JW, Gur R and Wadden TA (2017). “Changes in Fasting and Prandial Gut and Adiposity Hormones Following Vertical Sleeve Gastrectomy or Roux-en-Y-Gastric Bypass: an 18-Month Prospective Study.” Obesity Surgery 27(6): 1563–1572. [DOI] [PubMed] [Google Scholar]
- Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H and Scopinaro N (2017). “Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014.” Obesity Surgery: 1–11. [DOI] [PMC free article] [PubMed]
- Badaoui A, De Saeger C, Duchemin J, Gihousse D, de Timary P and Starkel P (2008). “Alcohol dependence is associated with reduced plasma and fundic ghrelin levels.” Eur J Clin Invest 38(6): 397–403. [DOI] [PubMed] [Google Scholar]
- Ballsmider LA, Vaughn AC, David M, Hajnal A, Di Lorenzo PM and Czaja K (2015). “Sleeve Gastrectomy and Roux-en-Y Gastric Bypass Alter the Gut-Brain Communication.” Neural Plasticity 2015: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Tschöp M, Robinson SM and Heiman ML (2002). “Extent and Direction of Ghrelin Transport Across the Blood-Brain Barrier Is Determined by Its Unique Primary Structure.” Journal of Pharmacology and Experimental Therapeutics 302(2): 822–827. [DOI] [PubMed] [Google Scholar]
- Barazzoni R, Zanetti M, Nagliati C, Cattin MR, Ferreira C, Giuricin M, Palmisano S, Edalucci E, Dore F, Guarnieri G and de Manzini N (2013). “Gastric bypass does not normalize obesity-related changes in ghrelin profile and leads to higher acylated ghrelin fraction.” Obesity 21(4): 718–722. [DOI] [PubMed] [Google Scholar]
- Beckman LM, Beckman TR, Sibley SD, Thomas W, Ikramuddin S, Kellogg TA, Ghatei MA, Bloom SR, le Roux CW and Earthman CP (2011). “Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass surgery.” JPEN J Parenter Enteral Nutr 35(2): 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn AN, Hajnal A and Leggio L (2016). “The gut in the brain: the effects of bariatric surgery on alcohol consumption.” Addict Biol [DOI] [PMC free article] [PubMed]
- Blum K, Bailey J, Gonzalez AM, Oscar-Berman M, Liu Y, Giordano J, Braverman E and Gold M (2011). “Neuro-Genetics of Reward Deficiency Syndrome (RDS) as the Root Cause of “Addiction Transfer”: A New Phenomenon Common after Bariatric Surgery.” J Genet Syndr Gene Ther 2012(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko LCS, Selva JA, Merfeld EB, Fimmel AI, Goldberg SA and Currie PJ (2014). “Ghrelin alters the stimulatory effect of cocaine on ethanol intake following mesolimbic or systemic administration.” Neuropharmacology 85: 224–231. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Kirchner H, Wilson–Perez HE, Willency JA, Hale JE, Gaylinn BD, Thorner MO, Pfluger PT, Gutierrez JA, Tschöp MH, Sandoval DA and Seeley RJ (2013). “The Effects of Vertical Sleeve Gastrectomy in Rodents Are Ghrelin Independent.” Gastroenterology 144(1): 50–52.e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changchien EM, Woodard GA, Hernandez-Boussard T and Morton JM (2012). “Normal alcohol metabolism after gastric banding and sleeve gastrectomy: a case-cross-over trial.” J Am Coll Surg 215(4): 475–479. [DOI] [PubMed] [Google Scholar]
- Conason A, Teixeira J, Hsu CH, Puma L, Knafo D and Geliebter A (2013). “Substance use following bariatric weight loss surgery.” JAMA Surg 148(2): 145–150. [DOI] [PubMed] [Google Scholar]
- Cuellar-Barboza AB, Frye MA, Grothe K, Prieto ML, Schneekloth TD, Loukianova LL, Hall-Flavin DK, Clark MM, Karpyak VM, Miller JD and Abulseoud OA (2015). “Change in consumption patterns for treatment-seeking patients with alcohol use disorder post-bariatric surgery.” J Psychosom Res 78(3): 199–204. [DOI] [PubMed] [Google Scholar]
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K and Nakazato M (2002). “The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats.” Gastroenterology 123(4): 1120–1128. [DOI] [PubMed] [Google Scholar]
- Davis JF, Schurdak JD, Magrisso IJ, Mul JD, Grayson BE, Pfluger PT, Tschoep MH, Seeley RJ and Benoit SC (2012). “Gastric Bypass Surgery Attenuates Ethanol Consumption in Ethanol-Preferring Rats.” Biol Psychiatry [DOI] [PubMed]
- Davis JF, Tracy AL, Schurdak JD, Magrisso IJ, Grayson BE, Seeley RJ and Benoit SC (2013). “Roux en y gastric bypass increases ethanol intake in the rat.” Obes Surg 23(7): 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weijer BA, van de Giessen E, Janssen I, Berends FJ, van de Laar A, Ackermans MT, Fliers E, la Fleur SE, Booij J and Serlie MJ (2014). “Striatal dopamine receptor binding in morbidly obese women before and after gastric bypass surgery and its relationship with insulin sensitivity.” Diabetologia 57(5): 1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disse E, Bussier AL, Veyrat-Durebex C, Deblon N, Pfluger PT, Tschop MH, Laville M and Rohner-Jeanrenaud F (2010). “Peripheral ghrelin enhances sweet taste food consumption and preference, regardless of its caloric content.” Physiol Behav 101(2): 277–281. [DOI] [PubMed] [Google Scholar]
- Druce MR, Wren AM, Park AJ, Milton JE, Patterson M, Frost G, Ghatei MA, Small C and Bloom SR (2005). “Ghrelin increases food intake in obese as well as lean subjects.” Int J Obes (Lond) 29(9): 1130–1136. [DOI] [PubMed] [Google Scholar]
- Dunn JP, Cowan RL, Volkow ND, Feurer ID, Li R, Williams DB, Kessler RM and Abumrad NN (2010). “Decreased dopamine type 2 receptor availability after bariatric surgery: preliminary findings.” Brain Res 1350: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JP, Kessler RM, Feurer ID, Volkow ND, Patterson BW, Ansari MS, Li R, Marks-Shulman P and Abumrad NN (2012). “Relationship of dopamine type 2 receptor binding potential with fasting neuroendocrine hormones and insulin sensitivity in human obesity.” Diabetes Care 35(5): 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Morton J, Shepard E, Peebles R, Farrales-Nguyen S, Hammer L and Albanese C (2006). “Methamphetamine Use Following Bariatric Surgery in an Adolescent.” Obesity Surgery 16(6): 780–782. [DOI] [PubMed] [Google Scholar]
- Egecioglu E, Jerlhag E, Salome N, Skibicka KP, Haage D, Bohlooly YM, Andersson D, Bjursell M, Perrissoud D, Engel JA and Dickson SL (2010). “Ghrelin increases intake of rewarding food in rodents.” Addict Biol 15(3): 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertelt TW, Mitchell JE, Lancaster K, Crosby RD, Steffen KJ and Marino JM (2008). “Alcohol abuse and dependence before and after bariatric surgery: a review of the literature and report of a new data set.” Surg Obes Relat Dis 4(5): 647–650. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP and Benoit SC (2009). “Insulin, leptin, and food reward: update 2008.” Am J Physiol Regul Integr Comp Physiol 296(1): R9–R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK and Ogden CL (2012). “Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010.” JAMA 307(5): 491–497. [DOI] [PubMed] [Google Scholar]
- Fogger SA and McGuinness TM (2012). “The relationship between addictions and bariatric surgery for nurses in recovery.” Perspect Psychiatr Care 48(1): 10–15. [DOI] [PubMed] [Google Scholar]
- Fonseca AL, Schuster KM, Maung AA, Kaplan LJ and Davis KA (2013). “Routine nasogastric decompression in small bowel obstruction: is it really necessary?” Am Surg 79(4): 422–428. [PubMed] [Google Scholar]
- Gallo AS, Berducci MA, Nijhawan S, Nino DF, Broderick RC, Harnsberger CR, Lazar S, Echon C, Fuchs HF, Alvarez F, Sandler BJ, Jacobsen G and Horgan S (2015). “Alcohol metabolism is not affected by sleeve gastrectomy.” Surg Endosc 29(5): 1088–1093. [DOI] [PubMed] [Google Scholar]
- Garb J, Welch G, Zagarins S, Kuhn J and Romanelli J (2009). “Bariatric surgery for the treatment of morbid obesity: a meta-analysis of weight loss outcomes for laparoscopic adjustable gastric banding and laparoscopic gastric bypass.” Obes Surg 19(10): 1447–1455. [DOI] [PubMed] [Google Scholar]
- Garcia-Fuentes E, Garrido-Sanchez L, Garcia-Almeida JM, Garcia-Arnes J, Gallego-Perales JL, Rivas-Marin J, Morcillo S, Cardona I and Soriguer F (2008). “Different Effect of Laparoscopic Roux-en-Y Gastric Bypass and Open Biliopancreatic Diversion of Scopinaro on Serum PYY and Ghrelin Levels.” Obesity Surgery 18(11): 1424. [DOI] [PubMed] [Google Scholar]
- Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG and Pothos EN (2009). “Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity.” Neuroscience 159(4): 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK and Volkow ND (2007). “Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction?” Am J Psychiatry 164(1): 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop MR and Eysenck SB (1980). “A further investigation into the personality of drug addicts in treatment.” Br J Addict 75(3): 305–311. [DOI] [PubMed] [Google Scholar]
- Grabauskas G, Wu X, Lu Y, Heldsinger A, Song I, Zhou S-Y and Owyang C (2015). “KATP channels in the nodose ganglia mediate the orexigenic actions of ghrelin.” The Journal of Physiology 593(17): 3973–3989. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hagedorn JC, Encarnacion B, Brat GA and Morton JM (2007). “Does gastric bypass alter alcohol metabolism?” Surgery for Obesity and Related Diseases 3(5): 543–548. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Zharikov A, Polston JE, Fields MR, Tomasko J, Rogers AM, Volkow ND and Thanos PK (2012). “Alcohol reward is increased after Roux-en-Y gastric bypass in dietary obese rats with differential effects following ghrelin antagonism.” PLoS One 7(11): e49121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Tellez LA, Niu J, Medina S, Ferreira TL, Zhang X, Su J, Tong J, Schwartz GJ, van den Pol A and de Araujo IE (2016). “Striatal Dopamine Links Gastrointestinal Rerouting to Altered Sweet Appetite.” Cell Metab 23(1): 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankir MK, Ashrafian H, Hesse S, Horstmann A and Fenske WK (2015). “Distinctive striatal dopamine signaling after dieting and gastric bypass.” Trends Endocrinol Metab 26(5): 223–230. [DOI] [PubMed] [Google Scholar]
- Holt PR (2011). “Changes in alcohol metabolism after gastric bypass surgery.” Lancet 378(9793): 767–768. [DOI] [PubMed] [Google Scholar]
- Hsu LK, Benotti PN, Dwyer J, Roberts SB, Saltzman E, Shikora S, Rolls BJ and Rand W (1998). “Nonsurgical factors that influence the outcome of bariatric surgery: a review.” Psychosom Med 60(3): 338–346. [DOI] [PubMed] [Google Scholar]
- Inui A, Asakawa A, Bowers CY, Mantovani G, Laviano A, Meguid MM and Fujimiya M (2004). “Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ.” The FASEB Journal 18(3): 439–456. [DOI] [PubMed] [Google Scholar]
- Ivezaj V, Saules KK and Schuh LM (2014). “New-Onset Substance Use Disorder After Gastric Bypass Surgery: Rates and Associated Characteristics.” Obes Surg [DOI] [PubMed]
- Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L and Engel JA (2006). “Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward.” Addict Biol 11(1): 45–54. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L and Engel JA (2007). “Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens.” Addict Biol 12(1): 6–16. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Landgren S, Salomé N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL and Engel JA (2009). “Requirement of central ghrelin signaling for alcohol reward.” Proceedings of the National Academy of Sciences 106(27): 11318–11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Landgren S, Egecioglu E, Dickson SL and Engel JA (2011). “The alcohol-induced locomotor stimulation and accumbal dopamine release is suppressed in ghrelin knockout mice.” Alcohol 45(4): 341–347. [DOI] [PubMed] [Google Scholar]
- Jiang H, Betancourt L and Smith RG (2006). “Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers.” Mol Endocrinol 20(8): 1772–1785. [DOI] [PubMed] [Google Scholar]
- Kalivas PW and Volkow ND (2005). “The neural basis of addiction: a pathology of motivation and choice.” Am J Psychiatry 162(8): 1403–1413. [DOI] [PubMed] [Google Scholar]
- Katayama M, Nogami H, Nishiyama J, Kawase T and Kawamura K (2000). “Developmentally and regionally regulated expression of growth hormone secretagogue receptor mRNA in rat brain and pituitary gland.” Neuroendocrinology 72(6): 333–340. [DOI] [PubMed] [Google Scholar]
- Kazdin AE (1982). “Symptom substitution, generalization, and response covariation: implications for psychotherapy outcome.” Psychol Bull 91(2): 349–365. [PubMed] [Google Scholar]
- King WC, Chen J-Y, Courcoulas AP, Dakin GF, Engel SG, Flum DR, Hinojosa MW, Kalarchian MA, Mattar SG and Mitchell JE (2017). “Alcohol and other substance use after bariatric surgery: prospective evidence from a US multicenter cohort study.” Surgery for Obesity and Related Diseases [DOI] [PMC free article] [PubMed]
- King WC, Chen JY, Mitchell JE, Kalarchian MA, Steffen KJ, Engel SG, Courcoulas AP, Pories WJ and Yanovski SZ (2012). “Prevalence of alcohol use disorders before and after bariatric surgery.” JAMA 307(23): 2516–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockhoff H, Naslund I and Jones AW (2002). “Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery.” British Journal of Clinical Pharmacology 54: 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H and Kangawa K (1999). “Ghrelin is a growth-hormone-releasing acylated peptide from stomach.” Nature 402(6762): 656–660. [DOI] [PubMed] [Google Scholar]
- Koopmann A, von der Goltz C, Grosshans M, Dinter C, Vitale M, Wiedemann K and Kiefer F (2012). “The association of the appetitive peptide acetylated ghrelin with alcohol craving in early abstinent alcohol dependent individuals.” Psychoneuroendocrinology 37(7): 980–986. [DOI] [PubMed] [Google Scholar]
- Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, Taveras C, Schrope B and Bessler M (2009). “Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass.” Int J Obes (Lond) 33(7): 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren S, Simms JA, Hyytia P, Engel JA, Bartlett SE and Jerlhag E (2011). “Ghrelin receptor (GHS-R1A) antagonism suppresses both operant alcohol self-administration and high alcohol consumption in rats.” Addict Biol [DOI] [PubMed]
- Landgren S, Simms JA, Hyytia P, Engel JA, Bartlett SE and Jerlhag E (2012). “Ghrelin receptor (GHS-R1A) antagonism suppresses both operant alcohol self-administration and high alcohol consumption in rats.” Addict Biol 17(1): 86–94. [DOI] [PubMed] [Google Scholar]
- le Roux CW, Neary NM, Halsey TJ, Small CJ, Martinez-Isla AM, Ghatei MA, Theodorou NA and Bloom SR (2005). “Ghrelin Does Not Stimulate Food Intake in Patients with Surgical Procedures Involving Vagotomy.” The Journal of Clinical Endocrinology & Metabolism 90(8): 4521–4524. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, Nesci A, Miceli A, Malandrino N, Capristo E, Canestrelli B, Monteleone P, Kenna GA, Swift RM and Addolorato G (2012). “Ghrelin system in alcohol-dependent subjects: role of plasma ghrelin levels in alcohol drinking and craving.” Addict Biol 17(2): 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, Fricchione SR, Edwards SM, de la Monte SM, Swift RM and Kenna GA (2014). “Intravenous Ghrelin Administration Increases Alcohol Craving in Alcohol-Dependent Heavy Drinkers: A Preliminary Investigation.” Biol Psychiatry 76(9): 734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lent MR and Swencionis C (2012). “Addictive personality and maladaptive eating behaviors in adults seeking bariatric surgery.” Eat Behav 13(1): 67–70. [DOI] [PubMed] [Google Scholar]
- Maluenda F, Csendes A, De Aretxabala X, Poniachik J, Salvo K, Delgado I and Rodriguez P (2010). “Alcohol absorption modification after a laparoscopic sleeve gastrectomy due to obesity.” Obes Surg 20(6): 744–748. [DOI] [PubMed] [Google Scholar]
- Matzko ME, Argyropoulos G, Wood GC, Chu X, McCarter RJM, Still CD and Gerhard GS (2012). “Association of Ghrelin Receptor Promoter Polymorphisms with Weight Loss Following Roux-en-Y Gastric Bypass Surgery.” Obesity Surgery 22(5): 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JE, King WC, Courcoulas A, Dakin G, Elder K, Engel S, Flum D, Kalarchian M, Khandelwal S, Pender J, Pories W and Wolfe B (2014). “Eating behavior and eating disorders in adults before bariatric surgery.” Int J Eat Disord [DOI] [PMC free article] [PubMed]
- Niego SH, Kofman MD, Weiss JJ and Geliebter A (2007). “Binge eating in the bariatric surgery population: a review of the literature.” Int J Eat Disord 40(4): 349–359. [DOI] [PubMed] [Google Scholar]
- Ochner CN, Kwok Y, Conceicao E, Pantazatos SP, Puma LM, Carnell S, Teixeira J, Hirsch J and Geliebter A (2011). “Selective reduction in neural responses to high calorie foods following gastric bypass surgery.” Ann Surg 253(3): 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochner CN, Stice E, Hutchins E, Afifi L, Geliebter A, Hirsch J and Teixeira J (2012). “Relation between changes in neural responsivity and reductions in desire to eat high-calorie foods following gastric bypass surgery.” Neuroscience 209: 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund MP, Backman O, Marsk R, Stockeld D, Lagergren J, Rasmussen F and Naslund E (2013). “Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery.” JAMA Surg 148(4): 374–377. [DOI] [PubMed] [Google Scholar]
- Padwal R, Klarenbach S, Wiebe N, Hazel M, Birch D, Karmali S, Sharma AM, Manns B and Tonelli M (2011). “Bariatric surgery: a systematic review of the clinical and economic evidence.” J Gen Intern Med 26(10): 1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY, Okunade AL, Eagon JC, Bartholow BD, Bucholz K and Klein S (2015). “Effect of Roux-en-Y Gastric Bypass Surgery: Converting 2 Alcoholic Drinks to 4.” JAMA Surg 150(11): 1096–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, Woloszyn J, Yanagisawa M, Lutter M and Zigman JM (2010). “Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner.” Biol Psychiatry 67(9): 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polston JE, Pritchett CE, Tomasko JM, Rogers AM, Leggio L, Thanos PK, Volkow ND and Hajnal A (2013). “Roux-en-Y gastric bypass increases intravenous ethanol self-administration in dietary obese rats.” PLoS One 8(12): e83741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polston JE, Pritchett CE, Tomasko JM, Rogers AM, Leggio L, Thanos PK, Volkow ND and Hajnal A (2014). “Roux-en-Y Gastric Bypass Increases Intravenous Ethanol Self-Administration in Dietary Obese Rats.” PLOS ONE 8(12): e83741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prachand VN (2011). “The evolution of minimally invasive bariatric surgery.” World J Surg 35(7): 1464–1468. [DOI] [PubMed] [Google Scholar]
- Ramón JM, Salvans S, Crous X, Puig S, Goday A, Benaiges D, Trillo L, Pera M and Grande L (2012). “Effect of Roux-en-Y Gastric Bypass vs Sleeve Gastrectomy on Glucose and Gut Hormones: a Prospective Randomised Trial.” Journal of Gastrointestinal Surgery 16(6): 1116–1122. [DOI] [PubMed] [Google Scholar]
- Reddy IA, Wasserman DH, Ayala JE, Hasty AH, Abumrad NN and Galli A (2014). “Striatal dopamine homeostasis is altered in mice following Roux-en-Y gastric bypass surgery.” ACS Chem Neurosci 5(10): 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata I and Sakai T (2010). “Ghrelin Cells in the Gastrointestinal Tract.” International Journal of Peptides 2010: 945056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM, Ismail NA, Durighel G, Ahmed AR, Olbers T, Vincent RP, Alaghband-Zadeh J, Ghatei MA, Waldman AD, Frost GS, Bell JD, le Roux CW and Goldstone AP (2014). “Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding.” Gut 63(6): 891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin AC, Zheng H, Townsend RL, Sigalet DL and Berthoud HR (2010). “Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery.” Endocrinology 151(4): 1588–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA and Dickson SL (2011). “Ghrelin directly targets the ventral tegmental area to increase food motivation.” Neuroscience 180: 129–137. [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Shirazi RH, Hansson C and Dickson SL (2012). “Ghrelin interacts with neuropeptide Y Y1 and opioid receptors to increase food reward.” Endocrinology 153(3): 1194–1205. [DOI] [PubMed] [Google Scholar]
- Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, Kumar A, Brasic J and Wong DF (2010). “Alterations of central dopamine receptors before and after gastric bypass surgery.” Obes Surg 20(3): 369–374. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C and Small DM (2008). “Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele.” Science 322(5900): 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Zald D and Dagher A (2011). “Dopamine-based reward circuitry responsivity, genetics, and overeating.” Curr Top Behav Neurosci 6: 81–93. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Haimovici F and Chang G (2012). “Alcohol use disorders after bariatric surgery.” Obes Surg 22(2): 201–207. [DOI] [PubMed] [Google Scholar]
- Svensson P, Romeo S, Peltonen M, Sjöström L and Carlsson L (2012). “Alcohol Abuse After Bariatric Surgery In The Swedish Obese Subjects (sos) Study.” Obesity facts 5: 47. [Google Scholar]
- Svensson PA, Anveden A, Romeo S, Peltonen M, Ahlin S, Burza MA, Carlsson B, Jacobson P, Lindroos AK, Lonroth H, Maglio C, Naslund I, Sjoholm K, Wedel H, Soderpalm B, Sjostrom L and Carlsson LM (2013). “Alcohol consumption and alcohol problems after bariatric surgery in the Swedish obese subjects study.” Obesity (Silver Spring) 21(12): 2444–2451. [DOI] [PubMed] [Google Scholar]
- Tabakoff B and Hoffman PL (2013). “The neurobiology of alcohol consumption and alcoholism: an integrative history.” Pharmacol Biochem Behav 113: 20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari M, Catalano A, Pellitteri M, Di Francesco C, Marini F, Gerrard PA, Heidbreder CA and Melotto S (2007). “Correlation between serum ghrelin levels and cocaine-seeking behaviour triggered by cocaine-associated conditioned stimuli in rats.” Addict Biol 12(1): 22–29. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Subrize M, Delis F, Cooney RN, Culnan D, Sun M, Wang GJ, Volkow ND and Hajnal A (2012). “Gastric bypass increases ethanol and water consumption in diet-induced obese rats.” Obes Surg 22(12): 1884–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K and Matsukura S (2001). “Upregulation of Ghrelin Expression in the Stomach upon Fasting, Insulin-Induced Hypoglycemia, and Leptin Administration.” Biochemical and Biophysical Research Communications 281(5): 1220–1225. [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL and Heiman ML (2000). “Ghrelin induces adiposity in rodents.” Nature 407(6806): 908–913. [DOI] [PubMed] [Google Scholar]
- Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E and Heiman ML (2001). “Circulating ghrelin levels are decreased in human obesity.” Diabetes 50(4): 707–709. [DOI] [PubMed] [Google Scholar]
- van der Zwaal EM, de Weijer BA, van de Giessen EM, Janssen I, Berends FJ, van de Laar A, Ackermans MT, Fliers E, la Fleur SE, Booij J and Serlie MJ (2016). “Striatal dopamine D2/3 receptor availability increases after long-term bariatric surgery-induced weight loss.” Eur Neuropsychopharmacol 26(7): 1190–1200. [DOI] [PubMed] [Google Scholar]
- Vanderlinden LA, Saba LM, Kechris K, Miles MF, Hoffman PL and Tabakoff B (2013). “Whole brain and brain regional coexpression network interactions associated with predisposition to alcohol consumption.” PLoS One 8(7): e68878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND and Baler RD (2013). “Brain imaging biomarkers to predict relapse in alcohol addiction.” JAMA Psychiatry 70(7): 661–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N and Fowler JS (2001). “Brain dopamine and obesity.” Lancet 357(9253): 354–357. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M, Zhu W, Wong CT, Pappas NR, Geliebter A and Fowler JS (2004). “Exposure to appetitive food stimuli markedly activates the human brain.” Neuroimage 21(4): 1790–1797. [DOI] [PubMed] [Google Scholar]
- Woodard GA, Downey J, Hernandez-Boussard T and Morton JM (2011). “Impaired alcohol metabolism after gastric bypass surgery: a case-crossover trial.” J Am Coll Surg 212(2): 209–214. [DOI] [PubMed] [Google Scholar]
- Wurst FM, Rasmussen DD, Hillemacher T, Kraus T, Ramskogler K, Lesch O, Bayerlein K, Schanze A, Wilhelm J, Junghanns K, Schulte T, Dammann G, Pridzun L, Wiesbeck G, Kornhuber J and Bleich S (2007). “Alcoholism, Craving, and Hormones: The Role of Leptin, Ghrelin, Prolactin, and the Pro-Opiomelanocortin System in Modulating Ethanol Intake.” Alcoholism: Clinical and Experimental Research 31(12): 1963–1967. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB and Elmquist JK (2006). “Expression of ghrelin receptor mRNA in the rat and the mouse brain.” J Comp Neurol 494(3): 528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]


