Abstract
Objectives: The present study takes a culture-centered approach to better understand how the experiences of culture affect patient’s perception of type 2 diabetes mellitus (T2DM). This study explores personal models of T2DM and compares personal models across regional and race/ethnicity differences. Methods: In a practice-based research network, a cross-sectional survey was distributed to patients diagnosed with T2DM at medical centers in Nevada and Georgia. In analyses of covariance, controlling for age, health literacy, and patient activation, geographic location, and race/ethnicity were tested onto 5 dimensions of illness representation. Results: Among 685 patients, race/ethnicity was significantly associated with lower reported understanding diabetes (P < .01) and less perceived longevity of diabetes (P < .001). Geographic location was significantly associated with seriousness of the disease (P < .005) and impact of diabetes (P < .001). Conclusion: Non-Hispanic White Americans report greater understanding and perceive a longer disease course than non-Hispanic Black Americans and Asian Americans. Regionally, patients in Nevada perceive T2DM as more serious and having more impact on their lives than patients living in Georgia. Primary care physicians should elicit patient perceptions of diabetes within the context of the patient’s ethnic and geographic culture group to improve discussions about diabetes self-management. Specifically, primary care physicians should address the seriousness of a diabetes diagnosis and the chronic nature of the disease with patients who belong to communities with a higher prevalence of the disease.
Keywords: diabetes, cultural differences, psychosocial, race and ethnicity
Introduction
The growing prevalence of type 2 diabetes mellitus (T2DM) in the United States, at an estimated 30.3 million Americans or 9.4% of the US population,1 has likely affected the public’s perceived susceptibility to the disease. As the prevalence of a disease increases and the disease becomes common (socially normative), individual perceptions of severity can decrease. The Health Belief Model2 posits that individuals’ perceived susceptibility to a disease, and resulting complications, together with their perceptions of severity of a disease combine to determine their likelihood of enacting self-management behaviors. However, the potentially inverse relationship of susceptibility and severity may challenge how health communicators motivate behavior change among patients diagnosed with T2DM.
An explanatory model of disease shared across group members can function as a cultural model of the illness.3 When exploring illness representation among cultural groups, the explanatory model shared by patients diagnosed with diabetes is more similar to the general community’s explanatory model than to physicians’ explanatory model of the illness.3 In populations that experience disease at a higher rate, the cultural model of an illness can propagate narratives of that disease and alter social norms so that cultural members develop an expectation of illness rather than an expectation of health. For example, among South Asian patients living with diabetes, one cultural model of illness interprets the genetic nature of the disease as a patient’s “fate” or being ordained by God.4
Two specific dimensions of culture have been shown to play a role in the disparities in diabetes: race/ethnicity and geography. First, rates of diagnosed diabetes are approximately 7% for White Americans, 12% for Black Americans, and 14% for Mexican Americans and Puerto Ricans.5 Among patients carrying the diagnosis of diabetes, glycemic control tends to be worse among these same minority groups putting them at greater risk of complications and death from diabetes.6 In one study of African Americans’ experience with diabetes, participants described the diagnosis as expected because of the high prevalence of disease among their family and friends.7 Another study within the African American community demonstrated a connection between disease severity and label of the disease: patients who believed they had “sugar” (as opposed to “diabetes”) believed their condition was less serious.8 These findings point to a culturally bound perception of diabetes.
A second factor of culture that is linked to T2DM is geography. Geographically, diabetes is significantly increased in both White and Black Americans in the Stroke Buckle (North Carolina, South Carolina, and Georgia) as compared with residents in the remainder of the United States.9 This is likely a function of cultural factors linked to geography such as diet and physical activity social norms. Both race and region have independent and interaction effects on nutrient intake.10 These relationships led the Centers for Disease Control and Prevention (CDC) to identify a geographic region of the United States as the Diabetes Belt.11 This region has a higher prevalence of the disease, regardless of age, ethnicity, and obesity. The authors of the CDC study hypothesized that this association may be related to social, cultural, and genetic factors within the region.
The present study explores patients’ personal models of the T2DM diagnosis and compares those personal models of disease across geographic and race/ethnicity differences.
Methods
Two medical centers were selected in different US geographical regions: Augusta, Georgia, and Las Vegas, Nevada. Both of these sites meet the US Census definition of an urbanized area.12 These 2 geographic locations were selected to compare patient perceptions from within the Diabetes Belt to those outside the region. Inside the Diabetes Belt, Augusta is a Southern US city in Richmond County, Georgia, within which the CDC estimates that 13.5% of adults have diagnosed diabetes.13 Outside the Diabetes Belt, Las Vegas is a Western US city in Clark County, Nevada, within which the CDC estimates that 9.2% of adults have diagnosed diabetes.13 This study was approved by the first author’s institutional review board.
In September 2016, research staff mailed surveys to patients who met the inclusion criteria: age 25 to 64 years with a diagnosis of T2DM (HbA1c ≥ 6.5%). Patients were identified through the electronic medical record database by age and by the diagnostic coding as recorded by clinicians. Exclusion criteria included diagnosis of end-stage renal disease, diagnosis of schizophrenia, or currently receiving treatment for cancer.
The survey included demographics (age, gender, race/ethnicity) and measures of health literacy, patient activation, and personal model of disease. To capture the patient’s personal model of disease, the primary measures were the 5 dimensions of the Diabetes Illness Representations Questionnaire (DIRQ).14 The instrument uses Likert-style statements to evaluate understanding of diabetes (illness coherence), personal responsibility for influencing diabetes (personal responsibility), seriousness of diabetes (seriousness), chronicity or longevity of the disease (timeline), and impact on daily life (impact).
Included in this cross-sectional survey as covariates were patient activation and health literacy. Patients who are highly active in the management of their diabetes know and perceive the risks associated with the disease, which prompts them to develop the confidence and skills necessary to enact self-management behaviors. Patient activation was measured using the Patient Activation Measure (PAM),15 a proprietary scoring system that uses 13 Likert-style items to create a continuous patient activation measure on a scale of 0 to 100. Health literacy was assessed with 3 health literacy screening questions: “How confident are you filling out forms by yourself,” “How often do you have someone (like a family member, friend, hospital/clinic worker or caregiver) help you read hospital materials,” and “How often do you have problems learning about your medical condition because of difficulty understanding written information.” Responses to the three items were summed for a 3-to-15 point scale of health literacy.16
To collect information about race/ethnicity, the survey asked 2 questions. First, one question followed the US Census race categories. Patients could mark multiple categories to indicate mixed race. Second, a question asked the respondent to identify as Hispanic or non-Hispanic. The research team collapsed responses to the following categories, as has been done in previous research17: “non-Hispanic Black” refers to individuals of African descent, “non-Hispanic White” refers to nonminority individuals, “Hispanic American” refers to those of Mexican, South American, Cuban, or Puerto Rican descent born and/or residing in the United States, “Asian American” refers to individuals of South Asian, East Asian, Southeast Asian, and Pacific Island descent born and/or residing in the United States, and “Native American” refers to American Indians and Alaska Natives.
Results
Of a potential 3316 patients who were mailed surveys, 50 were recorded as not meeting inclusion criteria (38 mailings were returned for undeliverable address; 7 patients reported they were no longer patients of the system; 2 patients were reported deceased; and 3 patient caregivers reported that the patient was unable to complete the survey). Of the remaining mailed surveys assumed to meet inclusion criteria, 773 returned surveys (23.7% response rate). Casewise deletion was used for cases with missing demographic data; 44 cases were missing race/ethnicity information, 6 were missing age data.
For race, 11 participants marked multiple races. Four respondents indicated American Indian and Black American; for this analysis, these cases were coded as Black American. Three respondents indicated both White American and Black American; for this analysis, these cases were coded as black American. One respondent indicated American Indian and Asian American; for this analysis, that case was coded as Asian American. Three respondents indicated White American and Asian American; for this analysis, these cases were coded as Asian American.
For comparative analysis, only race/ethnic groups of more than 100 respondents were included; therefore, 27 Hispanic American respondents and 11 Native American respondents are excluded from analysis. Thus, the total sample here is 685 (21.0% of those mailed surveys). See Table 1 for participant demographics by location.
Table 1.
Respondent Characteristics.
| Total (N = 685) | Georgia (n = 280) | Nevada (n = 405) | Significance | |
|---|---|---|---|---|
| Age, years, mean (SD) | 57.62 (5.76) | 56.87 (5.98) | 58.15 (5.54) | .004 |
| Gender: female, n (%) | 348 (50.8) | 143 (51.1) | 205 (50.6) | n.s. |
| Race/ethnicity, n (%) | .000 | |||
| Asian American | 161 (23.5) | 24 (8.6) | 137 (33.8) | |
| Non-Hispanic Black American | 228 (33.3) | 169 (60.4) | 59 (14.6) | |
| Non-Hispanic White American | 296 (43.2) | 87 (31.1) | 209 (51.6) | |
| Patient activation (PAM),mean (SD) | 67.23 (SD 16.15) | 67.51 (SD 16.79) | 67.04 (SD 15.71) | n.s. |
| Health literacy, mean (SD) | 13.43 (SD 2.29) | 13.43 (SD 2.34) | 13.44 (SD 2.29) | n.s. |
Abbreviations: PAM, Patient Activation Measure; n.s., not significant.
For covariate and outcome measures, less than 2% of cases had missing values, which is far less than the missing rate of 15% to 20% common in psychological studies.18 Tests detected no pattern of missingness. For the missing items, linear regression imputation was used on scaled measures.
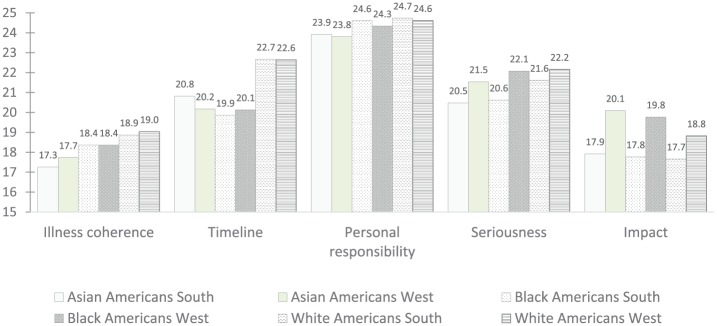
In 5 separate analyses of covariance (ANCOVA), controlling for age, health literacy, and patient activation, a full-factorial model of geographic location and race/ethnicity was tested onto the 5 dimensions of illness representation. See Figure 1 for group estimated marginal means.
Figure 1.
Estimated marginal means of dimensionsa of the Diabetes Illness Representations Questionnaire by race/ethnicityb and locationc.
aRanges: Illness coherence, 5-25; timeline, 6-30; personal responsibility, 6-30; seriousness, 6-30; impact, 7-35.
bRace/ethnicity significantly associated with illness coherence, F(2, 676) = 4.63, P < .01; and timeline, F (2, 676) = 23.42, P < .001.
cLocation significantly associated with seriousness, F(2, 676) = 9.98, P < .005; and impact of diabetes, F(2, 676) = 13.10, P < .001.
In the test on illness coherence, race/ethnicity was associated with understanding of the disease process, F(2, 675) = 4.55, P < .01. Health literacy (P < .05) and patient activation (P < .001) also had statistically significant associations with coherence. A post hoc Tukey revealed that White Americans reported significantly higher understanding than Asian Americans, P < .005.
A second ANCOVA revealed a significant main effect of race/ethnicity on longevity of illness, F(2, 675) = 23.34, P < .001. A post hoc Tukey revealed that White Americans perceived significantly greater longevity of the illness as compared with both Asian Americans, P < .001, and Black Americans, P < .001.
The ANCOVA was repeated onto seriousness of disease. Only geographic location had a significant main effect on seriousness, F(2, 675) = 9.59, P < .005. Patients living in Nevada perceived diabetes as more serious than patients living in Georgia.
In ANCOVA, geographic location also had a significant main effect on impact of diabetes, F(2, 675) = 12.60, P < .001. Patients in Nevada perceived greater impact on their lives than patients in Georgia. Age (P < .01), health literacy (P < .05), and patient activation (P < .001) also had statistically significant associations with impact.
No significant main effects were detected on personal responsibility for disease.
Discussion
Results here uncover race/ethnicity and geographic differences in how patients perceive diabetes. Even when controlling for health literacy and patient activation, Non-Hispanic White Americans report greater understanding and perceive a longer disease course than non-Hispanic Black Americans and Asian Americans. This result may be connected to previous research that ethnic culture influences disease perceptions.8 However, results extend the concept of culture to geographic groups. In our sample, patients in Nevada, outside the Diabetes Belt, perceive diabetes as more serious and having more impact on their lives than patients living in Georgia, inside the Diabetes Belt. This supports our hypothesis that widespread prevalence, whether among ethnic group or geographic area, may lessen the perceived severity of diabetes. Social norms of a place may influence the personal model of disease just as cultural norms do.
Results here, though statistically significant, cannot be considered clinically significant without more clinical inquiry applying the concept of personal models of disease to diabetes outcomes. Race/ethnicity is just one form of group membership that may influence the explanatory model of disease shared across group members.3 Outside the scope of this study, other group memberships may contribute to a personal model of disease, including religious affiliations, social networks, and work organizations. Limitations also include self-reported data, self-selection bias, and, as with any cross-sectional study, no sequence of events could establish causality among results. Notable among these limitations is the inability to compare survey responders to nonresponders on variables of race/ethnicity, age, health literacy, and patient activation.
These findings are actionable within the context of the Health Belief Model,2 which emphasizes the role of perceived susceptibility to and perceived severity of a disease. Studies have shown that culturally tailored diabetes interventions can improve glycemic control among ethnic minorities who are already managing their diabetes.19,20 Researchers should study perceptions of diabetes within the context of ethnic and geographic groups to improve messages about the disease and self-management. Moreover, health communicators should emphasize the seriousness of a diabetes diagnosis and the chronic nature of the disease with patients who belong to communities with a higher prevalence of the disease.
Acknowledgments
We thank Heather Rider, Angela Seehusen, and Jasmyne Womack for their assistance with recruitment and data collection. We also thank Jeremy Jackson, Military Primary Care Research Network, for editorial assistance.
Author Biographies
Christy J. W. Ledford, PhD, is an associate professor in the Department of Family Medicine at Uniformed Services University of the Health Sciences, where she also serves as Research Director for the Military Primary Care Research Network. As a communication scientist, she seeks to integrate communication principles into medical education and clinical practice. Dr. Ledford earned her PhD in health and strategic communication from George Mason University.
Dean A. Seehusen earned his medical degree from the University of Iowa and a Master of Public Health from the University of Washington. He is a graduate of the Tripler Army Medical Center Family Medicine Residency and the Madigan Army Medical Center Faculty Development Fellowship in Family Medicine. Dr. Seehusen is a professor in the Department of Family Medicine and is currently the associate dean for Graduate Medical Education at Augusta University in Augusta, Georgia.
Paul F. Crawford earned his medical degree from the Pennsylvania State University College of Medicine. He is a graduate of the Eglin Air Force Base Family Medicine Residency and the University of North Carolina Faculty Development Fellowship in Family Medicine. Dr. Crawford is a professor in the Department of Family Medicine, Uniformed Services University, and is currently the Director of Medical Education at the Mike O’Callaghan Military Medical Center, Nellis AFB, NV.
Footnotes
Authors’ Note: The views expressed within this publication do not represent those of the authors and do not reflect the official position of the US Air Force, US Army, Uniformed Services University of the Health Sciences, or the US Government, the Department of Defense at large.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Defense Medical Research and Development Program under Award No. FMBB100383695.
ORCID iD: Christy J. W. Ledford  https://orcid.org/0000-0001-5523-454X
https://orcid.org/0000-0001-5523-454X
References
- 1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. [Google Scholar]
- 2. Rosenstock IM. Historical origins of the health belief model. Health Educ Monogr. 1974;2:328-335. [Google Scholar]
- 3. Weller SC, Baer RD, de Alba Garcia JG, Rocha ALS. Explanatory models of diabetes in the US and Mexico: the patient-provider gap and cultural competence. Soc Sci Med. 2012;75:1088-1096. [DOI] [PubMed] [Google Scholar]
- 4. Fleming E, Gillibrand W. An exploration of culture, diabetes, and nursing in the South Asian community: a metasynthesis of qualitative studies. J Transcult Nurs. 2009;20:146-155. [DOI] [PubMed] [Google Scholar]
- 5. Black SA. Diabetes, diversity, and disparity: what do we do with the evidence? Am J Public Health. 2002;92:543-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Quandt SA, Bell RA, Snively BM, et al. Ethnic disparities in glycemic control among rural older adults with type 2 diabetes. Ethn Dis. 2005;15:656-663. [PMC free article] [PubMed] [Google Scholar]
- 7. Utz SW, Steeves RH, Wenzel J, et al. “Working hard with it”: self-management of type 2 diabetes by rural African Americans. Fam Community Health. 2006;29:195-205. [DOI] [PubMed] [Google Scholar]
- 8. Schorling JB, Saunders JT. Is “sugar” the same as diabetes? A community-based study among rural African-Americans. Diabetes Care. 2000;23:330-334. [DOI] [PubMed] [Google Scholar]
- 9. Voeks JH, McClure LA, Go RC, et al. Regional differences in diabetes as a possible contributor to the geographic disparity in stroke mortality: the REasons for Geographic And Racial Differences in Stroke Study. Stroke. 2008;39:1675-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Newby P, Noel SE, Grant R, Judd S, Shikany JM, Ard J. Race and region have independent and synergistic effects on dietary intakes in black and white women. Nutr J. 2012;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barker LE, Kirtland KA, Gregg EW, Geiss LS, Thompson TJ. Geographic distribution of diagnosed diabetes in the US: a diabetes belt. Am J Prev Med. 2011;40:434-439. [DOI] [PubMed] [Google Scholar]
- 12. US Census Bureau. Geography program. https://www.census.gov/geo/reference/urban-rural.html. Accessed October 27, 2018.
- 13. Centers for Disease Control and Prevention. Diabetes home: county data. https://www.cdc.gov/diabetes/data/county.html. Accessed March 26, 2019.
- 14. Skinner TC, Howells L, Greene S, Edgar K, McEvilly A, Johansson A. Development, reliability and validity of the Diabetes Illness Representations Questionnaire: four studies with adolescents. Diabet Med. 2003;20:283-289. [DOI] [PubMed] [Google Scholar]
- 15. Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40(6 pt 1):1918-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Int Med. 2008;23:561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13:814-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Enders CK. Using the expectation maximization algorithm to estimate coefficient alpha for scales with item-level missing data. Psychol Methods. 2003;8:322-337. [DOI] [PubMed] [Google Scholar]
- 19. Nam S, Janson SL, Stotts NA, Chesla C, Kroon L. Effect of culturally tailored diabetes education in ethnic minorities with type 2 diabetes: a meta-analysis. J Cardiovasc Nurs. 2012;27:505-518. [DOI] [PubMed] [Google Scholar]
- 20. Osborn CY, Amico KR, Cruz N, et al. A brief culturally tailored intervention for Puerto Ricans with type 2 diabetes. Health Educ Behav. 2010;37:849-862. [DOI] [PMC free article] [PubMed] [Google Scholar]