Abstract
Background
Statins may increase the risk of intracerebral haemorrhage (ICH) in individuals with previous stroke. It remains unclear whether this applies to individuals with no history of stroke. This study is the first to explore the statin-associated risk of ICH in stroke-free individuals while considering the timing of statin initiation.
Methods
We conducted a population-based, propensity score matched cohort study using information from five Danish national registers. We included all stroke-free individuals initiating statins in 2004–2013 and a propensity score matched group of non-users. Adjusted hazard ratios (aHRs) for ICH risk among statin users compared to non-users were calculated as a function of time since statin initiation.
Findings
519,894 stroke-free individuals initiating statins and their 1:5 matched stroke-free reference subjects were included and followed for up to ten years. During this period, 1409 ICHs occurred in statin users. Statin users had an overall aHR of 0.85 (95% confidence interval: 0.80–0.90) compared to non-users, but this risk was modified by time since statin initiation. Statin users and non-users had similar ICH risk during the first six months after statin initiation. Hereafter, statin users had a 22–35% lower risk throughout the study period.
Interpretation
Statin users had lower ICH risk than non-users from six months after statin initiation. This finding could not be explained by healthy initiator bias or differences between users and non-users in terms of sociodemographic characteristics, comorbidity, or parallel treatment regimens. Our study suggests that statin use in stroke-free populations is associated with reduced ICH risk.
Funding
The Novo Nordisk Foundation.
Keywords: Intracerebral haemorrhage, Ischaemic stroke, Statins, Epidemiology
Research in context
Evidence before this study
PubMed and Embase were systematically searched for all studies investigating the risk of intracerebral haemorrhage (ICH) associated with use of statins. We searched for studies in English published up until July 2018, and found no previous studies that consider the significance of the timing of statin initiation in stroke-free individuals. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial suggested that statins may increase the risk of ICH in individuals with previous stroke, but this could not be confirmed by two meta-analyses. Previous studies on statin-associated risk of ICH in stroke-free populations have been limited by small sample size, lack of generalisability due to selected populations, and no consideration of the potential significance of time since statin initiation. An association between even slightly increased ICH risk and statin intake in individuals with no prior history of stroke would have major public health implications. Nevertheless, the question of whether statins increase the risk of ICH in a general population of stroke-free individuals remains unanswered.
Added value of this study
To our knowledge, this is the first large-scale population-based propensity score matched cohort study to evaluate the association between statin initiation and risk of ICH in individuals with no history of stroke while considering the influence of the timing of statin initiation. We included 519,894 individuals who initiated statins and followed them for up to ten years (2004–2013). Statin users and non-users had similar ICH risk during the first six months after statin initiation. Hereafter, statin users had a 22–35% lower risk throughout the study period. Our analysis on time since statin initiation allowed us to conclude that our findings were not explained by better health status in individuals initiating statins than in non-users. Compared to non-users, statin users had similar ICH risk (and increased risk of ischaemic stroke) instantaneously after statin initiation. Moreover, the reduction in ICH risk could not be explained by differences between users and non-users in terms of sociodemographic factors, comorbidity, or concomitant initiation of other selected agents that may influence risk of stroke.
Implications of all the available evidence
Our study suggests that statin use is associated with a reduced risk of ICH in stroke-free populations. These findings may have major public health implications for the vast population of statin users with no history of stroke.
Alt-text: Unlabelled Box
1. Introduction
Statins are associated with lower risk of ischaemic stroke (IS) [1], [2], [3]. However, post-hoc analyses of data from the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial suggested that statins may increase the risk of intracerebral haemorrhage (ICH) in individuals with previous stroke [1], [4], but these results could not be confirmed by two meta-analyses [5], [6] or a large register-based study [7]. Although clinical guidelines report to have insufficient data to recommend restrictions on the use of statins in ICH patients [8], a precautionary principle has been proposed; this principle advises against initiating statins in patients with a sustained ICH [9], [10] unless there are clear indications for secondary prevention of ischaemic events [10].
Either scenarios of even slightly increased risk of ICH associated with statins in individuals with no prior stroke or unfounded statin prescribing reticence for this group would have major public health implications. Nevertheless, the association between statins and ICH in a general population of stroke-free individuals remains unclear. Previous studies have been limited by small samples [3], [5], [6], [11], [12], [13], [14], lack of generalisability due to selected populations [5], [6], and lack of opportunity to study the significance of time since statin initiation [3], [11], [12], [13], [14].
To our knowledge, this large-scale population-based cohort study, which is based on data from 519,894 individuals initiating statins in 2004–2013 and followed for up to ten years, is the first to evaluate the association between statin initiation and risk of ICH among individuals with no history of stroke while taking into account time since statin initiation.
2. Methods
2.1. Study Design and Setting
We conducted a propensity score matched cohort study using information from Danish nationwide registers. The source population included all individuals in Denmark above the age of 50 years, living in Denmark since 1980, alive on 1 January 2004, and with no history of stroke since 1995. Hereof, the study population included all individuals who initiated statin treatment from 2004 through 2013 and a 1:5 matched reference group of non-users. Matching was performed on the day of statin initiation for exposed individuals (index date).
2.2. Data Sources
We obtained data on sex, age, cohabitation status, emigration, and death from the Danish Civil Registration System [15], data on education and income from Statistics Denmark [16], data on hospital diagnoses and examinations from the Danish National Patient Register (DNPR) [17], data on psychiatric hospital diagnoses from the Danish Psychiatric Central Research Register [18], and data on medication prescriptions from the Danish National Prescription Register [19]. All data on stroke diagnoses were drawn from public hospitals in Denmark, which must all report to the DNPR. Private hospitals account for less than 1% of the total number of beds and do not provide acute care in Denmark [20]. All data were accurately linked at the personal level using the unique personal identification number (encrypted by a third party) assigned to all Danes at birth or immigration [21]. A detailed description of the registers has been described elsewhere [22]. The study was approved by the Danish Data Protection Agency, the Danish Health Data Authority, and Statistics Denmark. Ethical approval and informed consent were not needed, as all personal identification numbers were encrypted by Statistics Denmark prior to analysis.
2.3. Stroke
Our primary outcome of interest was ICH, but the temporal statin-associated risk of IS was also included as a reference measure for the general health of the study population. Therefore, we defined ‘incident stroke’ as a primary diagnosis of ICH (ICD-10: I61) or IS (including unspecified stroke) (ICD-10: I63 and I64) or a secondary diagnosis of ICH/IS along with a primary diagnosis of rehabilitation (ICD-10: Z50) during an inpatient or outpatient hospital contact (excluding emergency room contacts). We identified all cases of incident stroke with a registered code for brain scan with magnetic resonance imaging (MRI) or computed tomography (CT) within seven days before or after registration of the stroke diagnosis (Appendix 1 and 2). If an individual had both an IS and an ICH stroke diagnosis within a period of 14 days, this outcome was included in the IS category. We defined ‘previous stroke’ as a primary or secondary diagnosis of ICH or IS (including unspecified stroke) during an inpatient, outpatient, or emergency room hospital contact.
2.4. Exposure
We identified all redemptions of statin prescriptions from any Danish pharmacy since 1995 (Appendix 3). We considered individuals to be continuous ‘users’ if refilling their statin prescription before the discontinuation date, which was defined as the last redemption date plus a number of days corresponding to the number of redeemed pills plus a grace period of 33% extra days to include some leeway when refilling prescriptions [23]. The user period was extended each time a user redeemed a new prescription. After ending a period of use, individuals were suspended for a period of 365 days (and this ‘wash-out period’ was reset in case of a new redemption) before considered statin-free. Thus, these individuals could serve as references or once again be included due to initiation of a new treatment course [23].
2.5. Covariates
The following sociodemographic factors were included at index date: age, sex, cohabitation status, education, and income (Appendix 4). All diagnoses relevant for the risk of stroke were assessed at index date by a modified version of a previously developed algorithm (Appendix 5) [24]. The following medical, mental, and neurological comorbidities were included: hypertension, atrial fibrillation (AF), ischaemic heart disease, congestive heart failure, peripheral artery occlusive disease, cerebrovascular disease, diabetes, chronic obstructive pulmonary disease, chronic liver disease, coagulation defects, anaemia, cancer, epilepsy, Parkinson's disease, mood, stress and anxiety-related disorder, alcohol problems, substance abuse, bipolar affective disorder, schizophrenia/schizoaffective disorder, and dementia. We assessed whether individuals had redeemed prescriptions for agents that could influence stroke risk within 120 days before index date (Appendix 6). The following medications were included: antithrombotic agents (i.e., anticoagulant and antiplatelet agents), antihypertensive agents and other selected agents that could influence stroke risk (i.e., non-steroidal anti-inflammatory drugs (NSAIDs), systemic glucocorticoids, and selective serotonin reuptake inhibitors (SSRIs)). Use of these agents was divided into ‘newly initiated’ if treatment was initiated within 120 days before index date and ‘long-term use’ if initiated more than 120 days before index date.
2.6. Statistical Analysis
To account for non-random assignment of statin treatment in the study population, individuals initiating statins were primarily matched with non-users on age, sex, and calendar period, and they were subsequently matched on a continuously updated propensity score (i.e., propensity for initiating statin treatment). The propensity score was estimated in a Poisson model, which included information on socioeconomic factors and comorbidities based on information of the source population [25]. To avoid introducing bias, matching was performed in such a way that non-users could serve as references for more than one statin user [26].
Using Cox regression models stratified on matched groups, we calculated hazard ratios (HRs) for the risk of ICH among statin users compared to non-users. The HRs were adjusted in five nested models. The first model adjusted separately for each of the components of the propensity score and included intrinsic adjustment for age, sex, and calendar period imposed by the stratified Cox regression model. The second model additionally adjusted for antithrombotic agents. The third model additionally adjusted for antihypertensive agents. The fourth model additionally adjusted for other selected agents that could influence stroke risk, and the fifth model additionally adjusted for the interaction between AF and anticoagulant agents and between hypertension (diagnosis) and antihypertensive agents.
We plotted the fully adjusted HRs (aHRs) for the risk of ICH among statin users compared to non-users as a function of time since statin initiation. In addition, this risk was evaluated in strata of use of other medications (also handled time-dependently) to assess whether the risk of ICH associated with statin use could be affected by concomitant initiation of other agents that could influence the risk of stroke.
Supplementary analyses on risk of IS associated with statins were also included as a reference outcome. We calculated aHRs in five nested models and plotted the fully adjusted HRs as a function of time since statin initiation for risk of IS among statin users compared to non-users, as described for the analyses on ICH risk.
We calculated the aHRs for the statin-associated risk of ICH for different types of statins as our exposure (atorvastatin versus other than atorvastatin and lipophilic versus hydrophilic statins) (Appendix 3).
We performed five sensitivity analyses on the statin-associated risk of ICH as a function of time since statin initiation. First, we evaluated the risk in subgroups according to demographics, socioeconomic factors, comorbidity groups, and number of initiations since the start of the study (i.e., first and ‘other than first’ initiation of statins). Second, we considered an expansive outcome definition, which included stroke diagnoses made during an emergency room contact, all secondary diagnoses of stroke (regardless of the primary diagnosis), and stroke diagnoses without an MRI or CT brain scan within seven days before or after the stroke diagnosis. Third, we restricted the follow-up period to the most recent calendar period (i.e., between 2009 and 2013). Fourth, assuming that treatment may have effect after discontinuation, we considered varying lengths of carryover periods in which we extended the follow-up period in the statin user group until 30, 60, or 90 days after the actual date of discontinuation (stopping). Fifth, we considered varying lengths of grace periods (i.e., 0%, 20%, and 50%) in the definition of statin use.
The time scale was time since index date, and individuals were censored by change in exposure status, registration of a stroke diagnosis, death, emigration, end of follow-up, or age of 100 years. To account for dependence between observations of the same individual in different strata, we estimated 95% confidence intervals (CIs) for all aHRs using cluster robust variance estimation with individuals as the level of clustering. Proportional hazards were assessed by evaluating interaction between each covariate and follow-up time. Although some were statistically significant, adjusting for these effects did not substantially change the estimates. We used two-sided significance tests for all analyses; the level of statistical significance was set at P < 0.05. All analyses were performed using Stata software, version 13, College Station, Texas, USA.
3. Results
We included 519,894 individuals initiating statin treatment and 1,222,185 matched non-users (some users and non-users were included more than once) in the study (Table 1). The mean number of days spent at risk in the study was 1330.2 days for statin users and 1416.2 days for non-users. Within the 120 days prior to index date, more statin users had redeemed antiplatelet agents (36.4% vs 13.2%), anticoagulant agents (4.8% vs 3.6%), and antihypertensive agents (67.0% vs 41.7%) compared to non-users (Table 1).
Table 1.
Baseline characteristics according to background variables at index datea for a population-based cohort in Denmark of 519,894 statin users and their 1:5 matched reference group, 2004–2013.
| No statin use (%) | Statin use (%) | |
|---|---|---|
| Total (%) | 83.3% | 16.7% |
| Demographic factors | ||
| Age | ||
| 50–60 years | 33.3% | 33.3% |
| 61–70 years | 38.2% | 38.2% |
| 71–80 years | 21.7% | 21.7% |
| 81–100 years | 6.8% | 6.8% |
| Sex | ||
| Women | 51.7% | 51.7% |
| Men | 48.3% | 48.3% |
| Calendar period | ||
| 2004–2005 | 19.1% | 19.1% |
| 2006–2007 | 23.3% | 23.3% |
| 2008–2009 | 22.5% | 22.5% |
| 2010–2011 | 19.0% | 19.0% |
| 2012–2013 | 16.1% | 16.1% |
| Socioeconomic factors | ||
| Cohabitation status | ||
| Living with a partner | 68.5% | 68.0% |
| Living alone | 31.5% | 32.0% |
| Education | ||
| > 15 years | 13.6% | 13.9% |
| 10–15 years | 43.0% | 42.8% |
| > 10 years | 41.1% | 40.8% |
| Unknown | 2.3% | 2.5% |
| Income | ||
| High | 28.2% | 28.5% |
| Medium | 58.3% | 58.0% |
| Low | 13.5% | 13.5% |
| Comorbidity | ||
| Hypertension (hospital diagnoses) | 20.1% | 19.7% |
| Atrial fibrillation | 4.5% | 5.0% |
| Ischaemic heart disease | 12.9% | 13.3% |
| Congestive heart failure | 2.1% | 2.9% |
| Peripheral artery occlusive diseases | 4.6% | 5.7% |
| Cerebrovascular disease | 1.1% | 1.3% |
| Diabetes mellitus | 17.4% | 17.0% |
| Chronic obstructive pulmonary disease | 7.1% | 7.5% |
| Chronic liver disease | 0.7% | 0.7% |
| Coagulation defects | 0.4% | 0.5% |
| Anaemias | 1.8% | 1.9% |
| Cancer | 4.4% | 4.5% |
| Epilepsy | 0.6% | 0.7% |
| Parkinson's disease | 0.2% | 0.2% |
| Mood, stress or anxiety-related disorder | 0.8% | 1.0% |
| Alcohol problems | 0.7% | 0.8% |
| Substance abuse | 0.1% | 0.1% |
| Bipolar affective disorder | 0.3% | 0.4% |
| Schizophrenia/schizoaffective disorder | 0.4% | 0.3% |
| Dementia | 0.6% | 0.6% |
| Medications | ||
| Antithrombotic agents | ||
| Antiplatelet agents | ||
| No use | 86.8% | 63.6% |
| Newly initiated use | 4.2% | 25.7% |
| Long-term use | 9.0% | 10.7% |
| Anticoagulant agents | ||
| No use | 96.4% | 95.2% |
| Newly initiated use | 0.9% | 2.0% |
| Long-term use | 2.7% | 2.8% |
| Antihypertensive agents | ||
| No use | 58.3% | 33.0% |
| Newly initiated use | 7.5% | 25.0% |
| Long-term use | 34.2% | 42.0% |
| Other selected agents that may influence stroke risk | ||
| NSAIDs | ||
| No use | 88.5% | 85.9% |
| Newly initiated use | 6.7% | 8.6% |
| Long-term use | 4.8% | 5.5% |
| Systemic glucocorticoids | ||
| No use | 96.5% | 96.1% |
| Newly initiated use | 2.0% | 2.4% |
| Long-term use | 1.5% | 1.5% |
| SSRIs | ||
| No use | 94.3% | 93.8% |
| Newly initiated use | 1.5% | 2.0% |
| Long-term use | 4.2% | 4.2% |
| No of initiations | ||
| First | Not applicable | 85.5% |
| Other than first | Not applicable | 14.5% |
Abbreviations: NSAID: non-steroidal anti-inflammatory drug; SSRI: selective serotonin reuptake inhibitor.
Index date: day of statin initiation for exposed individuals.
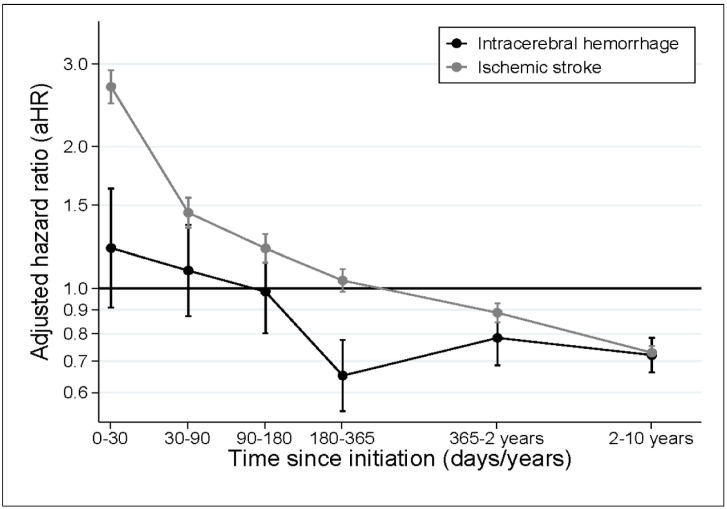
In total, 1409 (0.25%) ICHs occurred among statin users versus 0.30% among non-users. This corresponds to an overall aHR of 0.85 (95% CI: 0.80–0.90) for statin users compared to non-users when we adjusted for sociodemographic factors and comorbidity in model one (Table 2). This estimate was virtually unaffected when we adjusted for use of medications and corresponding interactions in models two–five (Table 2). However, the risk was modified by time since treatment initiation (Fig. 1).
Table 2.
Adjusted HRs and 95% CIs for the risk of intracerebral haemorrhage and ischaemic stroke among 519,894 individuals with use of statins compared to those with no use of statins for up to 10 years of follow-up.
| aHR (95% CI) | |
|---|---|
| ICH | |
| Model 1a | 0.85 (0.80; 0.90) |
| Model 2b | 0.75 (0.71; 0.80) |
| Model 3c | 0.77 (0.72; 0.82) |
| Model 4d | 0.77 (0.72; 0.82) |
| Model 5e | 0.77 (0.72; 0.82) |
| IS2 | |
| Model 1a | 0.96 (0.94; 0.99) |
| Model 2b | 0.92 (0.89; 0.94) |
| Model 3c | 0.90 (0.88; 0.92) |
| Model 4d | 0.90 (0.88; 0.92) |
| Model 5e | 0.90 (0.88; 0.92) |
Abbreviations: aHR: adjusted hazard ratio; CI: confidence interval.
Adjusted for age, sex, and calendar period, socioeconomic position (i.e., cohabitation status, education and income), comorbidity (ie hypertension, atrial fibrillation, ischaemic heart disease, congestive heart failure, peripheral artery occlusive disease, cerebrovascular disease, diabetes, chronic obstructive pulmonary disease, chronic liver disease, coagulation defects, anaemia, cancer, epilepsy, Parkinson's disease, mood, stress or anxiety-related disorder, alcohol problems, substance abuse, bipolar affective disorder, schizophrenia/schizoaffective disorder, and dementia).
Also adjusted for antithrombotic agents (i.e., anticoagulant agents and antiplatelet agents).
Also adjusted for antihypertensive agents.
Also adjusted for other selected agents that may influence stroke risk (ie non-steroidal anti-inflammatory drugs (NSAIDs), systemic glucocorticoids and selective serotonin reuptake inhibitors (SSRI)).
Also adjusted for the interaction between atrial fibrillation and anticoagulant agents and between hypertension and antihypertensive agents.
Fig. 1.
Adjusted HRs and 95% CIs for the risk of intracerebral haemorrhage and ischaemic stroke for statin users compared to references plotted as a function of time since initiation of statin exposure (ie index date).
Abbreviations: aHR: adjusted hazard ratio; CI: confidence interval.
The risk of ICH was similar for statin users and non-users during the first 180 days of follow-up (aHR90–180 days: 0.98, 95% CI: 0.80–1.21). Still, in the period from 180 days and up to ten years of follow-up, the risk of ICH was 22–35% lower among statin users (aHR180–365 days: 0.65, 95% CI: 0.55–0.78; aHR1–2 years: 0.78, 95% CI: 0.69–0.90; aHR2–10 years: 0.72, 95% CI: 0.66–0.78) (Fig. 1).
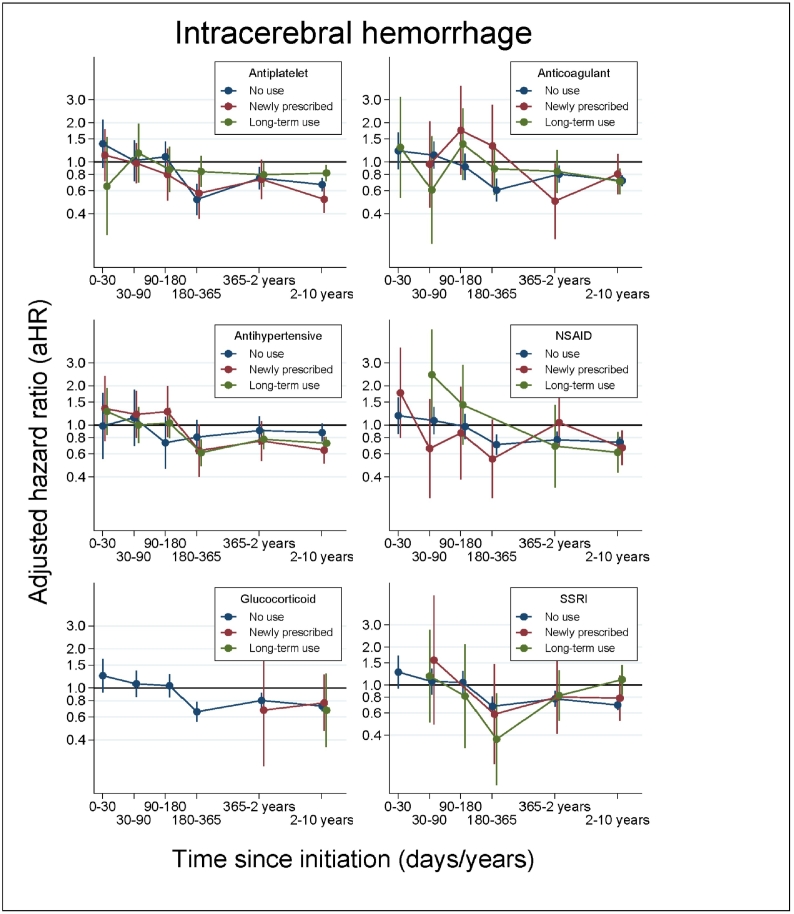
The temporal association between statins and ICH risk was not essentially modified by the concurrent use of other selected agents influencing risk of stroke (Fig. 2). Similarly, we found no substantial change in the relative risk of ICH for statin users compared to non-users in subgroups characterised by demographics, socioeconomic factors, comorbidities, and number of statin initiations (Supplementary Fig. 1A–B).
Fig. 2.
Adjusted HRs and 95% CIs for the risk of intracerebral haemorrhage for statin users compared to non-users plotted as a function of time since index date, stratified by use of selected medications that may influence risk of stroke.
Abbreviations: aHR: adjusted hazard ratio; CI: confidence interval; Antiplatelet: antiplatelet agents; Anticoagulant: anticoagulant agents; Antihypertensive: antihypertensive agents; NSAID: non-steroidal anti-inflammatory drug; Glucocorticoid: systemic glucocorticoids; SSRI: selective serotonin reuptake inhibitors.
Some values for the plots on NSAIDs, systemic glucocorticoids, and SSRIs were not displayed in case of less than five events due to privacy concerns.
Supplementary analyses on the association between risk of IS and statin use, including 11,402 IS events among statin users, showed considerable variation with time since treatment initiation; the risk of IS was markedly increased in the first 30 days after initiating statins (aHR: 2.68, 95% CI: 2.47–2.91). Hereafter, it consistently decreased to an 11% lower risk (aHR: 0.89, 95% CI: 0.85–0.93) in the period of one to two years of current use (Fig. 1).
Compared to non-use, the fully adjusted HR for risk of ICH was 0.98 (95% CI: 0.69–1.40) for use of atorvastatin, 0.76 (95% CI: 0.71–0.82) for use of statins other than atorvastatin, 0.86 (95% CI: 0.81–0.91) for use of lipophilic statins, and 0.81 (95% CI: 0.49–1.37) for use of hydrophilic statins.
The results were essentially unchanged in sensitivity analyses exploring statin use and risk of ICH when we used the extended outcome definition (Supplementary Fig. 2), the restricted follow-up period (Supplementary Fig. 3), various lengths of carryover periods (Supplementary Fig. 4), and various lengths of grace periods (Supplementary Fig. 5).
4. Discussion
This large-scale nationwide cohort study of more than 500,000 individuals initiating statins showed that statin users had similar ICH risk as propensity score matched non-users during the first 180 days of follow-up. Hereafter, statin users had a 22–35% lower ICH risk throughout the study period of up to ten years. The reduced ICH risk over time was not explained by sociodemographic factors, comorbidity, or concurrent treatment with other medications (e.g., antihypertensive or antiplatelet agents).
The underlying biological explanation for a lower risk of ICH among statin users is not known. In fact, statins inhibit platelet aggregation, enhance fibrinolysis, and reduce thrombosis, which theoretically could contribute to an increased risk of ICH associated with statins [10]. Contrarily, a statin-induced lower risk of IS might partly contribute to a reduced risk of ICH, as haemorrhagic transformation occurs in a significant proportion of patients following IS [27].
Previous research exploring the statin-associated risk of ICH have reported divergent results, which could be explained by lack of sufficient samples, lack of population-based settings, and lack of opportunity to study the significance of time since statin initiation. To the best of our knowledge, only five studies have evaluated the statin-associated risk of ICH in populations with no history of stroke, and none of these studies evaluated the significance of the timing of statin initiation [3], [11], [12], [13], [14]. In line with our results, two studies found a reduced risk of ICH to be associated with statins [11], [14]. A recent Swedish case–control study, including 7696 ICH cases and 14,670 controls from a stroke-free population, found that statin users had a 32% lower risk of ICH compared to non-users (OR: 0.68, 95% CI: 0.63–0.74) [11]. A case–control study, which explored the risk of ICH associated with level of cholesterol, found lower risk of ICH to be associated with use of statins (OR: 0.47 (95% CI: 0.23–0.95)) based on 12 haemorrhagic strokes among the limited fraction of the study population using statins (4–6%) [14]. Contrarily, three RCTs found no association between statin use and ICH in cohorts without a history of stroke (0.3% versus 0.5% [3] and OR: 1.18 (95% CI: 0.58–2.42) [13]) and no association in a mixed cohort including 10% with a history of stroke (OR: 0.55 (95% CI: 0.26–1.14) [12]). However, as these studies were significantly limited by small sample sizes (i.e., between 11 and 30 ICH events in the statin group) [3], [12], [13], these findings may not have yielded statistical significance due to lack of power. Several other studies exist, but they are not comparable to our study as they focus on participants with stroke and other selected patient groups (e.g., patients suffering from severe renal disease or atrial fibrillation and patients treated with antithrombotic agents or SSRI), do not include non-users as references, or are based on less than 30 ICH events [5], [6]. Noteworthy, of the five above-mentioned studies, only Åsberg and colleagues included a population-based cohort [11]. More importantly, none of the studies considered time since statin initiation and timing of concurrent treatment. In our study, these analyses revealed that the risk of ICH was similar for statin users and non-users within 6 months after initiating statins, but the risk decreased hereafter to a lower risk among statin users. Concurrent medication with antithrombotic and antihypertensive agents initiated at the time of statin initiation could not explain these findings.
Our study has several strengths. These include the large sample size, complete follow-up, sufficient observation time, and the population-based setting, which minimised potential selection bias and loss to follow-up. The information on stroke diagnoses had high validity, which limited the risk of information bias. The positive predictive value of IS and ICH was reported to be 97% and 74%, respectively, in a study validating these codes in the DNPR during 1998–1999 [28]. Compared to the validation study [28], the validity of ICH in our study is likely to be even higher, as we exclusively studied diagnoses of ICH in individuals registered with a concurrent brain scan during a more recent time period. Additionally, we used several statistical methods to reduce potential bias in our study. We explored the potential influence of time since statin initiation and concomitant initiation of other medications, which could influence stroke risk. We also matched on the propensity to receive a statin, which homogenised the cohort in terms of confounding factors. Moreover, we defined statin use based on a number of days corresponding to the number of pills redeemed plus a grace period, which ensured inclusion of newly initiated statin users and minimised the unintentional re-inclusion of long-term users.
Our study also has some limitations. First, the lower risk seen among statin users compared to non-users could potentially reflect a ‘healthy initiator bias’ [23]. This bias can arise through two distinct pathways: a selective initiation of preventive treatments among healthy and health-conscious patients and a selective channelling of treatments away from frail individuals at increased risk of adverse outcomes [23]. Generally, healthcare providers are more prone to initiate statin treatment in patients with an overall robust health [29], [30] and may specifically avoid initiating statins in case of a sustained ICH due to raised concern for harm [10]. Therefore, we cannot rule out that individuals initiating statins in our study were a selected group of more healthy individuals with a lower risk of ICH. However, if so, we would expect the risk of ICH to be lower for statin users in the 30-day period after initiating statins during which an instantaneous effect of statin seems unlikely. However, during this period, the risk of ICH was similar for statin users and non-users. In addition, the risk of IS was markedly elevated for statin users compared to non-users in the beginning of the study period. This could indicate that statins are given to persons at higher risk of cardiovascular events (i.e., confounding by indication) [23], which also speak against a healthy initiator bias. Second, there is always a risk of confounding in observational studies. Despite our meticulous efforts to minimise baseline confounding, we cannot conclude that time-varying confounding, such as a ‘healthy adherer bias’, is non-existent. Clinical guidelines advise to stop statin treatment in individuals with end-of-life status [30], and some clinicians may reconsider use of statins in individuals with high risk of ICH [10]. Thus, the group of statin users might be increasingly healthy over time if statin adherence is a proxy for advantageous lifestyle and health behaviour at the patient level and for selective stopping of treatment at the healthcare provider level. Therefore, the beneficial effect of statins on risk of ICH could potentially be exaggerated. Third, the lower risk of ICH associated with statins could be confounded by concurrent treatment with other medications that reduce the risk of ICH, such as antihypertensive agents. Hypertension is known to be highly associated with risk of ICH [31], and antihypertensive agents are likely to be initiated concurrently with statins in populations with high risk of cardiovascular disease [12]. Nevertheless, we found that the risk of ICH associated with statins was similar for those with and without use of antihypertensive agents, regardless of the timing of initiation of antihypertensive agents. Fourth, although we had detailed information on comorbidity and redeemed prescriptions, we lacked information on stroke diagnoses registered before 1995, comorbidities registered only in primary care (such as hypertension and heavy alcohol abuse), measures of frailty, estimated life expectancy, over-the-counter medicines (such as acetylsalicylic acid and NSAIDs for pain relief), blood pressure profiles, neuroimaging (on e.g., hypertension-related and cerebral amyloid angiopathy-related small-vessel disease and microbleeds), and lifestyle habits. Consequently, we cannot exclude residual confounding. Fifth, we only had information on redemptions of prescriptions and did not know if the patients actually adhered to the recommended treatment or discontinued treatment due to possible side effects (e.g., myalgia). However, although this could lead to exposure misclassification, it would also lead to conservative estimates as a potential beneficial effect of statins would be diluted by non-compliance. Finally, ICH is a heterogeneous entity that predominantly comprises ‘deep’ and ‘lobar’ ICHs, which have markedly different aetiology [5]. Unfortunately, our data did not allow us to determine whether statin use was associated with only specific types of ICHs.
5. Conclusions
Among individuals with no history of stroke, we found that statin users had a lower risk of ICH than non-users in the period from six months after initiating statins and throughout the study period. Our findings could not be explained by better health status of individuals initiating statins than of non-users; statin users had similar ICH risk and increased IS risk compared to non-users instantaneously after initiating statins. Moreover, the reduced ICH risk could not be explained by differences between users and non-users in terms of sociodemographic factors, comorbidity, other medications associated with risk of stroke, or parallel treatment regimens. In conclusion, our study suggests that statin use is associated with a reduced risk of ICH in stroke-free populations, and that potential statin prescribing reticence due to concerns about increased ICH risk seems to be unfounded in this population. These findings could have major public health implications for the vast population of statin users with no history of stroke.
Authors' Contributions
ARR and MV obtained the funding. All authors designed the study. ARR, CV, HSP and MFG acquired and analysed the data. ARR wrote the first draft of the manuscript. All authors interpreted the results, revised the manuscript, and approved the final version.
Conflict of Interest Statements
We declare no competing interests.
Funding
This study was funded by an unrestricted grant from the Novo Nordisk Foundation. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Ethical Considerations
The study was approved by the Danish Data Protection Agency, the Danish Health Data Authority, and Statistics Denmark. Ethical approval and informed consent were not needed, as all personal identification numbers were encrypted by Statistics Denmark prior to analysis.
Acknowledgements
This study was supported by an unrestricted grant from The Novo Nordisk Foundation donated to ARR. The authors would like to thank Lone Niedziella from the Research Unit for General Practice in Aarhus for language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.02.007.
Appendix A. Supplementary data
Supplementary material
References
- 1.Amarenco P., Bogousslavsky J., Callahan A., III High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 2.Baigent C., Blackwell L., Emberson J. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins R., Armitage J., Parish S., Sleight P., Peto R. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363(9411):757–767. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein L.B., Amarenco P., Szarek M. Hemorrhagic stroke in the stroke prevention by aggressive reduction in cholesterol levels study. Neurology. 2008;70(24):2364–2370. doi: 10.1212/01.wnl.0000296277.63350.77. Pt 2. [DOI] [PubMed] [Google Scholar]
- 5.Hackam D.G., Woodward M., Newby L.K. Statins and intracerebral hemorrhage: collaborative systematic review and meta-analysis. Circulation. 2011;124(20):2233–2242. doi: 10.1161/CIRCULATIONAHA.111.055269. [DOI] [PubMed] [Google Scholar]
- 6.McKinney J.S., Kostis W.J. Statin therapy and the risk of intracerebral hemorrhage: a meta-analysis of 31 randomized controlled trials. Stroke. 2012;43(8):2149–2156. doi: 10.1161/STROKEAHA.112.655894. [DOI] [PubMed] [Google Scholar]
- 7.Hackam D.G., Austin P.C., Huang A. Statins and intracerebral hemorrhage: a retrospective cohort study. Arch Neurol. 2012;69(1):39–45. doi: 10.1001/archneurol.2011.228. [DOI] [PubMed] [Google Scholar]
- 8.Hemphill J.C., 3rd, Greenberg S.M., Anderson C.S. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 9.Westover M.B., Bianchi M.T., Eckman M.H., Greenberg S.M. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68(5):573–579. doi: 10.1001/archneurol.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauer A., Greenberg S.M., Gurol M.E. Statins in intracerebral hemorrhage. Curr Atheroscler Rep. 2015;17(8):46. doi: 10.1007/s11883-015-0526-5. [DOI] [PubMed] [Google Scholar]
- 11.Åsberg S., Eriksson M. Statin therapy and the risk of intracerebral haemorrhage: a nationwide observational study. Int J Stroke. 2015;10:46–49. doi: 10.1111/ijs.12539. Suppl A100. [DOI] [PubMed] [Google Scholar]
- 12.Sever P.S., Dahlof B., Poulter N.R. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura H., Arakawa K., Itakura H. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 14.Tirschwell D.L., Smith N.L., Heckbert S.R., Lemaitre R.N., Longstreth W.T., Jr., Psaty B.M. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology. 2004;63(10):1868–1875. doi: 10.1212/01.wnl.0000144282.42222.da. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M., Pedersen L., Sorensen H.T. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 16.Denmark Statistics. 1991. The integrated database for longitudinal labour market research (Integreret database for arbejdsmarkedsforskning (IDA)) [Google Scholar]
- 17.Schmidt M., Schmidt S.A., Sandegaard J.L., Ehrenstein V., Pedersen L., Sorensen H.T. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mors O., Perto G.P., Mortensen P.B. The Danish psychiatric central research register. Scand J Public Health. 2011;39(7 Suppl):54–57. doi: 10.1177/1403494810395825. [DOI] [PubMed] [Google Scholar]
- 19.Kildemoes H.W., Sorensen H.T., Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen K.M., Christiansen T., Bech M. The Danish health care system: evolution--not revolution--in a decentralized system. Health Econ. 2005;14(Suppl. 1):S41–S57. doi: 10.1002/hec.1028. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen C.B. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 Suppl):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 22.Ribe A.R., Laursen T.M., Charles M. Long-term risk of dementia in persons with schizophrenia: a Danish population-based cohort study. JAMA Psychiat. 2015:1–7. doi: 10.1001/jamapsychiatry.2015.1546. [DOI] [PubMed] [Google Scholar]
- 23.Lund J.L., Richardson D.B., Sturmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221–228. doi: 10.1007/s40471-015-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prior A., Fenger-Gron M., Larsen K.K. The association between perceived stress and mortality among people with multimorbidity: a prospective population-based cohort study. Am J Epidemiol. 2016;184(3):199–210. doi: 10.1093/aje/kwv324. [DOI] [PubMed] [Google Scholar]
- 25.Brookhart M.A., Schneeweiss S., Rothman K.J., Glynn R.J., Avorn J., Sturmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heide-Jorgensen U., Adelborg K., Kahlert J., Sorensen H.T., Pedersen L. Sampling strategies for selecting general population comparison cohorts. Clin Epidemiol. 2018;10:1325–1337. doi: 10.2147/CLEP.S164456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sussman ES, Connolly ES, Jr. Hemorrhagic transformation: a review of the rate of hemorrhage in the major clinical trials of acute ischemic stroke. Front Neurol 2013; 4: 69. [DOI] [PMC free article] [PubMed]
- 28.Krarup L.H., Boysen G., Janjua H., Prescott E., Truelsen T. Validity of stroke diagnoses in a national register of patients. Neuroepidemiology. 2007;28(3):150–154. doi: 10.1159/000102143. [DOI] [PubMed] [Google Scholar]
- 29.O'Mahony D., O'Sullivan D., Byrne S., O'Connor M.N., Ryan C., Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–218. doi: 10.1093/ageing/afu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavan AH, Gallagher P, Parsons C, O'Mahony D. STOPPFrail (Screening tool of older persons prescriptions in frail adults with limited life expectancy): consensus validation. Age Ageing 2017; 46(4): 600–7. [DOI] [PubMed]
- 31.Ariesen M.J., Claus S.P., Rinkel G.J., Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34(8):2060–2065. doi: 10.1161/01.STR.0000080678.09344.8D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material