Abstract
Background: Women with preterm birth (PTB) have excess risk of cardiovascular disease (CVD) and metabolic dysregulation after delivery, but vascular mechanisms are poorly understood. We considered that women with PTB may have evidence of subclinical atherosclerosis after delivery, perhaps related to cardiometabolic risk factors.
Materials and Methods: The Pregnancy Outcomes and Community Health Moms (POUCHmoms) study followed women from pregnancy through 7 to 15 years after delivery (n = 678). Women underwent B-mode ultrasound to measure the average intima-media thickness (IMT) across the common carotid, bulb, and internal carotid artery segments at follow-up (n = 605). Linear regression estimated the overall and segment-specific difference in IMT between women with preterm and term births.
Results: Women were, on average, 38 years old (SD 5.7) at the follow-up visit. Those with a prior preterm versus term birth had thicker mean IMT (average of eight segments, 0.592 mm vs. 0.575, p = 0.04). Differences persisted after accounting for age, race, smoking, and body mass index (difference = +0.018 mm, p = 0.019) and were attenuated after adjustment for blood pressure, medication use, and total cholesterol (difference = +0.014, p = 0.052). Thicker mean bulb IMT in women with PTB was robust to cardiovascular risk factor adjustments (fully adjusted difference = +0.033, p = 0.029). Excluding cases of prepregnancy hypertension or preeclampsia did not change results.
Conclusions: Mechanisms leading to subclinical atherosclerosis may link PTB with future CVD. PTB differences in maternal vessel remodeling in the carotid bulb, an arterial segment more prone to early development of atherosclerosis, were independent of traditional risk factors suggesting that novel processes may be involved.
Keywords: preterm birth, atherosclerosis, cardiometabolic risk factors
Introduction
Women with preterm birth (PTB), defined as delivery occurring before 37 weeks' gestation, have excess risk of cardiovascular disease (CVD) and metabolic dysregulation after delivery,1–4 but vascular mechanisms are poorly understood. Very few studies evaluate subclinical vascular features, and recent evidence suggests that the PTB-CVD association may not be due to shared risk factors such as blood pressure, lipids, and adiposity.5 Further complicating these studies, PTB is a heterogeneous condition, with cases typically classified according to clinical circumstances as spontaneous (preterm labor or preterm premature rupture of membranes) and medically indicated (early C-section or labor induction due to maternal or fetal conditions). Despite concerns with this approach,6–8 these clinical phenotypes are often represented as pathophysiologically distinct. And yet, there is compelling evidence that the epidemiologic outcomes,2,4,9 metabolic changes,10,11 and placental abnormalities in indicated and spontaneous PTBs overlap, and thus, vascular mechanisms may also be shared.12,13
Studying the postpregnancy vascular health of women following PTB may help elucidate pathways and relevant subtypes that mark excess CVD risk. Atherosclerotic vascular disease is a chronic, inflammatory fibroproliferative disease of the large and medium-sized arteries fueled by lipids.14 The intima-media thickness (IMT) of the common carotid artery (CCA), the carotid bulb, and the internal carotid artery (ICA) have been used as markers of carotid atherosclerosis and are prognostic of cardiovascular events.15–19 Our group has previously reported that women with PTB had thicker carotid IMT measured 8 years after delivery, but results were not independent of traditional CVD risk factors.20 We also related PTB to carotid IMT in a study that relied on maternal report of pregnancy features and, thus, were not reliably able to distinguish clinical subtypes of PTB.21 Neither prior study examined carotid segment-specific changes, despite evidence that different carotid segments may carry differential CVD risk factor associations and risk prediction.
In a cohort recruited during pregnancy and followed up to 15 years after delivery, we considered that women with PTB would have evidence of subclinical atherosclerosis, explained, in part, by cardiometabolic risk factors. We also hypothesized that associations with the carotid bulb may be particularly strong in reproductive age women as this segment is most susceptible to atherogenesis and, thus, often the first to reflect atherosclerotic thickening.22
Materials and Methods
Designed to examine pathways leading to preterm delivery, the POUCH Study enrolled 3019 pregnant women from 1998 to 2004 in Michigan, United States. A subcohort of 1371 participants was created for in-depth pregnancy measures that was composed of all those delivering preterm (<37 weeks' gestation), those with higher risk of preterm delivery (African American race/ethnicity or those with elevated concentrations of maternal alpha-fetoprotein screening), and a random sample of those delivering at term (≥37 weeks' gestation). In all analyses sampling weights are used to account for this subcohort sampling strategy.
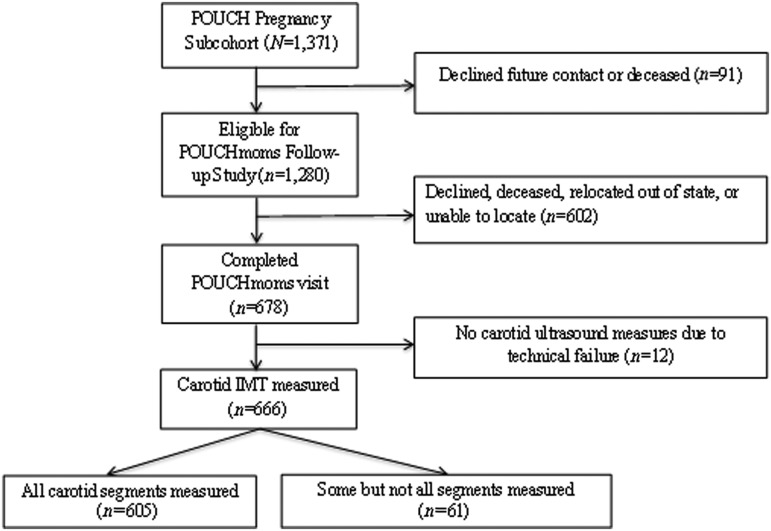
POUCHmoms was designed to examine early evidence of cardiovascular risk 7–15 years after the POUCH Study pregnancy. Women were invited to a one time follow-up visit, and 678 participated from 2011 to 2014 (Fig. 1). Women who attended the follow-up visit compared to those who did not were somewhat older, had higher levels of education, were less likely to have received Medicaid insurance at pregnancy, and were more likely to be of white or other race/ethnicity (all p < 0.05; Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/jwh). Frequency of PTB, hypertension in pregnancy, and prepregnancy body mass index (BMI) did not differ between those in the follow-up study compared to those not followed. All participating women provided written informed consent; the study was approved by the Michigan State University and University of Pittsburgh Institutional Review Boards. Of the women who attended the follow-up visit, 12 were excluded due to technical failures when measuring IMT. Another 61 women were excluded because they had incomplete IMT measures, for a final population of 605 included in the current analysis. Those with IMT measures were more likely to be of normal BMI and of white or other race/ethnicity compared to those without IMT measures (Supplementary Table S1).
FIG. 1.
Participants in POUCHmoms Study, 2011–2014. POUCHmoms, Pregnancy Outcomes and Community Health Moms.
Pregnancy features
Detailed pregnancy data were collected through structured interviews and medical record review at mid-pregnancy and after delivery. PTB included birth prior to 36 completed weeks' gestation. Gestational age was calculated from the last menstrual period (LMP) or from ultrasound (≤25 weeks' gestation) when this estimate differed from the LMP-based estimate by more than 2 weeks. Spontaneous PTBs were those following preterm labor or preterm premature rupture of membranes; medically indicated preterm deliveries were due to maternal or fetal indications. Hypertensive disorders were identified when explicitly diagnosed or when women met the diagnostic criteria in place during this time frame.23 Preeclampsia and gestational hypertension were defined as blood pressure ≥140 mmHg systolic or 90 mmHg diastolic on two occasions after 20 weeks of gestation, with preeclampsia requiring presence of proteinuria. Chronic hypertension was defined as blood pressure ≥140 mmHg systolic or 90 mmHg diastolic prior to pregnancy or before 20 weeks' gestation. Gestational diabetes status was determined by a failed 3-hour glucose tolerance test, failed glucose screen (>190 mg/dL) accompanied by a fasting glucose >95 mg/dL, or diagnosis in the medical records. Prepregnancy BMI and smoking status during pregnancy were self-reported at, on average, 22.4 weeks' gestation.
Carotid ultrasound
At the cardiovascular follow-up visit 7–15 years after delivery, B-mode ultrasound images of the right and left distal CCA, carotid bulb, and the first centimeter of the ICA were obtained in end diastole using the Terason t3000 Ultrasound System (Teratech Corp, Burlington, MA). Semiautomated edge-detection software (Artery Measurement System, Gothenburg, Sweden) was used to identify the lumen–intima and media–adventitia interfaces across 1 cm segments of the near and far walls of the CCA and the far wall of the bulb and ICA. IMT values across these eight sites were averaged to obtain mean average IMT. Site-specific mean IMT (CCA, bulb, ICA) was also evaluated. Reproducibility of the IMT measures was excellent, with an intra-class correlation coefficient between sonographers of ≥0.87 and between readers of 0.92.
Covariates
Race/ethnicity and age were reported during pregnancy. Other covariates were assessed at the follow-up visit, including education (<high school, high school, vocational or some college, ≥college), menopausal status (nonsurgical [no menstrual periods during the previous 12 months] and surgical [removal of both ovaries], or indeterminate), household income (<$20,000, $20,000–<$50,000, $50,000–<$90,000, ≥$90,000; missing for n = 10), family history of chronic health conditions, smoking (during pregnancy and current), lipid lowering or antihypertensive medication use, and the gestational age at delivery of all births before and after the POUCH Study birth. These interval births were classified as preterm or term based on maternal recall and considered as covariates. Numbers were too small to separately evaluate the group with two or more PTBs (n = 56). Insurance status was evaluated as Medicaid coverage during and/or after the POUCH Study pregnancy. Leisure physical activity was reported as hours/week using the Modifiable Activity Questionnaire,24,25 and diet quality was assessed using the Block Food Frequency Questionnaire.26 BMI (kg/m2) was calculated from measured height and weight at the follow-up visit. Waist circumference was assessed in centimeters with a Gulick tape measure, taken at the peak of expiration.
Blood pressure was measured thrice at the follow-up visit via a standard research protocol using either a Panasonic EW3109W (Panasonic Corp, Newark NJ) or an Omron Hem-907 (Omron Healthcare, Inc.; Lake Forest, IL) with an appropriately sized cuff. Readings from both digital monitors were comparable and were similar to manual readings. BP was the mean of the second and third measurements. Fasting blood samples were collected at the follow-up visit. Total cholesterol, high-density lipoprotein (HDL)-cholesterol, and triglycerides were measured under standard enzymatic procedures27–29 in the University of Pittsburgh Heinz Nutrition Laboratory. The coefficient of variation ranged from 1.3% to 6.5%. Low-density lipoprotein (LDL)-cholesterol was evaluated using the Friedewald calculation and measured directly when triglycerides were ≥400 mg/dL (n = 8).30
Statistical analysis
Maternal characteristics during pregnancy and at the follow-up visit were compared according to PTB status using chi square or t-tests. Associations between PTB and mean carotid IMT were estimated in linear regression models adjusted for age, race/ethnicity, smoking, and BMI at the follow-up visit. Models were then further adjusted for cardiovascular risk factors known to impact IMT or that differed according to PTB status (systolic blood pressure, antihypertensive medication use, and total cholesterol). Models were replicated for carotid segment-specific IMT (CCA, bulb, and ICA). Sensitivity analysis replicated models after excluding women with chronic hypertension (n = 20) or preeclampsia (n = 19), the dominant maternal conditions leading to medically indicated PTB. All analyses were conducted in SAS version 9.4 (Cary, NC), and sampling weights were applied to derive estimates reflective of the original source cohort.
Results
Women with a PTB (n = 146) were more likely to be of African American race/ethnicity, to have had prepregnancy hypertension or preeclampsia, to be primiparous during the POUCH Study, and to have had additional PTBs compared to women with term births (Table 1). At the follow-up visit 7–15 years after delivery (mean interval, 11 years) women with a prior PTB were more likely to be taking antihypertensive medication (16.8% vs. 10.1% p = 0.039) and had modestly higher total cholesterol than women with term births (189.7 [SE 3.8] vs. 181.2 [1.8]; p = 0.045).
Table 1.
Maternal Characteristics at Pregnancy and 7–15 Years Later, According to Pregnancy Outcomes and Community Health Study Preterm Birth Status
| Term birth | Preterm birth | ||
|---|---|---|---|
| N = 459 | N = 146 | pa | |
| POUCH pregnancy (1998–2004) | |||
| Maternal age at POUCH delivery (years), mean(se) | 26.8 (0.3) | 26.4 (0.5) | 0.465 |
| Prepregnancy BMI (kg/m2), mean(se) | 27.0 (0.4) | 26.2 (0.6) | 0.307 |
| African American, n (%) | 176 (23.6) | 39 (32.9) | 0.002 |
| Nulliparous before POUCH, n (%) | 193 (43.8) | 78 (54.0) | 0.042 |
| Hypertensive conditions, n (%) | 0.004 | ||
| None | 420 (92.2) | 120 (83.7) | |
| Gestational hypertension | 20 (3.7) | 6 (4.1) | |
| Preeclampsia | 8 (1.9) | 11 (7.4) | |
| Chronic hypertension | 11 (2.2) | 9 (4.8) | |
| Smoking during pregnancy, n (%) | 76 (17.0) | 34 (23.4) | 0.110 |
| Additional preterm births, n (%) | 68 (12.9) | 56 (39.5) | <0.0001 |
| Small for gestational age, n (%) | 59 (11.4) | 12 (9.2) | 0.095 |
| Postpregnancy visit (2011–2014) | |||
| Time to follow-up (years), mean(se) | 11.0 (0.1) | 11.1 (0.1) | 0.504 |
| Age (years), mean(se) | 37.8 (0.3) | 37.5 (0.5) | 0.567 |
| Years of regular smoking, mean(se) | 5.8 (0.5) | 6.9 (0.7) | 0.209 |
| Average leisure time physical activity (hours/week) | 4.3 (0.3) | 4.1 (0.4) | 0.734 |
| Diet quality | 53.3 (0.5) | 53.6 (1.0) | 0.804 |
| Smoking, n (%) | 127 (27.1) | 48 (34.2) | 0.105 |
| Insurance history, n (%) | 0.071 | ||
| Never on Medicaid | 204 (51.4) | 61 (40.0) | |
| Medicaid before POUCH only | 86 (17.5) | 28 (18.5) | |
| Medicaid after POUCH only | 26 (4.5) | 11 (8.0) | |
| Medicaid before/during and after POUCH | 142 (26.6) | 46 (33.5) | |
| Education, n (%) | 0.429 | ||
| <High school | 35 (6.9) | 13 (9.2) | |
| High school | 61 (12.7) | 18 (13.2) | |
| Vocational or some college | 228 (49.2) | 77 (53.5) | |
| ≥College graduate | 135 (31.2) | 38 (24.1) | |
| Menopause, n (%) | 0.054 | ||
| No | 418 (90.4) | 121 (83.8) | |
| Yes (surgical and nonsurgical) | 33 (7.4) | 22 (14.2) | |
| Indeterminate | 8 (2.2) | 3 (2.0) | |
| Family history, n (%) | |||
| Diabetes | 173 (35.7) | 64 (44.7) | 0.150 |
| Hypertension | 304 (64.9) | 105 (71.4) | 0.354 |
| Heart disease | 118 (25.0) | 48 (30.1) | 0.519 |
| Stroke | 68 (14.5) | 30 (21.8) | 0.153 |
| Gestational hypertension | 77 (16.8) | 26 (15.2) | 0.669 |
| Income, n (%) | 0.108 | ||
| <$20,000 | 119 (22.2) | 39 (28.7) | |
| $20,000–<$50,000 | 152 (33.8) | 49 (34.4) | |
| $50,000–<$90,000 | 105 (26.5) | 26 (16.8) | |
| ≥$90,000 | 74 (17.6) | 31 (20.0) | |
| Statin use, n (%) | 13 (3.2) | 7 (4.4) | 0.512 |
| Antihypertensive medication use, n (%) | 52 (10.1) | 24 (16.8) | 0.039 |
| Body mass index (kg/m2), mean(se) | 30.9 (0.5) | 29.8 (0.7) | 0.199 |
| Waist circumference (cm), mean(se) | 90.1 (0.9) | 88.4 (1.4) | 0.288 |
| Systolic blood pressure (mm Hg), mean(se) | 114.2 (0.7) | 115.2 (1.3) | 0.475 |
| Diastolic blood pressure (mm Hg), mean(se) | 75.3 (0.6) | 75.7 (1.0) | 0.734 |
| HDL cholesterol (mg/dL), mean(se) | 54.7 (0.8) | 55.0 (1.2) | 0.857 |
| LDL cholesterol (mg/dL), mean(se) | 105.2 (1.5) | 110.4 (2.8) | 0.102 |
| Triglycerides (mg/dL), median (IQR) | 86.3 (64.7) | 95.2 (66.4) | 0.378 |
| Total cholesterol (mg/dL), mean (se) | 181.2 (1.8) | 189.7 (3.8) | 0.045 |
Accounting for sampling weights; N = 605 except the following: n = 604 for insurance status; n = 595 for income; n = 597 for family history.
BMI, body mass index; HDL, high density lipoprotein; IQR, interquartile range; LDL, low density lipoprotein; POUCH, Pregnancy Outcomes and Community Health.
Women with a preterm compared to term birth had thicker carotid IMT averaged across all eight segments (0.592 [0.01] vs. 0.575 [0.00], p = 0.04; Table 2). Differences were most pronounced in the carotid bulb (0.629 [0.01] vs. 0.588 [0.01], p = 0.01). Thicker IMT according to PTB status persisted after accounting for maternal age, race/ethnicity, smoking, and BMI (adjusted difference +0.018 [0.01], p = 0.019; Table 3) and was attenuated after further adjusting for systolic blood pressure, antihypertensive medication use, and total cholesterol (adjusted difference +0.014 [0.01]; p = 0.052). Differences in the carotid bulb persisted after accounting for maternal factors and traditional cardiometabolic risk factors (fully adjusted difference +0.033 [0.01]; p = 0.029).
Table 2.
Carotid Intima-Media Thickness 7–15 Years After Pregnancy, According to Prior Preterm Birth Status
| Term | Preterm | ||
|---|---|---|---|
| (n = 459) | (n = 146) | p | |
| Overall carotid IMT, mm (mean, se) | 0.575 (0.00) | 0.592 (0.01) | 0.04 |
| Common carotid IMT, mm (mean, se) | 0.597 (0.00) | 0.606 (0.01) | 0.25 |
| Carotid bulb IMT, mm (mean, se) | 0.588 (0.01) | 0.629 (0.01) | 0.01 |
| Internal carotid IMT, mm (mean, se) | 0.516 (0.01) | 0.529 (0.01) | 0.30 |
N = 605; accounting for sampling weights.
IMT, intima-media thickness.
Table 3.
Difference in Carotid Intima-Media Thickness (mm) According to Preterm Birth and Clinical Subtypes; Women with Term Births Are the Referent
| All preterm births | Clinical presentation | Gestational age at delivery | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preterm (n = 146) | p | Spontaneous (n = 97) | p | Indicated (n = 49) | p | 34-< 37 weeks (n = 113) | p | <34 weeks (n = 33) | p | |
| Model 1a | ||||||||||
| Carotid IMT (all segments) | 0.018 (0.01) | 0.019 | 0.007 (0.01) | 0.411 | 0.040 (0.01) | 0.002 | 0.022 (0.01) | 0.010 | 0.004 (0.01) | 0.762 |
| Common carotid IMT | 0.010 (0.01) | 0.144 | −0.001 (0.01) | 0.869 | 0.032 (0.01) | 0.001 | 0.012 (0.01) | 0.106 | 0.003 (0.01) | 0.816 |
| Carotid bulb IMT | 0.039 (0.02) | 0.013 | 0.030 (0.02) | 0.081 | 0.056 (0.03) | 0.044 | 0.044 (0.02) | 0.014 | 0.021 (0.03) | 0.415 |
| Internal carotid IMT | 0.013 (0.01) | 0.265 | 0.002 (0.01) | 0.908 | 0.037 (0.02) | 0.061 | 0.020 (0.01) | 0.134 | −0.010 (0.02) | 0.620 |
| Model 2a | ||||||||||
| Carotid IMT (all segments) | 0.014 (0.01) | 0.052 | 0.005 (0.01) | 0.561 | 0.034 (0.01) | 0.009 | 0.018 (0.01) | 0.026 | 0.001 (0.01) | 0.965 |
| Common carotid IMT | 0.007 (0.01) | 0.300 | −0.003 (0.01) | 0.741 | 0.026 (0.01) | 0.010 | 0.009 (0.01) | 0.217 | 0.000 (0.01) | 0.999 |
| Carotid bulb IMT | 0.033 (0.01) | 0.029 | 0.024 (0.02) | 0.138 | 0.050 (0.03) | 0.075 | 0.038 (0.02) | 0.028 | 0.014 (0.02) | 0.554 |
| Internal carotid | 0.011 (0.01) | 0.336 | 0.0003 (0.01) | 0.980 | 0.034 (0.02) | 0.101 | 0.018 (0.01) | 0.171 | −0.012 (0.02) | 0.578 |
Model 1 adjusted for age, race/ethnicity, smoking, and BMI; Model 2 adjusted for factors in model 1 and systolic blood pressure, total cholesterol, and antihypertensive medication.
All models account for cohort sampling weights.
When evaluated according to PTB clinical circumstances, IMT averaged across all segments was most pronounced among women with medically indicated PTB (fully adjusted difference +0.034 [0.01], p = 0.009). It appeared that all carotid segments were affected in these women, although only the common carotid difference achieved statistical significance. In addition, those delivering preterm between 34 and <37 weeks had thicker overall IMT (fully adjusted difference +0.018 [0.01], p = 0.026); adjusted differences in the carotid bulb were particularly robust in this group (+0.038 [0.01], p = 0.028). Of note, differences were only modestly attenuated and remained statistically significant when women with chronic hypertension or preeclampsia were excluded from analysis (Table 4). Adjustment of models for self-reported PTBs that occurred before or after the POUCH Study pregnancy did not change any results. Results also were unaffected when postmenopausal women or those with indeterminate menopause status were excluded (n = 66).
Table 4.
Difference in Carotid Intima-Media Thickness (mm) According to Subtypes of Preterm Excluding Women with Prepregnancy Hypertension or Preeclampsia
| All preterm births | Clinical presentation | Gestational age at delivery | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preterm (n = 126) | p | Spontaneous (n = 91) | p | Indicated (n = 35) | p | 34-< 37 weeks (n = 99) | p | <34 weeks (n = 27) | p | |
| Carotid IMT (all segments)* | 0.014 (0.01) | 0.058 | 0.007 (0.01) | 0.442 | 0.034 (0.01) | 0.007 | 0.019 (0.01) | 0.023 | −0.002 (0.02) | 0.890 |
| Common carotid IMT | 0.007 (0.01) | 0.343 | −0.003 (0.01) | 0.722 | 0.031 (0.01) | 0.009 | 0.010 (0.01) | 0.182 | −0.006 (0.01) | 0.630 |
| Carotid bulb IMT | 0.031 (0.02) | 0.034 | 0.029 (0.02) | 0.093 | 0.038 (0.03) | 0.126 | 0.035 (0.02) | 0.037 | 0.019 (0.03) | 0.486 |
| Internal carotid IMT | 0.012 (0.01) | 0.329 | 0.003 (0.01) | 0.838 | 0.036 (0.02) | 0.107 | 0.019 (0.01) | 0.158 | −0.014 (0.02) | 0.548 |
Mean of the common, carotid bulb and internal carotid IMTs.
Discussion
Our results from a prospective pregnancy cohort recruited at mid-gestation and followed 7–15 years after delivery indicate that subclinical atherosclerosis may be a mechanism linking PTB with future CVD. The more robust associations with the carotid bulb suggest that early evidence of atherosclerosis in women with PTBs may not be entirely explained by traditional cardiometabolic risk factors. PTBs delivered between 34 and <37 weeks gestation and those following medical indication are likely driving these associations. Results persisted after excluding cases of preeclampsia and chronic hypertension, suggesting that other maternal morbidity and mechanisms may underlie these associations.
Our results are in line with those reported by the few other studies of subclinical vascular factors assessed in women with a prior PTB and extend them in several ways. First, our community-based cohort was recruited at mid-pregnancy, and thus, we had precise PTB information (rather than self-reported) derived from a cohort reflective of a general obstetric population. While the crude differences in overall IMT according to PTB that we detected were of a similar magnitude compared to other studies, adjustment for maternal and traditional cardiometabolic risk factors yielded IMT differences that were almost twofold higher (0.014 compared to 0.008 and 0.007).20,21 It is likely that the objective assessment of our pregnancy and postdelivery features enhanced the precision of our findings. In addition, the current study uniquely evaluated carotid segment-specific features that may be most relevant in reproductive age women and that may be more robustly linked to future cardiovascular events.31 Importantly, the carotid bulb vessel wall thickening detected in the current study persisted after accounting for traditional cardiometabolic risk factors, chronic hypertension, and preeclampsia. This finding raises the possibility that early atherosclerosis in women with a prior PTB may be due to novel factors, and this possibility warrants further study.
Women with medically indicated PTBs had the most severe IMT, and this was not explained by chronic hypertension or preeclampsia. Thus, occult maternal morbidity that may affect placentation and fetal growth may explain our findings. Large record linkage studies have reported that maternal CVD risk is elevated in women with spontaneous PTB compared to term uncomplicated births.1,3 Most women in our study were under 50 years of age (mean age 38 years), and thus, it is possible that longer follow-up of women with spontaneous PTB risk may reveal subclinical carotid vessel thickening.
Current guidelines for cardiovascular screening in women include history of hypertensive disorders of pregnancy and gestational diabetes as sex-specific risk markers.32 Our results suggest that PTB may also be a pregnancy feature that can mark women at excess risk for CVD who may benefit from postpartum and ongoing primary care follow-up.
Our study must be considered in the context of limitations. Although large, our cohort is not entirely representative of the source cohort. In addition, enrolled women with IMT measurement were, in general, less obese than those excluded due to incomplete visualization of the entire carotid artery, and thus, our estimates may be biased toward the null. In addition, our study population was too young to determine if the subclinical atherosclerosis we detected may contribute to cardiovascular events. Longer follow-up is needed to identify if PTB is linked to IMT progression and CVD events. We also had small numbers in some subgroups, such as early PTB (<34 weeks, n = 33), and thus were limited in our ability to detect true associations. We also chose to focus on the POUCH Study PTB, as the clinical circumstances of other PTBs could not be reliably reported via maternal recall.33 Future studies that can adjudicate these features are warranted. Strengths of our study include the prospective community cohort design, which enhances generalizability, and the comprehensive measurement of all carotid artery segments. We also collected pregnancy data prospectively and thus could relate precise pregnancy features to the maternal vascular profile a decade after delivery.
Conclusions
Our results indicate that subclinical atherosclerosis may be a mechanism linking PTB with future CVD. The more robust associations with the carotid bulb suggest that early evidence of atherosclerosis in women with PTBs is not entirely explained by traditional cardiometabolic risk factors. Women with PTB may identify a subset with accelerated progression to atherosclerotic coronary disease who may benefit from ongoing primary care surveillance after delivery.
Financial Support
This POUCHmoms Study was supported by the National Heart, Lung, and Blood Institute [R01-HL103825]. The POUCH Study was supported by the Perinatal Epidemiological Research Initiative Program Grant from the March of Dimes Foundation [20FY01-38 and 20FY04-37], the Eunice Kennedy Shriver National Institute for Child Health and Human Development and the National Institute of Nursing Research [R01-HD34543], the Thrasher Research Foundation [02816-7], and the Centers for Disease Control and Prevention [U01-DP000143-01].
Supplementary Material
Acknowledgments
The authors thank the sonographers, Tiffany Owen and Amy Cole, and the team at the University of Pittsburgh Ultrasound Research Laboratory who worked diligently to collect the excellent images required for this study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Heida KY, Velthuis BK, Oudijk MA, et al. Cardiovascular disease risk in women with a history of spontaneous preterm delivery: A systematic review and meta-analysis. Eur J Prev Cardiol 2016;23:253–263 [DOI] [PubMed] [Google Scholar]
- 2. Rich-Edwards JW, Klungsoyr K, Wilcox AJ, Skjaerven R. Duration of pregnancy, even at term, predicts long-term risk of coronary heart disease and stroke mortality in women: A population-based study. Am J Obstet Gynecol 2015;213:518 e511–e518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robbins CL, Hutchings Y, Dietz PM, Kuklina EV, Callaghan WM. History of preterm birth and subsequent cardiovascular disease: A systematic review. Am J Obstet Gynecol 2014;210:285–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Catov JM, Wu CS, Olsen J, Sutton-Tyrrell K, Li J, Nohr EA. Early or recurrent preterm birth and maternal cardiovascular disease risk. Ann Epidemiol 2010;20:604–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tanz LJ, Stuart JJ, Williams PL, et al. Preterm delivery and maternal cardiovascular disease in young and middle-aged adult women. circulation 2017;135:578–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldenberg RL, Gravett MG, Iams J, et al. The preterm birth syndrome: Issues to consider in creating a classification system. Am J Obstet Gynecol 2012;206:113–118 [DOI] [PubMed] [Google Scholar]
- 7. Kramer MS, Papageorghiou A, Culhane J, et al. Challenges in defining and classifying the preterm birth syndrome. Am J Obstet Gynecol 2012;206:108–112 [DOI] [PubMed] [Google Scholar]
- 8. Villar J, Papageorghiou AT, Knight HE, et al. The preterm birth syndrome: A prototype phenotypic classification. Am J Obstet Gynecol 2012;206:119–123 [DOI] [PubMed] [Google Scholar]
- 9. Hastie CE, Smith GC, Mackay DF, Pell JP. Maternal risk of ischaemic heart disease following elective and spontaneous pre-term delivery: Retrospective cohort study of 750 350 singleton pregnancies. Int J Epidemiol 2011;40:914–919 [DOI] [PubMed] [Google Scholar]
- 10. Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol 2007;166:1312–1319 [DOI] [PubMed] [Google Scholar]
- 11. Chatzi L, Plana E, Daraki V, et al. Metabolic syndrome in early pregnancy and risk of preterm birth. Am J Epidemiol 2009;170:829–836 [DOI] [PubMed] [Google Scholar]
- 12. Kelly R, Holzman C, Senagore P, et al. Placental vascular pathology findings and pathways to preterm delivery. Am J Epidemiol 2009;170:148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Romero R, Dey SK, Fisher SJ. Preterm labor: One syndrome, many causes. Science 2014;345:760–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Falk E. Pathogenesis of atherosclerosis. J Am College Cardiol 2006;47:C7–C12 [DOI] [PubMed] [Google Scholar]
- 15. Cao JJ, Arnold AM, Manolio TA, et al. Association of carotid artery intima-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality: The Cardiovascular Health Study. Circulation 2007;116:32–38 [DOI] [PubMed] [Google Scholar]
- 16. Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: The Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol 1997;146:483–494 [DOI] [PubMed] [Google Scholar]
- 17. Iglesias del Sol A, Bots ML, Grobbee DE, Hofman A, Witteman JC. Carotid intima-media thickness at different sites: Relation to incident myocardial infarction; The Rotterdam Study. Eur Heart J 2002;23:934–940 [DOI] [PubMed] [Google Scholar]
- 18. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007;115:459–467 [DOI] [PubMed] [Google Scholar]
- 19. Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: Prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke 2006;37:87–92 [DOI] [PubMed] [Google Scholar]
- 20. Catov JM, Dodge R, Barinas-Mitchell E, et al. Prior preterm birth and maternal subclinical cardiovascular disease 4 to 12 years after pregnancy. J Womens Health (Larchmt) 2013;22:835–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Catov JM, Lewis CE, Lee M, Wellons MF, Gunderson EP. Preterm birth and future maternal blood pressure, inflammation, and intimal-medial thickness: The CARDIA study. Hypertension 2013;61:641–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nature clinical practice. Cardiovasc Med 2009;6:16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 2002;77:67–75 [PubMed] [Google Scholar]
- 24. Kriska A. Physical activity and the prevention of type 2 diabetes mellitus: How much for how long? Sports Med 2000;29:147–151 [DOI] [PubMed] [Google Scholar]
- 25. Kriska AM, Knowler WC, LaPorte RE, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care 1990;13:401–411 [DOI] [PubMed] [Google Scholar]
- 26. Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 1990;43:1327–1335 [DOI] [PubMed] [Google Scholar]
- 27. Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem 1974;20:470–475 [PubMed] [Google Scholar]
- 28. Bucolo G, David H. Quantitive determination of serum triglycerides by the use of enzymes. Clin Chem 1973;19:476–482 [PubMed] [Google Scholar]
- 29. Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res 1978;19:65–76 [PubMed] [Google Scholar]
- 30. Friedewald W, Levy R, Fredrickson D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 31. Shore AC, Colhoun HM, Natali A, et al. Measures of atherosclerotic burden are associated with clinically manifest cardiovascular disease in type 2 diabetes: A European cross-sectional study. J Intern Med 2015;278:291–302 [DOI] [PubMed] [Google Scholar]
- 32. Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: A guideline from the American Heart Association. J Am Coll Cardiol 2011;57:1404–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carter E, Stuart J, Farland L, et al. Pregnancy complications as markers for subsequent maternal cardiovascular disease: Validation of a maternal recall questionnaire. J Womens Health 2015;24:702–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.