Abstract
Purpose: To report the results of a prospective, single-arm study to establish whether the initial treatment of acute or subacute limb ischemia (ALI and SLI, respectively) can be accomplished successfully using endovascular mechanical debulking of the target vessels to avoid the risks associated with thrombolysis and/or open surgery. Materials and Methods: From April 2009 to April 2015, 316 consecutive patients (mean age 70.9±12 years; 184 men) with ALI (202, 63.9%) or SLI (114, 36.1%) were enrolled; the only exclusion criterion was irreversible ischemia. The ALI group included 146 (72.3%) participants with category IIb ischemia and 56 (27.7%) with category IIa. Critical limb ischemia was diagnosed in 74 (64.9%) of the 114 patients with SLI. Target occlusions of thrombotic (n=256) or embolic (n=60) origin were located in the femoropopliteal segment (n=231), prosthetic or venous femoropopliteal bypass grafts (n=75), and the aortoiliac segment (n=35). The mean occlusion length was 22.9±14.8 cm. Results: The overall technical success (residual stenosis ≤30%) was 100% after debulking and adjunctive techniques (aspiration, dilation, stenting) at the level of the target lesions. No open surgical or thrombolytic modalities were necessary to bypass or recanalize the target vessels, and no death occurred in association with target occlusion therapy. Additional infrapopliteal interventions were performed in 195 (61.7%) patients (adjunctive thrombolysis in 29) to treat acute, subacute, and chronic lesions. Minor complications directly related to the debulking procedure occurred in 26 (8.2%) patients. Serious complications occurred in 11 (3.5%) patients, including hemorrhage in 8 (2.5%) patients (associated with infrapopliteal thrombolysis in 5). At 30 days, primary and secondary patency rates were 94.3% and 97.2%, respectively; mortality was 0.3% (1 fatal intracranial hemorrhage after adjunctive thrombolysis). Of 229 patients eligible for 1-year follow-up, amputation-free survival was estimated to be 87.4% in 199 patients with available data. Conclusion: In this all-comers study, mechanical debulking with the Rotarex alone or with adjunctive techniques is feasible as a primary therapy for occluded supratibial vessels in patients with ALI or SLI.
Keywords: acute limb ischemia, angioplasty, arterial occlusion, debulking, embolism, femoropopliteal segment, mechanical thrombectomy, thrombosis
Introduction
Acute lower limb ischemia (ALI) is a critical vascular emergency that both endangers the affected extremity and puts the patient’s life at risk. Duration of symptoms is 14 days or less. Similarly, subacute lower limb ischemia (SLI) can also threaten limb viability, but ischemic symptoms worsen gradually over a period of up to 3 months.1 Historically, open surgery and/or catheter-directed thrombolysis (CDT) have been front-line interventions in both ALI and SLI,2–6 but each therapy is associated with a significant incidence of complications.
Mechanical debulking offers advantages over CDT and surgery, with low invasiveness, prompt reperfusion, and the opportunity to immediately treat the underlying cause and concomitant lesions, with a low rate of bleeding complications and no need for an intensive care unit stay. However, mechanical removal (atherectomy, thromboembolectomy) of arterial occlusive material has not replaced the traditional treatments so far. Adoption of mechanical thrombectomy has been slow due to limited experience with the technique and the low standalone efficacy of some devices, necessitating concomitant use of thrombolysis.
Materials and Methods
Study Design
A prospective, single-arm, single-center, physician-initiated trial was undertaken to examine whether patients with ALI and SLI can be treated safely and effectively using endovascular mechanical debulking (concurrent atherectomy and thrombectomy) of supratibial occlusions as a feasible first-line therapy option. The University Hospital Královské Vinohrady and the Third Medical Faculty of the Charles University Centralized Institutional Review Board approved the study protocol and informed consent form (identifier: EK/IV-2/2009). The study was conducted in accordance with the Declaration of Helsinki, and all patients provided written informed consent. The trial was registered with the International Standard Randomized Controlled Trials Number registry (ISRCTN 154967770).
The inclusion criteria allowed enrollment of adult patients with ALI (symptom duration ≤14 days) or SLI (symptom duration ≤3 months) characterized by severe claudication, ischemic rest pain, and/or tissue defects (Rutherford categories 3–5). The only exclusion criterion was irreversible ischemia. Though vessel wall calcification was common at the site of target lesions, it was not considered an exclusion criterion in this study.
Study Population
From April 2009 to April 2015, 316 consecutive patients (mean age 70.9±12 years, range 23–96; 184 men) with ALI (202, 63.9%) or SLI (114, 36.1%) were enrolled in the study at a tertiary referral hospital. Overall, ischemic rest pain was present in 235 (74.4%) of the study cohort; 47 (14.9%) patients had gangrene or ulcerations. The ALI group (mean symptom duration 4.1±4.5 days, range 0.04–14) included 146 (72.3%) participants with category IIb ischemia and 56 (27.7%) with category IIa.7 The 114-patient SLI group (mean symptom duration 44.3±27.3 days, range 15–92) included 38 (33.3%) patients with Rutherford category 3 ischemia, 50 (43.9%) with category 4, and 26 (22.8%) with category 5. Critical limb ischemia was diagnosed in 74 (64.9%) patients according to established guidelines.3–5
As illustrated in Table 1, the study population was at high risk for developing cardiovascular disease. Patients with ALI were older and more often had arrhythmias and rest pain, while smoking history, gangrene, and ulcerations were more frequent in patients with SLI. The cause of ischemia (Table 2) was established on the basis of clinical manifestations and angiographic findings8: thrombotic occlusion was diagnosed in 256 (81.0%) patients and embolism in 60 (19.0%). Target lesions were located in the femoropopliteal segment (n=231; Figure 1), prosthetic or venous bypass grafts (n=75; Figure 2), and the aortoiliac segment (n=35; Figure 3). The mean lesion length was 22.9±14.8 cm. Besides target occlusions in the supratibial vessels and bypass grafts, 195 (61.7%) patients presented with additional lesions in the infrapopliteal arteries (Figure 1).
Table 1.
Risk Factors and Baseline Characteristics of 316 Subjects with Subacute vs Acute Limb Ischemia.a
| All Subjects (n=316) | Subacute Ischemia (n=114) | Acute Ischemia (n=202) | pb | |
|---|---|---|---|---|
| Age, y | 70.9±12.0 | 67.9±10.9 | 72.6±12.2 | <0.001 |
| Men | 184 (58.2) | 70 (61.4) | 114 (56.4) | 0.408 |
| Symptom duration, d | 70.9±12.0 | 44.3±27.3 | 4.13±4.47 | <0.001 |
| Hypertension | 236 (74.7) | 84 (73.7) | 152 (75.2) | 0.788 |
| Smoking history | 172 (54.4) | 73 (64.0) | 99 (49.0) | 0.013 |
| Cardiac disease | 133 (42.1) | 40 (35.1) | 93 (46.0) | 0.075 |
| Hyperlipidemia | 123 (38.9) | 47 (41.2) | 76 (37.6) | 0.550 |
| Diabetes mellitus | 97 (30.7) | 32 (28.1) | 65 (32.2) | 0.526 |
| Arrhythmias | 81 (25.6) | 19 (16.7) | 62 (30.7) | 0.007 |
| Cerebrovascular disease | 79 (25.0) | 22 (19.3) | 57 (28.2) | 0.081 |
| Renal insufficiency | 74 (23.4) | 20 (17.5) | 54 (26.7) | 0.074 |
| Malignant disease | 23 (7.3) | 9 (7.9) | 14 (6.9) | 0.823 |
| Gangrene, ulcerations | 47 (14.9) | 26 (22.8) | 21 (10.4) | 0.005 |
| Rest pain | 235 (74.4) | 66 (57.9) | 169 (83.7) | <0.001 |
Continuous data are presented as the mean ± standard deviation; categorical data are given as the number (percentage).
Acute vs subacute.
Table 2.
Procedure and Target Lesion Characteristics of 316 Subjects With Subacute vs Acute Limb Ischemia.a
| Characteristics | All Subjects (n=316) | Subacute Ischemia (n=114) | Acute Ischemia (n=202) | pb |
|---|---|---|---|---|
| Target occlusion length, cm | 22.9±14.8 | 20.2±13.7 | 24.5±15.2 | 0.016 |
| Thrombosis | 256 (81.0) | 104 (91.2) | 152 (75.2) | <0.001 |
| Embolism | 60 (19.0) | 10 (8.8) | 50 (24.8) | <0.001 |
| Femoropopliteal segment occlusion | 231 (73.1) | 90 (78.9) | 141 (69.8) | 0.087 |
| Femoropopliteal bypass occlusion | 72 (22.8) | 20 (17.5) | 52 (25.7) | 0.124 |
| Aortoiliac segment occlusion | 35 (11.1) | 7 (6.1) | 28 (13.9) | 0.040 |
| Deep femoral artery occlusion | 32 (10.1) | 6 (5.3) | 26 (12.9) | 0.033 |
| In-stent occlusion | 74 (23.4) | 25 (21.9) | 49 (24.3) | 0.680 |
| Additional infrapopliteal lesion | 195 (61.7) | 57 (50.0) | 138 (68.3) | 0.002 |
| Residual stenosis after RTX alone, % | 38.4±26.1 | 43.8±23.8 | 35.3±26.8 | 0.008 |
| Residual stenosis length after RTX alone, cm | 3.8±4.9 | 4.5±5.8 | 3.4±4.2 | 0.007 |
| Stented length, cm | 4.3±6.9 | 4.4±7.5 | 4.2±6.6 | 0.891 |
| RTX run time, min | 2.3±1.2 | 2.2±1.2 | 2.3±1.1 | 0.488 |
| Number of RTX passes | 3.4±1.1 | 3.3±1.2 | 3.4±1.1 | 0.130 |
| Dilation after RTX | 245 (77.5) | 102 (89.5) | 143 (70.8) | <0.001 |
| Stenting after RTX | 139 (44.0) | 51 (44.7) | 88 (43.6) | 0.906 |
| Aspirationc in target vessel | 25 (7.9) | 7 (6.1) | 18 (8.9) | 0.516 |
Abbreviation: RTX, Rotarex catheter.
Continuous data are presented as the mean ± standard deviation; categorical data are given as the number (percentage).
Acute vs subacute.
Percutaneous aspiration thromboembolectomy.
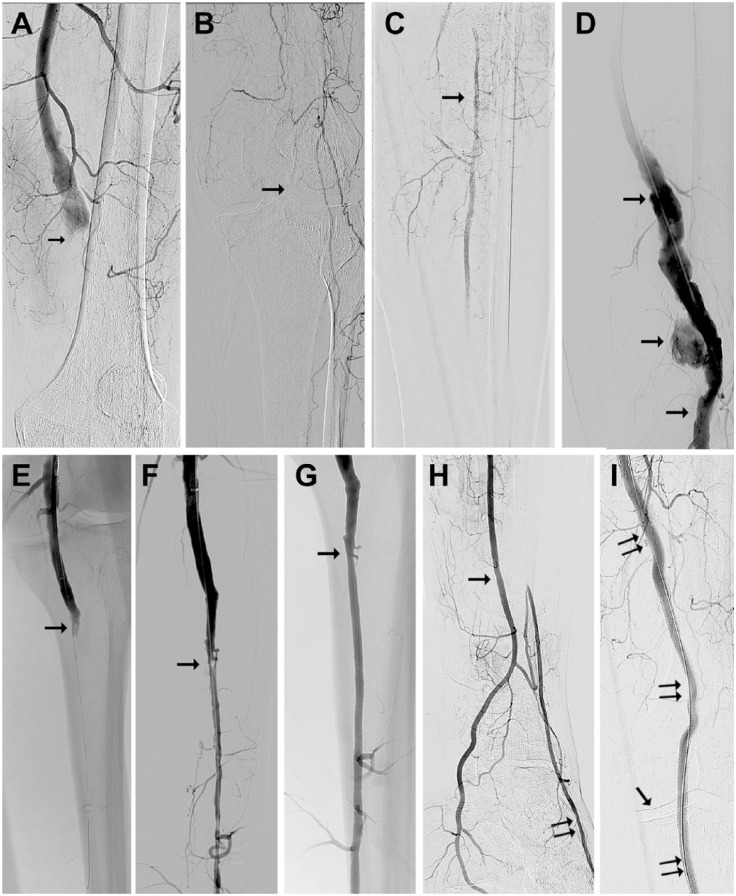
Figure 1.
(A) Digital subtraction angiography from a 56-year-old man with acute limb ischemia type IIb owing to popliteal aneurysm thrombotic occlusion (arrow). (B) The knee joint is depicted by the arrow. (C) Only one fragment (arrow) of the calf vessel is filled with contrast via collaterals. (D) After mechanical debulking of the popliteal artery with recanalized lumen (arrows). (E) The popliteal artery was recanalized to its periphery (arrow) with the Rotarex catheter. (F) The popliteal artery debulking opened the way for successful dilation of the peroneal artery with residual stenosis (arrow). (G) The stenosed area after dilation and stenting (arrow). (H) The peroneal artery (arrow) supplies the plantar vessels directly and the dorsal pedis artery via collaterals (double arrow). (I) The popliteal aneurysm was excluded using a Viabahn stent-graft (double arrows); the single arrow indicates the knee joint.
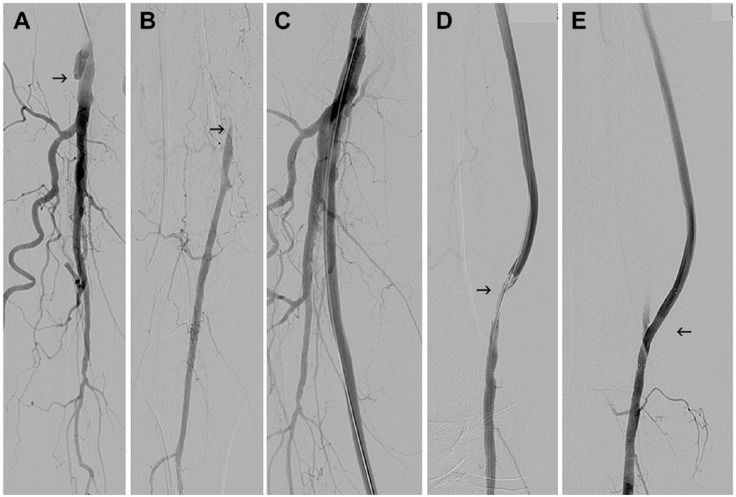
Figure 2.
(A) Occlusion of a prosthetic femoropopliteal bypass at its origin (arrow) in a 71-year-old man with category 3 subacute limb ischemia. (B) The popliteal artery (arrow) is filled via collaterals. (C) Angiogram after graft recanalization with the Rotarex. (D) Residual stenosis at the distal anastomosis (arrow). (E) Final angiogram after angioplasty and stenting (arrow).
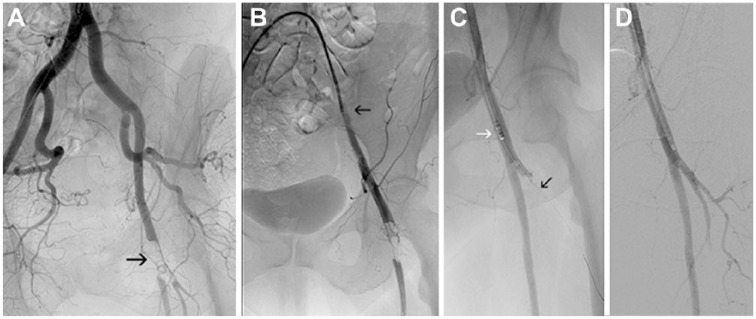
Figure 3.
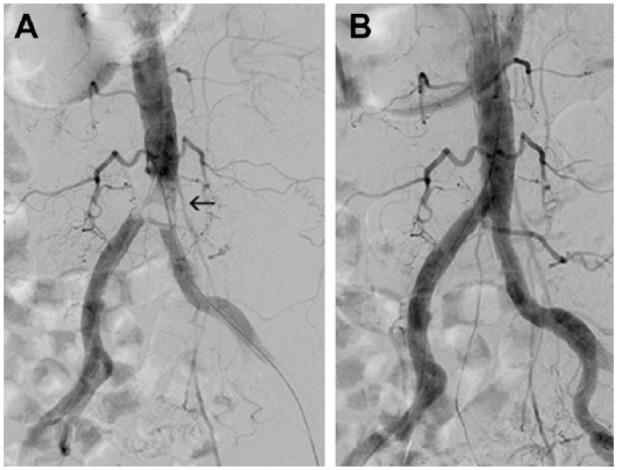
(A) Saddle embolus in the aortic bifurcation (arrow) in an 81-year-old woman with acute limb ischemia category IIb-III. (B) Angiogram after embolus removal with the Rotarex introduced from the left groin. During Rotarex activation, the balloon catheter stayed inflated in the right common iliac artery to protect the extremity from potential embolism.
Compared to patients with SLI, those with ALI had significantly longer target occlusions, more embolic occlusions, and greater frequencies of concomitant aortoiliac and infrapopliteal lesions. There was a higher percentage of thrombotic occlusion in patients with SLI. Compared with embolic occlusions (Table 3), thromboses were significantly longer and more often located in previously implanted stents. Femoropopliteal and deep femoral artery occlusions were more frequent in the embolic subgroup while femoropopliteal bypasses were more frequently occluded in patients with thrombosis. Embolism was more often associated with ALI IIb and angiographically significant infra-popliteal lesions.
Table 3.
Risk Factors and Baseline Characteristics Stratified by the Etiology of the Occlusion.a
| Characteristics | Thrombosis (n=256) | Embolism (n=60) | pb |
|---|---|---|---|
| Age, y | 70.2±11.3 | 73.7±14.2 | 0.005 |
| Men | 163/256 (63.7) | 21/60 (35.0) | <0.001 |
| Smoking history | 153/256 (59.8) | 19/60 (31.7) | <0.001 |
| Cardiac disease | 96/256 (37.5) | 37/60 (61.7) | 0.001 |
| Arrhythmias | 50/256 (19.5) | 31/60 (51.7) | <0.001 |
| Gangrene, ulcerations | 44/256 (17.2) | 3/60 (5.0) | 0.015 |
| Subacute ischemia | 104/256 (40.6) | 10/60 (16.7) | <0.001 |
| Acute ischemia IIa | 48/152 (31.6) | 8/50 (16.0) | 0.044 |
| Acute ischemia IIb | 104/152 (68.4) | 42/50 (84.0) | 0.044 |
| Femoropopliteal segment occlusion | 174/256 (68.0) | 57/60 (95.0) | <0.001 |
| Femoropopliteal bypass occlusion | 71/256 (27.7) | 1/60 (1.7) | <0.001 |
| Deep femoral artery occlusion | 18/256 (7.0) | 14/60 (23.3) | 0.001 |
| In-stent occlusion | 71/256 (27.7) | 3/60 (5.0) | <0.001 |
| Additional infrapopliteal lesion | 151/256 (59.0) | 44/60 (73.3) | 0.040 |
| Target occlusion length, mm | 246.6±150.4 | 155.0±111.7 | <0.001 |
| Antegrade (ipsilateral) approach | 232/256 (90.6) | 49/60 (81.7) | 0.035 |
| Residual stenosis after RTX alone, % | 42.3±24.5 | 21.6±26.4 | <0.001 |
| Residual stenosis length after RTX alone, cm | 4.2±5.1 | 1.9±2.9 | <0.001 |
| Dilation after RTX | 218/256 (85.2) | 27/60 (45.0) | <0.001 |
| Stenting after RTX | 123/256 (48.0) | 16/60 (26.7) | 0.004 |
| Stented length, cm | 4.8±7.4 | 1.9±3.6 | <0.001 |
Abbreviation: RTX, Rotarex catheter.
Continuous data are presented as the mean ± standard deviation; categorical data are given as the number (percentage).
Thrombosis vs embolism.
Study Device
The Rotarex (Straub Medical AG, Wangs, Switzerland) is a single lumen catheter equipped with a rotating head driven by a stainless steel helix rotating at up to 60,000 rpm. The Rotarex has 2 superimposed stainless steel cylinders, each with 2 lateral openings; the outer cylinder is connected to the rotating helix and the inner cylinder to the catheter shaft. The shape of the head facilitates the detachment and fragmentation of the occlusive material together with a strong vortex created by rotation. The displaced occlusion fragments are aspirated through the head openings and are shredded into debris that is transported to an external collection bag.9
Endovascular Procedure
The over-the-wire Rotarex device can recanalize vessels ≥3 mm in diameter; however, given the limited experience with the device at the beginning of this study, the device was applied to supratibial vessels ≥4 mm in diameter as a more conservative approach. Concomitant infra-popliteal lesions (>50%) were not managed using the Rotarex.
The number of patent runoff arteries was established angiographically before treatment. An ipsilateral approach (Figures 1 and 2) was used preferentially, but in a crossover access (Figure 4), a 6- to 8-F, 40-cm-long Flexor Check-Flo introducer (William Cook Europe ApS, Bjaeverskov, Denmark) was employed. Patients with ALI were fully anticoagulated with heparin before the endovascular procedure, but patients with SLI received intra-arterial heparin (5000 units) after wire passage through the target lesion without further coagulation control during the procedure. An angled, 0.035-inch, 260-cm-long Radifocus M stiff type guidewire with a 3-cm flexible tip (Terumo Europe NV, Leuven, Belgium) supported by a straight-flush angiographic catheter was used to penetrate the target lesion. The Rotarex was introduced over the wire close to the occlusion and activated. The number of passes was at the discretion of the operator. Though embolic protection filters can be used with the Rotarex, they are usually not necessary. In this study, filters were employed in only 2 crossover approaches where chances for potential emboli withdrawal were limited. Adjunctive techniques [angioplasty, stenting, percutaneous aspiration thromboembolectomy (PAT),10 and bioptome extraction11,12 (Bipal 7; Cordis Corporation, a Cardinal Health company, Milpitas, CA, USA)] were used to treat residual and underlying lesions (residual stenoses >30%) after debulking.
Figure 4.
(A) Left common femoral bifurcation embolism (arrow) in a 70-year-old woman with acute limb ischemia category IIb. (B) A sheath (arrow) was introduced from the contralateral approach, and (C) the superficial femoral artery was recanalized with the Rotarex (white arrow); subsequently, the deep femoral artery (DFA) was traversed with the guidewire (black arrow). (D) Final angiogram after DFA recanalization.
Concomitant infrapopliteal occlusions or stenoses (>50%) were treated during the same procedure immediately after mechanical debulking using PAT, dilation, and stenting. When these mechanical recanalization techniques were not able to establish adequate runoff, CDT was initiated in the infrapopliteal arteries at the discretion of the interventionist. In these cases, an end-hole catheter was positioned at the most distal part of the thrombus and retracted proximally as the recombinant tissue plasminogen activator (rtPA, Actilyse; Boehringer Ingelheim Pharma AG, Biberach, Germany) was delivered along the entire length of the thrombi. Initially, a dose up to 20 mg of rtPA was delivered during this bolus procedure, followed by a continuous infusion of rtPA (1 mg/h) to the trifurcation area. Heparin was infused to maintain the activated partial thromboplastin time at 2 to 3 times control. The rtPA infusion was stopped when a decline in the thrombus volume was no longer detected on angiography.
Antithrombotic Therapy
Pre- and postprocedural antiplatelet and anticoagulation therapy were not uniform and depended on the individual patient’s long-term antithrombotic medication, treatment efficacy, complications, and elimination of the embolism source. Typically, subcutaneous heparin (5000 units every 8 hours) was administered prophylactically for 24 hours after the procedure. Oral administration of acetylsalicylic acid (100 mg/d) was started or continued if previously prescribed. Patients with SLI received antiplatelet therapy (acetylsalicylic acid 100 mg/d) before endovascular treatment and thereafter as long-term therapy. If drug-eluting stents (DES) or drug-coated balloons (DCB) were used, clopidogrel was administered orally (75 mg/d) for 3 months then acetylsalicylic acid was prescribed after clopidogrel was discontinued.
Study Outcomes and Definitions
The patients were followed for adverse events through 30 days. Additional follow-up to ascertain death and limb amputation was performed at 12 to 13 months. All complications were monitored and classified by outcome as major or minor.2 The primary endpoint was the need for symptom-driven secondary interventions (thrombolytic and/or open). Secondary outcomes were mortality, amputation rate, amputation-free survival, and primary and secondary patency.1,2
Procedure duration was the time interval from vessel puncture to site closure or decision to use thrombolytic agents. Technical success for the Rotarex device was based on restoration of antegrade flow and residual stenosis ≤50%. Procedure success was based on technical success and a residual stenosis ≤30%. Major adverse limb events (MALE) included above-ankle amputation, major reintervention (new bypass graft or jump/interposition graft revision), and thrombectomy/thrombolysis. Clinical success was defined as procedure success and relief of acute ischemic symptoms at discharge and at 30 days. Hemodynamic success was documented by an increase in the ankle-brachial index (ABI).
Statistical Analysis
Continuous data are presented as the mean ± standard deviation; categorical data are given as the number (percentage). No imputation for missing data was used. Continuous variables were compared using a paired or unpaired t test or Wilcoxon rank sum test, as appropriate. Categorical variables were analyzed using a 2-sided chi-square or Fisher exact test, as appropriate. Freedom from death, amputation, and MALE were analyzed using the Kaplan-Meier method; the estimates were compared with the log-rank test. The threshold of statistical significance was p<0.05. All analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA).
Results
Procedure Outcomes
Rotarex debulking resulted in antegrade flow restoration in all target vessels (mean run time 2.3±1.2 minutes with an average 3.4±1.1 passes). The mean procedure duration was 69.5±37.0 minutes (range 20–240). The 8-F Rotarex was used for recanalization in 248 (78.5%) cases and the 6-F Rotarex in 76 (24.1%). Technical success (residual stenosis ≤50%) with the Rotarex device was achieved in 232 (73.4%) of 316 patients; among these, 140 patients had a residual stenosis ≤30% and 52 had no residual stenosis. Mean residual stenosis was 38.4% with a mean length of 38 mm.
Residual stenoses were managed with PAT (25, 7.9%), endomyocardial bioptome (26, 8.2%), balloon angioplasty (245, 77.5%), and stents (139, 44%). DES or DCBs were used in 147 patients. Procedure success (residual stenosis ≤30%) was 100% after debulking and adjunctive techniques at the level of the target lesions.
In the entire 316-patient population, infrapopliteal lesions were managed during the index procedure using PAT (103, 32.6%), angioplasty (78, 24.7%), and stenting (35, 11.1%). CDT was initiated in 29 (9.2%) patients with diffuse infra-popliteal occlusions resistant to mechanical removal and with poor or absent outflow. The frequency of thrombolytic procedures was higher in patients with ALI compared with the SLI subgroup (12.9% vs 2.6%, p<0.002). Infrapopliteal PAT was performed more frequently in patients with ALI (42.1% vs 15.8%, p<0.001) and in the embolic subgroup (51.7% vs 28.1%, <0.001). The mean number of patent tibial arteries before therapy was 0.9±1.1 vs 1.9±0.9 (p<0.001) after treatment (no significant stenoses, with good pedal outflow). It is worthy of note that 164 (52%) patients presented with no fully patent tibial arteries on angiography before the treatment, and it was the use of the Rotarex device that facilitated additional adjunctive therapies, resulting in only 22 (7%) patients with occlusions in tibial vessels.
Minor intraprocedural complications were seen in 63 (19.9%) patients. Peripheral embolization occurred in 40 (12.7%) patients, of which 19 (6%) cases were noted after Rotarex debulking and the remaining 21 (6.6%) after dilation or stenting. All emboli were immediately removed by Rotarex, PAT, and/or an endomyocardial biopsy device. Intraprocedural rethrombosis developed in previously cleared arterial segments in 18 (5.7%) patients with the need to repeat clot removal during the same procedure. Extravasation from the debulked segments was detected in 6 (1.9%) patients immediately after Rotarex action and in another 2 (0.6%) patients after dilation. These injuries were located in the popliteal artery in 6 cases and in a venous femoropopliteal bypass in 2 cases. Suspected mechanisms were vessel wall trauma and perforation caused by pressure injury (balloon angioplasty), poor vessel resistance to mechanical stress, and/or wall intrusion into the window of the Rotarex head. Extravasation was immediately controlled using a covered stent in 6 patients and prolonged balloon dilation (20 minutes) in 2 patients.
Minor clinically nonsignificant groin hematoma developed in 25 (7.9%) patients at the puncture site, with pseudo-aneurysm noted in 5 cases. Clinically insignificant vessel perforations with the guidewire were detected during difficult passage through infrapopliteal occlusions in 13 (4.1%) patients. These adverse events were not specifically related to the mechanical debulking technique.
Early Outcomes
Overall, there were no major complications associated solely with mechanical removal of occlusive material and no treatment-related complications causing amputation. However, 11 (3.5%) patients in the study cohort suffered serious sequelae, including 1 (0.3%) death due to intracerebral hemorrhage associated with infrapopliteal thrombolysis. In all, serious bleeding occurred in 8 (2.5%) patients (5 puncture sites, 2 retroperitoneal, and the fatal intracerebral); 5 of these patients underwent infrapopliteal thrombolysis as an adjunctive maneuver. Three (1%) patients with compartment syndrome required fasciotomy.
Reocclusions of target arteries occurred in 18 (5.7%) patients with the need for endovascular reintervention in 9 (2.8%). Primary and secondary patency rates at 30 days were 94.3% and 97.2%. Compartment syndrome developed in 7 (2.2%) participants without the need for fasciotomy.
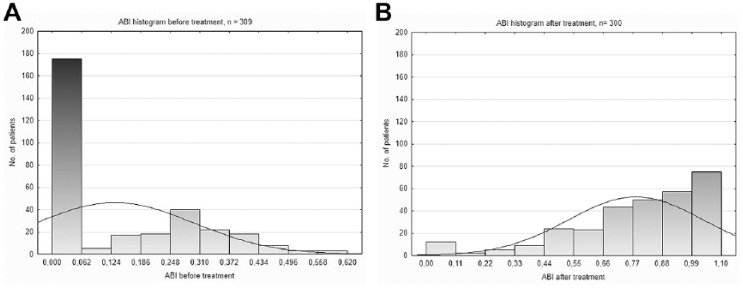
Open surgical revascularizations (bypass or embolectomy) were either not required or not feasible. Surgical removal of necrotic tissue was necessary in 13 patients, correction of retroperitoneal or groin bleeding in 7 patients, and the abovementioned fasciotomies in 3 patients. There were 15 (4.7%) limb amputations above the metatarsus for irreversible ischemia with major tissue loss, gangrene, or sepsis with no chance for further endovascular or surgical revascularization. Three (2.6%) limb amputations occurred in the 114-patient SLI subgroup, whereas 12 (5.9%) limbs were amputated in the 202-patient ALI subgroup. The amputation rate was significantly different between ALI patients with IIb and IIa ischemia (8.2% of 146 vs 0% of 56; p<0.02). Thirty-day amputation-free survival was 94.9% in the entire managed population: 93.6% in patients with ALI (91.1% in ALI IIb) and 97.4% in the SLI subgroup (p=0.139). Hemodynamic success was reflected in the ABI, which increased from 0.13±0.16 (range 0–0.62; n=309) before therapy to 0.78±0.25 (range 0–1.1; n=300) after treatment (p<0.001). The ABI increased ≥0.15 in 287 (95.7%) patients with pre/post data available (Figure 5). Clinical success was documented by the relief of acute ischemic symptoms in 182 (90.1%) of 202 patients with ALI.
Figure 5.
Ankle-brachial index (ABI) histograms before and after treatment. (A) The majority of patients presented with non-detectable distal pressures (ABI 0) before intervention. (B) The number of patients with ABI 0 dramatically decreased after treatment.
12-Month Follow-up
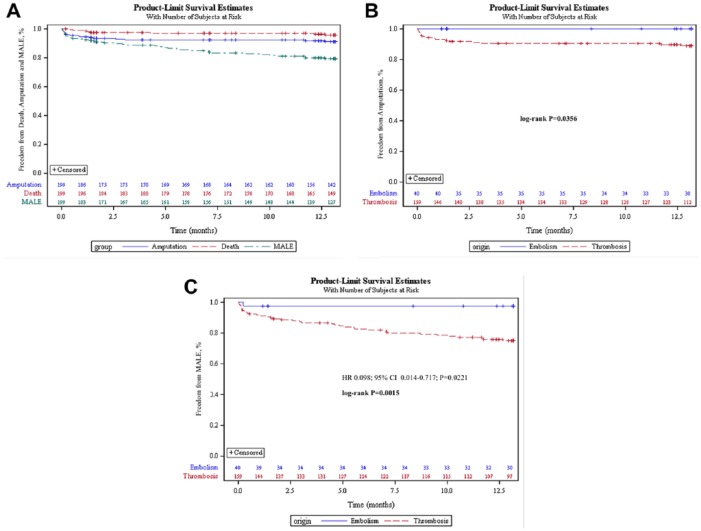
Of the 229 (72.5%) patients who were eligible for 12-month follow-up by the time of analysis, data were available in 199 cases. Eight (4%) deaths and 17 (8.5%) amputated limbs were recorded in follow-up (Figure 6A and B). Amputations occurred more frequently in patients with thrombosis compared with the embolic subgroup (10.7% vs 0%, p<0.03). Amputation-free survival was 87.4% in the 199 patients available for follow-up.
Figure 6.
Kaplan-Meier plots for (A) freedom from death, amputation, and major adverse limb events (MALE) in 199 patients with 12-month follow-up; (B) freedom from amputation of the index limb stratified by occlusion etiology; and (C) freedom from MALE involving the index limb stratified by occlusion etiology. The standard errors did not exceed 10% at any interval in any group.
Reocclusions occurred in 43 (21.6%) patients without a significant difference between the ALI and SLI subgroups, although there was a significant difference between patients with thrombosis and embolism (25.2% vs 7.5%, p<0.02). Twenty-six (13.1%) patients underwent endovascular reintervention and 12 (6%) patients required surgical reintervention, with no significant difference among either the etiology or severity subgroups (14.9% overall revascularization rate at 1 year). Time to event analysis of the MALE endpoint stratified by occlusion etiology is presented in Figure 6C.
Discussion
In this prospective cohort study, debulking alone converted a mean 229-mm-long target occlusion into a 38-mm residual stenosis in 2.9 minutes of Rotarex run time. Using additional mechanical methods, all target vessels were successfully recanalized with ≤30% residual stenosis. Importantly, since no patient was disqualified from the study due to clinical ischemia stage, concomitant disease, vessel calcification, runoff status, or contraindications to surgical or thrombolytic therapy, this study included a real-world mix of patients to test the feasibility of the Rotarex catheter as a primary treatment option for patients with ALI and SLI. Patients with ALI IIb, who typically are managed by vascular surgeons, represented more than a third of the treated population. The overall therapeutic success was negatively influenced by infrapopliteal artery status and the low potential for effective endovascular or surgical treatment in this runoff area.
In previous studies that used the Rotarex catheter to treat ALI and SLI,13–24 final technical success varied from 92% to 100% in studies with target occlusions in the infra-aortic arteries,14 femoropopliteal segment,13,15–18 and femoropopliteal bypass grafts19,20 despite having higher proportions of SLI and shorter lesions than the present study. Lower technical success was reported in subgroups with occluded bypass grafts (78%) and those treated via a crossover approach (56%).14
The frequency of concomitant thrombolytic therapy varied between 0%18 and 21.8%24 compared with 9.2% in the present study (used only for diffuse infrapopliteal occlusions). In our patients, additional thrombolytic treatment of target occlusions was not necessary. Kronlage et al22 compared 3 groups of patients (debulking with the Rotarex, thrombolysis, thrombolysis plus debulking) and identified a reduced rate of major bleeding, shorter hospitalization time, and lower costs when thrombolysis was not used.22 In the literature, the number of secondary surgical revascularization procedures ranged from 0%14,15,17–20 as in our study to 5.3%.13
Mortality in studies of the Rotarex varied between 0%13,15,18 and 1%14,17 at 30 days compared to 0.3% in this all-comers study. By comparison, in a meta-analysis of randomized studies comparing surgery to CDT,25 the 30-day mortality ranged from 4.6% to 8.2%. Reported 1-year mortality after Rotarex treatment varied from 0%15,19 to 15.8%13 without any association to mechanical debulking compared with traditional methods, in which the mortality was up to 42% in ALI patients treated with surgery or endovascular modalities.26
Complications directly related to the debulking procedure were classified as minor and occurred in ~8% of patients. Serious hemorrhage was the most frequent major complication and was associated with thrombolysis in most cases. In randomized studies,25 the incidence of major hemorrhage was 3.3% in open surgery vs 8.8% for CDT.
Limitations
Our study was nonrandomized so direct comparison with other therapeutic modalities is not possible. On the other hand, randomization into thrombolysis and surgery arms would exclude patients at high surgical risk or predisposed to bleeding, who represent the most threatened population. In addition, this study was confined to 30-day results for the primary endpoint, so further follow-up is required to get more detailed information about long-term outcomes. At the time of evaluation, 13% of our patients were lost from 12-month follow-up, so the 12-month data have limited value. Hypothetically, if all 30 patients lost to follow-up had undergone amputation or died, the amputation-free survival would be 76% rather than the 87% in 199 patients.
Conclusion
Mechanical debulking alone or with adjunctive techniques is feasible as a primary therapy option for occluded supratibial vessels in patients with ALI or SLI. Used as an initial treatment, mechanical debulking has a marked potential for reduced mortality and morbidity compared to studies of primary surgical or thrombolytic therapy. Despite the problems associated with a randomized clinical trial of traditional methods, a well-designed study of this nature might be able to evaluate effectively the superior safety and efficacy of mechanical debulking compared with CDT or surgery.
Acknowledgments
The authors thank Candance McClure (NAMSA, Toledo, OH, USA) for statistical expertise and to Lubomir Turek, MD, PhD (University of Iowa, Iowa City, IA, USA) for editorial assistance.
Footnotes
Authors’ Note: This study was presented at the Annual Scientific Meeting of the Society of Interventional Radiology (April 2–7 2016; Vancouver, British Columbia, Canada).
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Miroslav Bulvas was a proctor for Straub Medical AG.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Miroslav Bulvas  https://orcid.org/0000-0003-4211-6696
https://orcid.org/0000-0003-4211-6696
References
- 1. Patel N, Sacks D, Patel RI, et al. SIR reporting standards for the treatment of acute limb ischemia with use of transluminal removal of arterial thrombus. J Vasc Interv Radiol. 2003;14(9 pt 2):S453–S465. [DOI] [PubMed] [Google Scholar]
- 2. Rajan DK, Patel NH, Valji K, et al. Quality improvement guidelines for percutaneous management of acute limb ischemia. J Vasc Interv Radiol. 2009;20(7 suppl):S208–S218. [DOI] [PubMed] [Google Scholar]
- 3. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33(suppl 1):S1–S75. [DOI] [PubMed] [Google Scholar]
- 4. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease). Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239–1312. [DOI] [PubMed] [Google Scholar]
- 5. Tendera M, Aboyans V, Bartelink ML, et al. ESC guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. The Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2851–2906. [DOI] [PubMed] [Google Scholar]
- 6. Alonso-Coello P, Bellmunt S, McGorrian C, et al. Antithrombotic therapy in peripheral artery disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e669S–e690S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. [DOI] [PubMed] [Google Scholar]
- 8. O’Connell JB, Quiñones-Baldrich WJ. Proper evaluation and management of acute embolic versus thrombotic limb ischemia. Semin Vasc Surg. 2009;22:10–16. [DOI] [PubMed] [Google Scholar]
- 9. Schmitt HE, Jäger KA, Jacob AL, et al. A new rotational thrombectomy catheter: system design and first clinical experiences. Cardiovasc Intervent Radiol. 1999;22:504–509. [DOI] [PubMed] [Google Scholar]
- 10. Starck EE, McDermott JC, Crummy AB, et al. Percutaneous aspiration thromboembolectomy. Radiology. 1985;156:61–66. [DOI] [PubMed] [Google Scholar]
- 11. Bulvas M, Urbanová R, Klézlová R, et al. Markedly eccentric peripheral stenoses: percutaneous atherectomy with an endomyocardial biopsy device. Radiology. 2000;217:587–592. [DOI] [PubMed] [Google Scholar]
- 12. Bulvas M, Sommerová Z, Urbanová R. Acute popliteal and infrapopliteal arterial occlusions: endovascular therapy. In: Belch JJF, ed. Proceedings of the 16th European Chapter Congress of the International Union of Angiology, Glasgow, UK, October 25–27, 2005 Bologna, Italy: Medimond; 2006:43–49. [Google Scholar]
- 13. Duc SR, Schoch E, Pfyffer M, et al. Recanalization of acute and subacute femoropopliteal artery occlusions with the Rotarex catheter: one year follow-up, single center experience. Cardiovasc Intervent Radiol. 2005;28:603–610. [DOI] [PubMed] [Google Scholar]
- 14. Zeller T, Frank U, Bürgelin K, et al. Early experience with a rotational thrombectomy device for treatment of acute and subacute infra-aortic arterial occlusions. J Endovasc Ther. 2003;10:322–331. [DOI] [PubMed] [Google Scholar]
- 15. Wissgott C, Kamusella P, Richter A, et al. Mechanical rotational thrombectomy for treatment of acute and subacute occlusion of femoropopliteal arteries: retrospective analysis of the results from 1999 to 2005. Rofo. 2008;180:325–331. [DOI] [PubMed] [Google Scholar]
- 16. Staněk F, Ouhrabková R, Procházka D. Mechanical thrombectomy using the Rotarex catheter in the treatment of acute and subacute occlusions of peripheral arteries: immediate results, long-term follow-up. Int Angiol. 2013;32:52–60. [PubMed] [Google Scholar]
- 17. Freitas B, Steiner S, Bausback Y, et al. Rotarex mechanical debulking in acute and subacute arterial lesions: single-center experience with 525 patients. Angiology. 2017;68:233–241. [DOI] [PubMed] [Google Scholar]
- 18. Bérczi V, Deutschmann HA, Schedlbauer P, et al. Early experience and midterm follow-up results with a new, rotational thrombectomy catheter. Cardiovasc Intervent Radiol. 2002;25:275–281. [DOI] [PubMed] [Google Scholar]
- 19. Lichtenberg M, Kaeunicke M, Hailer B, et al. Percutaneous mechanical thrombectomy for treatment of acute femoropopliteal bypass occlusion. Vasc Health Risk Manag. 2012;8:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wissgott C, Kamusella P, Andresen R. Recanalization of acute and subacute venous and synthetic bypass-graft occlusions with a mechanical rotational catheter. Cardiovasc Intervent Radiol. 2013;36:936–942. [DOI] [PubMed] [Google Scholar]
- 21. Liu J, Li T, Huang W, et al. Percutaneous mechanical thrombectomy using Rotarex catheter in peripheral artery occlusion diseases—experience from a single center [published online November 20, 2018]. Vascular. doi: 10.1177/1708538118813239 [DOI] [PubMed] [Google Scholar]
- 22. Kronlage M, Printz I, Vogel B, et al. A comparative study on endovascular treatment of (sub)acute critical limb ischemia: mechanical thrombectomy vs thrombolysis. Drug Des Devel Ther. 2017;11:1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Staněk F, Ouhrabková R, Procházka D. Percutaneous mechanical thrombectomy in the treatment of acute and subacute occlusions of the peripheral arteries and bypasses. Vasa. 2016;45:49–56. [DOI] [PubMed] [Google Scholar]
- 24. Heller S, Lubanda JC, Varejka P, et al. Percutaneous mechanical thrombectomy using Rotarex S device in acute limb ischemia in infrainguinal occlusions. Biomed Res Int. 2017;2017:2362769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berridge DC, Kessel DO, Robertson I. Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database Syst Rev. 2013;6:CD002784. [DOI] [PubMed] [Google Scholar]
- 26. Baril DT, Ghosh K, Rosen AB. Trends in the incidence, treatment, and outcomes of acute lower extremity ischemia in the United States Medicare population. J Vasc Surg. 2014;60:669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]