Abstract
Objective
This nationwide cohort study evaluates seizure responses to immunotherapy and antiepileptic drugs (AEDs) in patients with anti-leucine-rich glioma-inactivated 1 (LGI1), anti-NMDA receptor (NMDAR), and anti-gamma-aminobutyric-acid B receptor (GABABR) encephalitis.
Methods
Anti-LGI1, anti-NMDAR, and anti-GABABR encephalitis patients with new-onset seizures were included. Medical information about disease course, AEDs and immunotherapies used, effects, and side effects were collected. Outcome measures were (1) seizure freedom while using AEDs or immunotherapy, (2) days to seizure freedom from start of AEDs or immunotherapy, and (3) side effects.
Results
Of 153 patients with autoimmune encephalitis (AIE) (53 LGI1, 75 NMDAR, 25 GABABR), 72% (n = 110) had epileptic seizures, and 89% reached seizure freedom. At least 53% achieved seizure freedom shortly after immunotherapy, and 14% achieved seizure freedom while using only AEDs (p < 0.0001). This effect was similar in all types (p = 0.0001; p = 0.0005; p = 0.013, respectively). Median time to seizure freedom from AEDs start was 59 days (interquartile range [IQR] 27–160), and 28 days from start of immunotherapy (IQR 9–71, p < 0.0001). Side effects were psychotic behavior and suicidal thoughts by the use of levetiracetam, and rash by the use of carbamazepine. Carbamazepine was more effective than levetiracetam in reducing seizures in anti-LGI1 encephalitis (p = 0.031). Only 1 patient, of 86 surviving patients, developed epilepsy after resolved encephalitis.
Conclusion
Epilepsy after resolved encephalitis was rare in our cohort of patients with AIE treated with immunotherapy. In addition, seizure freedom is achieved faster and more frequently after immunotherapy. Therefore, AEDs should be considered as add-on treatment, and similar to treatment of other encephalitis symptoms, immunotherapy is crucial.
The discovery of NMDA receptor (NMDAR) antibodies1 has led to the description of several other antibodies to extracellular neuronal antigens. Binding of these antibodies leads to cerebral dysfunction, which often manifests as limbic encephalitis characterized by cognitive decline, behavioral changes, and seizures. Seizures occur most frequently in autoimmune encephalitis (AIE) with leucine-rich glioma-inactivated 1 (LGI1),2 NMDAR antibodies,3 and gamma-aminobutyric-acid B receptor (GABABR) antibodies.4
The description of seizures in AIE has led to a new field of interest in epileptology with challenging issues in diagnosis and treatment. Concerning diagnosis, patients can present with seizures without other notable encephalitis signs,5–7 leading to diagnostic difficulties and treatment delay. Treatment delay is associated with a poorer outcome.3 Therefore, it is essential to consider an autoimmune etiology in presence of specific clinical clues. Moreover, faciobrachial dystonic seizures (FBDS)2 are considered pathognomonic for anti-LGI1 encephalitis. Alternatively, the subacute onset of drug-resistant seizures might be a common, but indiscriminative, feature.
Another challenging issue is to achieve seizure freedom rapidly. Seizures often seem unresponsive to antiepileptic drugs (AEDs), while responses to immunotherapy are considered good. Nevertheless, seizure freedom is not always achieved while using immunotherapy alone and AEDs are sometimes needed as well.
The overall efficacy of AEDs in these patients and whether any particular AEDs should be preferred is unclear. Therefore, the aim of this nationwide observational cohort study was to evaluate the responses to AEDs and immunotherapy in these syndromes, including safety, and to describe the risk for epilepsy after resolved encephalitis.
Methods
Patients
The department of neurology of the Erasmus MC University Medical Center is the national referral site for patients with suspected AIE and the department of immunology is the national referral site for antineuronal antibody testing. We identified all Dutch adults and children with AIE with LGI1, NMDAR, or GABABR antibodies. Patients were identified between August 1999 and May 2017, although 78% were identified after 2010. Antibodies were detected in serum or in CSF and confirmed with both cell-based assay and immunohistochemistry.8 Patients with new-onset seizures during their active disease course were included.
Standard protocol approvals, registrations, and patient consents
The medical ethics committee of the Erasmus MC University Medical Center approved this study. Written informed consent was obtained from all patients.
Seizures
Medical information about disease course, seizure type, status epilepticus, types of AEDs and immunotherapies used, and side effects of the different treatments were collected during a visit to our clinic (n = 77), from interviews with patients and relatives by phone (n = 27), and from medical files (n = 49). Clinical characteristics, including all encephalitis signs, of a part of the patients have been published before.9,10 To provide an overview of the clinical signs, we allocated patients into 2 groups: epileptic seizures plus and encephalitis. No patients had only epileptic seizures without any other neurologic symptoms at thorough examination. Epileptic seizures plus contained the patients with prominent seizures and only subtle other encephalitis signs, which were initially unrecognized or considered side effects of AEDs. Examples are mild cognitive complaints, behavioral disorders, or subtle movement disorders. Limbic encephalitis was defined as an encephalitis with predominant clinical involvement of the limbic system (short-term memory loss, difficulty forming new memories, behavioral disorder) or MRI fluid-attenuated inversion recovery/T2 abnormalities in the medial temporal lobes.11
The guidelines and new epilepsy classification of the International League Against Epilepsy (ILAE) were used to define epileptic seizures,12 epileptic seizures with an immune etiology,13 status epilepticus,14 and drug-resistant epileptic seizures,15 and to classify seizures.12,13,16 Epileptic seizures with an immune etiology were defined as at least 2 seizures, not provoked by other factors, occurring more than 24 hours apart resulting directly from an immune disorder, and with evidence of autoimmune-mediated CNS inflammation.12,16 Drug-resistant epileptic seizures were defined as failure to achieve seizure freedom, despite treatment with 2 tolerated, adequately dosed AEDs. Seizures were classified as focal or tonic–clonic. Moreover, focal seizures were classified as seizures with or without impaired awareness. FBDS were defined as frequent attacks (>8/d) with a dystonic posture of the arm, often combined with a facial contraction, lasting less than 30 seconds.2 Refractory status epilepticus was defined as status epilepticus continuing even after adequate treatment. Seizure freedom was defined as no clinical signs of seizures, meaning no seizures observed and no reporting of focal seizures (including auras) or tonic-clonic seizures by patients or physicians. At follow-up, seizures needed to be absent for at least 3 months.
Effectivity of AEDs was scored as ineffective, some effect, seizure freedom, or unknown effect. As this was no formal prospective study, some effects were difficult to assess precisely, and we could therefore not use frequently used variables like 50% seizure reduction. We only scored some effect when it was noted specifically as a considerable reduction. Level of functioning was measured with the modified Rankin Scale (mRS).17
Primary outcome measures were (1) seizure freedom achieved while using AEDs and while using immunotherapy, (2) days to seizure freedom from start of AEDs and from start of immunotherapy, (3) development of epilepsy after resolved encephalitis, and (4) reported side effects.
Statistics
Comparisons between 2 groups were performed with the Mann-Whitney U test (days to seizure freedom after start of epileptic seizures). Comparisons between multiple groups were performed with the Kruskal-Wallis test (age at onset, days to seizures after disease onset), the Fisher-Freeman-Halton test (comparing effects of different AEDs), and the one-way analysis of variance (sex, seizures presenting symptom, type of seizures at presentation and during disease course, and [refractory] status epilepticus).
The chances to achieve seizure freedom (during first disease episode) were compared by McNemar test, only in patients using both AED and immunotherapy before seizure freedom to avoid confounding by indication. For each patient individually, achievement of seizure freedom after the different treatments is shown visually in the figures. McNemar test was also used to compare AED treatment responses in patients receiving multiple AEDs. The Wilcoxon signed rank test was used to compare the days to seizure freedom from start of AEDs and from start of immunotherapy. For this test only responses of patients who were treated with both AEDs and immunotherapy before seizure freedom were evaluated.
p Values below 0.05 were considered significant. We used SPSS 21.0 (SPSS Inc., Chicago, IL) for Windows and Prism7 (GraphPad Software, La Jolla, CA) for Windows for statistical analysis.
Data availability statement
Any data not published within this article are available at the Erasmus MC University Medical Center. Patient-related data will be shared upon request from any qualified investigator, maintaining anonymization of the individual patients.
Results
Patient and seizure characteristics
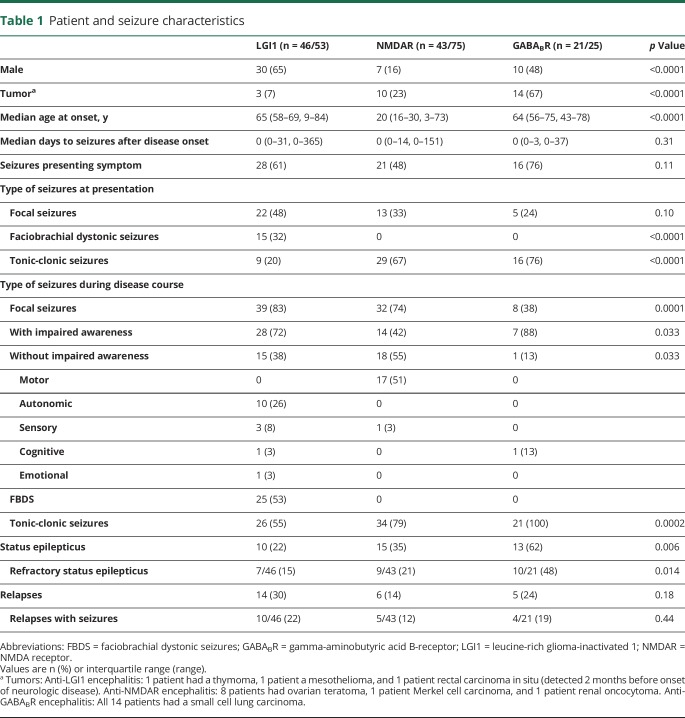
We identified 153 patients with AIE, including 53 patients with LGI1 antibodies, 75 patients with NMDAR antibodies, and 25 patients with GABABR antibodies. Among these cases, 72% of patients (n = 110) had epileptic seizures with an immune origin (87% LGI1, 57% NMDAR, 84% GABABR), while 14 additional patients (9%) had only one seizure. Table 1 shows seizure characteristics per antibody. Patients with NMDAR antibodies were younger (p < 0.0001) and only in this group there was a female predominance (p < 0.0001). Fourteen patients were categorized as having epileptic seizures plus (10/46 [22%] with LGI1 antibodies, 4/43 [9%] with NMDAR antibodies, and 0/21 with GABABR antibodies); the others had limbic encephalitis or panencephalitis.
Table 1.
Patient and seizure characteristics
FBDS only occurred in patients with LGI1 antibodies (53%). All patients with GABABR antibodies had tonic-clonic seizures, compared to 55% of patients with LGI1 antibodies (p = 0.0002), and 79% of patients with NMDAR antibodies. Status epilepticus occurred frequently (n = 38, 34%), in particular in patients with GABABR antibodies (62%, p = 0.006), of whom 26 (68%) had a refractory status epilepticus. Five patients (4%) died during status epilepticus.
Median follow-up time from onset of seizures was 27 months (interquartile range [IQR] 15–49, range 0–149 months); 24 patients had died (22%). Twenty-five patients (23%) had a relapse of the encephalitis; among them, 76% again had seizures (10 LGI1, 5 NMDAR, 4 GABABR). At last follow-up, 66% of patients had an mRS of 0–2 (LGI1 78%, NMDAR 74%, GABABR 24%).
Seizure treatment
Of all 110 patients with new-onset epileptic seizures with an immune origin, 91% were treated with 1 or more AEDs (LGI1 80%, NMDAR 98%, GABABR 100%). The median delay between seizure onset and start of AEDs was 3 days (IQR 0–31). This delay was higher in patients with anti-LGI1 encephalitis (median 64 days, IQR 0–178, p < 0.0001). During their disease course, patients were treated with a median of 2 AEDs (IQR 1–3, range 0–9). Moreover, 71 patients (65%) were treated with 2 or more AEDs. AEDs were continued for a median period of 8 months after diagnosis (IQR 4–18, range 0–102 months).
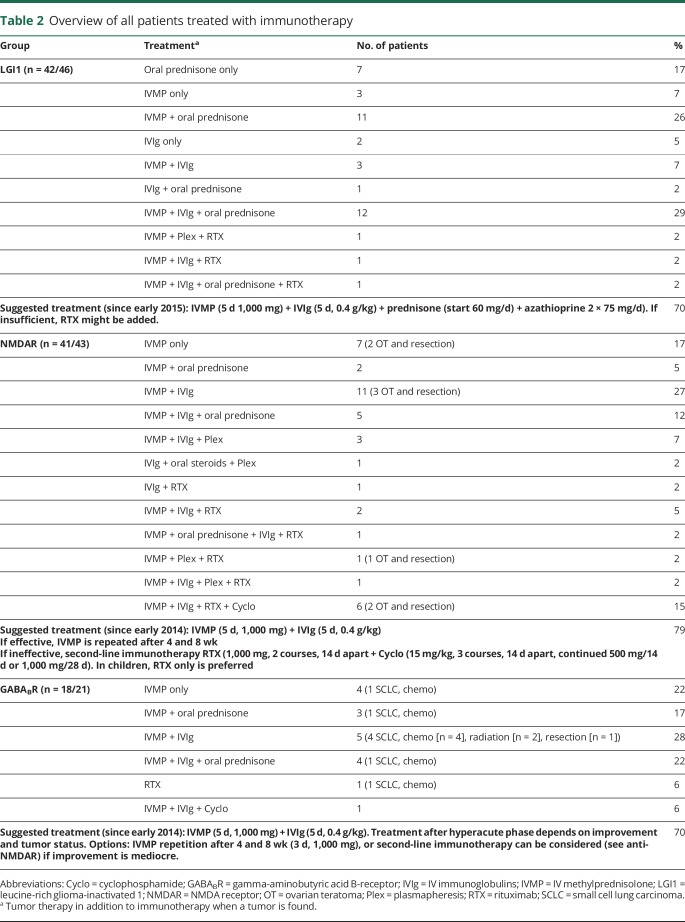
Most patients were treated with immunotherapy (92%), all but one with first-line immunotherapy (combination of methylprednisolone or IV immunoglobulins or plasmapheresis), and 17% with additional second-line immunotherapy (rituximab or cyclophosphamide; table 2). The patients not treated with immunotherapy received only AEDs (n = 9). Twenty-one percent of patients were treated with chronic immunotherapy, including azathioprine (n = 15) or mycophenolate (n = 8); of them, 19 (83%) had LGI1 antibodies. Fifteen of 19 anti-LGI1 patients were treated with chronic immunotherapy after the initial episode. Two of these anti-LGI1 patients (13%) developed a relapse, necessitating adaptation of the chronic immunotherapy. Thirty-one anti-LGI1 patients did not receive chronic immunotherapy after the initial episode. Of these, 11 developed a relapse (35%). Four patients had only started chronic immunotherapy after relapse. One of these 4 patients developed multiple relapses that halted after administration of rituximab.
Table 2.
Overview of all patients treated with immunotherapy
The majority of patients with anti-NMDAR and anti-GABABR encephalitis were treated with both AEDs and immunotherapy (NMDAR 93%, GABABR 81%). This percentage tended to be lower in patients with anti-LGI1 encephalitis (71%, p = 0.051). Among anti-LGI1 encephalitis patients, more were treated with immunotherapy (91%) than with AEDs (80%). The median treatment delay between symptom onset and start of immunotherapy was 30 days (IQR 11–93), which was highest in the anti-LGI1 group (median of 96 days, IQR 48–290, p < 0.0001). Patients with anti-LGI1 encephalitis and focal seizures had a longer treatment delay (p = 0.007) than patients without focal seizures, while this delay was not observed in patients with anti-LGI1 encephalitis and FBDS (p = 0.20).
Seizure freedom and treatment effects
Figures 1–3 visualize timelines of all patients with epileptic seizures per antibody. Seizure freedom was achieved in 89% of all 110 patients. Of these 98 patients, 14% (n = 14) achieved seizure freedom while using only AEDs, while in 52 patients seizure freedom was achieved shortly after the start of immunotherapy (53%). Comparing the 68 patients receiving both AEDs and immunotherapy before seizure freedom was reached, the chance to achieve seizure freedom was higher after the use of immunotherapy than after the use of AEDs (immunotherapy n = 44, AEDs n = 3, p < 0.0001). This also applied for the groups separately (LGI1, p = 0.0001; NMDAR, p = 0.0005; GABABR, p = 0.013).
Figure 1. Timelines (in days) of anti–leucine-rich glioma-inactivated 1 encephalitis patients with epileptic seizures.
The percentages shown on the left correspond to patients (1) reaching seizure freedom after the use of immunotherapy (green), (2) reaching seizure freedom probably after the use of immunotherapy (triple green), (3) reaching seizure freedom after the use of antiepileptic drugs (AEDs) (red), (4) reaching seizure freedom probably after the use of AEDs (double red), (5) who could not be categorized (gray stripes), and (6) who did not reach seizure freedom (black dots). If patients were treated with another immunomodulating treatment >1 month after the initial treatment (for example, IV immunoglobulin after prednisolone), this is shown as a new blue square. Treatment with an additional AED or dosage increase after >1 month is shown as a second purple diamond. Relapses are only shown if patients had seizures. Median time of follow-up from onset was 33 months (interquartile range [IQR] 19–52, range 8–119). Median time of seizure freedom was 23 months (IQR 14–40, range 4–102). The median interval between start of AEDs and start of immunotherapy was 57 days (IQR 27–152). **Timeline of the only patient who developed epilepsy after resolved encephalitis. The symbols in this timeline are not fitted to scale. The onset of seizures was in 2009, the patient was treated with prednisone (and AEDs), leading to reversibility of cognitive signs, but he still has temporal epilepsy. IT = immunotherapy.
Figure 2. Timelines (in days) of anti–NMDA receptor encephalitis patients with epileptic seizures.
See legend of figure 1. Median time of follow-up was 37 months (interquartile range [IQR] 15–59, range 1–149). Median time of seizure freedom was 31 months (IQR 15–58, range 4–129). The median interval between start of antiepileptic drugs (AEDs) and start of immunotherapy was 14 days (IQR 4–24). IT = immunotherapy.
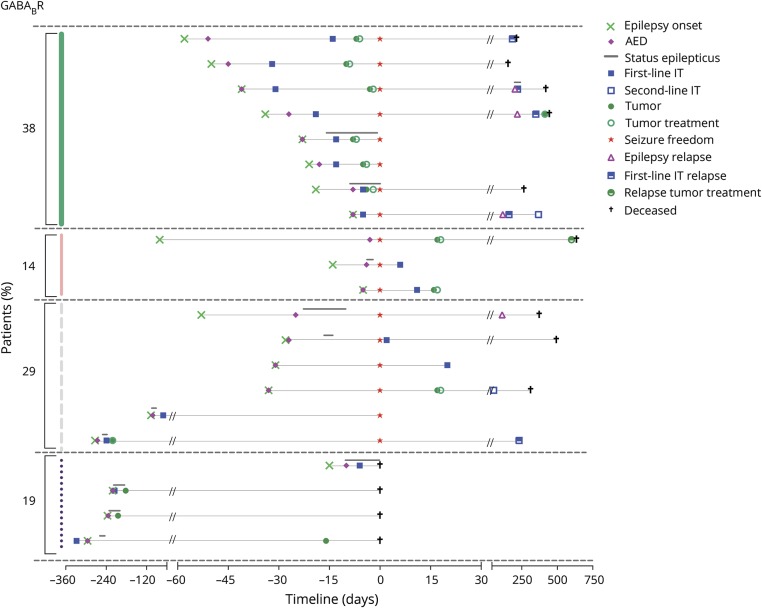
Figure 3. Timelines (in days) of anti–gamma-aminobutyric acid B-receptor encephalitis patients with epileptic seizures.
See legend of figure 1. Median time of follow-up was 15 months (interquartile range [IQR] 9–21, range 0–109). Median time of seizure freedom was 15 months (IQR 9–20, range 5–100). The median interval between start of antiepileptic drugs (AEDs) and start of immunotherapy was 10 days (IQR 7–28). IT = immunotherapy.
The median time to achieve seizure freedom after the start of AEDs was 59 days (IQR 27–160), and 28 days from start of immunotherapy (IQR 9–71, p < 0.0001). This decrease in days to seizure freedom after the use of immunotherapy was observed in all 3 syndromes (LGI1, p < 0.0001; NMDAR, p < 0.0001; GABABR, p = 0.001).
Seizure freedom was achieved faster in women than in men (p < 0.0001), attributed to patients with anti-NMDAR encephalitis (p = 0.038). No differences were observed in days to seizure freedom between patients with paraneoplastic18 (n = 27) or nonparaneoplastic encephalitis (n = 83; p = 0.085). In patients with focal seizures, it took longer to achieve seizure freedom (p < 0.0001), while presence of tonic-clonic seizures did not influence the interval to seizure freedom (p = 0.081). In patients with LGI1 antibodies, the presence of FBDS did not shorten the interval to seizure freedom (p = 0.20).
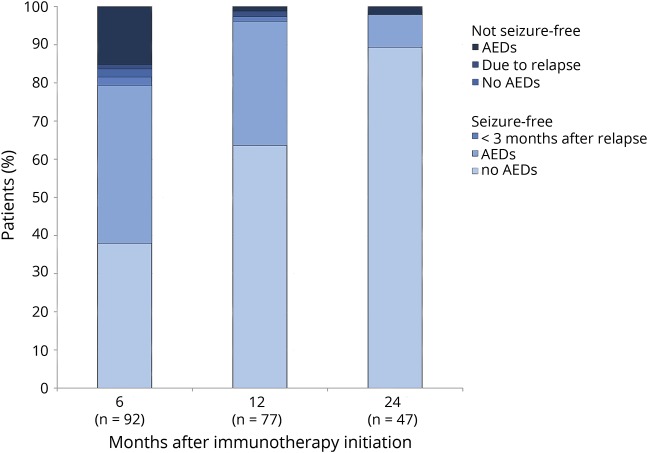
Eleven patients did not reach seizure freedom. Ten patients had died, due to the encephalitis, before reaching seizure freedom, while one patient with anti-LGI1 encephalitis (3% of surviving patients with seizures and anti-LGI1 encephalitis) developed temporal epilepsy after resolved encephalitis. Median time of seizure freedom in AIE patients (after initial episode or last relapse) was 22 months (IQR 14–45, range 4–129). Fourteen of these patients (14%) were still using AED, while seizure-free. We have evaluated the proportion of patients who continued to have seizures at 6, 12, and 24 months after the initiation of immunotherapy (figure 4). At 6 months, seizure freedom was achieved in 79% of patients; of these 73 patients, 38 (52%) still used AEDs. At 12 months, 96% of patients had reached seizure freedom, of whom 34% still used AEDs while seizure-free. At 24 months, only one patient had developed epilepsy after resolved encephalitis (2%); the other 46 patients (98%) were seizure-free, among them 4 (9%) treated with AEDs. Fourteen patients developed a relapse with epileptic seizures within these 2 years (7 while using AED), and 12 became seizure-free again within days or weeks after restarting immunotherapy.
Figure 4. Evaluation of the patients at risk to develop epilepsy after resolved encephalitis at 6, 12, and 24 months after the initiation of immunotherapy.
The figure shows the cumulative percentages of the patients who reached or did not reach seizure freedom and the use of antiepileptic drugs (AEDs). Patients with a relapse less than 3 months before the time point at 6, 12, or 24 months, or with a relapse at 6, 12, or 24 months, are also shown in the figure. Fourteen patients developed a relapse with epileptic seizures within 24 months after the start of immunotherapy, in 7 of them despite continuous AED treatment. At relapse, the median seizure duration was 12 days (interquartile range [IQR] 4–29, range 3–92). Eleven of these 14 patients became seizure-free within days or weeks after restarting immunotherapy, 2 patients became seizure-free after 3 months, and 1 patient developed epilepsy after resolved encephalitis.
AED effects and side effects
Prescribed AEDs were levetiracetam (66%), valproic acid (53%), carbamazepine (32%), phenytoin (30%), clobazam (15%), lacosamide (7%), oxcarbazepine (6%), and lamotrigine (5%). Topiramate and phenobarbital were only used sporadically.
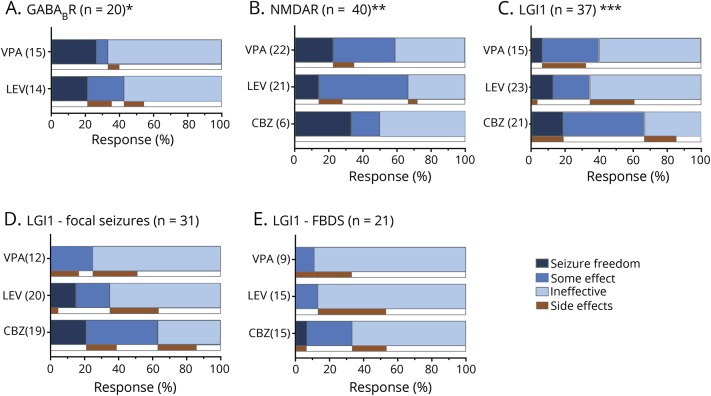
Responses to these most prescribed AEDs and side effects are visualized in figure 5. Although some response was seen in all 3 groups, seizure freedom was only infrequently achieved. Carbamazepine appeared to have the best effect to reduce focal seizure frequency in anti-LGI1 encephalitis (figure 5D), while FBDS hardly responded to AEDs (figure 5E). In those anti-LGI1 patients treated with both levetiracetam and carbamazepine (n = 15), carbamazepine appeared more effective to reduce seizure frequency than levetiracetam (p = 0.031).
Figure 5. Response percentages of most prescribed antiepileptic drugs (AEDs) and side effects.
Response percentages of most prescribed AEDs and side effects in patients with (A) anti–gamma-aminobutyric acid B-receptor (GABABR), (B) anti–NMDA receptor (NMDAR), or (C) anti–leucine-rich glioma-inactivated 1 (LGI1) encephalitis, (D) focal seizures in patients with anti-LGI1 encephalitis, and (E) faciobrachial dystonic seizures (FBDS) in patients with anti-LGI1 encephalitis.“Some effect” was scored if noted specifically as a considerable reduction of seizures. In some patients, responses to specific AEDs were not assessable, due to concomitant use of immunotherapy or missing data. *A total of 20/21 patients with anti-GABABR encephalitis were treated with levetiracetam (LEV) (n = 16) or valproic acid (VPA) (n = 15), 11 patients were treated with both LEV and VPA. Responses of 2 patients treated with LEV were not assessable. **A total of 40/42 patients with anti-NMDAR encephalitis were treated with LEV (n = 28), VPA (n = 24), or carbamazepine (CBZ) (n = 10); in 17 patients, these AEDs were combined. Responses of seizures of 2 patients treated with VPA, 7 patients treated with LEV, and 4 patients treated with CBZ were not assessable. ***A total of 37 patients with anti-LGI1 encephalitis were treated with LEV (n = 29), VPA (n = 19), or CBZ (n = 22); in 25 patients, combinations of these AEDs were used. Responses of seizures of 4 patients treated with VPA, 6 patients treated with LEV, and 1 patient treated with CBZ were not assessable. Comparing patients treated with both LEV and CBZ (most prescribed, n = 15), CBZ was more effective (p = 0.031). In the LGI1 group, only 4 patients were treated with oxcarbazepine, 1 patient reached seizure freedom, 1 patient showed some effect, and 2 had no effect. Treatment responses of patients with anti-LGI1 encephalitis are also shown for focal seizures (D) and FBDS (E). FBDS hardly responded to VPA, LEV, or CBZ, while focal seizures responded somewhat better to carbamazepine.
Side effects were frequently reported by patients with anti-LGI1 encephalitis (37%), and less by patients with anti-NMDAR (18%) and anti-GABABR (15%) encephalitis. Patients with LGI1 antibodies frequently had a rash by the use of carbamazepine (7/22, 32%). Most reported side effects by the use of valproic acid were memory deterioration (n = 3) and tremor (n = 2). Side effects of levetiracetam were rash (n = 3) and serious behavioral changes (n = 14; 19%), including 2 patients with anti-LGI1 encephalitis with severe psychotic behavior and suicidal thoughts.
Discussion
This nationwide observational cohort study evaluates seizure responses to immunotherapy and AEDs in patients with anti-LGI1, anti-NMDAR, and anti-GABABR encephalitis. We show that seizure freedom is achieved faster and more frequently after the use of immunotherapy than after the use of AEDs. In some patients, AEDs might decrease seizure frequency or lead to seizure freedom, but the effect is limited and incomparable to the effect of immunotherapy. After immunotherapy, the development of epilepsy after resolved encephalitis is rare in our cohort of AIE patients treated with immunotherapy.
These results emphasize the usefulness of immunotherapy in the treatment of epileptic seizures with an immune etiology caused by extracellular neuronal antibodies. In all groups there was a clear decrease in days to seizure freedom after the use of immunotherapy. It is customary to start AEDs before immunotherapy, so only comparing intervals between start of different treatments and seizure freedom would not be entirely fair. To avoid this confounding, we in addition compared the effects of AEDs and immunotherapy in patients who used both, and in which the responses to the individual treatment could be determined. This showed a clear preference for immunotherapy, which is in line with prior research in anti-LGI1 encephalitis, showing the positive effects of early immunotherapy on epileptic seizures and cognition.5,19
The effects of different treatment options were visualized (figures 1–3), showing that seizure freedom was frequently preceded directly by the initiation of immunotherapy and that patients treated earlier on in disease course seemed to reach seizure freedom faster. This effect was most remarkable in patients with anti-LGI1 encephalitis, wherein almost half of the patients became seizure-free within a week after immunotherapy, while they had been refractory to AEDs for longer periods. We did not analyze the effects of tumor treatment separately, because it was always accompanied by immunotherapy. Yet, we visualized that in patients with paraneoplastic encephalitis, both tumor treatment and immunomodulation often preceded seizure freedom. Mechanistically, both immunotherapy and tumor treatment are causal treatments, while AEDs are symptomatic treatments.
Our study shows that seizures of most patients were AED-resistant. Seizure freedom was achieved in the minority of patients while using only AEDs, and adjustments in treatment regimen or dosage increase of AEDs did not affect the chance to achieve seizure freedom. In addition, these patients often had a milder disease course without status epilepticus. The AED-resistant character of seizures is a confirmation of observations in other studies.6,19,20 In addition, the often accompanying (subtle) cognitive symptoms also favor treatment with immunotherapy. Therefore, it seems better to use AEDs only as add-on symptomatic treatment.
After treating the acute phase of the encephalitis, the continued use of AEDs is debatable. In our study, AED therapy was successfully discontinued in most patients after resolution of encephalitis. Chronic AED use does not appear to be necessary in most AIE patients long term. This is in line with previous studies studying separate subtypes of AIE.5,9,20 Although mesiotemporal sclerosis has been described in 25%–50% of follow-up MRIs in patients with anti-LGI1 encephalitis, only a few develop epilepsy after resolved encephalitis.9,21 For this reason, some argue against the implementation of the term epilepsy with immune origin13 (new ILAE classification) in the acute phase, reserving this for the situation after the encephalitis has been treated.22 In addition, side effects of AEDs, like memory disturbances, might disturb recovery after AIE, especially in combination with other drugs influencing brain functions, questioning even more the necessity for long-term AED use. Finally, half the patients who experienced a clinical relapse with epileptic seizures developed this relapse despite using AEDs and almost all patients became seizure-free again within days or weeks after restarting immunotherapy. However, prospective studies comparing different treatments in the chronic disease phase are lacking.
The AED-resistant character of seizures and crucial role of immunotherapy in treatment of seizures stress the importance of considering AIE as cause of epileptic seizures in patients with acquired drug-resistant seizures. Due to increased awareness, patients with a fulminant disease course with coma and status epilepticus, most frequently caused by GABABR or NMDAR antibodies, are regularly diagnosed early on in disease course. On the other hand, almost a quarter of the patients with anti-LGI1 encephalitis did not have a full-blown encephalitis, but seizures with only subtle encephalitis signs, which were often unrecognized by referring physicians. The unrecognition leads to diagnostic and treatment delay.9 In our study, this is reflected by (1) the longest treatment delay, (2) the longest interval between start of AEDs and immunotherapy, (3) a lower percentage of patients treated with AEDs, and (4) the observation that the presence of focal seizures extends the time to seizure freedom. As FBDS have gained much attention, better recognition and earlier treatment are to be expected. A longer delay until diagnosis and appropriate treatment in those with focal seizures shows that we should also look beyond FBDS to reduce delays and improve outcomes.
Concerning responses to most prescribed AEDs, in our cohort, physicians preferred the use of levetiracetam. However, patients often had serious behavioral changes and 2 patients with anti-LGI1 encephalitis developed a severe psychosis and suicidal thoughts. In addition, levetiracetam might exaggerate symptoms of AIE, especially behavioral disorders. Focal seizures of anti-LGI1 patients responded relatively better to carbamazepine, while FBDS hardly responded to any AED. Only a few patients were treated with oxcarbazepine, a drug with a comparable mechanism of action as carbamazepine. Individual results of treatment with oxcarbazepine seem promising and comparable to the effect of carbamazepine, but need confirmation in larger patient groups. Lacosamide, a similar drug, was only used infrequently and as add-on, therefore assessment of the effects was impossible. A recent study describes that only 10% of patients with voltage-gated potassium channel (VGKC) complex and glutamic acid decarboxylase 65 (GAD65) antibodies reached seizure freedom by the use of specific AEDs.23 Carbamazepine, lacosamide, and oxcarbazepine led most frequently to seizure-free outcome, while levetiracetam was ineffective in all patients. This is in line with our results, but difficult to compare as not all VGKC complex antibodies are pathogenic24 and as the pathogenicity of anti-GAD65 is unclear (incomparable to the pathogenicity of antibodies to extracellular antigens).25
Side effects were reported most frequently by patients with LGI1 antibodies, and less by the other patients, probably due to a more fulminant disease course in patients with anti-GABABR and anti-NMDAR encephalitis. One-third of patients with LGI1 antibodies treated with carbamazepine had a rash. Rash is a common side effect of carbamazepine and occurs most often in patients with specific proimmunogenic human leukocyte antigen (HLA) types.26 Recently, a strong correlation with specific HLA types (HLA DR7 and DRB4) was found in patients with LGI1 antibodies.27,28 Yet these types do not correspond to the HLA types of patients who are prone to rash by the use of carbamazepine. An alternative explanation for the high percentage of rash within the LGI1 group might be the rapid dosage increase because of frequent, drug-resistant seizures.
Although this is the largest cohort, and a nationwide study, regarding seizure responses to different treatments in patients with AIE and epileptic seizures, there are some limitations associated with the retrospective design of this study. Concerning data collection, effects and side effects were not always accurately documented. Patients were treated with a variety of AEDs and immunotherapies, and not per protocol, so comparisons are more difficult. However, the visualization of individual data in timelines is convincing that the differences between effects of AEDs and immunotherapy are real. We were not able to compare different treatment regimens (different AEDs and immunotherapies) due to small group sizes. Especially side effects are difficult to evaluate systematically in a retrospective design. Cognitive decline and behavioral disorders are hallmark symptoms of AIE, making it more difficult to categorize symptoms as disease progression or side effects of treatment. In addition, severe disease courses with coma and long-term intensive care stay make a proper evaluation of treatment effects (and side effects) difficult. Nevertheless, by treating these patients and by interviewing most patients, relatives, and treating physicians, important effects and side effects were still assessable and results from this study may help to compose treatment recommendations.
We would suggest using AEDs with sodium channel blocking properties (like carbamazepine or potentially oxcarbazepine) as first add-on next to immunotherapy in the symptomatic treatment of patients with anti-LGI1 encephalitis and seizures as it seems to have at least some effect in reducing focal seizures. However, due to the frequent occurrence of rash, often leading to discontinuation of therapy, it is essential to be cautious with rapid dosage increase. On the other hand, levetiracetam seems not preferable in the treatment of autoimmune epileptic seizures as the effects are limited and it can induce or exaggerate serious behavioral disorders.
From this nationwide study, we can conclude that immunotherapy is most important in the treatment of epileptic seizures in patients with anti-LGI1, anti-NMDAR, and anti-GABABR encephalitis. The overall effect of AEDs in the symptomatic treatment of epilepsy in these patients is limited and antibody-dependent. Specific AEDs should be considered to use as add-on therapy to control seizures, but not as primary and long-term treatment.
Acknowledgment
The authors thank the patients for their participation; all referring physicians, especially Prof. Dr. F.S.S. Leijten, Dr. J.A.E. van Asseldonk, and Dr. P.W. Wirtz; and Esther Hulsenboom and Mariska Nagtzaam for technical assistance.
Glossary
- AED
antiepileptic drug
- AIE
autoimmune encephalitis
- FBDS
faciobrachial dystonic seizures
- GABABR
gamma-aminobutyric acid B-receptor
- GAD65
glutamic acid decarboxylase 65
- HLA
human leukocyte antigen
- ILAE
International League Against Epilepsy
- IQR
interquartile range
- LGI1
leucine-rich glioma-inactivated 1
- mRS
modified Rankin Scale
- NMDAR
NMDA receptor
- VGKC
voltage-gated potassium channel
Footnotes
Editorial, page 877
Author contributions
M.A.A.M. de Bruijn: study design, acquisition of data, statistical analysis, interpretation of data, draft of the manuscript. A. van Sonderen: acquisition of data, revision of manuscript for content. M.H. van Coevorden-Hameete: acquisition of data, revision of manuscript for content. A.E.M. Bastiaansen: acquisition of data, revision of manuscript for content. M.W.J. Schreurs: acquisition of data, revision of manuscript for content. R.P.W. Rouhl: acquisition of data, revision of manuscript for content. C.A. van Donselaar: acquisition of data, revision of manuscript for content. M.H.J.M. Majoie: acquisition of data, revision of manuscript for content. R.F. Neuteboom: acquisition of data, revision of manuscript for content. P.A.E. Sillevis Smitt: acquisition of data, revision of manuscript for content. R.D. Thijs: acquisition of data, interpretation of data, revision of manuscript for content. M.J. Titulaer: study design, study supervision, interpretation of data, statistical analysis, revision of manuscript for content.
Study funding
M.J.T. was supported by an ErasmusMC fellowship, has received funding from the Netherlands Organization for Scientific Research (NWO, Veni incentive), from the Dutch Epilepsy Foundation (NEF, project 14–19), and from ZonMw (Memorabel program).
Disclosure
M. de Bruijn, A. van Sonderen, M.H. van Coevorden-Hameete, A.E.M. Bastiaansen, M.W.J. Schreurs, R.P.W. Rouhl, C.A. van Donselaar, M.H.J.M. Majoie, and R.F. Neuteboom report no disclosures relevant to the manuscript. P.A.E. Sillevis Smitt holds a patent for the detection of anti-DNER and received research support from Euroimmun. R.D. Thijs reports no disclosures relevant to the manuscript. M.J. Titulaer received research funds for serving on a scientific advisory board of MedImmune LLC and a travel grant for lecturing in India from Sun Pharma, India; has filed a patent for methods for typing neurologic disorders and cancer and devices for use therein; and has received research funds for serving on a scientific advisory board of MedImmune LLC, for consultation at Guidepoint Global LLC, and an unrestricted research grant from Euroimmun AG. Go to Neurology.org/N for full disclosures.
References
- 1.Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007;61:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 2011;69:892–900. [DOI] [PubMed] [Google Scholar]
- 3.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 2010;9:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irani SR, Stagg CJ, Schott JM, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 2013;136:3151–3162. [DOI] [PubMed] [Google Scholar]
- 6.Hoftberger R, Titulaer MJ, Sabater L, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology 2013;81:1500–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent A, Irani SR, Lang B. The growing recognition of immunotherapy-responsive seizure disorders with autoantibodies to specific neuronal proteins. Curr Opin Neurol 2010;23:144–150. [DOI] [PubMed] [Google Scholar]
- 8.Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol 2014;13:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Sonderen A, Thijs RD, Coenders EC, et al. Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology 2016;87:1449–1456. [DOI] [PubMed] [Google Scholar]
- 10.van Coevorden-Hameete MH, de Bruijn MA, et al. Autoantibodies to KCTD16 mark the presence of a tumor in patients with GABAb receptor encephalitis. Brain (in press 2019). doi: 10.1093/brain/awz094. [DOI] [Google Scholar]
- 11.van Sonderen A, Ariño H, Petit-Pedrol M, et al. The clinical spectrum of Caspr2 antibody-associated disease. Neurology 2016;87:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014;55:475–482. [DOI] [PubMed] [Google Scholar]
- 13.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia 2017;58:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus: report of the ILAE task force on classification of status epilepticus. Epilepsia 2015;56:1515–1523. [DOI] [PubMed] [Google Scholar]
- 15.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51:1069–1077. [DOI] [PubMed] [Google Scholar]
- 16.Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia 2017;58:522–530. [DOI] [PubMed] [Google Scholar]
- 17.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 18.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson J, Bi M, Murchison AG, et al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain 2018;141:348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Yan B, Wang R, et al. Seizure outcomes in patients with anti-NMDAR encephalitis: a follow-up study. Epilepsia 2017;58:2104–2111. [DOI] [PubMed] [Google Scholar]
- 21.Finke C, Prüss H, Heine J, et al. Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with leucine-rich, glioma-inactivated 1 antibodies. JAMA Neurol 2017;74:50–59. [DOI] [PubMed] [Google Scholar]
- 22.Spatola M, Dalmau J. Seizures and risk of epilepsy in autoimmune and other inflammatory encephalitis. Curr Opin Neurol 2017;30:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feyissa AM, López Chiriboga AS, Britton JW. Antiepileptic drug therapy in patients with autoimmune epilepsy. Neurol Neuroimmunol Neuroinflamm 2017;4:e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Sonderen A, Schreurs MW, de Bruijn MA, et al. The relevance of VGKC positivity in the absence of LGI1 and Caspr2 antibodies. Neurology 2016;86:1692–1699. [DOI] [PubMed] [Google Scholar]
- 25.Gresa-Arribas N, Arino H, Martinez-Hernandez E, et al. Antibodies to inhibitory synaptic proteins in neurological syndromes associated with glutamic acid decarboxylase autoimmunity. PLoS One 2015;10:e0121364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grover S, Kukreti R. HLA alleles and hypersensitivity to carbamazepine: an updated systematic review with meta-analysis. Pharmacogenet Genomics 2014;24:94–112. [DOI] [PubMed] [Google Scholar]
- 27.Kim TJ, Lee ST, Moon J, et al. Anti-LGI1 encephalitis is associated with unique HLA subtypes. Ann Neurol 2017;81:183–192. [DOI] [PubMed] [Google Scholar]
- 28.van Sonderen A, Roelen DL, Stoop JA, et al. Anti-LGI1 encephalitis is strongly associated with HLA-DR7 and HLA-DRB4. Ann Neurol 2017;81:193–198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any data not published within this article are available at the Erasmus MC University Medical Center. Patient-related data will be shared upon request from any qualified investigator, maintaining anonymization of the individual patients.