Abstract
Background
Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are assumed to be prognostic factors in many diseases such as inflammatory diseases, cardiovascular diseases and cancer. However, NLR and PLR are race specific, it is important to determine the reference values of NLR and PLR in different races. The study aimed to investigate the reference range of NLR and PLR in Chinese Han population from Chaoshan region in South China.
Methods
A retrospective study was conducted in the First Affiliated Hospital of Shantou University Medical College in South China. Five thousand healthy adults aged 20–69 years were included. NLR and PLR were determined.
Results
Of 5000 healthy adults, 2500 men and 2500 women were included. The mean NLR and PLR across all ages for men and women were 1.59 ± 0.59, 92.88 ± 28.70, 1.62 ± 0.64 and 108.02 ± 32.99, respectively. The 95% reference range of NLR in normal male and female are 0.43~2.75 and 0.37~2.87, PLR are 36.63~149.13 and 43.36~172.68, respectively. The female had a higher NLR at age 30~49 than the male while the NLR at age 60~69 was higher in male than in female. The PLR was higher in female than in male.
Conclusion
The study provides reference data on NLR and PLR from different age and sex groups in South China. NLR and PLR varied with age and sex.
Keywords: Neutrophil-to-lymphocyte ratio, Platelet-to-lymphocyte ratio, Reference range
Background
Complete blood count (CBC) test is a simple economic and extensively used basic hematological test which mainly included white blood cell (WBC) count, red blood cell (RBC) count and platelet count. The most abundant white blood cells in healthy humans are neutrophils, which play important roles during acute and chronic inflammation and may be potential therapeutic targets in cardiovascular diseases [1]. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are the proportions of absolute neutrophil to lymphocyte and platelet to lymphocyte counts retrieved from the CBC test. As markers of inflammation, various studies have demonstrated the correlation between NLR, PLR and many diseases such as inflammatory diseases [2], cardiovascular diseases [3], cancer [4] and long-term type 2 diabetes remission after metabolic surgery [5]. NLR and PLR are assumed to be prognostic factors. Although there have been extensive investigations on NLR and PLR, the normal ranges of NLR and PLR were less investigated. It was reported that the average NLR is 2.15 in the US population [6] and 1.65 in South Korea [7], which suggested that NLR is race specific. Therefore it is important to investigate the ranges of NLR and PLR for evaluating the prognostic role of NLR and PLR in different races. The aim of this study is to explore the reference values of NLR and PLR among the Han populations in Chaoshan District of Guangdong Province in South China.
Methods
The study was conducted retrospectively in the First Affiliated Hospital of Shantou University Medical College in South China. CBC tests between February 2018 and July 2018 were reviewed and collected consecutively from healthy persons aged 20–69 years without diagnosed diseases including acute or chronic infection, heart failure, renal failure, autoimmune or hematopoietic diseases. The healthy adults were divided into groups according to gender and age. A total of 5000 healthy adults were included. Neutrophil, lymphocyte and platelet counts were determined by the Coulter method. NLR and PLR were calculated as the ratio of neutrophil cell and platelet count to lymphocyte cell count, respectively. The samples were excluded with WBC less than 3.5 × 109/L or more than 9.5 × 109/L and platelet less than 125 × 109/L or more than 350 × 109/L. The study was approved by the ethics committee of Shantou University Medical College.
Data are presented as mean ± SD. Differences between group means were assessed by an unpaired Student’s t-test for single comparisons or by ANOVA for multiple comparisons using SPSS 16.0. P value < 0.05 was considered significant.
Results
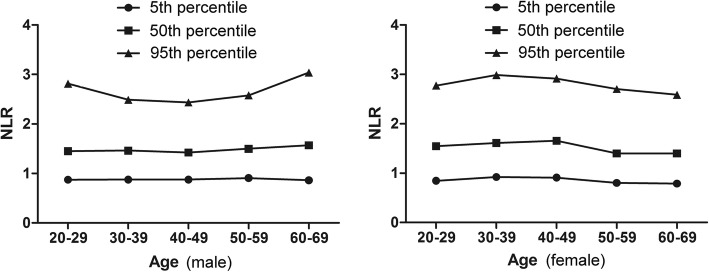
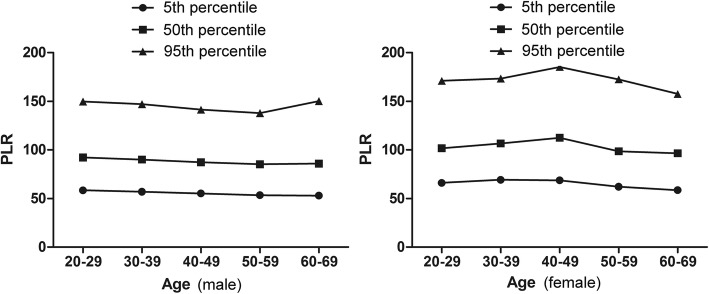
In present study, there are 5000 CBC tests. Two thousand five hundred men and 2500 women were included. As shown in the Table 1, the differences of age, WBC counts, neutrophils (NE) counts and RBC counts between the male and female were not significant. The male had a higher lymphocyte (LY) counts and hemoglobin (HGB) than the female while the female had a higher platelet (PLT) counts, NLR and PLR. The mean NLR and PLR across all ages for men and women were 1.59 ± 0.59, 92.88 ± 28.70, 1.62 ± 0.64 and 108.02 ± 32.99, respectively. The 95% reference range of NLR in normal male and female are 0.43~2.75 and 0.37~2.87, PLR are 36.63~149.13 and 43.36~172.68, respectively. NLR and PLR were analyzed based on sex and age (500 cases in each group), which were showed in Figs. 1 and 2 and Tables 2 and 3. The female had a higher NLR at age 30~49 than the male while the NLR at age 60~69 was higher in male than in female. The NLR was affected by age. In female the NLR increased with aging at age 20–49 while decreased in age groups of > 50 years. There is a sex difference in PLR at age 30~59, with higher in female than in male. The PLR decreased in women older than 50 years.
Table 1.
Main characteristics of the overall cohort based on sex
| Male | Female | P Value | |
|---|---|---|---|
| Number | 2500 | 2500 | |
| Age (years, mean ± SD) | 44.42 ± 14.02 | 44.45 ± 14.02 | 0.924 |
| Mean WBC (×109/L) | 6.55 ± 1.28 | 6.04 ± 1.25 | 0.059 |
| Mean NE (× 109/L) | 3.54 ± 0.93 | 3.34 ± 0.96 | 0.328 |
| Mean LY (× 109/L) | 2.35 ± 0.60 | 2.18 ± 0.56 | 0.001 |
| Mean NE/LY | 1.59 ± 0.59 | 1.62 ± 0.64 | 0.002 |
| 95% reference range | 0.43~2.75 | 0.37~2.87 | |
| Mean RBC (×109/L) | 4.96 ± 0.41 | 4.50 ± 0.40 | 0.186 |
| Mean HGB g/L | 152.88 ± 11.59 | 134.45 ± 12.76 | 0.013 |
| Mean PLT (×109/L) | 206.30 ± 40.81 | 222.22 ± 44.47 | 0.000 |
| Mean PLT/LY | 92.88 ± 28.70 | 108.02 ± 32.99 | 0.000 |
| 95% reference range | 36.63~149.13 | 43.36~172.68 |
Fig. 1.
The percentile nomogram for NLR in male and female
Fig. 2.
The percentile nomogram for PLR in male and female
Table 2.
Neutrophil-to-lymphocyte ratio at different groups
| Subgroup (age) | Neutrophil-to-lymphocyteratio (male) | Neutrophil-to-lymphocyteratio (female) | P value |
|---|---|---|---|
| 20~29 | 1.57 ± 0.61 | 1.64 ± 0.59 | 0.675 |
| 30~39 | 1.55 ± 0.56 | 1.72 ± 0.68# | 0.004 |
| 40~49 | 1.53 ± 0.52 | 1.74 ± 0.61# | 0.000 |
| 50~59 | 1.60 ± 0.55 | 1.52 ± 0.62## | 0.223 |
| 60~69 | 1.71 ± 0.68△ | 1.51 ± 0.64## | 0.005 |
△compared with group age 20~59 (P < 0.01) #compared with group age 20~29 (P < 0.05)
##compared with group age 20~49 (P < 0.01)
Table 3.
Platelet-to-lymphocyte ratio at different groups
| Subgroup (age) | Platelet-to-lymphocyte ratio (male) | Platelet-to-lymphocyte ratio (female) | P value |
|---|---|---|---|
| 20~29 | 96.16 ± 28.48 | 107.46 ± 31.15 | 0.176 |
| 30~39 | 94.91 ± 30.49 | 111.91 ± 32.93# | 0.017 |
| 40~49 | 91.84 ± 26.84 | 116.41 ± 34.49## * | 0.000 |
| 50~59 | 89.82 ± 26.72△ | 103.80 ± 32.83△△ | 0.001 |
| 60~69 | 91.67 ± 30.38 | 100.53 ± 31.11△△ | 0.922 |
△compared with group age 20~39 (P < 0.01) #compared with group age 20~29(P < 0.05)
△△compared with group age 30~49 (P < 0.01) ##compared with group age 30~39(P < 0.05)
*compared with group age 20~29 (P < 0.05)
Discussion
In present study we measured the NLR and PLR in 5000 Chinese healthy adults. We found that the 95% reference range of NLR in normal male and female are 0.43~2.75 and 0.37~2.87, PLR are 36.63~149.13 and 43.36~172.68, respectively. The NLR and PLR vary by sex and age.
CBC, an economic and extensively used basic hematological test, included a hemogram and differential WBC count. Though the CBC test was usually used to the diagnosis of anemia, certain cancers, infection and immunodeficiencies, it has been recently found that some parameter of CBC such as NLR and PLR are associated with activity, morbidity and mortality in different diseases. In active rheumatoid arthritis [8], systemic lupus erythematosus [9] and Takayasu’s arteritis [10], NLR and PLR were significantly increased than that in the control and can be used to evaluate disease activity. In patients with hepatocellular carcinoma after hepatectomy, postoperative NLR and PLR were associated with recurrence [11]. Additionally, admission NLR can be used to predict worse outcomes and hospital mortality in patients with acute type A aortic dissection [12–15].
Though NLR was used widely in many diseases, the cut off points for risk stratification were arbitrary used in these studies, which did not consider the factors affecting the NLR such as the disease category, age, and race of patients. In the studies from western countries a higher cutoff value was suggested than that in Asian or African. In fact the NLR in the United States population was generally higher than Asian races. It was reported that NLR is 2.24 in Whites and 1.76 in Blacks in the United States [6] while 1.65 in South Korea [7] and 1.72 in central China [16]. The effects of sex on NLR varied with race. There was no significant difference with NLR between in men and women in the United States population [6] while significant in Asian [7, 16]. In present study, we found that the mean NLR across all ages was higher in female than in male, which is consistent with studies in other Asian countries [7]. The mechanisms for sex-related differences in NLR are not well known. Sex hormones such as estrogen level may be attributed to the difference. The female had higher estrogen level than the male. It had been found that estrogen can delay neutrophil apoptosis [17], which leaded to higher NLR in female. Though NLR was different between sexes, a study from central China showed that it is higher in male than in female [16] while it is the reverse in present study, suggesting there is regional variations in NLR.
NLR can be also affected by age, especial in female. Estrogen decreased dramatically after menopause [18]. Thus, it was not surprising that the NLR in women is higher in age groups of < 50 years than > 50 years [7, 19], which was also verified in present study.
Unlike NLR, the PLR is less investigated. There is also a sex difference in PLR, with higher in women than in men [7]. The difference may be associated with the higher platelet counts in women. Many studies have found that females have higher platelet count than males [20–23]. The mechanisms of sex-related difference in platelet count are also not well known. One explanation is that there is lower serum iron in menstruating and elder women, which stimulates platelet production [24–26]. Additionally, sex hormonal difference such as estrogen level may be also play a role. It was reported that estrogens favour platelets formation in mouse [27]. Apart from sex, platelet count varies by age, being higher in youth than in old age [28, 29], which may be associated with hematopoietic stem cell. In elderly people a reduction in hematopoietic stem cell reserve would lead to reduction of the platelets formation.
Though the female has a higher PLR than the male, another study from central China showed no difference between gender groups in PLR [16], suggesting regional variation of PLR in China.
A few limitations were apparent in present study. First, the study is a retrospective study and routine blood analyses were collected from healthy populations in the checkup center of hospital, the effects of chronic concealed inflamation and smoking [30] on NLR and PLR can not be excluded. Secondly, owing to the geographic difference of platelet counts [21, 31], the reference range of PLR in healthy population in Chaoshan region may be different from other regions in China.
Conclusion
In summary, we found that the reference range of NLR and PLR in male was different from in female from Chaoshan region in South China. The NLR and PLR varied with age and sex.
Acknowledgements
Not applicable.
Abbreviations
- CBC
Complete blood count
- NLR
Neutrophil-to-lymphocyte ratio
- PLR
Platelet-to-lymphocyte ratio
- WBC
White blood cell
Authors’ contributions
LSW and SZ drafted the manuscript. CTW and XRT were involved in data collection. MY conceived of the study. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Raw data supporting the obtained results are available at the corresponding author.
Ethics approval and consent to participate
The study was approved by the ethics committee of Shantou University Medical College. The need for consent was waived because of the retrospective data. Dr. Min Yu granted administrative permissions to access the raw data.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lishan Wu and Shan Zou are equally to the work
Contributor Information
Lishan Wu, Email: stwulishan@sina.com.
Shan Zou, Email: stmedzou@163.com.
Cantian Wang, Email: cantianwang@sina.com.
Xuerui Tan, Email: stmedt2008@163.com.
Min Yu, Phone: +86-754-88905049, Email: 717146@sina.com.
References
- 1.Bonaventura A, Montecucco F, Dallegri F, Carbone F, Lüscher TF, Camici GG, et al. Novel findings in neutrophil biology and their impact on cardiovascular disease. Cardiovasc Res. 2019. 10.1093/cvr/cvz084 [Epub ahead of print]. [DOI] [PubMed]
- 2.Alan S, Tuna S, Türkoğlu EB. The relation of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume with the presence and severity of Behçet's syndrome. Kaohsiung J Med Sci. 2015;31(12):626–631. doi: 10.1016/j.kjms.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Zhang G, Jiang X, Zhu H, Lu Z, Xu L. Neutrophil to lymphocyte ratio in relation to risk of all-cause mortality and cardiovascular events among patients undergoing angiography or cardiac revascularization: a meta-analysis of observational studies. Atherosclerosis. 2014;234(1):206–213. doi: 10.1016/j.atherosclerosis.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Bowen RC, Little NAB, Harmer JR, Ma J, Mirabelli LG, Roller KD, et al. Neutrophil-to-lymphocyte ratio as prognostic indicator in gastrointestinal cancers: a systematic review and meta-analysis. Oncotarget. 2017;8(19):32171–32189. doi: 10.18632/oncotarget.16291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonaventura A, Liberale L, Carbone F, Vecchié A, Bonomi A, Scopinaro N, et al. Baseline neutrophil-to-lymphocyte ratio is associated with long-term T2D remission after metabolic surgery. Acta Diabetol. 2019. 10.1007/s00592-019-01345-2 Epub ahead of print. [DOI] [PubMed]
- 6.Azab B, Camacho-Rivera M, Taioli E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS One. 2014;9(11):e112361. doi: 10.1371/journal.pone.0112361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JS, Kim NY, Na SH, Youn YH, Shin CS. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine (Baltimore) 2018;97(26):e11138. doi: 10.1097/MD.0000000000011138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu H, Qin B, Hu Z, Ma N, Yang M, Wei T, et al. Neutrophil- and platelet-to-lymphocyte ratios are correlated with disease activity in rheumatoid arthritis. Clin Lab. 2015;61(3–4):269–273. doi: 10.7754/clin.lab.2014.140927. [DOI] [PubMed] [Google Scholar]
- 9.Qin B, Ma N, Tang Q, Wei T, Yang M, Fu H, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. 2016;26(3):372–376. doi: 10.3109/14397595.2015.1091136. [DOI] [PubMed] [Google Scholar]
- 10.Pan L, Du J, Li T, Liao H. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio associated with disease activity in patients with Takayasu's arteritis: a case-control study. BMJ Open. 2017;7(4):e014451. doi: 10.1136/bmjopen-2016-014451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Wen TF, Yan LN, Li B, Wang WT, Yang JY, et al. Postoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio predicts the outcomes of hepatocellular carcinoma. J Surg Res. 2015;198(1):73–79. doi: 10.1016/j.jss.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Lafçi G, Ciçek ÖF, Uzun HA, Yalçinkaya A, Diken Aİ, Turak O, et al. Relationship of admission neutrophil-to-lymphocyte ratio with in-hospital mortality in patients with acute type I aortic dissection. Turk J Med Sci. 2014;44(2):186–192. doi: 10.3906/sag-1301-136. [DOI] [PubMed] [Google Scholar]
- 13.Karakoyun S, Gürsoy MO, Akgün T, Öcal L, Kalçık M, Yesin M, et al. Neutrophil-lymphocyte ratio may predict in-hospital mortality in patients with acute type a aortic dissection. Herz. 2015;40(4):716–721. doi: 10.1007/s00059-014-4121-2. [DOI] [PubMed] [Google Scholar]
- 14.Sbarouni E, Georgiadou P, Analitis A, Voudris V. High neutrophil to lymphocyte ratio in type a acute aortic dissection facilitates diagnosis and predicts worse outcome. Expert Rev Mol Diagn. 2015;15(7):965–970. doi: 10.1586/14737159.2015.1042367. [DOI] [PubMed] [Google Scholar]
- 15.Kalkan ME, Kalkan AK, Gündeş A, Yanartaş M, Oztürk S, Gurbuz AS, et al. Neutrophil to lymphocyte ratio: a novel marker for predicting hospital mortality of patients with acute type a aortic dissection. Perfusion. 2017;32(4):321–327. doi: 10.1177/0267659115590625. [DOI] [PubMed] [Google Scholar]
- 16.Meng Xianchun, Chang Qian, Liu Yuying, Chen Ling, Wei Gaohui, Yang Jingjing, Zheng Peiguo, He Fucheng, Wang Wanhai, Ming Liang. Determinant roles of gender and age on SII, PLR, NLR, LMR and MLR and their reference intervals defining in Henan, China: A posteriori and big-data-based. Journal of Clinical Laboratory Analysis. 2017;32(2):e22228. doi: 10.1002/jcla.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9(12):911–922. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molloy EJ, O'Neill AJ, Grantham JJ, Sheridan-Pereira M, Fitzpatrick JM, Webb DW, et al. Sex-specific alterations in neutrophil apoptosis: the role of estradiol and progesterone. Blood. 2003;102(7):2653–2659. doi: 10.1182/blood-2003-02-0649. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Zhang Y, Zhao G, Chen C, Yang P, Ye S, et al. Difference in leukocyte composition between women before and after menopausal age, and distinct sexual dimorphism. PLoS One. 2016;11(9):e0162953. doi: 10.1371/journal.pone.0162953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bain BJ. Ethnic and sex differences in the total and differential white cell count and platelet count. J Clin Pathol. 1996;49(8):664–666. doi: 10.1136/jcp.49.8.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X, Zhao M, Pan B, Zhang J, Peng M, Wang L, et al. Complete blood count reference intervals for healthy Han Chinese adults. PLoS One. 2015;10(3):e0119669. doi: 10.1371/journal.pone.0119669.eCollection2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kibaya RS, Bautista CT, Sawe FK, Shaffer DN, Sateren WB, Scott PT, et al. Reference ranges for the clinical laboratory derived from a rural population in Kericho, Kenya. PLoS One. 2008;3(10):e3327. doi: 10.1371/journal.pone.0003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McIlhagger R, Gow AJ, Brett CE, Corley J, Taylor M, Deary IJ, et al. Differences in the haematological profile of healthy 70 year old men and women: normal ranges with confirmatory factor analysis. BMC Blood Disord. 2010;10:4. doi: 10.1186/1471-2326-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beguin Y. Erythropoietin and platelet production. Haematologica. 1999;84(6):541–547. [PubMed] [Google Scholar]
- 25.Kadikoylu G, Yavasoglu I, Bolaman Z, Senturk T. Platelet parameters in women with iron deficiency anemia. J Natl Med Assoc. 2006;98(3):398–402. [PMC free article] [PubMed] [Google Scholar]
- 26.Pirrie R. The influence of age upon serum iron in normal subjects. J Clin Pathol. 1952;5(1):10–15. doi: 10.1136/jcp.5.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagata Y, Yoshikawa J, Hashimoto A, Yamamoto M, Payne AH, Todokoro K. Proplatelet formation of megakaryocytes is triggered by autocrine-synthesized estradiol. Genes Dev. 2003;17(23):2864–2869. doi: 10.1101/gad.1128003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biino G, Santimone I, Minelli C, Sorice R, Frongia B, Traglia M, et al. Age- and sex-related variations in platelet count in Italy: a proposal of reference ranges based on 40987 subjects’ data. PLoS One. 2013;8(1):e54289. doi: 10.1371/journal.pone.0054289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biino G, Gasparini P, D'Adamo P, Ciullo M, Nutile T, Toniolo D, et al. Influence of age, sex and ethnicity on platelet count in five Italian geographic isolates: mild thrombocytopenia may be physiological. Br J Haematol. 2012;157(3):384–387. doi: 10.1111/j.1365-2141.2011.08981.x. [DOI] [PubMed] [Google Scholar]
- 30.Gumus F, Solak I, Eryilmaz MA. The effects of smoking on neutrophil/lymphocyte, platelet/ /lymphocyte ratios. Bratisl Lek Listy. 2018;119(2):116–119. doi: 10.4149/BLL_2018_023. [DOI] [PubMed] [Google Scholar]
- 31.Hong J, Min Z, Bai-shen P, Jie Z, Ming-ting P, Xian-zhang H, et al. Investigation on reference intervals and regional differences of platelet indices in healthy Chinese Han adults. J Clin Lab Anal. 2015;29(1):21–27. doi: 10.1002/jcla.21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data supporting the obtained results are available at the corresponding author.