Short abstract
Preoperative anxiety is common in patients undergoing elective surgery and is closely related to postoperative hyperalgesia. In this study, a single prolonged stress model was used to induce preoperative anxiety-like behavior in rats 24 h before the surgery. We found that single prolonged stress exacerbated the postoperative pain and elevated the level of serum corticosterone. Previous studies have shown that glucocorticoid is associated with synaptic plasticity, and decreased spinal GABAergic activity can cause hyperalgesia in rodents. Here, single prolonged stress rats’ lumbar spinal cord showed reduced glutamic acid decarboxylase-65, glutamic acid decarboxylase-67, GABA type A receptor alpha 1 subunit, and GABA type A receptor gamma 2 subunit, indicating an impairment of GABAergic system. Furthermore, neuronal PAS domain protein 4 was also reduced in rats after single prolonged stress stimulation, which has been reported to promote GABAergic synapse development. Then, intraperitoneal injection of RU486 (a glucocorticoid receptor antagonist) rather than spironolactone (a mineralocorticoid receptor antagonist) was found to relieve single prolonged stress-induced hyperalgesia and reverse neuronal PAS domain protein 4 reduction and the impairment of GABAergic system. Furthermore, overexpressing neuronal PAS domain protein 4 could also restore the damage of GABAergic system caused by single prolonged stress while interfering with neuronal PAS domain protein 4 caused an opposite effect. Finally, after stimulation of rat primary spinal cord neurons with exogenous corticosterone in vitro, neuronal PAS domain protein 4 and GABAergic markers were also downregulated, and RU486 reversed that. Together, our results demonstrated that preoperative anxiety led to GABAergic system impairment in spinal cord and thus caused hyperalgesia due to glucocorticoid-induced downregulation of neuronal PAS domain protein 4.
Keywords: Preoperative anxiety, postoperative pain, glucocorticoid receptor, neuronal PAS domain protein 4, GABAergic markers
Introduction
Postoperative pain is common in clinical practice, and there are many factors that can affect postoperative pain, such as nerve injury, edema, and incision infection.1 Although potent drugs like opioids and nonsteroidal anti-inflammatory drugs, the current state in treating postoperative pain remains unsatisfactory. In addition, patients with postoperative pain are usually accompanied by negative emotions such as anxiety and depression. Thus, it may be speculated that negative emotions also have effect on postoperative pain. Indeed, studies have found that patients with preoperative anxiety are more likely to experience postoperative pain, and the pain is more severe, than the general population.2–4 Similarly, more studies have shown that emotion, as a psychological stress, exert potent, but complex, modulatory influences on pain.5–7 However, the mechanism of preoperative anxiety-induced postoperative hyperalgesia remains to be elusive.
After stress, activation of the hypothalamic-pituitary-adrenal axis causes the adrenal cortex to release large amounts of glucocorticoids (GCs), known as cortisol in humans and corticosterone (CORT) in rodents. Several studies have demonstrated that GC is involved in pain modulation. For example, long-term subcutaneous injection of CORT in rats lead to visceral hyperalgesia, while adrenalectomy in rats eliminate forced swimming stress-induced hyperalgesia.8,9 In addition, GCs-induced microglia activation and synaptic plasticity changes contribute to the development of neuropathic pain. And the neuropathic pain is significantly relieved after treatment with RU486 (glucocorticoid receptor (GR) antagonist) or GR antisense oligonucleotide.10–12
Maintaining normal neural network function requires a balance between neuronal excitability and inhibition. Neuronal inhibition is primarily GABAergic-mediated, and impaired GABAergic system lead to neurological disorders including pain.13–15 GABA receptor agonists like baclofen and muscimol are effective in relieve pain. Conversely, bicuculline, a GABA receptor antagonist, aggravates pain.16,17 It has been revealed that stress leads to a decrease in GABA release, and stress can also cause GABA functional neurons apoptosis, which further leads to reduced GABAergic neurotransmission in brain and spinal cord.15,18–20 In fact, many factors are involved in the modulation of GABAergic plasticity, and neuronal PAS domain protein 4 (Npas4) has been shown to facilitate the development of inhibitory synapse and maintain the homeostasis of neural circuit.21–24 Npas4 is a neuronal PAS domain protein, belonging to the basic Helix-Loop-Helix (bHLH)–PAS family, and is involved in many essential physiological and pathological processes. Interestingly, stress can also reduce Npas4 in the prefrontal cortex and hippocampus.25–27
The purpose of this study was to test the hypothesis that preoperative anxiety-induced GC signaling downregulated Npas4, which then led to impaired spinal GABAergic system and ultimately contributing to postoperative hyperalgesia. Therefore, we applied a single prolonged stress (SPS) to rat followed by a plantar incision to simulate preoperative anxiety-induced postoperative hyperalgesia and furtherly examined the relationship between GC signaling, Npas4, and GABAergic system in it.
Materials and methods
Animals
Adult male Sprague Dawley rats, weighing 200 to 250 g, were supplied by the Laboratory Animal Center of Nanjing Drum Tower Hospital. The animal study was performed in accordance with the Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee of the Medical School of Nanjing University. The rats were caged individually in a temperature-controlled facility with a 12-h dark/light cycle and water and laboratory rodent chow ad libitum.
SPS procedure
SPS procedure were performed as described previously.28 Briefly, rats were acutely restrained in plastic animal holders for 2 h, followed immediately by a 20-min forced swim individually in an acrylic tank with clean water (24°C). After swimming, the rats were allowed to rest for 15 min and then exposed to the inhalation anesthetic ether until they lost consciousness. Meanwhile, the control rats had no treatment in an adjacent room.
Plantar incision
According to the previous studies,29 rats were anesthetized with 2% to 3% sevoflurane delivered via a nose cone. The plantar aspect of the right hind paw was prepared, and a 1-cm longitudinal incision was made through the skin, fascia, and muscle of the plantar aspect of the hind paw. The wound edges were sutured with a 5-0 nylon thread and then covered with antibiotic ointment.
Drug administration
About 30 min prior to SPS, rats were injected with RU486 (GR antagonist) (30 mg/kg; intraperitoneal (i.p.); Toronto Research Chemicals, North York, Canada), or spironolactone (mineralocorticoid receptor antagonist) (30 mg/kg; i.p.; Tocris Biochemicals). All solutions were freshly prepared. The doses of drugs used were based on the previous studies.30 For vehicle treatment, a similar volume of dimethyl sulfoxide was used. For the in vitro experiment, rat spinal cord primary neurons were treated with CORT (0.1/1/10/100 μm) or RU486 (25 μm).
Behavioral testing
Rats were placed individually on an elevated transparent plexiglass chamber (20-cm height, 25-cm width, 15-cm length) and allowed to acclimate. Mechanical allodynia was examined using the Dynamic Plantar Aesthesiometer (Ugo Basile, Italy). The metal filament were applied underneath the mesh (grid 1 × 1 cm) to the center of the palmar surface of the right hind paw, and upward force was increased from 1 to 50 g over 7 s. The value of the right hind paw retraction was recorded, and each rat model received four values following a 5-min stimulation interval.
Viral vector construction and injection
Rat rAAV-Npas4 vectors were generated by Shanghai R&S Biotechnology Co., Ltd. The sequences were utilized as follows: 5′-ATGTACCGATCCACCAAGGGC-3′ (forward) and 5′-TCAAAACGTTGGTTCCCCTCCACTT-3′ (reverse) for rAAV-Npas4 and 5′-GTTTTGGCCACTGACTGAC-3′ (forward) and 5′-GTCAGTCAGTGGC CAAAAC-3′ (reverse) for rAAV-Npas4 RNAi. We then injected viral vectors into two-month old male SD rats, as previously done by Guo et al.31 Briefly, when the Hamilton syringe penetrated the skin between the horizontal vertebrae of the cauda equina, a 10 μl viral solution containing rAAV (titer: 4 × 1010 TU/mL) was injected once observed the reflexive tail flick. After injection, the needle was taken in place for ∼1 min, and then the rat was placed into cage.
Western blot
Spinal lumbar enlargements were harvested from anesthetized animals. For whole-cell protein extraction, tissues were homogenized in ice-cold RIPA lysis buffer (Beyotime, Shanghai, China) with phenylmethylsulfonyl fluoride (Thermo Scientific, Waltham, MA, USA), then sonicated on ice and centrifuged at 12,000× g for 15 min at 4°C to isolate the supernatant part. The protein concentrations of all samples were detected with a BCA Protein Assay kit (Pierce Biotechnology, Rockford, IL, USA). After blocking in 5% nonfat milk for 1 h at room temperature (RT), the membranes were incubated with primary antibodies overnight at 4°C. The specific antibodies used in this study were as follows: anti-Npas4 (1:1000, NBP2-59332; Novus) and anti-actin ((1:4000; Abcam; USA). Subsequently, blots were incubated with horseradish peroxidase-linked secondary antibodies for 1 h at RT. After an extensive washing, protein bands were finally detected by electrochemiluminescence solution (Millipore) exposed to film, and ImageJ software (NIH, Bethesda, MD, USA) was used to measure the gray value of each band.
Immunohistochemistry
Rats were deeply anesthetized and perfused through the aorta via ice cold saline followed by 4% paraformaldehyde. Then, the lumbar segments of the spinal cords were harvested, postfixed in 4% paraformaldehyde, and placed into 30% sucrose until equilibration. Serial frozen sections were cut into 20-μm thick slides by a freezing microtome. For double-labeling immunohistochemistry analysis, the spinal cord sections were incubated overnight at 4°C with a mixture of mice anti-Npas4 (1:100, Novus) and rabbit monoclonal antineuronal nuclear antigen (NeuN, 1:500, Cell Signaling Technology). The sections were then incubated with Alexa Fluor 488 (1:3000, Thermo Fisher) and Alexa Fluor 594 (1:3000, Thermo Fisher) for 1 h at 37°C. After that the sections were rinsed in 0.01 M phosphate buffered saline, coverslips were applied. Images were captured by using an Olympus BX53F fluorescence microscope.
Quantitative reverse transcription polymerase chain reaction
Total mRNA from spinal cord tissues or primary spinal cord neurons were isolated using Trizol reagent (Invitrogen, USA), and cDNA was synthesized using HiScript II First Strand cDNA Synthesis Kit (Vazyme, Nanjing, China). All reverse transcription reactions were performed with cDNA and ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) and run in an ABI StepOnePlus Real-Time PCR System (Applied Biosystems, Foster, CA). The following primers were used in this study: 5′-GCTATACTCAGAAGGTCCAGAAGGC-3′ (forward) and 5′-TCAGAGAATGAGGGTAGCACAGC-3′ (reverse) for Npas4, 5′-CTCTTCCAGCCTTCCTTCCT-3′ (forward) and 5′-AGCACTGTGTTGGCGTACAG-3′ (reverse) for actin, 5′-CCTG GTTAGAGAGGAGGGACTGA-3′ (forward) and 5′-CATAGTGCTTATCTTGCTG AAAGAGGTA-3′ (reverse) for GAD65, 5′-TACAACCTTTGGCTGCATGT-3 (forward) and 5′-TGAGTTTGTGGCGATGCTT-3′ (reverse) for GAD67, 5′-GCAGATTGGATATTGGGAAGCA-3′ (forward) and 5′-GGTCCAGGCCCAA AGATAGTC-3 (reverse) for GABAA α1, and 5-AGAAAAACCCTCTTCTTCGG ATG-3 (forward) and 5-GTGGCATTGTTCATTTGAATGGT-3′ (reverse) for GABAA γ2.
Enzyme-linked immunosorbent assay
About 0.3 mL of blood samples were collected from the tail vein and kept at RT for 1 h and then centrifuged at 3000 r/min for 15 min to collect the serum. Collected serum samples were then stored at –80˚C for further analysis. A commercially available enzyme-linked immunosorbent assay kit (Senbeijia, Nanjing, China) was used to quantify the levels of CORT in the serum. To rule out the potential effect of circadian rhythm on rat hormone levels, blood samples were taken at the same time between 4:00 and 6:00 p.m.
Cell culture
Spinal neurons were prepared using timed-pregnant Sprague Dawley rats (E18, Laboratory Animal Center of Nanjing Drum Tower Hospital). Briefly, rats were anesthetized with sevoflurane, and the E18 embryos were removed. The fetal rat spinal cord was removed, stripped meninges, minced into 1 mm3 pieces, digested in 0.05% trypsin (10 U/mL, Sigma) for 20 min at 37°C, and then centrifuged at 1500 r/min for 5 min at RT. The cells were resuspended in Dulbecco’s modified eagle medium (Biological Industries) containing 10% fetal bovine serum (Gibco), 2 mM glutamine (Sigma), 25 mM glucose, and 1% penicillin/streptomycin (Gibco). Cells were plated onto poly-D-lysine-coated (Sigma) tissue culture plates at 1 × 106 cells/mL. Media were completely changed into serum-free neurobasal medium (Gibco) supplemented with 2% B27 (Gibco) supplement 4 h later. One-half medium changes were performed at day 2. Cultures were incubated at 37°C in a 5% CO2 incubator, and experiments were performed on days 7 to 9.
Data analysis
All data were expressed as the mean ± standard deviation. Data of behavioral tests for mechanical hyperalgesia were analyzed via two-way analysis of variance with repeated measures and followed by the Bonferroni post hoc test. The statistical analysis was conducted using SPSS 20.0 software, and the statistical significance was set at p < 0.05 in all cases. The differences of data from western blot and quantitative reverse transcription polymerase chain reaction between groups were compared using one-way analysis of variance followed by Bonferroni post hoc analysis.
Results
Preoperative SPS induces postoperative hyperalgesia and upregulates serum CORT levels
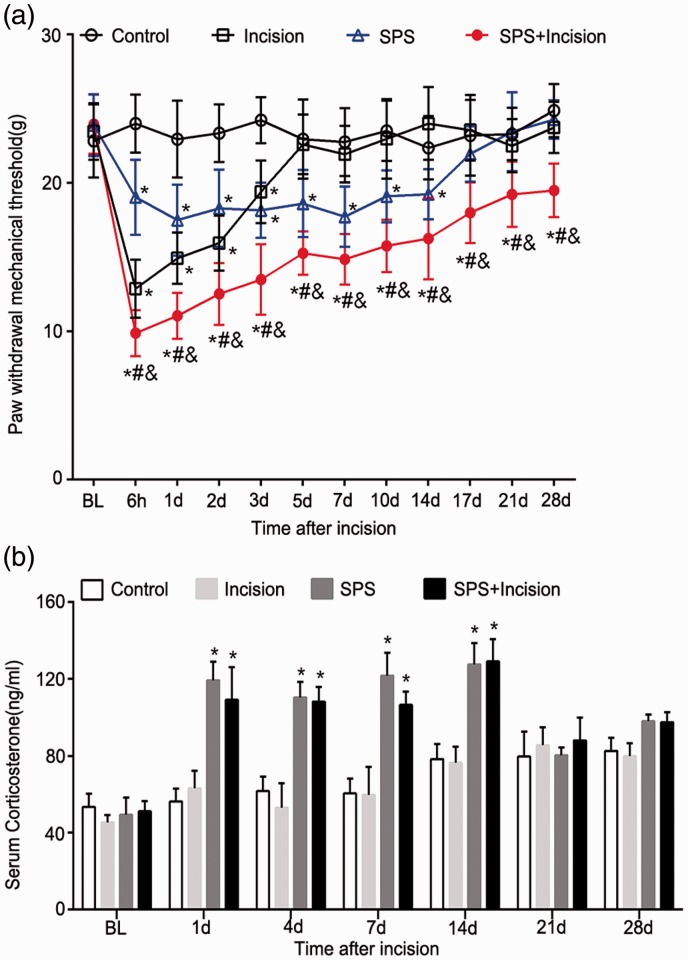
The effect of SPS on postoperative pain behavior in rats was observed (Figure 1(a)). There was no difference in the baseline level of the paw withdrawal mechanical threshold (PWMT) among each group (p > 0.05). SPS aggravated incision-induced pain in rats, which was consistent with our previous work. Compared with control group, the PWMT of SPS group decreased from 6 h to 14 days postoperatively (p < 0.05), and the decrease lasted for at least 28 days in SPS+ incision group after surgery (p < 0.05).
Figure 1.
Effects of SPS on paw withdrawal responses and CORT levels in rats after incision surgery. (a) The PWMT to Dynamic Plantar Aesthesiometer stimuli before SPS (baseline) and 6 h to 28 days after the incision surgery. Data are presented as mean ± SD (n = 8), *p < 0.05 versus control group; #p < 0.05 versus incision group; &p < 0.05 versus SPS group. Statistical analysis was performed using two-way repeated measures ANOVA followed by the Bonferroni test. (b) Serum CORT was measured 24 h before SPS and 1, 4, 7, 14, 21, and 28 days after plantar incision. Data are presented as mean ± SD (n = 3), *p < 0.05 versus control group; statistical analysis was performed using one-way ANOVA followed by the Bonferroni test. SPS: single prolonged stress; PWMT: paw withdrawal mechanical threshold; CORT: corticosterone; ANOVA: analysis of variance.
We next measured the effect of SPS on serum CORT via the enzyme-linked immunosorbent assay (Figure 1(b)). The results showed that serum CORT was significantly upregulated in both SPS group and SPS + incision group for at least 14 days compared with control group (p < 0.05), which was corresponded to the decrease of PWMT during the first 14 days. In contrast, the plantar incision operation had no effect on serum CORT (p > 0.05, incision group vs. control group). These data suggested that preoperative SPS aggravated postoperative pain and increased serum CORT levels.
Preoperative SPS downregulates GABAergic markers in spinal cord
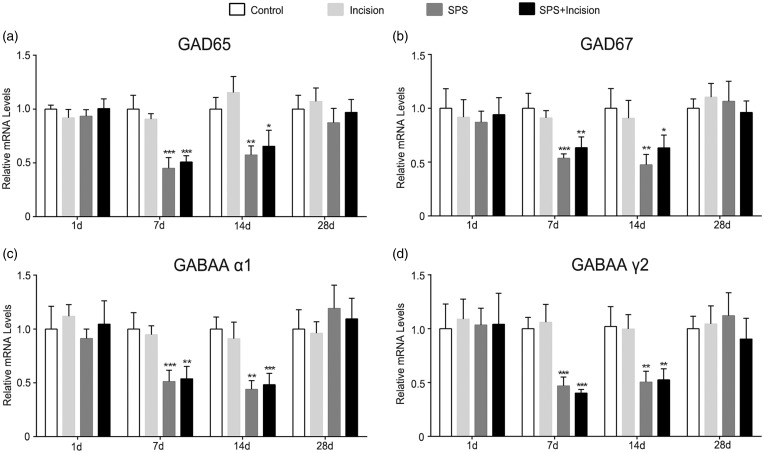
To explore the impact of SPS on GABAergic system activity, we collected spinal cord lumbar enlargement specimens at different time points (1, 7, 14, and 28 days after incision). The mRNA levels of GABA synthesizing enzymes (GAD65, GAD67) and GABA type A receptor subunits (α1, γ2) were then analyzed (Figure 2). Compared with control group, the mRNA levels of GAD65, GAD67, GABAA α1, and GABAA γ2 were decreased in SPS group and SPS + incision group (p < 0.05), whereas no significant change was observed in incision group (p > 0.05). These data indicated that SPS decreased the synthesis of GABA and altered the GABAA receptor composition.
Figure 2.
Relative mRNA levels of postoperative GABAergic markers in the lumbar spinal cord of SPS rats. (a–d) Sections correspond to GAD65, GAD67, GABAA receptor subunit α1 and γ2, respectively. Data are presented as mean ± SD (n = 4), ***p < 0.001 versus control group, **p < 0.01 versus control group, *p < 0.05 versus control group; statistical analysis was performed using one-way ANOVA followed by the Bonferroni test. SPS: single prolonged stress; ANOVA: analysis of variance; SD: standard deviation; ANOVA: analysis of variance.
Preoperative SPS reduces Npas4 in spinal cord
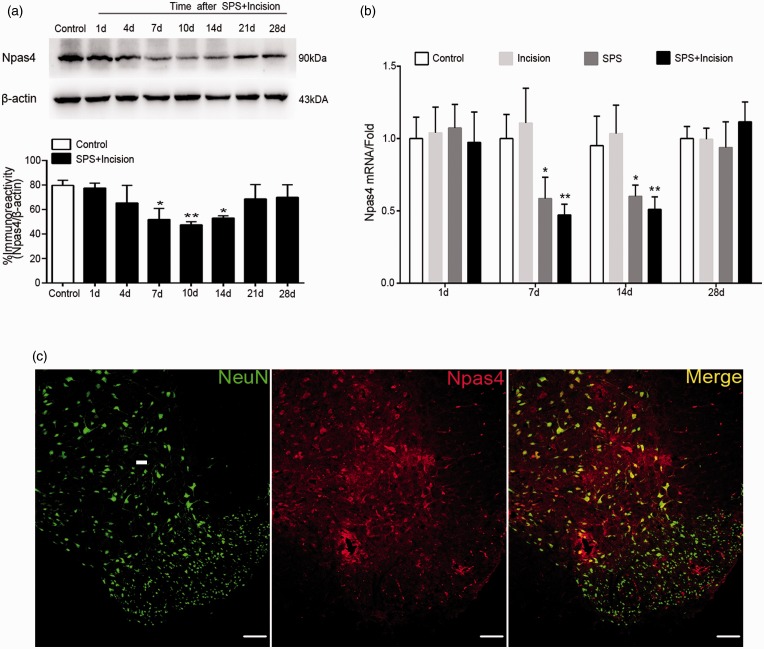
We then suspected whether Npas4 was involved in SPS-induced GABAergic markers inhibition. Our data showed that Npas4 protein was decreased in SPS + incision group at 7,10, and 14 days after incision (Figure 3(a)), p < 0.05) compared with control group. Consistent with western blot results, the relative mRNA levels of Npas4 were also reduced at 7 and 14 days after incision (p < 0.05) (Figure 3(b)). Next, the immunohistochemistry was performed to detect the localization of Npas4, and the results showed that Npas4 was expressed in spinal neuron (Figure 3(c)). These data suggested that SPS reduced postoperative spinal Npas4 between 7 and 14 days at least.
Figure 3.
Effects of SPS on Npas4 expression in spinal cord after incision surgery. (a) Protein levels of Npas4 in rats’ lumbar spinal cord were analyzed by western blotting. Npas4 protein levels were normalized by β-actin (n = 3). (b) Rats were sacrificed on days 1, 7, 14, and 28 after stress to measure the mRNA levels of Npas4 in the lumbar spinal cord (n = 4). (c) Double immunostain with NeuN (green) and Npas4 (red) in the dorsal horn of spinal cord (scale bar = 50 μm). Data are presented as mean ± SD. **p < 0.01, *p < 0.05 versus control group; statistical analysis was performed using one-way ANOVA followed by the Bonferroni test. SPS: single prolonged stress; Npas4: neuronal PAS domain protein 4; ANOVA: analysis of variance.
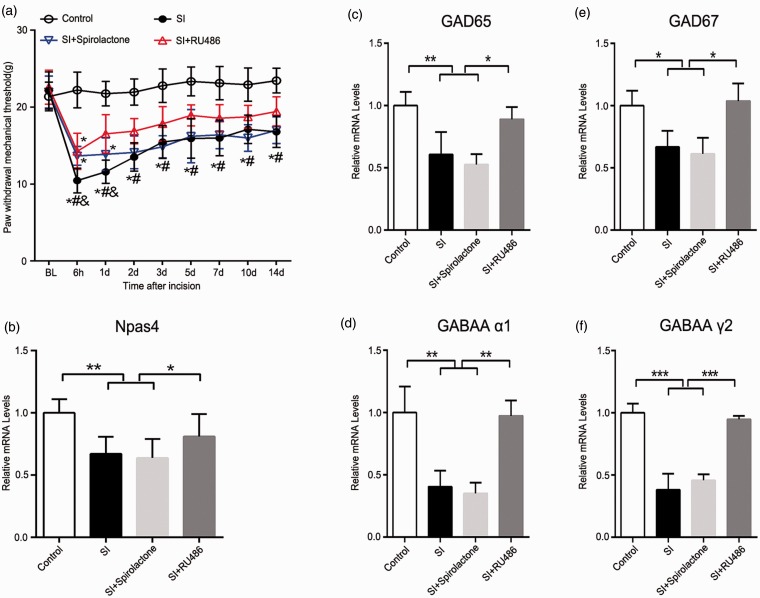
Application of RU486 attenuates SPS-induced postoperative hyperalgesia and reverses SPS-induced Npas4 and GABAergic markers reduction
In order to access the role of CORT in postoperative hyperalgesia, the MR (spironolactone) and GR (RU486) antagonists were used, respectively (i.p., 30 min before SPS) (Figure 4). We observed the pain behavior for 14 consecutive days, and the SPS rats pretreated with RU486 showed a significantly higher postoperative PWMT than SPS rats (p < 0.05). However, there was no significant PWMT change in SPS rats pretreated with spironolactone compared with SPS rats (p > 0.05) (Figure 4(a)).
Figure 4.
Effects of RU486 and spironolactone on pain behavior, Npas4, and GABAergic markers postoperatively in SPS rats. (a) The PWMT in response to Dynamic Plantar Aesthesiometer of the right hind paw was measured at 24 h prior to drug or vehicle injection (baseline) and at 6 h to 14 days after incisional surgery. Statistical analyses were performed using two-way repeated measures ANOVA, *p < 0.05 versus control group; #p< 0.05 versus SI + RU486 group; &p < 0.05 versus SI + spironolactone group (n = 8). (b) After drug or vehicle treatment, the lumbar spinal cord was homogenized on the 14th day after incision, and Npas4 mRNA level was analyzed. (c) to (f) Quantitative RT-PCR performed to detect the mRNA in spinal cord of rats treated with RU486 or spironolactone before SPS, and sections were corresponded to GAD65, GAD67, GABAA receptor subunit α1 and γ2, respectively (n = 4). Data are presented as mean ± SD, ***p < 0.001, **p < 0.01, and *p < 0.05; statistical analysis was performed using one-way ANOVA followed by the Bonferroni test. SPS: single prolonged stress; Npas4: neuronal PAS domain protein 4; SI: SPS + incision; PWMT: paw withdrawal mechanical threshold; ANOVA: analysis of variance; RT-PCR: real-time polymerase chain reaction.
To further investigate the effect of RU486 on SPS rats, the mRNA level of Npas4, GAD65, GAD67, GABAA α1, and GABAA γ2 in spinal cord were measured. The results showed that RU486 reversed the GABAergic markers reduction in SPS + incision group (Npas4: p < 0.05; GAD65: p < 0.05; GAD67: p < 0.05; GABAA α1: p < 0.01; GABAA γ2: p < 0.001) (Figure 4(b) and (c)). However, no significant effect was observed in spironolactone-treated rats (p > 0.05, spironolactone group vs. SPS + incision group). These data suggested that SPS-induced postoperative hyperalgesia was associated with activated GC signaling.
Results of intrathecal injection of rAAV-Npas4 or rAAV-Npas4 RNAi in spinal cord
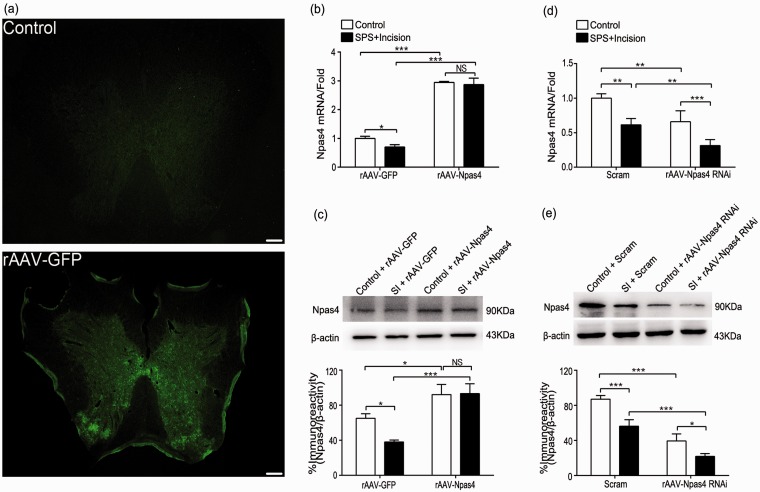
On 7th and 14th day after injection of viral solution (10 μL), the rat spinal cord was taken for analysis of frozen section fluorescence microscopy, quantitative reverse transcription polymerase chain reaction, and western blot (Figure 5). Under fluorescence microscope, the frozen spinal cord lumbar enlargement in rAAV-GFP group showed obvious spontaneous fluorescence with clear outline, while the control group did not see (Figure 5(a)). Compared with rAAV-GFP group, the mRNA and protein levels of Npas4 were higher in rAAV-Npas4 group (p < 0.001) (Figure 5(b) and (c)). Similarly, the mRNA and protein levels of Npas4 in rAAV-Npas4 RNAi group were lower compared with Scram group (p < 0.01, p < 0.001) (Figure 5(d) and (e)).
Figure 5.
Overexpressing Npas4 or interfering with Npas4 in vivo. (a) Representative photographs showing the control (top) and rAAV-GFP (bottom) intrathecal injection. (b) and (c) After injection of rAAV-Npas4, quantitative RT-PCR (n = 4) and immunoblot (n = 3) were performed to detect Npas4 levels from samples on the 14th day after incision (n = 4). (d) and (e) After injection of rAAV-Npas4 RNAi, quantitative RT-PCR (n = 4) and immunoblot (n = 3) were performed to detect Npas4 levels from samples on the 14th day after incision (n = 4). Data are presented as mean ± SD. ***p < 0.001, *p < 0.05 (scale bar = 200 μm). Statistical analysis was performed using two-way ANOVA followed by the Bonferroni test. SPS: single prolonged stress; Npas4: neuronal PAS domain protein 4; SI: SPS + incision; ANOVA: analysis of variance; RT-PCR: real-time polymerase chain reaction.
Intrathecal injection of rAAV-Npas4 or rAAV-Npas4 RNAi can relieve or aggravate postoperative hyperalgesia in SPS rats, respectively
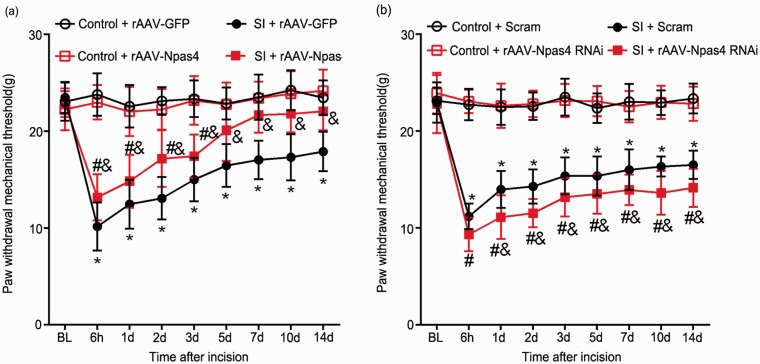
After overexpression of Npas4 or interference with Npas4, changes in pain behavior were observed to determine whether Npas4 participated in SPS-induced postoperative hyperalgesia. The results showed that overexpression of Npas4 alleviated postoperative mechanical hyperalgesia in SPS rats from 6 h to 14 days after incision (p < 0.05, Figure 6(a)). Conversely, interference with Npas4 led to increased pain compared with Scram group, but there was no significant difference in PWMT at 6 h after surgery (p < 0.05, Figure 6(b)). These data suggested that reduced Npas4 contributed to SPS-induced postoperative hyperalgesia.
Figure 6.
Overexpressing Npas4 or interfering with Npas4 changes response to postoperative mechanical stimuli in SPS rats. (a) rAAV-Npas4 vectors were injected seven days before SPS and increased Npas4 relieved SPS-induced postoperative hyperalgesia. *p < 0.05 versus control + rAAV-GFP group, #p < 0.05 versus control + rAAV-Npas4 group, &p < 0.05 versus SI + rAAV-GFP group. (b) Pretreatment with rAAV-Npas4 vectors aggravated SPS-induced postoperative hyperalgesia. *p < 0.05 versus control + Scram group, #p < 0.05 versus control + rAAV-Npas4 RNAi group, &p < 0.05 versus SI + Scram group. Statistical analyses were performed using two-way repeated measures ANOVA. SPS: single prolonged stress; Npas4: neuronal PAS domain protein 4; SI: SPS + incision; PWMT: paw withdrawal mechanical threshold; ANOVA: analysis of variance.
Intrathecal injection of rAAV-Npas4 enhances spinal GABAergic markers of SPS rats
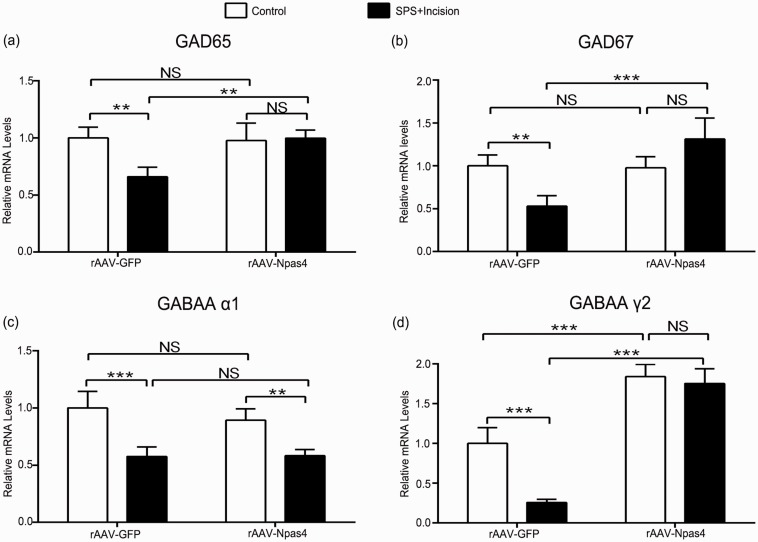
To assess whether overexpressing Npas4 improved SPS-induced GABAergic system damage, the levels of GABAergic markers were measured. Compared with rAAV-GFP group, Npas4 overexpression reversed the decrease of GABAergic markers (GAD65: p < 0.01; GAD67: p < 0.001; GABAA γ2: p < 0.001), except for GABAA α1 (p > 0.05) (Figure 7(a) to (d)). These results indicated that increased Npas4 contributed to restore SPS-induced spinal GABAergic markers reduction.
Figure 7.
Npas4 overexpression improves SPS-induced postoperative GABAergic markers decrease in spinal cord. (a–d) Sections corresponded to GAD65, GAD67, GABAA receptor subunit α1 and γ2, respectively. Data are expressed as mean ± SD (n = 4), ***p < 0.001, **p < 0.01, and *p < 0.05; statistical analyses were performed using two-way ANOVA followed by the Bonferroni test. Npas4: neuronal PAS domain protein 4; SPS: single prolonged stress; ANOVA: analysis of variance; NS: not significant.
Intrathecal injection of rAAV-Npas4 RNAi exacerbates preoperative SPS-induced spinal GABAergic markers reduction
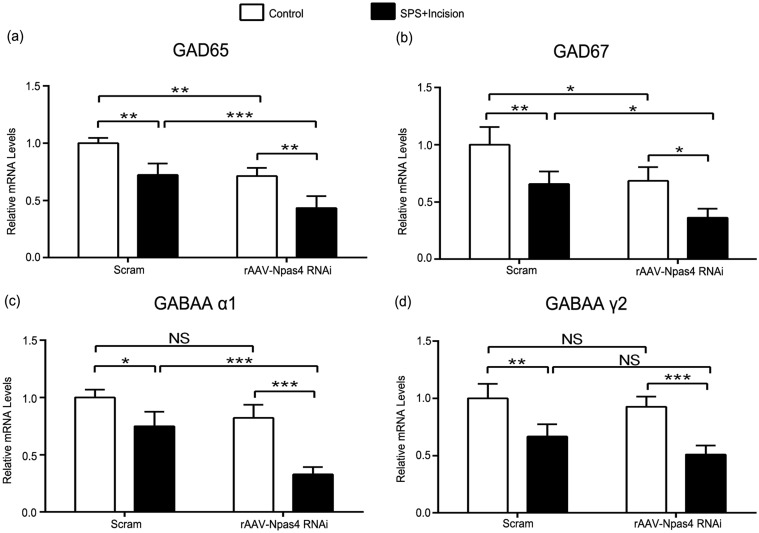
After interfering with Npas4 in SPS rats, it was found that GAD65, GAD67, and GABAA α1 in rAAV-Npas4 RNAi group were lower compared with Scram group (GAD65: p < 0.001; GAD67: p < 0.05; GABAA α1: p < 0.001) (Figure 8(a) to (c)). However, there was no significant difference in the amount of GABAA 2 between the two groups (p > 0.05) (Figure 8(d)). These data further supported the important regulatory role of Npas4 in preoperative SPS-induced spinal GABAergic system impairment.
Figure 8.
Interfering with Npas4 further reduces spinal GABAergic markers in SPS rats after incision. (a–d) Sections corresponded to GAD65, GAD67, GABAA receptor subunit α1 and γ2, respectively. Data are expressed as mean ± SD (n = 4), ***p < 0.001, **p < 0.01, and *p < 0.05; statistical analyses were performed using two-way ANOVA followed by the Bonferroni test. Npas4: neuronal PAS domain protein 4; SPS: single prolonged stress; ANOVA: analysis of variance; NS: not significant.
Figure 9.
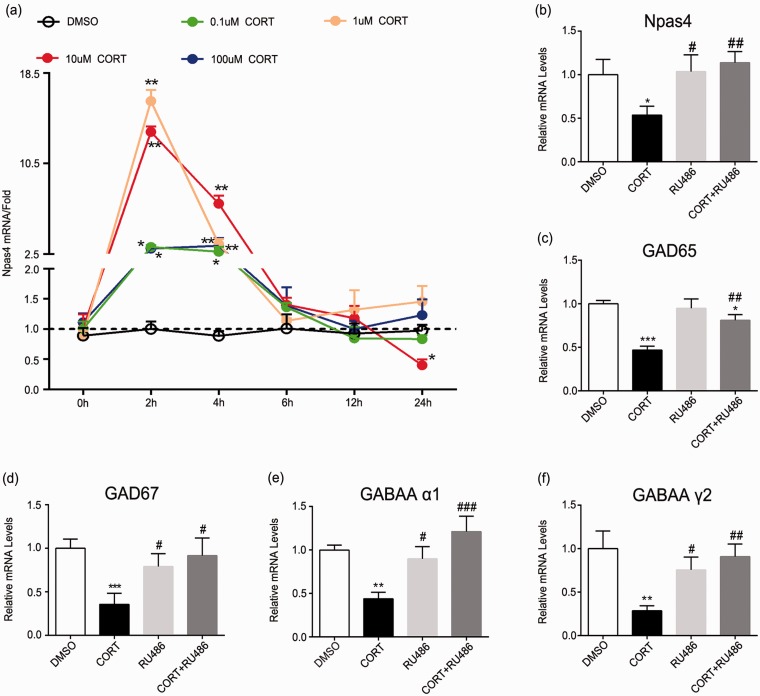
Effects of CORT, RU486, and their combination on Npas4 and GABAergic markers in vitro. (a) Npas4 was rapidly induced after the addition of corticosterone. Separate groups of neuron in vitro were harvested at 0 h, 2 h, 4 h, 8 h, 12 h, or 24 h after stimuli. (b–f) Neuron was harvested after stimulation with CORT or RU486 for 24 h, and mRNA levels of NPAS4, GAD65, GAD67, GABAA α1, and GABAA γ2 were analyzed by quantitative RT-PCR. Data are expressed as mean ± SD (n = 3), ***p < 0.001, **p < 0.01, and *p < 0.05 versus dimethyl sulfoxide group; ###p < 0.001, ##p < 0.01, and #p < 0.05 versus CORT group. Statistical analyses were performed using one-way ANOVA followed by the Bonferroni test. ANOVA: analysis of variance; CORT: corticosterone; Npas4: neuronal PAS domain protein 4; SD: standard deviation; DMSO: dimethyl sulfoxide; RT-PCR: real-time polymerase chain reaction.
CORT affects Npas4 and GABAergic markers in rat primary spinal cord neurons
To explore the direct effects of CORT on neurons, we subjected rat primary spinal cord neurons to different concentrations of CORT. The results indicated that Npas4 reduced significantly after 24 h treatment with 10 μm CORT (p < 0.05) (Figure 9(a)). It was also found that treatment with 10 μm CORT could reduce GABAergic markers. Compared with CORT group, cotreatment with RU486 and CORT reversed Npas4 and GABAergic markers reduction. (Npas4: p < 0.05; GAD65: p < 0.05; GAD67: p < 0.05; GABAA α1: p < 0.01; GABAA γ2: p < 0.001). No significant differences were found between dimethyl sulfoxide group and RU486 group (p > 0.05, Figure 9(b) to (f). Above data revealed that CORT could act directly on neurons and reduce Npas4 and GABAergic markers.
Discussion
In this study, we demonstrated that preoperative SPS-induced GC signaling impaired the GABAergic system in spinal cord, leading to postoperative hyperalgesia. In addition, GR antagonist RU486 attenuated the pain behaviors and reversed the loss of GABAergic markers. Meanwhile, we also found that Npas4 was involved in the damage of GC signaling to GABAergic system, and overexpressing Npas4 could prevent GABAergic markers decrease and therefore relief pain. Conversely, interference with Npas4 caused aggravation of hyperalgesia and further reduction of GABAergic markers. We next added exogenous CORT to rat primary spinal cord neurons, confirming that GABAergic system was regulated by GC signaling.
It has been reported that loss of spinal GABAergic inhibition promotes the occurrence and development of pain.14,17,32 As the neurotransmitter of GABAergic system, GABA is synthesized from glutamate through two GAD subtypes, GAD65 and GAD67, respectively. In current study, we observed reduced GAD65 and GAD67 in spinal cord of SPS rats, suggesting decreased presynaptic GABA synthesis. Although GAD65 and GAD67 have different subcellular localizations,33–35 both of them are involved in the balance of neural circuit homeostasis scaling. GABA release from presynaptic membrane usually binds to GABAA receptors (GABAARs) to exert fast synaptic inhibition.36 GABAARs are chloride ion permeable heteropentameric ligand-gated ion channels that are encoded by 19 different genes, which could be divided into eight subclasses based on the sequence homology (α1–6, β1–3, γ1–3, δ, ε, θ, π, and ρ1–3). The subsets of GABAARs at synapses are mainly composed of two α1, α2, or α3 subunits together with two β2 or β3 subunits and a single γ2 subunit. Among all these subunits, the α1 subunit is involved in binding of benzodiazepines,37 and the γ2 subunit is essential for postsynaptic clustering of GABAARs.28 It has also been reported that α1 subunit correlates with mediating downregulation of GABAAR and binding with GABA.38 Our findings also revealed decreased spinal GABAA α1 and GABAA γ2, suggesting a changed composition of GABAARs and a possibly decreased amount of GABAARs. Several lines of evidence have shown that both amount and composition ratio of GABAAR are involved in functional strength of GABAergic synapse.39,40 For example, clathrin adaptor AP2-mediated GABAAR endocytosis contributes to cerebral ischemia and epilepsy.41 However, in our study, only GABAA alpha 1 and gamma 2 were investigated, and further studies are warranted to explore the alteration and function of other GABAA receptor subunits.
As is known, the intact hypothalamic-pituitary-adrenal axis response after stress involves the release of corticotropin-releasing factor (CRF) from the hypothalamus, followed by CRF stimulation of the pituitary gland to release adrenocorticotropic hormone, and in turn inducing the adrenal cortex to secrete cortisol or CORT.42 Previous studies has demonstrated the clear role of CRF and its receptors in stress-induced hyperalgesia.43–46 Here, we found increased serum CORT in SPS rats, and blocking GR by RU486 could relief the hyperalgesia and improve GABAergic markers, implying the important role of GC signaling. There are many other studies of hyperalgesia caused by GC signaling.47 For example, the binding of GCs to GR induces the expression of pro-inflammatory mediators, such as IL-1β and ATP, to aggravate and maintain neuropathic pain.48–50 Furthermore, previous studies have demonstrated the effect of GC on synaptic plasticity. Anacker et al. find that high concentration of GCs inhibits synaptogenesis, resulting in atrophy of hippocampal neurons,51 while others find that GC is positively correlated with synaptic plasticity on learning and memory.52 Consistent with previous studies of GC-mediated neuroplasticity in brain, our finding validates this effect in spinal cord once again. However, GR is widespread in the central nervous system, not only in neurons, but also in all glia cells such as astrocytes and microglias.53,54 And our previous works have revealed that GCs can activate microglias and astrocytes in spinal cord, which contributes to postoperative hyperalgesia.55,56 While in this study, we also found that CORT downregulated the GABAergic markers of neurons in vitro, indicating the direct effects of GC on neurons.
What is the mechanism by which GCs impair GABAergic system in spinal cord? As an activity-dependent transcription factor, Npas4 has been demonstrated to promote the development of inhibitory synapses.21,22 Our data demonstrated that GCs-induced spinal GABAergic system impairment was Npas4 mediated. Previously, Npas4 is considered to be a brain neuron-specific transcription factor, which is later found in pancreatic β cells.21,57,58 Our finding showed that Npas4 also existed in spinal dorsal horn neurons. Our in vitro experiments also found that only 10 μM CORT caused a significant Npas4 decrease at 24 h under several concentration CORT conditions. In fact, previous studies have found that the effect of stress on Npas4 is related to the nature and strength of stress. It has been shown that chronic stress causes Npas4 reduction in hippocampus, which can impair learning and memory and cause mood disorders.25,59,60 However, experimental animals exposed to acute stress such as foot shock and forced swimming exhibit increased Npas4 in brain.61,62 Thus, these findings contribute to explain why CORT-stimulated neurons significantly increased Npas4 at 2 h and decreased with time, as well as why only the 10 μM CORT group significantly decreased Npas4 at 24 h. Although the exact role of Npas4 in modulating GABAergic inhibition remained unknown in our study, Lin et al. have demonstrated that Npas4 binds to BDNF promoters I and IV,21 whereas BDNF also played an important role in regulating GABAergic synapse.63,64
However, our experiment still has many limitations. First, the regulatory role of GC-GR in the transcriptional level of Npas4 was not studied here. Furukawa et al. reported that GC-GR complex binds to glucocorticoid response elements in the promoter region of Npas4 gene, thereby downregulating the expression of Npas4.65 We speculated that elevated CORT inhibited Npas4 transcription by binding to specific promoter sites, thereby impairing the homeostasis scaling of spinal GABAergic inhibition. Second, the level of CORT and Npas4 in SPS rats returned to normal at last 21 days after stress, while the pain behavior still existed. This inconsistency between phenotype and gene expression remained to be explored in our further studies. At last, several animal experiments have demonstrated that stress can activate glutamatergic system, including enhanced NMDA receptor function,10 augmented NMDA receptor numbers,66 and increased extracellular glutamate.67 Moreover, Li et al. furtherly find that the transition from acute to chronic postoperative pain caused by preoperative stress is related to the phosphorylation of AMPA receptors.68 Thus, the role of glutamatergic system in this study remains to be further explored.
In conclusion, we have identified GC signaling in the regulation of preoperative anxiety-induced postoperative hyperalgesia. Our present data show that preoperative anxiety activates GC signaling and, via inhibition of Npas4, induces GABAergic system impairment in spinal cord, thereby promoting postoperative hyperalgesia. Thus, our finding provides further insight into the mechanisms of preoperative anxiety-induced postoperative hyperalgesia and direction for the development of potential therapeutic strategy.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by National Natural Science Foundation of China (81471129, 81671087, and 81771142) and the grant from the Department of Health of Jiangsu Province of China (XK201140 and RC2011006).
References
- 1.Martinez V, Uceyler N, Ben Ammar S.Alvarez JC, Gaudot F, Sommer C, Bouhassira D and Fletcher D. Clinical, histological, and biochemical predictors of postsurgical neuropathic pain. Pain 2015; 156: 2390–2398. [DOI] [PubMed] [Google Scholar]
- 2.Papaioannou M, Skapinakis P, Damigos D.Mavreas V, Broumas G and Palgimesi A. The role of catastrophizing in the prediction of postoperative pain. Pain Med 2009; 10: 1452–1459. [DOI] [PubMed] [Google Scholar]
- 3.Granot M, Ferber SG. The roles of pain catastrophizing and anxiety in the prediction of postoperative pain intensity: a prospective study. Clin J Pain 2005; 21: 439–445. [DOI] [PubMed] [Google Scholar]
- 4.Richez B, Ouchchane L, Guttmann A.Mirault F, Bonnin M, Noudem Y, Cognet V, Dalmas AF, Brisebrat L, Andant N, Soule-Sonneville S, Dubray C, Duale C and Schoeffler P. The role of psychological factors in persistent pain after cesarean delivery. J Pain 2015; 16: 1136–1146. [DOI] [PubMed] [Google Scholar]
- 5.Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety 2009; 26: 888–901. [DOI] [PubMed] [Google Scholar]
- 6.Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol 2009; 88: 184–202. [DOI] [PubMed] [Google Scholar]
- 7.Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. NeuroImage 2009; 47: 987–994. [DOI] [PubMed] [Google Scholar]
- 8.Fereidoni M, Javan M, Semnanian S.and Ahmadiani A. Chronic forced swim stress inhibits ultra-low dose morphine-induced hyperalgesia in rats. Behav Pharmacol 2007; 18: 667–672. [DOI] [PubMed] [Google Scholar]
- 9.Hong S, Zheng G, Wu X.Snider NT, Owyang C and Wiley JW. Corticosterone mediates reciprocal changes in CB 1 and TRPV1 receptors in primary sensory neurons in the chronically stressed rat. Gastroenterology 2011; 140: 627–637.e624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander JK, DeVries AC, Kigerl KA.Dahlman JM and Popovich PG. Stress exacerbates neuropathic pain via glucocorticoid and NMDA receptor activation. Brain Behav Immun 2009; 23: 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puga DA, Tovar CA, Guan Z.Gensel JC, Lyman MS, McTigue DM and Popovich PG. Stress exacerbates neuron loss and microglia proliferation in a rat model of excitotoxic lower motor neuron injury. Brain Behav Immun 2015; 49: 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Lim G, Zeng Q. Sung B, Ai Y, Guo G, Yang L and Mao J. Expression of central glucocorticoid receptors after peripheral nerve injury contributes to neuropathic pain behaviors in rats. J Neurosci 2004; 24: 8595–8605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drew GM, Siddall PJ, Duggan AW. Mechanical allodynia following contusion injury of the rat spinal cord is associated with loss of GABAergic inhibition in the dorsal horn. Pain 2004; 109: 379–388. [DOI] [PubMed] [Google Scholar]
- 14.Moore KA, Kohno T, Karchewski LA.Scholz J, Baba H and Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci 2002; 22: 6724–6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quintero L, Cardenas R, Suarez-Roca H. Stress-induced hyperalgesia is associated with a reduced and delayed GABA inhibitory control that enhances post-synaptic NMDA receptor activation in the spinal cord. Pain 2011; 152: 1909–1922. [DOI] [PubMed] [Google Scholar]
- 16.Cao J, Yang X, Liu YN.Suo ZW, Shi L, Zheng CR, Yang HB, Li S and Hu XD. GABAergic disinhibition induced pain hypersensitivity by upregulating NMDA receptor functions in spinal dorsal horn. Neuropharmacology 2011; 60: 921–929. [DOI] [PubMed] [Google Scholar]
- 17.Yowtak J, Wang J, Kim HY.Lu Y, Chung K and Chung JM. Effect of antioxidant treatment on spinal GABA neurons in a neuropathic pain model in the mouse. Pain 2013; 154: 2469–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verkuyl JM, Hemby SE, Joels M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur J Neurosci 2004; 20: 1665–1673. [DOI] [PubMed] [Google Scholar]
- 19.Verkuyl JM, Karst H, Joels M. GABAergic transmission in the rat paraventricular nucleus of the hypothalamus is suppressed by corticosterone and stress. Eur J Neurosci 2005; 21: 113–121. [DOI] [PubMed] [Google Scholar]
- 20.Suarez-Roca H, Leal L, Silva JA.Pinerua-Shuhaibar L and Quintero L. Reduced GABA neurotransmission underlies hyperalgesia induced by repeated forced swimming stress. Behav Brain Res 2008; 189: 159–169. [DOI] [PubMed] [Google Scholar]
- 21.Lin Y, Bloodgood BL, Hauser JL.Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN and Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature 2008; 455: 1198–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloodgood BL, Sharma N, Browne HA.Trepman AZ and Greenberg ME. The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature 2013; 503: 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegel I, Mardinly AR, Gabel HW.Bazinet JE, Couch CH, Tzeng CP, Harmin DA and Greenberg ME. Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell 2014; 157: 1216–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshihara S, Takahashi H, Nishimura N.Kinoshita M, Asahina R, Kitsuki M, Tatsumi K, Furukawa-Hibi Y, Hirai H, Nagai T, Yamada K and Tsuboi A. Npas4 regulates Mdm2 and thus Dcx in experience-dependent dendritic spine development of newborn olfactory bulb interneurons. Cell Rep 2014; 8: 843–857. [DOI] [PubMed] [Google Scholar]
- 25.Yun J, Koike H, Ibi D.Toth E, Mizoguchi H, Nitta A, Yoneyama M, Ogita K, Yoneda Y, Nabeshima T, Nagai T and Yamada K. Chronic restraint stress impairs neurogenesis and hippocampus-dependent fear memory in mice: possible involvement of a brain-specific transcription factor Npas4. J Neurochem 2010; 114: 1840–1851. [DOI] [PubMed] [Google Scholar]
- 26.Coutellier L, Gilbert V, Shepard R. Npas4 deficiency increases vulnerability to juvenile stress in mice. Behav Brain Res 2015; 295: 17–25. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Fei P, Mu J.Wang H, Li W and Song J. Decreased expression of neuronal Per-Arnt-Sim domain protein 4 gene in the hippocampus of a post-stroke depression rat model. Exp Ther Med 2014; 7: 1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liberzon I, Krstov M, Young EA. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology 1997; 22: 443–453. [DOI] [PubMed] [Google Scholar]
- 29.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain 1996; 64: 493–501. [DOI] [PubMed] [Google Scholar]
- 30.Longden TA, Dabertrand F, Hill-Eubanks DC.Hammack SE and Nelson MT. Stress-induced glucocorticoid signaling remodels neurovascular coupling through impairment of cerebrovascular inwardly rectifying K+ channel function. Proc Natl Acad Sci U S A 2014; 111: 7462–7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Y, Wang D, Qiao T.Yang C, Su Q, Gao G and Xu Z. A single injection of recombinant adeno-associated virus into the lumbar cistern delivers transgene expression throughout the whole spinal cord. Mol Neurobiol 2016; 53: 3235–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichl S, Augustin M, Zahn PK.and Pogatzki-Zahn EM. Peripheral and spinal GABAergic regulation of incisional pain in rats. Pain 2012; 153: 129–141. [DOI] [PubMed] [Google Scholar]
- 33.Asada H, Kawamura Y, Maruyama K. Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T and Obata K. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A 1997; 94: 6496–6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Betley JN, Wright CV, Kawaguchi Y.Erdelyi F, Szabo G, Jessell TM and Kaltschmidt JA. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell 2009; 139: 161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci 1998; 19: 500–505. [DOI] [PubMed] [Google Scholar]
- 36.Smith KR, Kittler JT. The cell biology of synaptic inhibition in health and disease. Curr Opin Neurobiol 2010; 20: 550–556. [DOI] [PubMed] [Google Scholar]
- 37.Ali NJ, Olsen RW. Chronic benzodiazepine treatment of cells expressing recombinant GABA(A) receptors uncouples allosteric binding: studies on possible mechanisms. J Neurochem 2001; 79: 1100–1108. [DOI] [PubMed] [Google Scholar]
- 38.Essrich C, Lorez M, Benson JA.Fritschy JM and Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci 1998; 1: 563–571. [DOI] [PubMed] [Google Scholar]
- 39.Otis TS, De Koninck Y, Mody I. Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci U S A 1994; 91: 7698–7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin HC, Tseng YC, Mao SC.Chen PS and Gean PW. GABAA receptor endocytosis in the basolateral amygdala is critical to the reinstatement of fear memory measured by fear-potentiated startle. J Neurosci 2011; 31: 8851–8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith KR, Muir J, Rao Y.Browarski M, Gruenig MC, Sheehan DF, Haucke V and Kittler JT. Stabilization of GABA(A) receptors at endocytic zones is mediated by an AP2 binding motif within the GABA(A) receptor beta3 subunit. J Neurosci 2012; 32: 2485–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellavance MA, Rivest S. The HPA—immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front Immunol 2014; 5: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Posserud I, Agerforz P, Ekman R.Bjornsson ES, Abrahamsson H and Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut 2004; 53: 1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sagami Y. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut 2004; 53: 958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tache Y. Corticotropin releasing factor receptor antagonists: potential future therapy in gastroenterology? Gut 2004; 53: 919–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdelhamid RE, Kovacs KJ, Pasley JD.Nunez MG and Larson AA. Forced swim-induced musculoskeletal hyperalgesia is mediated by CRF2 receptors but not by TRPV1 receptors. Neuropharmacology 2013; 72: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rijsdijk M, van Wijck AJ, Kalkman CJ. and Yaksh TL. The effects of glucocorticoids on neuropathic pain: a review with emphasis on intrathecal methylprednisolone acetate delivery. Anesth Analg 2014; 118: 1097–1112. [DOI] [PubMed] [Google Scholar]
- 48.Cao J, Wang PK, Tiwari V.Liang L, Lutz BM, Shieh KR, Zang WD, Kaufman AG, Bekker A, Gao XQ and Tao YX. Short-term pre- and post-operative stress prolongs incision-induced pain hypersensitivity without changing basal pain perception. Mol Pain 2015; 11: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koyanagi S, Kusunose N, Taniguchi M.Akamine T, Kanado Y, Ozono Y, Masuda T, Kohro Y, Matsunaga N, Tsuda M, Salter MW, Inoue K and Ohdo S. Glucocorticoid regulation of ATP release from spinal astrocytes underlies diurnal exacerbation of neuropathic mechanical allodynia. Nat Commun 2016; 7: 13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Coz GM, Anton F, Hanesch U. Glucocorticoid-mediated enhancement of glutamatergic transmission may outweigh anti-inflammatory effects under conditions of neuropathic pain. PLoS One 2014; 9: e91393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anacker C, Cattaneo A, Luoni A.Musaelyan K, Zunszain PA, Milanesi E, Rybka J, Berry A, Cirulli F, Thuret S, Price J, Riva MA, Gennarelli M and Pariante CM. Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology 2013; 38: 872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liston C, Cichon JM, Jeanneteau F.Jia Z, Chao MV and Gan WB. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci 2013; 16: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sierra A, Gottfried-Blackmore A, Milner TA.McEwen BS and Bulloch K. Steroid hormone receptor expression and function in microglia. Glia 2008; 56: 659–674. [DOI] [PubMed] [Google Scholar]
- 54.Vielkind U, Walencewicz A, Levine JM. and Bohn MC. Type II glucocorticoid receptors are expressed in oligodendrocytes and astrocytes. J Neurosci Res 1990; 27: 360–373. [DOI] [PubMed] [Google Scholar]
- 55.Sun R, Zhao Z, Feng J. Bo J, Rong H, Lei Y, Lu C, Zhang X, Hou B, Sun Y, Liu Y, Ma Z and Gu X. Glucocorticoid-potentiated spinal microglia activation contributes to preoperative anxiety-induced postoperative hyperalgesia. Mol Neurobiol 2017; 54: 4316–4328. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Z, Wu H, Liu Y.Gu X, Zhang W and Ma Z. The GCs-SGK1-ATP signaling pathway in spinal astrocytes underlied presurgical anxiety-induced postsurgical hyperalgesia. Anesth Analg 2018. [DOI] [PubMed]
- 57.Bersten DC, Bruning JB, Peet DJ. and Whitelaw ML. Human variants in the neuronal basic helix-loop-helix/Per-Arnt-Sim (bHLH/PAS) transcription factor complex NPAS4/ARNT2 disrupt function. PLoS One 2014; 9: e85768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sabatini PV, Krentz NA, Zarrouki B.Westwell-Roper CY, Nian C, Uy RA, Shapiro AM, Poitout V and Lynn FC. Npas4 is a novel activity-regulated cytoprotective factor in pancreatic beta-cells. Diabetes 2013; 62: 2808–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Z, Fei P, Mu J.Li W and Song J. Hippocampal expression of aryl hydrocarbon receptor nuclear translocator 2 and neuronal PAS domain protein 4 in a rat model of depression. Neurol Sci 2014; 35: 277–282. [DOI] [PubMed] [Google Scholar]
- 60.Ibi D, Takuma K, Koike H. Mizoguchi H, Tsuritani K, Kuwahara Y, Kamei H, Nagai T, Yoneda Y, Nabeshima T and Yamada K. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurochem 2008; 105: 921–932. [DOI] [PubMed] [Google Scholar]
- 61.Drouet JB, Peinnequin A, Faure P.Denis J, Fidier N, Maury R, Buguet A, Cespuglio R and Canini F. Stress-induced hippocampus Npas4 mRNA expression relates to specific psychophysiological patterns of stress response. Brain Res 2018; 1679: 75–83. [DOI] [PubMed] [Google Scholar]
- 62.Luoni A, Fumagalli F, Racagni G. and Riva MA. Repeated aripiprazole treatment regulates Bdnf, Arc and Npas4 expression under basal condition as well as after an acute swim stress in the rat brain. Pharmacol Res 2014; 80: 1–8. [DOI] [PubMed] [Google Scholar]
- 63.Vyas A, Mitra R, Shankaranarayana Rao BS. and Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 2002; 22: 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drake-Baumann R. Activity-dependent modulation of inhibition in Purkinje cells by TrkB ligands. Cerebellum 2006; 5: 220–226. [DOI] [PubMed] [Google Scholar]
- 65.Furukawa-Hibi Y, Yun J, Nagai T. and Yamada K. Transcriptional suppression of the neuronal PAS domain 4 (Npas4) gene by stress via the binding of agonist-bound glucocorticoid receptor to its promoter. J Neurochem 2012; 123: 866–875. [DOI] [PubMed] [Google Scholar]
- 66.Wang S, Lim G, Yang L.Sung B and Mao J. Downregulation of spinal glutamate transporter EAAC1 following nerve injury is regulated by central glucocorticoid receptors in rats. Pain 2006; 120: 78–85. [DOI] [PubMed] [Google Scholar]
- 67.Wang S, Lim G, Zeng Q.Sung B, Yang L and Mao J. Central glucocorticoid receptors modulate the expression and function of spinal NMDA receptors after peripheral nerve injury. J Neurosci 2005; 25: 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li C, Yang Y, Liu S.Fang H, Zhang Y, Furmanski O, Skinner J, Xing Y, Johns RA, Huganir RL and Tao F. Stress induces pain transition by potentiation of AMPA receptor phosphorylation. J Neurosci 2014; 34: 13737–13746. [DOI] [PMC free article] [PubMed] [Google Scholar]