Abstract
Background: Preterm delivery has been linked to future maternal cardiovascular disease (CVD); however, research investigating clinical CVD risk factors is limited. We evaluated whether women who have delivered an infant preterm are at higher risk of developing CVD risk factors after adjustment for prepregnancy confounders.
Materials and Methods: We examined the association between preterm delivery and incident chronic hypertension, type 2 diabetes mellitus (T2DM), and hypercholesterolemia among 57,904 parous women in the Nurses' Health Study II. Multivariable Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between preterm delivery in first pregnancy and each CVD risk factor; adjusted cumulative incidence curves were computed using the Breslow estimator.
Results: Preterm delivery (<37 weeks) was associated with HRs of 1.11 (95% CI: 1.06–1.17) for chronic hypertension, 1.17 (95% CI: 1.03–1.33) for T2DM, and 1.07 (95% CI: 1.03–1.11) for hypercholesterolemia, adjusting for age, race/ethnicity, parental education, and prepregnancy confounders (e.g., body mass index, smoking, and family history). HRs were higher in women who delivered very preterm (<32 weeks) and in the first 10 years after first birth. The cumulative incidence of each risk factor was highest in women who delivered very preterm.
Conclusions: Women with a history of preterm delivery are at higher risk of developing chronic hypertension, T2DM, and hypercholesterolemia in the years after pregnancy. This increased risk was particularly pronounced in the first 10 years after a preterm delivery, indicating that it may be an important time period to implement lifestyle interventions.
Keywords: cardiovascular diseases, hypercholesterolemia, hypertension, premature birth, type 2 diabetes mellitus, women's health
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality in the United States, with over 400,000 deaths among women in 2014.1 The cardiometabolic stress of pregnancy may signal underlying CVD risk (e.g., endothelial dysfunction and vascular disease) through development of pregnancy complications.2–4 The American Heart and Stroke Associations (AHA, ASA) recognize gestational diabetes mellitus (GDM) and hypertensive disorders of pregnancy (HDP; preeclampsia and gestational hypertension) as CVD risk factors.5,6 HDP often result in preterm deliveries,7 and preterm delivery, even in the absence of HDP, is associated with increased risk of CVD.8–10 The AHA recommends that clinicians screen patients for prior preterm deliveries when obtaining a complete medical history.6 In addition, there have been calls to evaluate CVD risk factor trajectories after pregnancy for targeted prevention in women with a history of pregnancy complications.3,5
Preterm delivery impacts nearly 10% of pregnancies in the United States each year.11 Although it has been linked to future maternal CVD,9,10,12–18 research investigating clinical CVD risk factors (i.e., chronic hypertension, type 2 diabetes mellitus [T2DM], and hypercholesterolemia) is limited. Current literature suggests that women with a history of preterm delivery are at 1.2–2-fold higher risk of CVD risk factors, yet few studies have jointly adjusted for potential prepregnancy confounders, including body mass index (BMI), smoking, family history of CVD risk factors, and lifestyle.18–21 Finally, to our knowledge, all but one study have under 20 years of follow-up18–20,22–25 and only one, which evaluated T2DM, provides information on when these risk factors develop after preterm delivery.21 We provide new data to inform screening protocols in women who have delivered a preterm infant by providing more complete adjustment for confounding and describing the trajectory of risk factor development up to 50 years after preterm delivery.
Materials and Methods
Study population
The Nurses' Health Study II (NHSII) enrolled 116,429 female United States registered nurses aged 25–42 in 1989. Participants are mailed questionnaires every 2 years to collect sociodemographic, lifestyle, and behavior information, medication use, and incident disease. Pregnancy history was reported in 2001 and 2009. In 2001, 91,297 women were mailed a supplemental questionnaire about pregnancies lasting at least 12 weeks. As part of the 2009 biennial questionnaire, all women in the NHSII were asked to report their complete pregnancy history, including gestation length, pregnancy complications, and birth outcomes for all pregnancies, regardless of gestation length. This 2009 reproductive questionnaire is the primary source of our exposure data as it obtained a more complete pregnancy history. This study was approved by the Partners Human Research Committee (Institutional Review Board) of Brigham and Women's Hospital. Questionnaire return was considered informed consent.
Gestation length
Women reported the length of each pregnancy in the following categories of completed weeks: <8, 8–11, 12–19, 20–27, 28–31, 32–36, 37–39, 40–42, and 43+. For women who did not complete the 2009 questionnaire or were missing gestation length, data from the 2001 questionnaire were used (n = 10,644). Gestation length was dichotomized into preterm (≥20 to <37 weeks) or term (≥37 weeks) delivery. Preterm delivery was further split into moderate (≥32 to <37 weeks) and very (≥20 to <32 weeks) preterm. Pregnancies lasting <20 weeks were not included.26 A validation study conducted among 403 participants who reported preeclampsia/toxemia between 1991 and 2001 yielded an 81% sensitivity and 92% specificity for dichotomous preterm delivery and a Kappa statistic of 0.74 when gestation length was categorized into term, moderate preterm, and very preterm, compared to medical record data.
Cardiovascular risk factors
Participants self-reported physician diagnosed incident chronic hypertension, diabetes (not during pregnancy), and elevated cholesterol and the respective year of diagnosis in categories (e.g., on 1991 questionnaire: “before September 1989”; “September 1989 to May 1991”; or “after June 1, 1991”) on each biennial questionnaire. The midpoint of each category was assigned as the date of diagnosis for chronic hypertension and hypercholesterolemia. T2DM was confirmed and dated via supplemental questionnaire.
Chronic hypertension
Validation of self-reported hypertension in a random sample of NHSII participants resulted in 94% sensitivity and 85% specificity, indicating good agreement with medical records.27 Primary analyses used self-reported hypertension, while sensitivity analyses utilized an alternative definition additionally including antihypertensive medication use.
Type 2 diabetes mellitus
Women who reported a diabetes diagnosis were mailed a supplementary questionnaire to obtain information on symptoms, diagnostic tests, and hypoglycemic therapy. Definite and probable T2DM were based on criteria from the American Diabetes Association.28,29 Validation of these confirmation criteria in a similar cohort confirmed 98% of self-reported T2DM diagnoses.30 Primary analyses required a definite T2DM diagnosis, while sensitivity analyses additionally considered probable cases.
Hypercholesterolemia
Beginning in 1999, women reported cholesterol-lowering medication use and, from 1999 on, hypercholesterolemia was defined as self-report of elevated cholesterol or medication use. Validation of self-reported elevated cholesterol compared to medical records and measured blood samples in a similar cohort yielded a positive predictive value of 86% and negative predictive value of 85%.31
Covariates
The following covariates were selected a priori as potential confounders based on subject matter knowledge: age at first birth and at NHSII enrollment in 1989; sociodemographic variables, including race/ethnicity (white, African American, Latina, Asian, and other) and education of the nurses' mother and father (<9, 9–11, 12, 13–15, and ≥16 years); family history of hypertension (yes/no); family history of diabetes (yes/no); and prepregnancy BMI (in kg/m2: <18.5, 18.5 to <25, 25 to <30, and ≥30), smoking (never, past, and current), Alternative Healthy Eating Index (AHEI, quintiles) score, alcohol (none, <1 drink per week, 2–6 per week, and ≥1 per day), physical activity (none, 1–3, 4–6, 7–9, and 10–12 months per year), and oral contraceptive use (none, <2, 2 to <4, and ≥4 years). Missing indicators were used for the small amount of missing data in our covariates.
For women with first pregnancies before the start of the NHSII in 1989 (82%), baseline questionnaire items that asked about lifestyle and behavior from age 18 to cohort entry were used to assign prepregnancy covariate values. Women who delivered their first pregnancy after 1989 were assigned prepregnancy covariate values from the biennial questionnaire closest to, but preceding the pregnancy.
Exclusions
We excluded women who were nonresponders to both the 2001 and 2009 pregnancy history questionnaires (n = 28,945), nulliparous in 2009 (n = 15,556), missing gestation length or year of first birth (n = 324), or <18 or >45 years of age at first birth (n = 954). Women who reported chronic hypertension, hypercholesterolemia, myocardial infarction (MI), or stroke on the 1989 questionnaire without a date of diagnosis or with one before 1980 were also excluded (n = 1,366) as we were unable to precisely date these. We additionally excluded women who reported development of chronic hypertension, hypercholesterolemia, type 1 diabetes mellitus (T1DM) or T2DM, antihypertensive medication use, or those who had an MI or stroke before first pregnancy (n = 3,127). We excluded women who reported GDM or HDP in first pregnancy (n = 6,662) because both are indications for preterm delivery7,32 and are associated with CVD events and risk factors.33–48 Information on GDM and HDP was not collected in 2001, thus women for whom the 2001 questionnaire was the source of their pregnancy history were excluded if they reported GDM or HDP on any biennial questionnaire before 2001 (n = 1,553). Finally, because chronic hypertension before pregnancy can be incorrectly diagnosed as incident chronic hypertension after pregnancy, women who reported a chronic hypertension diagnosis within 1 year of first birth were additionally excluded (n = 38), resulting in an analytic sample of 57,904 women.
Statistical analysis
Characteristics of NHSII participants were age standardized and presented by preterm delivery status in first birth. Women entered the study at first birth and were followed until development of each CVD risk factor, MI or stroke, death, loss to follow-up, or June 2013 (end of follow-up). In the chronic hypertension analysis, women were additionally censored at report of antihypertensive medication use, as these women were no longer at risk. Similarly, in the T2DM analysis, women were censored at T1DM diagnosis as they were no longer at risk of developing T2DM. Multivariable-adjusted Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of preterm delivery with chronic hypertension, T2DM, and hypercholesterolemia. The proportional hazards assumption was assessed using likelihood ratio tests comparing models with and without interactions between preterm delivery and time since first birth. The assumption was violated for chronic hypertension and T2DM (both p < 0.04); therefore, we present HRs within 10-year intervals since first birth. For consistency, we also report HRs in 10-year intervals for hypercholesterolemia.
The Breslow estimator was used to obtain CVD risk profile-specific cumulative incidence curves49 for each CVD risk factor by preterm delivery in first birth. The low risk CVD profile was characterized as having the following characteristics before the first pregnancy: mean age at first birth (age = 27) and in 1989 (age = 35), white, ≥16 years of maternal and paternal education, healthy BMI (18.5–24.9 kg/m2), never smoker, healthiest diet (fifth quintile of AHEI), ≥1 alcoholic drink per day, 10–12 months of strenuous physical activity per year, no oral contraceptive use, and no family history of hypertension (for the chronic hypertension curves) or diabetes (for the T2DM curves). In contrast, the following characteristics represent the high risk CVD profile: mean age at first birth (age = 27) and in 1989 (age = 35), white, <9 years of maternal and paternal education, obese BMI (≥30 kg/m2), current smoker, unhealthiest diet (first quintile of AHEI), no alcoholic drinks per day, no physical activity, no oral contraceptive use, and family history of hypertension (for the chronic hypertension curves) or diabetes (for the T2DM curves). The characteristics used to create these risk profiles were measured on the 1989 baseline questionnaire or the questionnaire immediately preceding first pregnancy, depending on whether a woman's first pregnancy was before or after entry into the cohort in 1989.
To evaluate the association between recurrent preterm deliveries and each CVD risk factor, we characterized women by preterm delivery in first birth (term or preterm) and, subsequently, in all later births (all term, any preterm, or no later births), yielding six exposure categories. Follow-up for this analysis began at age 40 when most women (97%) had completed childbearing. Therefore, we additionally excluded women who had births at age 40 or later (n = 2,799); missing or invalid gestation length in a second or later pregnancy (n = 188); developed any of the CVD risk factors, T1DM, or reported antihypertensive medication use before age 40 (n = 10,033); or had a CVD event, died, or were lost to follow-up before age 40 (n = 122), leaving 44,762 women. Analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Approximately 6% of women delivered their first birth moderately preterm (≥32 to <37 weeks), while 2% delivered very preterm (<32 weeks). Women were generally similar across exposure groups; however, women with a very preterm delivery were more likely to have a family history of diabetes and achieve higher final parity (Table 1). Although 1% of first births in our study population resulted in stillbirth, 52.5% of very preterm first births were stillbirths.
Table 1.
Age-Standardized Baseline Characteristics of Study Participants by First Birth Preterm Delivery
| Term ≥37 weeks (n = 53,341) | Moderate preterm ≥32 to <37 weeks (n = 3,400) | Very preterm <32 weeks (n = 1,163) | |
|---|---|---|---|
| Age at first birth, years, mean (SD)a | 26.7 (4.5) | 27.2 (4.8) | 26.7 (5.2) |
| White | 93.0 | 91.3 | 91.6 |
| Education of the nurse's mother, more than high school | 27.6 | 26.2 | 25.8 |
| Education of the nurse's father, more than high school | 31.9 | 30.0 | 31.3 |
| Family history of hypertension | 51.3 | 51.1 | 52.0 |
| Family history of diabetes | 43.2 | 45.3 | 49.0 |
| Prepregnancy body mass index ≥30 kg/m2 | 2.0 | 2.0 | 2.9 |
| Strenuous physical activity, age 18–22 years | |||
| Never | 28.4 | 28.3 | 27.3 |
| 10–12 months/year | 11.2 | 11.6 | 14.4 |
| Prepregnancy alternative healthy eating index | |||
| First quintile (least healthy) | 20.0 | 19.9 | 16.8 |
| Fifth quintile (healthiest) | 19.4 | 20.1 | 23.4 |
| Prepregnancy smoking | |||
| Never smoker | 67.6 | 67.6 | 65.4 |
| Past smoker | 9.5 | 8.7 | 10.3 |
| Current smoker | 22.3 | 22.9 | 23.5 |
| Prepregnancy alcohol intake, drinks | |||
| None | 27.6 | 29.3 | 27.0 |
| ≥1/day | 5.7 | 6.5 | 5.7 |
| Duration of prepregnancy oral contraceptive use | |||
| None | 26.1 | 25.7 | 21.9 |
| <2 years | 23.9 | 21.8 | 25.3 |
| 2–4 years | 21.9 | 20.0 | 22.9 |
| ≥4 years | 28.1 | 32.5 | 29.9 |
| First pregnancy stillbirth | 0.4 | 1.7 | 52.5 |
| Final parity | |||
| 1 birth | 15.0 | 20.2 | 19.1 |
| 2 births | 49.0 | 47.9 | 28.1 |
| 3 births | 26.5 | 23.1 | 33.5 |
| ≥4 births | 9.5 | 8.8 | 19.3 |
Values are percentages unless otherwise noted and are standardized to the age distribution of the study population.
Values of polytomous variables may not sum to 100% due to rounding.
Value is not age adjusted.
SD, standard deviation.
Due to risk factor-specific censoring criteria, median length of follow-up differed by CVD risk factor. Median length of follow-up was 28 (interquartile range [IQR]: 21, 33) years for chronic hypertension, 32 (IQR: 27, 37) years for T2DM, and 26 (IQR: 19, 32) years for hypercholesterolemia. We observed 18,064 cases of chronic hypertension, 2,821 cases of T2DM, and 31,183 cases of hypercholesterolemia.
Table 2 shows multivariable-adjusted HRs for the association between preterm delivery and each CVD risk factor across follow-up (up to 49 years after first birth for chronic hypertension and hypercholesterolemia and up to 50 years for T2DM) and within 10-year intervals since first birth. Women who delivered their first infant preterm had an 11% (95% CI: 1.06–1.17) increased rate of chronic hypertension compared to women who delivered at term. The HR for chronic hypertension was highest in the first 10 years following a preterm delivery (1.43; 95% CI: 1.20–1.70) and appeared to be driven by very preterm delivery (2.07; 95% CI: 1.56–2.75). Women who delivered very preterm had significantly increased rates of chronic hypertension for 30 years following first birth, while in women who delivered moderately preterm, a significantly increased rate did not emerge until 31–40 years after first birth (Table 2).
Table 2.
Multivariable-Adjusted Hazard Ratios (95% Confidence Intervals) for Preterm Delivery in First Birth and Cardiovascular Disease Risk Factors in 10-Year Intervals Since First Birth
| Cases/person years | Term: ≥37 weeks (n = 53,341) | Preterm: <37 weeks (n = 4,563) | Moderate preterm: ≥32 to <37 weeks (n = 3,400) | Very preterm: <32 weeks (n = 1,163) | p-Trenda | |
|---|---|---|---|---|---|---|
| Chronic hypertension | ||||||
| Overallb | 18,064/1,574,176 | 1.00 (ref) | 1.11 (1.06–1.17) | 1.06 (1.00–1.13) | 1.28 (1.16–1.41) | <0.0001 |
| 1–10 years | 1,273/573,557 | 1.00 (ref) | 1.43 (1.20–1.70) | 1.22 (0.99–1.51) | 2.07 (1.56–2.75) | <0.0001 |
| 11–20 years | 5,471/520,984 | 1.00 (ref) | 1.07 (0.98–1.18) | 0.96 (0.86–1.08) | 1.45 (1.23–1.71) | 0.002 |
| 21–30 years | 7,809/351,212 | 1.00 (ref) | 1.08 (1.00–1.17) | 1.05 (0.96–1.16) | 1.17 (1.00–1.37) | 0.03 |
| 31–40 years | 3,241/117,903 | 1.00 (ref) | 1.10 (0.97–1.25) | 1.17 (1.01–1.34) | 0.92 (0.71–1.19) | 0.54 |
| Type 2 diabetes mellitus | ||||||
| Overallb | 2,821/1,832,561 | 1.00 (ref) | 1.17 (1.03–1.33) | 1.06 (0.91–1.24) | 1.48 (1.19–1.83) | 0.0007 |
| 1–10 yearsc | 41/560,826 | 1.00 (ref) | 2.83 (1.32–6.06) | 3.22 (1.39–7.47) | 1.94 (0.44–8.70) | 0.04 |
| 11–20 years | 528/567,508 | 1.00 (ref) | 1.33 (1.01–1.76) | 1.20 (0.86–1.67) | 1.77 (1.10–2.84) | 0.01 |
| 21–30 years | 1,300/462,511 | 1.00 (ref) | 1.11 (0.91–1.34) | 1.00 (0.79–1.26) | 1.40 (1.01–1.94) | 0.09 |
| 31–40 years | 885/201,537 | 1.00 (ref) | 1.13 (0.89–1.42) | 0.99 (0.74–1.32) | 1.46 (1.01–2.11) | 0.09 |
| Hypercholesterolemia | ||||||
| Overallb | 31,183/1,454,258 | 1.00 (ref) | 1.07 (1.03–1.11) | 1.06 (1.01–1.11) | 1.09 (1.01–1.18) | 0.002 |
| 1–10 years | 4,555/563,056 | 1.00 (ref) | 1.13 (1.02–1.25) | 1.10 (0.98–1.24) | 1.23 (1.01–1.50) | 0.01 |
| 11–20 years | 10,368/483,647 | 1.00 (ref) | 1.07 (1.00–1.15) | 1.05 (0.97 − 1.14) | 1.11 (0.97–1.27) | 0.05 |
| 21–30 years | 11,373/305,674 | 1.00 (ref) | 1.02 (0.95–1.09) | 1.01 (0.94–1.10) | 1.02 (0.89–1.17) | 0.66 |
| 31–40 years | 4,634/94,215 | 1.00 (ref) | 1.07 (0.96–1.19) | 1.09 (0.96–1.23) | 1.03 (0.84–1.25) | 0.39 |
Models are adjusted for age at first birth, age in 1989, race/ethnicity, parental education, family history of hypertension (for the chronic hypertension model), family history of type 2 diabetes mellitus (for the type 2 diabetes mellitus model), prepregnancy BMI, prepregnancy smoking, prepregnancy Alternative Healthy Eating Index (AHEI) score (in quintiles), prepregnancy alcohol intake, prepregnancy (age 18) physical activity, and prepregnancy oral contraceptive use.
Exposure (preterm delivery) was included in model as a continuous variable with 40 weeks representing term delivery, 36 weeks representing moderate preterm delivery, and 30 weeks representing very preterm delivery.
Includes all 49 years of follow-up for chronic hypertension and hypercholesterolemia and 50 years for type 2 diabetes mellitus.
Asian race and other race were combined as there were 0 diabetes cases in Asian women; women who were underweight (n = 1,260) or missing BMI (n = 501) were excluded because there were 0 cases in these BMI groups.
BMI, body mass index.
There was a 1.17-fold increased rate (95% CI: 1.03–1.33) of developing T2DM in women who delivered their first infant preterm compared to those who delivered at term. Similar to chronic hypertension, the fully-adjusted HR was strongest in the first 10 years (2.83; 95% CI: 1.32–6.06); the association generally attenuated over follow-up, but maintained statistical significance through 20 years after first birth. Women who delivered moderately preterm had an increased rate of T2DM only in the first 10 years after first birth (3.22; 95% CI: 1.39–7.47). In contrast, in women delivering very preterm, a significantly higher rate of T2DM was only observed from 11 to 40 years after first birth (Table 2).
For hypercholesterolemia, women who delivered preterm had a 1.07-fold increased rate (95% CI: 1.03–1.11) compared to women who delivered term. We also observed the highest HR for hypercholesterolemia in the first 10 years following preterm delivery (1.13; 95% CI: 1.02–1.25), which was attenuated thereafter. This increased rate appeared to be driven by the very preterm group, which had a 23% increased rate (95% CI: 1.01–1.50) of hypercholesterolemia in the first 10 years after first birth compared to women who delivered at term (Table 2).
To demonstrate the impact of adjustment for various prepregnancy lifestyle factors and family history, Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/jwh) shows HRs over all of follow-up with partial adjustment for confounding. For chronic hypertension and hypercholesterolemia, age- and fully-adjusted models were similar. However, for T2DM, we observed attenuation in the HRs for Model 2 compared to Model 1, driven primarily by adjustment for family history of T2DM.
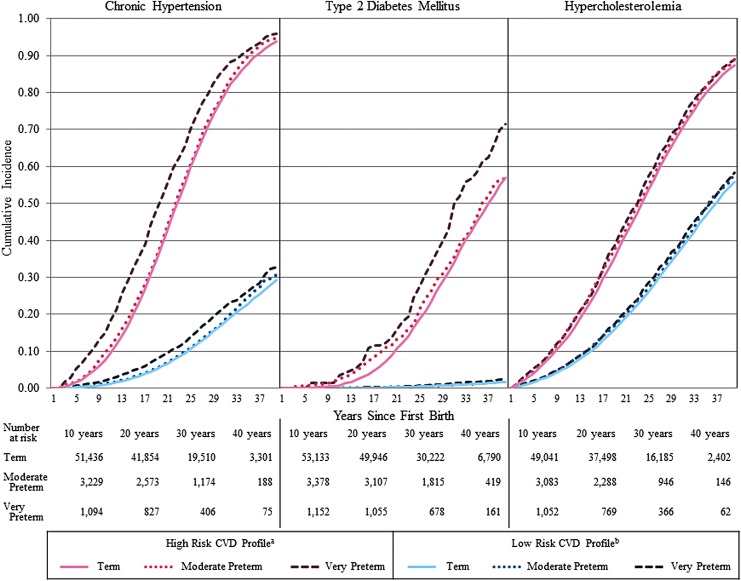
The cumulative incidence of chronic hypertension, T2DM, and hypercholesterolemia was highest in women who delivered very preterm for both the low- and high-risk CVD profiles (Fig. 1). Differences in absolute risk were more pronounced in the high-risk CVD profile group than the low-risk profile group for chronic hypertension and T2DM, but not for hypercholesterolemia. Divergence in the cumulative incidence curves between preterm delivery groups occurred immediately in the high-risk CVD profile for chronic hypertension and at 10 years for T2DM.
FIG. 1.
CVD risk profile-specific cumulative incidence curves for CVD risk factor development by preterm delivery in first birth. aHigh risk CVD profile denotes the following characteristics before first pregnancy: mean age at first birth (age = 27) and in 1989 (age = 35), white, <9 years of parental education, obese BMI (≥30 kg/m2), current smoker, unhealthiest diet (first quintile of AHEI), no alcohol consumption, no physical activity, no oral contraceptive use, and family history of hypertension (hypertension model) or diabetes (diabetes model). bLow risk CVD profile denotes the following characteristics before first pregnancy: mean age at first birth (age = 27) and in 1989 (age = 35), white, ≥16 years of parental education, healthy BMI (18.5–24.9 kg/m2), never smoker, healthiest diet (fifth quintile of AHEI), one alcoholic drink per day, 10–12 months of physical activity per year, no oral contraceptive use, and no family history of hypertension (hypertension model) or diabetes (diabetes model). CVD, cardiovascular disease; BMI, body mass index; AHEI, Alternative Healthy Eating Index.
Given that over half of the very preterm first births were stillbirths, we further evaluated the very preterm association separately in women whose first pregnancy resulted in a live birth (n = 546) and those with a stillbirth (n = 611). Risk patterns were generally similar for associations between very preterm live birth and stillbirth and each CVD risk factor; the relative risk primarily decreased over time, although the magnitudes varied between women who delivered live and stillbirths (Supplementary Table S2). However, in women whose first very preterm delivery resulted in stillbirth, the rate of T2DM increased over time compared to women who delivered at term, with the highest HR occurring 31–40 years after first birth (1.85; 95% CI: 1.18–2.90).
There was a 10% higher rate (95% CI: 1.01–1.19) of chronic hypertension in women who delivered their first pregnancy at term and had at least one additional preterm delivery compared to women who had at least two births all of which were term. There were otherwise no associations between recurrent preterm delivery and chronic hypertension, T2DM, or hypercholesterolemia (Table 3).
Table 3.
Multivariable-Adjusted Hazard Ratios (95% Confidence Intervals) for History of Preterm Deliveries and Cardiovascular Disease Risk Factors at Age 40 or Later, Among Women with No Births at Age 40 or Later
| Chronic hypertension | Type 2 diabetes mellitus | Hypercholesterolemia | |||||
|---|---|---|---|---|---|---|---|
| First pregnancy | Second or later pregnancies | Cases/person-years | HR (95% CI) | Cases/person-years | HR (95% CI) | Cases/person-years | HR (95% CI) |
| Term | All term | 9,247/504,927 | 1.00 (ref) | 1,198/614,820 | 1.00 (ref) | 15,341/475,246 | 1.00 (ref) |
| Term | Any preterm | 663/33,824 | 1.10 (1.01–1.19) | 105/41,622 | 1.23 (1.00–1.51) | 1,066/32,336 | 1.04 (0.98–1.11) |
| Term | No further births | 1,785/96,868 | 1.01 (0.96–1.07) | 244/118,769 | 1.02 (0.88–1.18) | 2,971/90,498 | 1.00 (0.95–1.04) |
| Preterm | All term | 445/23,042 | 1.09 (0.99–1.20) | 60/28,114 | 1.11 (0.86–1.44) | 708/21,798 | 1.04 (0.96–1.12) |
| Preterm | Any preterm | 353/18,036 | 1.06 (0.95–1.18) | 45/22,244 | 0.89 (0.66–1.19) | 581/17,026 | 1.03 (0.95–1.12) |
| Preterm | No further births | 214/11,089 | 1.07 (0.93–1.22) | 35/13,851 | 1.24 (0.88–1.76) | 359/10,588 | 1.04 (0.94–1.16) |
Models are adjusted for age at first birth, age in 1989, race/ethnicity, parental education, family history of hypertension (for the chronic hypertension model), family history of type 2 diabetes mellitus (for the type 2 diabetes mellitus model), prepregnancy BMI, prepregnancy smoking, prepregnancy Alternative Healthy Eating Index (AHEI) score (in quintiles), prepregnancy alcohol intake, prepregnancy (age 18) physical activity, prepregnancy oral contraceptive use, and parity at age 40.
HR, hazard ratio; CI, confidence interval.
Sensitivity analyses
The earliest date a woman could report a diagnosis of chronic hypertension or hypercholesterolemia was 1982 (based on the 1989 questionnaire categories). Therefore, women whose first births were before 1982 contributed person-time during which, by design, they could not have developed chronic hypertension or hypercholesterolemia (i.e., immortal person-time). To test the impact of including this person-time in our primary analyses, we excluded person-time contributed before 1982 (198,461 person-years for chronic hypertension and 199,650 person-years for hypercholesterolemia) and obtained similar results.
In addition, because preterm delivery was retrospectively reported in 2001 or 2009, we performed a purely prospective analysis with follow-up only from 2009 to 2013, when women were a median of 28 (IQR: 23, 33) years since first birth. We found no significant associations between preterm delivery and the CVD risk factors, which are generally consistent with the attenuation of the relative risk 21–40 years after first birth in the primary analysis.
Analyses using an alternative clinical hypertension outcome (including antihypertensive medication use, as well as self-reported high blood pressure), including probable cases of T2DM in addition to definite cases, and excluding multiples yielded similar results to primary analyses (data not shown). Finally, additional adjustment for family history of diabetes or hypertension in the hypercholesterolemia models, family history of hypertension in the T2DM models, and family history of diabetes in the chronic hypertension models also did not change our results (data not shown).
Discussion
Women with a history of preterm delivery in first birth were at higher risk of developing chronic hypertension, T2DM, and hypercholesterolemia later in life; this increased risk was generally stronger for women who delivered very preterm and within the first 10 years following preterm delivery. For women with a high-risk CVD profile before pregnancy, risk curves diverged immediately following a preterm delivery for chronic hypertension and 10 years after delivery for T2DM, while there was no separation between preterm groups for hypercholesterolemia.
While some studies have reported higher blood pressure in women who delivered an infant preterm, those that account for HDP are generally null.22–25 The exception is a registry-based study that followed women 12–15 years after delivery and reported 1.3- to 1.5-fold increased risk of hypertension for moderate and very preterm delivery, respectively.18 Our study, which excluded pregnancies complicated by HDP, found increased risks of similar magnitude in the first 10 years after preterm delivery, which disappeared in the moderate preterm group, but persisted in the very preterm group for up to 30 years.
Our analysis of preterm delivery and T2DM builds on an earlier NHSII study by incorporating the more comprehensive 2009 reproductive questionnaire data and extending maximum follow-up to 50 years.21 The results from the current analysis are similar to the prior study. However, with more follow-up, the increased risk of T2DM in women who delivered very preterm now persists through 40 years after pregnancy. Although studies examining the associations between preterm delivery and insulin or glucose levels following pregnancy are inconsistent,19,24,25 studies that instead evaluate T2DM demonstrate elevated risk in women with a history of preterm delivery,18,20 which are consistent with our results.
To our knowledge, no studies have specifically evaluated preterm delivery and hypercholesterolemia, but studies looking at changes in lipid levels after pregnancy are largely consistent with our results.19,22–25 We found a significant increased risk of hypercholesterolemia only in the first 10 years after a very preterm delivery. Three studies report no differences in total and low-density lipoprotein cholesterol and triglycerides.23–25 Since moderate preterm delivery is more common than very preterm delivery,11 these null results may be driven by moderate preterm delivery, which, in our data, was not associated with hypercholesterolemia. Consistent with our results, two studies with follow-up of <12 years since pregnancy show higher lipid levels and odds of hypertriglyceridemia, particularly in women who delivered very preterm.19,22
One of the primary contributions of our study was our ability to provide thorough adjustment for potential prepregnancy confounders, including BMI, smoking, and family history of hypertension and diabetes, which could underlie both a higher risk of preterm delivery and subsequent CVD risk factors. To our knowledge, this has not previously been done, as many studies on preterm delivery and CVD come from administrative databases, which typically lack information from before pregnancy. Interestingly, we did not observe substantial attenuation of our results with thorough adjustment for lifestyle factors. While there may be unmeasured confounders (e.g., genetics) that could explain the relationship between preterm delivery and CVD risk factors, our analysis represents the most complete control of confounding currently available for these associations.
The causes of preterm delivery are multifactorial and include inflammation, infection, and vascular disease.50 Preterm delivery is likely a marker of underlying subclinical CVD risk rather than a cause of vascular and inflammatory changes resulting in a faster trajectory to the development of CVD risk factors and events. Regardless, preterm delivery provides early insight into a woman's future CVD risk and is generally observed before a woman develops clinical CVD risk factors. Thus, clinicians may be able to use information regarding a woman's history of preterm delivery to inform targeted prevention strategies and screening protocols that could be implemented soon after a preterm delivery. Linkage of obstetric medical records with primary care records would additionally allow clinicians to easily glean information on a woman's pregnancy history to facilitate the implementation of these protocols.
Previous research suggests that <20% of the increased risk of CVD among women who delivered a preterm infant is explained by development of CVD risk factors postpartum.10 Thus, for some women with a history of preterm delivery, the development of CVD risk factors is a primary pathway through which they go on to develop CVD; targeted prevention and screening may ultimately reduce risk of CVD in these women. In addition, prevention of hypertension, T2DM, and hypercholesterolemia may have benefits beyond CVD risk reduction, including impacts on kidney disease and dementia, among others, as well as a reduction in the economic burden associated with CVD risk factors.1,51–53 Regardless of whether CVD risk factors may be potential mediators on the pathway between preterm and CVD for a given woman, history of preterm delivery provides an important risk marker of adverse CVD health in the years and decades following pregnancy. Therefore, this pregnancy complication presents an opportunity for primordial prevention.
The primary weaknesses of our study are exposure and outcome misclassification as gestation length, chronic hypertension, and hypercholesterolemia were self-reported. However, validation studies of preterm delivery and chronic hypertension in the NHSII and hypercholesterolemia in a similar nurse cohort suggest good accuracy.27,31 Lack of information on statin use before 1999 may have resulted in additional misclassification of hypercholesterolemia. However, we expect nondifferential misclassification of the exposure and outcome, yielding bias toward the null. In addition, the exact timing of the development of chronic hypertension and hypercholesterolemia was not known, but sensitivity analyses using parametric models for interval censored outcomes yielded similar conclusions to our primary analyses.
Since participants had to survive to 2001 or 2009 to report their pregnancy history, there is the potential that women who were lost to follow-up or died before this time were at higher risk of both preterm delivery and CVD risk factors. However, 98.3% of NHSII participants were alive in 2009, and our analysis with follow-up starting in 2009 obtained similar results to the primary analysis. We were also unable to separate preterm delivery into spontaneous or medically induced; however, as HDP and GDM are both indications for preterm delivery,7,32 and women reporting these were excluded, many preterm deliveries in our analysis were likely to be spontaneous. As we could not determine which preterm births were small-for-gestational age (SGA) in our cohort, we were unable to stratify by SGA. Our results are subject to residual or unmeasured confounding; however, this study represents the most complete control of confounding for the preterm-CVD risk factor associations in the literature. We excluded women with GDM and HDP and, thus, our results may not be generalizable to women with both a history of preterm delivery and other pregnancy complications. However, given that GDM and HDP are already recognized as CVD risk factors by AHA and ASA,5,6 restricting our analysis to women without these complications allowed us to investigate the associations between preterm delivery and CVD risk factors in women who would not be captured as having a higher risk under the current guidelines. Finally, 93% of our study population was white, limiting generalizability, particularly because preterm delivery is more common in African Americans and may result from different underlying causes.50
Despite these limitations, this longitudinal cohort study included 57,904 women with median follow-up between 26 and 32 years after first birth depending on the CVD risk factor and thorough adjustment for important prepregnancy shared CVD risk factors, including smoking, BMI, family history, and diet. It is also the first study to describe the trajectories of chronic hypertension and hypercholesterolemia development after preterm delivery, which may inform prevention and screening protocols.
Further investigation of the association between preterm delivery and the development of clinical CVD risk factors in a more diverse population is warranted as the incidence and causes of preterm delivery vary by race/ethnicity.50 In addition, evaluations of spontaneous and medically induced preterm delivery, as well as preterm premature rupture of membranes, may reveal different relationships with CVD risk and provide insight into mechanisms linking preterm delivery and CVD. Finally, the utility of including preterm delivery in established CVD risk prediction scores has not yet been assessed and may yield improvements in CVD risk prediction in women.
Conclusions
Women who delivered an infant preterm were at higher risk of developing chronic hypertension, T2DM, and hypercholesterolemia after pregnancy. This increased risk was particularly pronounced in the first 10 years after a preterm delivery, which may be an important time period to implement lifestyle interventions to delay or prevent the development of CVD risk factors.
Supplementary Material
Acknowledgments
The authors thank the Nurses' Health Study II participants and staff for their important contributions. The study was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute under the Ruth L. Kirschstein National Research Service Award (F31 HL131222 to L.J.T.). This work was also supported by grants from the National Heart, Lung, and Blood Institute (T32 HL098048 to L.J.T. and J.J.S.), the American Heart Association (12PRE9110014 to J.J.S. and 13GRNT17070022 to J.W.R.-E.), and the National Institutes of Health, National Cancer Institute (UM1 CA176726).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Benjamin EJ, Virani SS, Callaway CW, et al. . Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation 2018;137:e67–e492 [DOI] [PubMed] [Google Scholar]
- 2. Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: Opportunities for intervention and screening? BMJ 2002;325:157–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rich-Edwards JW, McElrath TF, Karumanchi SA, Seely EW. Breathing life into the lifecourse approach: Pregnancy history and cardiovascular disease in women. Hypertension 2010;56:331–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rich-Edwards JW. Reproductive health as a sentinel of chronic disease in women. Womens Health (Lond) 2009;5:101–105 [DOI] [PubMed] [Google Scholar]
- 5. Bushnell C, McCullough LD, Awad IA, et al. . Guidelines for the prevention of stroke in women: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:1545–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mosca L, Benjamin EJ, Berra K, et al. . Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: A guideline from the American Heart Association. J Am Coll Cardiol 2011;57:1404–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sibai BM. Preeclampsia as a cause of preterm and late preterm (near-term) births. Semin Perinatol 2006;30:16–19 [DOI] [PubMed] [Google Scholar]
- 8. Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: Population based cohort study. BMJ 2001;323:1213–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Catov JM, Wu CS, Olsen J, Sutton-Tyrrell K, Li J, Nohr EA. Early or recurrent preterm birth and maternal cardiovascular disease risk. Ann Epidemiol 2010;20:604–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanz LJ, Stuart JJ, Williams PL, et al. . Preterm delivery and maternal cardiovascular disease in young and middle-aged adult women. Circulation 2017;135:578–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: Final data for 2015. Natl Vital Stat Rep 2017;66:1. [PubMed] [Google Scholar]
- 12. Smith GCS, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: A retrospective cohort study of 129,290 births. Lancet 2001;357:2002–2006 [DOI] [PubMed] [Google Scholar]
- 13. Pell JP, Smith GC, Walsh D. Pregnancy complications and subsequent maternal cerebrovascular events: A retrospective cohort study of 119,668 births. Am J Epidemiol 2004;159:336–342 [DOI] [PubMed] [Google Scholar]
- 14. Bonamy AK, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: Effects of gestational age and fetal growth. Circulation 2011;124:2839–2846 [DOI] [PubMed] [Google Scholar]
- 15. Hastie CE, Smith GC, Mackay DF, Pell JP. Maternal risk of ischaemic heart disease following elective and spontaneous pre-term delivery: Retrospective cohort study of 750,350 singleton pregnancies. Int J Epidemiol 2011;40:914–919 [DOI] [PubMed] [Google Scholar]
- 16. Kessous R, Shoham-Vardi I, Pariente G, Holcberg G, Sheiner E. An association between preterm delivery and long-term maternal cardiovascular morbidity. Am J Obstet Gynecol 2013;209:368, e1–368.e8 [DOI] [PubMed] [Google Scholar]
- 17. Rich-Edwards JW, Klungsoyr K, Wilcox AJ, Skjaerven R. Duration of pregnancy, even at term, predicts long-term risk of coronary heart disease and stroke mortality in women: A population-based study. Am J Obstet Gynecol 2015;213:518, e1–518.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lykke JA, Paidas MJ, Damm P, Triche EW, Kuczynski E, Langhoff-Roos J. Preterm delivery and risk of subsequent cardiovascular morbidity and type-II diabetes in the mother. BJOG 2010;117:274–281 [DOI] [PubMed] [Google Scholar]
- 19. Catov JM, Dodge R, Yamal JM, Roberts JM, Piller LB, Ness RB. Prior preterm or small-for-gestational-age birth related to maternal metabolic syndrome. Obstet Gynecol 2011;117:225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. James-Todd T, Wise L, Boggs D, Rich-Edwards J, Rosenberg L, Palmer J. Preterm birth and subsequent risk of type 2 diabetes in black women. Epidemiology 2014;25:805–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. James-Todd TM, Karumanchi SA, Hibert EL, et al. . Gestational age, infant birth weight, and subsequent risk of type 2 diabetes in mothers: Nurses' health study II. Prev Chronic Dis 2013;10:E156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Catov JM, Dodge R, Barinas-Mitchell E, et al. . Prior preterm birth and maternal subclinical cardiovascular disease 4 to 12 years after pregnancy. J Womens Health (Larchmt) 2013;22:835–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Catov JM, Lewis CE, Lee M, Wellons MF, Gunderson EP. Preterm birth and future maternal blood pressure, inflammation, and intimal-medial thickness: The CARDIA study. Hypertension 2013;61:641–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fraser A, Nelson SM, Macdonald-Wallis C, et al. . Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: The Avon longitudinal study of parents and children. Circulation 2012;125:1367–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perng W, Stuart J, Rifas-Shiman SL, Rich-Edwards JW, Stuebe A, Oken E. Preterm birth and long-term maternal cardiovascular health. Ann Epidemiol 2015;25:40–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. ACOG. Practice bulletin no. 102: Management of stillbirth. Obstet Gynecol 2009;113:748–761 [DOI] [PubMed] [Google Scholar]
- 27. Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension 2008;52:828–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 29. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 30. Manson JE, Rimm EB, Stampfer MJ, et al. . Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–778 [DOI] [PubMed] [Google Scholar]
- 31. Colditz GA, Martin P, Stampfer MJ, et al. . Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol 1986;123:894–900 [DOI] [PubMed] [Google Scholar]
- 32. Spong CY, Mercer BM, D'Alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstet Gynecol 2011;118:323–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: Systematic review and meta-analysis. Eur J Epidemiol 2013;28:1–19 [DOI] [PubMed] [Google Scholar]
- 35. McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: A systematic review and meta-analyses. Am Heart J 2008;156:918–930 [DOI] [PubMed] [Google Scholar]
- 36. Wu P, Haththotuwa R, Kwok CS, et al. . Preeclampsia and future cardiovascular health: A systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2017;10:pii: [DOI] [PubMed] [Google Scholar]
- 37. Heida KY, Franx A, van Rijn BB, et al. . Earlier age of onset of chronic hypertension and type 2 diabetes mellitus after a hypertensive disorder of pregnancy or gestational diabetes mellitus. Hypertension 2015;66:1116–1122 [DOI] [PubMed] [Google Scholar]
- 38. Mannisto T, Mendola P, Vaarasmaki M, et al. . Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation 2013;127:681–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension 2009;53:944–951 [DOI] [PubMed] [Google Scholar]
- 40. Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol 2009;114:961–970 [DOI] [PubMed] [Google Scholar]
- 41. Feig DS, Shah BR, Lipscombe LL, et al. . Preeclampsia as a risk factor for diabetes: A population-based cohort study. PLoS Med 2013;10:e1001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Drost JT, Arpaci G, Ottervanger JP, et al. . Cardiovascular risk factors in women 10 years post early preeclampsia: The Preeclampsia Risk EValuation in FEMales study (PREVFEM). Eur J Prev Cardiol 2012;19:1138–1144 [DOI] [PubMed] [Google Scholar]
- 43. Marin R, Gorostidi M, Portal CG, Sanchez M, Sanchez E, Alvarez J. Long-term prognosis of hypertension in pregnancy. Hypertens Pregnancy 2000;19:199–209 [DOI] [PubMed] [Google Scholar]
- 44. Wilson BJ, Watson MS, Prescott GJ, et al. . Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: Results from cohort study. BMJ 2003;326:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kaul P, Savu A, Nerenberg KA, et al. . Impact of gestational diabetes mellitus and high maternal weight on the development of diabetes, hypertension and cardiovascular disease: A population-level analysis. Diabet Med 2015;32:164–173 [DOI] [PubMed] [Google Scholar]
- 46. Carr DB, Utzschneider KM, Hull RL, et al. . Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care 2006;29:2078–2083 [DOI] [PubMed] [Google Scholar]
- 47. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care 2008;31:1668–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tobias DK, Hu FB, Forman JP, Chavarro J, Zhang C. Increased risk of hypertension after gestational diabetes mellitus: Findings from a large prospective cohort study. Diabetes Care 2011;34:1582–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Julien M, Hanley JA. Profile-specific survival estimates: Making reports of clinical trials more patient-relevant. Clin Trials 2008;5:107–115 [DOI] [PubMed] [Google Scholar]
- 50. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and dementia—a comprehensive review. Ther Adv Neurol Disord 2009;2:241–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zelnick LR, Weiss NS, Kestenbaum BR, et al. . Diabetes and CKD in the United States Population, 2009–2014. Clin J Am Soc Nephrol 2017;12:1984–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.