Abstract
Background:
Recent animal and retrospective human studies have demonstrated that Schistosoma mansoni infection may have potential to protect against development of metabolic syndromes. Thus, the aim of this study was to assess metabolic panel among S. mansoni egg positives and egg negatives in stool examinations. This study was a cross-sectional study, conducted involving 120 participants from S. mansoni endemic town (Kemise) and 61 from non-endemic town (Kombolcha), Northeast Ethiopia. Stool samples were collected and examined for S. mansoni and other helminths using Kato-Katz method. Furthermore, blood samples were collected and used for determination of blood sugar, lipid profile tests, insulin, and C-reactive protein. Data were analyzed using SPSS software version 20. Chi-square test, independent mean t-test, and logistic regression models were employed on data. P values less than .05 were considered as statistically significant.
Results:
S. mansoni infected participants (n = 41; all from Kemise) had significantly lower levels of fasting blood sugar, low prevalence of dyslipidemia (at least one or more abnormal lipid profile tests; total cholesterol, low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], and triglycerides) as compared with controls (n = 79 in Kemise and 61 in Kombolcha). Moreover, logistic regression model indicated that with the adjusted odds ratios, there was significant inverse association between S. mansoni infection and impaired fasting glucose (adjusted odds ratio −0.181, 95% confidence interval: 0.042-0.774).
Conclusions:
Low fasting blood sugar and reduced prevalence of dyslipidemia in S. mansoni egg positive participants might suggest inverse association of S. mansoni infection and development of metabolic syndromes. Furthermore, large-scale studies are recommended to assess the role of S. mansoni egg and/or worm antigens in modulating the host metabolic profile and reducing the risk of metabolic syndromes, including diabetes mellitus and cardiovascular diseases.
Keywords: Schistosoma mansoni, metabolic syndrome, impaired fasting blood glucose, dyslipidemia
Introduction
Schistosomiasis caused by Schistosoma mansoni and other schistosome species (S. japonicum, S. interculatum, and S. haematobium) is the second most important parasitic disease worldwide following malaria.1 Studies suggest that around 779 million people are at risk of acquiring schistosomiasis worldwide.2 In sub-Saharan Africa, where more than 80% of Schistosomiasis occurs, around 393 million people are at risk of infection while about 54 million are currently infected.3
On the other hand, metabolic syndrome (MetS) is one of the complicated noncommunicable disease, affected millions of people worldwide. According to the definitions of International Diabetes Federation (IDF)4 and National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATPIII),5 to declare individual develops MetS expected to fulfill three or more of the following five criteria: arterial blood pressure ⩾130/85 mmHg; central obesity (waist circumference, male <102 cm; female <88 cm); serum triglyceride level ⩾150 mg/dL (1.7 mmol/L); serum high-density lipoprotein cholesterol (HDL-C) level <40 mg/dL (1.03 mmol/L) in male or <50 mg/dL (1.29 mmol/L) in female; fasting glucose ⩾110 mg/dL (6.1 mmol/L).
In the last decades, animal model and human epidemiological studies showed the prevalence of S. mansoni infection to be associated with reduced occurrence of MetS, such as diabetes mellitus (DM) and cardiovascular diseases (CVDs).6,7 In Ethiopia, S. mansoni infection is widespread in most parts of the country and the prevalence in endemic areas, such as Kemise administrative zone in the northeastern Ethiopia, reaches up to 89.6%.8 On the other hand, MetS, such as DM, is relatively low in the country. For example, according to reports of the IDF, the prevalence of DM in Ethiopia in 2012 was estimated to be around 3.32%.9
Epidemiological data from the World Health Organization (WHO) as well as from the IDF indicate that MetS, such as DM, is relatively less prevalent in most African and Asian populations where communicable diseases are more common, as compared with the developed world.10,11 Most of the data on the beneficial effect of helminthic infections against DM and CVDs came from animal studies,12,13 while the few studies on humans demonstrated an association between previous history of S mansoni infection and reduced risk of DM and cardiovascular disorders.14,15 In the present study, we assessed MetS profiles of S. mansoni infected individuals in comparison with S. mansoni stool negative individuals from S. mansoni endemic and non-endemic sites in Northeast Ethiopia.
Materials and Methods
Study design and participants
A cross-sectional study was conducted from February to May 2014, in Kemise and Kombolcha, which are located in the North Eastern part of Ethiopia at 325 and 376 km from Addis Ababa, respectively. The S. mansoni endemic area, Kemise, is special woreda of Oromia in Administrative Zone of Amhara Regional State. The city is located at 325 km northeast of Addis Ababa, with altitude of 1450 m, and surrounded by Borkena river basin. Incomes sources of the people are from trade, agriculture, and livestock productions. Irrigation is practiced for vegetables and chat cultivations. Studies indicated that in Kemise, prevalence of S. mansoni reaches up to 89.6%.8 Both communities have fairly comparable socio-demographic characteristics, but differ in endemicity of S. mansoni infection. Kemise is endemic for S. mansoni, whereas Kombolcha is nonendemic for S. mansoni infection.
Data collection
The inclusion criteria include individuals who were living in the specific areas (Kemise or Kombolcha) at least 5 years or more, age greater than 18 years, and not taking anti-helmints drugs in the last 6 months, while study participants had known MetS, including DM, CVDs, and other chronic noncommunicable diseases, such as cancer was excluded. A community health worker and a nurse at each study site were trained how to collect data. The health extension workers were given an assignment of going house to house, to identify eligible participants, and invite those consenting for interview.
After obtaining informed consent, data on socio-demographic characteristics were collected using a structured questionnaire. Stool samples were collected from a total of 181 study participants and processed using the wet mount saline slide and Kato-Katz thick smear technique (one wet mount and two Kato-Katz slides for each study participant). As shown in different parasitological method comparison studies, Kato-Katz has better yield on helminthes egg identification, and most recommend concentration method.16,17
Then blood samples were collected for biochemical tests. Fasting blood sugar was determined using a blood glucose meter (“mylife Pura,” Burgdorf, UK), at the study sites, and blood samples were further processed to determine additional biochemical parameters, such as insulin levels, and lipid profiles at the clinical chemistry laboratory of the Ethiopian Public Health Institute. Quality control samples were run and validated for biochemical tests, before running our study samples on cobas 6000 c501 (Roche Diagnostics GmbH, Mannheim, Germany).
Statistical analysis
Statistical analysis of the data was performed using SPSS version 20 software. The associations between S mansoni infection and metabolic profiles were determined by applying descriptive statistics, including chi-square test, independent mean t tests, and logistic regression, both binomial and multiple regression tests. P values less than .05 were considered as statistically significant.
Ethical considerations
The study protocol was reviewed and ethical approval obtained from the Institutional Review Board, Aklilu Lemma Institute of Pathobiology, Addis Ababa University and from National Ethical Clearance Committee of the Ethiopia Science and Technology Commission. Moreover, the aim of the study was explained and informed consent obtained from all study participants. Appropriate treatment was offered to participants who were found positive for S. mansoni or other helminth infections.
Results
Of the total 181 study participants, 120 were from S. mansoni endemic area (Kemise) and 61 were from S. mansoni nonendemic area (Kombolcha). The prevalence of S. mansoni was 34.2% (41 out of 120) in Kemise whereas none of the 61 participants from Kombolcha were positive and were included as non-endemic negative controls. Those S. mansoni negative participants from Kemise were also taken as endemic negative controls. The mean age (SD) of the study participants was 36.8 (14.6) years and 59.1% (107 out of 181) were male. There was no significant difference in socio-demographic factors between individuals from the two study sites. Moreover, determination of body mass index (BMI) and biochemical analysis revealed that S. mansoni positive study participants had statistically significantly higher levels of mean and lowered compared with of BMI, fasting blood sugar (FBS), HDL, and low-density lipoprotein (LDL) values, as compared with S. mansoni negative counterparts (Table 1). Percentage of individuals who had C-reactive protein (CRP) values greater than the upper limit of 6 mg/dL was higher in S. mansoni positives than in S. mansoni negatives in Kemise and Kombolcha (46.7% vs 27.4% and 34.4%, respectively), as shown in Table 1.
Table 1.
Socio-demographic characteristics and biochemical features of study participants with or without Schistosoma mansoni infection, in Kemise and Kombolcha, Northeast Ethiopia, 2014.
| Variablea |
S. mansoni positive, Kemise
(n = 41) Mean (SD) |
S. mansoni negative, Kemise
(n = 79) Mean (SD) |
S. mansoni negative, Kombolcha
(n = 61) Mean (SD) |
|---|---|---|---|
| Age, year | 44.2 (11.6) | 39.9 (16.4) | 28.1 (8.6) |
| Male, n % | 24 (58.5%) | 40 (50.6%) | 43 (70.5%) |
| BMI, kg/m2 | 20.4 (4.0)b,c | 23.5 (3.7) | 24.3 (3.9) |
| FBS, mg/dL | 94.0 (16.4)b,c | 114.3 (36.9) | 105.2 (26.5) |
| Insulin, µmol/mL | 15.2 (11.6) | 12 (19.8) | 11.1 (10.4) |
| CRP >6 mg/dLd | 14 (46.7%) | 20 (27.4%) | 11 (34.4%) |
| Systolic BP | 127.1 (12.5) | 129.1 (24.5) | 125.0 (15.9) |
| Diastolic BP | 82.8 (9.5) | 80.5 (13.7) | 80.6 (14.2) |
| T-Chol, mg/dL | 175.6 (156.5) | 176.8 (47.9) | 199.4 (81.3) |
| HDL-C, mg/dL | 32.2 (7.9)b,c | 36.9 (10.4) | 38.2 (15.5) |
| LDL-C, mg/dL | 96.4 (38.3)b,c | 116.3 (43) | 106.6 (39.3) |
| Trig, mg/dL | 133.6 (89.4)c | 118.2 (75.3)e | 240.1 (243.1) |
Abbreviations: BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; FBS, fasting blood sugar; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; T-Chol, total cholesterol; Trig, triglycerides.
All continuous variables were presented as mean ± SD, whereas categorical variables were presented as number (proportions).
Significant difference between Kemise S. mansoni positive and Kemise S. mansoni negatives (P < .05).
Significant difference between Kemise S. mansoni positive and Kombolcha S. mansoni negatives (P < .05).
HumaTex CRP test kit, Human Gesellschaft für Biochemica und Diagnostica mbH Germany.
Significant difference between Kemise S. mansoni negatives and Kombolcha S. mansoni negatives (P < .05).
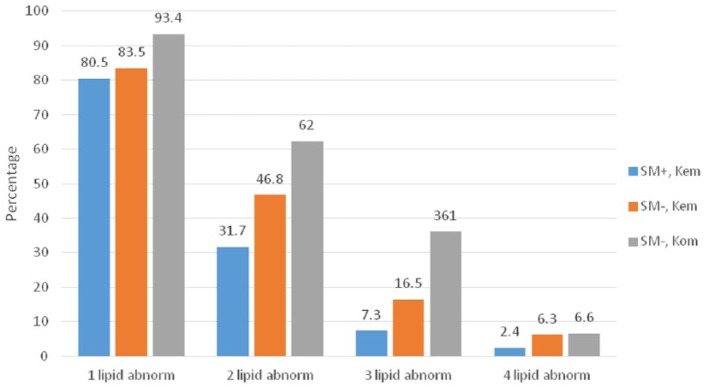
Dyslipidemia is defined as the presence of one or more abnormal lipid profiles (total cholesterol, HDL-C, LDL-C, and triglycerides) among study participants. Accordingly, at least one or more lipid profile abnormalities (dyslipidemia) was relatively more common among study participants who are S. mansoni negatives, regardless of study site, as compared with the S. mansoni infected participants from Kemise (Figure 1). These abnormalities in lipid profile parameters were markedly different in S. mansoni positives from Kemise compared with S. mansoni negatives from Kombolcha (P < .05).
Figure 1.
Occurrence of dyslipidemia among study participants with or without Schistosoma mansoni infection from Kemise and Kombolcha, Northeast Ethiopia, 2014. The occurrence of one lipid abnormality (total cholesterol, HDL-C, LDL-C, or triglycerides) in the groups was compared with the occurrence of 2, 3, or 4 lipid abnormalities within the different groups. SM indicates S. mansoni; Kem, Kemise; Kom, Kombolcha; abnorm, abnormality.
*Significant difference between SM+, Kem and SM–, Kom in 1, 2, and 3 lipid abnormalities (P < .05).
Results from logistic regression analysis demonstrated that individuals infected with S. mansoni were less likely to have impaired fasting glucose (IFG) results, COR = 0.208, 95% confidence interval (CI): 0.06-0.64, compared with their S. mansoni negative counterparts. This inverse association of S. mansoni infection and occurrence of IFG remained statistically significant after adjusting separately for age, sex, BMI, and lipid profiles (total cholesterol, HDL-C, LDL-C, and triglycerides). Moreover, in a multivariate logistic regression model that took account of all the pre-specified variables together (ie, age, sex, BMI, and Lipid profile), there was also significant inverse association between S. mansoni infection and IFG (0.181, 95% CI: 0.042-0.774) (Table 2).
Table 2.
Crude and adjusted effects of S. mansoni infection in Kemise on the likelihood of being diabetic.
| Variables | OR (95% CI) | P-value |
|---|---|---|
| Crude effect of S. mansoni infection on IFG | 0.208 (0.06-0.64) | .007 |
| The effect of S. mansoni on
occurrence of IFG after adjusting for: | ||
| Age | 0.209 (0.065-0.672) | .009 |
| Sex | 0.214 (0.069-0.666) | .008 |
| BMI | 0.237 (0.074-0.752) | .015 |
| T-Chol | 0.211 (0.068-0.659) | .007 |
| LDL-C | 0.257 (0.081-0.812) | .021 |
| HDL-C | 0.226 (0.072-0.706) | .011 |
| Trig | 0.184 (0.05-0.588) | .004 |
| Age, sex, BMI, and lipid profile (T-Chol, HDL-C, LDL-C, Trig) | 0.181 (0.042-0.774) | .021 |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; T-Chol, total cholesterol; Trig, triglycerides.
Discussion
The current study demonstrated that S. mansoni egg positive study participants showed reduced FBS, HDL-C, and LDL-C as compared with individuals who were negative for S. mansoni from both S. mansoni endemic and non-endemic sites. Differences in the blood sugar levels between S. mansoni positive and negative individuals were statistically significant, and these differences were still significant after adjusting for sex, age, BMI, and lipid profile (total cholesterol, HDL-C, LDL-C, and triglycerides). On this study, all samples were freshly collected and directly evaluated the association of blood sugar and lipid profile tests in S. mansoni stool positives versus stool negative study participants.
The finding of this study was in line with a study conducted in China, by Chen et al,18 although their study was carried out on previous history of S. mansoni infection. The inverse association of S. mansoni infection and blood sugar values is mostly immunological. S. mansoni infections are generally divided into two broad stages: an acute stage and chronic stage. The acute stage, which occurs from exposure to cercariae, migration of immature worms to tissue deposition of eggs, whereas the chronic stage, starts after egg laying at around 12 weeks of infection, and may last up to 40 years.19 As studies indicated, inflammatory cytokines (Th1) produced during acute schistosomiasis, as well as cytokines produced during pre-diabetes stages of DM (tumor necrosis factor [TNF], interleukin 1 [IL-1], and IL-6), have similarity. But, during the chronic stage of schistosomiasis, the concentration of Th1 cytokines is gradually decreased and substituted by Th2 cytokines, including IL-4, IL-10, and IL-13, that do have regulatory, and antagonist effect to Th1 cytokines.20,21 Thus, chronic schistosome infections may have a potential to reduce blood sugar levels and hence either delay or prevent occurrence of MetS, as well as DM, by antagonizing or suppressing the respective subclinical production of pro-inflammatory cytokines. Moreover, a study by Hussaarts et al22 also indicated that schistosomal soluble egg antigen (SEA) stimulates white adipose tissues and shift immune balance into anti-inflammatory macrophages (M2) pathways, which improves insulin sensitivity and maintains homeostasis of glucose; this in turn might protect occurrence of MetS.
Moreover, the occurrence of dyslipidemia in the current study was relatively low in S. mansoni infected participants as compared with non-infected ones. This concurs with studies done by Dimenstein et al,23 Assaad-Khalil et al,24 and Martins da Fonseca et al,25 which showed occurrence of low lipid profile or dyslipidemia in schistosomiasis patients as compared with control groups. Since there is no de novo synthesis of fatty acids by schistosomes, the parasite fully depends on the host for its fatty acid supply. Nevertheless, once the fatty acids enter into the parasite from the host cell, schistosomes prepare their own unique fatty acid and lipid. Among the different roles of these modified fatty acids and lipids in schistosomes, stimulation of immune regulatory cytokines, such as IL-10 by host cells, is mentioned.26 Moreover, since schistosomes are solely dependent on fatty acid utilization from the human host, this may help the host from excess lipid accumulation in blood vessels, and so may prevent occurrence of atherosclerosis.27 Thus, S. mansoni being fully dependent for its fatty acid and lipid sources on human host, indirectly favor the host by protecting MetS, including type II DM.
Conclusions
In general, the present study demonstrated that occurrence of low fasting blood glucose and reduced prevalence of dyslipidemia in S. mansoni egg positive participants might suggest a protective role of S. mansoni infection against the development of MetS. Furthermore, large-scale studies are recommended to assess the role of S. mansoni egg and/or worm antigens in modulating the host metabolic profile and reducing the risk of MetS, including DM and CVDs.
Acknowledgments
The authors gratefully acknowledge the study participants, and the support provided by Aklilu Lemma Institute of Pathobiology (ALIPB), AAU, Ethiopia; CIS (VU University Amsterdam, the Netherlands) to IVD, the Netherlands; Oslo University Hospital-Ullevål, Center for Imported and Tropical Diseases, Oslo, Norway.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grants from graduate students research grant, Addis Ababa University (AAU), Ethiopia; CIS (VU University Amsterdam, the Netherlands) to IVD; and Oslo University Hospital-Ullevål, Center for Imported and Tropical Diseases, Oslo, Norway.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MW: design the project, monitor data collection, and prepare the manuscript; NB, AT, IVD: main advisors of the project, and participate in conception and designing, as well revising the manuscript critically for important intellectual content; FC: participated in MetS panels test and result interpretations and involved in the designing of the project; GM: advise the biostatistics part of the project, and participate in designing, data analysis, and interpretation. In addition, all authors are agreed to be accountable for all aspects of the work. All authors have read and approved the final manuscript.
Availability of Data: The dataset supporting the conclusions of this article is included within the article.
Ethical Approval and Consent to Participate: Ethical clearance for the study was obtained from Aklilu Lemma Institute of Pathobiology, Addis Ababa University, and from the Ethiopian Ministry of Science and Technology. Moreover, support letters to collect data (sample) from study participants were secured from Kemise zone, and Kombolcha city health bureau. Written informed consent was obtained from the study participants after explaining about the study including their right to withdraw from the study anytime. Moreover, confidentiality of data was maintained throughout the study by keeping hard copies locked and electronic files password protected.
References
- 1. Campino S, Kwiatkowski D, Dessein A. Mendelian and complex genetics of susceptibility and resistance to parasitic infections. Semin Immunol. 2006;18:411–422. [DOI] [PubMed] [Google Scholar]
- 2. Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water Resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. 2006;6:411–425. [DOI] [PubMed] [Google Scholar]
- 3. Van der Werf MJ, De Vlas SJ, Brooker S, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. [DOI] [PubMed] [Google Scholar]
- 4. The IDF Concensus Worldwide Definition of the Metabolic Syndrome. Brussels: International Diabetes Federation; 2006:1–2. [Google Scholar]
- 5. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 6. Versini M, Jeandel PY, Bashi T, Bizzaro G, Blank M, Shoenfeld Y. Unraveling the Hygiene Hypothesis of helminthes and autoimmunity: origins, pathophysiology, and clinical applications. BMC Med. 2015;13:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zaccone P, Fehervari Z, Jones FM, et al. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol. 2003;33:1439–1449. [DOI] [PubMed] [Google Scholar]
- 8. Aemero M, Berhe N, Erko B. Status of Schistosoma mansoni prevalence and intensity of infection in geographically apart endemic localities of Ethiopia: a comparison. Ethiop J Health Sci. 2014;24:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. International Diabetes Federation (IDF). Diabetes Atlas. 5th ed. Brussels: IDF; 2012. [Google Scholar]
- 10. Perzanowski MS, Ng’ang’a LW, Carter MC, et al. Atopy, asthma, and antibodies to Ascaris among rural and urban children in Kenya. J Pediatr. 2002;140:582–588. [DOI] [PubMed] [Google Scholar]
- 11. Kamradt T, Goggel R, Erb KJ. Induction, exacerbation and inhibition of allergic and autoimmune diseases by infection. Trends Immunol. 2005;26:260–267. [DOI] [PubMed] [Google Scholar]
- 12. Zaccone P, Burton OT, Gibbs S, et al. Immune modulation by Schistosoma mansoni antigens in NOD mice: effects on both innate and adaptive immune systems. J Biomed Biotechnol. 2010;2010:795210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stanley RG, Jackson CL, Griffiths K, Doenhoff MJ. Effects of Schistosoma mansoni worms and eggs on circulating cholesterol and liver lipids in mice. Atherosclerosis. 2009;207:131–138. [DOI] [PubMed] [Google Scholar]
- 14. Shen SW, Lu Y, Li F, et al. Potential long-term effects of previous schistosome infection may reduce the atherogenic index of plasma in Chinese men. Int J Parasitol. 2015;45:289–294. [DOI] [PubMed] [Google Scholar]
- 15. Shen SW, Lu Y, Li F, et al. The potential long-term effect of previous schistosome infection reduces the risk of metabolic syndrome among Chinese men. Parasite Immunol. 2015;37:333–339. [DOI] [PubMed] [Google Scholar]
- 16. Yimer M, Hailu T, Mulu W, Abera B. Evaluation performance of diagnostic methods of intestinal parasitosis in school age children in Ethiopia. BMC Res Notes. 2015;8:820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Endris M, Tekeste Z, Lemma W, Kassu A. Comparison of the Kato-Katz, Wet Mount, and Formol-Ether concentration diagnostic techniques for intestinal Helminth infections in Ethiopia. ISRN Parasitol. 2012;2013:180439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen Y, Lu J, Huang Y, et al. Association of previous schistosome infection with diabetes and metabolic syndrome: a cross-sectional study in rural China. J Clin Endocrinol Metab. 2013;98:E283–E287. [DOI] [PubMed] [Google Scholar]
- 19. Wynn TA, Thompson RW, Cheever AW, Mentink-Kane MM. Immunopathogenesis of schistosomiasis. Immunol Rev. 2004;201:156–167. [DOI] [PubMed] [Google Scholar]
- 20. Dunne DW, Cooke A. A worm’s eye view of the immune system: consequences for evolution of human autoimmune disease. Nat Rev Immunol. 2005;5:420–426. [DOI] [PubMed] [Google Scholar]
- 21. Van Die I, Cummings RD. Glycans modulate immune responses in helminth infections and allergy. Chem Immunol Allergy. 2006;90:91–112. [DOI] [PubMed] [Google Scholar]
- 22. Hussaarts L, García-Tardón N, van Beek L, et al. Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2-macrophages and improve insulin sensitivity in obese mice. FASEB J. 2015;29:3027–3039. [DOI] [PubMed] [Google Scholar]
- 23. Dimenstein R, Carvalho VC, Oliveira DN, Gillett MP. Alteration in the levels and lipid compositions of plasma lipoproteins [VLDL, LDL and HDL] in Brazilian patients with hepatosplenic schistosomiasis mansoni. Braz J Med Biol. 1992;25:1091–1102 [PubMed] [Google Scholar]
- 24. Assaad-Khalil SH, Lachine N, Sidrak M, Amara F, Jacotot B, Fahmy MH. Immuno-metabolic factors in schistosomal hepatic-fibrosis modulating atherogenesis. Ann Biol Clin. 1992;50:697–701. [PubMed] [Google Scholar]
- 25. Martins da, Fonseca CS, Pimenta Filho AA, dos Santos BS, et al. Human plasma lipid modulation in schistosomiasis mansoni depends on apolipoprotein E polymorphism. PLoS ONE. 2014;9:e101964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kleij DVD, Latz E, Brouwers JFHM, et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidyl serine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277:48122–48129. [DOI] [PubMed] [Google Scholar]
- 27. Doenhoff MJ, Stanley RG, Griffiths K, Jackson CL. An anti-atherogenic effect of Schistosoma mansoni infections in mice associated with a parasite-induced lowering of blood total cholesterol. Parasitology. 2002;125:415–421. [DOI] [PubMed] [Google Scholar]