Abstract
Objective. Chemotherapy-induced peripheral neuropathy (CIPN) syndrome causes significant pain as an adverse effect of treatment, with few nonpharmacological interventions tested. A somatic yoga and meditation (SYM) intervention on functional outcomes and quality of life (QOL) was investigated. Design and methods. Individuals diagnosed with CIPN were enrolled in an open-label, single-arm, mixed-methods feasibility trial. Participants and Setting. In an outpatient rehabilitation center, ten participants with median age 64.4 years (47-81) attended 61% of the sessions with no adverse events. Intervention. SYM twice a week for 8 weeks for 1.5 hours, with home program and journaling. Main outcome measures. Primary functional outcomes included Sit and Reach (SR), Functional Reach (FR), and Timed Up and Go (TUG). Self-reported Patient Neurotoxicity Questionnaire (PNQ) and Functional Assessment of Cancer Therapy—Neurotoxicity (FACT-GOG-NTX) were secondary CIPN outcomes. Biomarkers included salivary cortisol (stress) and bioesthesiometer (vibration). Results: Quantitative findings. Significant improvements were found in flexibility (SR; P = .006); balance (FR; P = .001) and fall risk (TUG; P = .004). PNQ improved significantly (P = .003) with other measures improving non-significantly. Qualitative findings. Five themes emerged: (1) vacillation of CIPN pain perception over time; (2) transferability of skills to daily activities; (3) improvement in physical function; (4) perceived relaxation as an effect of SYM; and (5) group engagement provided a social context for not feeling isolated with CIPN. Conclusion. Preliminary data suggest SYM may improve QOL, flexibility, and balance in cancer survivors with CIPN, with a fully powered randomized controlled trial indicated.
Trial registration: NCT03786055
Keywords: cancer survivorship, neuropathy, somatic yoga, fall risk, function, quality of life
Introduction
Chemotherapy induced peripheral neuropathy (CIPN) is a prevalent side effect of certain commonly used chemotherapies and reduces quality of life (QOL) in individuals diagnosed with cancer, including breast, colon, and lung cancer and lymphoma.1 CIPN is a particularly essential adverse effect to address because the importance of curing the cancer limits the ability to reduce the serious, long-lasting, and even permanent debility that may result. In addition, the pharmacological agents used for pain have negative side effects on balance.2-4 Unfortunately, CIPN cannot be prevented, and research has focused on pharmacological therapies aimed at reducing CIPN pain, which does not treat the loss of sensation and motor weakness.
The incidence of CIPN varies by the type of chemotherapeutic agent(s), the total dose, and patient-related factors such as race and genomics.5-10 Almost 50% of women with breast cancer experience CIPN symptoms many years after treatment, with reduced function, greater disability, and higher risk of falls, which is complicated by the aging process.11 Acute oxaliplatin neurotoxicity occurs in 65% to 98% of patients with colorectal cancer.11,12
Clinically, CIPN presents as deficits in sensory function, but also motor and autonomic dysfunction, which develop in a glove and stocking distribution.13 Although chemotherapy improves the likelihood of disease-free survival, CIPN significantly decreases walking speed and balance, which can be observed after the first chemotherapy cycle and progresses with cumulative exposure. These functional deficits are mirrored with increased patient-reported symptom severity, including fall risk and disability.14,15 Few nonpharmacological interventions have been tested for their impact despite the fact that CIPN is routinely assessed during administration of toxic chemotherapy. This study explores the feasibility of a one-arm pilot somatic movement–based yoga program to reduce these side effects with the goal of developing optimal survivorship care plans.
Yoga and meditation as a combined intervention is often used by cancer survivors for symptom management, and a recent systematic review of randomized controlled trials (RCTs) showed that these combination interventions have strong beneficial effects on the QOL of oncological patients.16,17 Several studies demonstrate the positive effects of yoga with meditation on pain, sleep, fatigue, biomarkers,18 and QOL in cancer survivors.19,20 The Society of Integrative Oncology guidelines addressed the use of integrative therapies for the management of adverse treatment effects, and an American Society of Clinical Oncology (ASCO) expert panel endorsed yoga and meditation for stress management and depression or mood disorders and improved QOL.19 These studies informed the use of yoga for pain management in neuropathic pain. However, no study has rigorously investigated its effects on CIPN and the associated functional outcomes.21
Yoga studies conducted in other patient populations and healthy individuals have shown beneficial effects on psychological and somatic symptoms as well as other aspects of physical function.22 In our previous studies, we found that yoga with a brief meditation improved pain and reduced musculoskeletal symptoms of the treatment side effects of aromatase inhibitors.23 We also demonstrated the safety and feasibility of structured yoga in an elderly population at risk for falls.24,25 Therefore, we used the principles of these previous studies in a preliminary exploration of the therapeutic effects of a somatic-based yoga program with a meditation component on functional outcomes and QOL among individuals with CIPN in an open-label, single-arm, mixed-methods pilot study.
Procedures
Participant Recruitment and Inclusion/Exclusion Criteria
Recruitment occurred via referral by medical personnel and self-referral to reduce channeling bias. To reduce selection bias in the sample, multiple methods of recruitment were used. Flyers were placed in local oncology-related physician offices and emailed to physicians and office managers (including primary care, medical oncology, radiation oncology, surgery, and plastic surgery). Flyers were made available to the public via multiple social media and electronic platforms, in physical print in community centers and public establishments, and in auditory form on local radio. This variety of publicity was chosen to reduce age, socioeconomic, literacy, and physical biases. This pilot feasibility trial projected inclusion of a minimum of 10 with a maximum of 15 participants as the sample size because of the physical limitations of the outpatient clinical setting. Stockton University and Bacharach Institute for Rehabilitation Institutional Review Board approved the study protocol (#2018.19), and all participants signed informed consent. This trial was registered with ClinicalTrials.gov (NCT03786055).
Criteria for enrollment included cancer survivors 18 years or older; any type or stage of cancer; all treatment completed with no current evidence of disease; and at least mild peripheral neuropathy symptoms (sensory and/or motor) as rated on the Patient Neurotoxicity Questionnaire (PNQ), administered via telephone. The PNQ captured clinically important and quantifiable information on 2 symptom areas relevant to CIPN.26,27 The 2 questions specifically quantify sensory (eg, tingling, pain, and numbness) and motor (eg, weakness, walking, eating) areas to assess interference and noninterference in daily activities.26,27 The PNQ total score ranges from 2 to 10, with a high total score indicating severe CIPN symptoms.28-30
Exclusion criteria included active cancer disease or current cancer treatment, because those under active treatment may have continued cumulative effects of chemotherapy, and comorbidities such as diabetes or prior history of peripheral neuropathy from any cause. This is to ensure that the sample population under study has CIPN specifically from previous cancer treatment(s) to reduce confounding. All participants were screened in advance of eligibility testing to determine the safety of exercise based on health history, current symptoms, and risk factors. Administration of the Physical Activity Readiness Questionnaire, a brief, 7-question screening tool recommended by the American College of Sports Medicine, focuses on symptoms of possible heart disease while identifying musculoskeletal problems that should be evaluated and addressed for safe participation in any exercise program.31 For those who responded yes to any question, a physician clearance was obtained prior to the start of the trial intervention.
Data Collection and Primary Clinical Outcome Measurements
Primary Clinical Outcomes
The Timed Up and Go (TUG) is a standardized test that measures physical function in sitting to standing, walking, turning, and returning to the seated position.32 Participants were instructed to stand up from a chair, walk 3 m as quickly and safely as possible, turn around, walk back, and sit down. The test is timed in seconds, and longer time indicates worse performance; 9 s is the cutoff for high risk of falls.33
The Functional Reach (FR) test examines the balance of individuals with respect to the patient’s limit of stability. Each participant is instructed to flex the test arm forward to 90° and to reach forward as far as possible before taking a step. The reach is determined by the total excursion of the third metacarpal from the starting point to the point just before balance is lost. An average of 3 measurements in centimeters is used as the final score.32,34 FR has demonstrated strong reliability and validity for measuring postural control in reaching forward during standing.35 The FR test has shown criterion validity, predictive validity, test-retest reliability, and interrater reliability for younger and older adults.36 It has also been shown to possess predictive validity for falls.37 People with reaches less than 25.4 cm are 8 times more likely to fall than those with a reach >25.4 cm.38 Both tests (FR and TUG) can be used to track changes in balance performance and fall risk over time.
The Sit and Reach Test (SR) is the most widely used measure of hamstring and low back flexibility, examining the maximal reach an individual can make in a long-sitting position.39,40 Use of this test in most health-related fitness studies is consequent to the belief that maintaining this flexibility may prevent acute and chronic musculoskeletal injuries, low back problems, postural deviations, gait limitations, and risk of falling.41 The SR has established norms by the American College of Sports Medicine, with a score of 16 cm and 15 cm for adults 46 to 55 and 56 to 65 years old, respectively, in the 50th percentile.42,43 Participants are asked to maintain a long-sit position on the floor while safely reaching forward as far as possible with their shoes removed, feet flat against the table, and legs straight, with distance measured in centimeters.41,44
The TUG,32 FR,32,34 and SR39,40 were evaluated by research assistants and clinically assessed at baseline and 8 weeks. Interrater reliability was assessed between 2 investigators (85% κ coefficient). These measures served as primary clinical outcomes, have been used in previous yoga pilot studies and other clinical trials of yoga, and are sensitive to functional changes over time. Primary clinical outcome measures, participant-reported outcome surveys, and physiological measures were administered at baseline and at week 8.
Secondary/Patient-Reported Outcome Measures
Pain was measured with the Brief Pain Inventory (BPI),45 the PNQ,46 and the Functional Assessment of Cancer Therapy—Neurotoxicity (FACT-GOG-NTX47). Stress was measured with the Perceived Stress Scale (PSS),48 sleep with the Pittsburgh Sleep Quality Index (PSQI),29,30 spirituality with the Functional Assessment of Chronic Illness Therapy—Spiritual Well-being Scale (FACIT-SP),49 and fear of falling with the Falls Efficacy Scale (FES).50 All are valid and reliable patient-reported outcomes. The FACT-GOG-NTX47 and PNQ46 were used as the CIPN evaluation method.
Vibration sense was measured on the feet through a calibrated bioesthesiometer at baseline and at 8 weeks.51 Participants were placed in a relaxed position in a chair and tested with the same script and application of the vibration unit to the proximal lower extremity where sensation is intact. The midplantar pad and the tip of the great toe were tested via 2 trials per protocol. The amplitude is directly proportional to the square of the applied voltage, with results measured in microvolts.
Salivary cortisol was used as a biological and systemic/local inflammatory stress marker. Salivary cortisol was collected at baseline and 8 weeks and followed previous protocol.52 Samples were collected using the Salivette collecting device and standardized methodology, a commonly used saliva sampling technique with minimum invasiveness (Salimetrics, Inc State College, PA). Results were reported in micrograms per deciliter.
To address the qualitative perspectives of this mixed-methods approach, research assistants and yoga instructors described weekly observations and encounters with participants and recorded shared experiences in an Excel spreadsheet. A final focus group met for 1.5 hours during the last session and was transcribed. Participants were asked to write in journals to reflect on class and home practice. Guiding questions were adapted from previous qualitative research in cancer survivors23,24:
What was the most enjoyable aspect of the yoga session?
What was the most difficult aspect of yoga?
Has your pain changed (improved or worsened) since the last session?
What are your experiences in the home practice?
Do you feel that these yoga sessions are beneficial in managing your pain symptoms and function?
Study Intervention
Hatha yoga is widely recognized as a form of yoga that has a reproducible format; combines postures (asanas), breathwork (pranayama), and meditation; and can be used therapeutically for a wide variety of conditions. Yoga interventions for individuals in cancer recovery emphasize engaging the parasympathetic nervous system (PNS), which elicits the relaxation response. This relaxation response is an important component in the Hatha tradition; it counters the deleterious effects of the sympathetic nervous system (SNS) or stress response, allowing multisystem body and mind integration and healing.53-56
Yoga postures and breathwork do not all affect the body and mind in the same way. Some upregulate and stimulate the SNS, whereas others upregulate and stimulate the PNS. The protocol in this study included only those postures and breathwork that engage the PNS.57 Emphasis was placed on the importance of connection of movement with breath called dynamic movement.57 Dynamic movement leads to improvement in flexibility, strength, stability, and balance, all of which are frequently negatively affected with cancer treatment.58
In addition, the movement protocol for this trial was inspired greatly by the principles of Hanna Somatics (Thomas Hanna) and Somatic Yoga (Eleanor Criswell), both rooted in Hatha Yoga.59-61 Stresses and traumas create reflex patterns in the body that lead to chronic muscular contractions, which, over time, cannot be voluntarily relaxed. These states of contraction cause many ongoing symptoms such as poor posture, decreased flexibility and balance, debilitating pain, and overall decreased QOL.59 The contractions are a result of continuous subcortical brainstem-level impulses sent to the motor units.62 The return to cortical control allows voluntary relaxation of muscle fibers and relief of symptoms. Somatics movements use pandiculation, voluntary muscular contraction, and slow controlled decontraction (eccentric contraction), with the constant focus on sensation, to increase the resting length of muscles.63 Movements are performed slowly and gently with the least possible effort and are never forced.59
iRest yoga nidra-inspired guided meditations from the Integrative Restoration Institute were chosen for this protocol because this type of meditation has been shown to decrease the stress hormone cortisol, which drives the upregulation of the SNS.64 In 2010, iRest was endorsed by the US Army Surgeon General and Defense Centers of Excellence as treatment for chronic pain. These meditations, developed by Dr Richard Miller, are trauma informed and evidence based, supporting healing, personal growth, and well-being over a broad range of populations, including individuals with cancer (Table 1).65
Table 1.
Yoga Class Structure and Components.
| Asana/Activity | Minutes |
|---|---|
| Seated | |
| Verbal check in, address inquiries | 5 minutes |
| Supine | |
| Baseline body and breath scan, Diaphragmatic breath, set intention | 2 minutes, 4 minutes |
| Somatic movements | |
| (1) Arch/flatten back (2) Arch/curl (3) Twists (4) Hip series |
15 minutes |
| Knees to chest, knee circles | 1 minute |
| Seated Sukasana (cross-legged) cleansing breaths | 1 minute |
| Finger fan (activation of intrinsic muscles) | 1 minute |
| Chin mudra with kaki (beak exhale breath) | 2 minutes |
| Sun breaths with neck stretches | 2 minutes |
| Dandasana (staff pose)—joint freeing series ankles/feet, forward folds | 4 minutes |
| Switch sukasana (cross-legged)—joint freeing wrist/elbow/shoulder | 4 minutes |
| Side bends | 2 minutes |
| Marjariasana (table top) | |
| Cat/cow to center, left, right | 2 minutes |
| Chakravakasana (sunbird) to garbhasasana (childs pose) | 2 minutes |
| Tadasana (standing mountain pose) | |
| Toe fan (activation of the intrinsic muscles) | 1 minute |
| Dancing warrior series (virabhadrasana) | 4 minutes |
| Tree pose (vriksasana) | 4 minutes |
| Shake tree qi gong | 1 minute |
| Cleansing breaths | 1 minute |
| Supine (or seated variation) | |
| Twists | 2 minutes |
| Bridge | 1 minute |
| iRest® Yoga Nidra inspired guided meditation | 20 minutes |
| Savasana | 5 minutes |
| Postpractice body and breath scan noting changes | 2 minutes |
| Seated | |
| Mandala (circle) mudra with affirmation “I am complete and whole exactly as I am” | 1 minute |
| Sun breath, Anjali mudra, aum, namaste | 1 minute |
Participants met twice per week for 8 weeks, and sessions were taught by certified yoga instructors. The yoga program lasted 90 minutes per class and was structured precisely as shown in Table 1. Previous yoga studies for cancer survivors include a minimum of 8 weeks to effectuate change from various treatment inteventions.23,24,66 Our protocol was created by a physician and certified yoga therapist (RT), who holds specialty certifications with Integrative Yoga Therapy, Novato Institute for Somatic Research and Training, Trauma Informed Yoga Therapy, Yoga 4 Cancer, and The Integrative Restoration Institute and who trained our team of yoga instructors. They ensured the safety and comfort of each participant throughout each class, including assisting with the use of yoga props such as blocks, mats, blankets, chairs, and pillows. Participants were encouraged to honor how they were feeling in each moment and to only move and breathe in a way that felt comfortable. They were supported in choices to use props as needed during the study, with guidance to reflect any self-led progression in decreasing use of props to assume greater challenges in the yoga protocol. Overall, this program was designed to decrease the symptoms of CIPN, including pain, numbness, and tingling in the extremities, with resultant improvement in flexibility, pain, proprioception, agility balance, and kinesthetic sense.
Abbreviated versions of the yoga and meditation program were introduced for home practice during week 1 of the structured sessions. Recorded audio files were emailed to participants. Support was offered by the research team to verify that all participants were able to access the 4 files. Two recordings were an abbreviated home yoga practice to be used with or without a chair depending on the needs of the individual. The 2 additional recordings were short and long iRest® yoga nidra–inspired guided meditations.64,67 Adding the home program teaches participants to become increasingly self-sufficient. Additionally, this small amount of daily practice has been used in other mind-body therapy trials.68,69 Adherence to the home-based yoga program was measured weekly by discussion with a research assistant. Additionally, participants recorded their daily practice in a journal and provided insight into their experiences with the yoga classes and home practices.
Data Analysis
SPSS 24.0 was used to examine paired t-tests and Wilcoxon rank sum tests for all variables. For the qualitative data, content analysis was used and involved individual coding based on variables identified. Journals were reviewed, and codes were assigned to portions of the text and entered. The process is iterative and code categories were created, revised, and expanded. The qualitative data recorded from weekly research assistant discussion and yoga instructor observations were entered into Microsoft Word. After the journals were read in detail twice by all the researchers, main themes were identified and coded. Corresponding quotes were entered into Microsoft Excel to create tables identifying the main themes. The research group compared each result to establish good interrater reliability (κ 90%) within the recurring themes. There were no outliers or insignificant themes, and the researchers came to consensus on each identified theme. The investigators’ notes taken during the weekly encounters were coalesced and reviewed by the research team for any additional relevant information and confirmatory statements. A member check was performed to enhance the internal validity of the results. The ability to triangulate with journals, weekly encounters, and observation by the yoga instructors and researchers further confirmed these findings.
Results
Patient Characteristics
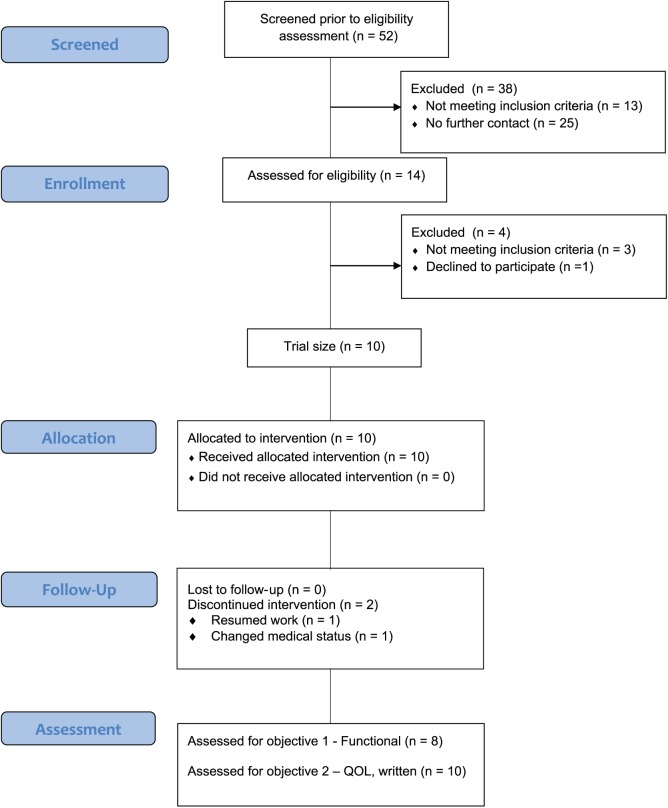
Between May 2018 and July 2018, 52 individuals personally responded to flyers or publicity about the study or were directly referred by medical providers because of CIPN symptoms. Of this group, 14 initially passed telephone screening for participation. Three were excluded prior to the start of the study because of ineligibility based on reoccurrence or change in medical status. One participant did not complete intake testing and opted not to participate, because of a family emergency. Ten participants consented to participation in the study, signed the institutional review board approved consent form, and completed all study testing (Figure 1).
Figure 1.
Flowchart.a
aEldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239.
The median age of the participants was 64.4 years, ranging from 47 to 81 years (Table 2). Seven participants self-identified as white/non-Hispanic, 1 as black/African American, 1 as more than 1 race/ethnicity/Hispanic, and 1 as more than 1 race/ethnicity. Nine participants were female and 1 male. Five participants had a diagnosis and treatment for breast cancer, 2 with colon cancer, 1 with ovarian cancer, 1 with uterine cancer, and 1 with bladder cancer. Five participants stated that they were at stage III disease, 2 at stage II, and 1 at stage I, and 2 did not report a stage or were uncertain. Four participants reported their most recent dose of chemotherapy as “less than 6 months ago,” 3 as “less than 5 years ago,” and 3 as “greater than 5 years ago.” Two participants were currently employed full-time, 2 were employed part-time, and 6 were retired or not currently employed.
Table 2.
Demographic and Clinical Characteristics of Clinical Trial Participants (n = 10).
| Median age, years (minimum-maximum) | 64.4 (47-81) |
|---|---|
| Race | n (%) |
| White | 7 (70%) |
| Black/African American | 1 (10%) |
| Hispanic | 1 (10%) |
| More than 1 race | 1 (10%) |
| Employment | |
| Full-time | 2 (20%) |
| Part-time | 2 (20%) |
| Not currently employed | 6 (60%) |
| Gender | |
| Male | 1 (10%) |
| Female | 9 (90%) |
| Type of cancer | |
| Ovarian | 1 (10%) |
| Breast | 5 (50%) |
| Colon | 2 (20%) |
| Uterine | 1 (10%) |
| Bladder | 1 (10%) |
| Stage of cancer | |
| Stage I | 1 (10%) |
| Stage II | 2 (20%) |
| Stage III | 5 (50%) |
| Uncertain | 2 (20%) |
| Last dose of chemotherapy | |
| <6 Months | 4 (40%) |
| <5 Years | 3 (30%) |
| 5 + Years | 3 (30%) |
| Patient Neurotoxicity Questionnaire (PNQ) sensory symptoms | |
| No numbness, pain, or tingling | 0 (0%) |
| Mild numbness, pain, and tingling | 0 (0%) |
| Moderate numbness, pain, and tingling | 4 (40%) |
| Moderate to severe numbness, pain, and tingling | 6 (60%) |
| Weakness symptoms | |
| No perceived weakness | 1 (10%) |
| Mild weakness | 1 (10%) |
| Moderate weakness | 3 (30%) |
| Moderate to severe weakness | 5 (50%) |
At baseline, 4 participants rated their sensory symptoms (eg, pain, numbness, burning, and/or tingling) as “moderate,” with 6 rating them as moderate to severe on the PNQ. Additionally, 5 self-rated muscular weakness as moderate to severe, 3 as moderate, 1 as mild, and 1 as no perceived weakness as defined by the PNQ (Table 2). Of the 10 participants, 8 were noted to be at high risk for falls as measured by the FR, whereas 5 of the 10 were identified with high fall risk as measured by the TUG at baseline. Two of the participants resumed working full-time during the course of the study. One participant completed 3 sessions, reported resuming full-time employment, and completed written poststudy outcome measures. One participant withdrew in the last week because of illness, with completion of written poststudy outcomes measures. Intention to treat was used for all data analyses.
Overall attendance was 61%, ranging from 3 to 16 sessions. In all, 80% trialed the home program (meditation/breath work and/or yoga-based movement). Seven performed the home program component at least once per week, with two performing the home program at least thrice per week.
Safety
One participant had bilateral lower-extremity mixed etiology lymphedema at baseline that was self-managed with knee-high compression garments, which was reported as a possible side effect of chemotherapy. There was no worsening of the condition based on patient self-report and clinician exam prestudy and poststudy. There were no adverse responses or events during the course of the study.
Improvements in Primary Clinical Outcome Measures
Participants had significant improvement in flexibility measured by SR (mean increase = 8.53; SD = 7.52; P = .006) and in balance measured by FR (mean increase = 14.32; SD = 8.78; P = .001). Of the 10 participants, 40% were at high risk and 40% were medium risk for falls as measured by FR, with none at fall risk at the end of the intervention. The TUG improved significantly, indicating improved mobility and balance and reduction in fall risk (mean reduction = 2.11, SD = 1.75, P = .004; Table 3). Five of the 10 participants were at high risk for falls using the TUG at intake, with only 1 participant remaining at fall risk by the end of the study.
Table 3.
Change in Function and Other Symptom Outcomes (n = 10).
| Measure | Baseline, Mean (SD) | Week 8, Mean (SD) | P Valuea |
|---|---|---|---|
| Clinical functional measures | |||
| Functional Reach (cm) | 20.87 (5.81) | 36.92 (6.82) | .001a |
| Sit and Reach (cm) | 13.01 (5.75) | 23.94 (7.68) | .006a |
| Timed Up and Go (s) | 9.50 (3.11) | 6.44 (3.38) | .004a |
| Patient-reported outcomes | |||
| Perceived Stress Scale | 18.8 (7.61) | 14.8 (8.56) | .56 |
| Brief Pain Inventory (BPI) items (Pain Severity) | 4.835 (1.92) | 2.9 (1.97) | .041a |
| BPI items (Pain Interference) | 4.769 (2.13) | 3.175 (2.07) | .011a |
| FACT-NTX-Total | 95.9 (22.0) | 104.4 (27.3) | .445 |
| Patient Neuropathy Questionnaire | 6.8 (1.47) | 5.3 (1.49) | .003a |
| Falls Efficacy Scale | 32.7 (11.96) | 28.8 (10.88) | .072 |
| FACIT-SP | 103 (26.7) | 110 (33.8) | .510 |
| Pittsburgh Sleep Quality Index (PSQI) | 10.5 (4.81) | 8.9 (4.93) | .238 |
| Clinician-measured biomarkers | |||
| Vibration | |||
| Right midplantar (µV) | 30.60 (15.2) | 28.65 (17.2) | .634 |
| Left midplantar (µV) | 29.70 (15.5) | 27.25 (17.6) | .533 |
| Right great toe (µV) | 33.1 0 (10.6) | 24.9 (9.9) | .035a |
| Left great toe (µV) | 33.75 (14.4) | 25.55 (14.6) | .088 |
| Stress measure | |||
| Cortisol (µg/dL) | 0.131 (0.06) | 0.118 (0.6) | .116 |
Abbreviations: FACIT-SP, Functional Assessment of Chronic Illness Therapy—Spiritual Well-being Scale; FACT-NTX, Functional Assessment of Cancer Therapy—Neurotoxicity; PSQI, Pittsburgh Sleep Quality Index.
P < .05 significance level.
Changes in Secondary Outcome Measures, Including Patient-Reported Symptoms Related to Pain and QOL
Participants experienced a significant reduction in BPI pain severity (4.84 to 2.90, P = .041) and in pain interference (4.77 to 3.18, P = .011). CIPN symptoms measured by PNQ showed significant improvement in sensory symptoms and muscular weakness (6.8 to 5.3, P = .003). The second measure of CIPN symptoms, the FACT-GOG-NTX, although not significant demonstrated trends of improvement (95.90 to 104.4, P = .445). Stress (PSS) was reduced (18.8 to 14.8, P = .056), with sleep quality (PSQI) nonsignificantly improved (10.5 to 8.9, P = .23). Spirituality as measured by FACIT-SP also improved nonsignificantly (103 to 110, P = .510), and fear of falling was nonsignificantly reduced (FES; 37.7 to 28.8, P = .07; Table 3). The salivary cortisol measures demonstrated a decreasing trend without statistical significance (0.131 to 0.118, P = .12). Sensation as measured via bioesthesiometer also demonstrated trends of improvement in all tested locations on the feet, with a single location (tip of right great toe) revealing statistically significant improvement (mean improvement = 8.2, SD = 10.6, P = .035; Table 3).
Qualitative Data Results
Five themes emerged from journal entries, weekly in-person checks, or through phone contact or email and final focus group meeting transcription and was substantiated among the participants through member checks: (1) vacillation of CIPN pain perception over time; (2) transferability of skills to daily activities learned throughout the yoga intervention; (3) improvement in physical function, leading to return to various work and hobbies; (4) perceived relaxation as an effect of the yoga and meditation; and (5) group engagement providing a social context for not feeling isolated with CIPN.
Theme 1: Vacillation of CIPN Pain Perception Over Time
Nine of the participants reported evolving neurogenic changes throughout the study. They indicated that the yoga practice resulted in new sensations of their hands and feet. All participants echoed sentiments that CIPN is a long-lasting side effect of treatment that was downplayed or ignored in medical visits.
“From the first day I came in, simply the acknowledgement that this [CIPN symptoms] is real, I mean, I had my oncologist say, ‘This is as good as it’s going to get,’ and ‘You have neuropathy.’ But the acknowledgement that the effect that it has [on your life], is so real. I can tell you how that has changed me.”—Participant 5
“Pain in feet was a little worse, and bad leg (right) was a little sore the day after. . . . The tingling and asleep feeling in my feet is bad sometimes, I wonder if the nerves are coming alive. I know it’s a process.”—Participant 10
“Around weeks 2 or 3, I was waking up with a lot of burning. I was telling myself it was nerve regeneration. And then it got better.”—Participant 5
“I didn’t think about my feet burning for the first two hours after the first session, when the pain suddenly returned with its usual crazy sensations, like a big boat being driven through my left heel.”—Participant 7
Theme 2: Transferability of Skills Learned Throughout the Yoga Intervention to Daily Activities
Six participants found the instruction and practice in breath work an essential aspect of their daily lives as a skill to assist in self-management of symptom exacerbation, daily activities, and anxiety. Three participants opted to use the breathing exercises as a sleep aid, whereas the other 4 participants used it to alleviate stress. Of the 9 participants who completed the journals, 5 participants asked for community yoga classes to attend after the study was over for continued sustainability of improvements.
“I believe it is not a cure all for my problems, but it has opened my eyes to what I am capable of doing when I put my mind to it.”—Participant 6
“Concentration on the belly breathing has cooled some of the sensation of fire in my feet—lasting a little longer after each breathing session. . . . I am very grateful for the relief I experience for long periods after practice and whenever I breathe through my nose.”—Participant 7
“My balance is better, my core stronger, and I have a better feeling about where I am in my life after cancer. I will never be the same person I was, but I can tell you that yoga has opened my eyes and mind about someone I can be again.”—Participant 9
Theme 3: Improvement in Physical Function Leading to Improvement or Return to Various Work and Hobbies
Six participants reported an improvement in their balance and flexibility as a result of the yoga sessions. Three participants indicated more confidence in performing daily tasks (standing up, cleaning, showering) as a result of more stability and balance. Six found improvement in their sleep from the breathing techniques and yoga exercises.
“Overall, I feel better—physically and mentally—less hesitant/afraid of doing things.”—Participant 5
“My biggest fear is falling in the shower . . . and instead of just throwing the washcloth on the bottom of the tub and washing it, the last couple weeks I’ve been picking one foot up and, you know, washing it by myself, instead of what I’ve been doing the last 3 or 4 years. So, I’m feeling a little bit more confident . . .”—Participant 9
“Played golf for the first time in a very long time . . . and actually played well.”—Participant 9
“Noticing my balance is improving. In the shower when I close my eyes to shampoo, I previously touched the side because I was immediately wobbly. I concentrate on mountain pose and find it not so wobbly.”—Participant 1
Theme 4: Relaxation as an Effect of the Yoga and Meditation
Eight participants consistently reported relaxation after the group sessions and/or home practice and were able to perceive improvements throughout daily function. Application of yoga techniques for relaxation contained some aspect of breathing exercises as a tool to aid sleep and relaxation.
“After practice, I am not focused on the numbness or pain, my mind is elsewhere.”—Participant 9
“The yoga sessions are wonderful and relaxing, and I feel like I’m accomplishing something. . . . The meditation is a wonderful cleansing process of letting everything go, which I need to make more time for.”—Participant 6
“The best part of class is the movement and how my body feels after class. I always feel relaxed and very mindful leaving the building.”—Participant 8
Theme 5: Group Engagement Provided a Social Context for Not Feeling Alone With CIPN
Nine participants indicated that they received “support” from the other trial participants, via validation of CIPN symptoms and cancer survivorship experience, with empathy, understanding, and support. Camaraderie was cultivated through group practice and, at times, was easier or more beneficial than home practice. Four indicated that they appreciated the acknowledgment of their CIPN symptoms and the long-standing impact on their function and QOL. Five participants expressed that the group dynamic allowed them to feel less alone with their pain/experience, whereas other participants truly understood the felt experience of CIPN more than friends or families.
“I enjoy other participants. There is a level of comfort that the others are experiencing symptoms similar to mine.”—Participant 5
“I feel better and love the people I’ve met. The empathy, acceptance, and sense of community or a private club. Not one I would have joined willingly, but at least there is good company. . . . I’m glad to be doing something normal.”—Participant 10
“But nobody in my circle of friends has anything, like the people in this room do and it’s good to know that I’m not the only one out there and I’m not alone. And we’re all working for something and you know it’s really important that I know that you guys are out doing it and I’m doing it. It makes you want to move forward with any kind of program and try to improve what we have.”—Participant 9
Discussion
Mind-Body Therapies: Promising Intervention for Functional Outcomes Associated With CIPN
The extensive use of integrative medicine among cancer survivors is well documented in the literature and may reduce both chronic pain and health care use.70-72 Yoga, an ancient practice originating in India 5000 years ago, is common among the general population and among cancer survivors.73,74 Yoga combines postures (asanas), breathing exercises (pranayama), and meditation to cultivate connection between the mind and body. Although the putative mechanism of these interventions for improving musculoskeletal and neurological conditions is unknown, the conceptual model proposed by Sherman et al75 suggests that posture training may improve strength and flexibility, meditation may decrease stress/anxiety associated with pain, and the relaxation response related to breathing and overall practice of yoga may result in modulation of the neuroendocrine system through the hypothalamic-pituitary-adrenal axis.75 Furthermore, the group activities and active engagement of the whole person in a movement meditation practice may change cognitive appraisal and improve self-efficacy related to pain.76
Whereas evidence of the efficacy of yoga and meditation for symptom management in cancer survivors is emerging, especially for sleep, fatigue, social function, and pain,40-42,75,77-82 we found no study that has addressed these interventions for musculoskeletal-related functional outcomes in CIPN. The existing literature in yoga for neurological conditions in the general population is also limited. In a study of participants with diabetic neuropathy, Malhotra et al83 reported that a 40-day yoga program improved nerve conduction velocity in the median nerve. An 8-week yoga program improved balance, balance confidence, and occupational performance in participants with diabetic neuropathy.84 Although studies in other populations experiencing neuropathy is promising, no published studies have assessed the effects of yoga with meditation on CIPN.
In this trial, we demonstrated the feasibility and safety of a somatic yoga and meditation (SYM) intervention for CIPN as well as preliminary effects of functional outcome. Yoga and meditation were well tolerated, and no adverse events occurred during the study. This somatic-based yoga appears to improve flexibility, balance, and QOL. Participants also reported improvements in various functional activities of daily living in addition to reduction in pain severity. Prior studies have identified decreased salivary cortisol levels following muscle relaxation and a connection between yoga and decreased salivary cortisol levels in cancer survivors.85 Additionally, research has shown decreased salivary cortisol levels following muscle relaxation.86 In somatic yoga, muscles are voluntarily relaxed, which may reduce cortisol levels.87
Vibration sense was enhanced with somatic yoga because the emphasis was placed on body awareness, with greater perception of the intrinsic musculature of the foot and sensation perception. We assume that increased simultaneous antagonistic muscle activation may be used as a safety strategy in the presence of joint stiffness to compensate for neuromuscular degradation.88 Thus, sensorimotor training has the potential to influence neuromuscular mechanisms in order to improve sensorimotor perception and, ultimately, balance performance. Therefore, yoga focusing on somatic awareness should be evaluated as a possible treatment strategy for CIPN.
CIPN causes balance impairments and leads to changes in elicitability and sensitivity of spinal reflex circuitry associated with postural instability. The use of cortisol as a stress biomarker in functional performance and perception of pain may confirm physiological relaxation through yoga and meditation. The improved trends in perceived stress, quality of sleep, fear of falling, and QOL may confirm stress reduction as measured by cortisol. Further research is needed to explore the utility of cortisol as a biomarker in cancer survivors with CIPN.
Our qualitative findings support previous research by Tofthagen,89 where similar participants initially described variability in neuropathic symptoms, muscle weakness, and loss of balance. This interfered with activities of daily living, and participants voiced feelings of frustration and loss of enjoyable activities.89 Our trial was able to demonstrate the renewed interest in activities and overall improvement in function. Thus, our quantitative findings of improved function through balance, flexibility, and mobility were confirmed through qualitative themes. Furthermore, the translation of these skills was noted in everyday activities, thereby improving QOL overall. Whereas the BPI was notably reduced, the qualitative theme articulates a unique pain pattern perception of CIPN through this intervention. Further research is needed to explore the underlying mechanisms for optimal pain management. Punch biopsy may provide physiological insight into the description of vacillation of pain over time.90
The FR test of balance was used in published research in persons with chronic low back pain and in other studies of cancer survivors, with improvements noted postinterventions.23,24,91 People with reduced ability to reach are 8 times more likely to fall than those with a reach of more than 25.4 cm.34 In our study for CIPN, baseline functional measures showed that 80% of the participants had a reach of <25.4 cm and were, thus, at risk for falls. After the 8-week yoga intervention, none remained at risk. Other associated comorbidities may affect balance, including postural instability.92 Balance and fall risk have a direct correlation to functional outcomes and potential disabilities and warrant further investigation.
Our participants reported chemotherapeutic treatment of their cancers with interventions, including taxanes,93 platinum-based drugs, and vinca alkaloids; 50% of all leukemia, lymphoma, colorectal, and breast cancer patients experience peripheral neuropathy.94-96 The ASCO provides evidence-based guidance on the optimum prevention and treatment approaches in the management of CIPN in adult cancer survivors. There are no agents recommended for the prevention of CIPN, and the best available data support a moderate recommendation for treatment with duloxetine for those with CIPN. Drug trials, including tricyclic antidepressants, gabapentin, and a compounded topical gel containing baclofen, amitriptyline, and ketamine, are inconclusive regarding treatment for other neuropathic pain syndromes.97 Thus, nonpharmacological approaches, such as SYM, for CIPN management, are warranted. Yoga and meditation may be a feasible and accessible cost-effective intervention for management of these drug toxicities, with further research into home versus studio practice efficacy indicated based on our participant feedback.
Fall risk increases with each cycle of chemotherapy, and patients receiving taxanes may be at greater risk than patients receiving neurotoxic platinum-based drugs. Patients who report muscle weakness, loss of balance, and long-term persistence of decreased mobility may be at risk.14,98,99 Our study showed that fall risk was reduced through yoga and meditation. Use of our primary clinical functional measures (FR, SR, and TUG) provided reasonable, low-cost analysis of functional change over time, although future studies must include a control group with blinding of assessors to reduce bias in measurement. Similar to previous research,100 we did not find significant effects on fear of falling. Given the duration of CIPN symptoms in our population, perhaps sustained use of yoga is necessary to enhance self-confidence in mobility.
Use of evidence-based traditional exercise in conjunction with mindful movement practices, including yoga and meditation may be beneficial for individuals suffering from CIPN. Traditional exercise interventions can improve static and dynamic balance, increase strength, and reduce CIPN symptoms of pain and paresthesia, which could reduce falls and improve QOL.101,102 Sensorimotor exercise during chemotherapy as measured by posturography has a positive effect on physical and psychological parameters in breast cancer patients undergoing neurotoxic chemotherapy with paclitaxel.102,103
A multimodal exercise program, including balance and strength training, increased QOL for stage IV colorectal cancer patients with CIPN.104 Use of lower-limb closed kinematic chain exercise is also effective in reducing symptoms of neuropathy and improving balance.105 A recent systematic review found that specific exercise, including endurance, strength, and sensorimotor training, for cancer patients with CIPN undergoing chemotherapy should be recommended because these interventions appear to be feasible and have been demonstrated to be useful strategies to counteract some CIPN-related limitations.106 However, if traditional exercise is not feasible or attainable by individuals living with CIPN, this yoga and meditation protocol may provide mitigation of pain and improved function as a precursor to a multimodal exercise regimen.
Yoga positions and relaxation breathing have been found to significantly reduce fatigue and pain levels, in addition to increasing levels of vigor, acceptance, and relaxation.107-109 Our pilot research shows that our participants experienced several physiological benefits from SYM. However, a randomized clinical trial is necessary to confirm the true impact of yoga for CIPN.
Limitations
There are several limitations of the study, including lack of diversity, no assessor blinding, small sample size, and lack of a control group. Our sample size did meet our original intent, and we enrolled 10 of 14 eligible participants, which is appropriate for a small group intervention that must accommodate the particular needs of participants with various comorbidities. In terms of retention, all 10 participants completed written patient-reported outcomes, including journaling, with 8 of 10 completing clinical outcome measures. Success in retention requires a multimodal approach via regular communication and support; we are thus unable to rule out the impact of group social support in our findings along with the Hawthorne effect.
This open-label, single-arm pilot study aimed at establishing feasibility and preliminary effects. A diverse community-based recruitment is challenging, and despite outreach to physicians in the area, most recruitment response was garnered through social media, fliers, and email correspondences. The lack of a control group negates the ability to exclude that findings were a result of placebo effect or regression to the mean. We did not measure long-term follow-up data after the intervention was completed to determine the sustainability of the structured sessions and home yoga-based program, and this would be important for future studies. Whereas recruitment and retention were demonstrated to be feasible, attendance was difficult for some participants, and a larger RCT may afford opportunities for further subanalysis for efficacy of home versus in-studio practice benefits.
There is a risk of bias because assessors of the clinical measures were not blinded; however, we allocated different assessors at the completion of the study to reduce clinician bias. Research assistants were not privy to baseline data information during pretest/posttest data collection. Therefore, future designs should include an adequate sample size for an RCT, with control group and blinding of assessors. To increase diversity, future recommendations include representation of all gender identities, an equal balance of cancer diagnoses with CIPN, and inclusion of all socioeconomic levels and ethnic representation.
We are unable to discern which aspects of the bundled SYM protocol had greatest impact, which would need a multiarm study. Recommendations for protocol revision would include increased options for variations in bilateral somatic hip movements to better accommodate various functional ability levels. Further suggestions include use of a separate video recording for the home program because our participants were most adherent to this aspect of the home practice and expressed a desire for a visual reference. This type of early-phase yoga trial offers an important opportunity to test recruitment and retention strategies and to refine the intervention protocol. Only by accomplishing such a step can we appropriately design and power a randomized controlled study of yoga and meditation for pain management and measurement of functional outcomes and QOL for individuals with CIPN.
In summary, we have conducted a successful feasibility trial of SYM to treat CIPN and its associated effects on function, balance, and fall risk. Few mind-body intervention studies have been performed to address this clinical problem, and we believe that our effort is an important first step in demonstrating the feasibility of rigorous evaluation of yoga and meditation for this indication. Future RCTs are needed to establish the comparative efficacy of SYM to address CIPN-related fall risk, function, and QOL, which affect many cancer survivors.
Conclusion
Cancer survivors with CIPN have sensory and motor deficits leading to inappropriate proprioceptive feedback, impaired postural control, and fall risk. SYM may improve clinical measures and patient-reported symptoms in cancer survivors with CIPN. Although results should be interpreted cautiously because of the small sample size, this pilot trial has shown preliminary evidence that yoga and meditation with a focus on somatic awareness has positive therapeutic effects for cancer survivors with CIPN. This can enhance QOL and produce significant improvement in vibration sense and functional measures of flexibility, balance, and mobility outcomes. A fully powered, randomized clinical trial is needed to confirm these results.
Acknowledgments
A special thank you to our yoga instructors, Naida Burgess, Elaine Sherma, and Salena Coaxum, who provided dynamic interaction with our participants. Thanks to Irvin Rodriquez for his translations of our fliers and documents and Awilda Colon, MSW, PhD, for her community support. Many thanks to Joyce Glick, PT, VP of Outpatient Services at Bacharach Institute for Rehabilitation, with efforts and support for recruitment and publicity, and to Mitzi Ciavarella, RN, for accommodating and promoting this initiative. For the use of the bioesthesiometer, we thank Dr. David Kietrys, Associate Professor, Rutgers University. We are grateful to the patients, oncologists, nurse practitioners, patient navigators, staff of our local and regional cancer centers, and staff of Gilda’s Club South Jersey, especially Gloria Hamlett, MSW, Program Manager, for their support of this study.
Footnotes
Authors’ Note: Trial registration: NCT03786055
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was hosted by Bacharach Institute for Rehabilitation and funded by Stockton University Research and Professional Development Grant and the Stockton Center for Successful Aging. We thank Nikita Lively, Grant Administrative Assistant, for her tireless efforts.
References
- 1. Stubblefield MD, Burstein HJ, Burton AW, et al. NCCN task force report: management of neuropathy in cancer. J Natl Compr Cancer Netw. 2009;7(suppl 5):S1-S28. doi: 10.6004/jnccn.2009.0078 [DOI] [PubMed] [Google Scholar]
- 2. Wonders KY, Reigle BS, Drury DG. Treatment strategies for chemotherapy-induced peripheral neuropathy: potential role of exercise. Oncol Rev. 2010;4:117-125. doi: 10.1007/s12156-010-0044-1 [DOI] [Google Scholar]
- 3. Kaley TJ, DeAngelis LM. Therapy of chemotherapy-induced peripheral neuropathy. Br J Haematol. 2009;145:3-14. doi: 10.1111/j.1365-2141.2008.07558.x [DOI] [PubMed] [Google Scholar]
- 4. Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9-17. doi: 10.1007/PL00007853 [DOI] [PubMed] [Google Scholar]
- 5. Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22:150-169. doi: 10.1159/000118611 [DOI] [PubMed] [Google Scholar]
- 6. Jakesz R, Jonat W, Gnant M, et al. ; ABCSG and the GABG. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455-462. doi: 10.1016/S0140-6736(05)67059-6 [DOI] [PubMed] [Google Scholar]
- 7. Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793-1802. doi: 10.1056/NEJMoa032312 [DOI] [PubMed] [Google Scholar]
- 8. Baum M, Budzar AU, Cuzick J, et al. ; ATAC Trialists’ Group. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131-2139. doi: 10.1016/S0140-6736(02)09088-8 [DOI] [PubMed] [Google Scholar]
- 9. Coombes CR, Hall E, Gibson LJ, et al. ; Intergroup Exemestane Study. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081-1092. doi: 10.1056/NEJMoa040331 [DOI] [PubMed] [Google Scholar]
- 10. Burstein HJ, Winer EP. Aromatase inhibitors and arthralgias: a new frontier in symptom management for breast cancer survivors. J Clin Oncol. 2007;25:3797-3799. doi: 10.1200/JCO.2007.11.9529 [DOI] [PubMed] [Google Scholar]
- 11. Wildes TM, Depp B, Colditz G, Stark S. Fall-risk prediction in older adults with cancer: an unmet need. Support Care Cancer. 2016;24:3681-3684. doi: 10.1007/s00520-016-3312-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Argyriou AA, Cavaletti G, Briani C, et al. Clinical pattern and associations of oxaliplatin acute neurotoxicity: a prospective study in 170 patients with colorectal cancer. Cancer. 2013;119:438-444. doi: 10.1002/cncr.27732 [DOI] [PubMed] [Google Scholar]
- 13. Starobova H, Vetter I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci. 2017;10:174. doi: 10.3389/fnmol.2017.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Monfort SM, Pan X, Patrick R, et al. Gait, balance, and patient-reported outcomes during taxane-based chemotherapy in early-stage breast cancer patients. Breast Cancer Res Treat. 2017;164:69-77. doi: 10.1007/s10549-017-4230-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winters-Stone KM, Horak F, Jacobs PG, et al. Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol. 2017;35:2604-2612. doi: 10.1200/JCO.2017.73.6207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tolia M, Tsoukalas N, Nikolaou M, et al. Utilizing yoga in oncologic patients treated with radiotherapy: review. Indian J Palliat Care. 2018;24:355-358. doi: 10.4103/IJPC.IJPC_112_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zimmermann FF, Burrell B, Jordan J. The acceptability and potential benefits of mindfulness-based interventions in improving psychological well-being for adults with advanced cancer: a systematic review. Complement Ther Clin Pract. 2018;30:68-78. doi: 10.1016/J.CTCP.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 18. Djalilova DM, Schulz PS, Berger AM, Case AJ, Kupzyk KA, Ross AC. Impact of yoga on inflammatory biomarkers: a systematic review. Biol Res Nurs. 2019;21:198-209. doi: 10.1177/1099800418820162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kleckner IR, Dunne RF, Asare M, et al. Exercise for toxicity management in cancer—a narrative review. Oncol Hematol Rev. 2018;14:28-37. [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma M, Lingam VC, Nahar VK. A systematic review of yoga interventions as integrative treatment in breast cancer. J Cancer Res Clin Oncol. 2016;142:2523-2540. doi: 10.1007/s00432-016-2269-2 [DOI] [PubMed] [Google Scholar]
- 21. Lyman GH, Greenlee H, Bohlke K, et al. Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol. 2018;36:2647-2655. doi: 10.1200/JCO.2018.79.2721 [DOI] [PubMed] [Google Scholar]
- 22. Lurie DI. An integrative approach to neuroinflammation in psychiatric disorders and neuropathic pain. J Exp Neurosci. 2018;12:1179069518793639. doi: 10.1177/1179069518793639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Galantino ML, Desai K, Greene L, DeMichele A, Stricker CT, Mao JJ. Impact of yoga on functional outcomes in breast cancer survivors with aromatase inhibitor–associated arthralgias. Integr Cancer Ther. 2011;11:313-320. doi: 10.1177/1534735411413270 [DOI] [PubMed] [Google Scholar]
- 24. Galantino ML, Green L, Decesari JA, et al. Safety and feasibility of modified chair-yoga on functional outcome among elderly at risk for falls. Int J Yoga. 2012;5:146-150. doi: 10.4103/0973-6131.98242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saravanakumar P, Higgins IJ, van der Riet PJ, Marquez J, Sibbritt D. The influence of tai chi and yoga on balance and falls in a residential care setting: a randomised controlled trial. Contemp Nurse. 2014;48:76-87. doi: 10.1080/10376178.2014.11081929 [DOI] [PubMed] [Google Scholar]
- 26. Cleeland CS, Farrar JT, Hausheer FH. Assessment of cancer-related neuropathy and neuropathic pain. Oncologist. 2010;15(suppl 2):13-18. doi: 10.1634/theoncologist.2009-S501 [DOI] [PubMed] [Google Scholar]
- 27. Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33:15-49. doi: 10.1053/J.SEMINONCOL.2005.12.010 [DOI] [PubMed] [Google Scholar]
- 28. Kuroi K, Shimozuma K, Ohashi Y, et al. A questionnaire survey of physicians’ perspectives regarding the assessment of chemotherapy-induced peripheral neuropathy in patients with breast cancer. Jpn J Clin Oncol. 2008;38:748-754. doi: 10.1093/jjco/hyn100 [DOI] [PubMed] [Google Scholar]
- 29. Akman T, Yavuzsen T, Sevgen Z, Ellidokuz H, Yilmaz AU. Evaluation of sleep disorders in cancer patients based on Pittsburgh Sleep Quality Index. Eur J Cancer Care (Engl). 2015;24:553-559. doi: 10.1111/ecc.12296 [DOI] [PubMed] [Google Scholar]
- 30. Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage. 2004;27:140-148. doi: 10.1016/J.JPAINSYMMAN.2003.12.002 [DOI] [PubMed] [Google Scholar]
- 31. Warburton DER, Gledhill N, Jamnik VK, et al. Evidence-based risk assessment and recommendations for physical activity clearance: Consensus Document 2011. Appl Physiol Nutr Metab. 2011;36(suppl 1):S266-S298. doi: 10.1139/h11-062 [DOI] [PubMed] [Google Scholar]
- 32. Lin MR, Hwang HF, Hu MH, Wu HDI, Wang YW, Huang FC. Psychometric comparisons of the timed up and go, one-leg stand, functional reach, and Tinetti balance measures in community-dwelling older people. J Am Geriatr Soc. 2004;52:1343-1348. doi: 10.1111/j.1532-5415.2004.52366.x [DOI] [PubMed] [Google Scholar]
- 33. Makizako H, Shimada H, Doi T, et al. Predictive cutoff values of the five-times sit-to-stand test and the timed “up & go” test for disability incidence in older people dwelling in the community. Phys Ther. 2017;97:417-424. doi: 10.2522/ptj.20150665 [DOI] [PubMed] [Google Scholar]
- 34. Chevan J, Atherton HL, Hart MD, Holland CR, Larue BJ, Kaufman RR. Nontarget-and target-oriented functional reach among older adults at risk for falls. J Geriatr Phys Ther. 2003;26:22-25. doi: 10.1519/00139143-200308000-00004 [DOI] [Google Scholar]
- 35. Banerjee B, Vadiraj HS, Ram A, et al. Effects of an integrated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integr Cancer Ther. 2007;6:242-250. doi: 10.1177/1534735407306214 [DOI] [PubMed] [Google Scholar]
- 36. Vadiraja HS, Rao MR, Nagarathna R, et al. Effects of yoga program on quality of life and affect in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Complement Ther Med. 2009;17:274-280. doi: 10.1016/J.CTIM.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 37. Chandwani KD, Thornton B, Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8:43-55. doi: 10.2310/7200.2010.0002 [DOI] [PubMed] [Google Scholar]
- 38. Galantino ML, Kietrys DM, Parrott JS, Stevens ME, Stevens AM, Condoluci DV. Quality of life and self-reported lower extremity function in adults with HIV-related distal sensory polyneuropathy. Phys Ther. 2014;94:1455-1466. doi: 10.2522/ptj.20130337 [DOI] [PubMed] [Google Scholar]
- 39. Armstrong C, Swarbrick CM, Pye SR, O’Neill TW. Occurrence and risk factors for falls in rheumatoid arthritis. Ann Rheum Dis. 2005;64:1602-1604. doi: 10.1136/ard.2004.031195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gansler T, Kaw C, Crammer C, Smith T. A population-based study of prevalence of complementary methods use by cancer survivors: a report from the American Cancer Society’s studies of cancer survivors. Cancer. 2008;113:1048-1057. doi: 10.1002/cncr.23659 [DOI] [PubMed] [Google Scholar]
- 41. Mao JJ, Palmer SC, Straton JB, et al. Cancer survivors with unmet needs were more likely to use complementary and alternative medicine. J Cancer Surviv. 2008;2:116-124. doi: 10.1007/s11764-008-0052-3 [DOI] [PubMed] [Google Scholar]
- 42. Desai K, Bowman MA, Galantino ML, et al. Predictors of yoga use among patients with breast cancer. Explore (NY). 2010;6:359-363. doi: 10.1016/J.EXPLORE.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sherman KJ, Cherkin DC, Erro J, Miglioretti DL, Deyo RA. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849-856. doi: 10.7326/0003-4819-143-12-200512200-00003 [DOI] [PubMed] [Google Scholar]
- 44. Streckmann F, Kneis S, Leifert JA, et al. Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann Oncol. 2014;25:493-499. doi: 10.1093/annonc/mdt568 [DOI] [PubMed] [Google Scholar]
- 45. Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003;4:2-21. doi: 10.1054/JPAI.2003.1 [DOI] [PubMed] [Google Scholar]
- 46. Kuroi K, Shimozuma K, Ohashi Y, et al. Prospective assessment of chemotherapy-induced peripheral neuropathy due to weekly paclitaxel in patients with advanced or metastatic breast cancer (CSP-HOR 02 study). Support Care Cancer. 2009;17:1071-1080. doi: 10.1007/s00520-008-0550-x [DOI] [PubMed] [Google Scholar]
- 47. Haryani H, Fetzer SJ, Wu CL, Hsu YY. Chemotherapy-induced peripheral neuropathy assessment tools: a systematic review. Oncol Nurs Forum. 2017;44:E111-E123. doi: 10.1188/17.ONF.E111-E123 [DOI] [PubMed] [Google Scholar]
- 48. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385. doi: 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 49. Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tinetti ME, Richman D, Powell L. Falls efficacy as a measure of fear of falling. J Gerontol. 1990;45:P239-P243. doi: 10.1093/geronj/45.6.P239 [DOI] [PubMed] [Google Scholar]
- 51. Gin H, Rigalleau V. Screening for peripheral neuropathy: which tools? [in French]. Diabetes Metab. 2002;28:250-254. [PubMed] [Google Scholar]
- 52. Dorn LD, Lucke JF, Loucks TL, Berga SL. Salivary cortisol reflects serum cortisol: analysis of circadian profiles. Ann Clin Biochem. 2007;44(pt 3):281-284. doi: 10.1258/000456307780480954 [DOI] [PubMed] [Google Scholar]
- 53. Tran MD, Holly RG, Lashbrook J, Amsterdam EA. Effects of Hatha yoga practice on the health-related aspects of physical fitness. Prev Cardiol. 2001;4:165-170. doi: 10.1111/j.1520-037X.2001.00542.x [DOI] [PubMed] [Google Scholar]
- 54. Jacobs GD. Clinical applications of the relaxation response and mind-body interventions. J Altern Comple-ment Med. 2001;7(suppl 1):S93-S101. doi: 10.1089/107555301753393850 [DOI] [PubMed] [Google Scholar]
- 55. Jacobs GD. The physiology of mind–body interactions: the stress response and the relaxation response. J Altern Complement Med. 2001;7(suppl 1):S83-S92. doi: 10.1089/107555301753393841 [DOI] [PubMed] [Google Scholar]
- 56. Benson H, Beary JF, Carol MP. The relaxation response. Psychiatry. 1974;37:37-46. doi: 10.1080/00332747.1974.11023785 [DOI] [PubMed] [Google Scholar]
- 57. Dhruva A, Miaskowski C, Abrams D, et al. Yoga breathing for cancer chemotherapy–associated symptoms and quality of life: results of a pilot randomized controlled trial. J Altern Complement Med. 2012;18:473-479. doi: 10.1089/acm.2011.0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Visovsky C. Muscle strength, body composition, and physical activity in women receiving chemotherapy for breast cancer. Integr Cancer Ther. 2006;5:183-191. doi: 10.1177/1534735406291962 [DOI] [PubMed] [Google Scholar]
- 59. Hanna T. Somatics: Reawakening the Mind’s Control of Movement, Flexibility, and Health. Boston, MA: Da Capo Press; 1988. [Google Scholar]
- 60. Criswell E. How Yoga Works: An Introduction to Somatic Yoga. Novato, CA: Freeperson Press; 1987. [Google Scholar]
- 61. Hanna T. The Body of Life: Creating New Pathways for Sensory Awareness and Fluid Movement. Rochester, VT: Healing Arts Press; 1979. [Google Scholar]
- 62. Stults-Kolehmainen MA, Bartholomew JB, Sinha R. Chronic psychological stress impairs recovery of muscular function and somatic sensations over a 96-hour period. J Strength Cond Res. 2014;28:2007-2017. doi: 10.1519/JSC.0000000000000335 [DOI] [PubMed] [Google Scholar]
- 63. Peterson MV. Move Without Pain. New York, NY: Sterling; 2011. [Google Scholar]
- 64. Wahbeh H, Nelson M. iRest meditation for older adults with depression symptoms: a pilot study [published online October 24, 2018]. Int J Yoga Therap. doi: 10.17761/2019-00036 [DOI] [PubMed] [Google Scholar]
- 65. Pritchard M, Elison-Bowers P, Birdsall B. Impact of integrative restoration (iRest) meditation on perceived stress levels in multiple sclerosis and cancer outpatients. Stress Health. 2010;26:233-237. doi: 10.1002/smi.1290 [DOI] [Google Scholar]
- 66. Bower JE, Greendale G, Crosswell AD, et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology. 2014;43:20-29. doi: 10.1016/J.PSYNEUEN.2014.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li L, Shu W, Li Z, et al. Using yoga Nidra recordings for pain management in patients undergoing colonoscopy. Pain Manag Nurs. 2019;20:39-46. doi: 10.1016/J.PMN.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 68. Ferreira-Vorkapic C, Borba-Pinheiro CJ, Marchioro M, Santana D. The impact of yoga Nidra and seated meditation on the mental health of college professors. Int J Yoga. 2018;11:215-223. doi: 10.4103/ijoy.IJOY_57_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Livingston E, Collette-Merrill K. Effectiveness of integrative restoration (iRest) yoga Nidra on mindfulness, sleep, and pain in health care workers. Holist Nurs Pract. 2018;32:160-166. doi: 10.1097/HNP.0000000000000266 [DOI] [PubMed] [Google Scholar]
- 70. Tillery R, McGrady ME. Do complementary and integrative medicine therapies reduce healthcare utilization among oncology patients? A systematic review of the literature and recommendations. Eur J Oncol Nurs. 2018;36:1-8. doi: 10.1016/J.EJON.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 71. Lu W, Rosenthal DS. Oncology acupuncture for chronic pain in cancer survivors: a reflection on the American Society of Clinical Oncology chronic pain guideline. Hematol Oncol Clin North Am. 2018;32:519-533. doi: 10.1016/J.HOC.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 72. Yeung KS, Hernandez M, Mao JJ, Haviland I, Gubili J. Herbal medicine for depression and anxiety: a systematic review with assessment of potential psycho-oncologic relevance. Phytother Res. 2018;32:865-891. doi: 10.1002/ptr.6033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev. 2017;(1):CD010802. doi: 10.1002/14651858.CD010802.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vollbehr NK, Bartels-Velthuis AA, Nauta MH, et al. Hatha yoga for acute, chronic and/or treatment-resistant mood and anxiety disorders: a systematic review and meta-analysis. PLoS One. 2018;13:e0204925. doi: 10.1371/journal.pone.0204925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sherman KJ, Cherkin DC, Cook AJ, et al. Comparison of yoga versus stretching for chronic low back pain: protocol for the yoga exercise self-care (YES) trial. Trials. 2010;11:36. doi: 10.1186/1745-6215-11-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Boon HS, Olatunde F, Zick SM. Trends in complementary/alternative medicine use by breast cancer survivors: comparing survey data from 1998 and 2005. BMC Womens Health. 2007;7:4. doi: 10.1186/1472-6874-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cassileth BR, Vickers AJ. High prevalence of complementary and alternative medicine use among cancer patients: implications for research and clinical care. J Clin Oncol. 2005;23:2590-2592. doi: 10.1200/JCO.2005.11.922 [DOI] [PubMed] [Google Scholar]
- 78. Mao JJ, Stricker C, Bruner D, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009;115:3631-3639. doi: 10.1002/cncr.24419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Duncan MD, Leis A, Taylor-Brown JW. Impact and outcomes of an Iyengar yoga program in a cancer centre. Curr Oncol. 2008;15(suppl 2):s109.es72-8. doi: 10.3747/co.v15i0.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rao MR, Raghuram N, Nagendra HR, et al. Anxiolytic effects of a yoga program in early breast cancer patients undergoing conventional treatment: a randomized controlled trial. Complement Ther Med. 2009;17:1-8. doi: 10.1016/J.CTIM.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 81. Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007;25:4387-4395. doi: 10.1200/JCO.2006.06.6027 [DOI] [PubMed] [Google Scholar]
- 82. Danhauer SC, Tooze JA, Farmer DF, et al. Restorative yoga for women with ovarian or breast cancer: findings from a pilot study. J Soc Integr Oncol. 2008;6:47-58. doi: 10.2310/7200.2008.0008 [DOI] [PubMed] [Google Scholar]
- 83. Malhotra V, Singh S, Tandon OP, Madhu SV, Prasad A, Sharma SB. Effect of yoga Asanas on nerve conduction in type 2 diabetes. Indian J Physiol Pharmacol. 2002;46:298-306. [PubMed] [Google Scholar]
- 84. Boslego LAW, Phillips CEM, Atler KE, Tracy BL, Van Puymbroeck M, Schmid AA. Impact of yoga on balance, balance confidence and occupational performance for adults with diabetic peripheral neuropathy: a pilot study. Br J Occup Ther. 2017;80:155-162. doi: 10.1177/0308022616680364 [DOI] [Google Scholar]
- 85. Chandwani KD, Perkins G, Nagendra HR, et al. Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J Clin Oncol. 2014;32:1058-1065. doi: 10.1200/JCO.2012.48.2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Moraes LJ, Miranda MB, Loures LF, Mainieri AG, Mármora CHC. A systematic review of psychoneuroimmunology-based interventions. Psychol Health Med. 2018;23:635-652. doi: 10.1080/13548506.2017.1417607 [DOI] [PubMed] [Google Scholar]
- 87. Pascoe MC, Thompson DR, Ski CF. Yoga, mindfulness-based stress reduction and stress-related physiological measures: a meta-analysis. Psychoneuroendocrinology. 2017;86:152-168. doi: 10.1016/j.psyneuen.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 88. Kneis S, Wehrle A, Freyler K, et al. Balance impairments and neuromuscular changes in breast cancer patients with chemotherapy-induced peripheral neuropathy. Clin Neurophysiol. 2016;127:1481-1490. doi: 10.1016/J.CLINPH.2015.07.022 [DOI] [PubMed] [Google Scholar]
- 89. Tofthagen C. Patient perceptions associated with chemotherapy-induced peripheral neuropathy. Clin J Oncol Nurs. 2010;14:E22-E28. doi: 10.1188/10.CJON.E22-E28 [DOI] [PubMed] [Google Scholar]
- 90. Ebenezer GJ, Hauer P, Gibbons C, McArthur JC, Polydefkis M. Assessment of epidermal nerve fibers: a new diagnostic and predictive tool for peripheral neuropathies. J Neuropathol Exp Neurol. 2007;66:1059-1073. doi: 10.1097/nen.0b013e31815c8989 [DOI] [PubMed] [Google Scholar]
- 91. Galantino M, Bzdewka T, Eissler-Russo JL, et al. The impact of modified Hatha yoga on chronic low back pain: a pilot study. Altern Ther Health Med. 2004;10:56-59. [PubMed] [Google Scholar]
- 92. Wampler MA, Topp KS, Miaskowski C, Byl NN, Rugo HS, Hamel K. Quantitative and clinical description of postural instability in women with breast cancer treated with taxane chemotherapy. Arch Phys Med Rehabil. 2007;88:1002-1008. doi: 10.1016/J.APMR.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 93. Kober KM, Mazor M, Abrams G, et al. Phenotypic characterization of paclitaxel-induced peripheral neuropathy in cancer survivors. J Pain Symptom Manage. 2018;56:908-919.e3. doi: 10.1016/J.JPAINSYMMAN.2018.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Miaskowski C, Mastick J, Paul SM, et al. Chemotherapy-induced neuropathy in cancer survivors. J Pain Symptom Manage. 2017;54:204-218.e2. doi: 10.1016/J.JPAINSYMMAN.2016.12.342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Visovsky C, Daly BJ. Clinical evaluation and patterns of chemotherapy-induced peripheral neuropathy. J Am Acad Nurse Pract. 2004;16:353-359. doi: 10.1111/j.1745-7599.2004.tb00458.x [DOI] [PubMed] [Google Scholar]
- 96. Streckmann F, Balke M, Lehmann HC, et al. The preventive effect of sensorimotor and vibration exercises on the onset of oxaliplatin- or vinca-alkaloid induced peripheral neuropathies—STOP. BMC Cancer. 2018;18:62. doi: 10.1186/s12885-017-3866-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hershman DL, Lacchetti C, Loprinzi CL. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline summary. J Oncol Pract. 2014;10:e421-e424. doi: 10.1200/JOP.2014.001776 [DOI] [PubMed] [Google Scholar]
- 98. Tofthagen C, Overcash J, Kip K. Falls in persons with chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2012;20:583-589. doi: 10.1007/s00520-011-1127-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hile ES, Fitzgerald GK, Studenski SA. Persistent mobility disability after neurotoxic chemotherapy. Phys Ther. 2010;90:1649-1657. doi: 10.2522/ptj.20090405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Schwenk M, Grewal GS, Holloway D, Muchna A, Garland L, Najafi B. Interactive sensor-based balance training in older cancer patients with chemotherapy-induced peripheral neuropathy: a randomized controlled trial. Gerontology. 2016;62:553-563. doi: 10.1159/000442253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Brayall P, Donlon E, Doyle L, Leiby R, Violette K. Physical therapy–based interventions improve balance, function, symptoms, and quality of life in patients with chemotherapy-induced peripheral neuropathy. Rehabil Oncol. 2018;36:161-166. doi: 10.1097/01.REO.0000000000000111 [DOI] [Google Scholar]
- 102. Vollmers PL, Mundhenke C, Maass N, et al. Evaluation of the effects of sensorimotor exercise on physical and psychological parameters in breast cancer patients undergoing neurotoxic chemotherapy. J Cancer Res Clin Oncol. 2018;144:1785-1792. doi: 10.1007/s00432-018-2686-5 [DOI] [PubMed] [Google Scholar]
- 103. Cammisuli S, Cavazzi E, Baldissarro E, Leandri M. Rehabilitation of balance disturbances due to chemotherapy-induced peripheral neuropathy: a pilot study. Eur J Phys Rehabil Med. 2016;52:479-488. [PubMed] [Google Scholar]
- 104. Zimmer P, Trebing S, Timmers-Trebing U, et al. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: a randomized controlled trial. Support Care Cancer. 2018;26:615-624. doi: 10.1007/s00520-017-3875-5 [DOI] [PubMed] [Google Scholar]
- 105. Fernandes J, Kumar S. Effect of lower limb closed kinematic chain exercises on balance in patients with chemotherapy-induced peripheral neuropathy: a pilot study. Int J Rehabil Res. 2016;39:368-371. doi: 10.1097/MRR.0000000000000196 [DOI] [PubMed] [Google Scholar]
- 106. Duregon F, Vendramin B, Bullo V, et al. Effects of exercise on cancer patients suffering chemotherapy-induced peripheral neuropathy undergoing treatment: a systematic review. Crit Rev Oncol Hematol. 2018;121:90-100. doi: 10.1016/J.CRITREVONC.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 107. DiStasio SA. Integrating yoga into cancer care. Clin J Oncol Nurs. 2008;12:125-130. doi: 10.1188/08.CJON.125-130 [DOI] [PubMed] [Google Scholar]
- 108. Smith BW, Shelley BM, Dalen J, Wiggins K, Tooley E, Bernard J. A pilot study comparing the effects of mindfulness-based and cognitive-behavioral stress reduction. J Altern Complement Med. 2008;14:251-258. doi: 10.1089/acm.2007.0641 [DOI] [PubMed] [Google Scholar]
- 109. Smith KB, Pukall CF. An evidence-based review of yoga as a complementary intervention for patients with cancer. Psychooncology. 2009;18:465-475. doi: 10.1002/pon.1411 [DOI] [PubMed] [Google Scholar]