Abstract
Objective
To assess whether hippocampal sclerosis (HS) severity is mirrored at the level of large-scale networks.
Methods
We studied preoperative high-resolution anatomical and diffusion-weighted MRI of 44 temporal lobe epilepsy (TLE) patients with histopathologic diagnosis of HS (n = 25; TLE-HS) and isolated gliosis (n = 19; TLE-G) and 25 healthy controls. Hippocampal measurements included surface-based subfield mapping of atrophy and T2 hyperintensity indexing cell loss and gliosis, respectively. Whole-brain connectomes were generated via diffusion tractography and examined using graph theory along with a novel network control theory paradigm that simulates functional dynamics from structural network data.
Results
Compared to controls, we observed markedly increased path length and decreased clustering in TLE-HS compared to controls, indicating lower global and local network efficiency, while TLE-G showed only subtle alterations. Similarly, network controllability was lower in TLE-HS only, suggesting limited range of functional dynamics. Hippocampal imaging markers were positively associated with macroscale network alterations, particularly in ipsilateral CA1-3. Systematic assessment across several networks revealed maximal changes in the hippocampal circuity. Findings were consistent when correcting for cortical thickness, suggesting independence from gray matter atrophy.
Conclusions
Severe HS is associated with marked remodeling of connectome topology and structurally governed functional dynamics in TLE, as opposed to isolated gliosis, which has negligible effects. Cell loss, particularly in CA1-3, may exert a cascading effect on brain-wide connectomes, underlining coupled disease processes across multiple scales.
Temporal lobe epilepsy (TLE) is the most common drug-resistant epilepsy in adults, with hippocampal sclerosis (HS) at its core.1 MRI has provided important insights in the understanding of histopathologic variability across patients, with measures of atrophy and T2 signal providing increasingly accurate surrogates of cell loss and gliosis.2–4 MRI studies have also been instrumental in revealing whole-brain structural compromise of multiple gray matter regions5,6 and white matter tracts.7–9 Together with graph theoretical investigations showing perturbed topology of brain networks,10–12 findings collectively indicate that TLE affects multiple brain systems.13–15
Despite the widely accepted conceptualization of TLE as a network disorder, surprisingly little work has addressed the interplay between hippocampal and connectome-level disease effects. Analyses of structural connectomes based on diffusion MRI have mainly focused on preoperative markers,10 disregarding the severity of hippocampal pathology. Even at the level of imaging, no study has systematically related hippocampal in vivo markers to white matter connectome features. Finally, and despite the general consensus that structural networks largely constrain functional connectivity,16 it remains unknown whether TLE-related structural connectome alterations affect functional dynamics. Methodologic advances in network science have extended conventional graph theoretical descriptive analysis of topology17 by approaches such as network control theory that address structure–function links mechanistically, specifically in predicting how the brain moves between functional states drawn from white matter network organization. By simulating consequences of structural connectome reorganization on brain dynamics, this novel framework lends a novel perspective to examine structurally governed macroscale dysfunction in TLE.18,19
To address the interplay between hippocampal and whole-brain anomalies in TLE, we combined hippocampal subfield imaging with diffusion MRI connectomics in patients with histologically confirmed HS and healthy controls. Given the dense connectivity of the hippocampus, particularly to default and temporo-limbic networks,20 we hypothesized that interpatient variations in HS severity would be reflected in the degree of white matter connectome reorganization. We parametrized network topology using graph theory and simulated structurally governed brain dysfunction using network control theory.18,19 To assess specificity, we compared effects of networks centered on the hippocampus to other intrinsic networks and repeated analyses after correcting for effects of cortical atrophy. Findings were validated across parcellation schemes and alternate topologic measures.
Methods
Participants
From a database of patients referred to our hospital for the investigation of drug-resistant TLE, we selected a consecutive cohort of 44 TLE patients (17 male; 18–53 years, mean ± SD age 33 ± 9 years) based on the following inclusion criteria: (1) patients underwent surgery; (2) the resected specimen was suitable for histologic analysis21; and (3) patients had a research-dedicated high-resolution 3T MRI prior to surgery that included anatomical and diffusion MRI. Demographic and clinical data were obtained through interviews with patients and their relatives. TLE diagnosis and lateralization of the seizure focus into left TLE (n = 20) and right TLE (n = 24) were determined by a comprehensive evaluation including detailed history, neurologic examination, review of medical records, video-EEG recordings, and clinical MRI evaluation in all patients. Notably, no patient had a mass lesion (malformations of cortical development, tumor, vascular malformations) or a history of traumatic brain injury or encephalitis.
Mean follow-up time after surgery was 80 ± 20 months (36–120 months). We determined postsurgical seizure outcome according to Engel modified classification.22 Thirty-three (75%) patients had a Class I outcome (i.e., free of disabling seizures). Residual seizures were noted in the remaining 11 cases (6 Class II, 4 Class III, 1 Class IV). Based on histopathologic criteria,21 25 patients showed hippocampal cell loss and gliosis (TLE-HS), including 15 with marked cell loss in both CA1 and CA3 (type 1), 7 with CA1-predominant (type 2), and 3 with CA4-predominant cell loss (type 3). Nineteen patients presented with isolated gliosis (TLE-G).
Comparing demographic and clinical measures (using Student t tests and Fisher exact tests, respectively), we noted no significant differences in age (TLE-HS: 32 ± 9 years; TLE-G: 34 ± 10 years; p > 0.4), male/female distribution (TLE-HS: 12/13; TLE-G: 5/14; p > 0.20), left/right seizure foci (TLE-HS: 12/13; TLE-G: 8/11; p > 0.4), and duration of epilepsy (19 ± 11/15 ± 10 years; p > 0.2). While likelihood of postoperative seizure freedom was somewhat higher in TLE-HS than in TLE-G, the difference was not significant (Class I vs II–IV in TLE-HS: 21/4; TLE-G: 12/7; p = 0.16). Similarly, patients with TLE-HS had a tendency for an earlier disease onset (13 ± 10 vs 18 ± 12 years; p = 0.09) and a higher proportion of patients experiencing generalized tonic-clonic seizures (TLE-HS: 19/6 vs TLE-G: 8/11; p < 0.04). Notably, all analyses were repeated after controlling for age, sex, duration of epilepsy, generalized tonic-clonic seizures, and age at onset to verify the robustness of network differences between TLE-HS vs TLE-G against subthreshold differences in these variables. The proportion of patients with childhood febrile convulsions was higher in TLE-HS (TLE-HS: 14/11 vs TLE-G: 2/17; p < 0.005), in keeping with prior pathologic1 and imaging findings.23 The control group consisted of 25 age- and sex-matched healthy individuals (12 male; 21–53 years; mean ± SD age 32 ± 8 years). See the table for sociodemographic and clinical information on the included study cohorts.
Table.
Demographic and clinical information of the temporal lobe epilepsy cohorts and healthy controls
Standard protocol approvals, registrations, and patient consents
The Ethics Committee of the Montreal Neurological Institute and Hospital approved the study and written informed consent to undergo research-dedicated MRI investigations was obtained from all participants.
MRI acquisition
MRI in patients and controls were acquired on a 3T Siemens (Erlangen, Germany) TimTrio scanner using a 32-channel head coil. For hippocampal subfield analysis, we acquired submillimetric 3D magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted MRI (repetition time [TR] 3,000 ms, echo time [TE] 4.32 ms, inversion time [TI] 1,500 ms, flip angle 7°, matrix 336 × 384, field of view [FOV] 201 × 229 mm2, 240 axial slices with 0.6 mm thickness, resulting in 0.6 × 0.6 × 0.6 mm3 voxels); to increase signal-to-noise ratio, 2 acquisitions were collected, coregistered, and averaged. T2-weighted MRI was obtained using a 2D turbo spin-echo sequence (TR 10,810 ms, TE 81 ms, flip angle 119°, matrix size 512 × 512, FOV 203 × 203 mm2, 60 coronal slices titled perpendicular to the hippocampal long axis, thickness 2.0 mm, resulting in 0.4 × 0.4 × 2.0 mm3 voxels). Whole-brain cortical surfaces were derived from millimetric 3D MPRAGE T1-weighted MRI (TR 2,300 ms, TE 2.98 ms, TI 900 ms, flip angle 9°, matrix 256 × 256, FOV 256 × 256 mm2, 176 sagittal slices with 1 mm thickness, resulting in 1 × 1 × 1 mm3 voxels). Finally, connectivity matrices were generated from a twice-refocused 2D echoplanar imaging diffusion MRI sequence (TR 8,400 ms, TE 90 ms, matrix size 128 × 128, FOV 256 × 256 mm2, 63 transverse slices with 2.0 mm thickness, resulting in 2.0 × 2.0 × 2.0 mm3 voxels; 64 diffusion directions with b = 1,000 seconds/mm2 along with 1 B0 image).
Network analysis
T1 preprocessing and brain parcellation
T1-weighted images underwent intensity nonuniformity correction and were linearly registered to Montreal Neurological Institute 152 template space. As in previous work,24,25 we extracted surfaces of the cortical mantle using automated procedures. Cortical surfaces were aligned with respect to sulco-gyral folding, improving interindividual correspondence in measurement points (henceforth, vertices). We nonlinearly registered each T1-weighted image to Colin27 single-subject template.26 The resulting deformation field was used to map the automated anatomical labeling (AAL) atlas27 to T1-weighted MRI, thereby parcellating the brain into 90 regions of interest (ROIs) (78 cortical, 12 subcortical; henceforth AALLR). We also generated a high-resolution parcellation, by subdividing each parcel into smaller patches with similar surface areas constrained by cortical sulcation, resulting in 294 cortical ROIs that were added to the original 12 subcortical labels (for a total of 306 areas, henceforth, AALHR).
Connectome construction
Diffusion MRI data underwent correction for geometric distortions, eddy currents, and head motion. Patients and controls did not differ with respect to head motion measures (translation: |t| < 0.7, p > 0.2; rotation: |t| < 0.52, p > 0.4). We estimated the shape of the diffusion tensor at each voxel and derived fractional anisotropy (FA) using trackvis (trackvis.org/). We mapped cortical surfaces and AAL parcellations from T1-weighted MRI to native diffusion space, using a nonlinear registration between the white matter partial volume classification derived from preprocessed T1-weighted images28 and the FA skeleton. For both parcellations (i.e., AALLR and AALHR), we propagated labels into the superficial white matter 1 mm below the gray–white matter boundary using our recent approach,29 where cortical vertices are moved towards the ventricles along a Laplacian field. We generated whole-brain structural connectomes for both spatial scales using streamline tractography based on fiber assignment by continuous tracking (FACT), an algorithm that accounts for multiple diffusion directions in a voxel.30 The propagation is constrained within the white matter and halted when reaching 1 mm below the white–gray matter interface or when streamlines exceed a curvature threshold of 60. Based on these streamlines, we sampled subject-wise average FA for each inter-regional pair and stored the data in the whole-brain connectivity matrix. As connectivity strength was quantified by FA and not numbers of streamlines, we did not control for distance between 2 given ROIs.31
Network topology
Using scripts from the Brain Connectivity Toolbox,32 we computed the clustering coefficient and characteristic path length for the whole connectome in controls and patients using standard formulas. Clustering coefficient is a marker of cliquishness and is correlated to local efficiency33; here we calculated clustering as the mean clustering coefficient across all nodes. After calculating the shortest path from every node to all other brain regions (or nodes), we computed the characteristic path length as the average of the shortest path length across all nodes. Long paths signify low global efficiency, which was calculated as the average of the inverse shortest path lengths.33
Network controllability
We leveraged a recently developed network control theory framework to calculate node- and network-wide controllability.18,19 Inputs to this approach are a structural connectome (as defined in the connectome construction section) and a model for the dynamics of neural processes based on prior work in human systems neuroscience; the latter is based on models linking structural brain networks to resting-state functional signals.16 Network control theory, in turn, defines a trajectory of an interconnected system as the temporal path that the system traverses through different states, where a state is defined as the magnitude of neurophysiologic activity across brain regions at a single time point. Trajectories can be understood as dynamic changes in brain-wide simulated functional activation patterns. Average controllability of a node indexes the ability of that node to influence others and is calculated as the average input energy needed to reach all possible states of the system (i.e., the overall magnitude of energy measured across all nodes).17 In nonrandom networks, regions with high average controllability generally also have high centrality, and thus serve as hubs playing an important role in maintaining macroscale connectome organization.34 Despite this resemblance, controllability metrics translate more directly into the overall functional dynamics than centrality measures. Parcel-wise controllability was sorted into ipsilateral and contralateral to the seizure focus following a z-normalization relative to controls,35 thereby accounting for interhemispheric asymmetry of healthy individuals.
Relation to hippocampal imaging measures
We first segmented hippocampal subfields36 using our previously developed patch-based multitemplate algorithm.37 Similar to our previous findings,38,39 TLE-HS presented with reduced global ipsilateral hippocampal volume compared to controls (t > 7.2, p < 0.001) that was consistent across all subfields albeit with highest effects in CA1-3 (t values for subiculum: 5.5, CA1–3: 7.0, CA4–DG: 4.8). No effects were seen contralaterally (|t| < 0.9). Similar effects were also seen when comparing TLE-HS to TLE-G ipsilaterally, with more pronounced global (t = 8.1) and subfield-level atrophy in the former (t > 5.4). We then generated medial sheet representations of these labels,40 and parameterized them using spherical harmonic shape descriptors. These surfaces, which run through the core of individual subfields, allow for vertex-wise computation of local features with minimal partial volume effects, and guarantee shape-inherent vertex correspondence across individuals.40 To assess atrophy, we estimated vertex-wise volumes between the subfield boundary and the medial surface40; for a given medial sheet vertex, columnar volume was calculated by multiplying the distance to the corresponding vertex on the outer subfield surface with the mean area of the triangles whose edges include both vertices. We computed normalized T2 signal intensity by dividing the intensity at a given voxel by the mean T2 signal in the CSF of the anterior horn of the lateral ventricles.40 Hippocampal measures in patients were z-scored relative to corresponding measures in controls and sorted into ipsilateral and contralateral to the seizure focus.
Statistical analysis
Statistical analyses were performed using SurfStat toolbox41 for MATLAB (R2017b; The Mathworks, Natick, MA).
Network-level group comparisons
We compared clustering coefficient, path length, and average controllability in TLE-HS vs healthy controls, TLE-G vs healthy controls, and TLE-HS vs TLE-G using linear models.
Correlation analysis between network markers and hippocampal features
We computed surface-wide multivariate correlations between the overall load of hippocampal MRI anomalies (i.e., volume, T2) and network indices (i.e., clustering coefficient, path length, controllability). In clusters of findings, we illustrated effects separately for volume and T2 via univariate post hoc correlations. To cover the range of pathology beyond histologic categories, correlations were also performed across all patients regardless of pathologic diagnosis.
Control for atrophy
To assess changes in connectome organization beyond effects of diffuse gray matter atrophy, we repeated the above graph and controllability analyses after statistically correcting for mean cortical thickness, measured as the distance between corresponding vertices on the inner and outer cortical surfaces.24,42
Multiple comparisons correction
Findings were corrected using false discovery rate (FDR) procedures.43,44
Data availability
Phenotypic and mean connectome data are available at doi: 10.6084/m9.figshare.7309214.
Results
Group differences in network topology and controllability
The main findings are based on the AALLR parcellation. In a separate reproducibility section (see below), we demonstrate consistency when using AALHR.
Network topology
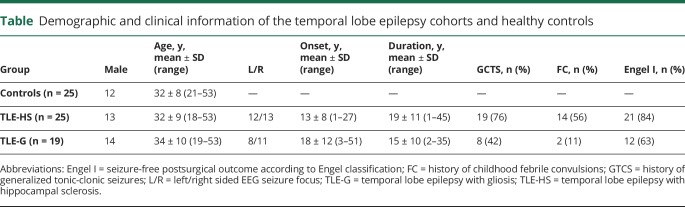
Considering whole-brain graph theoretical measures (figure 1), TLE-HS presented with markedly increased path length and decreased clustering coefficient compared to controls (FDR-corrected p value, pFDR < 0.05; Cohen d = 1.20–1.98), while TLE-G showed smaller effects (d = 0.39–0.88) that were only significant for path length after correction for multiple comparisons. Directly contrasting patient groups, we indeed observed lower clustering coefficient and longer paths in TLE-HS compared to TLE-G (pFDR < 0.05; d = 0.76–0.81).
Figure 1. Connectome analysis.
(A) Whole-brain structural connectomes in healthy controls, temporal lobe epilepsy patients with histologic confirmed hippocampal sclerosis (TLE-HS), and temporal lobe epilepsy patients with isolated gliosis (TLE-G). Connectomes were generated using systematic diffusion tractography between all regions, parcellated according to automated anatomical labeling. Letters in the matrix refer to regional groupings of the nodes (i.e., F = frontal, L = limbic, O = occipital, P = parietal, S = subcortical, T = temporal). (B) Graph theoretical topological measures of clustering coefficient and path length highlighting marked alterations in patients with TLE-HS, while those with TLE-G are only moderately affected compared to controls.
Network controllability
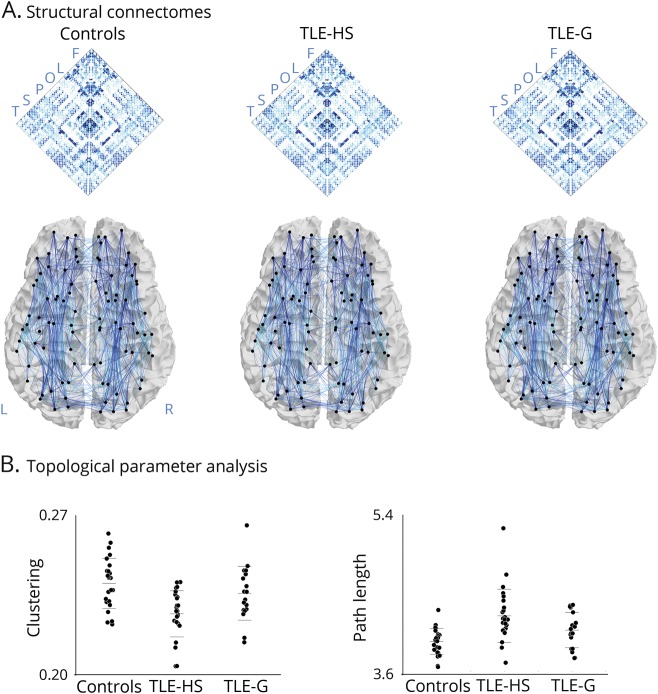
In controls (figure 2A), areas with high controllability included centro-parietal midline and superior frontal regions, replicating earlier findings in a separate cohort.17 These regions, which also display high centrality values, represent hubs.17 In fact, the rank of average controllability strongly correlated with the rank of degree centrality across parcels (r = 0.92, p < 1−15), an additional indication of deviation from a random network architecture (where such a relationship may not be seen).34
Figure 2. Controllability analysis.
(A) Controllability mapping in healthy controls. (A.a) Regional controllability ranked across areas. (A.b) Relation between rank of controllability and degree centrality, indicating that more central regions have larger influence on system dynamics. (B) Alterations in controllability in temporal lobe epilepsy with hippocampal sclerosis (TLE-HS) and temporal lobe epilepsy with gliosis (TLE-G) relative to controls. Maps show Cohen d effect size in pFDR < 0.05 corrected findings. FDR = false discovery rate.
Between-group comparisons of whole-brain controllability (figure 2B) showed a similar pattern as the graph theoretical analysis, supporting the notion that structural topology largely constrains functional dynamics. While controllability was decreased in both TLE-HS and TLE-G compared to controls (pFDR < 0.05), effects were more marked in the former (TLE-HS: d = 1.57, TLE-G: d = 0.64). Directly comparing patient cohorts, TLE-HS was indeed more severely affected than TLE-G (pFDR < 0.05; d = 0.69). Regional analysis mapped a specific pattern of alterations in TLE-HS, with lower controllability in ipsilateral anterior temporal and temporopolar, together with medial prefrontal and posterior midline regions, including retrosplenial cortex and precuneus (pFDR < 0.05). Contralateral decreases were confined to a small region in the posterior insula. Findings in TLE-G, on the other hand, did not survive correction for multiple comparisons.
Specificity of findings with respect to functional networks
For all group comparisons, we systematically compared topologic and controllability changes across several functional networks. In addition to selecting the network centered on the hippocampus/parahippocampus (based on the tractographic connections in healthy controls), we studied networks centered on somatomotor (pre/postcentral), visual (calcarine/inferior occipital), default (precuneus/medial prefrontal), frontoparietal (superior frontal, inferior parietal), and salience (anterior/midcingulate, insula). Notably, despite all networks being affected in TLE compared to healthy controls, effects were highest in hippocampal/parahippocampal networks (mean absolute Cohen d in hippocampal/parahippocampal = 0.83 compared to 0.45–0.75 in other networks) (figure e-1; doi.org/10.5061/dryad.v309h90).
Relation to hippocampal imaging measures
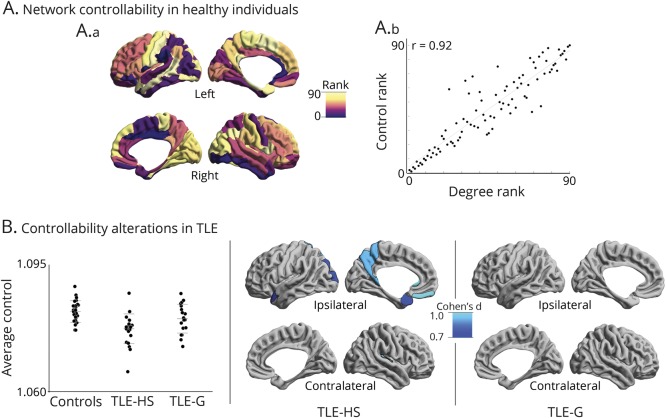
Surface-based multivariate analyses assessed correlations between whole-brain network markers (i.e., clustering coefficient, path length, and controllability) and the load of hippocampal anomalies, quantified through columnar volume and T2 intensity across TLE patients (figure 3). For all network measures, we observed a consistent correlation with hippocampal anomalies across all subfields bilaterally, particularly marked in average controllability (pFDR < 0.1). The most consistent areas of overlap were, however, in the ipsilateral CA1–3.
Figure 3. Relation between network markers and hippocampal subfield features.
(A) Surface-wide multivariate correlations between network features (clustering coefficient, path length, controllability) and local indices of structural subfield integrity (columnar volume, T2 intensity) across all patients. (B) Post hoc correlation analyses in clusters of findings showing effects for individual subfield markers (red dots: temporal lobe epilepsy with hippocampal sclerosis [TLE-HS]; gray dots: temporal lobe epilepsy with gliosis [TLE-G]). FDR = false discovery rate.
Post hoc univariate correlations in clusters of findings illustrated the association between each network measure and subfield marker. Specifically, patients with more marked hippocampal atrophy (i.e., less columnar volume) showed more severe network alterations (i.e., lower clustering, longer path lengths, lower controllability) compared to those with less marked volume loss. Associations between hippocampal T2 intensity and network markers followed an inverse pattern, with hyperintensity relating to decreased clustering, increased path length, and decreased controllability.
Relation to neocortical morphology
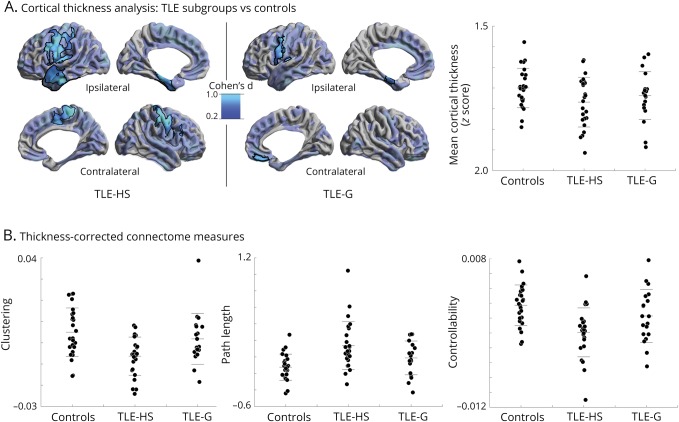
Surface-based cortical thickness analysis revealed marked atrophy in both TLE-HS and TLE-G in frontocentral, orbitofrontal, as well as mesiotemporal regions when compared to healthy controls (figure 4A). While effects were more diffuse in TLE-HS, differences between patient groups did not survive correction for multiple comparisons. Similar findings were seen at the level of mean cortical thickness, where effect sizes were greater in TLE-HS compared to healthy controls (Cohen d of TLE-HS/TLE-G vs controls: −0.33/−0.19), but the direct contrast was not significant (TLE-HS vs TLE-G: t = 0.86, p > 0.2). Notably, group differences in path length, clustering, and controllability remained consistent after correction for cortical thickness (pFDR < 0.05), suggesting that the between-cohort divergence in connectome organization was robust against effects of global atrophy (figure 4B).
Figure 4. Robustness of white matter connectome measures with respect to cortical thickness.
(A) Surface-wide group analysis of cortical thickness between temporal lobe epilepsy with hippocampal sclerosis (TLE-HS) and temporal lobe epilepsy with gliosis (TLE-G) compared to healthy controls. Maps show pFDR < 0.05 corrected findings (black outlines) superimposed on Cohen d effect size maps (thresholded at d > 0.2, semitransparent). Although effect sizes were larger in TLE-HS, there was no significant difference between patient subgroups after the correction for multiple comparisons. Whole-brain mean cortical thickness findings are displayed for the 3 cohorts. (B) Thickness-corrected measures of clustering coefficient, path length, and controllability in the 3 groups, highlighting robustness of the marked effect of TLE-HS on white matter connectome organization.
Reproducibility analysis
Efficiency measures
To assess reproducibility of topologic findings, we quantified local efficiency (with high values indicating high clustering) and global efficiency (with high values indicating short path length). Similar to clustering and path length, these measures showed marked reductions in TLE-HS (pFDR < 0.05; Cohen d > 1.65) and smaller effects in TLE-G (pFDR < 0.05; d > 0.68) (figure e-2; doi.org/10.5061/dryad.v309h90). Contrasting patient groups, structural connectomes in TLE-HS showed decreased global and local efficiency compared to TLE-G (pFDR < 0.05; d > 0.76).
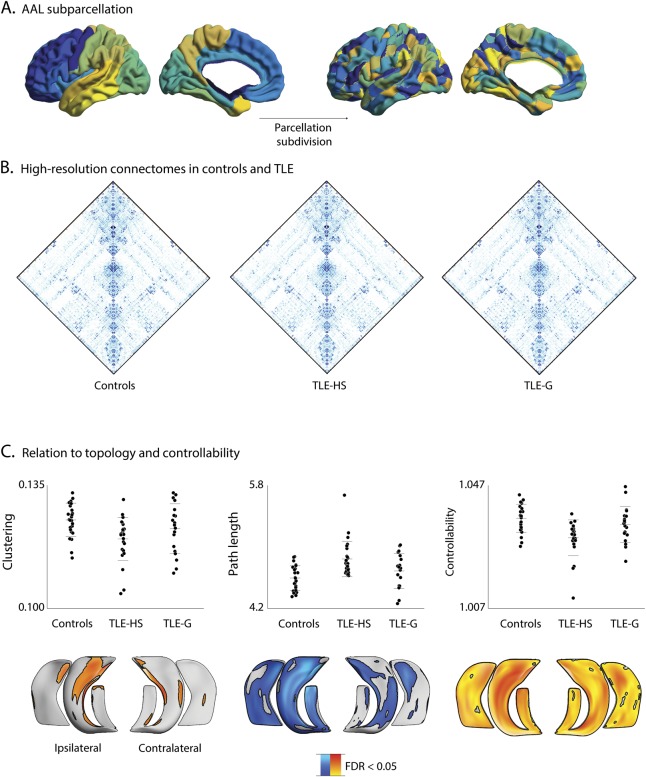
Spatial scale
Repeating topologic and control theory analysis (i.e., clustering, path length, controllability) using the alternative AALHR scheme (figure 5) resulted in similar findings, with decreases in TLE-HS compared to healthy controls for all measures (pFDR < 0.05), while only trends were found in TLE-G. Moreover, network indices derived from the AALHR parcellation correlated with subfield-level columnar volume and T2 intensity (pFDR < 0.05), suggesting that the association between large-scale network topology and hippocampal microstructure was consistent across spatial scales.
Figure 5. Reproducibility analysis using a high-resolution parcellation.
(A) Subdivision of AALLR into AALHR. (B) Connectomes in temporal lobe epilepsy (TLE) groups and controls. (C) Differences in clustering coefficient, path length, and controllability across the 3 groups and within-patient multivariate correlations between network measures and columnar volume and T2 intensity. AAL = automated anatomical labeling; FDR = false discovery rate; TLE-G = temporal lobe epilepsy with gliosis; TLE-HS = temporal lobe epilepsy with hippocampal sclerosis.
Clinical and demographic measures
We repeated network analyses after controlling for age, sex, duration of epilepsy, GTCS, and age at onset to verify whether network differences between TLE-HS vs TLE-G are robust against subthreshold differences in these variables. Indeed, marked alterations in TLE-HS compared to TLE-G were robust even when models additionally included for clinical and sociodemographic characteristics.
Head motion
We repeated our main analyses after including rotation/translation measures from the head motion correction of the diffusion MRI data. Again, we could replicate main results showing more marked alterations in TLE-HS than in TLE-G.
Discussion
This work combined 2 main streams in the neuroimaging of TLE, that is, high-resolution analysis of the hippocampal formation and whole-brain connectomics, including network control theory, a novel framework integrating network structure with models of neural function. Our study provides new evidence that variations in mesiotemporal pathology are main determinants of whole-brain structural network alterations and structurally governed functional dynamics. Indeed, compared to healthy controls, patients with TLE-HS present with marked alterations of network topology and reduced controllability, while TLE-G showed only subtle changes. Specificity for a contribution of hippocampal pathology to interpatient variability in large-scale white matter network reorganization and functional dynamics was suggested by several additional analyses, including a systematic subnetwork assessment revealing maximal changes in the hippocampal/parahippocampal circuity, high consistency of findings when controlling for overall neocortical thinning, and multivariate correlations between in vivo proxies of hippocampal pathology and network alterations.
A unique aspect of the connectome analysis was a categorization of patients into TLE-HS and TLE-G guided by postoperative histology. We additionally parameterized hippocampal subfield anomalies in vivo through high-resolution and multicontrast measurement of columnar volume and T2 intensity,40 the most widely studied MRI markers of HS.40 Correlative studies have shown associations of these markers with histologic grades, both at the whole-hippocampus2,3 and subfield-level,4,38 supporting their utility as a bridge towards pathology. Notably, multiparameter analysis in a surface-based reference frame that follows hippocampal folding can capture subtle changes that would evade global MRI volumetry,38,45 thereby increasing sensitivity in the evaluation of patients with TLE-G.40 Moreover, compared to histopathologic analysis, our in vivo framework provides a 3D and fully quantitative measurement of structural integrity along the entire extent of the hippocampus bilaterally.39,46
Connectome analysis was based on diffusion tractography allowing for the generation of subject-specific networks. Evaluations at different spatial scales showed increased path length in both TLE-G and TLE-HS, albeit with more marked effects in the latter. These findings, indicative of reduced global efficiency, likely reflect topology-level effects of previously reported diffusion anomalies in multiple long-range tracts in TLE.47,48 With regards to clustering, a measure of local efficiency, we could also observe reductions in patients, which were again more marked in TLE-HS. Given the relation between clustering and network segregation, these results suggest reduced connectivity, particularly within temporo-limbic networks heavily connected to the hippocampus. A stronger extent of white matter anomalies in the proximity of the mesiotemporal lobe is in line with prior network neuroscience studies,49 including tract-based assessments showing a tapering effect of diffusion metrics with increasing distance from the focus.47,48 As our connectivity matrices were weighted by FA, reductions could theoretically relate to increased fiber crossing in affected networks; however, given previous histopathologic evidence for associations of FA with axonal membrane circumference, myelin fraction, and axonal packing, our findings more likely relate to perturbations in tract microstructure.50
To address the functional implications of structural connectome reorganization, we harnessed a network control theory paradigm.17 Compared to graph theory's metrics describing the static organization of networks, control theory models neural dynamics upon network structure, allowing for mechanistic predictions on the influence of individual regions on network-wide function. Comparisons between patients and healthy controls showed severe controllability reductions in TLE-HS and only subtle effects in TLE-G, suggesting that the structural makeup in the former may have a limiting effect on the repertoire of whole-brain functional dynamics. Findings were complemented with region-specific analysis; in our controls, this approach mapped high average controllability in both midline parietal and medial prefrontal regions,17 suggesting that densely connected “hub” regions strongly influence large-scale functional dynamics. Specific reduction in the same regions in TLE-HS, alongside ipsilateral anterior and lateral temporal decreases, suggests again a selective effect of marked hippocampal neuronal loss on default and temporo-limbic networks. The former is one of the most reproducible networks showing activations when participants do not engage in an externally oriented task. Its relevance for resting-state paradigms likely relates to its participation in a broad class of self-generated cognition, including mind-wandering, self-referential thought, and future thinking.51 Notably, the mesiotemporal regions have been suggested to closely interact with the default network, representing an important subcomponent of it,20,52 possibly reflective of the relevance of mesiotemporal-mediated memory operations for self-generated thoughts,53 including spatial and semantic memory54 but also mental time travel.55 Therefore, our control theory simulations suggest that structural connectome rearrangements in patients with HS may compromise the capacity of the mesiotemporal subnetwork in flexibly driving large-scale functional states. Ultimately, these changes may relate to impairments in prospective as well as retrospective memory processes commonly seen in TLE,56–58 which are generally more prevalent in patients with marked hippocampal damage than in those with subtle pathology.59,60
Both TLE groups presented with bilateral cortical atrophy, in a similar frontocentral and lateral temporal distribution as those shown in our earlier work and a recent multisite meta-analysis.6,24 Importantly, despite tendencies for a more extensive pattern in TLE-HS compared to TLE-G, findings did not survive after correction for multiple comparisons, suggesting only little interdependence between white matter connectome reorganization and gray matter structural compromise. Indeed, between-cohort differences in TLE were virtually identical when controlling for cortical thickness. Previous human diffusion MRI data50,61 suggest Wallerian degeneration as a potential mediator for the interplay between hippocampal and macroscale connectome anomalies. As hippocampal pathologic grades largely reflect the degree and distribution of cell loss, rather than gliosis, it is plausible that a Wallerian cascade induces proximal and remote limbic deafferentation, which ultimately leads to macroscale network remodeling. Notably, in addition to segmental neuronal loss in the hippocampal formation, HS is also associated with abnormal migration of granule cells and reorganization of their axons, referred to as granule cell dispersion and mossy fiber sprouting. Pathologic reports have shown that patients with subtle HS show negligible mossy fiber sprouting, as compared to severe sprouting in those with marked neuronal loss.62,63 A remodeling of hippocampal internal circuitry may thus contribute to network-level findings at distance, such as those observed in our cohort. Given that the majority of patients included in our study underwent surgery shortly after the presurgical evaluation, we could not longitudinally evaluate white matter networks; such prospective designs may explore whether white matter connectome remodeling shows a progressive course similar to hippocampal and whole-brain gray matter changes in morphology previously reported in TLE.24,64,65
Our findings emphasize the importance of taking into consideration the modulatory effects of hippocampal structural alterations when considering TLE from a network perspective. Given recent evidence for network data to provide prognostic markers, particularly in relation to postsurgical outcome,12,66,67 it is therefore anticipated that approaches favoring a combination of connectome models, together with advanced structural imaging of the mesiotemporal lobe, will further our understanding of associated cognitive and comorbid psychiatric dysfunction.
Glossary
- AAL
automated anatomical labeling
- FA
fractional anisotropy
- FDR
false discovery rate
- FOV
field of view
- HS
hippocampal sclerosis
- MPRAGE
magnetization-prepared rapid gradient echo
- ROI
region of interest
- TE
echo time
- TI
inversion time
- TLE
temporal lobe epilepsy
- TLE-G
temporal lobe epilepsy with gliosis
- TLE-HS
temporal lobe epilepsy with hippocampal sclerosis
- TR
repetition time
Author contributions
N. Bernasconi and A. Bernasconi recruited patients and acquired the MRI data. B.C. Bernhardt, A. Bernasconi, and N. Bernasconi designed the study. M. Liu, F. Fadaie, B. Caldairou, and B.C. Bernhardt carried out the image processing and network analysis. D.S. Bassett and S. Gu consulted for the network analysis and provided the network control theoretical tools. B.C. Bernhardt wrote the paper and revised it with A. Bernasconi, N. Bernasconi, J. Smallwood, D.S. Bassett, and E. Jefferies.
Study funding
This work was funded by the Canadian Institutes of Health Research (CIHR MOP-57840 to A.B. and CIHR MOP-123520 to N.B.), Natural Sciences and Research Council (NSERC; Discovery-243141 to A.B. and 24779 to N.B.), Epilepsy Canada (Jay & Aiden Barker Breakthrough Grant in Clinical & Basic Sciences to A.B.), and Canada First Research Excellence Fund (HBHL-1a-5a-06 to N.B.). F.F. and B.C. received salary support from the Lloyd Carr-Harris Foundation. B.C.B. acknowledges salary support from FRQS and funding from CIHR (FDN-154298) and NSERC (Discovery-1304413), as well as SickKids (NI17-039). D.S.B. acknowledges support from Alfred P. Sloan and John D. and Catherine T. MacArthur Foundations, as well as from NIH (NS099348-02).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Thom M. Review: hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathol Appl Neurobiol 2014;40:520–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briellmann RS, Kalnins RM, Berkovic SF, Jackson GD. Hippocampal pathology in refractory temporal lobe epilepsy: T2-weighted signal change reflects dentate gliosis. Neurology 2002;58:265–271. [DOI] [PubMed] [Google Scholar]
- 3.Cascino GD, Jack CR Jr, Parisi JE, et al. Magnetic resonance imaging-based volume studies in temporal lobe epilepsy: pathological correlations. Ann Neurol 1991;30:31–36. [DOI] [PubMed] [Google Scholar]
- 4.Goubran M, Bernhardt BC, Cantor-Rivera D, et al. In vivo MRI signatures of hippocampal subfield pathology in intractable epilepsy. Hum Brain Mapp 2016;37:1103–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kemmotsu N, Girard HM, Bernhardt BC, et al. MRI analysis in temporal lobe epilepsy: cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia 2011;52:2257–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whelan CD, Altmann A, Botia JA, et al. Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain 2018;141:391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campos BM, Coan AC, Beltramini GC, et al. White matter abnormalities associate with type and localization of focal epileptogenic lesions. Epilepsia 2015;56:125–132. [DOI] [PubMed] [Google Scholar]
- 8.Concha L, Beaulieu C, Gross DW. Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann Neurol 2005;57:188–196. [DOI] [PubMed] [Google Scholar]
- 9.Focke NK, Yogarajah M, Bonelli SB, Bartlett PA, Symms MR, Duncan JS. Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage 2008;40:728–737. [DOI] [PubMed] [Google Scholar]
- 10.van Diessen E, Zweiphenning WJ, Jansen FE, Stam CJ, Braun KP, Otte WM. Brain network organization in focal epilepsy: a systematic review and meta-analysis. PLoS One 2014;9:e114606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu M, Chen Z, Beaulieu C, Gross DW. Disrupted anatomic white matter network in left mesial temporal lobe epilepsy. Epilepsia 2014;55:674–682. [DOI] [PubMed] [Google Scholar]
- 12.Bonilha L, Helpern JA, Sainju R, et al. Presurgical connectome and postsurgical seizure control in temporal lobe epilepsy. Neurology 2013;81:1704–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernhardt BC, Bonilha L, Gross DW. Network analysis for a network disorder: the emerging role of graph theory in the study of epilepsy. Epilepsy Behav 2015;50:162–170. [DOI] [PubMed] [Google Scholar]
- 14.Richardson MP. Large scale brain models of epilepsy: dynamics meets connectomics. J Neurol Neurosurg Psychiatry 2012;83:1238–1248. [DOI] [PubMed] [Google Scholar]
- 15.Gleichgerrcht E, Kocher M, Bonilha L. Connectomics and graph theory analyses: novel insights into network abnormalities in epilepsy. Epilepsia 2015;56:1660–1668. [DOI] [PubMed] [Google Scholar]
- 16.Honey CJ, Sporns O, Cammoun L, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA 2009;106:2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu S, Pasqualetti F, Cieslak M, et al. Controllability of structural brain networks. Nat Commun 2015;6:8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiles L, Gu S, Pasqualetti F, et al. Autaptic connections shift network excitability and bursting. Sci Rep 2017;7:44006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Betzel RF, Gu S, Medaglia JD, Pasqualetti F, Bassett DS. Optimally controlling the human connectome: the role of network topology. Sci Rep 2016;6:30770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron 2010;65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blumcke I, Thom M, Aronica E, et al. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia 2013;54:1315–1329. [DOI] [PubMed] [Google Scholar]
- 22.Engel J Jr. Update on surgical treatment of the epilepsies. Neurology 1993;43:1612–1617. [DOI] [PubMed] [Google Scholar]
- 23.Theodore WH, Kelley K, Toczek MT, Gaillard WD. Epilepsy duration, febrile seizures, and cerebral glucose metabolism. Epilepsia 2004;45:276–279. [DOI] [PubMed] [Google Scholar]
- 24.Bernhardt BC, Bernasconi N, Concha L, Bernasconi A. Cortical thickness analysis in temporal lobe epilepsy: reproducibility and relation to outcome. Neurology 2010;74:1776–1784. [DOI] [PubMed] [Google Scholar]
- 25.Hong SJ, Bernhardt BC, Caldairou B, et al. Multimodal MRI profiling of focal cortical dysplasia type II. Neurology 2017;88:734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins DL, Evans AC. Animal: validation and applications of nonlinear registration-based segmentation. Intern J Pattern Recognit Artif Intell 1997;11:1271–1294. [Google Scholar]
- 27.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 28.Kim JS, Singh V, Lee JK, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage 2005;27:210–221. [DOI] [PubMed] [Google Scholar]
- 29.Liu M, Bernhardt BC, Hong SJ, Caldairou B, Bernasconi A, Bernasconi N. The superficial white matter in temporal lobe epilepsy: a key link between structural and functional network disruptions. Brain 2016;139:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999;45:265–269. [DOI] [PubMed] [Google Scholar]
- 31.Hagmann P, Cammoun L, Gigandet X, et al. Mapping the structural core of human cerebral cortex. PLoS Biol 2008;6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010;52:1059–1069. [DOI] [PubMed] [Google Scholar]
- 33.Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett 2001;87:198701. [DOI] [PubMed] [Google Scholar]
- 34.Muldoon SF, Pasqualetti F, Gu S, et al. Stimulation-based control of dynamic brain networks. PLoS Comput Biol 2016;12:e1005076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu M, Bernhardt BC, Bernasconi A, Bernasconi N. Gray matter structural compromise is equally distributed in left and right temporal lobe epilepsy. Hum Brain Mapp 2015;37:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kulaga-Yoskovich J, Bernhardt BC, Hong S, et al. Multi-contrast submillimetric 3-Tesla hippocampal subfield segmentation protocol and dataset. Sci Data 2015;2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caldairou B, Bernhardt BC, Kim H, Mansi T, Bernasconi N, Bernasconi A. A surface patch-based segmentation method for hippocampal subfields. Med Image Comput Comput Assist Interv 2016;9901:379–387. [Google Scholar]
- 38.Bernhardt BC, Bernasconi A, Liu M, et al. The spectrum of structural and functional imaging abnormalities in temporal lobe epilepsy. Ann Neurol 2016;80:142–153. [DOI] [PubMed] [Google Scholar]
- 39.Bernhardt BC, Hong SJ, Bernasconi A, Bernasconi N. Magnetic resonance imaging pattern learning in temporal lobe epilepsy: classification and prognostics. Ann Neurol 2015;77:436–446. [DOI] [PubMed] [Google Scholar]
- 40.Kim H, Bernhardt BC, Kulaga-Yoskovitz J, Caldairou B, Bernasconi A, Bernasconi N. Multivariate hippocampal subfield analysis of local MRI intensity and volume: application to temporal lobe epilepsy. Med Image Comput Comput Assist Interv 2014;17:170–178. [DOI] [PubMed] [Google Scholar]
- 41.Worsley K, Taylor JE, Carbonell F, et al. SurfStat: a Matlab toolbox for the statistical analysis of univariate and multivariate surface and volumetric data using linear mixed effects models and random field theory. Neuroimage 2009;47. [Google Scholar]
- 42.Hong S, Bernhardt BC, Schrader DV, Bernasconi N, Bernasconi A. Whole-brain MRI phenotyping of dysplasia-related frontal lobe epilepsy. Neurology 2016;86:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 1995;57:289–300. [Google Scholar]
- 44.Zeisel A, Zuk O, Domany E. FDR control with adaptive procedures and FDR monotonicity. Ann Appl Stat 2011;5:943–968. [Google Scholar]
- 45.Hogan RE, Moseley ED, Maccotta L. Hippocampal surface deformation accuracy in T1-weighted volumetric MRI sequences in subjects with epilepsy. J Neuroimaging 2015;25:452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maccotta L, Moseley ED, Benzinger TL, Hogan RE. Beyond the CA1 subfield: local hippocampal shape changes in MRI-negative temporal lobe epilepsy. Epilepsia 2015;56:780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Concha L, Kim H, Bernasconi A, Bernhardt BC, Bernasconi N. Spatial patterns of water diffusion along white matter tracts in temporal lobe epilepsy. Neurology 2012;79:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keller SS, Glenn GR, Weber B, et al. Preoperative automated fibre quantification predicts postoperative seizure outcome in temporal lobe epilepsy. Brain 2017;140:68–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonilha L, Nesland T, Martz GU, et al. Medial temporal lobe epilepsy is associated with neuronal fibre loss and paradoxical increase in structural connectivity of limbic structures. J Neurol Neurosurg Psychiatry 2012;83:903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Concha L, Livy DJ, Beaulieu C, Wheatley BM, Gross DW. In vivo diffusion tensor imaging and histopathology of the fimbria-fornix in temporal lobe epilepsy. J Neurosci 2010;30:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 52.Vos de Wael R, Lariviere S, Caldairou B, et al. Anatomical and microstructural determinants of hippocampal subfield functional connectome embedding. Proc Natl Acad Sci USA 2018;115:10154–10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schooler JW, Smallwood J, Christoff K, Handy TC, Reichle ED, Sayette MA. Meta-awareness, perceptual decoupling and the wandering mind. Trends Cogn Sci 2011;15:319–326. [DOI] [PubMed] [Google Scholar]
- 54.Sormaz M, Jefferies E, Bernhardt BC, et al. Knowing what from where: hippocampal connectivity with temporoparietal cortex at rest is linked to individual differences in semantic and topographic memory. Neuroimage 2017;152:400–410. [DOI] [PubMed] [Google Scholar]
- 55.Karapanagiotidis T, Bernhardt BC, Jefferies E, Smallwood J. Tracking thoughts: exploring the neural architecture of mental time travel during mind-wandering. Neuroimage 2017;147:272–281. [DOI] [PubMed] [Google Scholar]
- 56.Helmstaedter C, Elger CE. Chronic temporal lobe epilepsy: a neurodevelopmental or progressively dementing disease? Brain 2009;132:2822–2830. [DOI] [PubMed] [Google Scholar]
- 57.Tai XY, Bernhardt B, Thom M, et al. Review: neurodegenerative processes in temporal lobe epilepsy with hippocampal sclerosis: clinical, pathological and neuroimaging evidence. Neuropathol Appl Neurobiol 2018;44:70–90. [DOI] [PubMed] [Google Scholar]
- 58.Adda CC, Castro LH, Alem-Mar e Silva LC, de Manreza ML, Kashiara R. Prospective memory and mesial temporal epilepsy associated with hippocampal sclerosis. Neuropsychologia 2008;46:1954–1964. [DOI] [PubMed] [Google Scholar]
- 59.Bell B, Lin JJ, Seidenberg M, Hermann B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol 2011;7:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alessio A, Bonilha L, Rorden C, et al. Memory and language impairments and their relationships to hippocampal and perirhinal cortex damage in patients with medial temporal lobe epilepsy. Epilepsy Behav 2006;8:593–600. [DOI] [PubMed] [Google Scholar]
- 61.Beaulieu C. The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed 2002;15:435–455. [DOI] [PubMed] [Google Scholar]
- 62.Proper EA, Jansen GH, van Veelen CW, van Rijen PC, Gispen WH, de Graan PN. A grading system for hippocampal sclerosis based on the degree of hippocampal mossy fiber sprouting. Acta Neuropathol 2001;101:405–409. [DOI] [PubMed] [Google Scholar]
- 63.Schmeiser B, Li J, Brandt A, Zentner J, Doostkam S, Freiman TM. Different mossy fiber sprouting patterns in ILAE hippocampal sclerosis types. Epilepsy Res 2017;136:115–122. [DOI] [PubMed] [Google Scholar]
- 64.Coan AC, Appenzeller S, Bonilha L, Li LM, Cendes F. Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology 2009;73:834–842. [DOI] [PubMed] [Google Scholar]
- 65.Bernhardt BC, Kim H, Bernasconi N. Patterns of subregional mesiotemporal disease progression in temporal lobe epilepsy. Neurology 2013;81:1840–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gleichgerrcht E, Munsell B, Bhatia S, et al. Deep learning applied to whole-brain connectome to determine seizure control after epilepsy surgery. Epilepsia 2018;59:1643–1654. [DOI] [PubMed] [Google Scholar]
- 67.Bonilha L, Jensen JH, Baker N, et al. The brain connectome as a personalized biomarker of seizure outcomes after temporal lobectomy. Neurology 2015;84:1846–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Phenotypic and mean connectome data are available at doi: 10.6084/m9.figshare.7309214.