Abstract
Purpose: To assess feasibility and preliminary efficacy of a mobile/online-based (mHealth) mindfulness intervention for cancer patients and their caregivers to reduce distress and improve quality of life (QoL). Material and Methods: Two-arm randomized controlled trial within Kaiser Permanente Northern California targeting cancer patients who received chemotherapy and their informal caregivers. The intervention group received a commercially available mindfulness program for 8 weeks. The wait-list control group received usual care. We assessed feasibility using retention and adherence rates and obtained participant-reported data on distress, QoL, sleep, mindfulness, and posttraumatic growth before and immediately after the intervention. Results: Ninety-seven patients (median age 59 years; female 69%; 65% whites) and 31 caregivers (median age 63 years; female 58%; 77% whites) were randomized. Among randomized participants, 74% of the patients and 84% of the caregivers completed the study. Among those in the intervention arm who initiated the mindfulness program, 65% practiced at least 50% of the days during the intervention period. We observed significantly greater improvement in QoL among patients in the intervention arm compared with controls. Caregivers in the intervention group experienced increased mindfulness compared with controls. Participants appreciated the convenience of the intervention and the mindfulness skills they obtained from the program. Conclusion: We demonstrated the feasibility of conducting a randomized trial of an mHealth mindfulness intervention for cancer patients and their informal caregivers. Results from fully powered efficacy trials would inform the potential for clinicians to use this scalable intervention to help improve QoL of those affected by cancer and their caregivers.
Keywords: mindfulness, cancer, clinical trial, chemotherapy, caregivers, quality of life
Introduction
A cancer diagnosis is associated with high levels of distress, particularly anxiety, depression, fear of recurrence, and low quality of life (QoL), in both patients and caregivers.1-3 The National Comprehensive Cancer Network (NCCN) definition of distress is “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”4 Screening for psychosocial distress became a Commission on Cancer accreditation requirement in 2015.5 Due to this requirement, it is likely that more patients will be identified as “distressed,” and providers will need efficient and affordable strategies to offer support to those who are in need. Research indicates that cancer patients’ levels of psychosocial stress and depression not only affect QoL but also survival and prognosis.6 Distress extends along a continuum, and although an estimated 35% of cancer patients experience such psychological distress,7 clinicians are often at a loss as to how to address the QoL of cancer patients and their families. To help cope with the distress associated with the diagnosis, disease symptoms, and treatment, cancer patients are increasingly turning to integrative medicine,8-12 including mindfulness practices or meditation.
Mindfulness, a psychological process of bringing one’s attention to the present moment with an attitude of nonjudgment, acceptance, and openness,13-15 appears to be an effective intervention for reducing symptoms of depression and anxiety in many populations.16-20 Through mindfulness meditation and practices, individuals are encouraged to turn toward and observe negative experiences, which they may have previously avoided, thus improving their ability to tolerate negative experiences such as cancer symptoms and anxiety about the future. Previous meta-analyses of mindfulness interventions in cancer patients/survivors have demonstrated that these interventions result in significant improvement in mood, anxiety, and QoL.21-26 Most of the previously studied mindfulness interventions used programs based on Jon Kabat-Zinn’s Mindfulness-Based Stress Reduction (MBSR) program or its variant, Mindfulness-Based Cancer Recovery program (MBCR),27 and often targeted early-stage breast cancer patients who had completed active treatment. Despite the established efficacy, logistical requirements of these traditional class-based mindfulness trainings reduce their potential to help distressed cancer patients who are sick or undergoing active treatment because these programs require patients to attend over 30 hours of in-person sessions together with 45 minutes of home practice daily. Caregivers, often overburdened with caregiving duties, may lack the time or energy to attend these in-person classes.28,29 In addition to physical challenges, logistical barriers such as distance, lack of transportation, and scheduling conflicts have been reported as reasons for nonadherence or refusal to participate in behavioral intervention research studies.30-33 In addition, in-person classes are costly for the patients and the health care system and not readily available in rural areas, which are other important barriers against wide dissemination. Therefore, it is necessary to identify effective, low-cost, and convenient interventions that meet patients’ and caregivers’ needs.
Mobile health (mHealth) technology is emerging as an important platform for the delivery of behavioral interventions. mHealth interventions offer an innovative mode of treatment delivery using mobile technologies such as cell phones, handheld tablets, and other wireless devices. Mobile phones are nearly ubiquitous, with 73% of Americans aged 50 to 64 years owning a smartphone.34 The widespread prevalence of smartphones underscores their potential to deliver cost-effective interventions that can be easily integrated into the lives of sick cancer patients or busy caregivers who are unable to attend regular in-person classes. Importantly, many of the app-based mindfulness programs provide shorter but more frequent programs, making it more feasible for users than intense, in-person programs. For instance, the program we used for this study requires a 10- to 20-minute daily practice each day, while MBSR requires 2 to 2.5 hours of in-person instruction per week along with 45 minutes of daily home practice. In addition, mHealth interventions can integrate text messaging and self-monitoring tools that are acceptable and enjoyable to patients.35 Finally, mHealth devices can collect objective and accurate measures of adherence without troubling participants to keep diaries. We previously demonstrated the feasibility of offering an mHealth mindfulness intervention for cancer patients actively undergoing chemotherapy and their primary caregivers.36 Based on the promising results from the pilot study, we conducted a pilot randomized controlled trial of an mHealth mindfulness program using a commercially available mindfulness mobile app within an integrated health care delivery system.
Methods
Setting
The POEM (Practice Of Embracing each Moment) study was conducted within Kaiser Permanente Northern California (KPNC), an integrated health care delivery system that provides comprehensive health care to a large, diverse community-based population of over 4.1 million individuals. KPNC provides coverage for approximately 31% of the northern California population, and its membership is similar demographically, ethnically, and socioeconomically to the area’s overall population. The only exception is with regard to income; KPNC members underrepresent the very poor and the very wealthy.37,38 Participants for the POEM study were recruited from 7 KPNC oncology clinics in the San Francisco Bay Area.
Participants
We included patients with a diagnosis of cancer who were currently receiving or had received chemotherapy, targeted therapies, or immunotherapy in the prior 6 months. The inclusion criteria for both patients and caregivers included age ≥18 years; owning a smartphone, tablet, or computer with internet access; and understanding English. Primary informal caregivers of the patients were also eligible and invited to participate. We excluded persons who regularly meditated or prayed at least 3 times per week, were currently participating in another type of stress reduction program, were severely hearing impaired, or had severe mental illness.
Procedures
This 2-arm randomized controlled pilot trial was conducted between October 2017 and November 2018. Participants completed an online informed consent form, and study protocols and procedures were approved by the KPNC Institutional Review Board. The study was registered on ClinicalTrials.gov (NCT03078608).
Recruitment
Patients were recruited using several strategies, including clinic referrals from oncologists, oncology social workers, and nurses; brochures at each clinic; and invitation emails, followed up with phone calls. Eligible patients were identified using the KPNC electronic health record. Caregivers were recruited by patient participant referral. Patients could participate with or without caregiver participation, and caregivers could also participate without an enrolled patient. Participants who completed the study received a $40 gift card and a year’s subscription to the mindfulness program used for this trial.
Randomization
Participants were allocated to the 2 study groups using simple balanced blocked randomization, stratified by facility. Randomization was implemented by use of allocation assignment concealed in a set of sequentially numbered opaque envelopes filled by research personnel not affiliated with the trial. Caregivers who enrolled with a patient participant were assigned to the same arm as the patient.
Intervention
Participants in the intervention arm received free access to a commercially available mindfulness program, HeadspaceTM (www.headspace.com), for 8 weeks. Headspace is a self-paced program that provides guided mindfulness meditation instructions via a website or mobile application (iOS and Android). A recent review identified Headspace as the most user-friendly commercial mindfulness mobile application.39 Recent studies in various populations have found use of the Headspace app improved mood40,41 and positive affect,40 decreased stress,41,42 improved general well-being,42 and increased mindfulness.43
Research staff emailed participants randomized to the intervention arm device-specific instructions for downloading the Headspace app and information about the program. Research staff checked in with participants over the phone a few days after emailing the instructions and provided support for setting up Headspace over the phone, if needed.
Participants were asked to complete Headspace sessions on a daily basis during the 8-week intervention. They were encouraged to first complete the 30-day Foundation Course, which teaches users the basics of mindfulness meditation, then the cancer pack, which was designed specifically for individuals affected by cancer. They also had the option to choose other 10- to 30-day courses that are more condition- or situation-specific, such as “Anxiety,” “Stress,” “Acceptance,” “Relationships,” or “Sleep,” or single meditation sessions. All Headspace courses teach mindfulness using various basic techniques, including breathing exercises, body scan (to mindfully pay attention to different parts of one’s body—one of the most basic exercises in the MBSR program), noting (being aware of any emotions that may be arising at the moment), and visualization (visualizing images such as sun shining on the entire body). The length of the sessions can be set from 10 to 20 minutes. The length was initially set to 10 minutes for all participants. In addition to the daily, progressive audio instruction, there are short (1-2 minutes) lecture videos every several days designed to increase the understanding of mindfulness and to encourage its integration into daily life. Headspace can be set up to send reminders using push notifications, and study staff made phone calls if an intervention participant completed fewer than 3 Headspace sessions in a week.
Wait-list Control Arm
Participants randomized to the wait-list control arm received usual care and were asked not to start any stress reduction programs during the study period. On completion of the 8-week survey, the control participants were provided with a year’s subscription to Headspace and instructions on downloading the app.
Outcome Measures
Retention
The retention rate was measured as the proportion of enrolled participants who completed the 8-week survey (the primary outcome time point).
Adherence
The Headspace program automatically collects adherence data, including date, time, length, and name of each session to which participants listened, identified by a study ID. Headspace transferred these adherence data to the researchers monthly during the study. Using these data, we calculated the proportion of days during the 8-week intervention period that each participant used the program for any amount of time.
Participant-Reported Measures
We collected outcome data using self-administered online surveys using DatStat software (DatStat Ilume, version 6.1.18.19, Seattle, WA) at the time of consent (baseline survey) and immediately post-intervention (8-week survey) using validated questionnaires as follows. Score ranges for each item are shown in Table 2.
Table 2.
Baseline and postintervention outcome measures of patients.
| Possible Score Range | Intervention Arm (n =
40) |
Wait-List Control Arm (n =
32) |
Intervention Effect |
Effect Size, Cohen’s d | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline, Mean (SD) | Post-Intervention, Mean (SD) | Baseline, Mean (SD) | Post-Intervention, Mean (SD) | F | P | |||
| Distress Thermometer | 0-10 | 4.9 (2.3) | 4.2 (2.6) | 4.9 (1.7) | 4.4 (2.0) | 0.18 | .67 | −0.11 |
| Hospital Anxiety and Depression Scale | ||||||||
| Depression | 0-21 | 5.7 (3.5) | 4.6 (3.6)* | 4.9 (3.2) | 4.9 (3.1) | 2.58 | .11 | −0.401 |
| Anxiety | 0-21 | 8.7 (4.3) | 7.2 (3.9)* | 8.0 (3.1) | 6.6 (3.3)* | 0 | 1.00 | 0.01 |
| PROMIS Pain scales | ||||||||
| Pain Intensity | 0-10 | 3.2 (2.4) | 2.4 (2.1)* | 2.4 (2.2) | 2.5 (2.4) | 3.24 | .08 | −0.427 |
| Pain Interference | 8-40 | 19.2 (7.2) | 16.8 (8.1) | 18.1 (7.3) | 18.3 (7.7) | 1.36 | .25 | −0.364 |
| PROMIS Sleep Disturbance | 8-40 | 22.1 (8.8) | 20.5 (8.3) | 21.0 (7.2) | 21.2 (7.7) | 1.35 | .25 | −0.276 |
| Brief Fatigue Inventory | 0-10 | 4.0 (2.5) | 3.5 (2.4) | 3.2 (2.2) | 3.2 (2.2) | 1.41 | .24 | −0.281 |
| Functional Assessment of Cancer Therapy General Scale (Quality of Life) | ||||||||
| Physical well-being | 0-28 | 18.7 (5.6) | 20.3 (5.9)* | 19.3 (5.4) | 19.6 (5.4) | 1.89 | .17 | 0.339 |
| Social/family well-being | 0-28 | 22.2 (5.2) | 22.5 (5.2) | 21.1 (5.8) | 20.3 (5.5) | 1.88 | .18 | 0.325 |
| Emotional well-being | 0-24 | 14.3 (5.3) | 16.4 (5.2)* | 17.1 (4.0) | 17.2 (4.3) | 5.18 | .03 | 0.542 |
| Functional well-being | 0-28 | 17.2 (5.6) | 18.4 (5.7) | 18.1 (4.9) | 18.2 (5.2) | 1.25 | .27 | 0.265 |
| Overall well-being | 0-108 | 72.4 (18.4) | 77.8 (19.0)* | 75.6 (14.2) | 75.4 (16.0) | 4.88 | .03 | 0.537 |
| Posttraumatic Growth Inventory | ||||||||
| Relating to others | 0-35 | 20.6 (8.5) | 22.3 (8.8) | 22.1 (7.4) | 22.9 (6.6) | 0.54 | .46 | 0.186 |
| New possibilities | 0-25 | 9.7 (6.2) | 12.0 (6.3)* | 10.3 (4.7) | 11.1 (5.5) | 2.14 | .15 | 0.362 |
| Personal strength | 0-20 | 9.5 (5.0) | 10.8 (5.9) | 12.5 (5.0) | 12.2 (5.0) | 2.41 | .13 | 0.393 |
| Spiritual change | 0-10 | 3.7 (3.1) | 4.6 (3.5)* | 4.2 (3.4) | 4.2 (3.0) | 3.30 | .07 | 0.467 |
| Appreciation of life | 0-15 | 9.9 (3.7) | 10.1 (4.0) | 10.3 (2.7) | 10.5 (2.9) | 0.01 | .94 | 0.019 |
| Total | 0-105 | 52.5 (23.2) | 59.5 (26.0)* | 59.4 (19.0) | 61.0 (18.8) | 2.79 | .10 | 0.454 |
| Five Facet Mindfulness Questionnaire | ||||||||
| Observing | 4-20 | 14.3 (3.8) | 15.1 (2.8) | 14.4 (3.6) | 14.6 (3.9) | 0.74 | .39 | 0.226 |
| Describing | 5-25 | 17.5 (4.4) | 18.1 (3.7) | 17.2 (3.2) | 17.2 (3.6) | 0.56 | .46 | 0.182 |
| Acting with awareness | 5-25 | 17.2 (3.8) | 18.5 (3.5)* | 17.4 (3.5) | 17.2 (3.2) | 3.74 | .06 | 0.434 |
| Nonjudging of inner experience | 5-25 | 17.3 (4.9) | 18.4 (4.2)* | 17.5 (4.3) | 17.4 (4.5) | 2.30 | .13 | 0.373 |
| Nonreactivity | 5-25 | 14.9 (3.7) | 16.6 (3.3)* | 16.1 (3.2) | 16.6 (3.3) | 2.94 | .09 | 0.451 |
Within group difference P ≤ .05; and bolded values indicate statistically significant differences in change between baseline and post-intervention between intervention and control groups.
Distress
The NCCN Distress Thermometer was used to assess current distress level.44,45 Respondents were asked to rate their level of distress during the past week by choosing a number, with 0 indicating no distress and 10 extreme distress.
Anxiety and depression
We used the 14-item Hospital Anxiety and Depression Scale (HADS) to assess anxiety and depression.46 Higher scores indicate greater anxiety and depression.
Pain
The PROMIS Pain Intensity numeric rating scale asks participants to rate average pain level in the past 7 days on a scale of 0 (“no pain”) to 10 (“worst imaginable pain”). The 8-item PROMIS Pain Interference scale assesses extent to which pain interfered with functional activities during the past 7 days.47 Higher scores indicate more interference due to pain.
Sleep quality
The 8-item PROMIS Sleep Disturbance scale assesses sleep disturbance during the past 7 days.48 A higher summary score indicates worse sleep disturbance.
Quality of life
We used the 27-item Functional Assessment of Cancer Therapy General Scale (FACT-G) to assess QoL in patients. FACT-G asks respondents to rate their QoL in 4 domains (physical, social/family, emotional, and functional well-being).49,50 Caregiver QoL was determined using the Caregiver Quality of Life Index–Cancer (CQOLC) scale.51 The CQOLC gauges the daily and overall impact caregiving has on respondents’ QoL. Higher scores indicate better QoL on both scales.
Fatigue
The 9-item Brief Fatigue Inventory assesses the severity and impact of fatigue on various aspects of life in the past 24 hours.52 Higher scores indicate greater fatigue.
Posttraumatic growth
The 21-item Posttraumatic Growth Inventory (PTGI) assesses 5 factors of posttraumatic growth, positive change experienced because of a traumatic event or crisis—relating to others, new possibilities, personal strength, spiritual change, and appreciation of life. Respondents are asked to rate to what extent they have seen the listed changes as a result as a crisis in their lives. We modified the wording to ask about changes as a result of their (patients) or their loved one’s (caregivers) cancer diagnosis.53 Higher scores indicate greater post-traumatic growth.
Mindfulness
The 24-item Five Facet Mindfulness Questionnaire–Short Form (FFMQ-SF) measures 5 factors representing elements of mindfulness: observing, describing, acting with awareness, nonjudging of inner experience, and nonreactivity to inner experience. Higher scores indicate greater mindfulness.54
Post-Intervention Interview
We conducted phone interviews with participants after they completed the 8-week intervention period to obtain qualitative feedback regarding the study. Question topics included benefits of using Headspace, things they liked about Headspace or thought could be improved, suggestions for improving the experience of study participants, and experience being in the wait-list control arm.
Data Analyses
Data from patient participants and caregiver participants were analyzed separately. We described baseline distributions between arms. Retention rates were calculated by dividing the number of participants who completed the 8-week surveys by the number of randomized participants. We used the program usage data collected by Headspace to calculate intervention adherence rates—the percent of days within the 8-week intervention period that participants engaged in at least one Headspace session of any length.
To obtain preliminary efficacy results, we performed repeated measures analysis of variance tests comparing change in outcome measures between baseline and 8-week follow-up survey between intervention and control arms using all participants who completed both surveys. Cohen’s d effect size was calculated by taking the difference between group mean change scores and dividing by the pooled standard deviation. All analyses were intent-to-treat. As a pilot study, this randomized clinical trial was not adequately powered to detect statistically significant differences between groups. As such, standardized effect sizes were calculated to demonstrate effects and trends. Effect sizes of approximately 0.2, 0.5, and 0.8 are generally considered small, medium, and large effects, respectively.55
As a supplementary analysis, we conducted dose-response per-protocol analyses, repeating the analysis but stratifying intervention participants by level of adherence to the mindfulness intervention protocol (percent of days during intervention period used Headspace: <50% and ≥50%) and comparing with the control participants.
Interviews were transcribed and uploaded into Nvivo 12 software. Inductive thematic analysis was employed to identify and develop codes on themes related to mindfulness benefits, the interface experience, the cancer patients’/caregivers, perspective of mindfulness. One primary coder (MM) initially coded each interview and met with a secondary coder (AA) to discuss the coding, identify disagreements, and ensure accuracy of codes. This article focuses on the feasibility component of the qualitative interviews.
Results
Participant Characteristics
Table 1 describes the baseline demographic and clinical characteristics of all individuals who completed the study. Patients in the intervention arm were more likely to be men, have postgraduate education, and have higher income compared with controls. Most patients were diagnosed with breast or a hematologic cancer, such as non-Hodgkin’s lymphoma. About 54% were receiving or had received supportive chemotherapy, and 50% were diagnosed within a year of their enrollment in the current study.
Table 1.
Baseline demographic and clinical characteristics of participants.
| Patients |
Caregivers |
|||
|---|---|---|---|---|
| Intervention Arm (n = 54) | Control Arm (n = 43) | Intervention Arm (n = 17) | Control Arm (n = 14) | |
| Age, mean (SD) | 59.3 (14.1) | 56.7 (14.7) | 57.1 (17.4) | 58.2 (18.6) |
| Female, n (%) | 33 (62.3) | 33 (76.7) | 9 (52.9) | 9 (64.3) |
| Marital status, n (%) | ||||
| Married | 38 (71.7) | 23 (57.5) | 14 (82.4) | 7 (53.9) |
| Living as married/domestic partner | 1 (1.9) | 2 (5.0) | 1 (5.9) | 3 (23.1) |
| Widowed | 3 (5.7) | 1 (2.5) | 0 (0) | 0 (0) |
| Separated/divorced | 6 (11.3) | 9 (22.5) | 0 (0) | 1 (7.7) |
| Never married | 5 (9.4) | 5 (12.5) | 2 (11.8) | 2 (15.4) |
| Race, n (%) | ||||
| White | 36 (66.7) | 27 (62.8) | 15 (88.2) | 9 (64.3) |
| African American | 5 (9.3) | 1 (2.3) | 0 (0) | 1 (7.1) |
| Asian | 4 (7.4) | 3 (7.0) | 0 (0) | 2 (14.3) |
| Other | 8 (14.8) | 9 (20.9) | 2 (11.8) | 2 (14.3) |
| Unknown/not reported | 1 (1.9) | 3 (7.0) | 0 (0) | 0 (0) |
| Education, n (%) | ||||
| Some college or less | 18 (34.0) | 19 (44.2) | 6 (35.3) | 5 (35.7) |
| College graduate | 15 (28.3) | 18 (41.9) | 6 (35.3) | 4 (28.6) |
| Postgraduate degree | 20 (37.7) | 6 (14.0) | 5 (29.4) | 5 (35.7) |
| Income, n (%) | ||||
| Less than $75 000 | 11 (22.9) | 21 (53.9) | 2 (13.3) | 3 (30.0) |
| $75 000 to $99 999 | 7 (14.6) | 5 (12.8) | 3 (20.0) | 3 (30.0) |
| $100 000 to $149 999 | 18 (37.5) | 8 (20.5) | 5 (33.3) | 1 (10.0) |
| $150 000 or more | 12 (25.0) | 5 (12.8) | 5 (33.3) | 3 (30.0) |
| Primary delivery of the mindfulness program, n (%) | ||||
| iOS (iPad or iPhone) | 13 (39.4) | 8 (61.5) | ||
| Android | 4 (12.1) | 1 (7.7) | ||
| Computer with internet | 16 (48.5) | 4 (30.8) | ||
| Cancer type, n (%) | ||||
| Breast | 13 (25.5) | 15 (38.5) | ||
| Hematologic | 13 (25.5) | 8 (20.5) | ||
| Gastrointestinal | 10 (19.6) | 2 (5.1) | ||
| Genitourinary | 5 (9.8) | 7 (17.9) | ||
| Head and neck | 4 (7.8) | 1 (2.6) | ||
| Skin | 3 (5.9) | 1 (2.6) | ||
| Lung | 0 (0.0) | 3 (7.7) | ||
| Other | 2 (3.9) | 1 (2.6) | ||
| Sarcoma | 1 (2.0) | 1 (2.6) | ||
| Time since diagnosis, n (%) | ||||
| <1 year | 24 (47.1) | 21 (53.8) | ||
| 1+ year | 27 (52.9) | 18 (46.2) | ||
| Intent of chemotherapy, n (%) | ||||
| Curative | 24 (44.4) | 21 (48.8) | ||
| Palliative | 30 (55.6) | 22 (51.2) | ||
| Caregiver relationship to the patient | ||||
| Spouse/partner | 8 (47.1) | 11 (78.6) | ||
| Child | 4 (23.5) | 1 (7.1) | ||
| Other relative | 2 (11.8) | 0 (0.0) | ||
| Friend | 3 (17.6) | 2 (14.3) | ||
Participant Enrollment and Flow
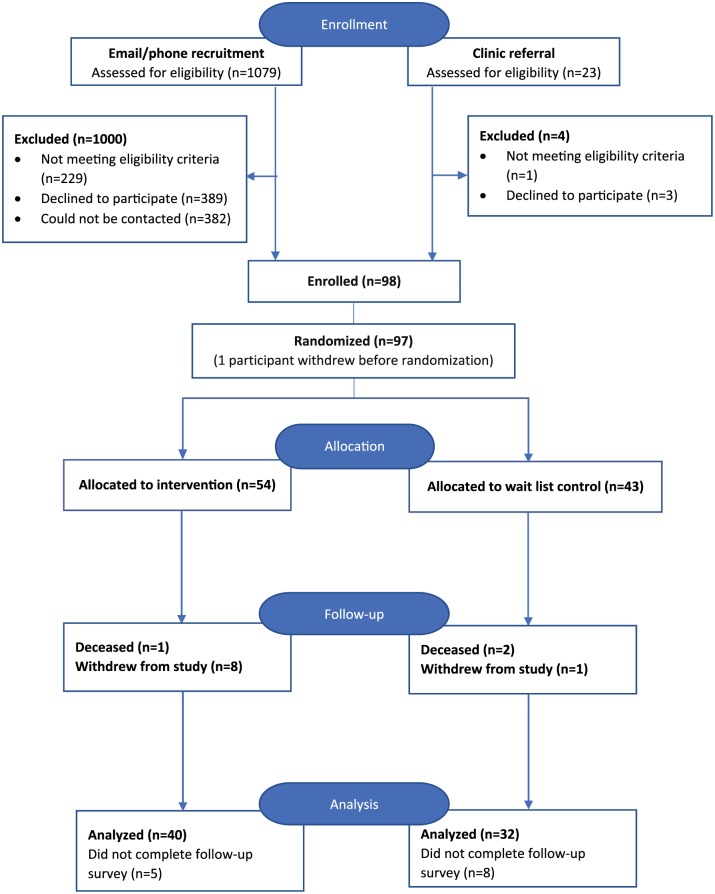
Figure 1 shows the overall flow of participants through the study. The most common reasons for ineligibility included already having a meditation practice, not having cancer or not having received chemotherapy in the past 6 months, not speaking English, and being uncomfortable using technology. The most common reasons given for declining to join the study were being too busy and lack of interest. Only one potential participant declined participation in the study because of the possibility that she would be randomized to the wait-list control arm.
Figure 1.
Overall flow of patient participants through the study.
Thirty-five (36.1%) participants identified a potential caregiver to participate in the study with them. In addition, 3 caregivers joined the study without a patient after we attempted to recruit the patient by phone. Another 3 caregivers self-referred after seeing a study brochure. Ten of the caregivers approached declined participation in the study; the most common reason was lack of interest in participating.
Retention Rate
A total of 97 patients and 31 caregivers enrolled in the study; 72 patients (74%; intervention n = 40, control n = 32) and 26 caregivers (84%; intervention n = 13, control n = 13) completed the study. Similar proportions of patients discontinued study participation in the control and intervention arms (25.9% and 25.6%, respectively). Reasons provided for discontinuation included the following: too sick from the side effects of chemotherapy; never established a meditation practice; illness progressed; caregiving responsibility increased; holiday schedules interfered; and death. No participants reported discontinuing study participation because they were randomized to the wait-list control group.
Adherence
Among the participants in the intervention arm who completed the study, 20 (50%) patients and 8 (61.5%) caregivers practiced the mindfulness program at least 50% of the days during the 8-week study period, and 13 (32.5%) patients and 5 (38.5%) caregivers practiced at least 70% of the days. Nine (22.5%) patients and 1 (7.7%) caregiver did not initiate the Headspace program. Of the 31 patients who completed at least 1 Headspace session, 20 (64.5%) and 13 (41.9%) practiced the mindfulness program at least 50% and at least 70% of the days during the 8-week study period, respectively. Of the 12 caregivers who actively used Headspace, 8 (66.7%) completed at least 50% and 5 (41.7%) completed at least 70% of the days. In addition, approximately 70% of patients and all caregivers continued to use the program after the completion of the study.
Participant-Reported Outcomes
Patient baseline and post-intervention scores for the outcome measures by study arm are shown in Table 2. Compared with controls, patients in the intervention arm had statistically significantly greater improvements between baseline and post-intervention in the FACT-G emotional well-being (P = .03) and overall well-being scores (P = .03). Although the results were of borderline significance, patients in the intervention arm experienced greater improvements on the PROMIS pain base scale (P = .08), PTGI spiritual change (P = .07), and FFMQ acting with awareness (P = .06) and nonreactivity scores (P = .09) compared with controls. No statistically significant differences in change in anxiety, depression, sleep, and fatigue were observed between study arms.
Baseline and post-intervention outcome measure scores for caregivers in each arm are shown in Table 3. The only significant difference in change between baseline and post-intervention between the treatment arms was in the FFMQ observing mindfulness domain score (P = .03). Caregiver participants in the intervention arm also had greater improvements compared with controls in the PTGI new possibilities (P = .06), personal strength (P = .06), and overall scores (P = .05), although these differences were of borderline significance.
Table 3.
Baseline and postintervention outcome measures of caregivers.
| Possible Score Range | Intervention Arm (n =
13) |
Wait-List Control Arm (n =
13) |
Intervention Effect |
Effect Size, Cohen’s d | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline, Mean (SD) | Post-Intervention, Mean (SD) | Baseline, Mean (SD) | Post-Intervention, Mean (SD) | F | P | |||
| Distress Thermometer | 0-10 | 4.7 (2.1) | 4.0 (2.1) | 5.0 (1.9) | 5.1 (2.7) | 0.53 | .47 | −0.259 |
| Hospital Anxiety and Depression Scale | ||||||||
| Depression | 0-21 | 5.1 (3.4) | 4.2 (2.6) | 5.6 (2.9) | 5.7 (3.3) | 1.17 | .29 | −0.421 |
| Anxiety | 0-21 | 8.1 (2.9) | 8.2 (3.3) | 8.7 (3.0) | 8.5 (3.6) | 0.07 | .79 | −0.265 |
| PROMIS Pain scales | ||||||||
| Pain Intensity | 0-10 | 1.5 (1.5) | 1.9 (1.5) | 2.5 (1.9) | 2.3 (1.9) | 0.92 | .35 | 0.402 |
| Pain Interference | 8-40 | 12.9 (5.5) | 14.4 (7.7) | 12.9 (5.3) | 12.4 (3.8) | 0.8 | .39 | 0.489 |
| PROMIS Sleep Disturbance | 8-40 | 20.0 (5.9) | 19.2 (7.6) | 20.5 (5.2) | 20.8 (6.3) | 0.17 | .69 | −0.16 |
| Brief Fatigue Inventory | 0-10 | 3.2 (2.1) | 2.4 (1.8)* | 2.6 (1.9) | 2.6 (1.9) | 1.54 | .23 | −0.458 |
| Caregiver Quality of Life Index–Cancer | 0-140 | 92.8 (10.0) | 93.7 (19.4) | 91.5 (13.9) | 94.6 (14.6) | 0.33 | .59 | 1.139 |
| Posttraumatic Growth Inventory | ||||||||
| Relating to others | 0-35 | 14.3 (6.3) | 14.5 (8.0) | 18.0 (7.4) | 15.0 (7.1) | 1.84 | .19 | 0.527 |
| New possibilities | 0-25 | 5.0 (3.2) | 5.5 (3.8) | 7.5 (5.2) | 4.8 (4.5) | 3.93 | .06 | 0.73 |
| Personal strength | 0-20 | 5.9 (4.9) | 7.0 (4.8) | 8.9 (3.8) | 6.7 (4.9) | 3.9 | .06 | 0.741 |
| Spiritual change | 0-10 | 2.5 (2.7) | 2.7 (2.7) | 3.6 (3.3) | 2.8 (3.0) | 0.52 | .48 | 0.238 |
| Appreciation of life | 0-15 | 6.9 (3.3) | 7.3 (3.8) | 8.5 (3.6) | 7.2 (4.5) | 1.28 | .27 | 0.426 |
| Total | 0-105 | 33.6 (15.8) | 38.3 (20.2) | 45.2 (20.1) | 37.8 (20.5) | 4.29 | .05 | 0.864 |
| Five Facet Mindfulness Questionnaire | ||||||||
| Observing | 4-20 | 13.6 (1.8) | 13.6 (2.0) | 14.3 (3.0) | 12.6 (4.0)* | 5.53 | .03 | 0.922 |
| Describing | 5-25 | 18.5 (3.1) | 18.6 (2.7) | 17.7 (3.5) | 17.8 (3.7) | 0 | .95 | −0.031 |
| Acting with awareness | 5-25 | 16.0 (3.3) | 16.4 (3.3) | 16.5 (3.5) | 16.1 (4.6) | 0.59 | .45 | 0.302 |
| Nonjudging of inner experience | 5-25 | 18.1 (3.1) | 18.6 (3.5) | 17.1 (4.2) | 19.2 (5.1)* | 1.17 | .29 | −0.47 |
| Nonreactivity | 5-25 | 14.9 (2.7) | 15.2 (1.6) | 15.4 (2.8) | 15.6 (2.6) | 0.02 | .89 | −0.211 |
Within group difference P ≤ .05; and bolded values indicate statistically significant differences in change between baseline and post-intervention between intervention and control groups.
Exploratory Dose-Response Analyses
We explored whether the effect sizes differed by differences in intervention adherence rates. Compared with controls, participants in the intervention group who had practiced mindfulness at least 50% of the days in the intervention period showed greater improvements in FACT-G emotional well-being (P = .01; Supplemental Figure 2, available online), PROMIS pain interference (P = .02; Supplemental Figure 3), HADS depression (P = .04; Supplemental Figure 4), PTGI spiritual change (P = .05, data not shown), FFMQ nonjudging of inner experience (P = .03, data not shown), and FACT-G overall well-being (P = .03, data not shown) scores compared with those who practiced mindfulness less frequently (<50%).
Post-Intervention Interview
During the interviews, participants commented on several factors related to study feasibility. Of the 82 participants who participated in the interviews, 74 reported that they had not engaged in any stress reduction practices other than the intervention during the study period. None of the wait-list control participant had engaged in stress reduction practices during the waiting period. Participants raised several suggestions for improving the study, including providing an in-person orientation to Headspace and assistance downloading the app at the chemotherapy clinic; sending daily meditation reminders; and providing assistance in creating a meditation schedule. Though most participants appreciated the personalized practice and flexibility of the Headspace app, some participants also noted wanting a more social experience. Eleven of 57 participants (19.3%) said that they would be interested in participating in a webinar-based mindfulness program for this reason. Based on this input, we have added a mindfulness webinar option in the next phase of our study, which targets advanced cancer patients. The results from this study will provide further information regarding the acceptability of and adherence to an online, teacher-led mindfulness classes.
Many individuals noted that the reminder calls from study staff were helpful in encouraging them to pursue their meditation practice. Nearly all intervention participants found the Headspace app to be useful (76 of 82 respondents) and would recommend the program to other cancer patients and caregivers (75 of 82 respondents). When asked about their experience waiting 8 weeks for the mindfulness program, wait-list control participants did not express that this wait was distressing or a barrier to participating although about half would have preferred to start the program earlier.
Discussion
To our knowledge, this is one of the first studies investigating the feasibility of conducting a randomized trial using a commercially available online mindfulness program to improve QoL of cancer patients who recently underwent chemotherapy and their primary caregivers. Our findings demonstrate that it is feasible to conduct a randomized trial using a mobile-based, self-paced mindfulness program in this population. Although not designed as an efficacy trial, the preliminary results suggested improvement in QoL among patients and mindfulness among caregivers. Participants who practiced mindfulness more frequently reported greater improvements in QoL, pain interference, and depression compared with intervention participants who practiced less frequently. In post-intervention interviews, participants stated that they appreciated the convenience of using a self-paced program that could be easily incorporated into their daily lives that were dominated by medical appointments and the stress associated with cancer treatment. Our protocol and results provide a practical strategy for conducting a larger, fully powered comparative effectiveness trial.
Our study extends existing knowledge in the area of mindfulness interventions for cancer patients in several ways. First, we used an mHealth intervention and targeted cancer patients who are actively undergoing or recently underwent chemotherapy treatment. Because the majority of previous mindfulness intervention studies targeting cancer patients included only early-stage breast cancer patients who had completed treatment, accessibility of in-person mindfulness classes was less of a concern. However, the few previous in-person mindfulness studies that included patients with advanced cancers or those undergoing active treatment reported that recruitment and retention were challenging due to patients’ difficulties in committing to an 8-week, in-person schedule30,56 and concluded that there is a need to tailor interventions to make them less intensive and more accessible for sick cancer patients.30 Our studies, including our previous pilot trials using CD- and MP3-based mindfulness programs,57 provided preliminary evidence that self-paced, technology-delivered mindfulness training could fill this gap because of the greater accessibility and lower intensity. Even though the traditional in-person programs such as MBSR or MBCR may be considered the “gold standard,” if participants cannot adhere to the program, it is unlikely the intervention will result in beneficial outcomes for patients. Our study participants greatly appreciated the ease of using the mobile application, especially during blocks of time when they might otherwise be unoccupied and anxious, such as time spent in waiting rooms to see their oncologists and while receiving chemotherapy infusions.
Second, our study differs from previous studies because we also included informal caregivers of the cancer patients.58-61 Even though it is known that caregivers of patients with cancer sometimes report greater distress than the patients themselves,62 overburdened caregivers seldom seek mental health care to promote emotional well-being, and clinical support is seldom offered.62-64 Higher distress may lead to poorer health among caregivers, reducing their ability to effectively provide care for the patients.65,66 Given the significant reciprocal emotional relationship between caregivers and patients, it is imperative to offer caregivers psychosocial and educational support to improve their own QoL and physical and mental health.67
Third, our sample population included a diverse group of patients: we successfully recruited men, non-whites, and patients with different types of cancer; 30% of patients were male, 31% were non-white, and over 60% of patients had cancers other than breast cancer. This may be at least partially because of KPNC’s diverse membership, but it could also be due to the greater accessibility of an mHealth mindfulness intervention. Ongoing treatment and concerns about the disease course itself cause substantial distress for both patients of advanced cancer and their loved ones, and these populations have been comparatively understudied.1-3,68 Because previous in-person mindfulness studies primarily included white females with early-stage breast cancer, little is known regarding the efficacy of mindfulness interventions for males, non-whites, or those with cancers other than breast or advanced stage diseases. A recent study reported that many Americans suffering from depression, stress, or anxiety prefer internet-based mindfulness training compared with in-person sessions.31,32 Our mHealth delivery of the mindfulness intervention may have helped recruit some of these understudied populations because of greater accessibility and the private nature of the program. Mobile applications are also more interactive and easier to use than CDs or MP3s, which previous self-paced mindfulness studies used.57
Last, although this study was not powered to examine dose-effect or mediation, our data suggested that those who adhered to the mindfulness intervention experienced greater benefits than those who had lower adherence. Future studies may aim to increase adherence to achieve greater efficacy of the intervention and to examine this potential dose-effect relationship in more detail. Regarding the role of mindfulness, we only observed significant improvement in mindfulness (observing) in caregivers. In the patients, the associations were of borderline significance, though still suggesting that improvement in mindfulness may mediate the association between the intervention and the outcomes. It is possible that we did not observe significant change in mindfulness measures because patients’ baseline mindfulness was high. We conducted a subanalysis using only patients with low mindfulness (below median) and saw significant improvements in the FFMQ describing (P = .022) and nonreactivity (P = .018) scores. A larger study to formally test the mechanism is warranted.
Several limitations should be considered. First, because this study was conducted within a large integrated health care delivery system where everyone had access to health care, the rate of access to a mobile device or the internet may be higher than in the general population. However, mobile devices and the internet are increasingly used by all segments of the population, including those in lower socioeconomic status and minority populations.69 Second, we used a usual-care control group instead of a time- and attention-matched or active control group. Active control utilizes an intervention that mimics the theoretically inactive elements (eg, placebo) but not the active elements of the intervention of interest.70 Although placebo controls are perfectly appropriate for pharmaceutical trials, many active-control group options are not appropriate for mind-body research because there are rarely obvious placebo or “sham” interventions that mimic mindfulness.71 Even when a satisfactory placebo exists, employing usual-care controls would be of great value for pragmatic trials evaluating treatments to improve clinical care.72 A final limitation is that rather than having 2 researchers independently code the post-intervention interview transcripts, we had one person primarily coding, with another investigator verifying the assigned codes, which may increase the risk of bias in the results.
Conclusions
Despite these limitations, our study provides encouraging preliminary evidence regarding the feasibility of conducting a randomized clinical trial using a commercially available self-paced mindfulness program for cancer patients undergoing or who have recently completed chemotherapy. Unlike in-person mindfulness programs, mHealth programs do not require health care delivery systems to hire teachers or secure physical locations for classes. Such benefits make mHealth mindfulness programs widely scalable and cost-effective. This easy access to support for psychosocial distress benefits patients with cancer, caregivers, and providers, especially those in remote locations. Fully powered trials are needed to establish the efficacy of the intervention.
Supplemental Material
Supplemental material, Supplemental_Figures_2-4_ for A Randomized Controlled Trial of mHealth Mindfulness Intervention for Cancer Patients and Informal Cancer Caregivers: A Feasibility Study Within an Integrated Health Care Delivery System by Ai Kubo, Elaine Kurtovich, MegAnn McGinnis, Sara Aghaee, Andrea Altschuler, Charles Quesenberry, Tatjana Kolevska and Andrew L. Avins in Integrative Cancer Therapies
Acknowledgments
We thank participants of the POEM (Practice of Embracing Each Moment) study, clinicians at Kaiser Permanente Medical Centers who helped us recruit patients, and Amy J. Markowitz, JD, University of California, San Francisco Clinical and Translational Research Career Development Program, for editorial help with the manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the American Cancer Society (128952-PEP-16-056-01-PCSM).
Supplemental Material: Supplemental material for this article is available online.
ORCID iD: Ai Kubo  https://orcid.org/0000-0002-1035-828X
https://orcid.org/0000-0002-1035-828X
References
- 1. Artherholt SB, Fann JR. Psychosocial care in cancer. Curr Psychiatry Rep. 2012;14:23-29. [DOI] [PubMed] [Google Scholar]
- 2. Bower JE. Cancer-related fatigue—mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fann JR, Ell K, Sharpe M. Integrating psychosocial care into cancer services. J Clin Oncol. 2012;30:1178-1186. [DOI] [PubMed] [Google Scholar]
- 4. National Comprehensive Cancer Network. Distress management clinical practice guidelines in oncology version 1. https://www.nccn.org/professionals/physician_gls/default.aspx. Published 2009. Accessed 2014.
- 5. Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120:2946-2954. [DOI] [PubMed] [Google Scholar]
- 6. Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29:413-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19-28. [DOI] [PubMed] [Google Scholar]
- 8. Greenlee H, Kwan ML, Ergas IJ, et al. Complementary and alternative therapy use before and after breast cancer diagnosis: the Pathways Study. Breast Cancer Res Treat. 2009;117:653-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shumay DM, Maskarinec G, Gotay CC, Heiby EM, Kakai H. Determinants of the degree of complementary and alternative medicine use among patients with cancer. J Altern Complement Med. 2002;8:661-671. [DOI] [PubMed] [Google Scholar]
- 10. Yates JS, Mustian KM, Morrow GR, et al. Prevalence of complementary and alternative medicine use in cancer patients during treatment. Support Care Cancer. 2005;13:806-811. [DOI] [PubMed] [Google Scholar]
- 11. Huebner J, Prott FJ, Micke O, et al. ; PRIO (Working Group Prevention and Integrative Oncology—German Cancer Society). Online survey of cancer patients on complementary and alternative medicine. Oncol Res Treat. 2014;37:304-308. [DOI] [PubMed] [Google Scholar]
- 12. Huebner J, Muenstedt K, Prott FJ, et al. Online survey of patients with breast cancer on complementary and alternative medicine. Breast Care (Basel). 2014;9:60-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York, NY: Delacorte; 1990. [Google Scholar]
- 14. Creswell JD. Mindfulness interventions. Annu Rev Psychol. 2017;68:491-516. [DOI] [PubMed] [Google Scholar]
- 15. Pagnini F, Philips D. Being mindful about mindfulness. Lancet Psychiatry. 2015;2:288-289. [DOI] [PubMed] [Google Scholar]
- 16. Vieten C, Astin J. Effects of a mindfulness-based intervention during pregnancy on prenatal stress and mood: results of a pilot study. Arch Womens Ment Health. 2008;11:67-74. [DOI] [PubMed] [Google Scholar]
- 17. Guardino CM, Schetter CD, Bower JE, Lu MC, Smalley SL. Randomised controlled pilot trial of mindfulness training for stress reduction during pregnancy. Psychol Health. 2014;29:334-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dimidjian S, Goodman SH, Felder JN, Gallop R, Brown AP, Beck A. Staying well during pregnancy and the postpartum: a pilot randomized trial of mindfulness-based cognitive therapy for the prevention of depressive relapse/recurrence. J Consult Clin Psychol. 2016;84:134-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor BL, Cavanagh K, Strauss C. The effectiveness of mindfulness-based interventions in the perinatal period: a systematic review and meta-analysis. PLoS One. 2016;11:e0155720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khoury B, Sharma M, Rush SE, Fournier C. Mindfulness-based stress reduction for healthy individuals: a meta-analysis. J Psychosom Res. 2015;78:519-528. [DOI] [PubMed] [Google Scholar]
- 21. Carmody J, Crawford S, Churchill L. A pilot study of mindfulness-based stress reduction for hot flashes. Menopause. 2006;13:760-769. [DOI] [PubMed] [Google Scholar]
- 22. Carmody JF, Crawford S, Salmoirago-Blotcher E, Leung K, Churchill L, Olendzki N. Mindfulness training for coping with hot flashes: results of a randomized trial. Menopause. 2011;18:611-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCabe Ruff K, Mackenzie ER. The role of mindfulness in healthcare reform: a policy paper. Explore (NY). 2009;5:313-323. [DOI] [PubMed] [Google Scholar]
- 24. Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. JAMA. 2008;300:1350-1352. [DOI] [PubMed] [Google Scholar]
- 25. Zhang J, Xu R, Wang B, Wang J. Effects of mindfulness-based therapy for patients with breast cancer: a systematic review and meta-analysis. Complement Ther Med. 2016;26:1-10. [DOI] [PubMed] [Google Scholar]
- 26. Zainal NZ, Booth S, Huppert FA. The efficacy of mindfulness-based stress reduction on mental health of breast cancer patients: a meta-analysis. Psychooncology. 2013;22:1457-1465. [DOI] [PubMed] [Google Scholar]
- 27. Carlson LE, Doll R, Stephen J, et al. Randomized controlled trial of mindfulness-based cancer recovery versus supportive expressive group therapy for distressed survivors of breast cancer. J Clin Oncol. 2013;31:3119-3126. [DOI] [PubMed] [Google Scholar]
- 28. Shrank WH, Liberman JN, Fischer MA, et al. Are caregivers adherent to their own medications? J Am Pharm Assoc (2003). 2011;51:492-498. [DOI] [PubMed] [Google Scholar]
- 29. Lee S, Colditz GA, Berkman LF, Kawachi I. Caregiving and risk of coronary heart disease in U.S. women: a prospective study. Am J Prev Med. 2003;24:113-119. [DOI] [PubMed] [Google Scholar]
- 30. Eyles C, Leydon GM, Hoffman CJ, et al. Mindfulness for the self-management of fatigue, anxiety, and depression in women with metastatic breast cancer: a mixed methods feasibility study. Integr Cancer Ther. 2015;14:42-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wahbeh H, Lane JB, Goodrich E, Miller M, Oken BS. One-on-one mindfulness meditation trainings in a research setting. Mindfulness (N Y). 2014;5:88-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wahbeh H, Oken BS. Internet mindfulness meditation intervention for the general public: pilot randomized controlled trial. JMIR Hental Health. 2016;3:e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schapira MM, Mackenzie ER, Lam R, et al. Breast cancer survivors willingness to participate in an acupuncture clinical trial: a qualitative study. Support Care Cancer. 2014;22:1207-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pew Research Center. Mobile fact sheet. http://www.pewinternet.org/fact-sheet/mobile/. Accessed November 12, 2018.
- 35. Gotsis M, Wang H, Spruijt-Metz D, Jordan-Marsh M, Valente TW. Wellness partners: design and evaluation of a web-based physical activity diary with social gaming features for adults. JMIR Res Protoc. 2013;2:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kubo A, Altschuler A, Kurtovich E, et al. A pilot mobile-based mindfulness intervention for cancer patients and their informal caregivers. Mindfulness. 2018;9:1885-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gordon N. A Comparison of Sociodemographic and Health Characteristics of the Kaiser Permanente Northern California Membership Derived from Two Data Sources: The 2008 Member Health Survey and the 2007 California Health Interview Survey. Oakland, CA: Kaiser Permanente Division of Research; 2012. [Google Scholar]
- 38. Gordon NP. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2011 California Health Interview Survey. Oakland, CA: Kaiser Permanente Northern California Division of Research; 2015. https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/chis_non_kp_2011.pdf. Accessed May 3, 2019. [Google Scholar]
- 39. Mani M, Kavanagh DJ, Hides L, Stoyanov SR. Review and evaluation of mindfulness-based iPhone apps. JMIR Mhealth Uhealth. 2015;3:e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Howells A, Ivtzan I, Eiroa-Orosa FJ. Putting the “app” in happiness: a randomised controlled trial of a smartphone-based mindfulness intervention to enhance wellbeing. J Happiness Stud. 2016;17:163-185. [Google Scholar]
- 41. Economides M, Martman J, Bell MJ, Sanderson B. Improvements in stress, affect, and irritability following brief use of a mindfulness-based smartphone app: a randomized controlled trial. Mindfulness (N Y). 2018;9:1584-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang E, Schamber E, Meyer RML, Gold JI. Happier healers: randomized controlled trial of mobile mindfulness for stress management. J Altern Complement Med. 2018;24:505-513. [DOI] [PubMed] [Google Scholar]
- 43. Wylde CM, Mahrer NE, Meyer RM, Gold JI. Mindfulness for novice pediatric nurses: smartphone application versus traditional intervention. J Pediatr Nurs. 2017;36:205-212. [DOI] [PubMed] [Google Scholar]
- 44. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30:1160-1177. [DOI] [PubMed] [Google Scholar]
- 45. Holland JC, Andersen B, Breitbart WS, et al. Distress management. J Natl Compr Canc Netw. 2013;11:190-209. [DOI] [PubMed] [Google Scholar]
- 46. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69-77. [DOI] [PubMed] [Google Scholar]
- 47. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2011;10:6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570-579. [DOI] [PubMed] [Google Scholar]
- 50. Broderick JE, DeWitt EM, Rothrock N, Crane PK, Forrest CB. Advances in patient-reported outcomes: the NIH PROMIS® measures. EGEMS (Wash DC). 2013;1:1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weitzner MA, Jacobsen PB, Wagner H, Friedland J, Cox C. (1999). The Caregiver Quality of Life Index–Cancer (CQOLC) scale: development and validation of an instrument to measure quality of life of the family caregiver of patients with cancer. Quality of Life Research, 1999;8(1-2):55-63. [DOI] [PubMed] [Google Scholar]
- 52. Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186-1196. [DOI] [PubMed] [Google Scholar]
- 53. Tedeschi RG, Calhoun LG. The Posttraumatic Growth Inventory: measuring the positive legacy of trauma. J Trauma Stress. 1996;9:455-471. [DOI] [PubMed] [Google Scholar]
- 54. Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27-45. [DOI] [PubMed] [Google Scholar]
- 55. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 56. Lengacher CA, Kip KE, Barta M, et al. A pilot study evaluating the effect of Mindfulness-Based Stress Reduction on psychological status, physical status, salivary cortisol, and interleukin-6 among advanced-stage cancer patients and their caregivers. J Holist Nurs. 2012;30:170-185. [DOI] [PubMed] [Google Scholar]
- 57. Atreya CE, Kubo A, Borno HT, et al. Being Present: a single-arm feasibility study of audio-based mindfulness meditation for colorectal cancer patients and caregivers. PLoS One. 2018;13:e0199423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Birnie K, Garland SN, Carlson LE. Psychological benefits for cancer patients and their partners participating in mindfulness-based stress reduction (MBSR). Psychooncology. 2010;19:1004-1009. [DOI] [PubMed] [Google Scholar]
- 59. Schellekens MP, van den Hurk DG, Prins JB, et al. Study protocol of a randomized controlled trial comparing mindfulness-based stress reduction with treatment as usual in reducing psychological distress in patients with lung cancer and their partners: the MILON study. BMC Cancer. 2014;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van den Hurk DG, Schellekens MP, Molema J, Speckens AE, van der Drift MA. Mindfulness-Based Stress Reduction for lung cancer patients and their partners: results of a mixed methods pilot study. Palliat Med. 2015;29:652-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schellekens MPJ, van den Hurk DGM, Prins JB, et al. Mindfulness-based stress reduction added to care as usual for lung cancer patients and/or their partners: a multicentre randomized controlled trial. Psychooncology. 2017;26:2118-2126. [DOI] [PubMed] [Google Scholar]
- 62. Braun M, Mikulincer M, Rydall A, Walsh A, Rodin G. Hidden morbidity in cancer: spouse caregivers. J Clin Oncol. 2007;25:4829-4834. [DOI] [PubMed] [Google Scholar]
- 63. Given BA, Given CW, Kozachik S. Family support in advanced cancer. CA Cancer J Clin. 2001;51:213-231. [DOI] [PubMed] [Google Scholar]
- 64. Ringdal GI, Ringdal K, Jordhoy MS, Ahlner-Elmqvist M, Jannert M, Kaasa S. Health-related quality of life (HRQOL) in family members of cancer victims: results from a longitudinal intervention study in Norway and Sweden. Palliat Med. 2004;18:108-120. [DOI] [PubMed] [Google Scholar]
- 65. Schulz R, Sherwood PR. Physical and mental health effects of family caregiving. Am J Nurs. 2008;108(9 suppl):23-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a meta-analysis. Psychol Aging. 2003;18:250-267. [DOI] [PubMed] [Google Scholar]
- 67. Glajchen M. Physical well-being of oncology caregivers: an important quality-of-life domain. Semin Oncol Nurs. 2012;28:226-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med. 2017;51:199-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Statista. Share of adults in the United States who owned a smartphone from 2011 to 2017, by ethnicity. https://www.statista.com/statistics/195001/percentage-of-us-smartphone-owners-by-ethnicity/. Accessed November 5, 2018.
- 70. Popp L, Schneider S. Attention placebo control in randomized controlled trials of psychosocial interventions: theory and practice. Trials. 2015;16:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kinser PA, Robins JL. Control group design: enhancing rigor in research of mind-body therapies for depression. Evid Based Complement Alternat Med. 2013;2013:140467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Avins AL, Cherkin DC, Sherman KJ, Goldberg H, Pressman A. Should we reconsider the routine use of placebo controls in clinical research? Trials. 2012;13:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Figures_2-4_ for A Randomized Controlled Trial of mHealth Mindfulness Intervention for Cancer Patients and Informal Cancer Caregivers: A Feasibility Study Within an Integrated Health Care Delivery System by Ai Kubo, Elaine Kurtovich, MegAnn McGinnis, Sara Aghaee, Andrea Altschuler, Charles Quesenberry, Tatjana Kolevska and Andrew L. Avins in Integrative Cancer Therapies