Abstract
A mechanism that describes the progression of traumatic brain injury (TBI) to end-stage chronic traumatic encephalopathy (CTE) is offered in this article. This mechanism is based upon the observed increase in the concentration of both tau protein and of human leukocyte antigen (HLA) class I proteins; the HLA increase is expressed on the cell membrane of neural cells. These events follow the inflammatory responses caused by the repetitive TBI. Associated inflammatory changes include macrophage entry into the brain parenchyma from increased permeability of the blood-brain barrier (BBB) and microglial activation at the base of the sulci. The release of interferon gamma from the microglia and macrophages induces the marked increased expression of HLA class I proteins by the neural cells and subsequent redistribution of the tau proteins to the glial and neuronal surface. In those individuals with highly expressed HLA class I C, the high level of HLA binds tau protein electrostatically. The ionic region of HLA class I C (amino acid positions 50-90) binds to the oppositely charged ionic region of tau (amino acid positions 93-133). These interactions thereby shift the cellular localization of the tau and orient the tau spatially so that the cross-linking sites of tau (275-280 and 306-311) are aligned. This alignment facilitates the cross-linking of tau to form the intracellular and extracellular microfibrils of tau, the primary physiological characteristic of tauopathy. Following endocytosis of the membrane HLA/tau complex, these microfibrils accumulate and produce a tau-storage-like disease. Therefore, tauopathy is the secondary collateral process of brain injury, resulting from the substantial increase in tau and HLA expression on neural cells. This proposed mechanism suggests several potential targets for mitigating the clinical progression of TBI to CTE.
Keywords: Traumatic brain injury, chronic traumatic encephalopathy, major histocompatibility complex, Human Leukocyte Antigen, tauopathy, tau protein, electrostatic binding, protein-protein interaction
Introduction
Interest in the molecular mechanisms involved in the progression of traumatic brain injury (TBI) to end-stage disease of chronic traumatic encephalopathy (CTE), diagnosed at postmortem, has increased as a result of the significant numbers of athletes and military personnel exhibiting signs of cognitive deficits and behavioral disabilities at an early age (second to fourth decade of life).1–3 TBI is the diagnosis given when the living patient presents with clinical signs following closed or penetrating head injuries from forceful head and abdominal impacts.1,4–7 Our consideration will focus on closed head injuries. Chronic traumatic encephalopathy, on the other hand, is the end-stage manifestation of TBI as determined from postmortem studies on brain tissue.2,8–12
Although TBI may occur as a result of a single forceful impact, the primary initiators of TBI are most often repetitive forceful impacts and subsequent rapid deceleration of the head and abdomen. There is a clinically significant variation in the symptomatic development of TBI due to external factors, such as the magnitude of the force of the impact, the number of such impacts, and the interval between these impacts.1,2,8,11,13 Earlier reports from our laboratory indicate that the development of persistent TBI results from an interaction between intrinsic vulnerability factors of the host to brain injury and the external forces acting on the head and abdomen.14,15 The internal susceptibility factors include a predisposition of the host to autoimmune disease,7,14 arteriovenous malformations, the degree of neuronal connectivity of the brain as indicated by fractional anisotropy determined from magnetic resonance imaging (MRI) studies,16 as well as the metabolic oxidative capacity of the brain.17,18
With repetitive non-penetrating forceful impacts on the head and abdomen, the clinical signs of confusion, vestibular imbalance, photophobia, loss of focus, and situation awareness increase markedly over a period of 10 to 20 years.11,12,19 The clinical signs include dementia, mood instability in the form of rage, anger, and depression, which become dominant factors in the patient’s ability to function socially.1,3,12,19,20
Studies on animals which experienced forceful head and abdominal impacts reveal a series of physiological changes including the release of macrophages and leukocytes from the spleen,7,21,22 increased permeability of the blood-brain barrier (BBB),23,24 entry of the splenic cells into the brain parenchyma, release of gamma interferon from microglia (an inflammatory marker), and the subsequent marked increased expression of human leukocyte antigen (HLA)/major histocompatibility complex (MHC) markers on neurons and glial cells, resulting in the silencing and death of the nerve cells.25,26 At the time of the impact, increased shearing force at the base of the sulci causes injury to the membranes of the neuronal and glial cells. This results in the leakage of proteins from these cells into the cerebrospinal fluid and serum, thereby causing the body to produce an immune response to the released neural proteins. The repetition of such forceful impacts causes an anamnestic response to the neural antigens that are not present in other organ systems. This anamnestic response entails the generation of antibodies against self proteins (eg, glial fibrillary acid protein, neuron-specific enolase, S-100, myelin basic protein, and neurofilament proteins, among others). The increased permeability of the BBB also results in an inflammatory response to the injury following forceful impacts to the head and abdomen.14,26
Studies of the pathology of end-stage TBI8,11,27 have identified several characteristics that define the postmortem state of the brain. One of the most prominent signs is an accumulation of a protein, tau protein, involved in stabilizing the structure of microtubules of neurons. There is a marked increased expression of a second protein, HLA/MHC, on neural cells resulting from inflammatory responses by microglia and the release of gamma interferon.25,26,28 The tau protein is a microtubule-associated protein (MAP tau) that binds the tubulin protein components of the microtubules and thereby maintains the stability and structure of the tubules. These tubules serve as pathways for the transport of macromolecules from the cell body to the distal parts of the neuron (dendrite and axon terminals).29
With the observation that there are marked increases in MHC levels and tau levels in neural cells observed in postmortem tissue from patients diagnosed with CTE, the question arose whether these 2 proteins interact with each other in vivo and through this interaction lead to increased deposition of tau on the cell surface and within the affected cells.19 To answer this question, the major insight in developing the proposed model was the recognition that electrostatic forces (Coulombic forces) are the major driver in protein-protein interactions. The mechanism by which structural changes in the brain resulting from TBI transition to those characteristics of the postmortem brain seen in end-stage disease (ie, CTE) is the focus of this article.
Method of Analysis
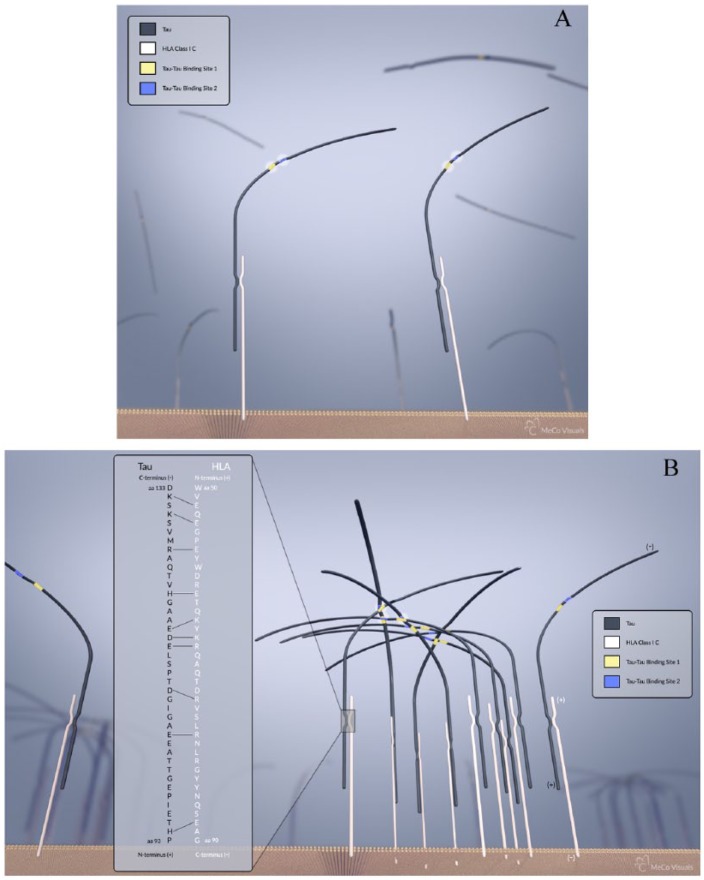
It has been demonstrated that polar group interactions are the primary force leading to stable interactions between 2 proteins.30,31 Xu et al described a positive and significant correlation between binding affinity and the number of ionic interactions at the interface of 2 interacting proteins. A measure of this attraction between oppositely charged ions is that the ionic (Coulombic) forces diminish as a function of the square of the distance between the charges, whereas van der Waals forces diminish as a sixth power of the distance (magnitudes weaker per dipole). There is significant literature demonstrating the interaction of cells with polyelectrolyte polypeptides.32–34 As a result of this background, the authors explored whether there were strong ionic pairings identifiable on class I MHC and tau protein. The amino acid sequences of tau35 and of HLA class I/MHC36 are known. Upon inspection of the amino acid sequences of these 2 proteins, the authors identified a 40-amino acid sequence on each of these 2 proteins that had 10 ionic amino acid pairs that could be aligned to form a strong protein-protein coupling. The alignment was facilitated by the recognition that there were 3 adjacent anionic amino acids (glutamate, aspartate, glutamate) on tau protein (positions 115, 116, 117) that could bind to 3 cationic amino acids (arginine, lysine, lysine) on HLA class I C positions (65, 67, 68) as shown in Figure 1B. The remaining 7 ionic pairs were readily identified from the known amino acid sequences of these proteins. The binding site of the MHC class I was on the extracellular surface of the cell toward the amino terminal end. The binding sequence of the tau protein was also toward the amino terminal end of that protein.
Figure 1.
(A) Illustration of neural membrane with low levels of HLA class I C expression. The HLA protein is depicted in white, the tau protein in black, and the membrane bilayer in orange. Tau has 2 sites that serve in binding other tau (yellow) and (blue). (B) Illustration of a neural membrane with high levels of expression of HLA class I C resulting from interferon gamma release from microglia. As a result of high HLA expression, the tau protein binds at a fixed height above the membrane, permitting binding of large amounts of tau to HLA and close proximity of tau to form cross-links with other tau. The cross-linked tau form a web-like structure seen in tauopathy. The 2 sites of cross-linking are differentiated using colors as shown. In addition, the 40-amino acid sequence that engages in electrostatic interactions between the HLA class I C and tau is shown in the inset. The 10 electrostatic interactions between amino acid pairings are highlighted with solid lines as shown. The positions of the amino acids on both the N- and C-terminal regions are presented. In the area of the HLA and tau interaction, the amino proximate end and the carboxy end are shown. Blurred HLA/tau represent these molecules at a plane further from the leading membrane edge. The HLA and tau proteins are depicted in a linear form rather than in alpha helical or beta pleated sheet conformation. HLA indicates human leukocyte antigen.
Results
Binding of the tau protein to the HLA class I C and the HLA class II DR beta
The amino acid sequence of HLA class I has been described by Reche and Reinherz,36 whereas the sequence of tau protein has been described by Kolarova et al.35 We propose that there is a 40-amino acid sequence on the HLA class I C that binds to a 40-amino acid sequence on the tau protein via the electrostatic binding of 10 amino acid binding pairs. These electrostatic interactions are located with the amino acid positions 50 to 90 of the HLA class I sequence, and the amino acid sequence from 93 to 133 of the tau protein. This is illustrated in Figure 1. Figure 1A illustrates the condition of low HLA expression present prior to inflammatory events associated with TBI. In this case, the tau proteins bound to HLA are too distant to form cross-links. Figure 1B illustrates the condition following repetitive forceful head impacts leading to inflammatory responses and the subsequent high expression of HLA, thereby bringing the bound tau proteins into close proximity where they become cross-linked. It is important to note that the 2 6-amino-acid-long cross-linking sites on tau protein (Val, Gln, Ileu, Ileu, Asn, Lys) and (Val, Gln, Ileu, Val, Tyr, Lys) that leads to the formation of tau microfibrils37 are located at amino acid positions 275 to 280 and 306 to 311, respectively.38 The binding site of the tau to the HLA class I is therefore approximately 140 amino acids distant from the cross-linking sites of the tau protein. The cross-linking sites within tau are approximately 30 amino acids apart. As a result of the binding of HLA to the planar surface of the neuronal membrane, there is a fixed alignment of HLA to tau. Steric hindrance does not impede the interactions of tau with HLA class I because there is a constant distance between the binding site of tau to HLA and the 2 cross-linking sites of tau. The HLA is attached to the membrane through a 20-transmembrane amino acid sequence. This consistent alignment between adjacent tau proteins, in addition to the close proximity between tau as a result of an increase in membrane-bound HLA expression, results in the formation of intricate tau microfibril formations, the primary clinical feature of tauopathy. This linear orientation of the binding of tau to HLA class I C, as well as the tau-to-tau interaction in microfibril tangles, is illustrated in Figure 1B.
In addition, there is a 29-amino acid sequence on the HLA class II DR beta that can bind to a 29-amino acid sequence on the tau protein through ionic pairs. The HLA DR sequence39 extends from amino acid position 65 to position 94, whereas the tau sequence is from position 93 to 122. The 3 adjacent anionic amino acids on tau (positions 115, 116, 117) are bound to 3 adjacent cationic amino acids (arginine, lysine, arginine) of HLA class II DR at positions 70, 71, and 72. In this domain of 29 amino acids, there are 7 electrostatic binding pairs that provide significant orientation of the tau protein on the membrane surface. The portion of the HLA that binds the tau is on the extracellular surface of the neuronal membrane. This location enables the binding of the extracellular tau40,41 to the HLA extracellular domain.
Endocytosis of HLA/tau complexes
It has been demonstrated that the clustering of class I MHC/HLA on cell membranes leads to endocytosis of the HLA-bound sites and ultimate fusion of the membrane vesicles with lysosomal bodies and endoplasmic reticulum.42 The induced clustering of HLA class II DR also leads to endocytosis of the HLA on the membrane into intracellular vesicles.43 As a result of this mechanism, the authors postulate that the cell-surface-bound tau protein and the intracellular accumulation of tau protein observed in TBI and CTE result from the binding of extracellular tau to the HLA/MHC and subsequent endocytosis of the HLA clusters. Electron microscopic studies have demonstrated the accumulation of microfibrillary tau and phosphorylated tau in intracellular compartments within neural cells.19,43,44 Such cross-linking also precludes the recycling of the HLA region back to the membrane.45 The cross-linked tau stabilizes the tau within the endoplasmic reticulum and lysosome and diminishes the recycling of the HLA vesicles with the plasmalemma.45 The transition of TBI to CTE is proposed here to result in the increased expression of HLA and tau protein complexes and the accumulation of HLA/tau complexes in neural cells. This increased accumulation of fibrillar aggregates that block recycling and are resistant to protease degradation in cells leads to cell dysfunction and death over protracted time periods. The phenomenon can be visualized as a tau storage disease, analogous to lipid storage disease such as Tay-Sachs disease.46
Summary
A mechanism that describes the progression of TBI to end-stage CTE is offered in this article. This mechanism is based upon the observed increase in the concentration of tau protein and of HLA class I proteins; the increased HLA is expressed on the cell membrane of neural cells. These events follow the inflammatory responses caused by the repetitive TBI. The impact-induced shear, at the gray matter interface with the white matter, increases microbleeds that are detected as hematin deposits. The associated inflammatory changes include macrophage entry into the brain parenchyma from increased permeability of the BBB and microglial activation at the base of the sulci. The release of interferon gamma from the microglia and macrophages induces the marked increased expression of HLA class I proteins by the neural cells and subsequent redistribution of the tau proteins to the glial and neuronal surface. In those individuals with highly expressed HLA class I C, the high level of HLA binds tau protein electrostatically. The ionic region of HLA class I C (amino acid positions 50-90) binds to the oppositely charged ionic region of tau (amino acid positions 93 to 133). These interactions thereby shift the cellular localization of the tau and orient the tau spatially so that the cross-linking sites of tau (275-280 and 306-311) are aligned. This alignment facilitates the cross-linking of tau to form the intracellular and extracellular microfibrils of tau, the primary physiological characteristic of tauopathy. Following endocytosis of the membrane HLA/tau complex, these microfibrils accumulate and produce a tau-storage-like disease. Therefore, tauopathy is the secondary collateral process of brain injury, resulting from the substantial increase in HLA expression on neural cells. The proposed mechanism provides several potential targets for mitigating the clinical progression of TBI to CTE.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MD and SK contributed in similar manner to the conception of the report, the data acquisition and the writing of the manuscript.
ORCID iD: Steven Kornguth  https://orcid.org/0000-0002-3709-0359
https://orcid.org/0000-0002-3709-0359
References
- 1. DeKosky ST, Ikonomovic MD, Gandy S. Traumatic brain injury: football, warfare, and long-term effects. N Engl J Med. 2010;363:1293–1296. [DOI] [PubMed] [Google Scholar]
- 2. Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4:134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358:453–463. [DOI] [PubMed] [Google Scholar]
- 4. Mansell JL, Tierney RT, Higgins M, McDevitt J, Toone N, Glutting J. Concussive signs and symptoms following head impacts in collegiate athletes. Brain Inj. 2010;24:1070–1074. [DOI] [PubMed] [Google Scholar]
- 5. Shahim P, Tegner Y, Gustafsson B, et al. Neurochemical aftermath of repetitive mild traumatic brain injury. JAMA Neurol. 2016;73:1308–1315. [DOI] [PubMed] [Google Scholar]
- 6. Shahim P, Gren M, Liman V, et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep. 2016;6:36791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nizamutdinov D, Shapiro LA. Overview of traumatic brain injury: an immunological context. Brain Sci. 2017;7:E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA. 2017;318:360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shively S, Scher AI, Perl DP, Diaz-Arrastia R. Dementia resulting from traumatic brain injury: what is the pathology? Arch Neurol. 2012;69:1245–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeKosky ST, Kochanek PM, Clark RS, Ciallella JR, Dixon CE. Secondary injury after head trauma: subacute and long-term mechanisms. Semin Clin Neuropsychiatry. 1998;3:176–185. [PubMed] [Google Scholar]
- 11. McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015;25:350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Omalu B, Bailes J, Hamilton RL, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery. 2011;69:173–183; discussion 183. [DOI] [PubMed] [Google Scholar]
- 13. Zhang TG, Satapathy SS, Dagro AM, McKee PJ. Numerical study of head/helmet interaction due to blast loading. Paper presented at: ASME 2013 International Mechanical Engineering Congress & Exposition; November 15-21, 2013; San Diego, CA. [Google Scholar]
- 14. Kornguth S, Rutledge N, Perlaza G, Bray J, Hardin A. A proposed mechanism for development of CTE following concussive events: head impact, water hammer injury, neurofilament release, and autoimmune processes. Brain Sci. 2017;7:E164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kornguth S, Rutledge N. Integration of biomarkers into a signature profile of persistent traumatic brain injury involving autoimmune processes following water hammer injury from repetitive head impacts. Biomark Insights. 2018;13:808216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kornguth S, Steinberg R, Schnyer D, Trujillo L. Integrating the human into the total system: degradation of performance under stress. Nav Eng J. 2013;125:85–90. [Google Scholar]
- 17. Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J. SARM1 activation triggers axon degeneration locally via NAD(+) destruction. Science. 2015;348:453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Essuman K, Summers DW, Sasaki Y, Mao X, DiAntonio A, Milbrandt J. The SARM1 Toll/interleukin-1 receptor domain possesses intrinsic NAD(+) cleavage activity that promotes pathological axonal degeneration. Neuron. 2017;93:1334e5–1343.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McKee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol. 2015;127:45–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanielian TL, Jaycox L; RAND Corporation. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008:xliii, 453. [Google Scholar]
- 21. Kim CC, Nakamura MC, Hsieh CL. Brain trauma elicits non-canonical macrophage activation states. J Neuroinflammation. 2016;13:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao H. The role of spleen-derived immune cells in ischemic brain injury. In: Chen J, Zhang J, Hu X, eds. Non-neuronal Mechanisms of Brain Damage and Repair After Stroke. Cham: Springer; 2016:189–199. [Google Scholar]
- 23. Gonzalez-Marrero I, Castaneyra-Ruiz L, Gonzalez-Toledo JM, et al. High blood pressure effects on the blood to cerebrospinal fluid barrier and cerebrospinal fluid protein composition: a two-dimensional electrophoresis study in spontaneously hypertensive rats. Int J Hypertens. 2013;2013:164653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito U, Ohno K, Yamaguchi T, Takei H, Tomita H, Inaba Y. Effect of hypertension on blood-brain barrier. Change after restoration of blood flow in post-ischemic gerbil brains. An electron microscopic study. Stroke. 1980;11:606–611. [DOI] [PubMed] [Google Scholar]
- 25. Neumann H, Cavalie A, Jenne DE, Wekerle H. Induction of MHC class I genes in neurons. Science. 1995;269:549–552. [DOI] [PubMed] [Google Scholar]
- 26. Neumann H, Schmidt H, Cavalie A, Jenne D, Wekerle H. Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-gamma and tumor necrosis factor (TNF)-alpha. J Exp Med. 1997;185:305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10:S242–S253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kornguth S, Bersu E, Mack K. MHC class I gene expression. Science. 1995;270:720–721. [PubMed] [Google Scholar]
- 29. Weingarten MD, Lockwood AH, Hwo SY, Kirschner M. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72:1858–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sheinerman FB, Norel R, Honig B. Electrostatic aspects of protein-protein interactions. Curr Opin Struct Biol. 2000;10:153–159. [DOI] [PubMed] [Google Scholar]
- 31. Xu D, Lin SL, Nussinov R. Protein binding versus protein folding: the role of hydrophilic bridges in protein associations. J Mol Biol. 1997;265:68–84. [DOI] [PubMed] [Google Scholar]
- 32. Kornguth S, Anderson M, Turski P, et al. Glioblastoma multiforme: MR imaging at 1.5 and 9.4 T after injection of polylysine-DTPA-Gd in rats. AJNR Am J Neuroradiol. 1990;11:313–318. [PMC free article] [PubMed] [Google Scholar]
- 33. Kornguth SE, Kalinke T, Robins HI, Cohen JD, Turski P. Preferential binding of radiolabeled poly-L-lysines to C6 and U87 MG glioblastomas compared with endothelial cells in vitro. Cancer Res. 1989;49:6390–6395. [PubMed] [Google Scholar]
- 34. Kornguth SE, Stahmann MA. Effect of polylysine on the leakage and retention of compounds by Ehrlich ascites tumor cells. Cancer Res. 1961;21:907–912. [PubMed] [Google Scholar]
- 35. Kolarova M, Garcia-Sierra F, Bartos A, Ricny J, Ripova D. Structure and pathology of tau protein in Alzheimer disease. Int J Alzheimers Dis. 2012;2012:731526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reche PA, Reinherz EL. Sequence variability analysis of human class I and class II MHC molecules: functional and structural correlates of amino acid polymorphisms. J Mol Biol. 2003;331:623–641. [DOI] [PubMed] [Google Scholar]
- 37. Giannetti A, Lindwall G, Kohlstaedt L, Chau MF, Radeke M, Feinstein S. Fibers of tau fragments, but not full length tau, exhibit a cross β-structure: implications for the formation of paired helical filaments. Protein Sci. 2000;9:2427–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simic G, Babic Leko M, Wray S, et al. Tau protein hyperphosphorylation and aggregation in Alzheimer’s disease and other tauopathies, and possible neuroprotective strategies. Biomolecules. 2016;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Larhammar D, Schenning L, Gustafsson K, et al. Complete amino acid sequence of an HLA-DR antigen-like beta chain as predicted from the nucleotide sequence: similarities with immunoglobulins and HLA-A, -B, and -C antigens. Proc Natl Acad Sci U S A. 1982;79:3687–3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamada K, Holth JK, Liao F, et al. Neuronal activity regulates extracellular tau in vivo. J Exp Med. 2014;211:387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gomez-Ramos A, Diaz-Hernandez M, Cuadros R, Hernandez F, Avila J. Extracellular tau is toxic to neuronal cells. FEBS Lett. 2006;580:4842–4850. [DOI] [PubMed] [Google Scholar]
- 42. Huet C, Ash JF, Singer SJ. The antibody-induced clustering and endocytosis of HLA antigens on cultured human fibroblasts. Cell. 1980;21:429–438. [DOI] [PubMed] [Google Scholar]
- 43. Karagiannis SN, Warrack JK, Jennings KH, et al. Endocytosis and recycling of the complex between CD23 and HLA-DR in human B cells. Immunology. 2001;103:319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perreault S, Bousquet O, Lauzon M, Paiement J, Leclerc N. Increased association between rough endoplasmic reticulum membranes and mitochondria in transgenic mice that express P301L tau. J Neuropathol Exp Neurol. 2009;68:503–514. [DOI] [PubMed] [Google Scholar]
- 45. Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer’s disease. J Cell Biol. 1998;143:777–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Terry RD, Weiss M., Studies in. Tay-Sachs disease. II. Ultrastructure of the cerebrum. J Neuropathol Exp Neurol. 1963;22:18–55. [DOI] [PubMed] [Google Scholar]