Abstract
Inflammatory bowel disease (IBD) is a spectrum of immune-mediated inflammatory disorders with a complex multifactorial pathogenesis, where different pathways may predominate in different individuals. This complexity will most likely require a panoply of drugs targeting different pathways if one wants to treat to steroid-free sustained remission and mucosal healing. Presently, the mainstay of medical management of IBD is based on 5-aminosalicylates, corticosteroids, thiopurines, methotrexate, antitumor necrosis factor, anti-alpha4 beta7 (α4β7) integrin and anti-interleukin (IL)-12/IL-23 therapies. The discovery of new pathways involved in the pathogenesis of IBD resulted in new drugs targeting Janus kinase/signal transducers and activators of transcription, IL-6, spingosine-1-phosphate, and phosphodiesterase 4, among others. These new therapies might result in more advantageous safety profiles. Several of these new drugs have already been successfully tested in other inflammatory disorders, such as psoriasis or rheumatoid arthritis. In this review, evidence from phase II and phase III randomized controlled clinical trials in patients with IBD involving new biologicals and small molecules are summarized.
Keywords: inflammatory bowel disease, Crohn’s disease, ulcerative colitis, small molecules, biologicals, therapy, JAK inhibitor, S1P modulator, anti-integrin
Introduction
Crohn’s disease and ulcerative colitis are the two extreme phenotypes of the spectrum of inflammatory bowel diseases (IBDs). These are chronic relapsing diseases, originating mostly during adolescence and young adulthood, and characterized by chronic inflammation along the gastrointestinal tract with invalidating symptoms of bloody diarrhea, abdominal pain, weight loss and fatigue.1
Being a systemic disease, IBD can also be associated with extra-intestinal manifestations. Between 6% and 47% of patients with IBD suffer at least from one extra-intestinal manifestation, being dermatological (e.g. pyoderma gangrenosum, erythema nodosum), ocular (e.g. uveitis, conjunctivitis), rheumatological (e.g. spondyloarthropathy, arthralgia) or hepatic disorders (e.g. primary sclerosing cholangitis, PSC), among others.2
The pathogenesis of IBD is intricate and has yet to be fully understood. A complex interaction between genetic, immunological, microbial and environmental factors may explain the increasing incidence of IBD around the world.3,4
The therapeutic options for Crohn’s disease or ulcerative colitis include corticosteroids, 5-aminosalicylates, thiopurines, methotrexate, calcineurin inhibitors, and biological therapies. The choice between the different medical therapies depends on several factors such as disease location and severity, medical and surgical history, age, comorbidities, extra-intestinal manifestations, local guidelines, pricing, and treatment availability.5,6
Currently, there are three classes of biologicals available to treat IBD. These are antagonists to tumor necrosis factor (TNF), anti-integrins and inhibitors of interleukin (IL) 12/IL-23. Unfortunately, primary nonresponse is observed in 20–30% of patients, and another 30% of patients become refractory due to secondary loss of response.
In this context, several new drugs have recently been tested in phase II or III trials in patients with IBD (Table 1).
Table 1.
New drugs tested in patients with IBD.
| Class | Drug | Admin. | Mechanism of action | Clinical trials |
|
|---|---|---|---|---|---|
| CD | UC | ||||
| JAK inhibitor | Filgotinib | Oral | Small molecule that blocks JAK1 | Phase II | – |
| Peficitinib | Oral | Small molecule that blocks JAK1, JAK2 and JAK3 | – | Phase II | |
| Tofacitiniba | Oral | Small molecule that preferentially blocks JAK1 and JAK3 | Phase IIb | Phase III | |
| Upadacitinib | Oral | Small molecule that blocks JAK1 | Phase II | Phase II | |
| Anti-IL-6 | PF-04236921 | s.c. | Fully human mAb targeting IL-6 | Phase II | – |
| S1P modulator | Etrasimod | Oral | Selective S1P1 modulator | – | Phase II |
| Ozanimod | Oral | Agonist of the S1P receptor subtypes 1 and 5 | – | Phase II | |
| Laquinimod | Oral | Inhibition APC and T cells | Phase II | – | |
| Anti-integrin | Abrilumab | s.c. | Fully human mAb targeting α4β7 | Phase II | Phase II |
| AJM-300 | Oral | Small molecule that blocks the α4 integrin subunit | – | Phase II | |
| Etrolizumab | s.c. | Humanized mAb targeting the β7 subunit | – | Phase II | |
| SHP647 (PF-00547659) | s.c. | Fully human mAb targeting MAdCAM-1 | Phase II | Phase II | |
| PTG-100 | Oral | Small molecule that targets the α4β7 integrin | – | Phase II | |
| Anti-IL-23 | Brazikumab | i.v. and s.c. | Fully human mAb targeting the p19 subunit of IL-23 | Phase II | – |
| Briakinumab | i.v. and s.c. | Fully human mAb targeting the p40 subunit of IL-12/IL-23 | Phase IIb | – | |
| Mirikizumab | i.v. and s.c. | Humanized mAb targeting the p19 subunit of IL-23 | – | Phase II | |
| Risankizumab | i.v. and s.c. | Humanized mAb targeting the p19 subunit of IL-23 | Phase II | – | |
| Ustekinumaba | i.v. and s.c. | Fully human mAb targeting the p40 subunit of IL-12/IL-23 | Phase III | Phase II | |
| PDE4 inhibitor | Apremilast | Oral | Inhibitor of PDE4 | – | Phase II |
α, alpha; Admin, administration; APC, antigen-presenting cell; β, beta; CD, Crohn’s disease; IBD, inflammatory bowel disease; IL, interleukin; i.v., intravenous; JAK, Janus kinase; mAb, monoclonal antibody; MAdCAM, mucosal vascular addressin cell-adhesion molecule 1; PDE4, phosphodiesterase type 4; S1P, sphingosine-1-phosphate; s.c., subcutaneous; UC, ulcerative colitis.
Already approved by the US Food and Drug Administration and the European Medicines Agency.
Negative trial.
Janus kinase inhibitors
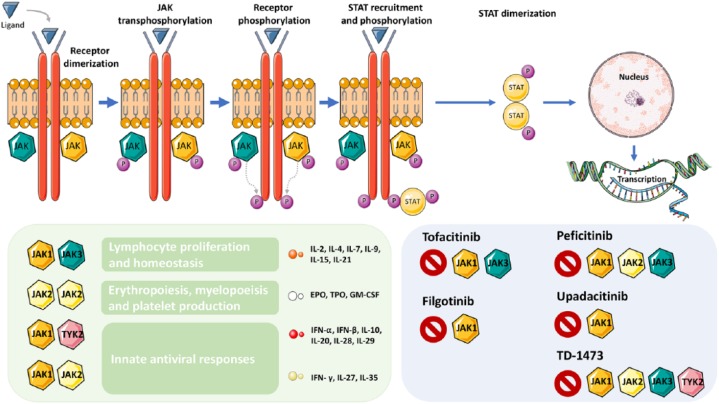
Janus kinases (JAKs) are intracellular nonreceptor tyrosine kinases that play a key role in signaling transduction for several extracellular molecules such as cytokines and growth factors. JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2) are the four JAKs belonging to this family. The activation of cell-membrane receptors by circulating cytokines results in the phosphorylation of signal transducers and activators of transcription (STATs) by the JAK family members.7 Phosphorylated STATs will translocate to the nucleus and bind to specific DNA elements allowing for direct transcription. The JAK/STAT pathway is involved in an array of essential processes such as cell proliferation, growth, differentiation and migration (Figure 1).
Figure 1.
The JAK-STAT signaling pathway is involved in several processes.
This figure was made with Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License: https://smart.servier.com.
The presence of the ligand leads to receptor dimerization. Subsequently, JAK transphosphorylation takes place which allows for STAT recruitment and phosphorylation. Once phosphorylated, STATs will make dimers, which will translocate and promote transcription in the nucleus. Different JAK combinations will lead to different effects. Several JAK inhibitors are being studied in inflammatory bowel disease.
EPO, erythropoietin; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; JAK, Janus kinase; P, phosphorylated; STAT, signal transducer and activator of transcription protein; TPO, thrombopoietin.
Blocking the JAK/STAT pathway results in inhibition of several pro-inflammatory cytokines. Hereby, the JAK/STAT pathway has become a very attractive target in IBD. However, as JAK/STAT is also involved in important biological processes, such as erythropoiesis, immune response and tolerance, and protection against tumors, selectivity is important and vigilance for side effects is needed.8
The small molecule tofacitinib is an oral pan-JAK inhibitor, with preferential inhibition of JAK1 and JAK3. The OCTAVE trials were three multicenter, double-blind, randomized controlled trials (RCTs) in patients with moderately to severely active ulcerative colitis. OCTAVE 1 and 2 were two identical phase III induction trials. The remission rates at 8 weeks were 18.5% in the tofacitinib group (10 mg twice a day, BID) versus 8.2% in the placebo group in OCTAVE 1, and 16.6% versus 3.6% in OCTAVE 2. Centrally assessed mucosal healing was more frequent in patients taking tofacitinib than in patients taking placebo (OCTAVE 1: 31.3% versus 15.6%; OCTAVE 2: 28.4% versus 11.6%). Patients with clinical response in the induction trials were eligible for the follow-up trial OCTAVE Sustain. At 52 weeks, more patients taking tofacitinib were in remission than patients taking placebo (remission rate of 34.3% in the 2 × 5 mg tofacitinib group, 40.6% in the 2 × 10 mg tofacitinib group, and 11.1% in the placebo group).9 Tofacitinib was also tested in two phase II multicenter, double-blind RCTs in Crohn’s disease but failed to demonstrate efficacy.10,11 The reasons for the lack of efficacy in Crohn’s disease as opposed to ulcerative colitis may be multiple and explained by different disease characteristics, patient characteristics (high steroid intake), and trial design (no central endoscopic reading). In the OCTAVE 1 and 2 studies, tofacitinib was associated with higher risk for infection. A higher risk for herpes zoster infection was reported in the OCTAVE Sustain study. Both in the induction and maintenance trials, abnormal lipid and creatine kinase levels were more frequent in the tofacitinib group. Tofacitinib has now been approved by the European Medicine Agency and by the US Food and Drug Administration (FDA) for patients with ulcerative colitis, rheumatoid arthritis, and psoriatic arthritis.
Recent blinded data from a trial in patients with rheumatoid arthritis showed a fivefold increase in pulmonary embolisms in those patients treated with 2 × 10 mg tofacitinib compared with patients treated with infliximab. Of note, all patients included in this trial needed to have a significant cardiovascular risk and data on the absolute risk are currently unknown. Although the FDA alerted the public, they did not force a stop of the higher dose of 2 × 10 mg tofacitinib in other patient populations, including ulcerative colitis.
Filgotinib is a selective JAK1 inhibitor that has been tested in patients with Crohn’s disease. The FITZROY study, a phase II double-blind RCT, included patients with moderate-to-severe Crohn’s disease based on centrally read endoscopies. Clinical remission was significantly more frequent after 10 weeks of treatment in patients taking filgotinib (200 mg once daily, QD) than in patients taking placebo (47% in the filgotinib group versus 23% in the placebo group). Interestingly, recruitment based on centrally read endoscopies resulted in a high rate of screening failure (44%), mainly due to insufficient endoscopic severity, reflecting the importance of patient selection in RCTs. The safety profile of filgotinib was acceptable; however, patients taking filgotinib were more prone to serious infections.12 Phase III trials are ongoing in patients with Crohn’s disease and ulcerative colitis [ClinicalTrials.gov identifiers: NCT02914561 and NCT02914522, respectively].
Another selective JAK1 inhibitor, upadacitinib, has recently been tested in two dose-ranging phase II RCTs. In the CELEST trial, patients with moderate-to-severe active Crohn’s disease were randomized to receive placebo or one of five doses of upadacitinib (3, 6, 12 or 24 mg BID, or 24 mg QD). Clinical and endoscopic improvements were observed in patients exposed to upadacitinib, with a significant dose–response relationship.13 Patients who completed the 16-week induction phase were rerandomized to receive upadacitinib at 3 mg BID, 12 mg BID or 24 mg QD for 36 weeks. During the trial, the 24 mg QD arm was replaced by a 6 mg BID arm to study an intermediate maintenance dose. At week 52, a dose–response relationship was observed in clinical and endoscopic remission.14 In patients with moderate-to-severe ulcerative colitis, upadacitinib was given at four different doses (U-ACHIEVE trial: 7.5 mg QD, 15 mg QD, 30 mg QD, 45 mg QD) that differed from the ones administered in the CELEST trial. At doses higher than 15 mg per day, upadacitinib induced significant clinical (14.3% versus 0%) and endoscopic remission (30.6% versus 2.2%) after 8 weeks of treatment. These effects were more evident with the highest dose of 45 mg per day, confirming a significant dose–response relationship. Adverse events rates were similar across all groups. Phase III trials in patients with Crohn’s disease [ClinicalTrials.gov identifiers: NCT03345836 and NCT03345849] and ulcerative colitis [ClinicalTrials.gov identifiers: NCT03653026, NCT02819635, and NCT03006068] are ongoing.
In addition to the abovementioned JAK inhibitors, peficitinib has also been tested in patients with ulcerative colitis. A recently published phase II dose-ranging RCT in patients with moderate-to-severe ulcerative colitis evaluated the efficacy and safety of peficitinib at 25 mg QD, 75 mg QD, 75 mg BID, and 150 mg QD versus placebo.15 After 8 weeks of treatment, a trend toward increased clinical and endoscopic remission was observed at doses > 75 mg per day. However, no significant dose–response relationship was observed in the patients taking peficitinib. The safety profile of peficitinib was comparable with the placebo group. Future trials are needed to evaluate peficitinib at higher doses.
While the safety profile of the JAK inhibitors is generally acceptable, long-term safety studies in rheumatologic populations and in patients with ulcerative colitis taking tofacitinib, reported a higher risk for reactivation of herpes zoster.9,16 This increased risk may be a class effect of all JAK inhibitors, as it is hypothesized to be related to the inhibition of interferon and IL-15.17 The increase in selectivity of some JAK inhibitors is expected to result in better safety profiles. Nevertheless, whether the increased selectivity of newer JAK inhibitors will lead to a better safety profile still needs to be demonstrated. The data supporting the inhibition of one specific JAK above the other is still lacking. A phase II RCT comparing a JAK1-inhibitor, a JAK3-inhibitor, and placebo in patients with ulcerative colitis is ongoing [ClinicalTrials.gov identifier: NCT02958865].
Anti-IL6 therapy
The pleiotropic cytokine IL-6 plays an important role in several pro-inflammatory pathways, acting through the induction of proliferation and differentiation of T cells, differentiation of B cells and regulation of the production of acute-phase reactants (e.g. C-reactive protein, CRP).18 Therefore, IL-6 is a potential therapeutic target in inflammatory disorders. PF-04236921 is a subcutaneously administered fully human monoclonal antibody that binds to IL-6. In the ANDANTE I trial, a multicenter, double-blind phase II RCT, patients with moderate-to-severe Crohn’s disease who failed anti-TNF therapy were randomized to receive subcutaneous placebo or PF-04236921 10, 50 or 200 mg, on days 1 and 28. In a separate trial investigating the efficacy and safety of PF-04236921 in patients with systemic lupus erythematosus, three deaths were reported in the arm receiving 200 mg QD.19 This led to the early termination of patient enrolment in the 200 mg arm. The primary endpoint of the ANDANTE I trial [Crohn’s Disease Activity Index (CDAI)-70 response rate at week 8 or 12] was met for the 50 mg QD group. Endoscopic endpoints were not assessed as endoscopy was not mandatory. Patients who completed the 12-week induction phase were included in the ANDANTE II, an open-label extension trial designed for safety analysis. In this extension study, patients received subcutaneous PF-04236921 50 mg every 8 weeks. The development of PF-04236921 was halted after reports of intestinal gastrointestinal perforations in different patient populations.
S1P modulators
Naïve T lymphocytes play a key role in immune surveillance. Activation of these lymphocytes occurs in secondary lymphoid organs, such as the spleen, lymph nodes and Peyer’s patches. The chemoattractant sphingosine 1-phosphate (S1P) guides lymphocyte circulation through these lymphoid organs in a gradient-dependent manner.20 S1P is also involved in other processes such as angiogenesis, vascular permeability, and cancer growth and metastasis. There are five S1P receptors subtypes (S1P1–5) that modulate the various actions of S1P.21 Ozanimod, amiselimod and etrasimod are agonists of S1P1, blocking the S1P gradient-dependent egress of lymphocytes from the lymph nodes (Figure 2).
Figure 2.
S1P mechanisms of action.
This figure was made with Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License: https://smart.servier.com.
(a) S1P binds to five known receptors (S1P1, S1P2, S1P3, S1P4, and S1P5), each promoting distinct functions; (b) dendritic cells migrate to lymph nodes and present antigens to T cells. The binding of S1P to the S1P receptors promotes the egress of activated T cells from the lymph nodes to the lymph, following the S1P gradient. S1P modulators block this binding, resulting in a decrease of circulating blood lymphocytes. Ozanimod blocks the binding of S1P to receptor S1P1 and S1P5. Etrasimod selectively blocks receptor S1P1.
DC, dendritic cell; NK cell, natural-killer cell; S1P, sphingosine-1-phosphate; TC, T cell; TH17, T-helper cell 17.
Ozanimod is an oral S1P1 and S1P5 agonist that was previously tested in patients with relapsing multiple sclerosis. In a double-blind phase II RCT, the efficacy and safety of ozanimod in patients with moderate-to-severe ulcerative colitis was evaluated. Clinical remission at week 8 was observed in 16% of patients receiving ozanimod 1 mg QD, 14% of patients receiving ozanimod 0.5 mg QD, and in 6% of patients receiving placebo. The difference between the 1.0 mg and placebo groups was statistically significant. Patients receiving ozanimod 1 mg QD achieved histologic remission more frequently than patients receiving placebo (22% versus 11 %). Adverse events were similar in all groups.22 Phase III trials for Crohn’s disease [ClinicalTrials.gov identifiers: NCT03440372, NCT03440385 and NCT03464097] and ulcerative colitis [ClinicalTrials.gov identifiers: NCT02435992 and NCT02531126] are ongoing.
Etrasimod is an S1P1, S1P4, and S1P5 modulator. In the phase II dose-ranging OASIS study, patients with moderate-to-severe ulcerative colitis were given etrasimod 1 mg QD, etrasimod 2 mg QD or placebo. A dose-dependent significant response was observed in the primary (change from baseline in three components of the Mayo Clinic score) and secondary endpoints (endoscopic improvement, clinical response, and clinical remission). In the group receiving etrasimod 2 mg QD, a decrease of 57% in the lymphocyte count in comparison with baseline was observed at week 12. Adverse event rates were similar among all groups.23 Phase II and III studies in patients with Crohn’s disease and ulcerative colitis are underway.
S1P modulators are also used for the treatment of multiple sclerosis. Owing to their action in the central nervous system, the risk of progressive multifocal leukoencephalopathy (PML) has been closely followed. The risk of PML is low in the patients treated with fingolimod. Ozanimod and etrasimod are more selective than fingolimod, and no cases of PML have been reported so far in patients taking ozanimod or etrasimod.
Laquinimod
Initially developed for relapsing–remitting multiple sclerosis, laquinimod is an oral small molecule with immunomodulatory effects.24 Although the mechanism of action of laquinimod is yet to be fully understood, the drug may work through downregulation of pro-inflammatory cytokines.25 Due to its anti-inflammatory profile, laquinimod has been tested in patients with active moderate-to-severe Crohn’s disease in a double-blind dose-ranging phase II RCT. Laquinimod was given in four different daily doses: 0.5 mg QD, 1 mg QD, 1.5 mg QD and 2 mg QD. Within all treatment groups, the dose of laquinimod 0.5 mg daily resulted in the safest and most efficacious in inducing clinical remission in this patient population.26 The development of laquinimod in multiple sclerosis has been recently abandoned,27 following the disappointing results in the BRAVO28 and CONCERTO29 phase III trials. Phase III trials in Crohn’s disease of ulcerative colitis have not been initiated.
Anti-integrins
Intestinal inflammation depends on the migration of circulating leukocytes from blood into the intestinal site of inflammation. This immune-cell trafficking into the intestine is mediated by adhesion molecules, such as mucosal addressin cell-adhesion molecule-1 (MAdCAM-1). The interaction between MAdCAM-1 and its integrin ligand, α4ß7, results in the recruitment of lymphocytes into the gut or gut-associated lymphoid tissue.30 Natalizumab, an anti-α4 integrin antibody, and vedolizumab, an anti-α4ß7 integrin antibody, are efficacious in the treatment of IBD.31–33 However, reports of PML in patients treated with natalizumab34–36 led to the restriction of its use in IBD. Through the selective targeting of the α4 integrin subunit, natalizumab blocks the interaction between α4ß1 and α4ß7 with vascular cell-adhesion molecule 1 (VCAM-1) and MAdCAM-1, respectively. VCAM-1 is expressed in the central nervous system, hereby playing an important role in the immune surveillance. The John Cunningham virus (JC virus) is a human polyoma virus that plays a key role in the pathogenesis of PML. Considering the majority of the population in Europe and the United States is seropositive for the JC virus, a decreased immune surveillance in the central nervous system results in an unacceptable increased risk for PML in patients with IBD.
Despite these concerns of nonselective targeting, a new, oral, small-molecule anti-α4 integrin, AJM-300, has been developed. The efficacy and safety of AJM-300 was assessed in a double-blind phase II RCT. Patients with moderately active ulcerative colitis were given AJM-300 or placebo thrice daily for 8 weeks. Clinical remission (23.5% versus 3.9%), clinical response (62.7% versus 25.5%) and mucosal healing (local endoscopic assessment: 58.8% versus 29.4%) were significantly more frequent in patients taking AJM-300 than in patients taking placebo. The rates of adverse events were similar between active-treatment and placebo groups. In this phase II RCT with 51 patients exposed to AJM-300, there was no case of PML observed.37 However, considering that AJM-300 might have a similar mechanism of action with natalizumab, the safety of AJM-300 in patients with IBD needs to be carefully addressed in the following phase III trial [ClinicalTrials.gov identifier: NCT03531892].
PTG-100 is an oral small molecule that targets the α4β7 integrin, hereby being gut selective. In the PROPEL study, a phase II RCT, patients with ulcerative colitis were given placebo or PTG-100 150 mg, 300 mg or 900 mg QD. An initial interim analysis of 65 patients indicated that PTG-100 was not better than placebo in inducing remission in patients with moderate-to-severe ulcerative colitis. A post hoc analysis of the centrally read endoscopic data, triggered by a higher-than-expected placebo effect, showed that PTG-100 induced more clinical and endoscopic improvements than placebo. A dose–response relationship was observed with higher responses being observed in the PTG-100 900 mg QD group. The full data of the PROPEL study is eagerly awaited.
Etrolizumab is a monoclonal antibody targeting the β7 subunits of the α4β7 and αEβ7 integrins, hereby blocking the α4β7-MAdCAM-1 and αEβ7-E–cadherin interactions. The αEβ7 integrin is involved in the epithelial retention of lymphocytes and it is mainly expressed by lymphocytes after extravasation into the epithelium and lamina propria.38 By blocking these interactions, etrolizumab is thought to impair the trafficking of gut-homing lymphocytes and the retention of these lymphocytes in the epithelium. A double-blind phase II RCT evaluated the efficacy and safety of subcutaneous etrolizumab, in patients with moderate-to-severe ulcerative colitis. Patients were randomized to two active-treatment groups (100 mg at weeks 0, 4, and 8; or 420 mg loading dose at week 0 followed by 300 mg at weeks 2, 4, and 8) or to the placebo group. At week 10, 21% of patients in the etrolizumab 100 mg group were in clinical remission, as compared with 10% in the etrolizumab 420/300 mg and 0% in the placebo group. A very strict secondary endpoint (Mayo endoscopic subscore of 0 and a rectal bleeding subscore of 0 at week 10) was met by 10 % of the patients in the etrolizumab 100 mg group and by 8% of patients in the etrolizumab 420/300 mg group. No patients in the placebo group met this secondary endpoint. Clinical remission rates were higher in patients with higher gene expression of integrin αE in colon biopsies taken at baseline.39 In a retrospective analysis, the expression of high levels of integrin αE and granzyme A at baseline identified patients most likely to benefit from etrolizumab.40 An extensive phase III program is ongoing [ClinicalTrials.gov identifiers: NCT02118584, NCT02100696, NCT02165215, NCT02394028, NCT02403323], including head-to-head trials with adalimumab [ClinicalTrials.gov identifiers: NCT02171429 and NCT02163759] and infliximab [ClinicalTrials.gov identifier: NCT02136069].
SHP647, former PF-00547659, is an anti-MAdCAM monoclonal antibody that selectively reduces lymphocyte homing to the intestinal tract. In the OPERA study, a dose-ranging phase II RCT, 265 patients with active moderate-to-severe Crohn’s disease were randomized to receive placebo or PF-00547659 22.5 mg, 75 mg or 225 mg subcutaneously at weeks 0, 4 and 8. The primary endpoint (clinical response at weeks 8 and 12, defined as decrease from baseline in CDAI ⩾ 70 points) was not statistically different between groups, probably due to high rates of clinical response in the placebo group (41.4% at week 8 and 44.4% at week 12).41 Unlike in Crohn’s disease, SHP647 was better than placebo in patients with ulcerative colitis. The TURANDOT trial evaluated the efficacy and safety of SHP647 in 357 patients with active ulcerative colitis. The primary endpoint was remission, defined as Mayo score ⩽ 2 with no individual subscore > 1 and rectal bleeding subscore ⩽ 1. At week 12, remission rates were higher in the active-treatment groups (11.3%, 16.77%, 15.5%, and 5.7% for 7.5 mg, 22.5 mg, 75 mg, and 225 mg, respectively) in comparison with the placebo group (2.7%), although the 225 mg group did not reach statistical significance.42 SHP647 was safe and well tolerated in both studies. Phase III programs in Crohn’s disease [ClinicalTrials.gov identifiers: NCT03627091, NCT03566823, and NCT03559517] and ulcerative colitis [ClinicalTrials.gov identifiers: NCT03259308, NCT03259334, and NCT03290781] are ongoing.
Abrilumab, formerly known as AMG-181 or MEDI 7183, is a monoclonal antibody against the α4β7 integrin. The phase IIb RCT with patients with moderate-to-severe Crohn’s disease failed to meet the primary endpoint (clinical remission, CDAI < 150, at week 8).43 In contrast, in the phase IIb RCT with 354 patients with moderate-to-severe ulcerative colitis, abrilumab had a favorable safety and efficacy profile. Patients randomized to the active-treatment groups of abrilumab 70 mg or 210 mg had significantly higher remission rates in comparison with the placebo group (13.5%, 13.4%, and 4.4%, respectively).44 Phase III programs in IBD populations are yet to be announced.
IL-12/IL-23 pathway
Adaptive immunity plays a key role in the pathogenesis of IBD. T-helper (TH) lymphocytes are cytokine-producing lymphocytes that potentiate or regulate immune responses by interacting with other immune cells such as macrophages, CD8+ T cells, eosinophils and basophils. Different cytokine milieus will induce TH1, TH2, TH17 or regulatory T-cell subsets.1
The heterodimeric pro-inflammatory cytokines IL-12 and IL-23 induce a TH1 and TH17 cell response, respectively. Consisting of the p40 and p35 subunits, IL-12 stimulates JAK2 and TYK2 activity. IL-23, composed of the p40 and p19 subunits, also activates the JAK/STAT pathway.45,46 The IL-12/IL-23 pathway has been identified in genome-wide association studies as an important player in the pathogenesis of IBD.47
Ustekinumab is a monoclonal antibody directed against the p40 subunit, therefore blocking the biologic activity of IL-12 and IL-23 simultaneously. The UNITI program recruited patients with Crohn’s disease and consisted of two phase III induction RCT (UNITI-1 and UNITI-2) and one phase III maintenance RCT (IM-UNITI). Ustekinumab was efficient both in the induction phase and in the maintenance phase in patients with Crohn’s disease. The safety profile of ustekinumab was acceptable.48
A phase III induction RCT in patients with moderate-to-severe ulcerative colitis (UNIFI trial) met the primary endpoint of clinical remission (Mayo score ⩽ 2 points, with no individual subscore > 1) at week 8. Patients were also given a single dose of intravenous (IV) ustekinumab 130 mg, ustekinumab 6 mg per kilogram of body weight, or placebo. After 8 weeks, 15.6%, 15.5%, and 5.3% of the patients were in clinical remission, respectively. A significant decrease of fecal calprotectin was also observed in patients who received ustekinumab [ClinicalTrials.gov identifier: NCT02407236].49
The development of briakinumab, a human anti-IL-12/23p40 monoclonal antibody, was stopped following the disappointing results from a phase II RCT in patients with Crohn’s disease.50
Selective targeting of IL-23 in patients with IBD might be advantageous, as IL-12 pathways are involved in immune responses and antitumor activity.51,52
Risankizumab is a monoclonal antibody targeting the p19 subunit, which is specific to IL-23. In a phase II RCT, 121 patients with moderate-to-severe Crohn’s disease (mostly refractory to anti-TNF) were randomized to receive IV risankizumab 200 mg, risankizumab 600 mg, or placebo, at weeks 0, 4, and 8. At week 12, clinical remission (CDAI < 150) was observed in 24.4%, 36.6%, and 15.4% of the patients, respectively. Adverse events were similar in all groups.53 Of those 108 patients, 6 were in deep remission (CDAI < 150 and Crohn’s Disease Endoscopic Index of Severity (CDEIS) ⩽ 4, or ⩽2 for patients with isolated ileitis) and entered a 12-week washout phase. The remaining 102 patients were not in deep remission and were offered risankizumab 600 mg every 4 weeks for another 12 weeks. One patient of the 102 declined to continue the study. The rate of patients achieving clinical remission at week 26 remission was significantly increased. Patients in clinical remission at week 26 continued maintenance therapy with subcutaneous risankizumab 180 mg every 8 weeks until week 52. Of the 56 patients entering the maintenance phase, 44 (71%) were in clinical remission at week 52.54 A phase III program is ongoing in Crohn’s disease [ClinicalTrials.gov identifiers: NCT03104413, NCT03105128, and NCT03105102] and ulcerative colitis [ClinicalTrials.gov identifiers: NCT03398148 and NCT03398135].
Formerly known as MEDI2070, brazikumab is a monoclonal antibody against the p19 subunit of IL-23. The safety and efficacy of brazikumab was evaluated in a double-blind phase II RCT in patients with moderate-to-severe Crohn’s disease. Patients were given 700 mg of IV brazikumab or placebo at weeks 0 and 4, followed by an open-label 210 mg of brazikumab for all patients. The primary endpoint (CDAI decrease of 100 points from baseline) occurred in 49.2% of patients receiving brazikumab compared with 26.7% of patients receiving placebo. IL-22 is a cytokine that is induced by IL-23. Patients with a higher serum concentration of IL-22 at baseline were more likely to respond to brazikumab than to placebo. Headache and nasopharyngitis were the most common adverse events.55 A phase III trial in patients with Crohn’s disease is ongoing. This trial includes a comparative arm with adalimumab [ClinicalTrials.gov identifier: NCT03759288]. A phase II trial in patients with ulcerative colitis with a comparative arm to vedolizumab is ongoing [ClinicalTrials.gov identifier: NCT03616821].
Mirikizumab is also a monoclonal antibody against the p19 subunit of IL-23. A recent phase II RCT in patients with moderate-to-severe ulcerative colitis demonstrated the efficacy of mirikizumab in the induction treatment of patients with moderate-to-severe ulcerative colitis.56 Patients were randomized into receiving placebo or mirikizumab 600 mg IV fixed dose, 50 mg IV exposure-based dose, or 200 mg IV exposure-based dose. The dose of mirikizumab in the exposure-based dose groups was increased if the serum trough concentrations were lower than a predefined cut off. Clinical remission was achieved in 4.8% of patients receiving placebo compared with 15.9%, 22.6%, and 11.5% of patients in the mirikizumab 50 mg, 200 mg, and 600 mg groups, respectively. Adverse event frequencies were similar among all treatment groups. A phase II in patients with Crohn’s disease is ongoing [ClinicalTrials.gov identifier: NCT02891226] and a phase III program in ulcerative colitis has been initiated [ClinicalTrials.gov identifier: NCT03518086, NCT03524092, and NCT03519945].
PDE4 inhibitors
Inflammation is a complex cascade of events that is regulated by several molecules. Cyclic AMP (cAMP) plays an important role in the regulation of the inflammatory response. Phosphodiesterases are enzymes that degrade cAMP, acting as pro-inflammatory enzymes. Increased expression of phosphodiesterase 4 (PDE4) has been reported in inflammatory diseases such as psoriasis.57 Apremilast is an oral small molecule that inhibits PDE4 and is already approved for the treatment of psoriatic arthritis and moderate-to-severe plaque psoriasis. A phase II RCT in patients with active ulcerative colitis studied apremilast 30 mg BID, apremilast 40 mg BID, or placebo BID for 12 weeks. Clinical remission (total Mayo score ⩽ 2, with no individual subscore > 1) was achieved in 31.6% and 21.8% in the apremilast 30 mg BID and apremilast 40 mg BID groups, respectively. Only 13.8% of patients receiving placebo achieved clinical remission. The safety profile of apremilast was acceptable, with headache being the most frequent adverse event.58 A phase III program in ulcerative colitis is expected to be launched soon.
Therapeutic pitfalls and future perspectives
Despite the undeniable positive impact of biological therapies, clinical remission is only achieved in a relatively small percentage of patients (usually less than 30%) treated with currently available biologicals. This clearly illustrates the existence of a therapeutic gap in the treatment of IBD. Another important knowledge gap in the treatment of IBD is posed by the relative lack of properly powered head-to-head trials comparing the different available biologicals and small molecules. One exception is the VARSITY trial, of which some data were recently made public. The VARSITY trial is a phase IIIb randomized, double-blind, multicenter, active-controlled study including 771 patients with moderate-to-severe ulcerative colitis. Patients were randomized to adalimumab (n = 386) or vedolizumab (n = 385) and followed up to 52 weeks. Significantly more patients from the vedolizumab group achieved clinical remission (primary outcome: 31.3% in the vedolizumab group versus 22.5% in the adalimumab group) and mucosal healing (secondary outcome: 39.7% in the vedolizumab group versus 27.7% in the adalimumab group). However, the proportion of patients with corticosteroid-free remission at week 52 was greater in the adalimumab group compared with the vedolizumab group (21.7% versus 12.6%, p = 0.688). The safety profile was comparable between the two groups.59 The peer-reviewed publication of the VARSITY trial is widely awaited.
Combination therapy with different classes of biologicals may improve the efficacy of biologicals and small molecules, although safety is an important issue when combining different immunosuppressive strategies. The treatment strategy involving biologicals urgently needs to be optimized through the use of biomarkers, such as IL13RA260 or TREM1,61,62 which can predict response to anti-TNF medications. The use of new molecules could be guided by IL-22, integrin αE/granzyme A, which allow the selection of patients more prone to respond to brazikumab or etrolizumab, respectively. For most biomarkers, however, formal validation in an independent cohort is still required. Notwithstanding, with the new wave of biologicals and small molecules, new biomarkers are needed in order to select the right drug for the right patient. Precision medicine, where treatment strategies are tailored to the individual patient, is expected to result in higher rates of clinical remission and a better quality of life.
Conclusion
Treating patients with IBD is a challenging task due to the complexity and severity of the disease, and due to the shortcomings of the available therapeutic options. Although anti-TNF, anti-integrins, and anti-IL-12/IL-23 are powerful available drugs to treat IBD, a considerable number of patients will not be helped in the long run. Newer, more efficacious and safer drugs are urgently needed to significantly change the course of IBD in many of our (therapy-refractory) patients. In recent years, several new drugs have been developed, which are now being tested in phase II or phase III trials. JAK inhibitors, spearheaded by tofacitinib, are the new treatment class available to treat IBD. This new class has a short half-life, a rapid onset of action and lacks immunogenicity associated with biologics. The expected abundance of new therapeutic options will trigger a new effort toward a personalized and mechanism-based IBD treatment. Ideally, biomarkers, such as the expression of integrin αE and granzyme A, IL-22, IL13RA2 or TREM1, will allow us to select the best therapy for a specific patient, as different pathways may play a role in the pathogenesis of IBD in different patients. Personalized medicine will change the paradigm of disease management in IBD. However, in order to achieve the widely awaited evidence-based personalized medicine, correctly designed RCTs are essential. These trials should include patients with objectively assessed active disease (e.g. endoscopy, CRP and fecal calprotectin), aim at objective and clinically significant endpoints (e.g. deep remission), and include clinically significant comparators (e.g. placebo and, even better, available biologicals or small molecules). Failing to do so may result in pursuing research of otherwise futile medicines.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: João Sabino has received speaker fees from AbbVie and Nestle Health Sciences.
Bram Verstockt has received financial support for research from Pfizer; lecture fees from AbbVie, Ferring, Takeda Pharmaceuticals, Janssen and R-Biopharm AG; consultancy fees from Janssen.
Marc Ferrante has received research grants from Janssen, Pfizer, and Takeda; consultancy fees from AbbVie, Boehringer-Ingelheim, Falk, Ferring, Janssen, Mitsubishi Tanabe, MSD, and Pfizer; speakers fees from AbbVie, Boehringer-Ingelheim, Chiesi, Falk, Ferring, Janssen, Mitsubishi Tanabe, MSD, Pfizer, and Tramedico.
Séverine Vermeire has received grant support from AbbVie, MSD, Pfizer, J&J, and Takeda; received speaker fees from AbbVie, MSD, Takeda, Ferring, Dr. Falk Pharma, Hospira, Pfizer Inc., and Tillots; and served as a consultant for AbbVie, MSD, Takeda, Ferring, Genentech/Roche, Robarts Clinical Trials, Gilead, Celgene, Prometheus, Avaxia, Prodigest, Shire, Pfizer Inc, Galapagos, Mundipharma, Hospira, Celgene, Second Genome, and Janssen.
ORCID iDs: João Sabino  https://orcid.org/0000-0002-8892-7075
https://orcid.org/0000-0002-8892-7075
Bram Verstockt  https://orcid.org/0000-0003-3898-7093
https://orcid.org/0000-0003-3898-7093
Contributor Information
João Sabino, Universitaire Ziekenhuizen Leuven, Belgium.
Bram Verstockt, Universitaire Ziekenhuizen Leuven, Belgium.
Séverine Vermeire, Universitaire Ziekenhuizen Leuven, Belgium.
Marc Ferrante, Universtaire Ziekenhuizen Leuven, Herestraat 49, Leuven B3000, Belgium.
References
- 1. De Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 2016; 13: 13–27. [DOI] [PubMed] [Google Scholar]
- 2. Ott C, Scholmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol 2013; 10: 585–595. [DOI] [PubMed] [Google Scholar]
- 3. Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology 2013; 145: 158–165.e152. [DOI] [PubMed] [Google Scholar]
- 4. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46–54.e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 5. Gomollon F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 6. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017; 11(7): 769–784. [DOI] [PubMed] [Google Scholar]
- 7. Boland BS, Vermeire S. Janus Kinase antagonists and other novel small molecules for the treatment of Crohn’s disease. Gastroenterol Clin North Am 2017; 46: 627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Villarino AV, Kanno Y, Ferdinand JR, et al. Mechanisms of Jak/STAT signaling in immunity and disease. J Immunol 2015; 194: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 376: 1723–1736. [DOI] [PubMed] [Google Scholar]
- 10. Panes J, Sandborn WJ, Schreiber S, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 2017; 66: 1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sandborn WJ, Ghosh S, Panes J, et al. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014; 12: 1485–1493.e1482. [DOI] [PubMed] [Google Scholar]
- 12. Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017; 389: 266–275. [DOI] [PubMed] [Google Scholar]
- 13. Sandborn WJ, Feagan BG, Panes J, et al. Safety and efficacy of ABT-494 (upadacitinib), an oral JAK1 inhibitor, as induction therapy in patients with Crohn’s disease: results from Celest. Gastroenterology 2017; 152: S1308–S1309. [Google Scholar]
- 14. Panes J, Sandborn WJ, Loftus EV, et al. Efficacy and safety of upadacitinib maintenance treatment for moderate to severe Crohn’s disease: results from the CELEST study. J Crohns Colitis 2018; 12: S238–S239. [Google Scholar]
- 15. Sands BE, Sandborn WJ, Feagan BG, et al. Peficitinib, an oral Janus kinase inhibitor, in moderate-to-severe ulcerative colitis: results from a randomised, phase 2 study. J Crohns Colitis 2018; 12: 1158–1169. [DOI] [PubMed] [Google Scholar]
- 16. Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017; 76: 1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choy EH. Clinical significance of Janus kinase inhibitor selectivity. Rheumatology (Oxford). Epub ahead of print 1 December 2018. DOI: 10.1093/rheumatology/key339. [DOI] [Google Scholar]
- 18. Van Snick J. Interleukin-6: an overview. Annu Rev Immunol 1990; 8: 253–278. [DOI] [PubMed] [Google Scholar]
- 19. Wallace DJ, Strand V, Merrill JT, et al. Efficacy and safety of an interleukin 6 monoclonal antibody for the treatment of systemic lupus erythematosus: a phase II dose-ranging randomised controlled trial. Ann Rheum Dis. 2017; 76: 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mendoza A, Fang V, Chen C, et al. Lymphatic endothelial S1P promotes mitochondrial function and survival in naive T cells. Nature 2017; 546: 158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nielsen OH, Li Y, Johansson-Lindbom B, et al. Sphingosine-1-phosphate signaling in inflammatory bowel disease. Trends Mol Med 2017; 23: 362–374. [DOI] [PubMed] [Google Scholar]
- 22. Sandborn WJ, Feagan BG, Wolf DC, et al. Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med 2016; 374: 1754–1762. [DOI] [PubMed] [Google Scholar]
- 23. Sandborn WJ, Trokan L, Zhang J, et al. A randomized, double-blind, placebo controlled trial of a selective, oral sphingosine 1-phosphate receptor modulator, Etrasimod (APD334), in moderate to severe ulcerative colitis: results from the OASIS study. Paper presented at United European Gastroenterology Week 2018, Vienna, Austria. [Google Scholar]
- 24. Polman C, Barkhof F, Sandberg-Wollheim M, et al. Treatment with laquinimod reduces development of active MRI lesions in relapsing MS. Neurology 2005; 64: 987–991. [DOI] [PubMed] [Google Scholar]
- 25. Bruck W, Wegner C. Insight into the mechanism of laquinimod action. J Neurol Sci 2011; 306: 173–179. [DOI] [PubMed] [Google Scholar]
- 26. D’Haens G, Sandborn WJ, Colombel JF, et al. A phase II study of laquinimod in Crohn’s disease. Gut 2015; 64: 1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Active Biotech regains global rights to development and commercialization of laquinimod, https://www.activebiotech.com/en/media/pressreleases/?id=2214295&date=1536132600 (2018, accessed 25 November 2018).
- 28. Vollmer TL, Sorensen PS, Selmaj K, et al. A randomized placebo-controlled phase III trial of oral laquinimod for multiple sclerosis. J Neurol 2014; 261: 773–783. [DOI] [PubMed] [Google Scholar]
- 29. Teva and Active Biotech announce CONCERTO trial of laquinimod in RRMS did not meet primary endpoint. https://tevapharm.com/news/teva_and_active_biotech_announce_concerto_trial_of_laquinimod_in_rrms_did_not_meet_primary_endpoint_05_17.aspx (2017, accessed 25 November 2018).
- 30. Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol 1997; 151: 97–110. [PMC free article] [PubMed] [Google Scholar]
- 31. Ghosh S, Goldin E, Gordon FH, et al. Natalizumab for active Crohn’s disease. N Engl J Med 2003; 348: 24–32. [DOI] [PubMed] [Google Scholar]
- 32. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369: 711–721. [DOI] [PubMed] [Google Scholar]
- 33. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]
- 34. Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med 2005; 353: 362–368. [DOI] [PubMed] [Google Scholar]
- 35. Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med 2005; 353: 369–374. [DOI] [PubMed] [Google Scholar]
- 36. Langer-Gould A, Atlas SW, Green AJ, et al. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 2005; 353: 375–381. [DOI] [PubMed] [Google Scholar]
- 37. Yoshimura N, Watanabe M, Motoya S, et al. Safety and efficacy of AJM300, an oral antagonist of alpha4 integrin, in induction therapy for patients with active ulcerative colitis. Gastroenterology 2015; 149: 1775–1783.e1772. [DOI] [PubMed] [Google Scholar]
- 38. Farstad IN, Halstensen TS, Lien B, et al. Distribution of beta 7 integrins in human intestinal mucosa and organized gut-associated lymphoid tissue. Immunology 1996; 89: 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vermeire S, O’Byrne S, Keir M, et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet 2014; 384: 309–318. [DOI] [PubMed] [Google Scholar]
- 40. Tew GW, Hackney JA, Gibbons D, et al. Association between response to etrolizumab and expression of integrin alphaE and Granzyme A in colon biopsies of patients with ulcerative colitis. Gastroenterology 2016; 150: 477–487.e479. [DOI] [PubMed] [Google Scholar]
- 41. Sandborn WJ, Lee SD, Tarabar D, et al. Phase II evaluation of anti-MAdCAM antibody PF-00547659 in the treatment of Crohn’s disease: report of the OPERA study. Gut 2018; 67: 1824–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vermeire S, Sandborn WJ, Danese S, et al. Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2017; 390: 135–144. [DOI] [PubMed] [Google Scholar]
- 43. Sandborn WJ, Cyrille M, Hansen MB, et al. OP035 efficacy and safety of abrilumab (AMG 181/MEDI 7183) therapy for moderate to severe Crohn’s disease. J Crohns Colitis 2017; 11(Suppl. 1): S22–S23. [Google Scholar]
- 44. Sandborn WJ, Cyrille M, Hansen MB, et al. OP034 Efficacy and safety of abrilumab in subjects with moderate to severe ulcerative colitis: results of a phase 2b, randomised, double-blind, multiple-dose, placebo-controlled study. J Crohns Colitis 2017; 11(Suppl. 1): S21–S22. [Google Scholar]
- 45. Teng MW, Bowman EP, McElwee JJ, et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med 2015; 21: 719–729. [DOI] [PubMed] [Google Scholar]
- 46. Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol 2018; 16: 185–196. [DOI] [PubMed] [Google Scholar]
- 47. Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012; 491: 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016; 375: 1946–1960. [DOI] [PubMed] [Google Scholar]
- 49. Sands BE, Sandborn WJ, Panaccione R, et al. Safety and efficacy of utekinumab induction therapy in patients with moderate to severe ulcerative colitis: results from the phase 3 UNIFI study. Paper presented at UEG Week 2018, Vienna, Austria. [Google Scholar]
- 50. Panaccione R, Sandborn WJ, Gordon GL, et al. Briakinumab for treatment of Crohn’s disease: results of a randomized trial. Inflamm Bowel Dis 2015; 21: 1329–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lin Y, Kuang W, Wu B, et al. IL-12 induces autophagy in human breast cancer cells through AMPK and the PI3K/Akt pathway. Mol Med Rep 2017; 16: 4113–4118. [DOI] [PubMed] [Google Scholar]
- 52. Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med 2017; 376: 1551–1560. [DOI] [PubMed] [Google Scholar]
- 53. Feagan BG, Sandborn WJ, D’Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 2017; 389: 1699–1709. [DOI] [PubMed] [Google Scholar]
- 54. Feagan BG, Panes J, Ferrante M, et al. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol Hepatol 2018; 3: 671–680. [DOI] [PubMed] [Google Scholar]
- 55. Sands BE, Chen J, Feagan BG, et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology 2017; 153: 77–86.e76. [DOI] [PubMed] [Google Scholar]
- 56. Sandborn WJ, Ferrante M, Bhandari BR, et al. 882 - Efficacy and safety of anti-interleukin-23 therapy with mirikizumab (Ly3074828) in patients with moderate-to-severe ulcerative colitis in a phase 2 study. Paper presented at Digestive Disease Week, Washington, DC; 2018. [Google Scholar]
- 57. Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal 2016; 28: 753–763. [DOI] [PubMed] [Google Scholar]
- 58. Danese S, Neurath M, Kopon A, et al. OP006 Apremilast for active ulcerative colitis: a phase 2, randomised, double-blind, placebo-controlled induction study. Paper presented at European Crohn’s and Colitis Organisation 2018, Vienna, Austria. [Google Scholar]
- 59. Schreiber S, Peyrin-Biroulet L, Loftus EV, et al. OP34 VARSITY: a double-blind, double-dummy, randomised, controlled trial of vedolizumab versus adalimumab in patients with active ulcerative colitis. J Crohns Colitis 2019; 13(Suppl. 1): S612–S613. [Google Scholar]
- 60. Verstockt B, Verstockt S, Creyns B, et al. Mucosal IL13RA2 expression predicts non-response to anti-TNF therapy in Crohn’s disease. Aliment Pharmacol Ther 2018; 49: 572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gaujoux R, Starosvetsky E, Maimon N, et al. Cell-centred meta-analysis reveals baseline predictors of anti-TNFalpha non-response in biopsy and blood of patients with IBD. Gut. Epub ahead of print 4 April 2018. DOI: 10.1136/gutjnl-2017-315494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Verstockt B, Verstockt S, Blevi H, et al. TREM-1, the ideal predictive biomarker for endoscopic healing in anti-TNF-treated Crohn’s disease patients? Gut 2018; pii: gutjnl-2018-316845. [DOI] [PubMed] [Google Scholar]