Abstract
Purpose:
To investigate the impact of intra- and inter-fractional esophageal motion on dosimetry and observed toxicity in a phase I dose escalation study of accelerated radiotherapy with concurrent chemotherapy for locally advanced lung cancer.
Methods and Materials:
Patients underwent computed tomography imaging for radiotherapy treatment planning (CT1 and 4DCT1) and at 2 weeks (CT2 and 4DCT2) and 5 weeks (CT3 and 4DCT3) after initiating treatment. Each computed tomography scan consisted of 10-phase 4DCTs in addition to a static free-breathing or breath-hold computed tomography. The esophagus was independently contoured on all computed tomographies and 4DCTs. Both CT2 and CT3 were rigidly registered with CT1 and doses were recalculated using the original intensity-modulated radiation therapy plan based on CT1 to assess the impact of interfractional motion on esophageal dosimetry. Similarly, 4DCT1 data sets were rigidly registered with CT1 to assess the impact of intrafractional motion. The motion was characterized based on the statistical analysis of slice-by-slice center shifts (after registration) for the upper, middle, and lower esophageal regions, respectively. For the dosimetric analysis, the following quantities were calculated and assessed for correlation with toxicity grade: the percent volumes of esophagus that received at least 20 Gy (V20) and 60 Gy (V60), maximum esophageal dose, equivalent uniform dose, and normal tissue complication probability.
Results:
The interfractional center shifts were 4.4 ± 1.7 mm, 5.5 ± 2.0 mm and 4.9 ± 2.1 mm for the upper, middle, and lower esophageal regions, respectively, while the intrafractional center shifts were 0.6 ± 0.4 mm, 0.7 ± 0.7 mm, and 0.9 ± 0.7 mm, respectively. The mean V60 (and corresponding normal tissue complication probability) values estimated from the interfractional motion analysis were 7.8% (10%), 4.6% (7.5%), 7.5% (8.6%), and 31% (26%) for grade 0, grade 1, grade 2, and grade 3 toxicities, respectively.
Conclusions:
Interfractional esophageal motion is significantly larger than intrafractional motion. The mean values of V60 and corresponding normal tissue complication probability, incorporating interfractional esophageal motion, correlated positively with esophageal toxicity grade.
Keywords: 4D CT, lung cancer, esophageal motion, IMRT, 4D computed tomography
Introduction
Conventional radiation therapy with concurrent chemotherapy is the current standard of care for locally advanced, inoperable nonsmall cell lung cancer.1-5 This standard treatment has improved long-term survival rate from approximately 5% with conventional radiation therapy alone to approximately 15% to 20% with combined modality therapy. These gains, however, come at a cost of increased toxicity, especially esophagitis.4 Given high rates of local failure after conventional treatment, radiation therapy dose intensification is attractive, which has the potential to improve local control and survival.4,6-9 However, the rapid proliferation kinetics of the esophageal mucosa makes dose intensification challenging due to excessive toxicity.6,10 Radiation Therapy Oncology Group protocol 0617 demonstrated that dose escalation from 60 to 74 Gy during conventional treatment led to more severe esophagitis which was associated with a higher risk of mortality.11
Intensity modulated radiation therapy (IMRT) is a state-of-art radiation delivery technique for improving dose conformality and sparing organs-at-risk, and it is effective for reducing the esophageal dose compared with conventional techniques.12,13 Using IMRT, we recently performed a phase 1 dose escalation study of accelerated radiation therapy with concurrent chemotherapy,14 which is one of the first prospective studies using dose intensification via IMRT. However, the ability of esophageal sparing with IMRT can be greatly impacted by esophageal motion.
In this study, we performed quantitative analyses for intrafractional and interfractional esophageal motion and assessed their impact on dosimetry and observed esophageal toxicity. While many studies have evaluated both intra-fractional motion15-20 and interfractional motion21-24 of the esophagus, few studies have evaluated the dosimetric impact of either.12,25 We sought to evaluate this further using data on esophageal motion obtained prospectively in patients with lung cancer.
Methods and Materials
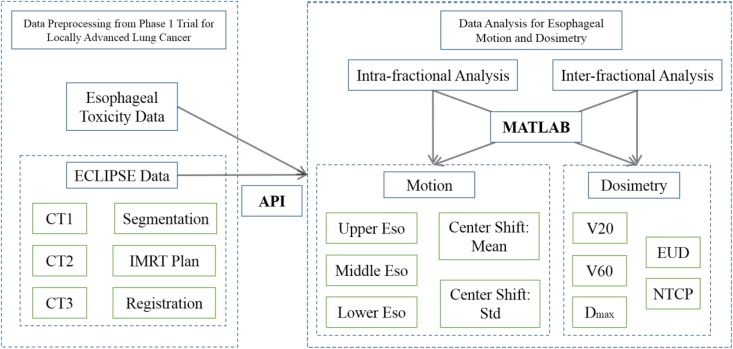
The methods for the esophageal motion analysis are summarized in Figure 1, which can be divided into data preprocessing (left panel) and data analysis (right panel).
Figure 1.
The flowchart of esophageal analysis. The data from phase 1 trial for locally advanced lung cancer were preprocessed using a treatment planning system (Varian ECLIPSE), and then automatically exported via Eclipse scripting API to MATLAB for intrafractional and interfractional motion and dosimetric analyses.
Data Preprocessing
Twenty-four patients were enrolled on this prospective dose-escalation study. The dose was delivered using 2 Gy fractions, 6 fractions per week (twice daily on fridays with a 6-hour interval). The dose was escalated from 58 Gy to a planned maximum dose of 74 Gy in 4 Gy increments in a standard 3 + 3 trial design. Computed tomography (CT) scans were obtained at 3 time points in all patients. Baseline scans (CT1 and 4DCT1) were obtained for radiation treatment planning with 2 subsequent scans obtained at 2 weeks (CT2 and 4DCT2) and 5 weeks (CT3 and 4DCT3) after beginning treatment, respectively. Each CT scan consisted of a 10-phase 4DCT in addition to a static free-breathing or breath-hold CT.
The internal target volume was expanded by 5 mm for the primary tumor, and 3 mm for the involved lymph nodes to generate a clinical target volume, which was expanded for another 3 mm to form the planning target volume. Intensity-modulated radiation therapy was utilized in all patients to minimize the dose to esophagus, with the planning goal to keep esophageal V20 < 50% and V60 < 25%. Here, V20 and V60 indicate the percent volumes in which the esophagus received at least 20 Gy and 60 Gy, respectively. The IMRT plan for each patient was based on CT1 utilizing data from both CT1 and 4DCT1 using the ECLIPSE planning system (Varian Medical Systems, Palo Alto, California). The esophagus was manually contoured for each patient on all image volumes independently, including both CTs and 4DCTs.
Each of the 10-phase 4DCT1 data sets were rigidly registered with CT1 for intrafractional motion analysis. Both CT2 and CT3 were rigidly registered with CT1 for interfractional motion analysis. Registration between any 2 sets of CT images was first manually aligned based on the bony landmarks (eg, vertebral and sternum bodies) and then automatically registered using the ECLIPSE planning system based on the thoracic region-of-interest (the extension of PTV slices ∼2 cm inferiorly and superiorly) followed by manual double checks. Such rigid registration was performed to account for couch shifts and rotation that could be corrected during treatment, to avoid the overestimation of esophageal motion and its dosimetric impact.
To account for dosimetric changes due to esophageal motion, the initial IMRT treatment plan that was calculated based on CT1 was reassigned to both CT2 and CT3 for dose calculation. Specifically, the same IMRT beam angles and multileaf collimator (MLC) apertures with the same isocenter and monitor units were used to recalculate the dose distribution based on new CT volumes that were registered to CT1. Next, the dosimetric parameters were extracted for the esophagus to assess respectively intrafractional dosimetric changes of 4DCT1 from CT1, and interfractional dosimetric changes of CT2 and CT3 from CT1.
To facilitate and ensure the accuracy of the above analyses, all relevant data including CT images, rigid registration (shifts and rotations), esophageal contours, and three-dimensional (3D) dose distributions for each CT were automatically exported from the ECLIPSE planning system via Eclipse Scripting API to MATLAB for detailed numerical analysis.
Esophageal toxicity data were available to study the correlation between dosimetric changes and grades of esophageal toxicity. Esophageal toxicity was prospectively assessed and graded using Common Terminology Criteria for Adverse Events version 4.03,14 including 5 grade-1, 13 grade-2, 3 grade-3 toxicity (one patient with grade-3 acute esophagitis, one patient with both grade-3 acute esophageal fistula esophagitis and grade-2 esophagitis, and one patient with both grade-3 esophageal obstruction and grade-2 esophagitis).
Motion Analysis
The esophagus was contoured from the sternal notch to the GE junction on mediastinal windows. The outer wall defined the esophageal border. For the motion analysis, the esophagus in the thoracic region was divided anatomically into upper (from the sternal notch to the lower border of the azygos vein), middle (from the lower border of the azygos vein to the inferior pulmonary veins), and lower (from the inferior pulmonary veins to the stomach) regions.
As the esophagus is relatively round in cross section and long in inferior-superior direction, the esophageal geometry was quantified as slice-by-slice esophageal centers, and then the center shift on each slice was used to quantify esophageal motion. Specifically, the esophageal center was computed for each axial slice from each CT volume as the center-of-mass for the pixels within the esophageal contour. These centers were then used to compute the center shifts between any 2 CT volumes slice-by-slice. Note that the calculation of center shifts was after rigid image registration. The final quantitative indicator between any 2 CTs for the motion analysis was the average of all center shifts for the slices within each esophageal region (ie, upper, middle, and lower esophagus), namely “regional shift mean,” and the standard deviation of these center shifts within each esophageal region, namely “regional shift deviation.”
To evaluate intrafractional esophageal motion, regional shift means and regional shift deviations of 3 esophageal regions were calculated between each of 10-phase 4DCT1 and CT1. However, since 4DCT1 had 10 phases, the mean and standard deviation of regional shift means and deviations were calculated among all 10 phases for each of 3 esophageal regions, for the purpose of statistical plots. In addition, the average of mean regional shift means (namely intra mean shift) and the average of mean regional shift deviations (namely intra mean standard deviation) among all patients were calculated for each esophageal region.
To evaluate interfractional esophageal motion, regional shift means and regional shift deviations of 3 esophageal regions were calculated for CT2 and CT3 from CT1. In addition, the average of regional shift means (namely inter mean shift) and the average of regional shift deviations among all patients (namely inter mean standard deviation) including both CT2 and CT3 were calculated for each esophageal region.
Dosimetric Analysis
For the dosimetric analysis, we calculated the following quantities for the entire esophagus: the percent volume of esophagus that received 20 Gy or more (V20), the percent volumes of esophagus receiving 60 Gy or more (V60), the maximum esophageal dose (Dmax), equivalent uniform dose (EUD), and normal tissue complication probability (NTCP). Equivalent uniform dose and NTCP were based on the Lyman–Kutcher–Burman model26,27 using the established model parameters for esophagus.28,29 Moreover, to evaluate intrafractional dosimetric changes for esophagus, the mean and standard deviation for each of above dosimetric quantities were calculated among all 10-phase 4DCT1.
Results
Esophageal Motion
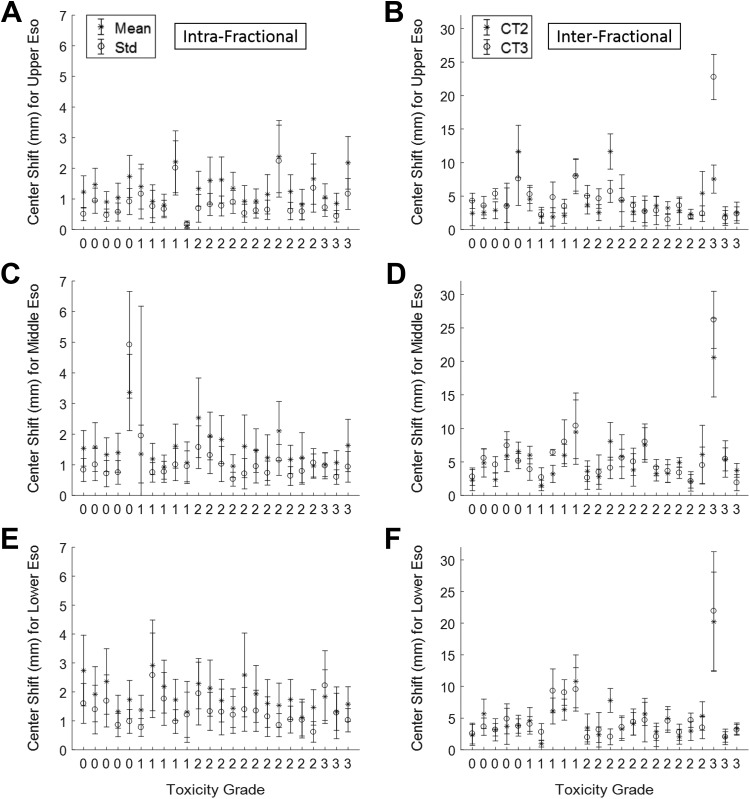
The intrafraction mean shift (average of mean regional shift means using all 10-phase 4DCT1 for all patients) and the intrafraction mean standard deviation (average of mean regional shift deviations using all 10-phase 4DCT1 for all patients) for each esophageal region were: 0.6 mm ± 0.4 mm, 0.7 mm ± 0.7 mm, and 0.9 mm ± 0.7 mm (shift ± standard deviation) for the upper, middle, and lower esophagus, respectively. On the other hand, the interfraction mean shift (average of regional shift means for CT2 and CT3 for all patients) and the interfraction mean standard deviation (average of regional shift deviations for CT2 and CT3 for all patients) for each esophageal region were: 4.4 mm ± 1.7 mm, 5.5 mm ± 2.0 mm, and 4.9 ± 2.1 mm for the upper, middle, and lower esophagus, respectively. The motion analysis results are shown in Figure 2.
Figure 2.
Esophageal motion analysis in correlation with toxicity data. The x-axis represents the patients in ascending order of esophageal toxicity grades (labeled).
The x-axis in Figure 2 (also in Figures 3 and 4) represents the patients in ascending order of esophageal toxicity grades. The same ordering is used for Figures 2 to 4 for the convenience of data interpretation: (1) the same coordinate on the x-axis indicates the same patient; (2) the magnitude of esophageal motion and its dosimetric impact can be assessed in correlation with toxicity grade.
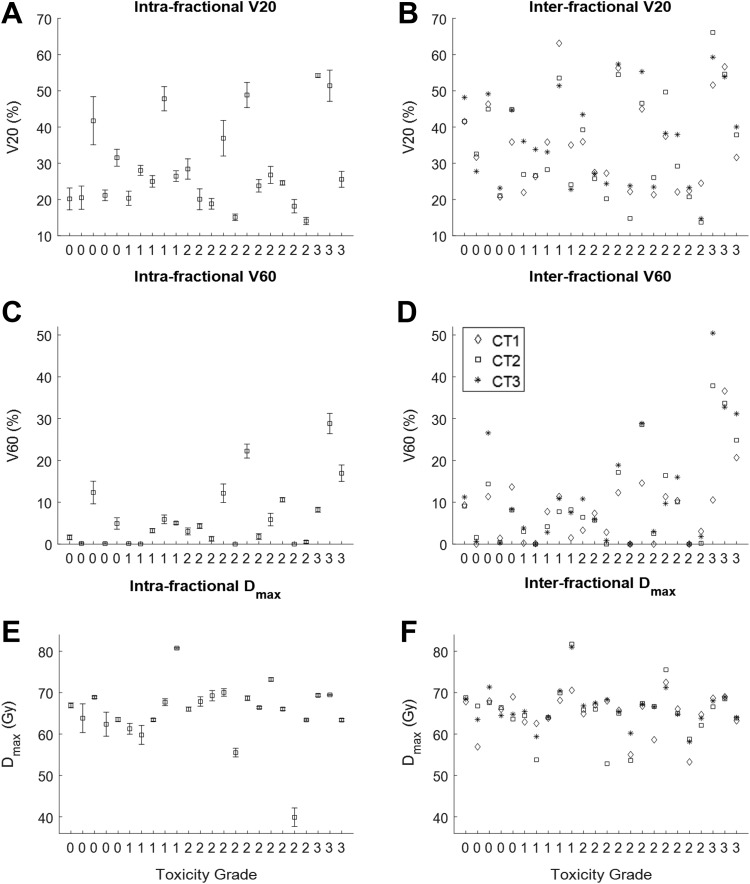
Figure 3.
V20, V60, and Dmax for both intrafractional (A, C, and E) and interfractional (B, D, and F) motion analyses. The x-axis represents the patients in ascending order of esophageal toxicity grades (labeled). Dmax indicates maximum esophageal dose.
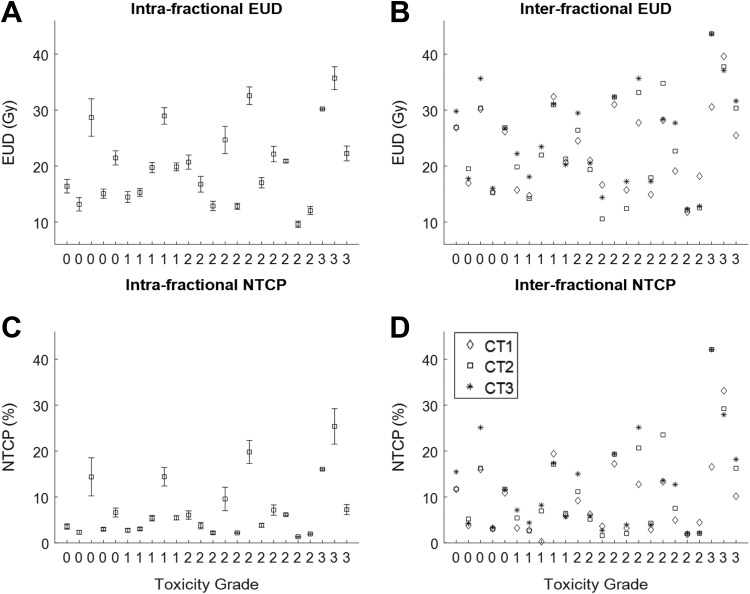
Figure 4.
Equivalent uniform dose and NTCP for both intrafractional (A and C) and interfractional (B and D) motion analyses. The x-axis represents the patients in ascending order of esophageal toxicity grades (labeled). NTCP indicates normal tissue complication probability.
The intrafractional results are plotted in Figures 2A, C, and E for the upper, middle, and lower esophagus, respectively. As explained above, the statistical plots here display (1) the mean and standard deviation of regional shift means among 10-phase 4DCT1 (star-marked statistical plots of Figures 2A, C, and E), and (2) the mean and standard deviation of regional shift deviations among 10-phase 4DCT1 (circle-marked statistical plots of Figures 2A, C, and E), to characterize the overall esophageal motion between 4DCT1 and CT1.
The interfractional results are plotted in Figures 2B, D, and F for upper, middle, and lower esophagus, respectively. The statistical plots here display (1) regional shift mean and deviation between CT2 and CT1 (star-marked statistical plots of Figures 2B, D, and F), and (2) regional shift mean and deviation between CT3 and CT1 (circle-marked statistical plots of Figures 2B, D, and F).
Dosimetric Impact
The dosimetric impacts due to intra- and interfractional esophageal motion were quantified using esophageal V20, V60, Dmax, EUD, and NTCP were plotted in Figures 3 and 4. The statistical plots for intrafractional dosimetry were based on the mean and standard deviation among 10-phase 4DCT1 for each dosimetric quantity.
Regarding interfractional changes of V60 (Figure. 3D), the esophageal V60 for all patients was originally under 25% (the IMRT planning constraint for V60) using CT1 except patient 23 (37%). Taking into account esophageal motion, 3 additional cases with equally weighted V60 from both CT2 and CT3 violated the planning constraint, that is, V60 increased from 14% to 29% in patient 16, from 11% to 44% in patient 22 (see Figure. 5), and from 21% to 28% in patient 24. On the other hand, V60 decreased from 37% to 33% in patient 23.
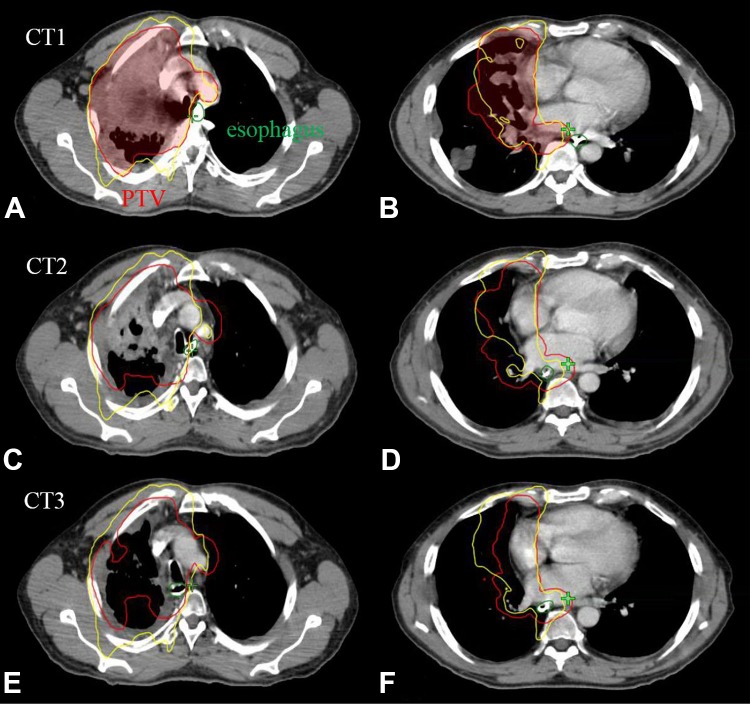
Figure 5.
Illustration of interfractional esophageal motion and its dosimetric impact for a patient with a grade-3 esophageal toxicity. An upper esophageal slice is shown in the left (A, C, and E), and a lower esophageal slice is shown in the right (B, D, and F) with 100% isodose line (yellow), PTV (red), and esophagus (green) contours.
For these 4 cases with V60 more than 25%, the interfractional average of equally weighted EUD (Figure 4C) from both CT2 and CT3 increased from 28 Gy to 34 Gy for patient 16, from 31 Gy to 44 Gy for patient 22 (see Figure 5), from 25 Gy to 31 Gy for patient 24, and decreased from 40 Gy to 37 Gy for patient 23; the interfractional average of equally weighted NTCP (Figure 4D) from both CT2 and CT3 increased from 13% to 22% for patient 16, from 17% to 42% for patient 22 (see Figure 5), from 10% to 17% for patient 24, and decreased from 33% to 29% for patient 23.
Correlation Between Toxicity and Dosimetry
There were 5 grade-1, 13 grade-2, 3 grade-3 toxicity in this study (one patient with grade-3 acute esophagitis, one patient with both grade-3 acute esophageal fistula esophagitis and grade-2 esophagitis, and one patient with both grade-3 esophageal obstruction and grade-2 esophagitis).
Figure 3D indicates that the patients with higher grade toxicity had higher interfractional V60 values. Figures 4B and D demonstrate that the patients with higher grade toxicity had higher interfractional NTCP values.
For all patients in this study, the dosimetric changes incorporating interfractional esophageal motion were positively correlated with the esophageal toxicity grade. It was found that high-interfractional mean values of V60 (Figure 3) and NTCP (Figure 4) from all CTs that took into account of esophageal motion correlated positively with high-grade toxicity. The mean V60 values for grade-0, grade-1, grade-2, and grade-3 were 7.8%, 4.6%, 7.5%, and 31%, respectively, and the mean NTCP values for grade-0, grade-1, grade-2, and grade-3 were 10%, 7.5%, 8.6%, and 26%, respectively.
Statistical analysis was performed to quantify the correlation between dosimetric quantities and toxicity grades, with the no-correlation null hypothesis. The results from Table 1 clearly indicate that the esophageal toxicity grade was positively correlated with V60 and NTCP, and such correlation was higher with more significance with respect to following up CTs (CT2 and CT3 with dosimetric changes induced by interfractional esophageal motion) than initial planning CT (CT1): correlation/P value was 0.37/.08 and 0.48/.02 for V60 on CT1 and follow-up CTs respectively, and 0.27/.21 and 0.36/.08 for NTCP on CT1 and follow-up CTs respectively.
Table 1.
Statistical Analysis of the Correlation Between Dosimetric Quantities and Toxicity Grades.a
| CT1 (Planning CT) | CT2 and CT3 (Follow-Up CTs) | |||
|---|---|---|---|---|
| Correlation | P Value | Correlation | P Value | |
| V20 | 0.09 | .68 | 0.14 | .50 |
| V60 | 0.37 | .08 | 0.48 | .02 |
| Dmax | −0.03 | .90 | −0.11 | .60 |
| EUD | 0.22 | .29 | 0.25 | .24 |
| NTCP | 0.27 | .21 | 0.36 | .08 |
Abbreviations: Dmax, maximum esophageal dose; EUD, equivalent uniform dose; NTCP, normal tissue complication probability.
a The statistical correlation and the corresponding P value are summarized for CT1 (planning CT) and CT2 and CT3 (follow-up CTs), respectively.
Noticeably, a patient with 20 to 30 mm interfractional esophageal center shifts (patient 22 that is the third from the right on the x-axis in Figure 2) developed grade-3 toxicity, as presented in Figure 5. For this patient, an upper esophageal slice is shown in the left of Figures 5A, C, and E for CT1, CT2, and CT3 scans, respectively, and a lower esophageal slice is shown in the right of Figures 5B, D and F for CT1, CT2, and CT3 scans, respectively. Note that PTV (red contour) and esophagus (green contour) were contoured on all CTs independently, although CT2 and CT3 images at display were after registration to CT1. Meanwhile, the 100% isodose lines (yellow contour) were plotted for each image to evaluate the dosimetric impact due to interfractional esophageal motion. The figures indicate that, although the esophagus was successfully excluded from 100% isodose line in the IMRT plan based on CT1, it fell inside 100% isodose line in both CT2 and CT3 as the tumor shrank during the treatment. Further, esophageal V60 values increased from 11% for CT1 to 38% for CT2 and 51% for CT3 respectively, which violated the planning constraint of V60 < 25%. Equivalent uniform dose values increased from 31 Gy for CT1 to 44 Gy for both CT2 and CT3, and NTCP values increased from 17% for CT1 to 42% for both CT2 and CT3.
Discussion
The accuracy of an esophageal motion analysis is primarily affected by 3 factors: the choice of imaging modality and assessment frequency, accurate generation of esophageal contours, and data analysis method. Most studies to date have utilized CT scans with high-image quality, while some utilized on-board cone-beam CT (CBCT),22 megavoltage CT,21 magnetic resonance imaging,20 or 2D projection images.24 Regarding CT-based esophageal contours or targets, motion analyses have been based on visual assessment,18 fiducial markers,19 gastroesophageal junction only,16 ,23 algorithm-based generation of esophageal contours,17 partial esophageal contours,15 or esophageal contours only on a reference volume.30 In terms of motion analysis method, all previous studies involved manual processes, which might render erroneous or biased analysis outcome.
Deformable image registration (DIR) may be more accurate than rigid registration in transferring contoured structures from planning CT to follow-up CT or CBCT, for the purpose of interfractional delivered dose estimation, when these structures are not manually contoured on the follow-up CT or CBCT. However, the relevant structures were already manually contoured on the follow-up CT in this study, and therefore there is no need for DIR. Moreover, manually contoured structures on the follow-up CT were as accurate as those on the planning CT by same clinical experts, and thus should be more accurate than the deformed structures from planning CT that would have been obtained with DIR. Moreover, the relative distance between vertebral bodies and esophagus using rigid body registration provides accurate displacement of esophagus at different times. The previous study also indicated that manually contoured structures on replanning CT served as the gold standard to evaluate the accuracy of deformed structures from some DIR methods.25 On the other hand, the accuracy of DIR and its potential distortion can be another complication factor for the use of DIR.
To assure the accuracy of esophageal analysis, this study utilized: (1) 3 sets of CT and 4DCT scans acquired throughout the treatments that should provide a more accurate estimation of intra- and interfractional anatomy of the patients; (2) manually contoured esophagus on all CT volumes; (3) automated export of esophageal contours together with 3D volumes from the treatment planning system followed by automated analysis by a computer program with predefined algorithms.
Our observations in regard to intrafractional motion of the esophagus were consistent with prior studies using 4DCT with partial esophageal contours at defined anatomic levels.15 Both suggested intrafractional esophageal motion could be approximately one centimeter in maximum displacement. However, the averaged intrafractional center shift on any 2D slice between 4DCT and free-breathing or breath-hold CT was considerably less than 1 cm, that is, approximately 1 mm. In terms of interfractional results, the averaged center shifts were consistent with prior CT based study,30 that is, around half centimeter. Note that 2 differences between this study and prior analyses are: (1) the esophagus was contoured on all axial slices on all 4D phases, and (2) the center shifts were calculated automatically using MATLAB programs.
From the dosimetric results in this study, intrafractional dosimetric variations due to esophageal motion seem to be minor, while the interfractional dosimetric changes can be significant. Thus, replanning based on esophageal motion may be necessary in selected patients to address such significant esophageal changes as was observed in one of our patients (as shown in Figure 5). CBCT scans can be routinely acquired before daily treatments and can be utilized to monitor for interfractional esophageal motion.25 The major challenge of using CBCT for lung radiotherapy is its poor reconstructed image quality. Due to the limitation of CBCT gantry speed, CBCT takes much longer imaging time than CT to acquire, which often accompanies significant breathing motion from scanned patients. Thus, it is technically challenging to reconstruct 4DCBCT images from often undersampled projection data in order to remove motion artifacts. However, our ultimate goal is to develop efficient adaptive planning techniques and establish effective procedures for patient-specific esophageal dose reduction to limit unnecessary toxicity, for which cine CBCT should be useful.31,32 On the other hand, a safety margin on esophagus, that is, planning organ at risk volume (PRV), should be useful to deal with intrafractional motion. However, due to significant interfractional motion range we have observed in this study, the benefit of esophageal PRV may be limited.
In this study, the averaged dosimetric quantities (ie, the averages from 10-phase 4DCT1 and CT1, CT2, and CT3) were used to characterize intra- and interfractional dosimetric changes. Although they may not correspond to the actual delivered dose, they meaningfully quantify the intra- and interfractional dosimetric changes based on CT images that are a fairly accurate representation of patient anatomy and its dynamic variation during the treatment.
Here we conclude the esophageal dosimetry (V60 and NTCP) incorporating interfractional esophageal motion correlated well with toxicity. On the other hand, it is possible that large interfractional esophageal motion does not contribute to esophageal dose and thus toxicity, such as these cases with large esophageal motion indicated in Figure 2.
We acknowledge that though the data set is relatively small, all of the scans were obtained uniformly and prospectively during this phase I study. Moreover, while we agree that more follow-up CTs would make the data more robust, the observations obtained from 2 follow-up CTs obtained during treatment provide valuable information on both inter/intrafractional motion, and specifically, how that motion can affect delivered dose. Note that the follow-up CTs were taken at week 2 and week 5 of treatment, approximating both early and later time points during treatment.
Conclusion
Using 4DCT and CT scans acquired throughout a phase 1 dose escalation trial for locally advanced lung cancer, we performed both intra- and interfractional esophageal motion analyses and assessed its dosimetric impact in correlation with esophageal toxicity. The results suggested that intrafractional esophageal motion was small but interfractional motion can be significant. This can result in significant dosimetric changes, which in turn may lead to increased toxicity. Indeed, high V60 and NTCP (the averaged values based on all CTs that take esophageal motion into account) correlated well with high-grade esophageal toxicity. For select patients, adaptive planning may be necessary to limit unnecessary toxicity.
Abbreviations
- CT
computed tomography
- DIR
deformable image registration
- Dmax
maximum esophageal dose
- EUD
equivalent uniform dose
- NTCP
normal tissue complication probability
- PRV
planning organ at risk volume
- RT
radiotherapy
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Hao Gao, PhD  https://orcid.org/0000-0003-1064-2149
https://orcid.org/0000-0003-1064-2149
References
- 1. Dillman RO, Herndon J, Seagren SL, Eaton WL, Jr, Green MR. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996;88(17):1210–1215. [DOI] [PubMed] [Google Scholar]
- 2. Le Chevalier T, Arriagada R, Quoix E, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: first analysis of a randomized trial in 353 patients. J Natl Cancer Inst. 1991;83(6):417–423. [DOI] [PubMed] [Google Scholar]
- 3. Sause W, Kolesar P, Taylor SI, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: radiation therapy oncology group, eastern cooperative oncology group, and southwest oncology group. Chest. 2000;117(2):358–364. [DOI] [PubMed] [Google Scholar]
- 4. Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III nonsmall- cell lung cancer. J Clin Oncol. 1999;17(9):2692–2699. [DOI] [PubMed] [Google Scholar]
- 6. Ball D, Bishop J, Smith J, et al. A randomised phase III study of accelerated or standard fraction radiotherapy with or without concurrent carboplatin in inoperable non-small cell lung cancer: final report of an Australian multi-centre trial. Radiother Oncol. 1999;52(2):129–136. [DOI] [PubMed] [Google Scholar]
- 7. Saunders M, Dische S, Barrett A, Harvey A, Griffiths G, Palmar M. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: mature data from the randomized multicentre trial. chart steering committee. Radiother Oncol. 1999;52(2):137–148. [DOI] [PubMed] [Google Scholar]
- 8. Bonner JA, McGinnis WL, Stella PJ, et al. The possible advantage of hyperfractionated thoracic radiotherapy in the treatment of locally advanced nonsmall cell lung carcinoma: Results of a North Central Cancer Treatment Group Phase III Study. Cancer. 1998;82(6):1037–1048. [PubMed] [Google Scholar]
- 9. Baumann M, Herrmann T, Koch R, et al. Final results of the randomized phase III CHARTWEL-trial (ARO 97 -1) comparing hyperfractionated-accelerated versus conventionally fractionated radiotherapy in non-small cell lung cancer (NSCLC). Radiother Oncol. 2011;100(1):76–85. [DOI] [PubMed] [Google Scholar]
- 10. Belani CP, Wang W, Johnson DH, et al. Phase III study of the Eastern Cooperative Oncology Group (ECOG 2597): Induction chemotherapy followed by either standard thoracic radiotherapy or hyperfractionated accelerated radiotherapy for patients with unresectable stage IIIA and B non-small-cell lung cancer. J Clin Oncol. 2005;23(16):3760–3767. [DOI] [PubMed] [Google Scholar]
- 11. Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niedzielski J, Bluett JB, Williamson RT, Liao Z, Gomez DR, Court LE. Analysis of esophageal sparing treatment plans for patients with high-grade esophagitis. J Appl Clin Med Phys. 2013;14(4):4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boyle J, Ackerson B, Lin G, Kelsey CR. Dosimetric advantages of intensity modulated radiation therapy in locally advanced lung cancer. Adv Radiat Oncol. 2017;2(1):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelsey CR, Das S, Gu L, Dunphy FR, 3rd, Ready NE, Marks LB. Phase 1 dose escalation study of accelerated radiation therapy with concurrent chemotherapy for locally advanced lung cancer. Int J Radiat Oncol Biol Phys. 2015;93(5):997–1004. [DOI] [PubMed] [Google Scholar]
- 15. Dieleman EM, Senan S, Vincent A, Lagerwaard FJ, Slotman BJ, van Sörnsen de Koste JR. Four-dimensional computed tomographic analysis of esophageal mobility during normal respiration. Int J Radiat Oncol Biol Phys. 2007;67(3):775–780. [DOI] [PubMed] [Google Scholar]
- 16. Liao Z, Bucci MK, Komaki R, et al. Evaluation of respiratory-induced target motion for esophageal tumors at the gastroesophageal junction. Radiother Oncol. 2007;84(3):283–289. [DOI] [PubMed] [Google Scholar]
- 17. Yaremko BP, Guerrero TM, McAleer MF, et al. Determination of respiratory motion for distal esophagus cancer using four-dimensional computed tomography. Int J Radiat Oncol Biol Phys. 2008;70(1):145–153. [DOI] [PubMed] [Google Scholar]
- 18. Patel AA, Wolfgang JA, Niemierko A, Hong TS, Yock T, Choi NC. Implications of respiratory motion as measured by four-dimensional computed tomography for radiation treatment planning of esophageal cancer. Int J Radiat Oncol Biol Phys. 2009;74(1):290–296. [DOI] [PubMed] [Google Scholar]
- 19. Yamashita H, Kida S, Sakumi A, et al. Four-dimensional measurement of the displacement of internal fiducial markers during 320-multislice computed tomography scanning of thoracic esophageal cancer. Int J Radiat Oncol Biol Phys. 2011;79(2):588–595. [DOI] [PubMed] [Google Scholar]
- 20. Lever FM, Lips IM, Crijns SP, et al. Quantification of esophageal tumor motion on cine-magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2014;88(2):419–424. [DOI] [PubMed] [Google Scholar]
- 21. Chen YJ, Han C, Liu A, et al. Setup variations in radiotherapy of esophageal cancer: evaluation by daily megavoltage computed tomographic localization. Int J Radiat Oncol Biol Phys. 2007;68(5):1537–1545. [DOI] [PubMed] [Google Scholar]
- 22. Yamashita H, Haga A, Hayakawa Y, et al. Patient setup error and day-to-day esophageal motion error analyzed by cone-beam computed tomography in radiation therapy. Acta Oncologica. 2010;49(4):485–490. [DOI] [PubMed] [Google Scholar]
- 23. Wang J, Lin SH, Dong L, et al. Quantifying the interfractional displacement of the gastroesophageal junction during radiation therapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;83(2):e273–e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fukada J, Hanada T, Kawaguchi O, et al. Detection of esophageal fiducial marker displacement during radiation therapy with a 2-dimensional on-board imager: Analysis of internal margin for esophageal cancer. Int J Radiat Oncol Biol Phys. 2013;85(4):991–998. [DOI] [PubMed] [Google Scholar]
- 25. Pham AHN, Yorke E, Rimner A, et al. Potential for interfraction motion to increase esophageal toxicity in lung SBRT. Technol Cancer Res Treat. 2017. doi:10.1177/1533034617711353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res. 1985;104:S13–S19. [PubMed] [Google Scholar]
- 27. Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method gerald. Int J Radiat Oncol Biol Phys. 1989;16(6):1623–1630. [DOI] [PubMed] [Google Scholar]
- 28. Belderbos J, Heemsbergen W, Hoogeman M, Pengel K, Rossi M, Lebesque J. Acute esophageal toxicity in non-small cell lung cancer patients after high dose conformal radiotherapy. Radiother Oncol. 2005;75(2):157–164. [DOI] [PubMed] [Google Scholar]
- 29. Werner-Wasik M, Yorke E, Deasy J, Nam J, Marks LB. Radiation dose-volume effects in the esophagus. Int J Radiat Oncol Biol Phys. 2010;76(suppl 3):S86–S93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen RJ, Paskalev K, Litwin S, Price RA, Jr, Feigenberg SJ, Konski AA. Esophageal motion during radiotherapy: quantification and margin implications. Dis Esophagus. 2010;23(6):473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cai JF, Jia X, Gao H, Jiang SB, Shen Z, Zhao H. Cine cone beam CT reconstruction using low-rank matrix factorization: algorithm and a proof-of-principle study. IEEE Trans. Med. Imaging. 2014;33(8):1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao H, Zhang Y, Ren L, Yin FF. Principal component reconstruction (PCR) for cine CBCT with motion learning from 2D fluoroscopy. Med Phys. 2018;45(1):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]