Abstract
Since the beginning of human civilization, plants have been used in alleviating the human distress and it was recorded for about thousands of years ago that the plants are being used for medicinal purposes. Natural bioactive compounds called phytochemicals are obtained from medicinal plants, vegetables, and fruits, which functions to combat against various ailments. There is dire need to explore the plant biodiversity for its medicinal and pharmacological potentials. Different databases such as Google scholar, Medline, PubMed, and the Directory of Open Access Journals were searched to find the articles describing the cardioprotective function of medicinal plants. Various substances from a variety of plant species are used for the treatment of cardiovascular abnormalities. The cardioprotective plants contain a variety of bioactive compounds, including diosgenin, isoflavones, sulforaphane, carotinized, catechin, and quercetin, have been proved to enhance cardioprotection, hence reducing the risk of cardiac abnormalities. The present review article provides the data on the use of medicinal plants particularly against cardiac diseases and to explore the molecules/phytoconstituents as plant secondary metabolites for their cardioprotective potential.
Keywords: cardioprotection, phytochemicals, cardiotoxicity, phytotherapeutic
Introduction
Heart attack, also called myocardial infarction (MI), and related complications are the main causes of deaths throughout the world.1 The use of herbal antioxidants is increasing as defensive agents against number of cardiovascular abnormalities. The bioactive agents from natural sources have gained fundamental importance in modern system of medicines, reducing the risks of cardiac ailments by scavenging the free radicals formation.2 Herbal medicines play considerable role in health care to a large proportion of world’s population and have been regarded as component of cultural heritage of various tribes. Polyphenols perform cardioprotective activity by inhibiting the oxidation of low-density lipoprotein.3 Most of the pharmacologically important drugs are derived from plants. Plant derivatives as drugs play significant role in health-care systems around the globe for animals and humans. They not only used for the management of disease condition but also to maintain proper health. Since long, medicinal plants have been used for the treatment of ischemic heart diseases. Accumulation of phytochemical, biological, and clinical data during past decade of 20th century revealed that plant-based herbal remedies are the emerging choice for the treatment of various ailments. Medicinal plants such Daucus carota Linn, Nerium oleander (NO) Linn, Amaranthus viridis, Ginkgo biloba, Terminalia arjuna, Tinospora cordifolia, Hydrocotyle asiatica Linn, Mucuna pruriens, and Cichorium intybus are known to have cardioprotective potential. Large number of important phytochemicals has been identified from plant sources by the scientists. This review article provides useful information for researchers and clinicians using natural products as therapy for clinical management of cardiovascular abnormalities, leading to the development of more efficient therapeutic drugs. For example, Digitalis lanata is one of the oldest medicinal plants widely used for the treatment of cardiac diseases and the most active constituent present in it is a steroid glycoside called digoxin. Digoxin was also found to be used for the treatment of arrhythmia.4 Another plant Atropa belladonna contains atropine, which is being used for slow heart rate (bradycardia).5 Most of the widely used modern medicines for the treatment of cardiovascular abnormalities have side effects and there is dire need to search for alternative therapies with fewer or no side effects.
Plants are pivotal source of traditional medicines being used in treating different ailments. About 4 22 000 flowering plants have been reported all over the world, out of which above 50 000 plants are of medicinal importance that are being used for pharmaceutical purposes.6 About 80% of worldwide populations rely on traditional medicines for primary health-care needs.7 Remedies of medicinal plants are most often being used as an alternative to allopathic medicines. Pakistan is blessed with an exceptional biodiversity expanded along the 9 major ecological zones. The major parts of the country are fairly rich in medicinal herbs because of its healthy atmosphere.8 So far, in Pakistan about 6000 plant species have been reported with documented ethnomedicinal knowledge of only 600 plant species. There is a tremendous demand to safeguard the precious traditional knowledge of medicinally important plants. Preservation and promotion of indigenous knowledge about medicinally important plants leads to the discovery of new drugs in addition to rescuing the global traditional medicinal systems.6
Medicinal Plants With Cardioprotective Potential
Medicinal plants are used to prepare many drugs, but the phytochemical compounds present on original plant material are more efficient with less side effects than their pharmaceutical derivatives. Variety of plants and their bioactive phytoconstituents are well known for their minimal side effects, providing alternative therapeutic potential against cardiac diseases. Some of the plants having cardioprotective molecules/agents are given below, and the plants having cardioprotective potential against cardiotoxicity induced by various agents are given in Table 1.
Table 1.
Reported Cardioprotective Activities of Medicinal Plants Against Cardiotoxicity Produced by Various Agents.
| Common Name | Plant Names | Family | Parts Used | Dosage Form | Chemical Constituents | Actions | References |
|---|---|---|---|---|---|---|---|
| Against isoproterenol-induced cardiotoxity | |||||||
| Fountain tree, African tulip tree, pichkari or nandi flame | Spathodea campanulata | Bignoniaceae | Bark | Ethanol extract | Saponin, flavonoids, steroid, alkaloids, glycoside, tannin, phenol, phlobatanin, terpenoids, and anthraquinone | Antimalarial, anti-HIV, hypoglycemic, cardioprotective | 9 |
| Garlic | Allium sativum | Liliaceae | Bulb | Garlic oil | Alkaloids, flavonoids, tannins, saponins, and cardiac glycosides | Antimicrobial, antihyperlipidemic, and cardioprotective | 10 |
| Hairy fig | Ficus hispida | Moraceae | Leaves | Methanol | Alkaloids, terpenes, saponins, glycosides, mucilage, gums, flavonoids, phenols, sterols, amino acids, β-amyrine acetate, protein, carbohydrates, n-triacontanol, lupeol acetate, β-sitosterol, gluanol, and β-amyrin | Cardioprotective, antipyretic, hepatoprotective, anti-inflammatory | 11 |
| Kerala ginseng, ginseng of Kani tribes | Trichopus zeylanicus | Trichpodaceae | Leaves | Ethanol | Alkaloids, glycosides, flavonoids, steroids, tannins, steroids, terpenoids | Cardioprotective, adoptogenic, aphrodisiac | 12 |
| Gokhru, kharkhask, caltrop | Tribulus terrestris | Zygophyllaceae | Fruit | Aqueous | Flavonol, flavonoids, alkaloids, glycosides, and steroidal saponins | Cardioprotective, antilithiatic, diuretic, hypouricemic, anti-inflammatory, aphrodisiac | 13,14 |
| Baladur, billar, bhilavan | Semecarpus anacardium | Anacardiaceae | Dried nuts | Ethanol | Bhilwanols, phenolic compounds, biflavonoids, sterols, glycosides, ursuhenol, anacardoside, semecarpetin, nallaflavanone, jeediflavanone, semecarpuflavanone | Cardioprotective, antioxidant, anticancer, antidiabetic | 15 |
| Kalmegh | Andrographis paniculata | Acanthaceae | Leaves | Methanol | Andrographolide, diterpenoids, flavonoids, quinic acid, xanthones, noriridoids, and andrographidoids A, B, C, D, and E | Cardioprotective, gastroprotective, antioxidant | 16 |
| Zafran, Saffron | Crocus sativus | Iridaceae | Flowers | Aqueous | Carotenoid compounds, crocetin, crocin, safranal, glucoside picrocrocin, anthocyanins, delphinidin, petunidin | Cardioprotective, hypnotic, anxiolytic, anticancer | 17 |
| Tulsi | Ocimum sanctum | Lamiaceae | Seeds | Hydroalcohol | Alkaloids, saponins, tannin, steroid, flavonoids, terpenoid | Cardioprotective, antioxidant, hypolipidemic, hypoglycemic | 18 |
| Basil, Saint-Joseph’s-wort | Ocimum basilicum | Lamiaceae | Aerial parts | Ethanol | Flavonoids, phenolic compounds | Antibacterial, antifungal, antioxidant, and cardioprotective | 19 |
| Moringa, drumstick tree | Moringa oleifera | Moringaceae | Leaves | Hydroalcohol | Tannins, saponins, alkaloids, terpenes, carbohydrates, flavonoids, and cardiac glycosides | Anticancer, anti-inflammatory, antipyretic, and cardioprotective | 20,21 |
| Bottle gourd | Lagenaria siceraria | Cucurbitaceae | Fruit | Juice | Sterols, flavonoids, terpenoids, and saponin | Antioxidant, antihyperlipidemic, and cardioprotective | 22 |
| Picrorhiza, kutki, katuka | Picrorhiza kurroa | Scrofulariaceae | Rhizome | Ethanol | Sterols, glycosides, phenolic compounds, cucurbitacins (triterpenoids), and iridoid glycosides | Antioxidant, anti-inflammatory, and cardioprotective | 23 |
| Ban tulasi, raan tulas | Croton sparsiflorus | Euphorbiaceae | Leaves | Methanol | Terpenoids, saponins, tannins, phenols, flavonoids, alkaloids | Antinociceptive, anti-inflammatory, and cardioprotective | 24 |
| Against isoprenaline hydrochloride–induced cardiotoxicity | |||||||
| Neem tree, Indian lilac | Azadirachta indica | Meliaceae | Aqueous | Leaves | Reducing sugar, tannins, flavonoids, steroids, terpenoids, glycosides, and alkaloids | Cardioprotective, chemopreventive, antiplasmodial, anti-inflammatory | 25 |
| Gander | Coleus forskohlii | Lamiaceae | Roots | Ethanol | Forskolin hydrochloride, demethylcryptojaponol, α-amyrin, betulic acid, α-cedrol and β-sitosterol, diterpene glycosides, and diterpenoids forskolin | Antihypertensive, antithrombotic, antiobesity, and cardioprotective | 26 |
| Kokum, red mango tree | Garcinia indica | Clusiaceae | Fruit | Aqueous | Garcinol, isoxanthochymol, xanthochymol, hydroxycitric acid, phenolic acids, flavonoids, benzophenones, isogarcinol, anthocyanins, and tannins | Cardioprotective, antibacterial, hepatoprotective, antioxidant | 27 |
| Against ischemia-reperfusion–induced cardiotoxicity | |||||||
| Hawthorn | Crataegus oxyacantha | Rosaceae | Berries | Ethanol | Flavonoids, oligomeric procyanidins, triterpenes, phenolic acids, fatty acids, and sterols | Anti-inflammatory, antiapoptotic, and cardioprotective | 28 |
| Lemon guava, Guava | Psidium guajava | Myrtaceae | Leaves | Aqueous | Phenolic, carotenoid, flavonoid, terpenoid, and triterpene. | Cardioprotective, antispasmodic, antidiabetic | 29 |
| Gotu kola, Brahmi | Hydrocotyle asiatica | Umbelliferae | Whole plant | Alcohol | Alkaloids, flavonoids, and glycosides | Cardioprotective, antipsoriatic, neuroprotective | 30 |
| Maqui berry | Aristotelia chilensis | Elaeocarpaceae | Fruits | Methanol | Phenolic compounds, anthocyanidins, flavonoids, delphinidin, cyanidin, gallate, gallocatechin gallate, quercetin, rutin, myricetin, and catechin action | Cardioprotective, antioxidant, analgesic, anti-inflammatory | 31 |
| Arjuna or arjun tree | Terminalia arjuna | Combretaceae | Bark | Alcohol | Lactones, phytosterol, flavonoids, phenolic compounds, glycosides, and tannins | Antioxidant, antihyperlipidemic, and cardioprotective | 32 |
| Against doxorubicin-induced cardiomyopathy | |||||||
| Bottle brush | Callistemon lanceolatus | Myrtaceae | Leaves | Ethanol | Phenolic compounds, carbohydrates, saponins, alkaloids, flavonoids, glycosides, phytosterols, and tannins | Anti-inflammatory, antioxidant, and cardioprotective | 33 |
| Stone breaker, Black katnip | Phyllanthus niruri | Phyllanthaceae | Whole plant | Aqueous | Flavonoids, terpenoids, alkaloids, lignans, tannins, polyphenols, coumarins, and saponins | Cardioprotective, anticancer, antimicrobial, hypolipidemic, antihepatotoxic | 34 |
| Turmeric | Curcuma longa | Zingiberaceae | Rhizome | Ethanol | Curcumin, ar-turmerone, β-sesquiphellandrene, curcumenol, sesquiterpenes, and phenolic constituents | Cardioprotective, anti-inflammatory, antioxidant | 35 |
| Shershir | Tribulus macropterus | Zygophyllaceae | Aerial parts | Methanol | Flavonoids, saponins, alkaloids, glycosides, and flavonol | Cytotoxic and cardioprotective | 36 |
| Olive | Olea europaea | Oleaceae | Aerial parts | Methanol | Flavonoids, iridoids, secoiridoids, flavanones, benzoic acid derivatives, and triterpenes | Antidiabetic, anticancer, antimicrobial, and cardioprotective | 36 |
| Athel tree, Athel pine, and saltceda | Tamarix aphylla | Tamaricaceae | Aerial parts | Methanol | Alkaloids, tannins, glycosides, phenolic compounds, and saponins. | Antidiabetic, anticholinsterase, antioxidant, and cardioprotective | 36 |
| Sorrel | Hibiscus sabdariffa | Malvaceae | Petals | Aqueous | Tannins, saponnins, phenols, glycosides, alkaloids, and flavonoids | Antihypertensive, antioxidant, and cardioprotective | 37 |
| Malta fungus | Cynomorium coccineum | Cynomoriaceae | Aerial parts | Methanol | Alkaloids, glycosides, anthraquinones, flavonoids, tannins, saponins, and terpenoids | Antioxidant, antihypertensive, and cardioprotective | 36 |
| Assyrian plum | Cordia myxa | Boraginaceae | Fruit | Methanol | Flavonoids, saponins, and tannin | Anti-inflammatory, analgesic, and cardioprotective | 36 |
| Calligonum comosum | Polygonaceae | Aerial parts | Methanol | Flavonoids, anthraquinones, and dehydrodicatechin | Cytotoxic, anticancer, and cardioprotective | 36 | |
| Camellia sinensis | Theaceae | Leaves | Aqueous | Tannins, flavonoids, steroids, and flavonoids | Antioxidant, antiobesity, and cardioprotective | 38 | |
| Withania somnifera | Solanaceae | Leaves and roots | Herbal tablet | Alkaloids, steroids, glycosides, hentriacontane, dulcitol, withaniol, withananine, withananinine, pseudo-withanine, tannins, and flavonoids | Anti-inflammaory, analgesic, immunomodulant, antirhematic, and cardioprotective | 39 | |
| Ficus racemosa | Moraceae | Bark | Acetone | Flavonoids, triterpenoids, alkaloids, tannins, kaempferol and coumarin, glycoside | Antioxidant, hepatoprotective, and cardioprotective | 40 | |
| Onion | Allium cepa | Alliaceae | Leaves | Methanol | Flavonoids, triterpenic acids, amino acids, steroids | Cardioprotective, antibacterial, antioxidant, hypouricemic | 41 |
| Against cigarette smoke–exposed rats | |||||||
| Sesbania grandiflora | Fabaceae | Leaves | Aqueous suspension | Alkaloids, flavonoids, glycosides, tannin, anthraquinone, steroid, pholobatannins, and terpenoids | Antibacterial, anxiolytic, and cardioprotective | 42 | |
| Against glucose-induced oxidative stress in H9C2 cardiomyocytes | |||||||
| Syzygium cumini | Myrtaceae | Seeds | Methanol | Anthocyanins, ellagic acid, glucoside, kaemferol isoquercetin, alkaloids, myrecetin, glycosides, and jambosine | Antidiabetic, antioxidant, and cardioprotective | 43 | |
| Against Naja sputatrix (Javan spitting cobra) venom | |||||||
| Velvet bean, Cowhage | Mucuna pruriens | Fabaceae | Seeds | Aqueous | Alkaloids, sterols, saponins, alkylamines, 6-methoxyharman, mucunain, mucunadine, and mucunine | Cardioprotective, antidepressant, neuroprotective | 44 |
Daucus carota
Daucus carota is a white-flowering herb belongs to Apiaceae plant family and is generally recognized as wild carrot. This plant is native to temperate regions of Southeast Asia and Europe. Parts used for medicinal preparations are roots and seeds. The phytochemicals present in this plant include daucosol, xanthophylls, carotene, sesquiterpenoids, and daucoside.45,46 Muralidharan et al47 studied the cardioprotective potential of D carota Linn’s aqueous extract in isoproterenol-induced MI in rats. They studied the cardioprotection by determining the activity of cardiac enzymes like transaminases, lipid peroxidases, cardiac protein, and lactate dehydrogenase (LDH).
Nerium oleander
Nerium oleander (NO) from Apocynaceaefamily is an ever green shrub that grows primarily in the Easter Mediterranean regions, northern America, and Anatolia. By boosting antioxidant components against oxidative stress, NO concentrate has been experimentally shown to serve as a cardioprotective agent.48 Parts used for pharmaceutical preparations are leaves, flowers, roots, and root bark. Phytochemicals present in this plant includes tannic acid, oleanolic acid, uzarigenin, neriodorein, oleandrose, karabin, neriodin, nerium D, nerium F, oleanolic acid, digitoxigenin, gitoxigenin, neriantin, odoroside, adyresin, ursolic acid, oleandrin, scopolin, scopoletin, oleandrigenin, 16-acetyl gitoxigenin, deacetyloleandrin, and dambonitol.49 Gayathri et al50 studied the cardioprotective potential of NO flowers in rats using isoproterenol for the induction of myocardial oxidative stress and found good cardioprotective activity of this plant.
Amaranthus viridis
Amaranthus viridis Linn is commonly known as slender amaranth in English while it was called as never fading flower in Greek. It is an annual herb.51,52 Various parts such as leaves, roots, and whole plants are used for pharmacological purpose. Active phytoconstituents are quercetin and rutin.53 This plant also contains variety of amino acids, including leucine, lysine, isoleucine, arginine, cystine, histidine, valine, phenylalanine, methionine, threonine, tryptophan, and tyrosine.54 Various studies reported the cardioprotective potential of A viridis Linn in rats. In a study conducted by Saravanan et al55 to evaluate the cardioprotective potential of this plant using isoproterenol to induce MI at a dose concentration of 20 mg/kg body weight subcutaneously for 2 consecutive days. They observed significant variation in cardiac enzymes. Amaranthus viridis was orally administered at dose concentrations of 100, 200, and 300 mg/kg body weight for 45 days. Lower levels of cardiac enzymes were observed in plant-treated groups of rats, showing its cardioprotective activity. Amaranthus viridis was found more effective at a dose of 300 mg/kg body weight.55
Ginkgo biloba
Ginkgo biloba L belongs to Ginkgoaceae plant family. This plant is also known as “living fossils” because of its existence among the oldest seed plants. It contains Ginkgolides, flavones glycosides, flavonol, ascorbic acid, diterpen lactones, catechin, sesquiterpenes, and iron-based superoxide dismutase. Variety of biological activities of this plant have been reported, including antioxidants, antimicrobial, anti-inflammatory, memory enhancer, hepatoprotective, antidepressant, anticoagulant, antiulcer, cytotoxic, antiaging, and antistress activities. This plant is also popular for its medicinal and nutritional potential.56 Ginkgo biloba also contains many other phytoconstituents, including fatty acids, resins, essential oils, tannins, carotenoids, quercetin, and myricetin.57 Ginkgo biloba extract was observed in improving the blood flow, preventing hypoxia, improving blood rheology, causing platelet aggregation, and reducing the capillary permeability due to the release of prostaglandins and NO.58
Leaves and seeds of G biloba have been reported to have cardioprotective effect. Panda59 in his study administered Ocimum sanctum and G biloba extract in isoproterenol-induced myocardial necrosis rats. He found an increase in serum enzymes of isoproterenol-induced myocardial necrosis rats compared with serum enzymes of normal rats. The rats were given O sanctum (50 and 75 mg/kg body weight) and G biloba (100 mg/kg body weight) orally for 1 month. Isoproterenol at a dose of 85 mg/kg body weight was administered through subcutaneous route. Serum enzymes level was reduced significantly on the 29th and 30th days of therapy. Ocimum sanctum (100 mg/kg body weight) and G biloba (50 mg/kg body weight) showed significant cardioprotection as compared to combined administration of O sanctum (50 mg/kg body weight) and G biloba (100 mg/kg body weight).59
Terminalia arjuna
Terminalia arjuna is a large ever green tree with an average height of about 60 to 80 feet. This plant belongs to family Combretaceae. It is found most abundantly all around the sub-Himalayan tracts in India. It’s bark outer covering is gray brown while the inside is red. Arjuna plant contains variety of phytoconstituents, including flavonoids, triterpenes, and tannins.60 Leaves and barks of Arjuna plant have cardioprotective activity. Phytoconstituents are arjunetin, polyphenols, β-sitosterol, freidelin, arjunic acid, and triterpenes.61 Cardioprotective potential of T arjuna alcoholic extract was investigated against isoproterenol-induced myocardial injury in Wistar rats by administering extract dose concentrations orally for a period of 28 days. After 4 weeks treatment period, the rats were administered subcutaneously with isoproterenol (85 mg/kg body weight) for 2 consecutive days to all the treated animals, except control group rats (normal untreated rats) to induce myocardial injury. Results of the study showed that T arjuna restored the myocardial ischemic–reperfusion injury induced by isoproterenol protecting the myocardium.31 In another study conducted for investigating the cardioprotective effect of T arjuna bark aqueous extract on mice model against DOX-induced cardiotoxicity. The study concluded that T arjuna aqueous extract is a relatively safe and promising cardiotonic with cardioprotective potential. The bark extract of this plant is beneficial for healthy heart that can be used as cardioprotective agent in adjuvant chemotherapy for patients with cancer.62
Picrorhiza kurroa
Picrorhiza kurroa from Scrophulariaceae plant family is a high-value medicinal plant commonly known as “Kutki.” This plant grows mainly in Kashmir to Kum in the northern western Himalayan region while in India’s Garhwal and Sikkim regions; it grows above mean sea level at an altitude of 3000 to 4500 miles. The genus Picrorhiza in recent past has attracted the great interest and the promising role of P kurroa formulations has been revealed in many chemical and pharmacological investigations.63,64 Picrorhiza kurroa is considered a bitter drug that is rich in iridoid glycosides with many biological activities such as antioxidant, anticholestatic, anti-inflammatory, immunomodulatory, and hepatoprotective activities.64 Chemical constituents found in this plant are berberine, kurrin, picrorhizetin, kutkisterol, sesquiterpene, apocynin, cathartic acid, and kutkin.65 Cardioprotective activity of P kurroa ethanolic extract was investigated in isoproterenol-induced MI using rats model. Significant cardioprotective activity of P kurroa extract was observed at dose concentration of 80 mg/kg body weight.23
Salvia miltiorrhiza
Salvia miltiorrhiza belongs to Labiatae plant family that is widely used against cardiovascular abnormalities for disease prevention and treatment.66 This plant has a long history of its medicinal use as well as healthy food and is considered as an essential herbal plant widely used in Chinese traditional medicinal system. In Asia, Europe, and the United States, the rhizome and roots of this plant are extensively used in treating cerebrovascular and cardiovascular diseases in the form of tablets, injection solutions, oral liquid, capsule, and slow-release formulation.67
The active ingredients of this plant include both the lipid-soluble and water-soluble substances. The lipophilic substances are tanshinones, which includes tanshinone I, dihydrotanshinone I, tanshinone IIA, cryptotanshinone, and tanshinone IIB.67,68 While the water-soluble constituents are phenolic acids such as caffeic acid, danshenu, salvianolic acid A, salvianolic acid B, and rosmarinic acid. Salvia miltiorrhiza phenolic acids showed many biological actions including antithrombotic, antioxidant, antitumor, anticoagulant, anti-HIV, and anti-blood coagulation activities.67 Cardioprotective potential of S miltiorrhiza extract was investigated in experimental rats against isoproterenol-induced MI. Isoproterenol-treated rats showed reduction in left ventricular systolic pressure with increased serum glutamic oxaloacetic transaminase (SGOT) level and elevated ST-segment. Antioxidant enzymes such as superoxide dismutase and glutathione peroxidase activity were reduced in isoproterenol-treated rats. Salvia miltiorrhiza extract was administered orally at dose of 29.76 or 59.52 mg/kg body weight. Salvia miltiorrhiza extract reversed the isoproterenol-induced hemodynamic and biochemical changes, indicating cardioprotection against isoproterenol-induced MI.69
Tinospora cordifolia
Tinospora cordifolia (Wild) from genus Tinospora is a climbing shrub and is well known as “amrita” in Sanskrit and Hindi while “amudamor chindle” in Tamil. It is found throughout the tropical India. The roots and stem of this plant are extremely important in Ayurvedic and tribal medicinal systems. Its preparations are useful for the cure of jaundice, fever, diabetes, respiratory disorders, rheumatism, and neurological abnormalities.70 The leaves, fruits, roots, and stem of T cordifolia possess cardioprotective activity. Phytoconstituents are tinosporin, tinosporic acid, tinosporol, giloin, giloinin, gilosterol, columbin, chasmanthin, palmarin, steroids, glycosides, sesquiterpenoids, diterpenoid lactones, and berberine.70,71 Cardioprotective activity of T cordifolia alcoholic extract was investigated in a study using rat models. Surgical occlusion of coronary artery was performed to induce myocardial ischemia and then reperfusion for 4 hours. Results showed that T cordifolia treatment reduces the infarct size and decreased the lipid peroxide level compared to control group, indicating the cardioprotective activity of this plant.72
Hydrocotyle asiatica
Phytochemicals found in H asiatica whole plant are asiaticoside, tannic acid, and vallarin. Cardioprotective activity of H asiatica alcoholic extract (100-1000 mg/kg body weight) has been investigated against ischemia-reperfusion–induced MI in rats by oral administration of plant extract for 1 week. Dose-dependent response was observed. Results showed considerable decrease in infarct size in extract treated rats as compared to normal untreated rats.30
Bombax ceiba
Bombax ceiba L belongs to Bombacaceae plant family generally known as kapok tree or red silk cotton that grows in India and other countries such as Sri Lanka, Myanmar, and Indonesia.73 Pharmacologically active parts are leaves, flowers, fruits, buds, barks, gums, seeds, and roots. This plant contains tannins, flavonoids, β-sitosterol, lupeol, glycosides naphthoquinone, n-triacontanol, and sesquiterpenoids.74 Patel et al75 reported the cardioprotective potential of B ceiba flowers aqueous extract against cardiotoxicity induced by adriamycin as compared to vitamin E.
Centella asiatica
Centella asiatica (L) belongs to Apiaceae plant family generally known as Asiatic pennywort. It is regularly used as medicinal herb or a culinary vegetable in many Asian countries, including Sri Lanka, India, China, and Thailand.76 Phytoconstituents found in this plant includes tannins, phenols, vallarine, sitosterol, hersaponin, hydrocotylin, bacogenin, triterpenes, asiaticoside, and asiatic acid.77 Gnanapragasam et al.78 investigated the effect of C asiatica on cardiac and antioxidant enzymes of experimental animals with adriamycin-induced cardiomyopathy. Induction of myocardial damage due to adriamycin (2.5 mg/kg body weight intraperitoneal) was evident from elevated levels of serum enzymes, including aspartate aminotransferase, alanine aminotransferase, LDH, and creatine phosphokinase. Centella asiatica (200 mg/kg body weight) treatment prevented these alterations and restored the enzyme activities to normal, indicating cardioprotective activity of this plant.
Sonchus asper
Sonchus species are herbaceous plants extensively distributed in Asia, Europe, and Africa. Sonchus species aerial parts are rich in essential amino acids, minerals, vitamins, and protein that help reduce hypoalimentation-associated abnormalities. These plant species are generally used in decoctions or infusions administered externally or orally in treating cancer, acute icterohepatitis, inflammation, diarrhea, snake venom poisoning, and rheumatism.79 This plant contains phenols, flavonoids, flavonols, alkaloids, riboflavins, thiamine, niacin, tannins, sesquiterpenes, and proanthocyanidin.80 Khan et al81 studied the cardioprotective effect of Sonchus asper methanolic extract against oxidative damage induced by KBrO3 in cardiac tissues of Sprague-Dawley male rats. They found significant cardioprotective activity of S asper methanolic extract (100 and 200 mg/kg body weight) against KBrO3-induced oxidative stress.
Mucuna pruriens
Mucuna pruriens (L) DC Is commonly called as velvet bean and is native to the East India and China.82 Chemical constituents are tannins, iron, zinc, calcium, aluminum, steroids, tetrahydroisoquoline, and glycosides.83 Mucuna pruriens seeds are rich in l-3,4-dihydroxy phenylalanine (l-dopa). This L-DOPA is the precursor of a neurotransmitter dopamine most often used in treating Parkinson disease.82 Fung et al.84 studied the cardioprotective potential of M pruriens against Naja sputatrix (Javan spitting cobra) venom in experimental rats. Cardiorespiratory and neuromuscular depressant activity of N sputatrix was attenuated through pretreatment with Mucuna extract, which might be due to cobra venom toxins neutralization by antibodies elicited with M pruriens extract.
Andrographis paniculata
Andrographis paniculata (AP) belongs to Acanthaceae family and is well known due to its medicinal importance. It is widely used for medicinal purposes throughout the world, including China, India, Bangladesh, Pakistan, Thailand, Hong Kong, Malaysia, Philippines, and Indonesia as traditional herbal medicine. It is one of the most commonly used medicinal plant in Ayurvedic and Unani medicines.85 Chemical constituents found in this plant are sodium, potassium, glycosides, flavonoids, tannic acid, diterpene lactone andrographolide, kalmeghin, 14-deoxy andrographolide, and 14-deoxy-11,12-didehydro andrographolide.85,86 Woo et al87 reported cardioprotective activity of AP against reoxygenation/hypoxic injury in neonatal rat cardiomyocytes, upregulating the antioxidant enzyme activities and reduced cellular glutathione level.
Cichorium intybus
Cichorium intybus from genus Cichorium belongs to Asteraceae plant family. The genus consists of 6 species mainly distributed in Asia and Europe. Cichory plant contains a number of phytocompounds of medicinal importance, such as flavonoids, coumarins, vitamins, inulin, volatile compounds, esculin, and lactones.88 Cichory contains volatile oils, phenolics, flavonoids, alkaloids, glycosides, saponins, tannins, fatty acids, emodine, triterpenoids, and anthracene.89 Nayeemunnisa et al90 studied the C intybus for its cardioprotective potential against aging myocardium in albino rats by administering the plant powder for 30 days. They concluded that aging caused an increase in taurine and glutathione level while decreasing the catalase activity in heart. Treatment with cichory plant ameliorated the oxidative damage and aging-induced injury of the heart.
Sesbania grandiflora
Sesbania grandiflora belongs to Fabaceae family. This plant is native to Southeast Asia.91 Phytoconstituents are vitamin A, C, riboflavin, nicotinic acid, amino acids, and minerals.92 Ramesh et al42 conducted a study to investigate the S grandiflora cardioprotective effect in adult male Wistar-Kyoto rats exposed to cigarette smoke for 90 days to induce oxidative damage. The rats were given S grandiflora (1000 mg/kg body weight) aqueous suspension orally for 3 weeks and an increase in LDH activity with reduction in catalase, glutathione peroxidase, glucose-6-phosphate dehydrogenase, glutathione-S-transferase, glutathione reductase, and cardiac superoxide dismutase activities in cigarette smoke–exposed rats. The study concluded that S grandiflora reduces the oxidative stress protecting the heart from cigarette smoke–induced oxidative damage.
Phytoconstituents
Various phytoconstituents found in plants having cardioprotective potential are given below and listed in Table 2.
Table 2.
Phytochemicals Responsible for Cardioprotective Activity.
| Botanical Name | Family | Part Used | Phytoconstituents | References |
|---|---|---|---|---|
| Allium sativum | Liliaceae | Bulb | Allicin, sulfur compounds | 93 |
| Anacardium occidentale | Anacardiaceae | Stem bark | Flavonoids, carotenoids | 94 |
| Buxus microphylla | Buxaceae | Leaves | Cyclovirobuxine D | 95 |
| Antiaris toxicaria | Moraceae | Bark | Cardiac glycosides | 96 |
| Asparagus racemosus | Asparagaceae | Roots | Saponin-shatavarins 1-1V | 97 |
| Ganoderma lucidum | Ganodermataceae | Fruit | Triterpenes | 98 |
| Leptadenia pyrotechnica | Asclepiadaceae | Aerial parts | Triterpenoids | 98,99 |
| Digitalis purpurea | Scrophulariaceae | Leaves | Cardiac glycosides | 100 |
| Tinospora cordifolia | Menispermaceae | Whole plant | Bitter constituents including tinosporon, tinosporol, tinosporic acid, palmarin, chasmanthin, and columbin; alkaloidal constituents including berberine | 101 |
| Crataeva nurvala | Capparidaceae | Stem bark | Pentacyclic triterpene, lupeol and its ester | 102 |
| Raphanus sativus | Cruciferae | Fruit | Caffeic acid | 103 |
| Crocus sativus | Iridaceae | Flowers | Crocin | 104 |
| Glycyrrhiza glabra | Leguminaceae | Roots | Glycyrrhizic acid | 105 |
| Garcinia kola | Guttiferae | Seeds | Kolaviron | 106 |
| Garcinia mangostana | Guttiferae | Fruit | α Mangostin | 107 |
| Morus alba | Moraceae | Leaves | Morin | 108 |
| Aegle marmelos | Rutaceae | Fruit | Marmesin | 109 |
| Catharanthus roseus | Apocynaceae | Leaves | Vincristine | 110 |
| Moringa oleifera | Moringaceae | Leaves | Vincosamide | 111 |
| Zingiber officinale | Zingiberaceae | Rhizome | Zingerone | 112 |
Cyclovirobuxine D
It contains cyclovirobuxine-D, steroidal alkaloid, artemetin, 4′,5-dihydroxy-3,3′,7-tetra methoxy flavones, (−)-(Z)-buxenone, (−)-(E)-buxenone.113 Yu et al114 studied the Buxus microphylla to investigate its cardioprotective potential in experimental rats against left coronary artery occlusion-induced heart failure. The rats were given cyclovirobuxine D, derivative of B microphylla for a period of 4 weeks. Cardiac functions, hemodynamics, microcirculation, histology, and mortality assessments of experimental rats were recorded. They found that cyclovirobuxine D is useful for the management of cardiac failure due to occlusion of left coronary artery, leading to the development of new therapeutic agents for the treatment of cardiac failure.
Withanolides
Cardioprotective potential of Withania somnifera (300 mg/kg body weight) purified extract (withanolide 1.5%) was investigated using male Wistar rats. Rats were given doxorubicin (10 mg/kg body weight) to induce necrosis and apoptosis in cardiac tissues. Doxorubicin administration in rats causes elevation in protein carbonyl levels, catalase activity, and malondialdehyde due to oxidative stress. Total antioxidant capacity and superoxide dismutase activity were exhausted in heart tissues. The study concluded that W somnifera possess efficient cardioprotective potential against doxorubicin-induced cardiotoxicity.39
Silymarin
Cardioprotective efficacy of silymarin was carried out in experimental rats against ischemia-reperfusion–induced MI. Rats were given 2 different doses (100, 250, and 500 mg/kg body weight) of silymarin for 7 days. Occlusion of left anterior descending coronary artery was performed after 1 week of silymarin treatment for 30 minutes in control (ischemia–reperfusion) and test (silymarin-treated) group rats and then reperfused for 4 hours. Control group rats showed significant cardiac necrosis as evident from elevated serum enzyme levels (SGPT, SGOT, and LDH). Silylmarin administration resulted in the restoration of endogenous antioxidant enzyme activities, suppressed neutrophil infiltration, and reduced infarct area in test group rats as compared to control group rats.30
Flax lignin
Linum usitatissimum seeds extract (flax lignan concentrate) was studied for cardioprotective activity against isoprenaline-induced myocardial necrosis in rats.115 Male Wistar rats (200-230 g) were divided into 3 groups as control group, isoprenaline group, and test (flax lignin treated) group. Test group rats were administered with flax lignin concentrate (500 mg/kg body weight) for 8 days, while isoprenaline was given to rats except control rats at a dose of 5.25 and 8.5 mg/kg body weight subcutaneously during 9th and 10th day of therapy, respectively. Isoprenaline-induced cardiotoxicity was evident from the elevated cardiac enzymes level, while flax lignin concentrate restored the activities of cardiac enzymes by lowering the serum enzymes level in cardiotoxicity-induced rats. This study concluded that flax lignin concentrate has cardioprotective effect on isoprenaline-induced cardiotoxicity.
Cardioprotective Mechanism of Medicinal Plants
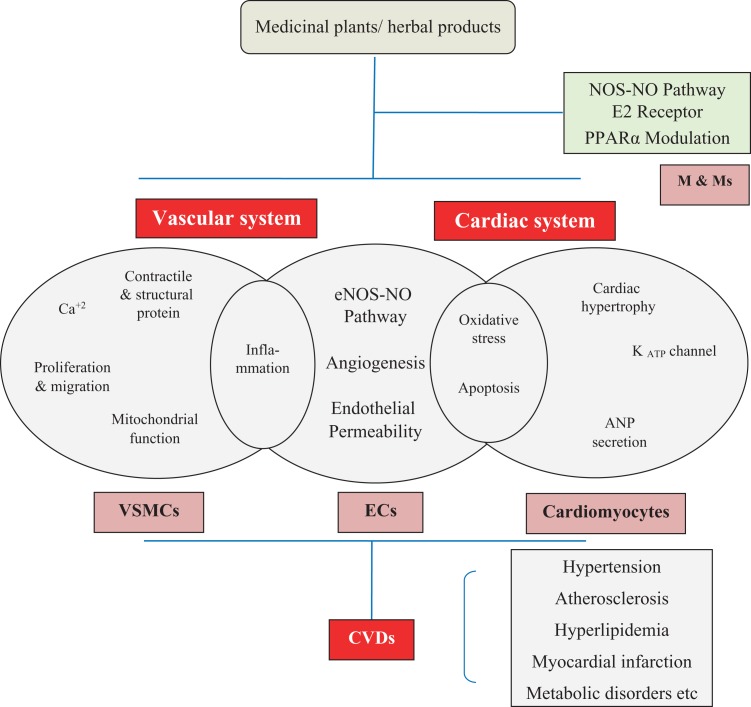
Since ancient times, numerous medicinal plants/herbal remedies have been used for the treatment of cardiovascular ailments. However, no scientific basis have been studied and reported the molecular mechanism of cardioprotective potential of medicinal plant remedies using cellular and molecular techniques. Medicinal plants discussed in this review article appear to show pharmacotherapeutic potential in vitro and in animal studies that may influence the cardiovascular ailments. These natural medicinal plants exert protective therapeutic effect through a series of processes, including the inhibiting, modulating, and regulating the expression of various proteins such as contractile and structural proteins, and glycoproteins, regulating the calcium levels and improvement in the functioning of mitochondria. The schematic mechanisms of cardioprotection of medicinal plants are presented in Figure 1.
Figure 1.
Cardioprotective mechanism of medicinal plants/herbal products on target sites during pathogenesis of cardiovascular abnormalities. ANP indicates atrial natriuretic peptide; CVDs, cardiovascular disorders; E2, estrogen; ECs, endothelial cells; M&Ms, macrophages and monocytes; NOS-NO, nitric oxide synthase-nitric oxide; PPARα, peroxisome proliferator activated receptor α; VSMCs, vascular smooth muscle cells.
The cardioprotective effect of medicinal plants/herbal products during cardiovascular ailments has been demonstrated by attenuating the damage in cardiac muscle cells, vascular smooth muscle cells (VSMCs), endothelial cells (ECs), and macrophages and monocytes. In cardiomyocytes, the protective effect of medicinal plants/herbal products has been shown by opening of KATP channel, increased secretion of atrial natriuretic peptide, cardiac hypertrophy, oxidative stress, and apoptosis. In ECs, beneficial effects of medicinal plants/herbal products have been shown by inflammation inhibition, oxidative stress & apoptosis, endothelial nitric oxide synthase-nitric oxide (NOS-NO) signaling pathway activation, angiogenesis induction, and endothelial permeability suppression. In VSMCs, medicinal plants/herbal products beneficial effects have been shown through expression inhibition, or inhibition of structural and contractile proteins activities, modulating the extracellular matrix proteins/glycoproteins expression, regulation of calcium levels, alleviating inflammation, attenuating proliferation and migrations, and mitochondrial functional improvements. In macrophages and monocytes, protective effect of medicinal plants/herbal products has been shown through estrogen receptor activation, NOS-NO signaling pathway inhibition, and the activation of nuclear receptor peroxisome proliferator activated receptor α.116
Conclusion
The current review concluded that therapeutic and prophylactic potential of plant phytoconstituents for the management of cardiovascular disorders have explored several ways in chemoprevention, although exact molecular mechanisms are still unclear. Apparently, phytoconstituents exert cardioprotective function by suppressing specific factors, inhibiting the key enzymes, and scavenging the oxygen-free radicals. It is described in this review that phytochemicals possess versatile cardioprotective functions. The nutraceutical and pharmaceutical industries can play a promising lead in drug designing and nutraceutical supplementations using medicinal plants. It could not be possible to include all the studies describing cardioprotective effect of medicinal plants or herbal agents in this review because of limited access to research articles and our search strategy. But the evidences presented in this review are strongly indicative of the notion that medicinal plants/herbal products are the source of emerging medicines for the prevention and treatment of cardiovascular ailments. One may predict the increasing attention of the use of herbal products as alternative medicine in coming years. Therefore, to develop more effective and safe agents from natural herbs is a promising way in preventing and treating cardiovascular abnormalities. However, documentation of criteria for clinical studies is essential for standardizing the evaluation of medicinal plants/herbal agents.
Future Prospects
Screening of indigenous medicinal plants from local flora should be carried out to explore specific plant constituents with therapeutic potential against cardiovascular ailments as an alternative to allopathic treatment regimens. Furthermore, characterization of specific isolated compounds from potent indigenous medicinal plants may be considerably helpful in novel drug designing and drug development for the therapy of cardiovascular disorders. International collaboration may be encouraged by the government through financial support for improving the quality of research.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Muhammad Akram  https://orcid.org/0000-0002-7457-8572
https://orcid.org/0000-0002-7457-8572
Muhammad Riaz  https://orcid.org/0000-0002-5524-7735
https://orcid.org/0000-0002-5524-7735
References
- 1. Roger VL. Epidemiology of myocardial infarction. Med Clin N Am. 2007;91(4):537–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang CZ, Mehendale SR, Yuan CS. Commonly used antioxidant botanicals: active constituents and their potential role in cardiovascular illness. Am J Chin Med. 2007;35(04):543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zern TL, Fernandez ML. Cardioprotective effects of dietary polyphenols. J Nutr. 2005;135(10):2291–2294. [DOI] [PubMed] [Google Scholar]
- 4. Dec GW. Digoxin remains useful in the management of chronic heart failure. Med Clin. 2003;87(2):317–337. [DOI] [PubMed] [Google Scholar]
- 5. Vukajlovic DD, Guettler N, Miric M, Pitschner HF. Effects of atropine and pirenzepine on heart rate turbulence. Ann Noninvas Electro. 2006;11(1):34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murad W, Azizullah A, Adnan M, et al. Ethnobotanical assessment of plant resources of Banda Daud Shah, District Karak, Pakistan. J Ethnobiol Ethnomed. 2013;9(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ullah R, Iqbal ZHZ, Hussain J, et al. Traditional uses of medicinal plants In Darra Adam Khel NWFP Pakistan. J Med Plants Res. 2010;4(17):1815–1821. [Google Scholar]
- 8. Abbasi AM, Khan MA, Ahmad M, et al. Ethnobotanical study of wound healing herbs among the tribal communities in Northern Himalaya ranges District Abbottabad, Pakistan. Pak J Bot. 2010;42(6):3747–3753. [Google Scholar]
- 9. Abubaker S, Shanmukha I, Jyoti T, Gupt K. Cardioprotective effect of Spathodea campanulata bark on isoproterenol-induced myocardial infarction in rats. Asian Pacific J Trop Dis. 2012;2:S1–S5. [Google Scholar]
- 10. Senthilkumar G, Moses M, Sengottuvelu S, Rajarajan T, Saravanan G. Cardioprotective activity of garlic (Allium sativum) in isoproterenol-induced rat myocardial necrosis: a biochemical and histoarchitectural evaluation. Int J Pharm Sci Nanotechnol. 2010;2(4):779–784. [Google Scholar]
- 11. Shanmugarajan T, Arunsundar M, Somasundaram I, Krishnakumar E, Sivaraman D, Ravichandiran V. Cardio protective effect of Ficus hispida Linn on cyclophosphamide provoked oxidative myocardial injury in a rat model. Int J Pharmacol. 2008;1:1–10. [Google Scholar]
- 12. Velavan S, Selvarani S, Adhithan A. Cardioprotective effect of Trichopus zeylanicus against myocardial ischemia induced by isoproterenol in rats. Bangladesh J Pharmacol. 2009;4:88–91. [Google Scholar]
- 13. Sailaja K, Shivaranjani VL, Poornima H, Rahamathulla SM, Devi KL. Protective effect of Tribulus terrestris L. fruit aqueous extracton lipid profile and oxidative stress in isoproterenol induced myocardial necrosis in male albino Wistar rats. EXCLI J. 2013;12:373. [PMC free article] [PubMed] [Google Scholar]
- 14. Riaz M, Shahid M, Jamil A, Saqib M. In vitro antioxidant potential of selected aphrodisiac medicinal plants. J Biol Regul Homeost Agents. 2017;31(2):419–424. [PubMed] [Google Scholar]
- 15. Chakraborty M, Asdaq SMB. Interaction of Semecarpus anacardium L. with propranolol against isoproterenol induced myocardial damage in rats. Indian J Exp Biol. 2011;49(3):200–206. [PubMed] [Google Scholar]
- 16. Sah DK, Nagarathana P. Screening of cardioprotective activity of leaves of Andrographis paniculata against isoproterenol induced myocardial infarction in rats. Int J Pharmacol Res. 2016;6:23–28. [Google Scholar]
- 17. Mehdizadeh R, Parizadeh MR, Khooei A-R, Mehri S, Hosseinzadeh H. Cardioprotective effect of saffron extract and safranal in isoproterenol-induced myocardial infarction in Wistar rats. Iran J Basic Med Sci. 2013;16(1):56. [PMC free article] [PubMed] [Google Scholar]
- 18. Sharma M, Kishore K, Gupta SK, Joshi S, Arya DS. Cardioprotective potential of Ocimum sanctum in isoproterenol induced myocardial infarction in rats. Mol Cell Biochem. 2001;225(1-2):75–83. [DOI] [PubMed] [Google Scholar]
- 19. Fathiazad F, Matlobi A, Khorrami A, et al. Phytochemical screening and evaluation of cardioprotective activity of ethanolic extract of Ocimum basilicum L.(basil) against isoproterenol induced myocardial infarction in rats. DARU. 2012;20(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kauser A, Shah SMA, Iqbal N, et al. In vitro antioxidant and cytotoxic potential of methanolic extracts of selected indigenous medicinal plants. Prog Nutr. 2018;20(4):706–712. [Google Scholar]
- 21. Nandave M, Ojha SK, Joshi S, Kumari S, Arya DS. Moringa oleifera leaf extract prevents isoproterenol-induced myocardial damage in rats: evidence for an antioxidant, antiperoxidative, and cardioprotective intervention. J Med Food. 2009;12(1):47–55. [DOI] [PubMed] [Google Scholar]
- 22. Upaganlawar A, Balaraman R. Cardioprotective effects of Lagenaria siceraria fruit juice on isoproterenol-induced myocardial infarction in wistar rats: a biochemical and histoarchitecture study. J Young Pharm. 2011;3(4):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumar SHS, Anandan R, Devaki T, Kumar MS. Cardioprotective effects of Picrorrhiza kurroa against isoproterenol-induced myocardial stress in rats. Fitoterapia. 2001;72(4):402–405. [DOI] [PubMed] [Google Scholar]
- 24. Beaulah A, Sadiq M, Sivakumar V, Santhi J. Cardioprotective activity of methanolic extract of Croton sparciflorus on isoproterenol induced myocardial infarcted Wistar albino rats. J Med Plants Stud. 2014;2:01–08. [Google Scholar]
- 25. Peer PA, Trivedi PC, Nigade PB, Ghaisas MM, Deshpande AD. Cardioprotective effect of Azadirachta indica A. Juss. on isoprenaline induced myocardial infarction in rats. Int J Cardiol. 2008;126(1):123–126. [DOI] [PubMed] [Google Scholar]
- 26. Ahsan F, Siddiqui H, Mahmood T, Srivastav RK, Nayeem A. Evaluation of cardioprotective effect of Coleus forskohlii against isoprenaline induced myocardial infarction in rats. Indian J Pharmaceut Biol Res. 2014;2(1):17. [Google Scholar]
- 27. Patel KJ, Panchasara AK, Barvaliya MJ, et al. Evaluation of cardioprotective effect of aqueous extract of Garcinia indica Linn. fruit rinds on isoprenaline-induced myocardial injury in Wistar albino rats. Res Pharm Sci. 2015;10(5):388. [PMC free article] [PubMed] [Google Scholar]
- 28. Swaminathan JK, Khan M, Mohan IK, et al. Cardioprotective properties of Crataegus oxycantha extract against ischemia–reperfusion injury. Phytomedicine. 2010;17(10):744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamashiro S, Noguchi K, Matsuzaki T, et al. Cardioprotective effects of extracts from Psidium guajava L. and Limonium wrightii, Okinawan medicinal plants, against ischemia–reperfusion injury in perfused rat hearts. Pharmacology. 2003;67(3):128–135. [DOI] [PubMed] [Google Scholar]
- 30. Pragada R, Veeravalli KK, Chowdary K, Routhu K. Cardioprotective activity of Hydrocotyle asiatica L. in ischemia–reperfusion induced myocardial infarction in rats. J Ethnopharmacol. 2004;93(1):105–108. [DOI] [PubMed] [Google Scholar]
- 31. Céspedes CL, El-Hafidi M, Pavon N, Alarcon J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), maqui. Food Chem. 2008;107(2):820–829. [Google Scholar]
- 32. Karthikeyan K, Bai BS, Gauthaman K, Sathish K, Devaraj SN. Cardioprotective effect of the alcoholic extract of Terminalia arjuna bark in an in vivo model of myocardial ischemic reperfusion injury. Life Sci. 2003;73(21):2727–2739. [DOI] [PubMed] [Google Scholar]
- 33. Firoz M, Bharatesh K, Nilesh P, Vijay G, Tabassum S, Nilofar N. Cardioprotective activity of ethanolic extract of Callistemon lanceolatus leaves on doxorubicin-induced cardiomyopathy in rats. Bangl J Pharmacol. 2011;6(1):38–45. [Google Scholar]
- 34. Thippeswamy A, Shirodkar A, Koti B, et al. Protective role of Phyllantus niruri extract in doxorubicin-induced myocardial toxicity in rats. Indian J Pharmacol. 2011;43(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. El-Sayed EM, El-azeem ASA, Afify AA, Shabana MH, Ahmed HH. Cardioprotective effects of Curcuma longa L. extracts against doxorubicin-induced cardiotoxicity in rats. J Med Plants Res. 2011;5(17):4049–4058. [Google Scholar]
- 36. Ashour OM, Abdel-Naim AB, Abdallah HM, Nagy AA, Mohamadin AM, Abdel-Sattar EA. Evaluation of the potential cardioprotective activity of some Saudi plants against doxorubicin toxicity. Z Naturforsch C. 2012;67(5-6):297–307. [DOI] [PubMed] [Google Scholar]
- 37. Obouayeba AP, Meite S, Boyvin L, Yeo D, Kouakou TH, N’Guessan JD. Cardioprotective and anti-inflammatory activities of a polyphenols enriched extract of Hibiscus sabdariffa petal extracts in Wistar rats. J Pharmacog Phytochem. 2015;4(1):57–63. [Google Scholar]
- 38. Khan G, Haque SE, Anwer T, Ahsan MN, Safhi MM, Alam M. Cardioprotective effect of green tea extract on doxorubicin-induced cardiotoxicity in rats. Acta Pol Pharm. 2014;71(5):861–868. [PubMed] [Google Scholar]
- 39. Hamza A, Amin A, Daoud S. The protective effect of a purified extract of Withania somnifera against doxorubicin-induced cardiac toxicity in rats. Cell Biol Toxicol. 2008;24(1):63–73. [DOI] [PubMed] [Google Scholar]
- 40. Ahmed F, Urooj A. Cardioprotective activity of standardized extract of Ficus racemosa stem bark against doxorubicin-induced toxicity. Pharm Biol. 2012;50(4):468–473. [DOI] [PubMed] [Google Scholar]
- 41. Alpsoy S, Aktas C, Uygur R, et al. Antioxidant and anti-apoptotic effects of onion (Allium cepa) extract on doxorubicin-induced cardiotoxicity in rats. J Appl Toxicol. 2013;33(3):202–208. [DOI] [PubMed] [Google Scholar]
- 42. Ramesh T, Mahesh R, Sureka C, Begum VH. Cardioprotective effects of Sesbania grandiflora in cigarette smoke-exposed rats. J Cardiovasc Pharm. 2008;52(4):338–343. [DOI] [PubMed] [Google Scholar]
- 43. Atale N, Chakraborty M, Mohanty S, et al. Cardioprotective role of Syzygium cumini against glucose-induced oxidative stress in H9C2 cardiac myocytes. Cardiovasc toxicol. 2013;13(3):278–289. [DOI] [PubMed] [Google Scholar]
- 44. Fung SY, Sim SM, Armugam A, Aguiyi JC, Tan NH. Prophylactic effect of Mucuna pruriens Linn (velvet bean) seed extract against experimental Naja sputatrix envenomation: gene expression studies. Indian J Exp Biol. 2014;52(9):849–859. [PubMed] [Google Scholar]
- 45. Fu HW, Zhang L, Yi T, Feng YL, Tian JK. Two new guaiane-type sesquiterpenoids from the fruits of Daucus carota L. Fitoterapia. 2010;81(5):443–446. [DOI] [PubMed] [Google Scholar]
- 46. Zaini R, Clench MR, Le Maitre CL. Bioactive chemicals from carrot (Daucus carota) juice extracts for the treatment of leukemia. J Med Food. 2011;14(11):1303–1312. [DOI] [PubMed] [Google Scholar]
- 47. Muralidharan P, Balamurugan G, Kumar P. Inotropic and cardioprotective effects of Daucus carota Linn. on isoproterenol-induced myocardial infarction. Bangl J Pharmacol. 2008;3(2):74–79. [Google Scholar]
- 48. Hitit M, Corum O, Corum DD, et al. A cardioprotective role of Nerium oleander with the expression of hypoxia inducible factor 2A mRNA by increasing antioxidant enzymes in rat heart tissue. Acta Sci Vet. 2018;46(1):1560. [Google Scholar]
- 49. Dey P, Roy S, Chaudhuri T. A. quantitative assessment of bioactive phytochemicals of Nerium indicum: an ethnopharmacological herb. Int J Res Pharm Sci. 2012;3(4):579–587. [Google Scholar]
- 50. Gayathri V, Ananthi S, Chandronitha C, Ramakrishnan G, Sundaram RL, Vasanthi HR. Cardioprotective effect of Nerium oleander flower against isoproterenol-induced myocardial oxidative stress in experimental rats. J Cardiovasc Pharm Ther. 2011;16(1):96–104. [DOI] [PubMed] [Google Scholar]
- 51. Khan M, Musharaf S, Ibrar M, Hussain F. Pharmacognostic evaluation of the Amaranthus viridis L. Res Pharmaceut Biotechnol. 2011;3(1):11–16. [Google Scholar]
- 52. Vakili SA, Talageri A, George A, Mathai B. Acute toxicity of petroleum ether extracts of Amaranthus viridis L. Int J Pharma Res Health Sci. 2018;6(3):2591–2593. [Google Scholar]
- 53. Bierman EL, Amaral JA, Belknap BH. Hyperlipemia and diabetes mellitus. Diabetes. 1966;15(9):675–679. [DOI] [PubMed] [Google Scholar]
- 54. Kumar B, Lakshman K, Swamy V, et al. Hepatoprotective and antioxidant activities of Amaranthus viridis linn. Macedonian J Med Sci. 2011;4(2):125–130. [Google Scholar]
- 55. Saravanan G, Ponmurugan P, Sathiyavathi M, Vadivukkarasi S, Sengottuvelu S. Cardioprotective activity of Amaranthus viridis Linn: effect on serum marker enzymes, cardiac troponin and antioxidant system in experimental myocardial infarcted rats. Int J Cardiol. 2013;165(3):494–498. [DOI] [PubMed] [Google Scholar]
- 56. Badore NS, Das PK, Pillai S, Thakur A. Role of Ginkgo biloba extract, against isoproterenol induced cardiac toxicity in rats. Indian J Pharm Educ. 2017;51(4):S691–S699. [Google Scholar]
- 57. Oyama Y, Fuchs PA, Katayama N, Noda K. Myricetin and quercetin, the flavonoid constituents of Ginkgo biloba extract, greatly reduce oxidative metabolism in both resting and Ca2+-loaded brain neurons. Brain Res. 1994;635(1-2):125–129. [DOI] [PubMed] [Google Scholar]
- 58. DeFeudis FV. Ginkgo biloba Extract (EGb 761): From Chemistry to the Clinic. Wesbaden: Ullstein Medical; 1998. [Google Scholar]
- 59. Panda VS, Naik SR. Evaluation of cardioprotective activity of Ginkgo biloba and Ocimum sanctum in rodents. Altern Med Rev. 2009;14(2):161. [PubMed] [Google Scholar]
- 60. Ahmed Q, Gupta N, Kumar A, Nimesh S. Antibacterial efficacy of silver nanoparticles synthesized employing Terminalia arjuna bark extract. Artif Cells Nanomed Biotechnol. 2017;45(6):1192–1200. [DOI] [PubMed] [Google Scholar]
- 61. Aqil F, Ahmad I, Mehmood Z. Antioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plants. Turk J Biol. 2006;30(3):177–183. [Google Scholar]
- 62. Bishop S, Liu SJ. Cardioprotective action of the aqueous extract of Terminalia arjuna bark against toxicity induced by doxorubicin. Phytomedicine. 2017;36:210–216. [DOI] [PubMed] [Google Scholar]
- 63. Bantawa P, Ghosh SK, Bhandari P, et al. Micropropagation of an elite line of Picrorhiza scrophulariiflora, pennell, an endangered high valued medicinal plant of the Indo-China Himalayan region. Med Aromat Plant Sci Biotechnol. 2010;4:1–7. [Google Scholar]
- 64. Sultan P, Rasool S, Hassan QP. Picrorhiza kurroa Royle ex Benth. A plant of diverse pharmacological potential. Ann Phytomed. 2017;6(1):63–67. [Google Scholar]
- 65. Shukla B, Visen P, Patnaik G, Dhawan B. Choleretic effect of picroliv, the hepatoprotective principle of Picrorhiza kurroa1. Planta Medica. 1991;57(01):29–33. [DOI] [PubMed] [Google Scholar]
- 66. Wang L, Li Y, Deng W, et al. Cardio-protection of ultrafine granular powder for Salvia miltiorrhiza Bunge against myocardial infarction. J Ethnopharmacol. 2018;222:99–106. [DOI] [PubMed] [Google Scholar]
- 67. Shi M, Huang F, Deng C, Wang Y, Kai G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit Rev Food Sci. 2019;59(6):953–964. [DOI] [PubMed] [Google Scholar]
- 68. Zhou W, Huang Q, Wu X, et al. Comprehensive transcriptome profiling of Salvia miltiorrhiza for discovery of genes associated with the biosynthesis of tanshinones and phenolic acids. Sci Rep U K. 2017;7(1):10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhou R, He LF, Li YJ, Shen Y, Chao RB, Du JR. Cardioprotective effect of water and ethanol extract of Salvia miltiorrhiza in an experimental model of myocardial infarction. J Ethnopharmacol. 2012;139(2):440–446. [DOI] [PubMed] [Google Scholar]
- 70. Mridula K, Parthibhan S, Kumar TS, Rao M. In vitro organogenesis from Tinospora cordifolia (Willd.) Miers—a highly valuable medicinal plant. S Afr J Bot. 2017;113:84–90. [Google Scholar]
- 71. Upadhay A, Kumar K, Kumar A, Mishra H. Tinospora cordifolia (Wild) Hook. F and Thoms.(Guduchi)–validation of the ayurvedic pharmacology through experimental and clinical studies. Int J Ayurveda Res. 2010;1:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sharma AK, Kishore K, Sharma D, et al. Cardioprotective activity of alcoholic extract of Tinospora cordifolia (Willd.) miers in calcium chloride-induced cardiac arrhythmia in rats. J Biomed Res. 2011;25(4):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chakraborty P. Airborne pollen of Bombax ceiba L.: an important source of aeroallergen from india. J Palynol Vol. 2017;53:139–147. [Google Scholar]
- 74. Nitika G, Meena A, Jaspreet N. Evaluation of physicochemical and preliminary phytochemical studies on the root of Bombax ceiba Linn. Int J Res Ayurveda Pharmacy. 2011;2(3):924–926. [Google Scholar]
- 75. Patel SS, Verma NK, Rathore B, Nayak G, Singhai AK, Singh P. Cardioprotective effect of Bombax ceiba flowers against acute adriamycin-induced myocardial infarction in rats. Rev Bras De Farmacogn. 2011;21(4):704–709. [Google Scholar]
- 76. Intararuchikul T, Teerapattarakan N, Rodsiri R, et al. Effects of Centella asiatica extract on antioxidant status and liver metabolome of rotenone-treated rats using GC–MS. Biomed Chromatogr. 2019;33(2):e4395. [DOI] [PubMed] [Google Scholar]
- 77. Hamid K, Ng I, Tallapragada VJ, et al. An investigation of the differential effects of ursane triterpenoids from Centella asiatica, and their semisynthetic analogues, on GABAA receptors. Chem Biol Drug Des. 2016;88(3):386–397. [DOI] [PubMed] [Google Scholar]
- 78. Gnanapragasam A, Ebenezar KK, Sathish V, Govindaraju P, Devaki T. Protective effect of Centella asiatica on antioxidant tissue defense system against adriamycin induced cardiomyopathy in rats. Life Sci. 2004;76(5):585–597. [DOI] [PubMed] [Google Scholar]
- 79. Li XM, Yang PL. Research progress of Sonchus species. Int J Food Prop. 2018;21(1):147–157. [Google Scholar]
- 80. Helal AM, Nakamura N, El-Askary H, Hattori M. Sesquiterpene lactone glucosides from Sonchus asper. Phytochemistry. 2000;53(4):473–477. [DOI] [PubMed] [Google Scholar]
- 81. Khan MR, Haroon J, Khan RA, Bokhari J, Shabbir M, Rashid U. Prevention of KBrO3-induced cardiotoxicity by Sonchus asper in rat. J Med Plants Res. 2011;5(12):2514–2520. [Google Scholar]
- 82. Sathyanarayana N, Pittala RK, Tripathi PK, et al. Transcriptomic resources for the medicinal legume Mucuna pruriens: de novo transcriptome assembly, annotation, identification and validation of EST-SSR markers. BMC Genomics. 2017;18(1):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kavitha C, Thangamani C. Amazing bean Mucuna pruriens: a comprehensive review. J Med Plants Res. 2014;8(2):138–143. [Google Scholar]
- 84. Fung SY, Tan NH, Sim SM, Marinello E, Guerranti R, Aguiyi JC. Mucuna pruriens Linn. seed extract pretreatment protects against cardiorespiratory and neuromuscular depressant effects of Naja sputatrix (Javan spitting cobra) venom in rats. Indian J Exp Biol. 2011;49(4):254–259. [PubMed] [Google Scholar]
- 85. Hossain M, Urbi Z, Sule A, Rahman K. Andrographis paniculata (Burm. F.) Wall. ex Nees: a review of ethnobotany, phytochemistry, and pharmacology. Sci World J. 2014;2014:274905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tan MCS, Oyong GG, Shen C-C, Ragasa CY. Chemical constituents of Andrographis paniculata (Burm. F.) Nees. Int J Pharmacogn Phytochem Res. 2016;8:1398–1402. [Google Scholar]
- 87. Woo AY, Waye MM, Tsui SK, Yeung ST, Cheng CH. Andrographolide up-regulates cellular-reduced glutathione level and protects cardiomyocytes against hypoxia/reoxygenation injury. J Pharmacol Exp Ther. 2008;325(1):226–235. [DOI] [PubMed] [Google Scholar]
- 88. Saxena R, Sulakhiya KB, Rathore M. Cichorium intibus Linn.: a review of pharmacological profile. Int J Curr Pharmaceutl Res. 2014;6(4):11–15. [Google Scholar]
- 89. Abbas ZK, Saggu S, Sakeran MI, Zidan N, Rehman H, Ansari AA. Phytochemical, antioxidant and mineral composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi J Biol Sci. 2015;22(3):322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nayeemunnisa, Rani MK. Cardioprotective effects of Cichorium intybus in ageing myocardium of albino rats. Curr Sci. 2003;84(7):941–943. [Google Scholar]
- 91. Bhoumik D, Mallik A, Berhe AH. Hepatoprotective activity of aqueous extract of Sesbania grandiflora Linn leaves against carbon tetrachloride induced hepatotoxicity in albino rats. Int J Phytomed. 2016;8(2):294–299. [Google Scholar]
- 92. Arun A, Karthikeyan P, Sagadevan P, Umamaheswari R, Peo RR. Phytochemical screening of Sesbania grandiflora (Linn). Int J Biosci Nanosci. 2014;1(2):33–36. [Google Scholar]
- 93. Isensee H, Rietz B, Jacob R. Cardioprotective actions of garlic (Allium sativum). Arznei Forschung. 1993;43(2):94–98. [PubMed] [Google Scholar]
- 94. Trox J, Vadivel V, Vetter W, et al. Bioactive compounds in cashew nut (Anacardium occidentale L.) kernels: effect of different shelling methods. J Agr Food Chem. 2010;58(9):5341–5346. [DOI] [PubMed] [Google Scholar]
- 95. Hu D, Liu X, Wang Y, Chen S. Cyclovirobuxine D ameliorates acute myocardial ischemia by KATP channel opening, nitric oxide release and anti-thrombosis. Eur J Pharmacol. 2007;569(1-2):103–109. [DOI] [PubMed] [Google Scholar]
- 96. Shi LS, Liao YR, Su MJ, et al. Cardiac glycosides from Antiaris toxicaria with potent cardiotonic activity. J Nat Prod. 2010;73(7):1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bopana N, Saxena S. Asparagus racemosus—ethnopharmacological evaluation and conservation needs. J Ethnopharmacol. 2007;110(1):1–15. [DOI] [PubMed] [Google Scholar]
- 98. Sheena N, Lakshmi B, Janardhanan K. Therapeutic potential of Ganoderma lucidum (Fr.) P. Karst. Nat Prod Rad. 2005;4:382–386. [Google Scholar]
- 99. Jain G, Jhalani S, Agarwal S, Jain K. Hypolipidemic and antiatherosclerotic effect of Leptadenia pyrotechnica extract in cholesterol fed rabbits. Asian J Exp Sci. 2007;21(1):115–122. [Google Scholar]
- 100. Žiberna L, Lunder M, Može Š, Vanzo A, Drevenšek G. Cardioprotective effects of bilberry extract on ischemia–reperfusion-induced injury in isolated rat heart. 15th Scientific Symposium of the Austrian Pharmacological Society (APHAR). Joint meeting with the Hungarian Society of Experimental and Clinical Pharmacology (MFT) and the Slovenian Pharmacological Society (SDF) Graz, Austria: Licensee BioMed Central Ltd; 2009. [Google Scholar]
- 101. Rao PR, Kumar VK, Viswanath RK, Subbaraju GV. Cardioprotective activity of alcoholic extract of Tinospora cordifolia in ischemia–reperfusion induced myocardial infarction in rats. Biol Pharm Bull. 2005;28(12):2319–2322. [DOI] [PubMed] [Google Scholar]
- 102. Sudharsan P, Mythili Y, Selvakumar E, Varalakshmi P. Cardioprotective effect of pentacyclic triterpene, lupeol and its ester on cyclophosphamide-induced oxidative stress. Hum Exp Toxicol. 2005;24(6):313–318. [DOI] [PubMed] [Google Scholar]
- 103. Zaman R. Study of cardioprotective activity of Raphanus sativus L. in the rabbits. Pak J Biol Sci. 2004;7(5):843–847. [Google Scholar]
- 104. Goyal S, Arora S, Sharma A, et al. Preventive effect of crocin of Crocus sativus on hemodynamic, biochemical, histopathological and ultrastuctural alterations in isoproterenol-induced cardiotoxicity in rats. Phytomedicine. 2010;17(3-4):227–232. [DOI] [PubMed] [Google Scholar]
- 105. Haleagrahara N, Varkkey J, Chakravarthi S. Cardioprotective effects of glycyrrhizic acid against isoproterenol-induced myocardial ischemia in rats. Int J Mol Sci. 2011;12(10):7100–7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Adaramoye OA, Lawal SO. Kolaviron, a biflavonoid fraction from Garcinia kola, protects against isoproterenol-induced injury by mitigating cardiac dysfunction and oxidative stress in rats. J Basic Clin Physiol Pharmacol. 2015;26(1):65–72. [DOI] [PubMed] [Google Scholar]
- 107. Devi Sampath P, Vijayaraghavan K. Cardioprotective effect of α-mangostin, a xanthone derivative from mangosteen on tissue defense system against isoproterenol-induced myocardial infarction in rats. J Biochem Mol Toxicol. 2007;21(6):336–339. [DOI] [PubMed] [Google Scholar]
- 108. Pogula BK, Maharajan MK, Oddepalli DR, Boini L, Arella M, Sabarimuthu DQ. Morin protects heart from beta-adrenergic-stimulated myocardial infarction: an electrocardiographic, biochemical, and histological study in rats. J Physiol Biochem. 2012;68(3):433–446. [DOI] [PubMed] [Google Scholar]
- 109. Vimal V, Devaki T. Linear furanocoumarin protects rat myocardium against lipidperoxidation and membrane damage during experimental myocardial injury. Biomed Pharmacother. 2004;58(6-7):393–400. [DOI] [PubMed] [Google Scholar]
- 110. Panda S, Kar A, Ramamurthy V. Cardioprotective effect of vincristine on isoproterenol-induced myocardial necrosis in rats. Eur J Pharmacol. 2014;723:451–458. [DOI] [PubMed] [Google Scholar]
- 111. Panda S, Kar A, Sharma P, Sharma A. Cardioprotective potential of N, α-l-rhamnopyranosyl vincosamide, an indole alkaloid, isolated from the leaves of Moringa oleifera in isoproterenol induced cardiotoxic rats: in vivo and in vitro studies. Bioorg Med Chem Lett. 2013;23(4):959–962. [DOI] [PubMed] [Google Scholar]
- 112. Hemalatha K, Prince PSM. Preventive effects of zingerone on altered lipid peroxides and nonenzymatic antioxidants in the circulation of isoproterenol-induced myocardial infarcted rats. J Biochem Mol Toxicol. 2015;29(2):63–69. [DOI] [PubMed] [Google Scholar]
- 113. Yan YX, Hu XD, Chen JC, et al. Cytotoxic triterpenoid alkaloids from Buxus microphylla. J Nat Prod. 2009;72(2):308–311. [DOI] [PubMed] [Google Scholar]
- 114. Yu B, Fang TH, Lü GH, Xu HQ, Lu JF. Beneficial effect of cyclovirobuxine D on heart failure rats following myocardial infarction. Fitoterapia. 2011;82(6):868–877. [DOI] [PubMed] [Google Scholar]
- 115. Zanwar A, Hegde M, Bodhankar S. Cardioprotective activity of flax lignan concentrate extracted from seeds of Linum usitatissimum in isoprenalin induced myocardial necrosis in rats. Interdiscip Toxicol. 2011;4(2):90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Huang CLaY. Chinese herbal medicine on cardiovascular diseases and the mechanisms of action. Front Pharmacol. 2016;7:469. [DOI] [PMC free article] [PubMed] [Google Scholar]