Abstract
Introduction. Limited data suggest that children with cancer in sub-Saharan Africa have poor survival. We aimed to describe the presentation, treatment outcomes, and factors associated with survival among children with cancer managed at Uganda Cancer Institute. Methods. We retrospectively evaluated patients with childhood cancer (age ≤19 years) from Kyadondo County treated at Uganda Cancer Institute from 2006 to 2009. Cox’s regression and Kaplan-Meier methods were used to study 1-year survival. Results. Among 310 patients studied, median age was 7 years (range = 0.25-19 years), 64% were boys, and 92% had histological confirmation of cancer diagnosis. The commonest diagnoses were Burkitt lymphoma (BL, N = 87), Kaposi sarcoma (KS, N = 68), non-BL non-Hodgkin lymphoma (NHL, N = 32), acute lymphoblastic leukemia (ALL, N = 28), Wilms (N = 28), and Hodgkin disease (HD, N = 20). Advanced disease at diagnosis was common for all cancers (ranging from 45% for KS to 83% for non-BL NHL). Overall, 33.2% abandoned treatment. One-year survival was 68% for HD (95% confidence interval [CI] = 11.3-40.6), 67% for KS (95% CI = 52.1-77.9), 55% for BL (95% CI = 42-66.9), 44% for Wilms (95% CI = 22.5-63), 43% for non-BL NHL (95% CI = 23.3-61.3), and 20% for ALL (95% CI = 6.4-38.7). In univariate and multivariate analysis, anemia and thrombocytopenia were associated with mortality for several cancers. Conclusion. Survival among children with cancer in Uganda is poor. Advanced stage disease and loss to follow-up likely contribute to poor outcomes. Anemia and thrombocytopenia may augment traditional staging methods to provide better prognostic factors in Uganda and warrant further evaluation.
Keywords: Uganda, children, follow-up, prognostic, treatment, Burkitt lymphoma, non-Hodgkin lymphoma, Hodgkin disease, leukemia, Wilms’ tumor, Kaposi sarcoma
Introduction
There are marked differences in the incidence and survival outcomes of childhood cancers in sub-Saharan Africa (sSA) compared with North America and Europe. First, the overall childhood cancer incidence is lower in sSA ranging between 139 per one million children (0-19 years) in Kyadondo County, Uganda, to 60 in Botswana, compared with 187 in the United States, 184 in Canada, and 233.9 in Naples, Italy.1 Second, infection-associated cancers like Burkitt lymphoma (BL) and Kaposi sarcoma (KS) predominate in sSA compared with acute lymphoblastic leukemia (ALL) and central nervous system tumors in North America.1,2 Third, limited data on KS, BL, and ALL show poor 1-year survival rates ranging from 25% to 75% in sSA compared with 5-year survival rates that exceed 80% for most childhood cancers in North America/Europe.3-7
However, data on presentation and outcomes of childhood cancer patients in sSA are lacking. To address this gap, we sought to describe the clinical presentation, treatment outcomes, and factors associated with survival of children with cancer from Kyadondo County in Uganda treated at Uganda Cancer Institute (UCI).
Methods
We included patients aged ≤19 years who were residents of Kyadondo County and diagnosed with cancer between 2006 and 2009; Kyadondo County includes Uganda’s Capital City, Kampala, and its neighboring suburbs. Standardized forms were used to abstract demographic, clinical, treatment, and follow-up information of patients from UCI medical records. Each patient received a detailed history and physical examination and completed investigations to stage their cancer and identify comorbidities. Histopathologic diagnosis was generally based on examination of hematoxylin and eosin–stained specimens; immunohistochemical staining was not routinely performed. Additional investigations performed typically included a complete blood count, HIV testing, chemistry, abdominal ultrasound, chest X-ray, bone marrow biopsy, lumbar puncture, computed tomography (CT) scan, bone scan, echocardiogram, and electrocardiogram as clinically indicated. Chemotherapy was administered at the UCI; surgery and radiotherapy were received from Mulago National Referral Hospital in Kampala, Uganda.
We categorized stage of cancer as localized (stages I and II) and advanced (stages III, IV, and V) based on the general staging criterion and disease-specific staging criteria. For staging, we used Ziegler staging for BL, AIDS Clinical Trials Group staging for KS, Ann Arbor staging for non-BL non-Hodgkin lymphoma (NHL) and Hodgkin disease (HD), and the SIOP staging for Wilms’ tumor.8-11 We stratified ALL into good risk and poor risk disease using age and white blood cell count at baseline.12 Tumor response to chemotherapy was assessed for solid tumors and lymphomas using the Response Evaluation Criteria in Solid Tumors (RECIST) and classified based on data available in the chart as complete (no tumor mass), partial (reduction in tumor mass by at least 30%), stable (no change), or progressive (increasing tumor mass by 25% or new disease).13 For leukemia, complete response was defined by evidence of ≤5% blast cells in the postinduction bone marrow biopsy on day 29; a finding of blasts >5% on postinduction bone marrow was classified as no response.14 Treatment abandonment was defined as failure to initiate or complete prescribed chemotherapy.15
Survival was calculated from the date of diagnosis to the date of death or last known date alive. A patient was considered alive at 1 year if he or she attended a clinic visit or vital status was confirmed at 1 year or more after the first visit using a scripted phone interview. If vital status at 1 year could not be determined, the patient was considered lost to follow-up and censored at the date of the last documented clinic visit.
Summary statistics were reported using percentages for categorical variables, and median and interquartile ranges (IQRs) for continuous variables. For each cancer type, Cox proportional hazards regression modeled the association between 1-year survival and baseline patient characteristics that included age, sex, fever, edema, HIV, CD4 percentage, antiretroviral therapy intake, hemoglobin, platelet count, lactate dehydrogenase (LDH), and stage. The multivariate model included covariates with a P < .1 from the univariate analysis, stage of cancer at baseline, and the number of cycles of chemotherapy received as a time-varying covariate. We used Kaplan-Meier curves to describe 1-year survival rates under 2 scenarios for patients lost to follow-up as either censored or dead at date of last follow-up. We used a statistical significance level of P < .05. All analyses were performed using STATA version 13 (STATA Corp, College Station, TX).
Makerere University School of Medicine Research and Ethics committee (REC REF 2011-153), Uganda National Council of Science and Technology (HS 995), and the Fred Hutchinson Cancer Research Center Institutional Review Board (IR file # 7545) approved the study. These committees waived the need for consent to collect, analyze, and publish retrospectively obtained and anonymized data for this observational study; they approved an oral script that was used to obtain verbal consent and updated vital status information from participants who had a telephone contact on record.
Results
UCI charts were identified for 316 participants, of which 6 were excluded because of conflicting histological diagnoses. Among the 310 patients, median age was 7 years (range = 0.25-19 years) and 36% were girls. The cancer diagnosis was confirmed by histology for 92% of patients, and the remainder were either clinically diagnosed or the histology reports were missing from the chart. The commonest diagnoses were KS (N = 68), BL (N = 87), non-BL NHL (N = 32), ALL (N = 28), Wilms (N = 28), and HD (N = 20; Figure 1).
Figure 1.
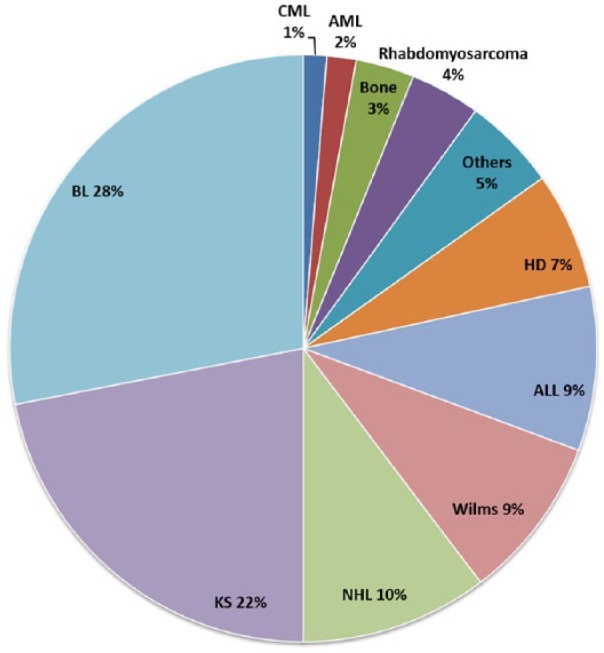
Distribution of childhood cancer diagnoses among patients from Kyadondo County at Uganda Cancer Institute (2006-2009).
AML, acute myeloid leukemia; CML, chronic myeloid leukemia; non-BL NHL, non-Burkitt lymphoma non-Hodgkin lymphoma. Others category included retinoblastoma (N = 5), germ cell tumors (N = 4), liver cancer (N = 1), fibrosarcomas (N = 2), and primitive neuroectodermal tumor (N = 1).
To ascertain the level of referral bias for our study, we compared the cases enumerated in the Kampala Cancer Registry (KCR) over the study period with our cohort. Two hundred and thirty-eight patients were identified both in KCR and UCI registry, 420 patients were identified from the KCR alone, and 78 patients were identified from the UCI alone. Compared with this cohort, patients in the KCR who did not access care at UCI were more likely to be female (35.5% vs 50.5%, P < .001), diagnosed in earlier years of the study (P = .02), and to have had a clinical diagnosis (8% vs 40%, P < .001; see Supplementary Table 4, available online).
Kaposi Sarcoma
Among the 68 KS cases, median age was 8 years (range = 0.25-11 years), 28% were girls and 99% had histological confirmation of KS (Table 1). At presentation, medium duration of symptoms was 12 weeks (IQR = 4-20). KS lesions involved the skin in 60%, lymph nodes in 53%, and viscera (oral mucosa and evidence of lung involvement on chest X-ray) in 25% of patients. The median hemoglobin level was 9 g/dL (IQR = 7.9-11). Fever was reported by 56% and 42% had edema. Seventy-five percent of patients had poor risk tumor (T1) and 70% had systemic symptoms (S1) based on ACTG staging. HIV antibody test was positive in 94%, negative in 3%, and unknown for 3% of patients. Among the 64 HIV-infected patients, the baseline median CD4 percentage was 11.5% (IQR = 6-17) and 44% were on antiretroviral therapy at the time of diagnosis.
Table 1.
Univariate and Multivariate Associations of 1-Year Mortality by Childhood Cancer Diagnosis at Uganda Cancer Institute.
| Characteristics | Summary Statistics | Kaposi Sarcoma (N = 68) |
Summary Statistics | Burkitt Lymphoma (N =
87) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||
| Age (years), median (range), per 1-year increase | 8 (0.25-17) | 1.01 (0.91-1.12) | .89 | 7 (0.33-18) | 1.06 (0.96-1.17) | .24 | ||||
| Female, n (%); vs males | 19 (27.9) | 2.07 (0.80-5.38) | .13 | 35 (40.2) | 0.63 (0.3-1.35) | .24 | ||||
| Histology, n (%) | 67 (98.5) | 68 (78.1) | ||||||||
| Fever, n (%); vs no fever | 38 (55.9) | 1.67 (0.63-4.46) | .3 | 44 (50.7) | 0.69 (0.33-1.43) | .32 | ||||
| Edema, n (%); vs no edema | 29 (42.7) | 1.63 (0.64-4.11) | .31 | 12 (13.7) | 5.52 (2.53-12.03) | <.001 | 5.51 (2.07-14.67) | .001 | ||
| Under weight for age, n (%) | 21 (45.7) | 2.2 (0.55-8.81) | .26 | 24 (33) | 1.38 (0.59-3.24) | .45 | ||||
| HIV positive, n (%); vs HIV negative | 64 (94.1) | 9 (10.3) | 1.01 (0.29-3.56) | .98 | ||||||
| ART experienced, n (%); vs naïve | 28 (43.8) | 0.48 (0.18-1.30) | .15 | |||||||
| CD4%, median (IQR), per 1% decreasea | 11.5 (6-17) | 1.18 (1.01-1.37) | .04 | |||||||
| Hb, median (IQR), per 1 g increase | 9.1 (7.9-11) | 0.71 (0.57-0.88) | .002 | 10.9 (9-12.1) | 0.88 (0.74-1.05) | .15 | ||||
| Hb <8 g/dL, n (%); vs ≥8g/dL | 17 (27.9) | 4.65 (1.78-12.19) | .002 | 3.12 (1.10-8.88) | .03 | 9 (11.4) | 4.29 (1.59-11.56) | .004 | 4.96 (1.67-14.72) | .004 |
| Plt <150 × 103/dL, n (%); vs ≥150 × 103/dL | 46 (75.41) | 3.02 (1.14-7.98) | .03 | 1.47 (0.50-4.28) | .48 | 8 (27.9) | 2.92 (1.11-7.74) | .03 | 1.87 (0.55-6.34) | .31 |
| Late stage, n (%); vs earlyb | 51 (75) | 1.48 (0.99-2.22) | .06 | 1.37 (0.91-2.07) | .13 | 60 (73.1) | 1.15 (0.51-2.59) | .7 | 0.75 (0.29-1.94) | .55 |
Abbreviations: HR, hazard ratio; CI, confidence interval; HIV, human immune deficiency virus; ART, antiretroviral therapy; CD4, cluster of differentiation 4; IQR, interquartile range; Hb, hemoglobin level; Plt, platelet count.
Not included in multivariate model because the limited number of subjects with CD4 percentage data.
Late-stage disease (stages III and IV, T1 for Kaposi sarcoma); early stage (stages III and IV); only AIDS Clinical Trials Group tumor criteria for Kaposi sarcoma staging used in model.
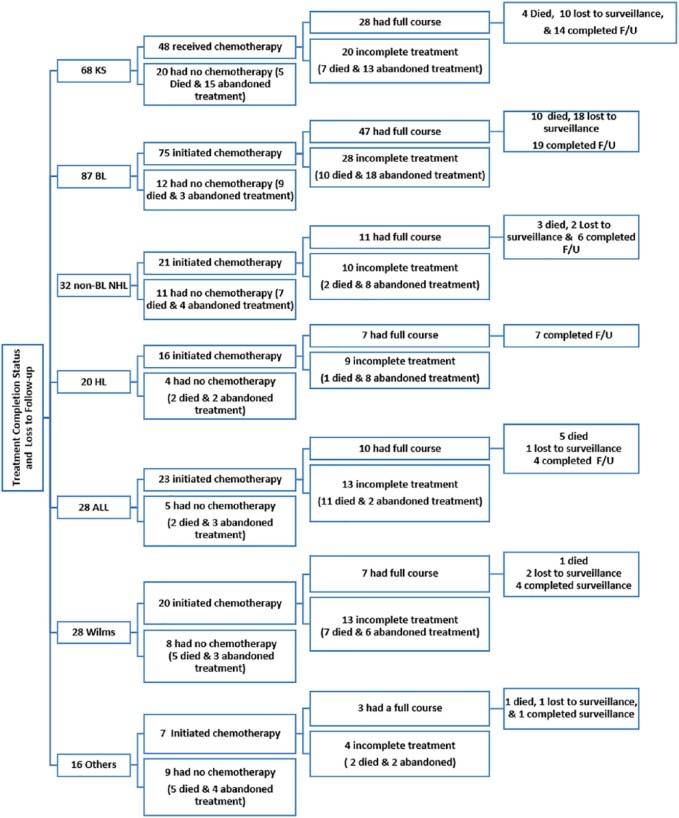
Seventy percent (48/68) of patients with KS received chemotherapy—30 patients received vincristine monotherapy weekly and 18 patients received a combination of vincristine and bleomycin given once every 3 weeks (Figure 2). Of the 48 patients treated, 42% did not complete treatment (7 patients died and 13 patients abandoned treatment). All HIV-infected patients received concurrent antiretroviral therapy with chemotherapy except one. Tumor response to chemotherapy was assessed in 24 patients: 33% had a complete response, 29% had a partial response, and 38% had a progressive or stable disease response.
Figure 2.
First-line chemotherapy receipt and completion status by cancer diagnosis.
BL, Burkitt lymphoma; KS, Kaposi sarcoma; non-BL NHL, non-BL non-Hodgkin lymphoma; ALL, acute lymphoblastic leukemia; HD, Hodgkin disease.
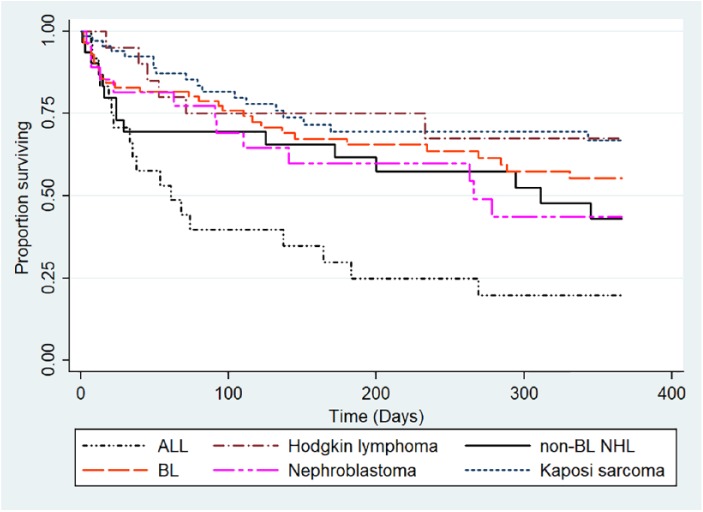
At the end of 1-year of follow-up, of the 68 patients with KS, 26% had died, 34% were alive, and for 40% vital status was unknown. The overall 1-year survival rate was 66.8% (95% confidence interval [CI] = 52.1-77.9) for KS patients (Figure 3); survival was significantly inferior when patients lost to follow-up were considered dead at date of last follow-up (supplementary Figure 4 available online). In multivariate analysis, patients with a hemoglobin level less than 8 g/dL at presentation had an increased risk of death compared with those with a hemoglobin level of 8 g/dL and higher (hazard ratio [HR] = 3.12, 95% CI = 1.10-8.88, P = .03; Table 1). Platelet count (P = .48), KS tumor stage (P = .13), and number of first-line chemotherapy cycles received (P = .18) were not associated with mortality.
Figure 3.
Kaplan-Meier estimation of 1-year survival by cancer diagnosis (patients lost to follow-up censored at date of last follow-up).
BL, Burkitt lymphoma; KS, Kaposi sarcoma; non-BL NHL, non-BL non-Hodgkin lymphoma; ALL, acute lymphoblastic leukemia; HD, Hodgkin disease.
Burkitt Lymphoma
Among the 87 patients with BL, the median age at diagnosis was 7 years (range = 0.33-18 years), 40% were girls, and 78% had histological confirmation of BL. At baseline, the median duration of symptoms was 6 weeks (IQR = 3-8). BL tumor involved the abdomen in 60% of patients, the jaw in 54%, and the gonads in 4%. Fifty percent reported fever and 10% had a positive HIV antibody test. Among the 82 BL patients who were staged, 20% had stage A, 7% stage B, 35% stage C, and 38% stage D; median hemoglobin level was 10.9 g/dL (IQR = 9-12.1).
Chemotherapy, consisting of a combination of cyclophosphamide, vincristine, and methotrexate (COM) given every 2 weeks, was administered to 86% (75/87) of patients with BL (Figure 2). Of the 75 patients treated, 37% did not receive the full course of COM (18 abandoned treatment and 10 died). Tumor response assessment after chemotherapy was available for 36 patients—39% had a complete response, 19% had a partial response, and 42% had progressive disease.
Of the 87 BL cases, 34% died, 28% were alive, and vital status was unknown for 48% at the end of 1-year of follow-up. The overall 1-year survival rate was 55.4% (95% CI = 42.0-66.9; Figure 3). In multivariate analysis, patients with a hemoglobin level <8 g/dL had an increased risk of death compared with those with a hemoglobin level of ≥8 g/dL (HR = 4.96, 95% CI = 1.67-14.72, P = .004). Similarly, patients with edema had an increased risk death compared with those without edema (HR = 5.51, 95% CI = 2.07-14.67, P = .001). Platelet count (P = .31), stage (P = .55), and number of first-line chemotherapy cycles received (P = .18) were not associated with survival (Table 1).
Non-BL Non-HL
Among the 32 patients with NHL, the median age at diagnosis was 8 years (range = 0.33-18 years); 32% were girls and all had histological confirmation of their NHL diagnosis. Patients had symptoms for a median duration of 12 weeks (IQR = 4-22) at presentation; 81% reported B symptoms. Median hemoglobin level was 8.4 g/dL (IQR = 7-10.3); LDH was normal in 16%, elevated in 50%, and unknown in 34%. Of the 32 NHL patients, 9% had stage 1, 9% had stage 2, 34% had stage 3, and 47% had stage 4 disease.
A combination of cyclophosphamide, vincristine, adriamycin, and prednisolone (CHOP) was administered to 66% (21/32) of patients with NHL (Figure 2). Of these, 11 received the full-prescribed course of CHOP, 2 died during treatment, and 8 were lost to follow-up. Of the 8 patients assessed for tumor response after completing chemotherapy, 2 had a complete response, 2 had a partial response, and 4 had progressive disease.
At the end of 1-year of follow-up, of the 32 patients with NHL, 47% had died, 28% were alive, and for 25% vital status was unknown. The overall 1-year survival rate was 43% (95% CI = 23.3-61.3) for NHL cases (Figure 3). In multivariate analysis, the risk of death was significantly increased among female patients compared with male patients (HR = 31.66, 95% CI = 1.32-759.33, P = .03). Self-report of fever (P = .2), platelet count (P = .32), stage (P = .83), and number of first-line chemotherapy cycles received (P = .16) were not associated with mortality (Table 2).
Table 2.
Univariate and Multivariate Associations of 1-Year Mortality by Childhood Cancer Diagnosis at Uganda Cancer Institute.
| Characteristics | Summary Statistics | Non-BL NHL (N = 32) |
Summary Statistics | Hodgkin Disease (N =
20) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||
| Age (years), median (range), per 1-year increase | 8 (1-19) | 0.95 (0.85-1.06) | .34 | 11 (7-14) | 1.05 (0.87-1.27) | .6 | ||||
| Females, n (%); vs males | 10 (31.3) | 2.46 (0.87-7.00) | .09 | 31.66 (1.32-759.33) | .03 | 5 (25) | 2.02 (0.36-11.23) | .42 | ||
| Histology, n (%) | 32 (100) | 20 (100) | ||||||||
| Fever, n (%); vs no fever | 24 (75.0) | 10.36 (1.34-80.17) | .03 | 9.68 (0.31-298.90) | .2 | 11 (55.0) | 1.82 (0.33-9.99) | .49 | ||
| Edema, n (%); vs no edema | 5 (15.6) | 3.12 (0.61-15.99) | .17 | 1 (5) | 5.82 (0.6-55.96) | .13 | ||||
| Under weight for age, n (%) | 4 (23.5) | 3 (18.7) | 4.02 (0.65-24.82) | .13 | ||||||
| HIV positive, n (%); vs HIV negative | 7 (21.8) | 2.04 (0.43-9.64) | .37 | 1 (5.0) | ||||||
| Hb, median (IQR), per 1 g increase | 8.4 (7-10.3) | 0.82 (0.65-1.04) | .1 | 9 (5.6-10.5) | 0.59 (0.36-0.95) | .03 | ||||
| Hb <8 g/dL, n(%); vs ≥8 g/dL | 14 (46.7) | 1.94 (0.63-5.96) | .25 | 8 (40) | 9.38 (1.09-80.66) | .04 | 6.22 (0.68-56.82) | .11 | ||
| Plt <150 × 103/dL, n (%); vs ≥150 × 103/dL | 20 (69.0) | 2.68 (0.87-8.23) | .09 | 4.61 (0.22-94.98) | .32 | 15 (75) | 11.68 (1.89-72.03) | .01 | 6.82 (1.07-43.41) | .04 |
| Late stage, n (%); late vs earlya | 26 (81) | 3.62 (0.48-27.61) | .2 | 1.33 (0.1-17.77) | .83 | 10 (50.0) | 1.21 (0.24-6.0) | .82 | ||
Abbreviations: non-BL NHL, non-Burkitt lymphoma non-Hodgkin lymphoma; HR, hazard ratio; CI, confidence interval; HIV, Human immune deficiency virus; IQR, interquartile range; Hb, hemoglobin level; Plt, platelet count.
Late-stage disease (stages III and IV); early-stage disease (stages III and IV).
Hodgkin Disease
Among 20 patients with HD, the median age at diagnosis was 11 years (range = 1.25-7 years), 75% were boys and all had a histological confirmation of HD (Table 2). Patients had symptoms for a median duration of 26 weeks (IQR = 6-78) at presentation; 55% reported at least one B-symptoms. The median hemoglobin level was 9 g/dL (IQR = 5.6-10.5) and the median platelet count was 297 000 platelets/dL (IQR = 195 000-431 000);
LDH was normal in 20%, elevated in 35%, and unknown in 45%. Of the 20 HL patients, 25% had stage 1, 30% had stage 2, 30% had stage 3, and 15% had stage 4 disease.
Among the 16 patients who were administered chemotherapy (a combination of adriamycin, bleomycin, vincristine, and dacarbazine), 7 completed treatment, 1 patient died during treatment, 8 were lost to follow-up (Figure 2). Of the 7 patients assessed for tumor response to chemotherapy, 4 had a complete response, 2 had a partial response, and 1 had progressive disease.
At end of 1-year of follow-up, of the 20 patients with HD, 6 had died, 10 were alive, and vital status was unknown for 4 patients. The overall 1-year survival rate was 67.5% (95% CI = 11.3-40.6) for HD cases (Figure 3). In multivariate analysis, the risk of death was significantly increased in participants with a platelet count <150 platelets/dL compared with those with a count ≥150 platelets/dL (HR = 6.82, 95% CI = 1.07-43.41, P = .04). Hemoglobin level (P = .11) was not associated with mortality (Table 2).
Leukemia
Thirty-seven patients had leukemia: 28 had acute lymphoblastic leukemia (ALL), 5 had acute myeloid leukemia, and 4 had chronic myeloid leukemia. Among patients with ALL, median age was 9 years (range = 1.5-19 years) and 43% were female (Table 3).
Table 3.
Univariate and Multivariate Associations of 1-Year Mortality by Childhood Cancer Diagnosis at Uganda Cancer Institute.
| Characteristics | Summary Statistics | Acute Lymphoblastic Lymphoma(N =
28) |
Summary Statistics | Wilms Tumor (N = 28) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||
| Age (years), median (range), per 1-year increase | 10 (5-14) | 1.05 (0.97-1.15) | .24 | 2.4 (1.5-3.5) | 1.12 (0.95-1.31) | .18 | ||||
| Females, n (%); vs males | 16 (57.1) | 0.77 (0.27-2.18) | .62 | 14 (50) | 1.09 (0.36-3.30) | .88 | ||||
| Histology, n (%) | 26 (98.3) | 26 (92.9) | ||||||||
| Fever, n(%); vs no fever | 26 (92.9) | 1.97 (0.26-14.93) | .51 | 19 (67.7) | 3.22 (0.71-14.57) | .13 | ||||
| Edema, n (%); vs no edema | 3 (10.7) | 3.45 (0.71-16.74) | .12 | 2 (7.1) | 0.75 (0.1-5.81) | .79 | ||||
| Under weight for age, n (%) | 7 (33.3) | 0.43 (0.15-1.28) | .13 | 7 (31.8) | 1.67 (0.4-6.93) | .48 | ||||
| HIV positive, n (%); vs HIV negative | 1 (3.6) | 0 (0) | ||||||||
| Hb, median (IQR), per 1 g increase | 6.5 (4.9-9.1) | 1.19 (0.97-1.47) | .09 | 1.47 (1.00-2.16) | .05 | 9.8 (7.6-11.5) | 0.87 (0.7-1.1) | .25 | ||
| Hb <8 g/dL, n (%); vs ≥8 g/dL | 17 (63) | 3.14 (1.02-9.67) | .05 | 7 (29) | 2.26 (0.72-7.14) | .17 | ||||
| Plt <150 × 103/dL, n (%); vs ≥150 × 103/dL | 18 (72.0) | 0.52 (0.20-1.38) | .19 | 2 (8.7) | 1.85 (0.23-15.23) | .57 | ||||
| Late stage, n (%); late vs earlya | 4 (14.3) | 1.21 (0.35-4.21) | .76 | 0.33 (0.05-2.07) | .24 | 15 (53.8) | 1.32 (0.17-10.23) | .78 | ||
Abbreviations: HR, hazard ratio; CI, confidence interval; HIV, Human immune deficiency virus; hb, hemoglobin level; IQR, interquartile range; Plt, platelet count.
Late-stage disease (stages III and IV); early stage (stages III and IV).
Patients with acute lymphoblastic leukemia had symptoms for a median duration of 4 weeks (IQR = 6-12) at presentation; 96% reported B-symptoms, 36% reported bone pain, and 54% had bled. Median hemoglobin level was 6.5 g/dL (IQR = 4.9-9.1), and median platelet count of 21 000 platelets/dL (IQR = 15 000-263 000). Seventy-nine percent of patients had high-risk ALL based on age and white blood cell count count.
Chemotherapy was administered to 83% (23/28) of patients with ALL, of whom 11 completed induction chemotherapy. Only 4 patients had morphological complete remission after induction (Figure 2). At the end of 1-year follow-up, of the 28 patients with ALL, 14% were alive, 68% had died, and for 18% vital status was unknown. The overall 1-year survival rate was 19.9% (95% CI = 6.4-38.7) for ALL cases (Figure 3). In multivariate analysis, the risk of death was inversely associated with hemoglobin level (HR = 1.47 per 1 g/dL decrease, 95% CI = 1.00-2.16, P = .05). Platelet count (P = .24), and the number of cycles of chemotherapy received (P = .14) were not associated with mortality.
Wilms Tumor
Among 28 patients with Wilms, median age was 2.4 years (range = 1.5-3.5 years), 50% were male, and 93% were confirmed by histology (Table 3). Patients had symptoms for a median duration of 8 weeks (IQR = 3-12) at presentation; 71% reported at least one B-symptom. Of the 28 Wilms patients, 14% had stage 1, 46% had stage 3, 36% had stage 4, and 4% had stage 5 disease. Median hemoglobin level was 9.8 g/dL (7.6-11.5).
Of the 28 patients, 11% had surgery alone, 36% had surgery and adjuvant chemotherapy, 36% had chemotherapy alone, and 17% had palliative care. Among the 20 patients who received chemotherapy (cyclophosphamide, vincristine, and dactinomycin/doxorubicin), 65% did not complete treatment (4 died during treatment and 9 were lost to follow-up; Figure 2). Of the 7 patients who received a full course of chemotherapy, only one patient had a complete response, one had a partial response, and 5 had progressive disease. At the end of 1-year of follow-up, of the 28 patients with tumor, 46% had died, 32% were alive, and the remaining patients were lost to follow-up. The overall 1-year survival rate was 43.6% (95% CI = 22.5-63.0) for patients with Wilms tumor (Figure 3). No factors reached statistical criteria for survival in multivariate modeling (Table 3).
Discussion
Cancer is increasingly been recognized as a threat to the health of persons in sub-Saharan Africa. As the accuracy and coverage of cancer registries in the region grows, we have gained valuable insights into adult malignancies. However, relatively little attention has been paid to trends and correlates of pediatric malignancies.
Using data from a well-established major cancer referral center in Uganda, we found the commonest cancer diagnoses presenting for care at the Uganda Cancer Institute were BL, KS, non-BL NHL, Wilms, ALL, and HD. Advanced-stage disease at presentation and treatment abandonment were common. Survival was poor for all cancers assessed. Poor survival was associated with low hemoglobin for KS and BL, and low platelets for BL, non-BL NHL, and HD. Female gender and fever were associated with poor survival for non-BL NHL.
Our finding that anemia and lower platelet count were associated with poor outcomes in several cancers is striking. Anemia is common among patients with childhood cancer, but the degree and prevalence of anemia vary by cancer type, and its role in mortality may be modified by the intensity of treatment.16,17 Experience from Europe/North America suggests that for various cancers, patients with anemia have poorer tumor control, quality of life, and survival but experience no excess toxicities compared with their counterparts without anemia.18 Differences in the etiologies, prevalence, treatment, and supportive care available to patients in higher-income countries compared with sSA motivate further studies in this area. There is a large unmet need for treatment of anemia among patients with cancer in sSA because of the scarcity of blood products, lack of access to erythropoietin analogues, and absence of locally relevant treatment guidelines in cancer patients.19 In addition, the anemia observed in our cohort may also be caused by co-morbid conditions that are prevalent in sSA, such as malnutrition, chronic inflammation, and worm infestation, and management of these conditions is an important component of overall cancer care.20
The association of a lower platelet count with morbidity suggests that patients with low cell counts were more prone to severe bone marrow suppression as a complication of chemotherapy and probably subsequent fatal infections compared with their counterparts with higher counts.21 Treatment options for chemotherapy-associated thrombocytopenia are limited and depend on the chemotherapy treatment goals and the bleeding risk of the patient.22 Chemotherapy dose reductions, treating underlying causes such as infection, and platelet transfusions can adequately be implemented in the sSA setting but may be limited by loss to follow-up and non-adherence to the treatment schedule.
The poor survival we observed in our cohort was like findings elsewhere in sSA but significantly inferior to outcomes observed in Europe and North America.16,23-25 The unexpected lack of association of stage with mortality is likely due to lack of enough cases with localized disease to detect a difference. In addition, the lower intensity treatment regimens used in Uganda may fail to eradicate cancer cells, resulting in disease progression even in cases with localized disease. The rates of advanced disease we observed are consistent with findings in other African countries such as South Africa, Malawi, and Zambia.26-28 Similarly, treatment abandonment contributes significantly to treatment failure in low-income countries.24,27,29 Emerging evidence suggests that addressing hypothesized gaps in care through payment for transport costs, educating and counseling parents, and ensuring availability of diagnostics and medicines reduces loss to follow-up significantly but with only modest gains in survival.30 This suggests that modifications to the treatment regimens are needed in addition to ongoing efforts to improve patient adherence and follow-up.
The distribution of childhood cancers in our cohort is consistent with findings of the population-based KCR in Uganda,1,31 but differs with findings of hospital-based cohorts from elsewhere in sSA.26,27,32,33 BL and KS accounted for about 50% of the patients in our cohort, which reflects the high incidence of these cancers in Uganda. The distribution of childhood cancers in our cohort at UCI differed from findings in a cohort of children with cancer seen at University Teaching Hospital in Malawi, in which retinoblastoma was more common than KS, and ALL was virtually absent.26 These differences could be due to referral bias, differences in cancer care structure, or a variation in the incidence childhood cancers across sSA. For example, the incidence of leukemia in Uganda is 10.3 per million children compared with 1.1 per 1 million in Malawi and 22.8 per 1 million in Zimbabwe.1 This difference in cancer incidence could reflect reporting bias or geographical differences in cancer risk factors such as malaria, HIV, and HHV-8, and interventions such as antiretroviral treatment and vaccinations.
Our study is limited by several factors: (1) Ascertainment bias because histology review with advanced IHC staining to verify cancer diagnosis was not done and therefore some cases, particularly lymphomas, may have been mis-diagnosed. (2) Referral bias resulting in the possible exclusion of the sicker patients would have biased our estimates and inflated the survival estimates. However, we attempted to mitigate referral bias by studying patients from Kyadondo County who are near the UCI. (3) The high burden of treatment abandonment made it difficult to assess treatment efficacy adequately. (4) We lacked information on competing causes of death and therefore could not ascertain the cause-specific mortality.
Despite these limitations, our study provides one of the first detailed descriptions of the clinical characteristic, treatment, and survival outcomes of patients with childhood cancer seen at a specialized cancer treatment center in sSA. Anemia and thrombocytopenia were associated with mortality for several cancers and may provide additional prognostic information in addition to cancer stage in Uganda and warrant further evaluation to augment existing staging criteria. We have also shown that presentation with advanced disease and treatment abandonment are common at the UCI and likely contribute to poor patient outcomes. Efforts to enhance early diagnose, retention of patients in care, and provision of higher intensity treatment regimens may improve overall survival rates among children with cancer in sSA.
Supplemental Material
Supplemental material, Figure_4_Graph_survival_black_22MAR18_Dead for Presentation and Outcomes of Childhood Cancer Patients at Uganda Cancer Institute by Innocent Mutyaba, Henry R. Wabinga, Jackson Orem, Corey Casper and Warren Phipps in Global Pediatric Health
Supplemental Material
Supplemental material, Supplementary_Table_4 for Presentation and Outcomes of Childhood Cancer Patients at Uganda Cancer Institute by Innocent Mutyaba, Henry R. Wabinga, Jackson Orem, Corey Casper and Warren Phipps in Global Pediatric Health
Footnotes
Author Contributions: All authors made substantial contributions to the research and manuscript writing. MI, CC, and WP designed the study; MI, JO, WH, and CC were supported data acquistion; IM analysed the data; IM, CC, and WP interpreted the results; IM and WP drafted the manuscript; and MI, WH, JO, CC, and WP critically reviewed the manuscript. The authors acknowledge Irene Nassozi, the Staff of Kampala Cancer Registry and Uganda Cancer Institute for supporting data acquistion, Elizabeth Krantz for advising on the statistical approach, and Rhoda Morrow and Anna Wald for reviewing the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors are grateful to The National Cancer Institute Center for Global Health Beginning Investigator Grant for Catalytic Research (BIGCAT 2010) and African Organization for Research and Training in Cancer, and The AIDS International Training and Research Program Project on HIV-assocaited malignancies at the University of Washington and Fred Hutchinson Cancer Research Center (NIH Grant D43 CA153720) for funding the research.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs: Innocent Mutyaba  https://orcid.org/0000-0001-5548-1209
https://orcid.org/0000-0001-5548-1209
Corey Casper  https://orcid.org/0000-0002-3609-661X
https://orcid.org/0000-0002-3609-661X
References
- 1. Steliarova-Foucher E, Colombet M, Ries LAG, et al. ; IICC-3 Contributors.International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol. 2017;18:719-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stefan DC. Patterns of distribution of childhood cancer in Africa. J Trop Pediatr. 2015;61:165-173. [DOI] [PubMed] [Google Scholar]
- 3. Cox CM, El-Mallawany NK, Kabue M, et al. Clinical characteristics and outcomes of HIV-infected children diagnosed with Kaposi sarcoma in Malawi and Botswana. Pediatr Blood Cancer. 2013;60:1274-1280. [DOI] [PubMed] [Google Scholar]
- 4. Chagaluka G, Carey P, Banda K, et al. Treating childhood acute lymphoblastic leukemia in Malawi. Haematologica. 2013;98:e1-e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stanley CC, Westmoreland KD, Heimlich BJ, et al. Outcomes for paediatric Burkitt lymphoma treated with anthracycline-based therapy in Malawi. Br J Haematol. 2016;173:705-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Israels T, Borgstein E, Pidini D, et al. Management of children with a Wilms tumour in Malawi, Sub Saharan Africa. Tijdschr Kindergeneeskunde. 2013;81(suppl 1):57. [DOI] [PubMed] [Google Scholar]
- 7. Orem J, Maganda A, Mbidde EK, Weiderpass E. Clinical characteristics and outcome of children with Burkitt lymphoma in Uganda according to HIV infection. Pediatr Blood Cancer. 2009;52:455-458. [DOI] [PubMed] [Google Scholar]
- 8. Ziegler JL, Bluming AZ, Morrow RH, Fass L, Carbone PP. Central nervous system involvement in Burkitt’s lymphoma. Blood. 1970;36:718-728. [PubMed] [Google Scholar]
- 9. D’Angio GJ. Pre- or post-operative treatment for Wilms tumor? Who, what, when, where, how, why, and which. Med Pediatr Oncol. 2003;41:545-549. [DOI] [PubMed] [Google Scholar]
- 10. Rosenberg S. Validity of the Ann Arbor staging classification for the non-Hodgkin’s lymphomas. Cancer Treat Rep. 1977;61:1023-1027. [PubMed] [Google Scholar]
- 11. Krown SE, Testa MA, Huang J. AIDS-related Kaposi’s sarcoma: prospective validation of the AIDS Clinical Trials Group staging classification. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol. 1997;15:3085-3092. [DOI] [PubMed] [Google Scholar]
- 12. Rabin KR, Poplack DG. Management strategies in acute lymphoblastic leukemia. Oncology (Williston Park). 2011;25:328-328. [PubMed] [Google Scholar]
- 13. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205-216. [DOI] [PubMed] [Google Scholar]
- 14. Pui CH, Campana D, Evans WE. Childhood acute lymphoblastic leukaemia—current status and future perspectives. Lancet Oncol. 2001;2:597-607. [DOI] [PubMed] [Google Scholar]
- 15. Weaver MS, Arora RS, Howard SC, et al. A practical approach to reporting treatment abandonment in pediatric chronic conditions. Pediatr Blood Cancer. 2015;62:565-570. [DOI] [PubMed] [Google Scholar]
- 16. Israels T, Borgstein E, Pidini D, et al. Management of children with a Wilms tumor in Malawi, sub-Saharan Africa. J Pediatr Hematol Oncol. 2012;34:606-610. [DOI] [PubMed] [Google Scholar]
- 17. Michon J. Incidence of anemia in pediatric cancer patients in Europe: results of a large, international survey. Med Pediatr Oncol. 2002;39:448-450. [DOI] [PubMed] [Google Scholar]
- 18. Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116:11-26. [DOI] [PubMed] [Google Scholar]
- 19. Butler EK, Hume H, Birungi I, et al. Blood utilization at a national referral hospital in sub-Saharan Africa. Transfusion. 2015;55:1058-1066. [DOI] [PubMed] [Google Scholar]
- 20. Israëls T, van de Wetering MD, Hesseling P, van Geloven N, Caron HN, Molyneux EM. Malnutrition and neutropenia in children treated for Burkitt lymphoma in Malawi. Pediatr Blood Cancer. 2009;53:47-52. [DOI] [PubMed] [Google Scholar]
- 21. Tamamyan G, Danielyan S, Lambert MP. Chemotherapy induced thrombocytopenia in pediatric oncology. Crit Rev Oncol Hematol. 2016;99:299-307. [DOI] [PubMed] [Google Scholar]
- 22. Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncology. 2015;29:282-282. [PubMed] [Google Scholar]
- 23. Stones DK, Hadley G, Wainwright R, Stefan DC. The impact of ethnicity on Wilms tumor: characteristics and outcome of a South African cohort. Int J Pediatr. 2015;706058. doi: 10.1155/2015/706058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paintsil V, David H, Kambugu J, et al. The Collaborative Wilms Tumour Africa Project; baseline evaluation of Wilms tumour treatment and outcome in eight institutes in sub-Saharan Africa. Eur J Cancer. 2015;51:84-91. [DOI] [PubMed] [Google Scholar]
- 25. Hesseling P, Broadhead R, Mansvelt E, et al. The 2000 Burkitt lymphoma trial in Malawi. Pediatr Blood Cancer. 2005;44:245-250. [DOI] [PubMed] [Google Scholar]
- 26. Slone JS, Chunda-Liyoka C, Perez M, et al. Pediatric malignancies, treatment outcomes and abandonment of pediatric cancer treatment in Zambia. PLoS One. 2014;9:e89102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arora RS, Eden T, Pizer B. The problem of treatment abandonment in children from developing countries with cancer. Pediatr Blood Cancer. 2007;49:941-946. [DOI] [PubMed] [Google Scholar]
- 28. De Boer J, Boellaard T, Parkinson S, Blanchard E, Heij H. Patient compliance in the treatment of Burkitt’s lymphoma in rural Zambia: a retrospective study on 80 Burkitt’s lymphoma patients in Katete, Zambia. Afr J Paediatr Surg. 2009;6:3-6. [DOI] [PubMed] [Google Scholar]
- 29. Njuguna F, Mostert S, Slot A, et al. Abandonment of childhood cancer treatment in Western Kenya. Arch Dis Child. 2014;99:609-614. [DOI] [PubMed] [Google Scholar]
- 30. McGoldrick SM, Omoding A, Gerdts SE, et al. Impact of a quality improvement project on pediatric endemic Burkitt lymphoma outcomes in Uganda. Am Soc Hematol. 2016;128:156. [Google Scholar]
- 31. Wabinga HR, Nambooze S, Amulen PM, Okello C, Mbus L, Parkin DM. Trends in the incidence of cancer in Kampala, Uganda, 1991-2010. Int J Cancer. 2014;135:432-439. [DOI] [PubMed] [Google Scholar]
- 32. Sinfield R, Molyneux E, Banda K, et al. Spectrum and presentation of pediatric malignancies in the HIV era: Experience from Blantyre, Malawi, 1998-2003. Pediatr Blood Cancer. 2007;48(5):515-520. [DOI] [PubMed] [Google Scholar]
- 33. Harif M, Barsaoui S, Benchekroun S, et al. Treatment of B-cell Lymphoma with LMB Modified protocols in Africa - report of the French-African Pediatric Oncology Group (GFAOP). Pediatr Blood Cancer. 2008;50(6): 1138-1142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Figure_4_Graph_survival_black_22MAR18_Dead for Presentation and Outcomes of Childhood Cancer Patients at Uganda Cancer Institute by Innocent Mutyaba, Henry R. Wabinga, Jackson Orem, Corey Casper and Warren Phipps in Global Pediatric Health
Supplemental material, Supplementary_Table_4 for Presentation and Outcomes of Childhood Cancer Patients at Uganda Cancer Institute by Innocent Mutyaba, Henry R. Wabinga, Jackson Orem, Corey Casper and Warren Phipps in Global Pediatric Health