Abstract
Aim. To evaluate the anti-invasive effect of ethanol extracts of rhizome of Dryopteris crassirhizoma (EEDC) in matrix invasion and formation of functional invadopodia and to determine the anti-tumor effect of EEDC in a mouse model of mandibular invasion by gingival squamous cell carcinoma (SCC). Methods. The rhizome of D crassirhizoma was extracted in ethanol. The anti-invasive effect of EEDC was analyzed with a Matrigel-coated transwell invasion and 3D culture system. Crucial factors related to the control of cancer cell invasion by EEDC were determined using a human protease array. Molecular evidence supporting the anti-invasive effect of EEDC in oral SCC (OSCC) cells used an invadopodia-mediated extracellular matrix (ECM) degradation; an in vivo athymic mouse model was also provided. Results. EEDC treatment (10 µg/mL) suppressed transwell migration and invasion of HSC-3 OSCC cells without cytotoxicity. Decreased levels of matrix metalloprotease (MMP)-7, kalikrein 10, cathepsin V, MMP-2, and cathepsin D were also found in EEDC-treated HSC-3 cells based on human protease array. The anti-invasive effects of EEDC involved the suppression of invadopodia-mediated ECM degradation via inhibition of globular-actin elongation. The anti-invasive effect resulting from disturbance of functional invadopodia formation by EEDC was observed even at a low concentration of 5 µg/mL. The phosphorylation of cortactin involved in functional invadopodia formation was decreased at EEDC concentrations that inhibited invadopodia formation. The anti-tumor effect of EEDC was also observed in a mouse xenograft model. Administration of EEDC resulted in inhibition of tumor growth and progression. Conclusions. EEDC represents a potential anti-invasive and anti-tumor agent in cancer control.
Keywords: Dryopteris crassirhizoma, invasion, invadopodia, extracellular matrix, actin polymerization, xenograft
Introduction
Intake of anticancer compounds via diet or dietary supplement is one of the best ways to prevent cancer growth and progression. Medicinal herbs have been actively studied in cancer prevention research. Limited or no cytotoxicity of anticancer drugs is desirable along with clinical efficacy. Therefore, products derived from natural sources are sought for prevention and treatment of cancer. Vincristine, vinblastine, irinotecan, etoposide, and paclitaxel are plant-derived natural compounds.1 They are currently used clinically or are undergoing clinical trials as chemotherapy drugs. Tea polyphenols, curcumin, capsaicin, isothiocyanate, resveratrol, lycopene, pomegranate, luteolin, and genistein have also been developed as promising natural compounds for cancer treatment.2,3
Invasion is necessary for cancer progression. Intensive extracellular matrix (ECM) degradation is closely related to cancer invasion and metastasis.4 Focalized matrix degradation facilitates cancer invasion and migration. Cellular protrusions readily observed in invasive cancer mainly consist of actin polymers.5 Invasive cancer cells polymerize globular-actin (G-actin), assemble scaffold components to filamentous-actin (F-actin), and build protrusive structures known as invadopodia. Completion of invadopodia formation leads to focalized degradation of regional matrix, thus facilitating invasion and migration. Therefore, invadopodia is a crucial system for cancer invasion. Targeting invadopodia is an appropriate strategy to develop anticancer drugs.
The objective of this study was to determine the anti-invasive and anti-tumor effects of ethanol extract of the rhizome of Dryopteris crassirhizoma (EEDC). The anti-invasive activity of EEDC was observed in terms of its effect on abnormal invadopodia formation. Its effect on actin polymerization and focalization of protease activity was also determined. EEDC also showed anti-tumor activity in the mouse model of mandibular invasion by gingival squamous cell carcinoma (SCC). This study elucidates the role of D crassirhizoma rhizome and its valuable phytochemicals as potential anti-invasive and anti-tumor agents.
Materials and Methods
Materials and Reagents
Dulbecco’s modified Eagle’s medium (DMEM), Ham’s F-12 nutrient mixture, and fetal bovine serum (FBS) were purchased from Gibco BRL Co (Rockville, MD). Cortactin (CTTN; clone 4F) was purchased from Millipore (Billerica, MA). Anti-CTTN (phospho Y466) and anti-β-actin were purchased from Abcam (Cambridge, MA). Alexa fluor 568 phalloidin was purchased from Molecular Probes (Eugene, OR). Oregon Green 488 gelatin was purchased from Molecular Probes (Carlsbad, CA). EEDC was provided by COSMAX Inc (Seongnam City, Republic of Korea).
Plant Material and Extraction
The rhizomes of D crassirhizoma were collected from Yeongcheon, Gangwon province, Korea, in 2014 by COSMAX R&I Center (COSMAX Inc). Taxonomic identification was done by botanist and herbalist (Ms Seok Kyun Yun, COSMAX R&I Center, COSMAX Inc). A voucher specimen (CH200) was deposited at COSMAX R&I Center. No approval or permission is required to collect plant samples. There is no formal restriction on the collection of plant samples for research because the place where the plant sample was collected is not a private property but a state-owned land. The rhizomes of D crassirhizoma were thoroughly washed with distilled water and dried under shade and ventilation. Dried leaves were ground in an electronic mill and extracted by stirring for 72 hours in 70% ethanol. The extract was then concentrated with a rotary evaporator under reduced pressure and stored in the refrigerator until use.
Cell Lines and Cultures
HSC-3 oral SCC (OSCC) cell line, immortalized gingival fibroblasts (IGFs), and normal gingival fibroblasts (NGFs) were obtained from the Oral Cancer Institute at Yonsei University College of Dentistry, Republic of Korea. Cells were cultured in DMEM/F12 (3:1 ratio) medium supplemented with 10% FBS, 1 × 10−10 M cholera toxin, 0.4 mg/mL hydrocortisone, 5 µg/mL insulin, 5 µg/mL apo-transferrin, and 2 × 10−11 M T3 in a humidified atmosphere of 5% CO2 at 37°C.6 Dimethyl sulfoxide (DMSO) or EEDC was treated in complete media.
MTT Assay
Cells (5 × 103 cells/well) in 96-well culture plates were treated with various concentrations of EEDC for 24 hours. Control cells were treated with 0.05% DMSO alone. After the culture medium was replaced with a fresh medium, 10 µL of MTT solution (5 mg/mL in PBS) was added to each well, and the plate was incubated for an additional 4 hours at 37°C. The medium was aspirated, and the formed formazan crystals were solubilized by adding 200 µL DMSO per well. Absorbance was measured at 570 nm with a microplate reader (Bio-Rad, Hercules, CA).
Transwell Invasion Assay
Polycarbonate nucleopore filter inserts with a pore size of 8 µm Transwell chamber (Corning Costar, Cambridge, MA) were coated with Matrigel (30 µg/well; Becton Dickinson, Lincoln Park, NJ) for 3 hours at 37°C. Then HSC-3 cells (5 × 103 cells/100 µL/well) were loaded into the upper part of the Matrigel-coated filter inserts, and a complete medium with or without EEDC was added to the lower chamber for 48 hours. Invaded cells on the lower surface of the filter were fixed with ethanol, and noninvasive cells were thoroughly removed with a cotton swab. Then, cells were stained with hematoxylin for 10 minutes. Invaded cells from 5 fields were counted under a microscope.
Migration Assay
Cells were seeded into a 6-well culture plate and allowed to grow to 90% confluence. One artificial wound was made by scratching the monolayer with a sterile micropipette tip. The debris and floating cells were removed by PBS washing. The width of the wound edge was photographed, and cells were cultured in complete media with or without EEDC overnight. Scratched areas at the identical location used for initial image were then photographed, and wound areas in each image were measured using ImageJ program (National Institutes of Health, Bethesda, MD).
Three-Dimensional Culture
Dermal equivalent was generated with a type I-A collagen mixture (Nitta Gelatin Inc, Osaka, Japan), 8 volumes of collagen solution, 1 volume of 10× reconstitution solution (0.022 g/mL NaHCO3, 0.0477 g/mL HEPES, 0.05 N NaOH), and 1 volume 10× DMEM/F12 (3:1) with IGF (1.5 × 105 cells). Collagen mixture was loaded onto filter inserts (3 µm pore size, 12 mm in diameter; Millipore) and placed into 6-well cell culture plates (Costar). The collagen mixture was solidified by 24 hours incubation at 37°C; cells (1 × 106 cells) were then loaded onto the collagen mixture. Complete medium in the presence or absence of EEDC was added to the chamber. During the experiment, an air-liquid interface microenvironment was generated by removing excess media from the top of the cell layer. Two weeks later, the dermal equivalent was removed from the filter insert and transferred to formalin solution and histologically examined by hematoxylin and eosin staining.
Western Blotting
Cells were treated with EEDC at different concentrations for 1 hour and then collected. Total protein was prepared with RIPA buffer containing protease inhibitor cocktail tablets. Equal quantities of protein were separated on 12% sodium dodecyl sulfate polyacrylamide gels and then transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). The membrane was blocked with 5% skim milk in PBS and subsequently incubated with primary antibody (1:1000 dilution) in 5% skim milk overnight at 4°C. Then, the membrane was incubated with respective horseradish peroxidase–conjugated secondary antibodies (1:3000 dilution) for 2 hours at room temperature. Targeted proteins were visualized using Enhanced Chemiluminescence Detection kit (Amersham Life Science, Parsippany, NJ).
Protease Array
Cells (3 × 106 cells) on 100-mm culture plates were treated with EEDC for 24 hours; 0.05% DMSO was treated for control. The medium was collected and centrifuged to remove debris and floating cells. Conditioned media with equal quantities of protein were then incubated with Proteome Profiler Human Protease Array (R&D Systems, Minneapolis, MN) for 24 hours at 4°C according to the manufacturer’s protocol. Relative expression levels of proteases were determined by comparing their signal intensities with Quantity One software using Gel Doc 2000 system (Bio-Rad) following the manufacturer’s protocol.
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA) according to the instructions provided by the manufacturer. Single-stranded cDNA was transcribed from total RNA using a RT System (Promega, Madison, WI). PCR was then performed using cDNA as template in a reaction mixture containing 25 mM magnesium chloride (MgCl2), dNTPs, reverse and forward primers, and Taq polymerase (Takara Bio, Shiga, Japan). The following primers were used for PCR: F-MMP7, 5′-TGAGCTACAGTGGGAACAGG-3′ and R-MMP7, 5′-CAAGGTGCTGGCTGAGTAGATC-3′; F-Kalikrein10, 5′-CTCTGGCGAAGCTGCTG-3′ and R-Kalikrein10, 5′-ATAGGCTTCGGGGTCCAA-3′; F-Cathepsin V, 5′-TGGAAGGCAACACACAGAAG-3′ and R-Cathepsin V, 5′-GAAGCCATGTTTCCCTTGG-3′ and F-MMP-2, 5′-TCTCCTGACATTGACCTTGGC-3′ and R-MMP-2, 5′-CAAGGTGCTGGCTGAGTAGATC-3′; F-Ca-thepsin D, 5′-CAACAGCGACAAGTCCAGC-3′ and R-Ca-thepsin D, 5′- CTGAATCAGCGGCACGGC-3′; F-GAPDH, 5′-CCCCCTACTGCCCACTGCCACCAC-3′ and R-GAPDH, 5′-TCCATCCACTATGTCAGCAGGTCC-3′. Ampli-fied PCR products were electrophoresed on 2% agarose gel in 1× Tris-Borate-EDTA buffer containing ethidium bromide. They were visualized using Quantity One software and Gel Doc 2000 system (Bio-Rad).
Invadopodia Formation
Cells were plated onto gelatin-coated chamber slides and cultured for 6 hours. Floating cells were then removed by PBS washing. Adherent cells were fixed in 4% paraformaldehyde, permeabilized with 0.5% Triton X-100/PBS, and stained with anti-CTTN and Alexa fluor 568 phalloidin. Fluorescein isothiocyanate (FITC)-goat anti-mouse IgG (H+L) was used as secondary antibody for anti-CTTN. Cells were visualized and photographed with an LSM 510 META confocal laser-scanning microscope (Carl Zeiss, Jena, Germany). Images were processed using Zeiss LSM image browser software. Invadopodia were identified as regions of CTTN-containing actin spots.
ECM Degradation Assay
FITC-conjugated gelatin-coated coverslips were prepared as described previously.7 Gelatin-coated coverslips were quenched for 1 hour with complete media at 37°C prior to cell plating. Cells were then plated on coverslips and cultured for 16 hours. Cells were fixed, permeabilized, and stained for actin with Alexa fluor 568 phalloidin. Dark areas lacking fluorescence in coverslip and actin-stained cells were observed with a LSM 510 META confocal laser-scanning microscope (Carl Zeiss). To quantify invadopodia-mediated ECM degradation, black and white images of gelatin degradation were analyzed using ImageJ program (NIH).
Actin Polymerization Assay
The actin polymerization assay was performed using Actin Polymerization Kit (Cytoskeleton Inc, Denver, CO) following the manufacturer’s protocol. Briefly, G-buffer with or without pyrene labeled muscle actin (0.4 mg/mL) was placed into a 96-well plate, and baseline fluorescence was read every 60 s for 3 minutes using a fluorimeter. Fluorescence readings were paused, EEDC was placed in the wells, and reading was continued for 10 minutes. After pausing the reading again, actin polymerization buffer with ATP was added, and the reading continued until fluorescent signal plateau was reached. The intensity of pyrene fluorescence on initiation of actin polymerization was monitored at 407 nm with an excitation wavelength of 350 nm using TECAN infinite M200 pro (Tecan Group Ltd, Männedorf, Switzerland). To identify any polymerization activities associated with EEDC, polymerization profiles were compared.
Animal Study and Analysis
Male Balb/c nu/nu mice (4 weeks of age, 12 ± 2 g body weight) were purchased from Orient Bio Inc (Seongnam, Korea) and maintained at 20°C to 22°C with a 12-hour light/dark cycle. All animal studies were performed in accordance with experimental protocols approved by Animal Ethics Committee of Eulji University (Approval #EUIACUC16-08). Mice were anesthetized, and HSC-3 cells (1 × 106 cells/0.1 mL in Hank’s Balanced Salt Solution, pH 7.3 HBSS) were injected into the masseter muscle within 30 minutes of harvesting. A total of 20 mice with xenografts were randomly divided into PBS-treated cancer group and EEDC-treated cancer group. For the EEDC treatment group, mice were administered with EEDC (5 mg/PBS/kg body weight) 3 times/wk by oral gavage for 5 weeks. Control mice received PBS alone. Tumor mass of mice was measured twice weekly using a caliper and calculated using the following formula: (Width in mm)2 × (Length in mm)/2. At the end of the experiment, mice were killed humanely by cervical dislocation, and alveolar bones of nude mice were scanned using a Skyscan 1076 high-resolution in vivo micro-computed tomography (SKYSCAN, Antwerpen, Belgium) at 100 kV, 140 µA current, rotation step 0.6°, and camera pixel size 35 µm. Tumors were collected for histopathological studies. Immunohistochemical staining was also performed using anti-CTTN and antiphosphor Y466 CTTN (Abcam, Cambridge, MA) at 1:100 dilution.
Statistical Analysis
All statistical analyses were conducted using InStat statistical software (GraphPad Software, Inc, San Diego, CA). Data are expressed as means ± standard errors. Asterisks were used to graphically indicate statistical significance. Repeated measures of 1-way ANOVA were used to analyze statistical significance in differences between groups. P values <.05 were considered statistically significant.
Results
EEDC Inhibits Invasion and Migration of HSC-3 Cells
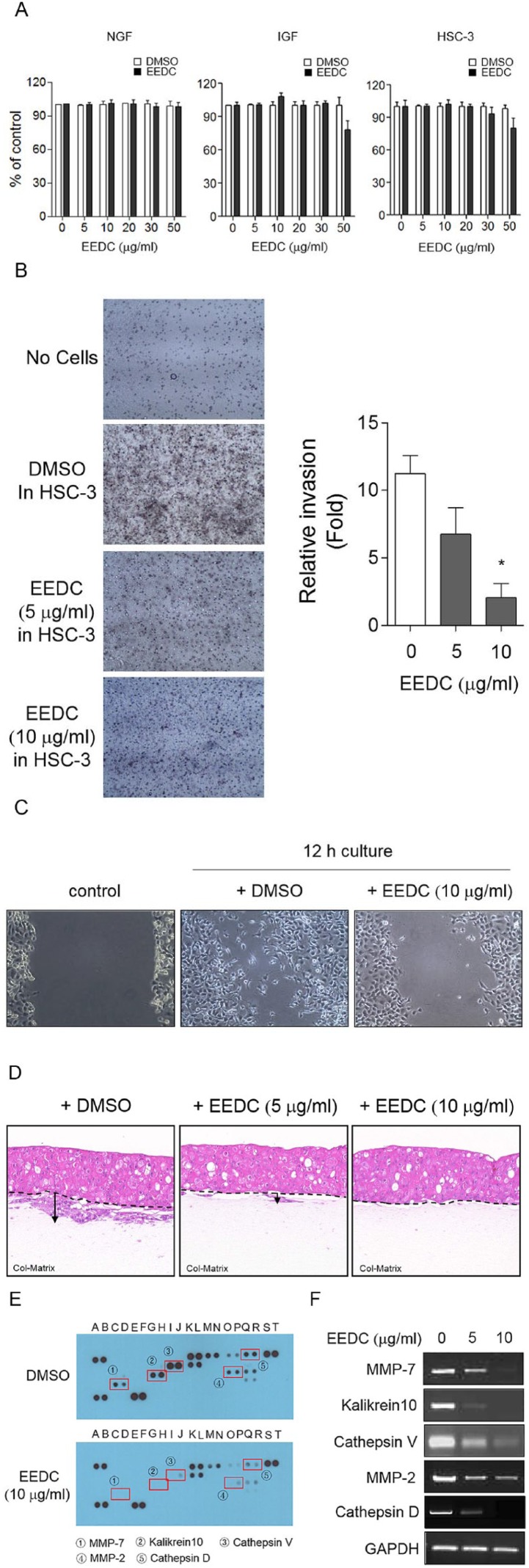
The MTT assay was performed to examine cytotoxicity of EEDC. IGF and HSC-3 OSCC were used for experiments. Treatment with EEDC at 5 and 30 µg/mL did not affect the viability of both cells (Figure 1A). The concentrations of EEDC required to cause a 50% inhibition of cell viability (IC50) for IGF and HSC-3 OSCC were 82.1 and 90 µg/mL at 24 hours. The viability of NGFs was unaffected by >130 µg/mL EEDC treatment at 24 hours. To determine the effect of EEDC on invasion and migration of HSC-3 cells, transwell assays were performed and relative invasion was analyzed. As shown in Figure 1B, Matrigel-coated transwell invasion activity of HSC-3 cells was significantly inhibited by EEDC treatment. Invasion was reduced 3.5-fold by 10 µg/mL EEDC. The inhibitory effect of EEDC was dose dependent. EEDC treatment also effectively decreased the motility of HSC-3 cells in scratch-wound assay (Figure 1C). Compared with the DMSO experiment, the cell-free area in the experiment with EEDC treatment was 2.51 times wider. Invasive areas into dermal equivalents were also observed using 3D collagen gels as a representative in vitro tissue model. The invasion index was calculated by multiplying the invasion depth by the invasive area. 3D culture was carried out independently 3 times, and the average of the invasion index was used for statistical analysis. Enlarged infiltrative HSC-3 cell growth into the dermal matrix was observed, but EEDC treatment significantly inhibited this infiltration (Figure 1D). Statistically significant difference in infiltration was found. Crucial factors related to the control of cancer cell invasion by EEDC were then determined. As shown in human protease profiles of HSC-3 cells, release levels of matrix metalloprotease (MMP)-7 (①), kalikrein 10 (②), cathepsin V (③), MMP-2 (④), and cathepsin D (⑤) were significantly suppressed by EEDC treatment (Figure 1E). RT-PCR analysis revealed that mRNA expression changes of these proteases were consistent with their protease profiles (Figure 1F). These results indicate that EEDC effectively inhibits the expression of several proteases, thereby suppressing the invasion and migration of cancer cells.
Figure 1.
EEDC inhibits invasion of HSC-3 cells: (A) cytotoxicity of EEDC to NGF, IGF, or HSC-3 was determined by the MTT assay. DMSO treatment was used as control. (B) Matrigel-coated transwell invasion was performed with or without EEDC. Changes in invasion activity of HSC-3 cells were detected by membrane staining with hematoxylin. Results represent the mean ± SE of 3 independent experiments. *P <.01 versus without EEDC control. (C) Cell motility assay was performed in confluent monolayer cell after wounding with a sterile pipette tip. EEDC was added to complete media, and images were captured after 12 hours of incubation. (D) Invasion into dermal equivalent was performed in matrix of type I-A collagen. EEDC was added to complete media. Media was replaced with fresh media containing EEDC every third day. Invasion pattern was observed with paraffin-embedded matrix section by hematoxylin and eosin staining. (E) Protease modulated by EEDC in HSC-3 cells: culture medium was harvested and analyzed by Proteome Profiler Human Protease Array. Altered factors are indicated with rectangles and circled numbers. (F) Inhibitory effects of EEDC on mRNA expression levels of MMP-7, kalikrein 10, cathepsin V, MMP-2, and cathepsin D by RT-PCR.
Abbreviations: DMSO, dimethyl sulfoxide; EEDC, ethanol extracts of rhizome of the Dryopteris crassirhizoma; IGF, immortalized gingival fibroblast; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; MMP, matrix metalloproteinase; NGF, normal gingival fibroblast; RT-PCR, reverse transcription polymerase chain reaction.
EEDC Impairs ECM Degradation Through Inhibiting Invadopodia Formation
HSC-3 cells displayed punctuate F-actin-enriched invadopodia (Figure 2A). When HSC-3 cells were cultured on FITC-gelatin-coated coverslips, proteolytic degradation of gelatin was observed underneath invadopodia (Figure 2B). However, EEDC treatment abolished matrix degradation capacity of HSC-3 cells. Inhibition of gelatin matrix degradation was observed even at the relatively low concentration of EEDC of 2.5 µg/mL. Multiple F-actin-enriched invadopodia were also repressed by EEDC treatment. Phosphorylation (Y466) of CTTN, one crucial event during functional invadopodia formation, was significantly inhibited by EEDC treatment (Figure 2C). To determine the anti-invasive effect of EEDC on invadopodia structure, fluorescence-based actin polymerization assay was performed using pyrene-labeled actin. Actin polymerization is a main event in protrusive membrane formation of invasive cancer cells. It contributes to the focalization of the release of matrix degradation protease, thus increasing the migration and invasion efficiency of cancer cells.8 G-actin readily polymerizes to form F-actin with concomitant hydrolysis of ATP. Fluorescence intensity was increased in the mixture of pyrene conjugated actin and ATP. However, reduction in the rate of actin polymerization was observed in reactions containing 5 or 10 µg/mL EEDC (Figure 3). This result indicates that EEDC can disturb actin filament elongation, thereby retarding actin polymerization. Therefore, interference of EEDC in invadopodia assembly can lead to impaired ECM degradation activity of HSC-3 cells.
Figure 2.
EEDC impairs ECM degradation through inhibiting invadopodia formation of HSC-3 cells: (A) Cells on gelatin-coated slips were fixed and double stained with actin (phalloidin, red) and cortactin (green) specific antibodies to identify invadopodia. Merged images show colocalization between actin and cortactin (arrow, original magnification 400×). (B) Cells were cultured on FITC (fluorescein isothiocyanate)-conjugated gelatin-coated slips (green) for 12 hours with or without EEDC, and F-actin was visualized with phalloidin (red). Degraded matrix regions are dark (original magnification 400×). A total of 350 cells were counted to quantify the number of invadopodia foci. The dark area was measured for gelatin degradation compared with the control. Results represent the mean ± standard error of 3 independent experiments. *P <.01 versus control. (C) Cells were treated with EEDC for 1 hour and analyzed by western blotting with specific antibody for total and phosphor(Y466) cortactin.
Abbreviations: ECM, extracellular matrix; EEDC, ethanol extracts of rhizome of the Dryopteris crassirhizoma.
Figure 3.
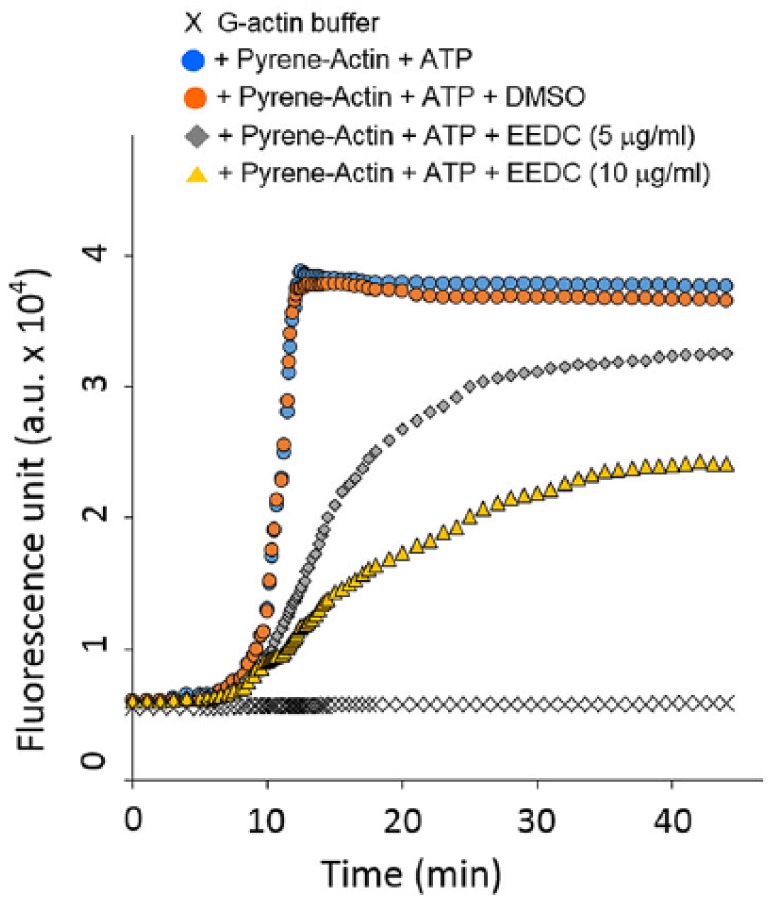
EEDC disturbs actin polymerization: In vitro actin polymerization assay was performed with or without EEDC in HSC-3 cells. G-buffer containing pyrene-actin and ATP was analyzed as positive control. DMSO was added as sample control instead of EEDC. This experiment was repeated 3 times independently, yielding similar results.
Abbreviations: ATP, adenosine triphosphate; DMSO, dimethyl sulfoxide; EEDC, ethanol extracts of rhizome of the Dryopteris crassirhizoma; G-actin, globular-actin.
EEDC Inhibits Tumor Growth and Progression
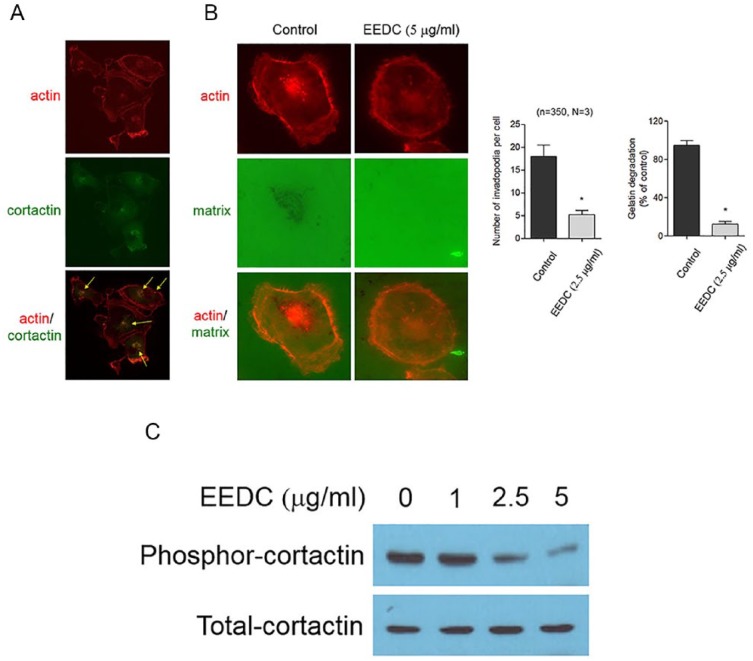
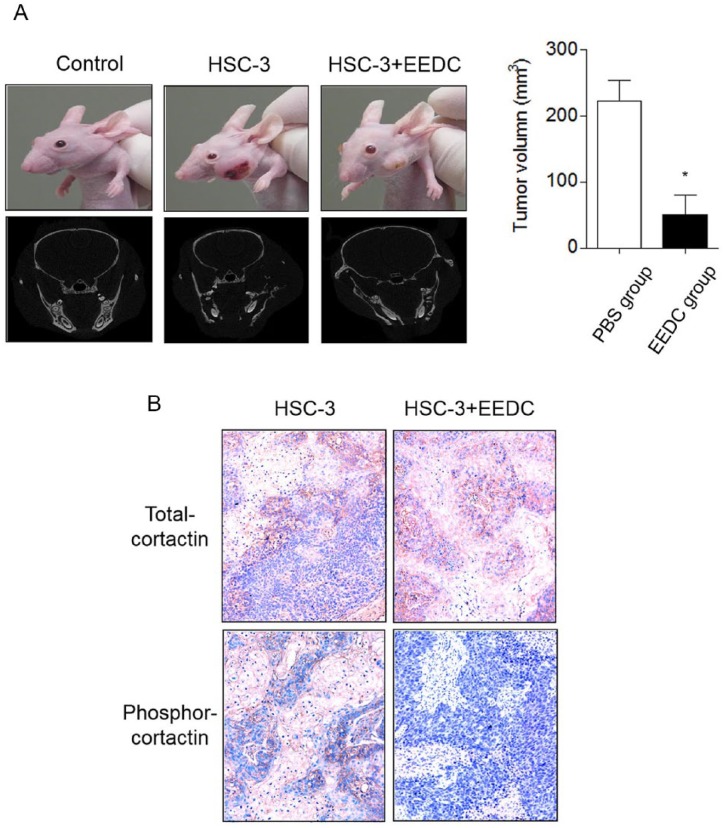
HSC-3 cells were inoculated into the left masseter region of nude mice. The effect of EEDC on tumor growth was then determined. Body weights of the EEDC-treated group were not significantly changed during the experimental period compared with those of the PBS-treated control group (data not shown). Tumor growth was steadily increased in the control group with extensive bone destruction around the mandibular bone on the mediolateral radiograph (Figure 4A). Osteolytic lesions occurred in cancellous and cortical bones on vertical projection. However, decreased tumor growth was observed in the EEDC-treated group for 5 weeks. Osteolytic mandibular bone lesions were also reduced. Phosphorylated CTTN was highly detected in tumor tissue of the control group mice (Figure 4B). However, limited CTTN phosphorylation was observed in tumor tissues of EEDC-treated group mice. This result verified that EEDC could suppress tumor growth and progression by inhibiting invadopodia formation in mice.
Figure 4.
EEDC inhibits tumor growth and progression: (A) HSC-3 cells were injected into the masseter muscle of athymic nude mice. Tumor growth was observed for 5 weeks. EEDC was orally administered 3 times a week at a dose of 5 mg/PBS/kg body weight. The head and neck region of mice were scanned using micro–computed tomography. The graph represents tumor volumes in each group. *P <.01 versus PBS group. (B) Immunohistochemical staining for total- and phosphor-cortactin were also performed (original magnification 100×).
Abbreviations: EEDC, ethanol extracts of rhizome of the Dryopteris crassirhizoma; PBS, phosphate buffered saline.
Discussion
The rhizomes of D crassirhizoma grow extensively throughout Korea. They have been traditionally used for medicinal purposes. The dried underground stem mainly has functional compounds. Properties of various compounds of the rhizomes of D crassirhizoma have been widely investigated. It has been reported that acylphloroglucinols isolated from D crassirhizoma have in vitro antibacterial and fatty acid synthase inhibitory activities.9 Sutchuenoside A and kaempferitrin constituents isolated from D crassirhizoma also possess antiparasitic activities in vivo.10 In addition, water extract of D crassirhizoma can inhibit bone loss by suppressing osteoclast differentiation and function.11
To determine the effect of D crassirhizoma on human gingival cancer, EEDC was prepared and its anti-invasive and anti-tumor effects were investigated. Inhibition of transwell-mediated migration and invasion of cancer cells by EEDC treatment was observed at noncytotoxic concentrations. The anti-invasive effect of the EEDC in human prostate cancer cells has been previously reported to be a result of its effect on cell cycle arrest and apoptosis.12 In this study, EEDC at 100 µg/mL was used to verify its effect on cell cycle arrest and apoptosis induction. Approximately 50% of cell proliferation was inhibited by EEDC at 50 µg/mL. Only around 10% of cells were proliferative after treatment with EEDC at 100 µg/mL. However, EEDC at 5 to 30 µg/mL showed no cytotoxicity to gingival cancer cells. At concentrations of 5 or 10 µg/mL, EEDC inhibited the migration and invasion of gingival cancer cells in the present study. These results indicate that EEDC has anti-invasive activity through suppressing cancer migration and invasion at relatively low doses. It will arrest cancer cell proliferation at higher doses.
Invasive OSCCs frequently metastasize to cervical lymph nodes and alveolar bone, leading to poor prognosis. For invasion, morphological changes to mesenchymal spindle shape are needed for invasion into the underlying matrix. Noninvasive cancer cells do not have such changes to increase their migratory and invasive ability. Invadopodia are cellular tools to infiltrate the basement membrane during invasion. Many kinds of components, such as the Arp2/3 complex, integrin, multiple signaling molecules, and matrix protease, are involved in invadopodia formation.5 Assembly of actin polymerization and adhesion complex can lead to nucleation of actin cytoskeleton and focalized matrix degradation, thereby increasing the efficiency of invasive growth. Incomplete assembly of invadopodia has an adverse effect on invasion and metastasis. Highly invasive cells have abundant invadopodia with relatively high matrix degradation. In addition, the invadopodia formation process is known to have no effect on overall cell mortality because the half-life of invadopodia is several seconds to a few minutes.13 Therefore, targeting of invadopodia might be an efficacious strategy for controlling cancer invasion and progression. It might have utility for drug discovery. In this study, actin-enriched multiple invadopodia colocalized with CTTN were found in cancer cells. However, significantly reduced invadopodia were observed after EEDC treatment. Their ECM degradation activity on FITC-gelatin slip was also inhibited by EEDC treatment. Decreased G-actin polymerization by EEDC caused diminution of invadopodial degradation. Decreased CTTN phosphorylation was also observed after EEDC treatment. Actin-binding CTTN is a crucial molecule during functional invadopodia formation. Tyrosine phosphorylated CTTN is particularly aggregated within actin-enriched spots during early phases of invadopodia formation. Proteases such as MT1-MMP and MMP-2 will join in these sites.14 These results indicate that the reduced ECM degradation after EEDC treatment might be a result of its suppression of invadopodia formation. Inhibition of actin polymerization and CTTN phosphorylation by EEDC treatment disturbs focalized release of proteases near the stroma, and thereby, invasion activity of cancer cells is impaired.
Nature has plentiful resources for developing anticancer drugs. Extensive cancer studies have been conducted to develop anticancer drugs. Various types of compounds from food sources and herbal medicine have been validated to have anticancer effects. Natural compounds without cytotoxicity to normal cells are known to be useful as effective anticancer drugs for cancer prevention and treatment. For example, tea polyphenols, isothiocyanate, resveratrol, lycopene, luteolin, genistein, and gingerol have been suggested to have potential in cancer prevention and treatment.2,3 More research is needed to increase the therapeutic efficacy of these compounds in order to use them in clinical practice. To elucidate functional compounds from traditional medicinal plants with anticancer activity, invadopodial matrix degradation was used as a screening tool in this study. Intense anti-invadopodia activity of EEDC was observed. This activity was displayed at a noncytotoxic dose of EEDC. Vincristine, vinblastine, irinotecan, etoposide, and paclitaxel are well-known plant-derived anticancer medicines. They target cell cycle–regulating molecules through different mechanisms of action such as interaction with microtubules, alkylation of DNA, and inhibition of topoisomerase.15,16 Although these can be effective drugs for cancer treatment and reduction, they also have side effects.
Orthotopic animal models reflect the morphology and character of their respective original tumors. Gingival mucosa is intimately adjacent to the alveolar bone. Malignant gingival SCC cells, therefore, frequently invade mandibular bone.17 A xenograft model of mandibular bone invasion of gingival SCC is appropriate for osteolytic gingival cancer studies. However, the rigidity of gingival mucosa limits the injection volume of suspended cancer cell solution and restricts analysis of cancer growth and regional bone invasion. It is not an appropriate animal model for the assessment of efficacy of anticancer drugs. Therefore, inoculation of cancer cells into the masseter region of mice is frequently used to establish an animal model of gingival SCC growth and bone invasion.18,19 We also used the masseter muscle injection method to demonstrate the anti-tumor effect of EEDC in this study. In the mouse model, tumors of the HSC-3 xenograft developed substantially and showed a well-differentiated SCC. However, oral administration of EEDC was efficacious in reducing cancer growth without triggering weight loss, significant hepatic disease, or nephrotoxicity. Less invasive SCC and decreased CTTN phosphorylation level were monitored immunohistochemically. Our results indicated that EEDC was a promising anti-tumor agent for control of OSCC growth and progression. In addition, extensive osteolytic lesions of HSC-3 xenografts were significantly diminished by oral administration of EEDC based on radiological evidence. As shown in Figure 1E, control of proteases such as MMP-7, kalikrein 10, cathepsin V, MMP-2, and cathepsin D by EEDC might be mediated via its effect on tumor growth and bone resorption. Tumor cells frequently overexpress proteases such as MMP, kallikrein, and cathepsin, and these have been correlated with invasive activity, metastatic potential, and/or poor prognosis in several cancers. MMP-7, kalikrein 10, cathepsin V, MMP-2, and cathepsin D are reported to be involved in cancer invasion, especially MMP-2 as an invadopodia-recruited protease that is involved in the matrix-degrading capability.14,20-24
Oral cancer is one of the most frequent types in head and neck cancers. SCC accounts for nearly 95% of all oral cancers.25 Oral hygiene is an important determinant of oral cancer. Accumulating evidence indicates that oral bacteria are intimately associated with oral cancer.26 Porphyromonas gingivalis disrupts the balance of immuno-inflammation in the oral microenvironment, leading to growth of pathogenic microbial communities such as Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans. The dysregulated immune responses in the oral microenvironment lead to prolonged inflammation and alveolar bone loss, ultimately causing periodontal disease and oral cancer.27 Decreasing the levels of these species can improve oral health. Several solvent extracts of D crassirhizoma have been reported to possess antibacterial activities.28,29 In addition, it has been reported that the water extract of D crassirhizoma prevents bone loss by suppressing osteoclast differentiation and function.11 Therefore, the use of D crassirhizoma has a beneficial effect not only in bacterial growth suppression, but also in the inhibition of cancer growth and progression.
Conclusion
EEDC inhibits cancer migration and invasion by suppressing functional invadopodia formation. Repression of protease levels and abnormal actin polymerization resulted in inhibition of cancer migration and invasion. Administration of EEDC led to inhibition of tumor growth and progression in mouse xenograft. Thus, EEDC is a potential anti-invasive and anti-tumor agent for prevention and therapy of OSCC. The active ingredients of EEDC need to be isolated and identified for pharmaceutical application.
Footnotes
Authors’ Note: All animal studies were performed in accordance with experimental protocols that were approved by the animal ethics committee of Eulji University (EUIACUC16-08).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Eulji University in 2017 and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2018R1D1A1B07042035).
ORCID iD: Young Sun Hwang  https://orcid.org/0000-0001-7012-3434
https://orcid.org/0000-0001-7012-3434
References
- 1. Bhanot A, Sharma R, Noolvi MN. Natural sources as potential anti-cancer agents: a review. Int J Phytomedicine. 2011;3:9-26. [Google Scholar]
- 2. Zhou Y, Zheng J, Li Y, et al. Natural polyphenols for prevention and treatment of cancer. Nutrients. 2016;8:E515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang H, Khor TO, Shu L, et al. Plants against cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem. 2012;12:1281-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eddy RJ, Weidmann MD, Sharma VP, Condeelis JS. Tumor cell invadopodia: invasive protrusions that orchestrate metastasis. Trends Cell Biol. 2017;27:595-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hastie EL, Sherwood DR. A new front in cell invasion: the invadopodial membrane. Eur J Cell Biol. 2016;95:441-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang X, Jung IH, Hwang YS. EGF enhances low-invasive cancer cell invasion by promoting IMP-3 expression. Tumour Biol. 2016;37:2555-2563. [DOI] [PubMed] [Google Scholar]
- 7. Bowden ET, Coopman PJ, Mueller SC. Invadopodia: unique methods for measurement of extracellular matrix degradation in vitro. Methods Cell Biol. 2001;63:613-627. [DOI] [PubMed] [Google Scholar]
- 8. Revach OY, Weiner A, Rechav K, Sabanay I, Livne A, Geiger B. Mechanical interplay between invadopodia and the nucleus in cultured cancer cells. Sci Rep. 2015;5:9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Na M, Jang J, Min BS, et al. Fatty acid synthase inhibitory activity of acylphloroglucinols isolated from Dryopteris crassirhizoma. Bioorg Med Chem Lett. 2006;16:4738-4742. [DOI] [PubMed] [Google Scholar]
- 10. Jiang B, Chi C, Fu YW, Zhang QZ, Wang GX. In vivo anthelmintic effect of flavonol rhamnosides from Dryopteris crassirhizoma against Dactylogyrus intermedius in goldfish (Carassius auratus). Parasitol Res. 2013;112:4097-4104. [DOI] [PubMed] [Google Scholar]
- 11. Ha H, Shim KS, Kim T, An H, Ma JY. Water extract of Dryopteris crassirhizoma attenuates bone loss by suppressing osteoclast differentiation and function. Evid Based Complement Alternat Med. 2013;2013:852648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang SH, Bae JH, Hong DP, et al. Dryopteris crassirhizoma has anti-cancer effects through both extrinsic and intrinsic apoptotic pathways and G0/G1 phase arrest in human prostate cancer cells. J Ethnopharmacol. 2010;130:248-254. [DOI] [PubMed] [Google Scholar]
- 13. Murphy DA, Courtneidge SA. The “ins” and “outs” of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jacob A, Prekeris R. The regulation of MMP targeting to invadopodia during cancer metastasis. Front Cell Dev Biol. 2015;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zulkipli IN, David SR, Rajabalaya R, Idris A. Medicinal plants: a potential source of compounds for targeting cell division. Drug Target Insights. 2015;9:9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song YH, Sun H, Zhang AH, Yan GI, Han Y, Wang XJ. Plant-derived natural products as leads to anti-cancer drugs. J Med Plant Herb Ther Res. 2014;2:6-15. [Google Scholar]
- 17. Okura M, Yanamoto S, Umeda M, et al. ; Japan Oral Oncology Group. Prognostic and staging implications of mandibular canal invasion in lower gingival squamous cell carcinoma. Cancer Med. 2016;5:3378-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cui N, Nomura T, Noma H, et al. Effect of YM529 on a model of mandibular invasion by oral squamous cell carcinoma in mice. Clin Cancer Res. 2005;11:2713-2719. [DOI] [PubMed] [Google Scholar]
- 19. Segawa E, Kishimoto H, Takaoka K, et al. Promotion of hematogenous metastatic potentials in human KB carcinoma cells with overexpression of cyclooxygenase-2. Oncol Rep. 2010;24:733-739. [DOI] [PubMed] [Google Scholar]
- 20. Basu S, Thorat R, Dalal S. MMP7 is required to mediate cell invasion and tumor formation upon plakophilin3 loss. PLoS One. 2015;10:e0123979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dong Y, Loessner D, Irving-Rodgers H, et al. Metastasis of ovarian cancer is mediated by kallikrein related peptidases. Clin Exp Metastasis. 2014;31:135-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Santamaría I, Velasco G, Cazorla M, Fueyo A, Campo E, López-Otín C. Cathepsin L2, a novel human cysteine proteinase produced by breast and colorectal carcinomas. Cancer Res. 1998;58:1624-1630. [PubMed] [Google Scholar]
- 23. Chen WT, Wang JY. Specialized surface protrusions of invasive cells, invadopodia and lamellipodia, have differential MT1-MMP, MMP-2, and TIMP-2 localization. Ann N Y Acad Sci. 1999;878:361-371. [DOI] [PubMed] [Google Scholar]
- 24. Zhang M, Wu J, Yang X, et al. Overexpression cathepsin D contributes to perineural invasion of salivary adenoid cystic carcinoma. Front Oncol. 2018;8:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noguti J, De Moura CF, De Jesus GP, et al. Metastasis from oral cancer: an overview. Cancer Genomics Proteomics. 2012;9:329-335. [PubMed] [Google Scholar]
- 26. Whitmore SE, Lamont RJ. Oral bacteria and cancer. PLoS Pathog. 2014;10:e1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Atanasova KR, Yilmaz O. Looking in the Porphyromonas gingivalis cabinet of curiosities: the microbium, the host and cancer association. Mol Oral Microbiol. 2014;29:55-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee HB, Kim JC, Lee SM. Antibacterial activity of two phloroglucinols, flavaspidic acids AB and PB, from Dryopteris crassirhizoma. Arch Pharm Res. 2009;32:655-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kwon DY, Kang OH, Choi JG, et al. Antibacterial effect of Dryopteris crassirhizoma against methicillin-resistant Staphylococcus aureus. Fitoterapia. 2007;78:430-433. [DOI] [PubMed] [Google Scholar]