Abstract
There is increasing evidence of a sustained state of systemic inflammation after pig-to-nonhuman primate (NHP) xenotransplantation (that has been termed systemic inflammation in xenograft recipients [SIXR]). Increases in inflammatory markers, e.g., C-reactive protein, histones, serum amyloid A, D-dimer, cytokines, chemokines, and a decrease in free triiodothyronine, have been demonstrated in the recipient NHPs. The complex interactions between inflammation, coagulation, and the immune response are well-recognized, but the role of inflammation in xenograft recipients is not fully understood. The evidence suggests that inflammation can promote the activation of coagulation and the adaptive immune response, but the exact mechanisms remain uncertain. If prolonged xenograft survival is to be achieved, anti-inflammatory strategies (e.g., the administration of anti-inflammatory agents, and/or the generation of genetically-engineered organ-source pigs that are protected from the effect of inflammation) may be necessary to prevent, control, or negate the effect of the systemic inflammation that develops in xenograft recipients. This may allow for a reduction in the intensity of exogenous immunosuppressive therapy. If immunological tolerance to a xenograft is to be obtained, then control of inflammation may be essential.
Keywords: Inflammation, Non-human primates, Pigs, Xenotransplantation
Introduction
Organ transplantation is one of the medical success stories of the past 70 years, but there remain insufficient organs from deceased human donors to treat all of the patients who might benefit. For example, in the USA at present there are approximately 120,000 patients awaiting an organ of one sort or another, and yet this year only approximately 10,000 deceased human donors will become available, providing an average of three or four organs per donor [1].
The lack of human organs could be obviated if a suitable animal source of organs were available. For a number of logistic and other reasons, the pig has been identified as a potential source of organs for clinical transplantation [2]. The field of xenotransplantation (cross-species transplantation) has therefore been extensively investigated during the past 35 years [3]. Although organs from wild-type (i.e., genetically-unmodified) pigs transplanted into humans or nonhuman primates (NHPs) are rejected within minutes [4], our ability to genetically-engineer the pig to protect its organs from the primate immune response has resulted in life-supporting kidney or heart graft survival in NHPs extending to many months or even more than a year [5–9]. One of the barriers that has had to be overcome, but continues to be problematic, is the inflammatory response to the presence of a pig organ.
Inflammation is part of the complex biological response of body tissues to harmful stimuli, and is observed in various diseases, e.g., inflammatory disease [10], infection [11], atherosclerosis [12]. The release of appropriate pro-inflammatory cytokines and chemokines is necessary for protective immunity, but production of these factors in excess can result in various pathological states [13]. An inflammatory response follows ischemia-reperfusion injury after organ transplantation [14]. This may play an important role in initiating the allo-immune response [15], and in the development of allograft vasculopathy [16].
There is increasing evidence of a systemic inflammatory response to the presence of a pig xenograft (‘systemic inflammation in xenograft recipients’ [SIXR]) [17–19]. Inflammation promotes activation of coagulation [17–21] and of the immune response [17, 18] that develop after xenotransplantation [22, 23]. In organ xenograft recipients, C-reactive protein (C-RP) increases before the development of consumptive coagulopathy or a T cell response [17, 18]. Infiltrating innate immune cells express tissue factor, which plays a role in initiating coagulation [24]. The development of T cell tolerance is inhibited by inflammation [22, 25].
We here review the evidence of a prolonged systemic inflammatory response to a xenograft, and consider what steps can be taken to prevent or reduce it. We have primarily drawn on our own observations, but have supplemented these by a review of the literature.
Evidence for a sustained inflammatory response in xenograft recipients (SIXR) (Table 1)
Table 1.
Evidence for systemic inflammation in xenograft recipients (SIXR)
| Indicators of inflammation | Change when associated with xenotransplantation | References | |
|---|---|---|---|
| In vivo | C-reactive protein (C-RP) | ↑ | [13, 19, 26] |
| Serum amyloid A (SAA) | ↑ | [26–28] | |
| Histones | ↑ | [26] | |
| D-dimer | ↑ | [6, 13, 19] | |
| Tumor necrosis factor-alpha (TNF-α) | ↑ | [18] | |
| Interferon-gamma (IFN-γ) | ↑ | [18] | |
| Interleukin-6 (IL-6) | ↑ | [13, 18] | |
| Interleukin-8 (IL-8) | ↑ | [13, 18] | |
| Interleukin-12 (IL-12) | ↑ | [18] | |
| Monocyte chemotactic protein-1 (MCP-1) | ↑ | [13, 18] | |
| Soluble CD40 ligand (sCD40L) | ↑ | [13, 29] | |
| Free triiodothyronine (fT3) | ↓ | [6, 30] | |
| In vitro | Platelet aggregation | ↑ | [26] |
| Endothelial cell apoptosis | ↑ | [26] |
C-reactive protein (C-RP) is an acute phase protein synthesized largely by hepatocytes in response to proinflammatory cytokines, in particular interleukin-6 (IL-6) [31]. C-RP provides the first line of defense to an invasive pathogen, and can promote activation of complement, bacterial capsular swelling, and phagocytosis [32]. It is a marker of early infection, and provides an easy objective parameter [33]. Moreover, C-RP mRNA expression increases in the presence of acute rejection of a renal allograft [34]. C-RP can contribute both to host defense against infection and enhancement of inflammatory tissue damage.
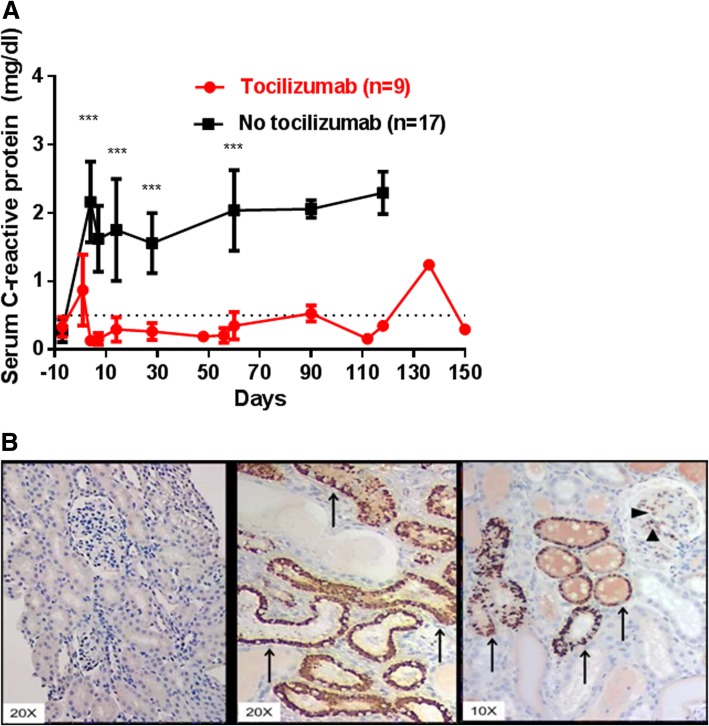
After pig-to-baboon organ transplantation, C-RP is increased for several months, suggesting a persisting inflammatory state [13, 19, 26] (Fig. 1a), and is deposited in the transplanted pig kidney [18] (Fig. 1b). Whether this is secondary to initial antibody binding remains uncertain.
Fig. 1.
a C-RP in baboons with pig artery patch (n = 9) or organ (n = 17) grafts. Levels of C-RP in baboons before (day 0) and after pig organ or artery patch transplantation. (Black line = without tocilizumab therapy; Red line = with tocilizumab therapy.) The mean level of C-RP in the tocilizumab-treated baboons remained < 0.5 mg/dL from day 4, which (on days 7, 14, 28, and 60) was significantly lower than in baboons not receiving tocilizumab (day 7, 0.2 vs 1.6 mg/dL, P < 0.001; day 14, 0.3 vs 1.8 mg/dL, P < 0.001; day 28, 0.3 vs 1.6 mg/dL, P < 0.001; and day 60, 0.3 vs 2.0 mg/dL, P < 0.01, respectively). (**P < 0.01; ***P < 0.001). Tocilizumab treatment therefore prevented an increase in C-RP after xenotransplantation. (The rise in C-RP on day 136 in one of the baboons in the tocilizumab-treatment group was associated with the onset of systemic infection.) (Reprinted with permission from ref. [26]). b C-RP deposition in pig kidneys transplanted into baboons, an indicator of the inflammatory response to the graft. (Left panel) At 30 min after reperfusion of an α1,3-galactosyltransferase gene-knockout (GTKO) pig kidney graft, no C-RP deposition was detected. In two different kidneys at the time of euthanasia (middle and right panels), C-RP deposition was detected in the glomeruli (arrow heads, right panel) and tubules (arrows, middle and right panels). Our data suggest that both the xenograft and the recipient contribute to C-RP production. (We detected minimal C-RP in NHPs undergoing heart allotransplantation [not shown]). (Reproduced with permission from ref. [18])
Serum amyloid A (SAA) is a major acute-phase protein and an inflammation-related marker in tuberculosis, rheumatoid arthritis, Crohn’s disease, and in various cancers [35, 36]. SAA is also a sensitive marker of acute allograft rejection [37]. Hepatocytes are a major source of SAA [38]. Elevated SAA results from increases in circulating serum interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) [39]. The inflammation-associated cytokines produced by endothelial cells (ECs), lymphocytes, specially-activated monocytes, and macrophages stimulate amyloid A synthesis [35, 40]. In turn, SAA may induce the release of some pro-inflammatory cytokines e.g., TNF-α, IL-1β, and the chemokine IL-8 [41, 42]. However, SAA can also induce the secretion of chemokines that might suppress inflammation locally [43], and mobilizes phospholipids and cholesterol for cell repair [44].
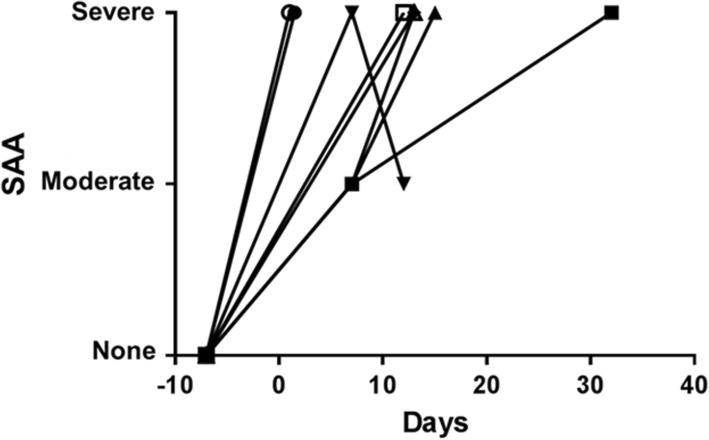
After pig-to-baboon organ xenotransplantation, significant increases in SAA have been observed during antibody-mediated rejection (Fig. 2) or when a consumptive coagulopathy or infection is developing [26, 27]. Amyloid A is deposited in the transplanted pig kidney [28]. Although the current method of measuring SAA is not fully quantitative, it is a simple and rapid indicator of the inflammatory state, allowing early investigation, e.g., for rejection, infection, or other complications.
Fig. 2.
Serum amyloid A (SAA) in baboons with pig kidney grafts that failed within the first post-transplant month. The SAA increased immediately after pig kidney transplantation, and never returned to pre-transplant levels. Other measurements indicated that a state of inflammation had developed
Extracellular histones play a key role in inflammation [45]. In vivo, they result in EC dysfunction (e.g., neutrophil margination, hemorrhage, thrombosis), and in vitro they are cytotoxic to ECs [45]. Five types of histones have been identified [46, 47]. Release of histones can be triggered by sepsis, trauma, chemical toxicity, transplant injury, and ischemia-reperfusion [48]. They bind to Toll-like receptors (TLRs) of various cells, e.g., platelets, red blood cells [49], which in turn induce NETosis (cell death, release of granular contents into the extracellular space). This in turn increases histone release and amplifies inflammation [50–57].
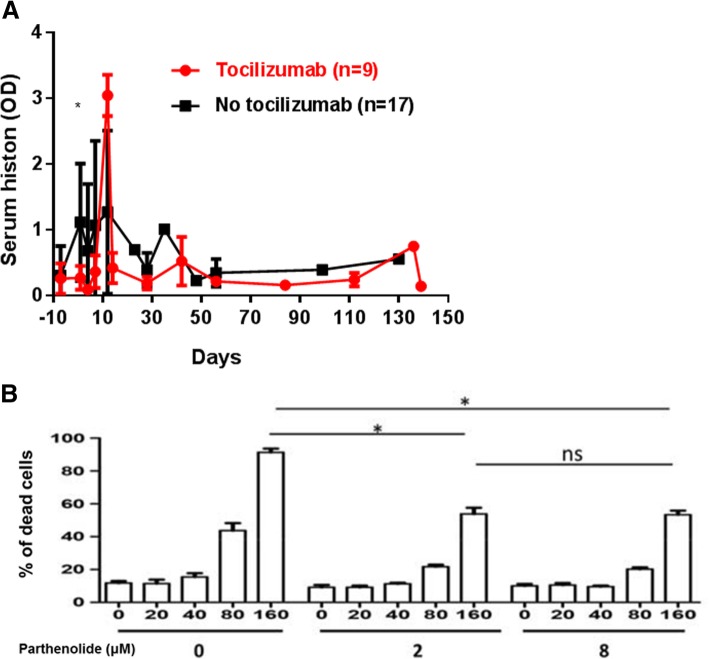
The direct prothrombotic activity of histone-DNA complexes increases inflammatory cytokine formation, and fosters thrombotic responses by activating TLRs 2, 4, and 9 [48]. Moreover, inflammatory cytokines downregulate thrombomodulin, induce tissue factor, and upregulate plasminogen activator inhibitor [48]. Histones can also cause direct platelet activation [53, 58]. Their levels increase in xenograft recipients when there is evidence for inflammation and coagulation dysfunction [26]. In the absence of IL-6-receptor blockade (with tocilizumab), the mean serum histone level after pig organ transplantation rises significantly [26] (Fig. 3a). A decrease in the number of neutrophils might reduce extracellular histone release [59, 60]. In in vitro studies, histone-induced porcine EC apoptosis/death was significantly reduced by an inhibitor of nuclear factor kappa B (NF-κB), parthenolide (Fig. 3b) [26]. EC apoptosis is observed in many inflammatory and immune disorders [61].
Fig.3.
a Serum extracellular histone levels in baboons with pig artery patch grafts. In the absence of tocilizumab therapy, the mean serum histone level was higher on post-transplant day 1 than in baboons receiving tocilizumab (day 1, 1.2 vs. 0.3, *P < 0.05), excluding 2 baboons that required euthanasia on day 12 for consumptive coagulopathy. (Black line = without tocilizumab therapy; Red line = with tocilizumab therapy.) Tocilizumab treatment appeared to prevent the sustained histone increase seen after xenotransplantation. (Reprinted with permission from ref. [26]). b In vitro histone-induced porcine endothelial cell apoptosis/death is influenced by NF-κB inhibition. The NF-κB inhibitor, parthenolide (at 2 and 8 μM), significantly reduced histone (160 μg/mL)-induced cell apoptosis/death (mean percentage apoptosis/death of 91.4% vs 54%, respectively; both P < 0.05). There was no significant difference in the protective effect of parthenolide at concentrations of 2 and 8 μM (mean percentage apoptosis/death of 54% at both concentrations). (Reprinted with permission from ref. [26])
D-dimer is a protein product of cross-linked fibrin degradation. An elevated blood concentration of D-dimer is observed in intravascular coagulation and thrombotic disease [62]. D-dimer may promote the inflammatory cascade by activating neutrophils and monocytes, inducing secretion of inflammatory cytokines (e.g., IL-6) [62–65].
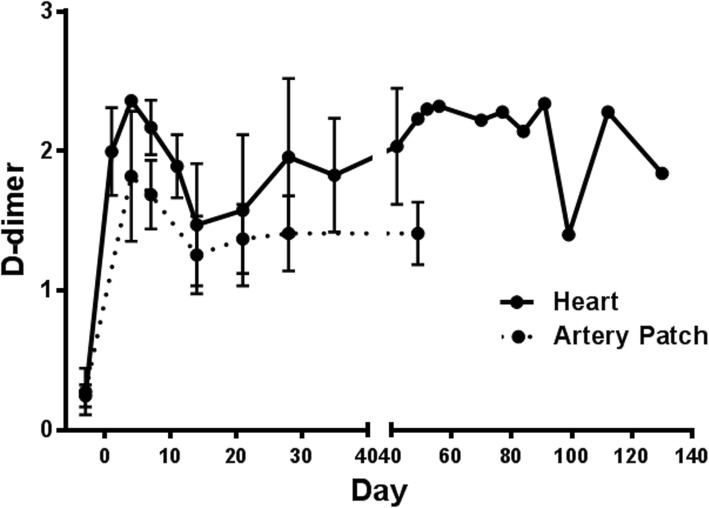
D-dimer may also be a marker of inflammation [19, 64, 66, 67], and may rise when a xenograft is failing (Fig. 4) [19].
Fig. 4.
Changes in D-dimer after pig-to-baboon heart (n = 4) or artery patch (n = 14) transplantation. In heart recipients, mean D-dimer increased from < 0.5μg/ml pre-transplant to > 2.0μg/ml (on post-transplant day 4) and was variable thereafter. In artery patch recipients, mean D-dimer increased from < 0.5μg/ml pre-transplant to 1.4μg/ml on post-transplant day 48 (P < 0.01). (Reprinted with permission from ref. [19])
Pro-inflammatory cytokines/chemokines help to resist infection, but may induce systemic inflammation [68, 69]. In in vitro studies, porcine IL-6, IL-1β, and TNF-α activated human umbilical vein ECs (HUVECs) [70]. Pig aortic ECs (pAECs) can be significantly activated by human IL-6, IL-17, IL-1β, and TNF-α [70]. For example, (i) human IL-17, IL-1β, and TNF-α increased the expression of adhesion molecule genes (e.g., E-selectin, VCAM-1, and ICAM-1), (ii) human IL-6, IL-17, IL-1β, and TNF-α induced chemokines (e.g., IL-8 and MCP-1) and increased tissue factor expression, and (iii) expression of swine leukocyte antigen (SLA) class-I was induced by human IL-1β and TNF-α [70]. All of the above cytokines/chemokines are likely to promote inflammation and coagulation in response to a xenograft.
In the absence of immunosuppressive therapy, increases in certain cytokine levels are seen after xenotransplantation, but not when immunosuppressive therapy is administered [18] (Fig. 5a,b).
Fig. 5.
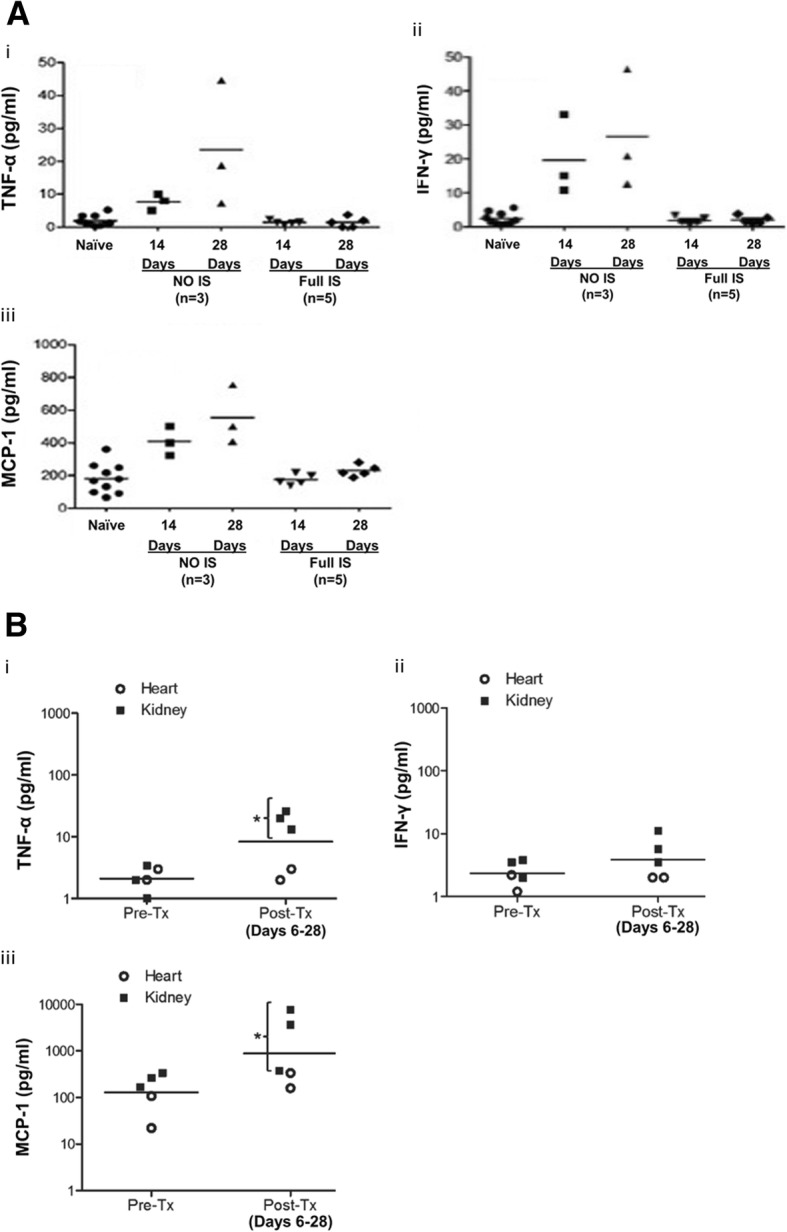
a Changes in the levels of selected serum cytokines after pig-to-baboon artery patch transplantation (n = 8) (i) TNF-α; (ii) IFN-γ; (iii) MCP-1. In baboons (n = 3) that did not receive immunosuppressive therapy after artery patch transplantation, levels of (i) TNF-α, (ii) IFN-γ, and (iii) MCP-1 were significantly higher on post-transplant days 14 and 28 than pre-transplant. When full immunosuppressive therapy was administered to the baboons (n = 5), no increase in any of the three cytokines was observed. (Reprinted with permission from ref. [18]). b Changes in the levels of selected cytokines after pig-to-baboon kidney (n = 3) or heart (n = 2) transplantation. (i) TNF-α; (ii) IFN-γ; (iii) MCP-1. Although the numbers of experiments were small, (i) TNF-α and (iii) MCP-1 were significantly higher in kidney recipients (*P < 0.05), but not in heart recipients. (ii) IFN-γ was not significantly increased in recipients after kidney or heart transplantation. (Reprinted with permission from ref. [18])
Inflammation plays a key role in platelet activation and aggregation [71], which in turn plays an important role in the dysregulation of coagulation seen after xenotransplantation [72]. Extracellular histones bind to TLRs, particularly to TLR2 and TLR4, on platelets, which results in platelet aggregation [51, 53]. In humans, the cytokine, IL-17, can promote platelet activation and aggregation through the ERK2 and P53 signaling pathways [73, 74], although the exact mechanism remains unclear [75]. Recipient platelets might also be activated by binding directly to pig ECs [76]. Human platelets can upregulate tissue factor expression after contact with pAECs in the absence of human serum or antibodies, which can lead to coagulation through thrombin production [77].
There is a relationship between a low plasma free triiodothyronine (fT3) and inflammation [78–82]. Plasma fT3 falls following brain death [83, 84], and major surgical procedures, especially heart surgery on cardiopulmonary bypass [85–89].
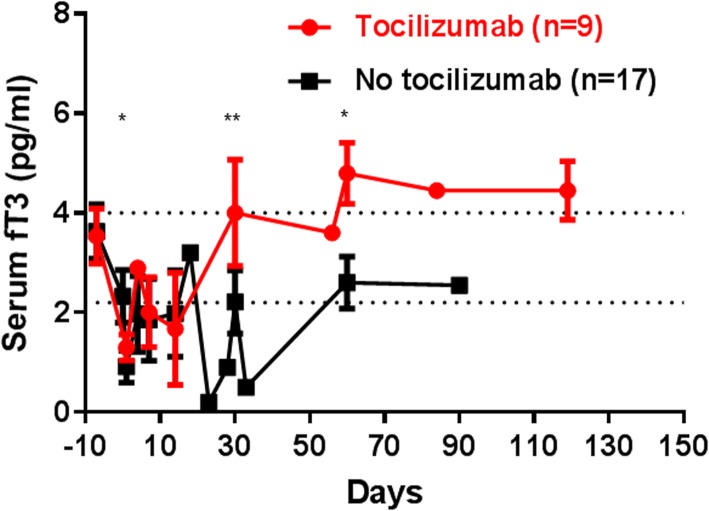
In recipient baboons undergoing pig heart, kidney, liver, and artery patch xenotransplants, fT3 falls rapidly, and takes several days to return to pre-transplant levels [26] (Fig. 6). A negative correlation between serum IL-6 and TNF-α with thyroid hormone concentrations has been reported [80]. A persisting low level is almost certainly associated with an inflammatory response to a xenograft [26].
Fig. 6.
Changes in serum free triiodothyronine (fT3) after pig-to-baboon organ or artery patch transplantation (n = 26). The serum fT3 showed an immediate and significant decrease (P < 0.001) in all baboons (n = 26) after pig organ transplantation. In baboons that received the IL-6R blocker, tocilizumab (n = 9), the fT3 recovered more rapidly and to a higher level than in baboons that did not receive tocilizumab (n = 17) (day 1, 1.3 vs 0.9 pg/mL, P < 0.05; day 30, 4.0 vs 2.2 pg/mL, P < 0.01; day 60, 4.8 vs 2.6 pg/mL, P < 0.05, respectively). We concluded that IL-6R blockade reduced inflammation, allowing the fT3 to recover more rapidly. (Reprinted with permission from ref. [26])
Evidence for the relationship between inflammation and coagulation in xenograft recipients
Until recently, a major barrier to successful pig organ transplantation in NHPs was dysregulation of coagulation resulting from excessive thrombin generation [90–93]. The activation of thrombin receptors amplifies production of the chemokine, CCL18, and the pulmonary activation-regulated chemokine by mature dendritic cells [94]. Thrombin can upregulate ICAM-1 mRNA and induce ICAM-1 expression on monocytes in vitro [95], and by activating NF-κB [96].
It is well-known that inflammation contributes to activation of coagulation dysfunction [17, 18, 70, 97, 98]. Tissue factor is not only a promoter of thrombin, but also a marker of inflammation [99, 100]. TNF-α [101], IL-6 [102], and C-RP [103] increase tissue factor expression on innate immune cells, which in turn promotes the activation of coagulation [100, 103]. There is an amplification circuit between coagulation and inflammation which results in activation of inflammatory mediators as well as procoagulant factors [20]. Therefore, therapeutic prevention of inflammation may be a major factor in minimizing coagulation dysregulation after pig organ xenotransplantation.
An important observation made recently indicates that, when pig vascular ECs expressing only natural pig thrombomodulin (which also has an anti-inflammatory effect) are activated by TNF-α, the expression of thrombomodulin is significantly downregulated (Fig. 7a) [98]. This suggests that, when a pig organ is exposed to inflammation (which is universal after a pig organ transplant into a NHP), thrombotic microangiopathy is likely to develop. The absence of the anti-inflammatory effect of human thrombomodulin may result in the early development of consumptive coagulopathy [6]. In contrast, transgenic expression of human thrombomodulin is not downregulated, thus maintaining both its anticoagulant and anti-inflammatory effects (Fig. 7b) [98].
Fig. 7.
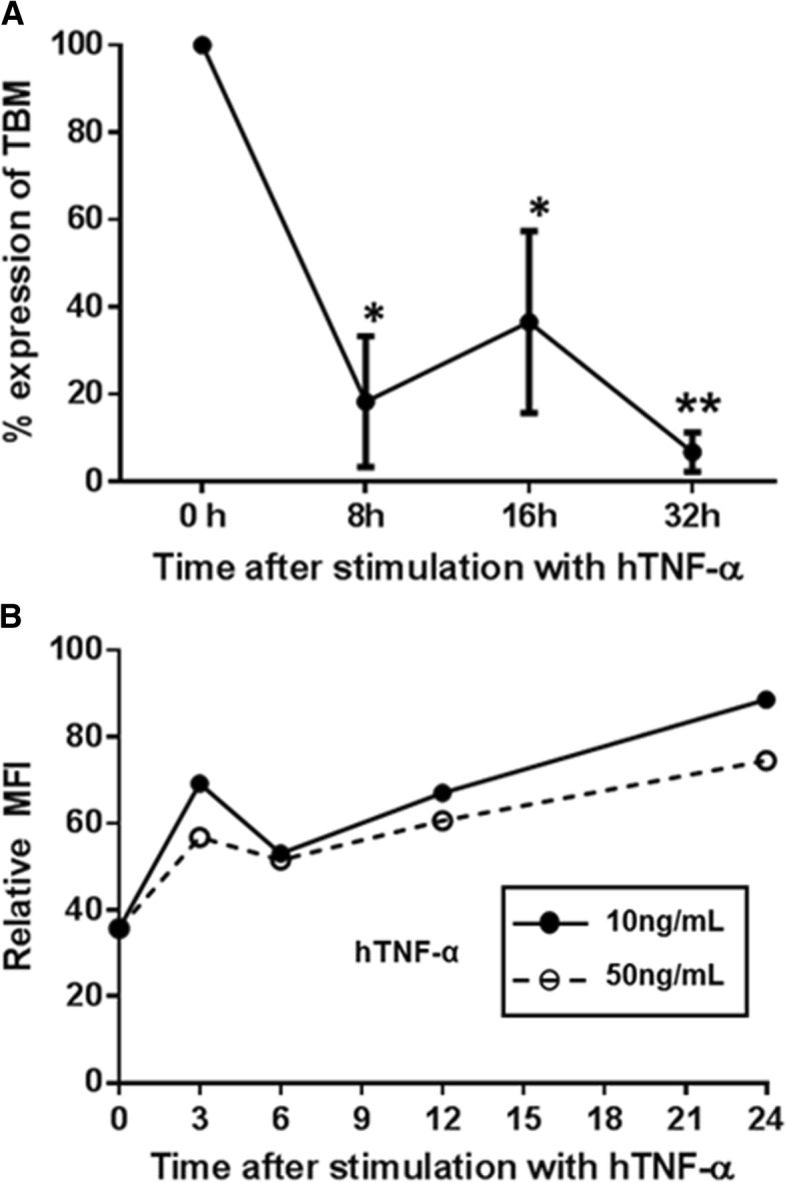
a Inflammation down-regulated expression of natural pig thrombomodulin (TBM) Expression of natural pig thrombomodulin was down-regulated after exposure to TNF-α, and was confirmed by real-time PCR (*P < 0.05, **P < 0.01). (The expression of pig thrombomodulin in GTKO/CD46 pig aortic endothelial cells [pAECs] was measured by real-time PCR. The PCR primer sequences used were: pTBM: Sense 5′- GAA GCT ATG AGG TCC AGC CC − 3′; Antisense 5′- CAG ACA GAC AGC GAA GAG CA − 3′.) (Details in ref. [104]). b Inflammation did not down-regulate expression of transgenic human TBM. The expression of transgenic human thrombomodulin was upregulated, confirmed by flow cytometry. Transgenically-expressed human thrombomodulin would appear to be resistant to down-regulation by inflammation. (The expression of human thrombomodulin in human thrombomodulin-transgenic pAECs was measured by flow cytometry (clone 1A4, BD Biosciences, San Jose, CA)
Evidence for the relationship between inflammation and the immune response in xenograft recipients
The significant increase in certain cytokines/chemokines after xenotransplantation likely results from innate immune cell activity, and may well be a causative factor in xenograft injury [17, 18]. Inflammation and the innate immune response augment the adaptive immune response [70, 98]. Systemic upregulation of inflammatory markers is related to inefficient blockade of the T cell-dependent adaptive immune response [105].
In an in vitro study, there was a significant increase in the human peripheral blood mononuclear cell (PBMC) proliferative response when pAECs were activated by pig IFN-γ, supporting the concept that inflammation augments the immune response to a xenograft [106] (Fig. 8a). The induction of T cell tolerance after transplantation is inhibited by inflammation [25]. By affecting the immune response, cytokine and chemokine secretions influence the outcome of allotransplantation [107, 108]. Increased IL-7, IL-8, and IFN-γ-induced protein 10, chemokine ligand 9, and chemokine ligands 2 and 5 are associated with early allograft dysfunction [109–111].
Fig. 8.
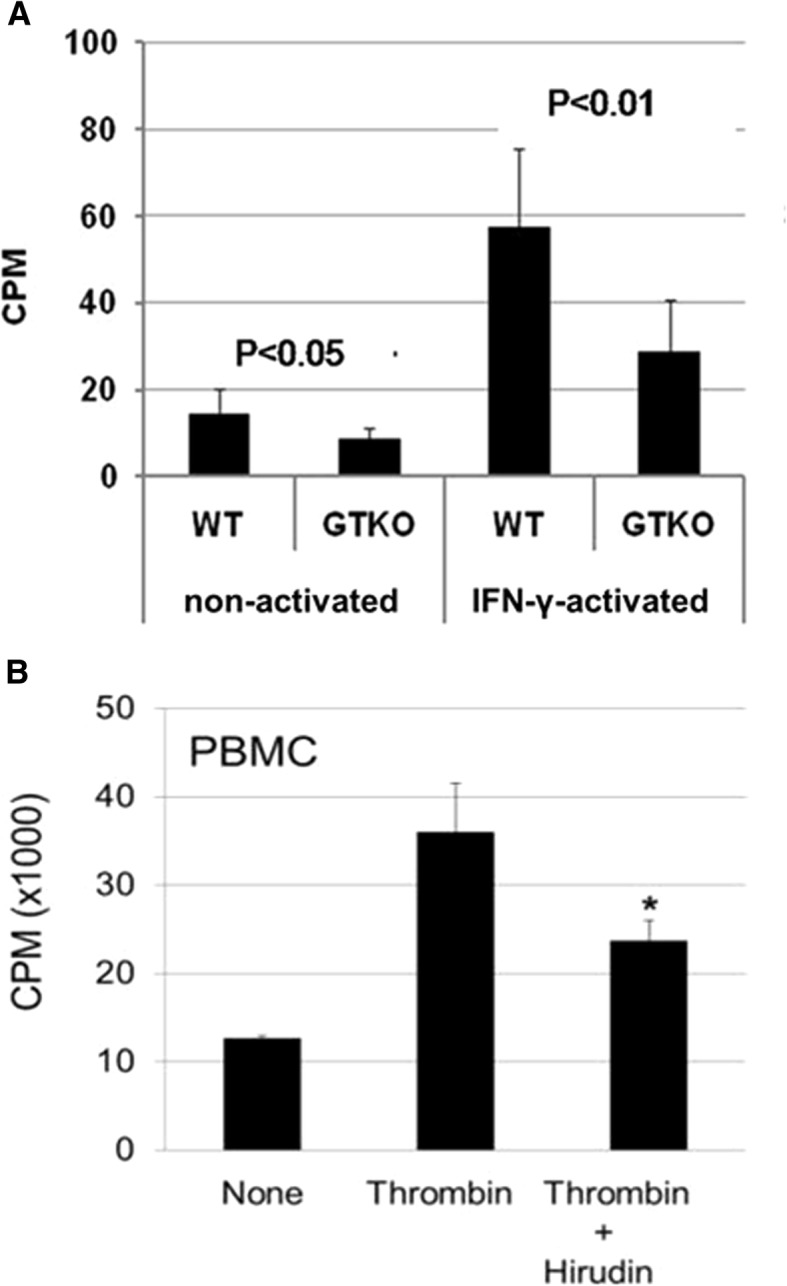
a IFN- γ-activation increases the proliferative response of human peripheral blood mononuclear cells (PBMCs) to wild-type (WT) and GTKO pig aortic endothelial cells (pAECs). When non-activated, the proliferative response to WT pAECs was greater than to GTKO pAECs (P < 0.05). There was an increase in the PBMC response when the pAECs were activated by IFN-γ, the response to WT pAECs again being significantly greater than to GTKO pAECs (P < 0.01) . The study illustrates how inflammation can increase the immune response to a xenograft. (CPM = counts per minute; SI = stimulation index). (Reproduced with permission from ref. [106]). b Thrombin activates T cell proliferation. The degree of activation of GTKO pig PBMCs by thrombin was comparable to that resulting from stimulation of the cells by porcine interferon-gamma (pIFN-γ). Thrombin-stimulated activation of the human cellular response was reduced by the addition of hirudin, confirming that thrombin was the stimulatory factor. (Reproduced with permission from ref. [97])
Inflammation, coagulation, and the immune response have a complex inter-relationship [23, 50]. For example, thrombin activates the human cellular response to pig cells in vitro, and induces a T cell proliferative response to the same extent as IFN-γ activation (Fig. 8b) [97].
Potential strategies to prevent inflammation in xenotransplantation recipients
Several strategies aimed at preventing or reducing excessive inflammation after xenotransplantation have been tested, some of which are clinically-approved.
Drug therapy (Table 2)
Table 2.
Anti-inflammatory agents that may prevent or reduce SIXR
| Agent | Results of therapy | References | |
|---|---|---|---|
| Anti-complement agents | IL-6, IL-8, MCP-1 | ↓ | [13] |
| IL-6 receptor blockade | fT3, IL-6, MCP-1 | ↑ | [6, 13, 19, 26, 30] |
| Histones, C-RP, SAA | ↓ | [19, 26] | |
| IL-6 inhibitor | C-RP | ↓ | [112] |
| TNF-α inhibitor | E-selectin, VCAM-1 | ↓ | [113] |
|
NF-κB inhibitor (parthenolide) |
Platelet aggregation, endothelial cell apoptosis | ↓ | [26, 114] |
| Alpha-1 antitrypsin (AAT) | IL-8, IL-1β, TNF-α | ↓ | [115] |
| Platelet inhibitor (aspirin) | IL-6 | ↓ | [116] |
| T3 | Adenosine phosphate, creatine phosphate, lactate | ↓ | [88, 117, 118] |
| Glycogen | ↑ | ||
| TNF-α, IL-6 | ↓ | [119] | |
Corticosteroids
Corticosteroids activate several genes, including inhibitors of NF-κB, which has an anti-inflammatory effect [120]. After their administration to pig heart xenograft recipients, the levels of IL-6, IL-8, and MCP-1 were reduced [13]. However, D-dimer remained increased, irrespective of corticosteroids and/or anti-inflammatory therapy, suggesting that an inflammatory response persisted [13].
Anti-complement agents
Although cobra venom factor (CVF) is primarily administered to deplete complement [121], MCP-1, IL-8, and IL-6 are reduced after its administration [13]. After cobra venom factor administration in baboons with pig artery patch grafts, IL-6, IL-8, and MCP-1 remained lower than, or comparable to, pre-transplant levels [13]. Eculizumab is an anti-C5 humanized monoclonal antibody and inhibits the terminal complement effector pathway by preventing its cleavage by the C5 convertase [122]. It modifies the cytokine profile by increasing IFN-γ and IL-17 and lowering IL-4. [123–125]. Cp40, a cyclic 14-amino acid peptide, is a complement inhibitor that inhibits the generation of pro-inflammatory effectors (e.g., TNF-α, IL-1β, and IL-17) through inhibiting the activation of C3 [126, 127]. C1-inhibitor is the only known plasma protein inhibitor of serine proteases, C1s and C1r, of the classical complement pathway. It decreases some pro-inflammatory cytokines (TNF-α, IL-18) and increases a protective cytokine (IL-10) [128, 129].
IL-6 receptor blockade, and IL-6 inhibitors
Treatment of the NHP recipient of a pig xenograft with the IL-6 receptor blockade agent, tocilizumab, results in greatly decreased levels of C-RP (Fig. 1a) [19] and serum histones (Fig. 3a) [26]. However, D-dimer remained elevated (Fig. 4) [13, 19]. Blockade of IL-6 receptors is also associated with more rapid recovery of the fall in the level of fT3 seen after xenotransplantation (Fig. 6) [26].
Tocilizumab has several other beneficial effects on the immune response to a graft. It reduces the number of memory B cells [130, 131]) and plasma cells [132], but increases regulatory B cells [133], and the ratio of regulatory T cells [134]. It also reduces monocytes and myeloid dendritic cells [135]. Recipients of kidney allografts treated with tocilizumab suffer less antibody-mediated rejection [136], and have reduced donor-specific antibody levels [137].
However, recent evidence indicates that tocilizumab, although binding to primate IL-6 receptors, does not bind to IL-6 receptors on the pig graft [70], and therefore may have no protective effect on the graft. The IL-6 inhibitor, siltuximab, has a therapeutic effect in Castleman disease and certain inflammatory diseases by neutralizing IL-6 production [138]. IL-6 neutralization with siltuximab resulted in sustained C-RP suppression in Castleman disease [112], but it is not completely effective in xenotransplantation [Zhang G, et al., manuscript in preparation].
Anti-histone antibodies
Extracellular histones and TLR pathways are major targets for treating a variety of inflammatory conditions. Anti-histone therapy has the potential to prevent histone-induced inflammation in xenotransplantation [26]. The administration of an anti-histone antibody (e.g., anti-histone H4 monoclonal antibody) inhibits cytokine production and has a protective effect on various inflammatory injuries [45, 56, 139–147]. The protective effects of rTBM against histone toxicity are mediated through both activated protein C-dependent and -independent ways [148]. Anti-histone antibodies have not yet been tested in in vivo models of xenotransplantation.
TNF-α inhibitors
EC activation is reduced by a TNF-α inhibitor [113]. A TNF-receptor fusion protein (TNF-RFP) has reduced inflammation in an in vivo xenoperfusion model, although the mechanism of its function is poorly understood [113].
NF-κB inhibitors
NF-κB plays a crucial role in enhancing the cellular responses to inflammation. Thrombin not only activates NF-κB, but also upregulates NF-κB-dependent genes [87]. As extracellular histones induce expression of tissue factor on ECs potentially through the NF-κB pathway, this amplifies thrombin generation [149]. The NF-κB inhibitor, parthenolide, reduced porcine EC apoptosis/death in vitro [26] (Fig. 3b). Parthenolide has also been reported to reduce endotoxic shock and prevent inflammation in immune glomerulonephritis [150]. It is used as prophylactic treatment for migraine, and has been reported to have a beneficial effect in clinical trials [151].
Alpha 1-antitrypsin (AAT)
AAT, a prototypic serine protease inhibitor, is abundant in human blood. Although mainly produced by hepatocytes [152], it is also produced by other cells (e.g., epithelial cells [153], monocytes [154], macrophages and neutrophils [155, 156], intestinal epithelial cells [157], alpha and delta cells of human pancreatic islets [158], and cancer cells [159]). Plasma levels of AAT increase during inflammation and infection [160].
AAT has anti-inflammatory, anti-leukocyte migratory, anti-apoptotic, and anti-thrombotic effects [161–166]. Treatment with AAT significantly decreases the levels of pro-inflammatory cytokines (IL-8, IL-1β, TNF-α) [115]. In monkeys with islet allotransplants, AAT prevented an inflammatory response [167] but, when baboons received artery patch grafts from genetically-engineered pigs, treatment with AAT had no effect on IL-8 and C-RP levels [13].
Platelet inhibitors
Aspirin is widely used as a preventative against vascular disease, and is associated with a reduction in myocardial infarction and stroke [168]. In addition, there is evidence that aspirin down-regulates some proinflammatory cytokines (e.g., IL-6) [116] and proinflammatory signaling pathways, including NF-κB [169–171].
Triiodothyronine (T3)
It remains uncertain whether, in the presence of a pig xenograft, the administration of T3 can suppress the inflammatory state [79], but T3 treatment reduces inflammatory cytokines (e.g., TNF-α, IL-6), improving glycemic control in diabetic rats [119]. Nevertheless, as there is a fall in fT3 in all baboons following pig organ transplantation [30], we have found it beneficial to administer T3 to increase fT3 levels.
Genetic modification of the organ-source pig (Table 3)
Table 3.
Genetic modifications of the organ-source pig that may be protective against the inflammatory response
| Genes | Function | References |
|---|---|---|
| Hemeoxygenase-1 (HO-1) |
anti-inflammatory, anti-apoptotic |
[14, 172–178] |
| A20 (tumor necrosis factor-α-induced protein) |
anti-inflammatory, anti-apoptotic |
[179–181] |
| Thrombomodulin (TBM) |
anticoagulation, anti-inflammatory |
[182–187] |
| Endothelial protein C receptor (EPCR) |
anticoagulation, anti-inflammatory |
[188] |
| Ectonucleoside triphosphate diphosphohydrolase-1 (CD39) |
anticoagulation, anti-inflammatory |
[189–191] |
| Tissue factor pathway inhibitor (TFPI) |
anticoagulation, anti-inflammatory |
[192, 193] |
Expression of hemeoxygenase-1 (HO-1)
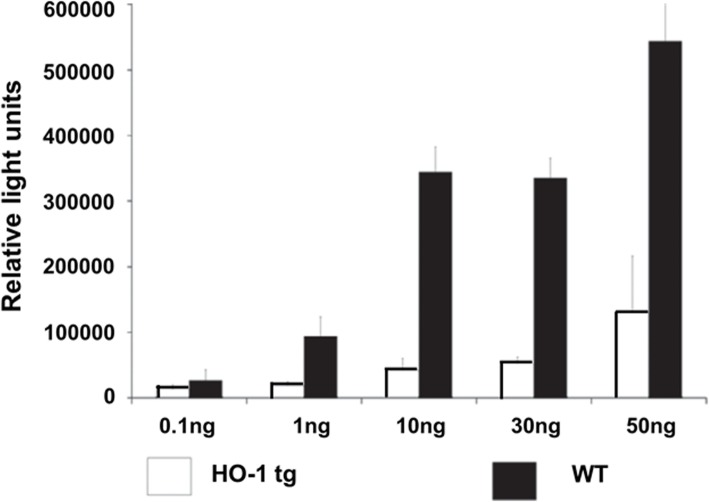
HO-1 is known to have an anti-inflammatory effect and reduces cell apoptosis [14, 172–178]. It is an anti-oxidant enzyme, which is regulated by the erythroid 2-related factor 2 (Nrf2) pathway [194]. The activation of HO-1 can prevent TNF-α-induced inflammatory and oxidative damage by up-regulating the Nrf2/HO-1 signaling pathway [195]. hHO-1 expression on porcine cells prevents TNFα- and cycloheximide-mediated apoptosis (Fig. 9) [173–176], and results in the downregulation of adhesion molecules, e.g., E-selectin, ICAM-1, and VCAM-1 [175]. Organs expressing hHO-1 were shown to be critical for prolonged survival of mouse cardiac xenografts in rats [173, 177], and expression of hHO-1 in pig islets prolonged their survival in mice, and decreased immune cell infiltration and islet cell apoptosis [178].
Fig. 9.
TNF-α-induced apoptosis was reduced by transgenic expression of hHO-1. Human hemeoxygenase-1 (hHO-1) transgenic pig aortic endothelial cells (pAECs) were protected against TNF-α-mediated apoptosis, measured by a caspase 3/7 assay. pAECs from hHO-1 transgenic pigs were better protected against TNF-α-mediated apoptosis compared to WT pAECs. (Modified from ref. [175])
Expression of A20
A20, a TNF-α-induced protein, has been shown to be anti-inflammatory and anti-apoptotic [179–181]. A20 is an important regulator of inflammatory signaling, which counteracts NF-κB activation. Several reports suggested that A20 plays a crucial role in inhibiting NF-κB signaling in response to TNF-α and microbial products [180, 181]. pAECs from hA20 transgenic pigs underwent significantly reduced apoptosis compared to wild-type pAECs [179]. hA20-transgenic pig hearts were partially protected against ischemia/reperfusion injury [179].
Expression of coagulation-regulatory proteins
Several coagulation-regulatory proteins have anti-inflammatory properties, e.g., thrombomodulin [182–187], endothelial protein C receptor (EPCR) [188], ectonucleoside triphosphate diphosphohydrolase-1 (CD39) [189–191], and tissue factor pathway inhibitor (TFPI) [192, 193]. The N-terminal lectin–like domain of thrombomodulin was reported to possess direct anti-inflammatory activity and to suppress complement activation [182]. Thrombomodulin also has anti-inflammatory effects through its capacity to promote generation of activated protein C [183–186], which exerts anticoagulant activity and has a direct cytoprotective effect [196]. Endothelial protein C receptor also elicits activated protein C-dependent and -independent anti-inflammatory effects [188]. CD39 is a major vascular nucleoside triphosphate diphosphohydrolase, and converts adenosine triphosphate (ATP), and adenosine diphosphate (ADP) to adenosine. CD39 was demonstrated to protect kidney grafts from ischemia-reperfusion injury via anti-inflammatory adenosine receptor signaling [189], and to protect islets from the instant blood-mediated inflammatory reaction (IBMIR) [190]. TFPI is an essential anticoagulant protein that acts by preventing the activation of the blood coagulation proteases, factor VII to VIIa (fVIIa) and factor X to Xa (fXa) [197]. In murine pneumococcal pneumonia, recombinant human TFPI reduces IL-6, TNF-α, MCP-1, IFN-γ, keratinocyte-derived cytokine, and macrophage-inflammatory protein-2, and increases the anti-inflammatory cytokine IL-10 [192].
Conclusions
Systemic inflammation may be playing a crucial role in pig organ xenotransplantation through activating the coagulation cascade and immune response. The administration of anti-inflammatory agents or the genetic modification of the organ-source pig by the introduction of human inflammation-regulatory transgenes may be beneficial to prevent or control inflammation. Control of inflammation is likely to allow a reduction in the intensity of exogenous immunosuppressive therapy. If immunological tolerance to a xenograft is to be obtained, then control of inflammation may be essential.
Acknowledgements
Not applicable.
Funding
Work on xenotransplantation at the University of Alabama at Birmingham is supported in part by NIH NIAID U19 grant AI090959 and by a grant from United Therapeutics, Silver Spring, MD, USA.
Availability of data and materials
Not applicable.
Abbreviations
- AAT
Alpha 1-antitrypsin
- C-RP
C-reactive protein
- EC
Endothelial cell
- fT3
Free triiodothyronine
- HO-1
Hemeoxygenase-1
- IL
Interleukin
- MCP-1
Monocyte chemotactic protein-1
- NF-kB
Nuclear factor kappa B
- NHP
Nonhuman primate
- SAA
Serum amyloid A
- SIXR
Systemic inflammation in xenograft recipients
- TFPI
Tissue factor pathway inhibitor
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor-alpha
Authors’ contributions
All authors were involved in drafting and editing, they all read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Juan Li, Email: juanli@uab.edu.
Hidetaka Hara, Email: hhara@uabmc.edu.
Yi Wang, Email: wangyi0108@gmail.com.
Charles Esmon, Email: Charles-Esmon@omrf.org.
David K. C. Cooper, Email: dkcooper@uabmc.edu
Hayato Iwase, Phone: 205-975-3938, Email: hiwase@uabmc.edu.
References
- 1.UNOS. https://unos.org/data/transplant-trends/ Accessed April 22, 2019.
- 2.Cooper DKC, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002;53:133–147. doi: 10.1146/annurev.med.53.082901.103900. [DOI] [PubMed] [Google Scholar]
- 3.Cooper DKC, Ezzelarab MB, Hara H, et al. The pathobiology of pig-to-primate xenotransplantation: a historical review. Xenotransplantation. 2016;23:83–105. doi: 10.1111/xen.12219. [DOI] [PubMed] [Google Scholar]
- 4.Lambrigts D, Sachs DH, Cooper DKC. Discordant organ xenotransplantation in primates - world experience and current status. Transplantation. 1998;66:547–561. doi: 10.1097/00007890-199809150-00001. [DOI] [PubMed] [Google Scholar]
- 5.Iwase H, Liu H, Wijkstrom M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22:302–309. doi: 10.1111/xen.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwase Hayato, Hara Hidetaka, Ezzelarab Mohamed, Li Tao, Zhang Zhongqiang, Gao Bingsi, Liu Hong, Long Cassandra, Wang Yi, Cassano Amy, Klein Edwin, Phelps Carol, Ayares David, Humar Abhinav, Wijkstrom Martin, Cooper David K. C. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation. 2017;24(2):e12293. doi: 10.1111/xen.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higginbotham L, Mathews D, Breeden CA, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22:221–230. doi: 10.1111/xen.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams AB, Kim SC, Martens GR, et al. Xenoantigen deletion and chemical immunosuppression can prolong renal xenograft survival. Ann Surg. 2018;268(4):564–573. doi: 10.1097/SLA.0000000000002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langin M, Mayr T, Reichart B, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018;564(7736):430–433. doi: 10.1038/s41586-018-0765-z. [DOI] [PubMed] [Google Scholar]
- 10.Ghighi M, Llorens A, Baroukh B, et al. Differences between inflammatory and catabolic mediators of peri-implantitis and periodontitis lesions following initial mechanical therapy: An exploratory study. J Periodontal Res. 2018;53:29–39. doi: 10.1111/jre.12483. [DOI] [PubMed] [Google Scholar]
- 11.Sato K, Meng F, Venter J, et al. The role of the secretin/secretin receptor axis in inflammatory cholangiocyte communication via extracellular vesicles. Sci Rep. 2017;7:11183. doi: 10.1038/s41598-017-10694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pant S, Deshmukh A, Gurumurthy GS, et al. Inflammation and atherosclerosis--revisited. J Cardiovasc Pharmacol Ther. 2014;19:170–178. doi: 10.1177/1074248413504994. [DOI] [PubMed] [Google Scholar]
- 13.Iwase Hayato, Liu Hong, Li Tao, Zhang Zhongquiang, Gao Bingsi, Hara Hidetaka, Wijkstrom Martin, Long Cassandra, Saari Ryan, Ayares David, Cooper David K. C., Ezzelarab Mohamed B. Therapeutic regulation of systemic inflammation in xenograft recipients. Xenotransplantation. 2017;24(2):e12296. doi: 10.1111/xen.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura K, Zhang M, Kageyama S, et al. Macrophage heme oxygenase-1-SIRT1-p53 axis regulates sterile inflammation in liver ischemia-reperfusion injury. J Hepatol. 2017;67:1232–1242. doi: 10.1016/j.jhep.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solhjou Z, Athar H, Xu Q, et al. Emerging therapies targeting intra-organ inflammation in transplantation. Am J Transplant. 2015;15:305–311. doi: 10.1111/ajt.13073. [DOI] [PubMed] [Google Scholar]
- 16.Labarrere CA, Woods JR, Hardin JW, et al. Early inflammatory markers are independent predictors of cardiac allograft vasculopathy in heart-transplant recipients. PLoS One. 2014;9(12):e113260. doi: 10.1371/journal.pone.0113260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ezzelarab MB, Cooper DK. Systemic inflammation in xenograft recipients (SIXR): a new paradigm in pig-to-primate xenotransplantation? Int J Surg. 2015;23:301–305. doi: 10.1016/j.ijsu.2015.07.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezzelarab MB, Ekser B, Azimzadeh A, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 2015;22:32–47. doi: 10.1111/xen.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwase H, Ekser B, Zhou H, et al. Further evidence for a sustained systemic inflammatory response in xenograft recipients (SIXR) Xenotransplantation. 2015;22:399–405. doi: 10.1111/xen.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strukova S. Coagulation-dependent inflammation and inflammation-dependent thrombosis. Front Biosci. 2006;11:59–80. doi: 10.2741/1780. [DOI] [PubMed] [Google Scholar]
- 21.Iwase H, Ekser B, Zhou H, et al. Platelet aggregation in humans and nonhuman primates: relevance to xenotransplantation. Xenotransplantation. 2012;19:233–243. doi: 10.1111/j.1399-3089.2012.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezzelarab M, Welchons D, Torres C, et al. Atorvastatin down-regulates the primate cellular response to porcine aortic endothelial cells in vitro. Transplantation. 2008;86:733–737. doi: 10.1097/TP.0b013e3181821cad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Lupu F, Esmon CT, et al. Inflammation, innate immunity and blood coagulation. Hamostaseologie. 2010;30:5–6, 8-9. doi: 10.1055/s-0037-1617146. [DOI] [PubMed] [Google Scholar]
- 24.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in alpha1, 3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong AS, Alegre ML. The impact of infection and tissue damage in solid-organ transplantation. Nat Rev Immunol. 2012;12:459–471. doi: 10.1038/nri3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li T, Lee W, Hara H, et al. An investigation of extracellular histone in pig-to-baboon organ xenotransplantation. Transplantation. 2017;101:2330–2339. doi: 10.1097/TP.0000000000001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Tao, Hara Hidetaka, Ezzelarab Mohamed B, Long Cassandra, Long Yongqi, Wang Yi, Cooper David KC, Iwase Hayato. Serum amyloid A as a marker of inflammation in xenotransplantation. European Journal of Inflammation. 2018;16:205873921878004. doi: 10.1177/2058739218780046. [DOI] [Google Scholar]
- 28.Zhang Guoqiang, Hara Hidetaka, Yamamoto Takayuki, Li Qi, Jagdale Abhijit, Li Yong, Cooper David K.C., Iwase Hayato. Serum amyloid a as an indicator of impending xenograft failure: Experimental studies. International Journal of Surgery. 2018;60:283–290. doi: 10.1016/j.ijsu.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ezzelarab MB, Ekser B, Isse K, et al. Increased soluble CD154 (CD40 ligand) levels in xenograft recipients correlate with the development of de novo anti-pig IgG antibodies. Transplantation. 2014;97:502–508. doi: 10.1097/TP.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 30.Iwase H, Ekser B, Hara H, et al. Thyroid hormone: relevance to xenotransplantation. Xenotransplantation. 2016;23:293–299. doi: 10.1111/xen.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An HJ, Jang JW, Bae SH, et al. Serum C-reactive protein is a useful biomarker for predicting outcomes after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2012;18:1406–1414. doi: 10.1002/lt.23512. [DOI] [PubMed] [Google Scholar]
- 32.Ansar W, Ghosh S. C-reactive protein and the biology of disease. Immunol Res. 2013;56:131–142. doi: 10.1007/s12026-013-8384-0. [DOI] [PubMed] [Google Scholar]
- 33.Neumaier M, Braun KF, Sandmann G, et al. C- reactive protein in orthopaedic surgery. Acta Chir Orthop Traumatol Cechoslov. 2015;82:327–331. [PubMed] [Google Scholar]
- 34.Jabs WJ, Lögering BA, Gerke P, et al. The kidney as a second site of human C-reactive protein formation in vivo. Eur J Immunol. 2003;33:152–161. doi: 10.1002/immu.200390018. [DOI] [PubMed] [Google Scholar]
- 35.Urieli-Shoval S, Linke RP, Matzner Y. Expression and function of serum amyloid a, a major acute-phase protein, in normal and disease states. Curr Opin Hematol. 2000;7:64–69. doi: 10.1097/00062752-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Ye RD, Sun L. Emerging functions of serum amyloid a in inflammation. J Leukoc Biol. 2015;98:923–929. doi: 10.1189/jlb.3VMR0315-080R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartmann A, Eide TC, Fauchald P, et al. Serum amyloid a protein is a clinically useful indicator of acute renal allograft rejection. Nephrol Dial Transplant. 1997;12:161–166. doi: 10.1093/ndt/12.1.161. [DOI] [PubMed] [Google Scholar]
- 38.Eklund KK, Niemi K, Kovanen PT, et al. Immune functions of serum amyloid a. Crit Rev Immunol. 2012;32:335–348. doi: 10.1615/CritRevImmunol.v32.i4.40. [DOI] [PubMed] [Google Scholar]
- 39.Dong Z, Wu T, Qin W, et al. Serum amyloid a directly accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Mol Med. 2011;17:1357–1364. doi: 10.2119/molmed.2011.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uhlar CM, Whitehead AS. Serum amyloid a, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 41.Furlaneto CJ, Campa A. A novel function of serum amyloid a: a potent stimulus for the release of tumor necrosis factor-alpha, interleukin-1beta, and interleukin-8 by human blood neutrophil. Biochem Biophys Res Commun. 2000;268:405–408. doi: 10.1006/bbrc.2000.2143. [DOI] [PubMed] [Google Scholar]
- 42.Ribeiro FP, Furlaneto CJ, Hatanaka E, et al. mRNA expression and release of interleukin-8 induced by serum amyloid a in neutrophils and monocytes. Mediat Inflamm. 2003;12:173–178. doi: 10.1080/0962935031000134897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Buck M, Gouwy M, Wang JM, et al. The cytokine-serum amyloid A-chemokine network. Cytokine Growth Factor Rev. 2016;30:55–69. doi: 10.1016/j.cytogfr.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kisilevsky Robert, Manley Paul N. Acute-phase serum amyloid A: Perspectives on its physiological and pathological roles. Amyloid. 2012;19(1):5–14. doi: 10.3109/13506129.2011.654294. [DOI] [PubMed] [Google Scholar]
- 45.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finch JT, Lutter LC, Rhodes D, et al. Structure of nucleosome core particles of chromatin. Nature. 1977;269:29–36. doi: 10.1038/269029a0. [DOI] [PubMed] [Google Scholar]
- 47.Luger K, Mäder AW, Richmond RK, et al. Crystal structure of the nucleosome core particle at 2.8 a resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 48.Esmon CT. Molecular circuits in thrombosis and inflammation. Thromb Haemost. 2013;109:416–420. doi: 10.1160/TH12-08-0634. [DOI] [PubMed] [Google Scholar]
- 49.Semeraro F, Ammollo CT, Esmon NL, et al. Histones induce phosphatidylserine exposure and a procoagulant phenotype in human red blood cells. J Thromb Haemost. 2014;12:1697–1702. doi: 10.1111/jth.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Esmon CT, Xu J, Lupu F. Innate immunity and coagulation. J Thromb Haemost. 2011;9:182–188. doi: 10.1111/j.1538-7836.2011.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuchs TA, Bhandari AA, Wagner DD, et al. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118:3708–3714. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Etulain J, Martinod K, Wong SL, et al. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015;126:242–246. doi: 10.1182/blood-2015-01-624023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Semeraro F, Ammollo CT, Morrissey JH, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118:1952–1961. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu J, Zhang X, Monestier M, et al. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol. 2011;187:2626–2631. doi: 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bosmann M, Grailer JJ, Ruemmler R, et al. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 2013;27:5010–5021. doi: 10.1096/fj.13-236380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allam R, Darisipudi MN, Tschopp J, et al. Histones trigger sterile inflammation by activating the NLRP3 inflammasome. Eur J Immunol. 2013;43:3336–3342. doi: 10.1002/eji.201243224. [DOI] [PubMed] [Google Scholar]
- 57.Allam R, Santhosh V. Extracellular histones in tissue injury and inflammation. J Mol Med. 2014;92:465–472. doi: 10.1007/s00109-014-1148-z. [DOI] [PubMed] [Google Scholar]
- 58.Esmon CT. Extracellular histones zap platelets. Blood. 2011;118:3456–3457. doi: 10.1182/blood-2011-07-364380. [DOI] [PubMed] [Google Scholar]
- 59.Ogata A, Hirano T, Hishitani Y, et al. Safety and efficacy of tocilizumab for the treatment of rheumatoid arthritis. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:27–42. doi: 10.4137/CMAMD.S7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka T, Narazaki M, Kishimoto T, et al. Anti-interleukin-6 receptor antibody, tocilizumab, for the treatment of autoimmune diseases. FEBS Lett. 2011;585:3699–3709. doi: 10.1016/j.febslet.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 61.Winn RK, Harlan JM. The role of endothelial cell apoptosis in inflammatory and immune diseases. J Thromb Haemost. 2005;3:1815–1824. doi: 10.1111/j.1538-7836.2005.01378.x. [DOI] [PubMed] [Google Scholar]
- 62.Shorr AF, Thomas SJ, Alkins SA, et al. D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest. 2002;121:1262–1268. doi: 10.1378/chest.121.4.1262. [DOI] [PubMed] [Google Scholar]
- 63.Edgington TS, Curtiss LK, Plow EF, et al. A linkage between the haemostatic and immune systems embodied in the fibrinolytic release of lymphocytic suppressive peptides. J Immunol. 1985;134:471–477. [PubMed] [Google Scholar]
- 64.Robson SC, Shephard EG, Kirsch RE, et al. Fibrin degradation product D-dimer induces the synthesis and release of biologically active IL-1 beta, IL-6 and plasminogen activator inhibitors from monocytes in vitro. Br J Haematol. 1994;86:322–326. doi: 10.1111/j.1365-2141.1994.tb04733.x. [DOI] [PubMed] [Google Scholar]
- 65.Rao KMK, Pieper CS, Currie MS, et al. Variability of plasma IL-6 and crosslinked fibrin D-dimer over time in community dwelling elderly subjects. Am J Clin Pathol. 1994;102:802–805. doi: 10.1093/ajcp/102.6.802. [DOI] [PubMed] [Google Scholar]
- 66.Bao W, Qi X, Li H, et al. Correlation of D-dimer level with the inflammatory conditions: a retrospective study. AME Med J. 2017;2:1–8. doi: 10.21037/amj.2017.01.01. [DOI] [Google Scholar]
- 67.Zhang J, Guo Z, Yang W, et al. D-dimer levels are correlated with disease activity in Crohn's patients. Oncotarget. 2017;8:63971–63977. doi: 10.18632/oncotarget.19250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ulloa L, Tracey KJ. The “cytokine profile”: a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 69.Wilson N, Djamali A, Redfield R. Elevation of peripheral IL-6 in kidney transplant recipients with antibody mediated rejection. Am J Transplant. 2016;16:707. [Google Scholar]
- 70.Gao Hanchao, Liu Lu, Zhao Yanli, Hara Hidetaka, Chen Pengfei, Xu Jia, Tang Jia, Wei Ling, Li Zesong, Cooper David K.C., Cai Zhiming, Mou Lisha. Human IL-6, IL-17, IL-1β, and TNF-α differently regulate the expression of pro-inflammatory related genes, tissue factor, and swine leukocyte antigen class I in porcine aortic endothelial cells. Xenotransplantation. 2017;24(2):e12291. doi: 10.1111/xen.12291. [DOI] [PubMed] [Google Scholar]
- 71.Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23:2131–2137. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 72.Lin CC, Ezzelarab M, Shapiro R, et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010;10:1556–1568. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maione F, Cicala C, Liverani E, et al. IL-17A increases ADP-induced platelet aggregation. Bioc Biop Res Commun. 2011;408:658–662. doi: 10.1016/j.bbrc.2011.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang S, Yuan J, Yu M, et al. IL-17A facilitates platelet function through the ERK2 signaling pathway in patients with acute coronary syndrome. PLoS One. 2012;7(7):e40641. doi: 10.1371/journal.pone.0040641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jing Y, Peiwu D, Miao Y, et al. IL-17 induces MPTP opening through ERK2 and p53 signaling pathway in human platelets. J Huazhong Univ Sci Technolog Med Sci. 2015;35:679–683. doi: 10.1007/s11596-015-1489-z. [DOI] [PubMed] [Google Scholar]
- 76.Bombeli T, Schwartz BR, Harlan JM, et al. Endothelial cells undergoing apoptosis become proadhesive for nonactivated platelets. Blood. 1999;93:3831–3838. [PubMed] [Google Scholar]
- 77.Lin CC, Chen D, McVey JH, et al. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008;86:702–709. doi: 10.1097/TP.0b013e31818410a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zoccali C, Tripepi G, Cutrupi S, Pizzini P, Mallamaci F. Low triiodothyronine: a new facet of inflammation in end-stage renal disease. J Am Soc Nephrol. 2005;16:2789–2795. doi: 10.1681/ASN.2005040356. [DOI] [PubMed] [Google Scholar]
- 79.Novitzky D, Cooper DK. Thyroid hormone and the stunned myocardium. J Endocrinol. 2014;223:R1–R8. doi: 10.1530/JOE-14-0389. [DOI] [PubMed] [Google Scholar]
- 80.Lee WY, Kang MI, OH KW, et al. Relationship between circulating cytokine levels and thyroid function following bone marrow transplantation. Bone Marrow Transplant. 2004;33:93–98. doi: 10.1038/sj.bmt.1704304. [DOI] [PubMed] [Google Scholar]
- 81.Paraskevaidis IA, Parissis JT, TH Kremastinos D, et al. Antiinflammatory and anti-apoptotic effects of levosimendan in decompensated heart failure: a novel mechanism of drug-induced improvement in contractile performance of the failing heart. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:243–247. doi: 10.2174/1568016054368232. [DOI] [PubMed] [Google Scholar]
- 82.Lubrano V, Pingitore A, Carpi A, et al. Relationship between triiodothyronine and proinflammatory cytokines in chronic heart failure. Biomed Pharmacother. 2010;64:165–169. doi: 10.1016/j.biopha.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 83.Novitzky D, Wicomb WN, Cooper DKC, et al. Electrocardiographic, hemodynamic and endocrine changes occurring during experimental brain death in the Chacma baboon. J Heart Transplant. 1984;4:63–69. [Google Scholar]
- 84.Novitzky D, Cooper DKC, Reichart B. Hemodynamic and metabolic responses to hormonal therapy in brain-dead potential organ donors. Transplantation. 1987;43:852–854. doi: 10.1097/00007890-198743060-00016. [DOI] [PubMed] [Google Scholar]
- 85.Bremner WF, Taylor KM, Baird S, et al. Hypothalamo-pituitary thyroid axis function during cardiopulmomary bypass. J Thorac Cardiovasc Surg. 1978;75:392–399. [PubMed] [Google Scholar]
- 86.Robuschi G, Medici D, Fesani F, et al. Cardiopulmonary bypass: a low T4 and T3 syndrome with blunted thyrotropinin (TSH) response to thyrotropin-releasing hormone (TRH) Horm Res. 1986;23:151–158. doi: 10.1159/000180311. [DOI] [PubMed] [Google Scholar]
- 87.Novitzky D, Human PA, Cooper DKC. Effect of triiodothyronine (T3) on myocardial high energy phosphates and lactate following ischemia and cardiopulmonary bypass - an experimental study in baboons. J Thorac Cardiovasc Surg. 1986;96:600–607. [PubMed] [Google Scholar]
- 88.Novitzky D, Human PA, Cooper DKC. Inotropic effect of triiodothyronine following myocardial ischaemia and cardiopulmonary bypass: an experimental study in pigs. Ann Thorac Surg. 1988;45:50–55. doi: 10.1016/S0003-4975(10)62396-X. [DOI] [PubMed] [Google Scholar]
- 89.Novitzky D, Cooper DKC, Swanepoel A. Inotropic effect of triiodothyronine (T3) following myocardial ischemia and cardiopulmonary bypass: initial experience in patients undergoing open heart surgery. Eur J Cardiothorac Surg. 1989;3:140–145. doi: 10.1016/1010-7940(89)90092-4. [DOI] [PubMed] [Google Scholar]
- 90.Buhler L, Basker M, Ip A, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000;70:1323–1331. doi: 10.1097/00007890-200011150-00010. [DOI] [PubMed] [Google Scholar]
- 91.Buhler L, Yamada K, KitamuraI H, et al. Pig kidney transplantation in baboons: anti-gal (alpha)1-3Gal IgM alone is associated with acute humoral xenograft rejection and disseminated intravascular coagulation. Transplantation. 2001;72:1743–1752. doi: 10.1097/00007890-200112150-00007. [DOI] [PubMed] [Google Scholar]
- 92.Cowan PJ, Aminian A, Barlow H, et al. Renal xenografts from triple-transgenic pigs are not hyperacutely rejected but cause coagulopathy in non-immunosuppressed baboons. Transplantation. 2000;69:2504–2515. doi: 10.1097/00007890-200006270-00008. [DOI] [PubMed] [Google Scholar]
- 93.Wang L, Cooper DKC, Burdorf L, Wang Y, Iwase H. Overcoming coagulation dysregulation in pig solid organ transplantation in nonhuman Primates: recent Progress. Transplantation. 2018;102(7):1050–1058. doi: 10.1097/TP.0000000000002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li X, Syrovets T, Paskas S, et al. Mature dendritic cells express functional thrombin receptors triggering chemotaxis and CCL18/pulmonary and activation-regulated chemokine induction. J Immunol. 2008;181:1215–1223. doi: 10.4049/jimmunol.181.2.1215. [DOI] [PubMed] [Google Scholar]
- 95.Clark P, Jordan F, Pearson C, et al. Intercellular adhesion molecule-1 (ICAM-1) expression is upregulated by thrombin in human monocytes and THP-1 cells in vitro and in pregnant subjects in vivo. Thromb Haemost. 2003;89:1043–1051. doi: 10.1055/s-0037-1613406. [DOI] [PubMed] [Google Scholar]
- 96.Anrather D, Millan MT, Palmetshofer A, et al. Thrombin activates nuclear factor-kappaB and potentiates endothelial cell activation by TNF. J Immunol. 1997;159:5620–5628. [PubMed] [Google Scholar]
- 97.Ezzelarab C, Ayares D, Cooper DKC, et al. Human T-cell proliferation in response to thrombin-activated GTKO pig endothelial cells. Xenotransplantation. 2012;19:311–316. doi: 10.1111/j.1399-3089.2012.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hara H, Iwase H, Miyagawa Y, et al. Reducing the inflammatory response by expressimng human thrombomodulin in pigs. Xenotransplantation. 2017;24:19–20. [Google Scholar]
- 99.Chu AJ. Tissue factor mediates inflammation. Arch Biochem Biophys. 2005;440:123–132. doi: 10.1016/j.abb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 100.Chu AJ. Tissue factor, blood coagulation, and beyond: an overview. Int J Inflam. 2011;2011:367284. doi: 10.4061/2011/367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kambas K, Markiewski MM, Pneumatikos IA, et al. C5a and TNF-alpha up-regulate the expression of tissue factor in intra-alveolar neutrophils of patients with the acute respiratory distress syndrome. J Immunol. 2008;180:7368–7375. doi: 10.4049/jimmunol.180.11.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kruithof EK, Mestries JC, Gascon MP, et al. The coagulation and fibrinolytic responses of baboons after in vivo thrombin generation–effect of interleukin 6. Thromb Haemost. 1997;77:905–910. doi: 10.1055/s-0038-1656076. [DOI] [PubMed] [Google Scholar]
- 103.Wu J, Stevenson MJ, Brown JM, et al. C-reactive protein enhances tissue factor expression by vascular smooth muscle cells: mechanisms and in vivo significance. Arterioscler Thromb Vasc Biol. 2008;28:698–704. doi: 10.1161/ATVBAHA.107.160903. [DOI] [PubMed] [Google Scholar]
- 104.Lee W, Miyagawa Y, Long C, Zhang M, Cooper DK, Hara H. Effect of rho-kinase inhibitor, Y27632, on porcine corneal endothelial cell culture, inflammation and immune regulation. Ocul Immunol Inflamm. 2016;24:579–593. doi: 10.3109/09273948.2015.1056534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pankratz S, Bittner S, Herrmann AM, et al. Human CD4+ HLA-G+ regulatory T cells are potent suppressors of graft-versus-host disease in vivo. FASEB J. 2014;28:3435–3445. doi: 10.1096/fj.14-251074. [DOI] [PubMed] [Google Scholar]
- 106.Wilhite T, Ezzelarab C, Hara H, et al. The effect of gal expression on pig cells on the human T-cell xenoresponse. Xenotransplantation. 2012;19:56–63. doi: 10.1111/j.1399-3089.2011.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim N, Yoon YI, Yoo HJ, et al. Combined detection of serum IL-10, IL-17, and CXCL10 predicts acute rejection following adult liver transplantation. Mol Cells. 2016;39:639–644. doi: 10.14348/molcells.2016.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tang J, Shi Y, Deng R, et al. Cytokine profile in calcineurin inhibitor-induced chronic nephrotoxicity in Chinese liver transplant recipients. Transplant Proc. 2016;48:2756–2762. doi: 10.1016/j.transproceed.2016.06.047. [DOI] [PubMed] [Google Scholar]
- 109.Hoffman SA, Wang L, Shah CV, et al. Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. Am J Transplant. 2009;9:389–396. doi: 10.1111/j.1600-6143.2008.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uchida Y, Ke B, Freitas MC, et al. T-cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in micein a TLR4-dependent manner. Gastroenterology. 2010;139:2195–2206. doi: 10.1053/j.gastro.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oweira H, Lahdou I, Daniel V, et al. Early post-operative acute phase response in patients with early graft dysfunction is predictive of 6-month and 12-month mortality in liver transplant recipients. Hum Immunol. 2016;77:952–960. doi: 10.1016/j.humimm.2016.07.234. [DOI] [PubMed] [Google Scholar]
- 112.Casper C, Chaturvedi S, Munshi N, et al. Analysis of inflammatory and anemia-related biomarkers in a randomized, double-blind, placebo-controlled study of siltuximab (anti-IL6 monoclonal antibody) in patients with multicentric castleman disease. Clin Cancer Res. 2015;21:4294–4304. doi: 10.1158/1078-0432.CCR-15-0134. [DOI] [PubMed] [Google Scholar]
- 113.Ramackers W, Klose J, Tiede A, et al. Effect of TNF-alpha blockade on coagulopathy and endothelial cell activation in xenoperfused porcine kidneys. Xenotransplantation. 2015;22:284–294. doi: 10.1111/xen.12179. [DOI] [PubMed] [Google Scholar]
- 114.Malaver E, Romaniuk MA, D'Atri LP, et al. NF-kappaB inhibitors impair platelet activation responses. J Thromb Haemost. 2009;7:1333–1343. doi: 10.1111/j.1538-7836.2009.03492.x. [DOI] [PubMed] [Google Scholar]
- 115.Griese M, Latzin P, Kappler M, et al. Alpha1-antitrypsin inhalation reduces airway inflammation in cystic fibrosis patients. Eur Respir J. 2007;29:240–250. doi: 10.1183/09031936.00047306. [DOI] [PubMed] [Google Scholar]
- 116.Hovens MM, Snoep JD, Groeneveld Y, Frölich M, Tamsma JT, Huisman MV. Effects of aspirin on serum C-reactive protein and interleukin-6 levels in patients with type 2 diabetes without cardiovascular disease: a randomized placebo-controlled crossover trial. Diabetes Obes Metab. 2008;10:668–674. doi: 10.1111/j.1463-1326.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 117.WicombI WN, Cooper DK, Novitzky D, et al. Impairment of renal slice function following brain death, with reversibility of injury by hormonal therapy. Transplantation. 1986;41:29–33. doi: 10.1097/00007890-198601000-00005. [DOI] [PubMed] [Google Scholar]
- 118.Novitzky D, Cooper DKC. The brain-dead organ donor: pathophysiology and management. New York: Springer; 2013. pp. 1–416. [Google Scholar]
- 119.Panveloski-Costa AC, Silva Teixeira S, Ribeiro IM, et al. Thyroid hormone reduces inflammatory cytokines improving glycaemia control in alloxan-induced diabetic wistar rats. Acta Physiol (Oxf) 2016;217:130–140. doi: 10.1111/apha.12647. [DOI] [PubMed] [Google Scholar]
- 120.Hall SE, Lim S, Witherden IR, et al. Lung type II cell and macrophage annexin I release: differential effects of two glucocorticoids. Am J Phys. 1999;276:L114–L121. doi: 10.1152/ajplung.1999.276.1.L114. [DOI] [PubMed] [Google Scholar]
- 121.Osipov AV, Mordvintsev DY, Starkov VG, et al. Naja melanoleuca cobra venom contains two forms of complement-depleting factor (CVF) Toxicon. 2005;46:394–403. doi: 10.1016/j.toxicon.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 122.Rother RP, Rollins SA, Mojcik CF, et al. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;5:1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 123.Longhi MP, Harris CL, Morgan BP, et al. Holding T cells in check—a new role for complement regulators? Trends Immunol. 2006;27:102–108. doi: 10.1016/j.it.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 124.Liu J, Liu T, Miwa, et al. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med. 2005;201:567–577. doi: 10.1084/jem.20040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heeger PS, Lalli PN, et al. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201:1523–1530. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qu H, Ricklin D, Bai H, et al. New analogues of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiology. 2013;218:496–505. doi: 10.1016/j.imbio.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mastellos DC, Yancopoulou D, Kokkinos P, et al. Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention. Eur J Clin Investig. 2015;45:423–440. doi: 10.1111/eci.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Davis AE, Lu F, Mejia P, et al. C1 inhibitor, a multi-functional serine protease inhibitor. Thromb Haemost. 2010;104:886–893. doi: 10.1160/TH10-04-0252. [DOI] [PubMed] [Google Scholar]
- 129.Kim KY, Kim MY, Choi HS, et al. Thrombin induces IL-10 production in microglia as a negative feedback regulator of TNF-alpha release. NeuroReport. 2002;13:849–852. doi: 10.1097/00001756-200205070-00022. [DOI] [PubMed] [Google Scholar]
- 130.Roll P, Muhammad K, Schumann M, et al. In vivo effects of the anti-interleukin-6 receptor inhibitor tocilizumab on the B cell compartment. Arthritis Rheum. 2011;63:1255–1264. doi: 10.1002/art.30242. [DOI] [PubMed] [Google Scholar]
- 131.Muhammad K, Roll P, Seibold T, et al. Impact of IL-6 receptor inhibition on human memory B cells in vivo: impaired somatic hypermutation in preswitch memory B cells and modulation of mutational targeting in memory B cells. Ann Rheum Dis. 2011;70:1507–1510. doi: 10.1136/ard.2010.141325. [DOI] [PubMed] [Google Scholar]
- 132.Illei GG, Shirota Y, Yarboro CH, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010;62:542–552. doi: 10.1002/art.27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Snir A, Kessel A, Haj T, et al. Anti-IL-6 receptor antibody (tocilizumab): a B cell targeting therapy. Clin Exp Rheumatol. 2011;29:697–700. [PubMed] [Google Scholar]
- 134.Samson M, Audia S, Janikashvili N, et al. Inhibition of IL-6 function corrects Th17/Treg imbalance in rheumatoid arthritis patients. Arthritis Rheum. 2012;64:2499–2503. doi: 10.1002/art.34477. [DOI] [PubMed] [Google Scholar]
- 135.Richez C, Barnetche T, Khoryati L, et al. Tocilizumab treatment decreases circulating myeloid dendritic cells and monocytes, 2 components of the myeloid lineage. J Rheumatol. 2012;39:1192–1197. doi: 10.3899/jrheum.111439. [DOI] [PubMed] [Google Scholar]
- 136.Vo AA, Choi J, Kim I, et al. A phase I/II trial of the interleukin-6 receptor-specific humanized monoclonal (tocilizumab) + intravenous immunoglobulin in difficult to desensitize patients. Transplantation. 2015;99:2356–2363. doi: 10.1097/TP.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 137.Choi J, Aubert O, Vo A, et al. Assessment of tocilizumab (anti-Interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am J Transplant. 2017;17:2381–2389. doi: 10.1111/ajt.14228. [DOI] [PubMed] [Google Scholar]
- 138.Rossi JF, Lu ZY, Jourdan M, Klein B. Interleukin-6 as a therapeutic target. Clin Cancer Res. 2015;21:1248–1257. doi: 10.1158/1078-0432.CCR-14-2291. [DOI] [PubMed] [Google Scholar]
- 139.Kusano T, Chiang KC, Inomata M, et al. A novel anti-histone H1 monoclonal antibody, SSV monoclonal antibody, improves lung injury and survival in a mouse model of lipopolysaccharide-induced sepsis-like syndrome. Biomed Res Int. 2015;2015:491649. doi: 10.1155/2015/491649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Monestier M, Fasy TM, Losman MJ, et al. Structure and binding properties of monoclonal antibodies to core histones from autoimmune mice. Mol Immunol. 1993;30:1069–1075. doi: 10.1016/0161-5890(93)90153-3. [DOI] [PubMed] [Google Scholar]
- 141.Kuriyama N, Isaji S, Hamada T, et al. Activated protein C prevents hepatic ischaemia-reperfusion injury in rats. Liver Int. 2009;29:299–307. doi: 10.1111/j.1478-3231.2008.01796.x. [DOI] [PubMed] [Google Scholar]
- 142.Huang H, Evankovich J, Yan W, et al. Endogenous histones function as alarmins in sterile inflammatory liver injury through toll-like receptor 9 in mice. Hepatology. 2011;54:999–1008. doi: 10.1002/hep.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Abrams ST, Zhang N, Manson J, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med. 2013;187:160–169. doi: 10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bosmann M, Ward PA. Protein based therapies for acute lung injury: targeting neutrophil extracellular traps. Expert Opin Ther Targets. 2014;18:703–714. doi: 10.1517/14728222.2014.902938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen R, Kang R, Fan XG, et al. Release and activity of histone in diseases. Cell Death Dis. 2014;5:e1370. doi: 10.1038/cddis.2014.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Daigo K, Takamatsu Y, Hamakubo T, et al. The protective effect against extracellular histones afforded by long-Pentraxin PTX3 as a regulator of NETs. Front Immunol. 2016;7:1–9. doi: 10.3389/fimmu.2016.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wildhagen K. C. A. A., Garcia de Frutos P., Reutelingsperger C. P., Schrijver R., Areste C., Ortega-Gomez A., Deckers N. M., Hemker H. C., Soehnlein O., Nicolaes G. A. F. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood. 2013;123(7):1098–1101. doi: 10.1182/blood-2013-07-514984. [DOI] [PubMed] [Google Scholar]
- 148.Nakahara M, Ito T, Kawahara K, et al. Recombinant thrombomodulin protects mice against histone-induced lethal thromboembolism. PLoS One. 2013;8:e75961. doi: 10.1371/journal.pone.0075961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang X, Li L, Liu J, et al. Extracellular histones induce tissue factor expression in vascular endothelial cells via TLR and activation of NF-κB and AP-1. Thromb Res. 2016;137:211–218. doi: 10.1016/j.thromres.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 150.Sheehan M, Wong HR, Hake PW, Malhotra V, O'Connor M, Zingarelli B. Parthenolide, an inhibitor of the nuclear factor-kappaB pathway, ameliorates cardiovascular derangement and outcome in endotoxic shock in rodents. Mol Pharmacol. 2002;61:953–963. doi: 10.1124/mol.61.5.953. [DOI] [PubMed] [Google Scholar]
- 151.Dey S, Sarkar M, Giri B. Anti-inflammatory and anti-tumor activities of parthenolide: An update. J Chem Biol Ther. 2016;1(2):107. doi: 10.4172/2572-0406.1000107. [DOI] [Google Scholar]
- 152.Rogers J, Kalsheker N, Wallis S, et al. The isolation of a clone for human alpha 1-antitrypsin and the detection of alpha 1-antitrypsin in mRNA from liver and leukocytes. Biochem Biophys Res Commun. 1983;116:375–382. doi: 10.1016/0006-291X(83)90532-6. [DOI] [PubMed] [Google Scholar]
- 153.Cichy J, Potempa J, Travis J, et al. Biosynthesis of alpha1-proteinase inhibitor by human lung-derived epithelial cells. J Biol Chem. 1997;272:8250–8255. doi: 10.1074/jbc.272.13.8250. [DOI] [PubMed] [Google Scholar]
- 154.Carroll TP, Greene CM, O’Connor CA, et al. Evidence for unfolded protein response activation in monocytes from individuals with alpha-1 antitrypsin deficiency. J Immunol. 2010;184:4538–4546. doi: 10.4049/jimmunol.0802864. [DOI] [PubMed] [Google Scholar]
- 155.Bergin DA, Reeves EP, Meleady P, et al. Alpha-1 antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J Clin Invest. 2010;120:4236–4250. doi: 10.1172/JCI41196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Du Bois RM, Bernaudin JF, Paakko P, et al. Human neutrophils express the alpha 1-antitrypsin gene and produce alpha 1-antitrypsin. Blood. 1991;77:2724–2730. [PubMed] [Google Scholar]
- 157.Molmenti EP, Perlmutter DH, Rubin DC. Cell-specific expression of alpha 1-antitrypsin in human intestinal epithelium. J Clin Invest. 1993;92:2022–2034. doi: 10.1172/JCI116797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bosco D, Meda P, Morel P, et al. Expression and secretion of alpha1-proteinase inhibitor are regulated by proinflammatory cytokines in human pancreatic islet cells. Diabetologia. 2005;48:1523–1533. doi: 10.1007/s00125-005-1816-1. [DOI] [PubMed] [Google Scholar]
- 159.Chen XL, Zhou L, Yang J, et al. Hepatocellular carcinoma-associated protein markers investigated by MALDI-TOF MS. Mol Med Report. 2010;3:589–596. doi: 10.3892/mmr_00000302. [DOI] [PubMed] [Google Scholar]
- 160.Perlmutter DH. Alpha-1-antitrypsin deficiency: diagnosis and treatment. Clin Liver Dis. 2004;8:839–859. doi: 10.1016/j.cld.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 161.Abbas AK, Corson JM, Carpenter CB, et al. Immunologic enhancement of rat renal allografts. III. Immunopathologic lesions and rejection in long-surviving passively enhanced grafts. Am J Pathol. 1975;79:255–270. [PMC free article] [PubMed] [Google Scholar]
- 162.Churg A, Dai J, Zay K, et al. Alpha-1-antitrypsin and a broad spectrum metalloprotease inhibitor, RS113456, have similar acute anti-inflammatory effects. Lab Investig. 2001;81:1119–1131. doi: 10.1038/labinvest.3780324. [DOI] [PubMed] [Google Scholar]
- 163.Jie Z, Cai Y, Yang W, et al. Protective effects of alpha 1-antitrypsin on acute lung injury in rabbits induced by endotoxin. Chinese Med J. 2003;116:1678–1682. [PubMed] [Google Scholar]
- 164.Lewis EC, Shapiro L, Bowers OJ, et al. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci U S A. 2005;102:12153–12158. doi: 10.1073/pnas.0505579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Petrache I, Fijalkowska I, Zhen L, et al. A novel antiapoptotic role for alpha1-antitrypsin in the prevention of pulmonary emphysema. Am J Respir Crit Care Med. 2006;173:1222–1228. doi: 10.1164/rccm.200512-1842OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Petrache I, Hajjar J, Campos M. Safety and efficacy of alpha-1-antitrypsin augmentation therapy in the treatment of patients with alpha-1-antitrypsin deficiency. Biologics. 2009;3:193–204. doi: 10.2147/btt.2009.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Koulmanda M, Sampathkumar RS, Bhasin M, et al. Prevention of nonimmunologic loss of transplanted islets in monkeys. Am J Transplant. 2014;14:1543–1551. doi: 10.1111/ajt.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Antithrombotic Trialists’ Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kopp E, Ghosh S. Inhibition of NF-κB by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 170.Grilli M, Pizzi M, Memo M, Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-κB activation. Science. 1996;274:1383–1385. doi: 10.1126/science.274.5291.1383. [DOI] [PubMed] [Google Scholar]
- 171.Morris T, Stables M, Hobbs A, et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183:2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 172.Cinti A, De Giorgi M, Chisci E, et al. Simultaneous overexpression of functional human HO-1, E5NT and ENTPD1 protects murine fibroblasts against TNF-α-induced injury in vitro. PLoS One. 2015;10:e0141933. doi: 10.1371/journal.pone.0141933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Soares MP, Lin Y, Anrather J, et al. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4:1073–1077. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- 174.Petersen B, Lucas-Hahn A, Lemme E, et al. Generation and characterization of pigs transgenic for human hemeoxygenase-1 (hHO-1) Xenotransplantation. 2010;17:102–103. doi: 10.1111/j.1399-3089.2010.00573_7.x. [DOI] [Google Scholar]
- 175.Petersen B, Ramackers W, Lucas-Hahn A, et al. Transgenic expression of human heme oxygenase-1 in pigs confers resistance against xenograft rejection during ex vivo perfusion of porcine kidneys. Xenotransplantation. 2011;18:355–368. doi: 10.1111/j.1399-3089.2011.00674.x. [DOI] [PubMed] [Google Scholar]
- 176.Yeom HJ, Koo OJ, Yang J, et al. Generation and characterization of human hemeoxygenase-1 transgenic pigs. PLoS One. 2012;7:e46646. doi: 10.1371/journal.pone.0046646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Camara NO, Soares MP. Heme oxygenase-1 (HO-1), a protective gene that prevents chronic graft dysfunction. Free Radic Biol Med. 2005;38:426–435. doi: 10.1016/j.freeradbiomed.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 178.Yan JJ, Yeom HJ, Jeong JC, et al. Beneficial effects of the transgenic expression of human sTNFalphaR-fc and HO-1 on pig-to-mouse islet xenograft survival. Transpl Immunol. 2016;34:25–32. doi: 10.1016/j.trim.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 179.Oropeza M, Petersen B, Carnwath JW, et al. Transgenic expression of the human A20 gene in cloned pigs provides protection against apoptotic and inflammatory stimuli. Xenotransplantation. 2009;16:522–534. doi: 10.1111/j.1399-3089.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- 180.Boone DL, Turer EE, Lee EG, et al. The ubiquitin-modifying enzyme A20 is required for termination of toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 181.Hitotsumatsu O, Ahmad R-C, Tavares R, et al. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity. 2008;28:381–390. doi: 10.1016/j.immuni.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Conway EM. Thrombomodulin and its role in inflammation. Semin Immunopathol. 2012;34:107–125. doi: 10.1007/s00281-011-0282-8. [DOI] [PubMed] [Google Scholar]
- 183.Conway EM, Van de Wouwer M, Pollefeyt S, et al. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways. J Exp Med. 2002;196(5):565–577. doi: 10.1084/jem.20020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Esmon CT. Crosstalk between inflammation and thrombosis. Maturitas. 2004;47:305–314. doi: 10.1016/j.maturitas.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 185.Van de Wouwer M, Plaisance S, De Vriese A, et al. The lectin-like domain of thrombomodulin interferes with complement activation and protects against arthritis. J Thromb Haemost. 2006;4:1813–1824. doi: 10.1111/j.1538-7836.2006.02033.x. [DOI] [PubMed] [Google Scholar]
- 186.Ikezoe T. Thrombomodulin/activated protein C system in specific disseminated intravascular coagulation. J Intensive Care. 2015;3(1). 10.1186/s40560-014-0050-7. [DOI] [PMC free article] [PubMed]
- 187.Kim H, Hawthorne WJ, Kang HJ, et al. Human thrombomodulin regulates complement activation as well as the coagulation cascade in xeno-immune response. Xenotransplantation. 2015;22:260–272. doi: 10.1111/xen.12173. [DOI] [PubMed] [Google Scholar]
- 188.Lee KF, Lu B, Roussel JC, et al. Protective effects of transgenic human endothelial protein C receptor expression in murine models of transplantation. Am J Transplant. 2012;12:2363–2372. doi: 10.1111/j.1600-6143.2012.04122.x. [DOI] [PubMed] [Google Scholar]
- 189.Crikis S, Lu B, Murray-Segal LM, et al. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant. 2010;10:2586–2595. doi: 10.1111/j.1600-6143.2010.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dwyer KM, Mysore TB, Crikis S, et al. The transgenic expression of human CD39 on murine islets inhibits clotting of human blood. Transplantation. 2006;82:428–432. doi: 10.1097/01.tp.0000229023.38873.c0. [DOI] [PubMed] [Google Scholar]
- 191.Imai M, Takigami K, Guckelberger O, et al. Recombinant adenoviral mediated CD39 gene transfer prolongs cardiac xenograft survival. Transplantation. 2000;70:864–870. doi: 10.1097/00007890-200009270-00003. [DOI] [PubMed] [Google Scholar]
- 192.Van den boogaard FE, Brands X, Schultz MJ, et al. Recombinant human tissue factor pathway inhibitor exerts anticoagulant, anti-inflammatory and antimicrobial effects in murine pneumococcal pneumonia. J Thromb Haemost. 2011;9:122–132. doi: 10.1111/j.1538-7836.2010.04089.x. [DOI] [PubMed] [Google Scholar]
- 193.Chinetti-Gbaguidi G, Copin C, Derudas B, et al. Peroxisome proliferator-activated receptor γ induces the expression of tissue factor pathway inhibitor-1 (TFPI-1) in human macrophages. PPAR Res. 2016;2016:2756781. doi: 10.1155/2016/2756781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nguyen T, Sherratt PJ, Huang HC, et al. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J Biol Chem. 2003;278:4536–4541. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- 195.Yang H, Zhao P, Tian S, et al. Clopidogrel protects endothelium by hindering TNFalpha-induced VCAM-1 expression through CaMKKbeta/AMPK/Nrf2 pathway. J Diabetes Res. 2016;2016:9128050. doi: 10.1155/2016/9128050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Isermann B, Vinnikov I, Madhusudhan T, et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13:1349–1358. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 197.Girard TJ, Warren LA, Novotny WF, et al. Functional significance of the Kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature. 1989;338:518–520. doi: 10.1038/338518a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.