Abstract
Background
Obstructive sleep apnoea (OSA) is a common comorbidity in patients with cardiovascular (CV) disease. We aimed to identify specific OSA clinical phenotypes relating to risks of serious CV events and response to continuous positive airway pressure (CPAP) treatment.
Methods
Post-hoc analyses of the Sleep Apnea Cardiovascular Endpoints (SAVE) study in participants with moderate-to-severe OSA and coronary artery disease (CAD) and/or cerebrovascular disease (CeVD) randomised to CPAP plus usual care or usual care alone. Latent class analysis (LCA) was used to identify OSA clinical phenotypes among 2649 (out of 2687 total) patients with complete data on 19 patient-centered variables, supported by Bayesian information criteria and clinical interpretability. Cox regression models were used to evaluate risks of composite cardiac and stroke outcome events in phenotype groups. Preferential response to CPAP treatment was evaluated using interaction terms as well as the Chi-square test.
Findings
LCA identified four OSA clinical phenotypes: CAD alone and with diabetes mellitus (CAD + DM), and CeVD alone and with DM (CeVD + DM), in 39%, 15%, 37% and 9% of participants, respectively. The rates of composite CV events were the highest in CAD + DM phenotype (HR 2.08, 95% CI 1.57–2.76) and for stroke were highest in CeVD + DM phenotype (HR 6.84, 95% CI 3.77–12.42). Adherence to CPAP treatment (nil or < 4 h vs ≥ 4 h in the first two years of the study) was shown to influence the risk of composite CV outcome in the phenotypes (P-interaction = 0.04); CPAP adherent patients of the CeVD + DM phenotype had the lowest risk of CV outcome (P = 0.02).
Interpretation
High risk clinical phenotypes were identified in relation to CV events and response to CPAP treatment, which may allow improved targeting of therapies in OSA patients.
Funding
The National Health and Medical Research Council (NHMRC) of Australia, Fisher & Paykel Healthcare, and ResMed.
Keywords: Obstructive sleep apnoea, Phenotype, Cardiovascular disease, Stroke, Latent class analysis
Research in context
Search strategy and selection criteria
We searched PubMed and Google Scholar for studies of obstructive sleep apnoea phenotypes published in English up to 4 April 2018, with the terms “obstructive sleep apnoea”, “phenotype” and “cardiovascular risk”. We did not impose any restrictions on language. Articles that were relevant to the topics were added to our reference list.
Evidence before this study
Studies which phenotype OSA patients with a view towards risk stratification are limited and it is unknown if any particular group of OSA patients benefit preferentially from CPAP treatment in terms of the prevention of recurrent cardiovascular events. One recent cross-sectional study by Zinchuk et al. used polysomnographic data to cluster OSA in 1247 US veterans to identify seven groups with distinguishing features and variable risk of cardiovascular event.
Added value of this study
In the present study, data from the largest clinical trial of continuous positive airway pressure (CPAP) in OSA patients, were used to identify phenotypes of OSA patients according to clinical features. Past cardiovascular history and diabetes mellitus were the strongest distinguishing features in the phenotype groups which predicted different risks of a composite cardiovascular outcome and stroke, as well as response to CPAP treatment.
Implication of the available evidence
High risk clinical phenotypes of OSA patients in relation to CV events and response to CPAP treatment may allow improved targeting of treatment.
Alt-text: Unlabelled Box
1. Introduction
Obstructive sleep apnoea (OSA) is a common disorder characterised by snoring and repetitive obstruction of the airways during sleep which affects up to two thirds of patients with cardiovascular (CV) risk factors [1], [2]. OSA is known to be associated with increased CV risks via multiple potential mechanisms that include autonomic activation, systemic inflammation, oxidative stress, endothelial dysfunction and insulin resistance [3], and CV event rates are particularly high in those with co-occurring OSA and established CV disease. While CPAP improves daytime sleepiness and quality of life [4], [5], studies including the international Sleep Apnea Cardiovascular Endpoints (SAVE) study have failed to show a clear effect of this therapy on CV protection [4], [6], [7], [8]. Due to the heterogeneous nature of OSA, there have been suggestions that certain subgroups of patients may benefit from CPAP treatment in terms of prevention of recurrent CV events. An improved understanding of the prognostic features of high risk OSA patients, could identify those who may benefit preferentially from targeted therapeutic strategies. Prior attempts to characterise OSA patients have primarily been based on physiological variables and daytime symptoms [9], [10], [11], [12], [13], with few studies considering CV risk factors [14], [15], [16] and their interplay in the clinical setting.
A novel methodological approach for identifying groups of individuals with wide ranging and potentially interacting clinical characteristics is latent class analysis (LCA). LCA has gained popularity in the recent years because of its probabilistic model-based approach and ability to yield goodness of fit estimates. In the recent years, LCA has been used for identifying subgroups of patients with OSA and heart failure, with a view towards outcome prediction and risk stratification [16], [17]. The aim of the present study was to use LCA to identify clinically meaningful OSA phenotypes with significantly different CV event rates and response to CPAP treatment among participants of the SAVE study [4].
2. Methods
2.1. Study Design
This was a post-hoc analysis of the SAVE study, an international, multicenter, randomised controlled trial that evaluated the effectiveness of CPAP on top of standard of care for OSA to reduce recurrent CV events [4]. In brief, 2687 adults (age 45 to 75 years) with a diagnosis of moderate-to-severe OSA and established coronary artery disease (CAD) and/or cerebrovascular disease (CeVD) were randomised to receive CPAP plus usual care or usual care alone at 89 clinical centers in 7 countries from December 2008 to November 2013. The diagnosis of OSA was based on a high oxygen desaturation index (ODI, ≥ 12 events per hour with ≥ 4% oxygen saturation dip), established through use of a home sleep screening device (ApneaLink, ResMed) and confirmed centrally by sleep specialists. The SAVE study was approved by all relevant ethics committees and written informed consent was obtained from participants or an appropriate surrogate. The study is registered at ClinicalTrials.gov number (NCT00738179).
2.2. Procedures and Outcomes
Demographic and clinical characteristics, medical history, medications and health behaviours of participants were recorded at the time of enrolment. After randomisation was undertaken in those who had successfully completed a 1-week run-in phase of sham CPAP, CV events and other CV and non-CV outcomes, serious adverse events, and adherence to CPAP therapy, were checked during follow-up assessments conducted at 1, 3, 6 and 12 months, and 6 monthly thereafter, for a mean of 3.7 years. CV events were defined according to standard definitions [4]. The primary endpoint was a composite of death from any CV cause, myocardial infarction, stroke, or hospitalisation for unstable angina, heart failure or, transient ischaemic attack. For these analyses, stroke occurrence was our secondary endpoint.
2.3. Statistical Analysis
LCA was used to identify discrete subgroups (or classes) within the study population based on a set of pre-selected indicator variables [18]. We chose 19 clinically relevant indicator variables associated with CV risk and OSA severity based on a review of the literature and through the use of crude logistic regression analysis of the SAVE dataset. The indicator variables for the LCA analysis included: older age (≥ 65), female sex, Asian ethnicity, current (some or every day) cigarette smoking, frequent (at least once a week) alcohol consumption, obesity (body mass index ≥ 30 kg/m2), high systolic blood pressure (SBP, ≥ 140 mmHg), high apnoea hypopnea index (AHI, ≥ 30 events/h), sleep time with oxygen saturation below 90% (ST90 ≥ 9.5), Epworth sleepiness scale (ESS) score > 10, history of any stroke (defined as past CeVD), CAD, revascularisation procedure (including percutaneous or bypass graft coronary intervention), hypertension, and diabetes mellitus (DM), and any use of antihypertensive, statin or other lipid lowering, antidiabetic, and anticoagulation medications. Using maximum likelihood estimation, the LCA calculated the (posterior) probability of individuals belonging to each constructed latent class and assigned individual patients membership to the class with the highest posterior probability. Models with between two and five latent classes were examined, with the optimal number of classes determined through meaningful clinical interpretability and parsimony based on the lowest Akaike information classification and Bayesian information classification indices, as well as an assessment of adequate entropy. LCA was performed using the poLCA library in the R statistical package (version 2.15.0, R Foundation for Statistical Computing, Vienna, Austria).
As the calculated average probability of class membership across all subjects was high at 0.99 (1 and 0 represent perfect classification and misclassification, respectively), a straight-forward ‘classify-analyse’ approach was used to evaluate associations between latent classes and clinical outcomes [19]. Association between OSA clinical phenotypes and the primary CV outcome and stroke events was undertaken using Cox proportional hazards models adjusted for posterior probability of latent class membership. Sensitivity analysis with adjustment for history of diabetes mellitus was also conducted. We further tested for a differential effect of CPAP treatment among each clinical phenotype using interaction terms in the Cox regression model and calculated the proportions of outcomes in each OSA phenotype group by level of adherence to CPAP (on average, < 4 vs. ≥ 4 h per night, in the first two years of the study). Also, we compared proportional variations in outcomes among the latent class by CPAP adherence time using the Chi-squared test. To ensure the robustness and reliability of the present findings, we repeated all analysis with alternative number of classes (three and five classes).
Data are reported with hazard ratios (HR) and 95% confidence intervals (CI). A two-sided P value of < 0.05 was considered statistically significant. Survival analyses were undertaken using SAS version 9.3 (SAS Institute, Cary, NC).
3. Results
3.1. Patient Characteristics in Four OSA Clinical Phenotypes
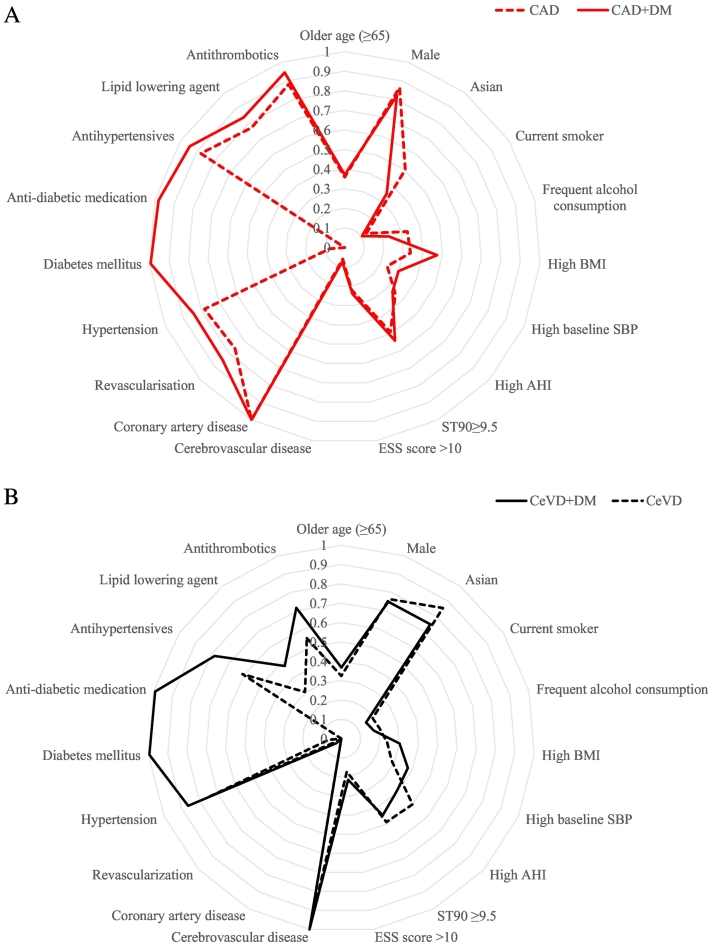
We included 2649 (97%) of the total study population that had complete information on 19 indicator variables (Supplemental Fig. S1). Based on both clinical interpretability and parsimony, we chose four groups of classification identified by LCA with varying clinical characteristics (Supplemental Tables S2–S4, Table 1, Fig. 1A and B). Latent class analysis with three to five classes consistently showed greatest inter-class difference in posterior probability of the indicator variables CAD, CeVD and DM. As such, we labelled the clinical phenotypes by those variables. The resulting OSA clinical phenotypes derived from these analyses were: CAD alone or with DM (CAD + DM), and CeVD alone or with DM (CeVD + DM), in 39%, 15%, 37% and 9% of participants, respectively.
Table 1.
Characteristics of patients with different OSA clinical phenotypes.
| All patients (n = 2649) |
CeVD + DM (n = 242) |
CAD + DM (n = 396) |
CeVD (n = 972) |
CAD (n = 1039) |
P value | |
|---|---|---|---|---|---|---|
| Older age (≥ 65) | 1332 (50) | 89 (37) | 147 (37) | 315 (32) | 374 (36) | 0.218 |
| Male | 2144 (81) | 181 (75) | 326(82) | 741 (76) | 896 (86) | < 0.001 |
| Asian | 1673 (63) | 181 (75) | 139 (35) | 832 (86) | 521 (50) | < 0.001 |
| Current smokera | 404 (15) | 37 (15) | 42 (11) | 189 (19) | 136 (13) | < 0.001 |
| Frequent alcohol consumptionb | 676 (26) | 42 (17) | 92 (23) | 200 (21) | 342 (33) | < 0.001 |
| Body mass index, > 30 kg/m2 | 843 (32) | 73 (30) | 187 (47) | 232 (24) | 351 (34) | < 0.001 |
| Systolic BP, > 140 mm Hg | 733 (28) | 91 (38) | 119 (30) | 277 (29) | 246 (24) | < 0.001 |
| AHI, ≥ 30 events/h | 1079 (41) | 95 (39) | 132 (33) | 488 (50) | 364 (35) | < 0.001 |
| ST90 ≥ 9.5 minc | 1317 (50) | 110 (45) | 215 (54) | 480 (49) | 512 (49) | 0.158 |
| ESS score > 10 | 544 (21) | 52 (21) | 94 (24) | 168 (17) | 230 (22) | 0.014 |
| Cerebrovascular disease | 1306 (49) | 242 (100) | 29 (7) | 972 (100) | 63 (6) | < 0.001 |
| Coronary artery disease | 1451 (55) | 0 (0) | 396 (100) | 16 (2) | 1039 (100) | < 0.001 |
| Revascularisation | 1146 (43) | 8 (3) | 336 (85) | 10 (1) | 792 (76) | < 0.001 |
| Hypertension | 2079 (78) | 209 (86) | 333 (84) | 724 (74) | 813 (78) | < 0.001 |
| Diabetes mellitus | 788 (30) | 241 (100) | 394 (99) | 65 (7) | 88 (8) | < 0.001 |
| Medications | ||||||
| Antihypertensive | 2069 (78) | 189 (78) | 374 (94) | 594 (61) | 912 (88) | < 0.001 |
| Statin or other lipid lowering | 1550 (59) | 115 (48) | 333 (84) | 296 (30) | 806 (78) | < 0.001 |
| Antidiabetic | 638 (24) | 242 (100) | 396 (100) | 0 (0) | 0 (0) | < 0.001 |
| Antithrombotic | 1997 (75) | 173 (71) | 374 (94) | 534 (55) | 916 (88) | < 0.001 |
Data are n (%) for categorical variables. AHI indicates apnoea-hypopnea index; BP, blood pressure; CPAP, continuous positive airway pressure; CAD, coronary artery disease; CeVD, cerebrovascular disease; DM, diabetes mellitus; ESS, Epworth Sleepiness Scale.
Smoking cigarettes at least some days during the week.
Drinking alcohol once a week or more.
Sleep time with oxygen saturation level below 90%.
Fig. 1.
Conditional probabilities of indicator variables by OSA clinical phenotype. A. Coronary artery disease (CAD, dashed line) and CAD plus diabetes mellitus (CAD + DM, solid line) phenotype groups. B. Cerebrovascular disease (CeVD, dashed line) and CeVD plus diabetes mellitus (CeVD + DM, solid line) phenotype groups. Lines indicate the proportion of participants in each group identified with each feature shown on a concentric y axis. AHI indicates apnea hypopnea index, BMI body mass index, CAD coronary artery disease, DM diabetes mellitus, OSA obstructive sleep apnea, SBP systolic blood pressure, ST90 sleep time with oxygen saturation below 90%, ESS Epworth Sleepiness Scale. High AHI (≥ 30 events/h); high BMI (> 30 kg/m2); high SBP (> 140 mm Hg).
A comparison between the CAD and CAD + DM phenotypes shows that the latter group had less patients from Asia, fewer who drank alcohol, had higher BMI and increased rates of CV comorbidities and usage of CV medications. The differences between the CeVD and CeVD + DM groups were similar to those of the CAD and CAD + DM phenotypes. Patients of the CeVD + DM phenotype had lower rates of severe OSA (i.e. AHI ≥ 30 events/h) and shorter ST90, however we also observed higher ESS score within the same phenotype. A comparison of the CAD and CeVD phenotypes shows a higher percentage of Asian females and more severe OSA (AHI ≥ 30) in the latter, whereas the CAD phenotypes were marked by greater numbers of non-Asian males, higher obesity rates, and more revascularisation and CV pharmacotherapy (Table 1).
3.2. OSA Clinical Phenotypes and CV Outcomes
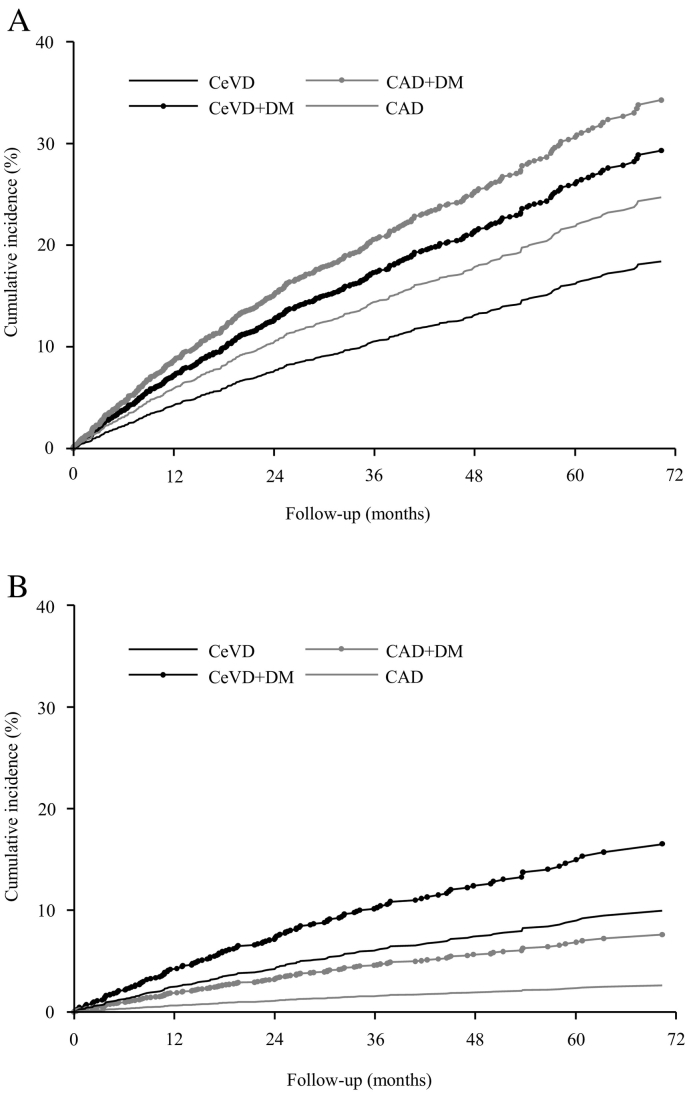
In models adjusted for posterior probability, the clinical phenotype CAD + DM was associated with the highest risk of the primary composite CV outcome (HR 2.08, 95% CI 1.57–2.76), followed by the CeVD + DM (HR 1.71, 95%CI 1.22–2.41) and CAD (HR 1.40, 95%CI 1.11–1.76) phenotypes in comparison to CeVD as reference group. The risk of stroke was higher in the CeVD + DM phenotype (HR 6.84, 95% CI 3.77–12.42) compared to the CAD clinical phenotype. The CAD + DM and CeVD phenotypes were also associated with increased risks of stroke (HR 2.99, 95% CI 1.56–5.73, and HR 3.97, 95% CI 2.39–6.60, respectively) as compared to the CAD phenotype (Table 2, Fig. 2). Similar associations between the phenotypes and the study outcomes were found in sensitivity analyses with adjustment for history of diabetes mellitus (Supplemental Table S5). Further analyses with three and five latent classes also showed that patients of the CAD + DM and CeVD + DM groups had the highest risk of primary outcome and stroke, respectively (Supplementary Tables S6–S7).
Table 2.
Risk of primary outcome and recurrent stroke by OSA clinical phenotype.
| Event | Phenotype | N (%) | HR | 95% CI | P valueb |
|---|---|---|---|---|---|
| Primary outcomea | CeVD + DM | 45 (19) | 1.71 | (1.22–2.41) | < 0.001 |
| CAD + DM | 81 (20) | 2.08 | (1.57–2.76) | ||
| CeVD | 124 (13) | 1 | Reference | ||
| CAD | 179 (17) | 1.40 | (1.11–1.76) | ||
| Stroke | CeVD + DM | 26 (11) | 6.84 | (3.77–12.42) | < 0.001 |
| CAD + DM | 18 (5) | 2.99 | (1.56–5.73) | ||
| CeVD | 69 (7) | 3.97 | (2.39–6.60) | ||
| CAD | 19 (2) | 1 | Reference |
HR indicates hazard ratio, CAD coronary artery disease, CeVD cerebrovascular disease, DM, diabetes mellitus, OSA, obstructive sleep apnoea.
The primary composite endpoint included death from cardiovascular causes, myocardial infarction, stroke, or hospitalisation for heart failure, unstable angina, and transient ischaemic attack.
Models adjusted for posterior probability.
Fig. 2.
Cumulative rate of cardiac and stroke outcomes by OSA clinical phenotype. (A) The primary endpoint was a composite of death from any CV cause, myocardial infarction, stroke, or hospitalisation for unstable angina, heart failure or, transient ischaemic attack. (B) Stroke events over time, according to phenotype group and adjusted for posterior probability. CAD coronary artery disease, CeVD cerebrovascular disease, CV cardiovascular, DM diabetes mellitus, OSA obstructive sleep apnoea.
3.3. Effects of CPAP by OSA Clinical Phenotype
There were significant interactions between adherence to CPAP treatment and the clinical phenotypes for the primary CV outcome (P = 0.04) but not stroke. Chi-squared test comparing proportion of outcome events by adherence to CPAP treatment showed a relationship between good adherence to CPAP treatment (≥ 4 h per night) and reduced risks of composite CV (P = 0.02) outcome in the CeVD + DM phenotype (Table 3). Sensitivity analysis with five latent classes also showed rates of primary outcome to be lower in CPAP adherent patients of the CeVD + DM phenotype (Supplemental Tables S8–S9).
Table 3.
Rates of primary composite CV outcome and recurrent stroke by OSA clinical phenotype and CPAP treatment.
| Primary outcomea % (95%CI) | Stroke event % (95%CI) | |||||
|---|---|---|---|---|---|---|
| Phenotype | No or < 4 h CPAP | ≥ 4 h CPAP | P valueb | No or < 4 h CPAP | ≥ 4 h CPAP | P valueb |
| CeVD + DM | 21.11 (15.65–27.44) | 4.88 (0.6–16.53) | 0.015 | 12.06 (7.88–17.41) | 2.44 (0.06–12.86) | 0.066 |
| CAD + DM | 19.59 (15.23–24.58) | 23.71 (15.66–33.42) | 0.384 | 4.73 (2.61–7.81) | 4.12 (1.13–10.22) | 0.804 |
| CeVD | 12.6 (10.34–15.15) | 13.47 (8.99–19.11) | 0.745 | 7.4 (5.65–9.48) | 6.22 (3.25–10.61) | 0.568 |
| CAD | 17.88 (15.28–20.71) | 15.35 (10.93–20.7) | 0.374 | 2.13 (1.24–3.38) | 0.88 (0.11–3.13) | 0.217 |
CAD denotes coronary artery disease, CI confidence interval, CPAP continuous positive airway pressure, CeVD, cerebrovascular disease, CV cardiovascular, DM diabetes mellitus, OSA obstructive sleep apnoea.
Missing 26 patients without information on adherence.
The primary composite endpoint included death from cardiovascular causes, myocardial infarction, stroke, or hospitalisation for heart failure, unstable angina, and transient ischaemic attack.
The P values are based on Chi-square test or Fisher's exact test as appropriate.
4. Discussion
In these secondary analyses of the large and heterogeneous SAVE clinical trial population, we were able to define four distinct clinical phenotypes that allowed risk stratification for recurrent CV events using an objective data-driven approach. The present findings support OSA being a complex and heterogeneous syndrome, and that those with co-occurring CV disease can be being categorised into several clinically meaningful prognostic groups. Although a wide range of clinically relevant variables including demographic details, lifestyle habits, clinical indices, comorbidities and medication use were used in the LCA to characterise individuals into clinically distinguishable subgroups, we found that ultimately prior CVD history and diabetes were the strongest distinguishing factors of the phenotypes. OSA patients were identified with high risks of CV events, and who thus have the potential to gain more than others from more intensive CV risk management and CPAP treatment, as the response to this treatment was not consistent among OSA clinical phenotypes.
The finding that the CAD groups were mainly non-Asian males, whereas CeVD groups were predominantly Asian and more often female, is consistent with ethnic differences in risks of CV disease, with higher rates of stroke and low rates of CAD in Asians compared to non-Asians [19]. As expected, patients in the CAD and CAD + DM groups more frequently reported obesity and use of CV medications, including antihypertensives, statins and anticoagulants, in comparison to the CeVD and CeVD + DM groups. Interestingly, more patients in the CeVD groups had severe OSA compared to CAD groups, which is consistent with studies showing a dose-dependent relationship of OSA severity and risk of stroke [21], [22], but not CAD [22].
Our study shows that CAD and CeVD patients with DM were at significantly higher risk of recurrent composite CV events and stroke respectively than individuals without DM. Although the significance of risk factors included in our analyses, such as sex, ethnicity, alcohol consumption and hypertension, are well established, the present findings suggest that having DM may both encompass and predominate other CV risk factors in the context of OSA [20], [21], [22], [23]. This practical and straightforward finding not only reinforces the importance of optimal DM management, but may also aid clinicians in readily stratifying OSA patients with various CV risk factors. Furthermore, as the phenotype groups with DM also displayed higher BMI and systolic BP, it presents opportunities for lifestyle and therapeutic management to lower future CV risk.
Although observational studies and small randomised trials have suggested benefits of CPAP on CV risk [6], [7], [24], [25], the SAVE trial showed no effects of this treatment on either the main composite or individual components of the primary CV outcome in a high risk OSA population. However, our current analysis suggests that CPAP may offer benefits in those at very high CV risk who are able to achieve high levels of adherence to CPAP. The long-term use of CPAP could yield metabolic regulatory benefits in terms of improved glycemic control and insulin sensitivity through the correction of pancreatic beta-cell structure [26], [27].
Prior attempts to phenotype OSA patients have primarily been based on symptoms and polysomnographic features in small, carefully selected series [10], [12], [14], [28], [29]. Strengths of our study are the large sample size, heterogeneous population in terms of risk factors and background health care settings, and detailed level of clinical data that were collected. Moreover, we used an approach to subgrouping that was data driven and person-centered rather than variable-centered, which allowed a greater focus on overall group characteristics rather than using a regression based approach with individual variables which cannot account for the numerous potential interactions. The intention was to derive clinical phenotypes with a view towards risk stratification for targeted or individualised therapy in OSA patients. We recognise, however, that there are limitations with our approach, in particular the inability to provide precise estimates of the effects of CPAP in each of the defined clinical phenotypes due to small numbers of CV events associated with subgroup analyses. Moreover, our study comprised a clinical trial population of less symptomatic patients with predominantly moderate–severe OSA, the findings may not be applicable to those with severe OSA symptoms with/without CV disease. Future investigations are needed to phenotype OSA in larger populations with additional pathophysiological and genetic variables.
In summary, our novel approach of applying LCA to common clinical variables identified four distinct clinical phenotypes of mildly symptomatic OSA patients who had significant differences in their CV event-free survival and response to CPAP treatment. Improved phenotyping of OSA patients may create a basis for more personalised therapies to be tailored to high-risk OSA patients. Further studies are required to validate our findings and accommodate a wider range of OSA patients and consider other relevant variables.
The following are the supplementary data related to this article.
Patient flowchart.
Supplementary material
Sources of Funding
This work was supported by National Health and Medical Research Council (NHMRC) of Australia Project Grants (1006501; 2011–2015) and (1060078; 2014–2016) and Respironics Sleep and Respiratory Research Foundation; Respironics Inc., Fisher&Paykel Healthcare; Enabling Grant to the Australasian Sleep Trials Network (343020; 2010–2012). Donations of equipment were from Respironics Inc. (CPAP devices) and ResMed (Sleep apnea diagnostic). RDM and CSA hold Practitioner and Senior Principal Research, Fellowships from the NHMRC, respectively. The funders were not involved in the paper design, data analysis, interpretation and writing of the manuscript.
Author Contributions
Drs Quan, Zheng, McEvoy and Anderson contributed to the concept and rationale for the study. Dr. Zheng undertook the statistical analyses. All the authors have contributed to the interpretation of results. Drs Quan and Zheng were responsible for the first draft. All authors participated in drafting and approval of the final manuscript, and take responsibility for its content and interpretation.
Disclosures
None.
Affiliations
From Rui Jin Hospital and Institute of Cardiovascular Diseases, Shanghai Jiao Tong University School of Medicine, Shanghai (WQ); The George Institute for Global Health, Faculty of Medicine, University of New South Wales, Sydney (DZ, QL, XW, JC, CSA); Neurology Department, Royal Prince Alfred Hospital, Sydney (CSA); Adelaide Institute for Sleep Health, Flinders University, Adelaide (RDM, KL, SM), Respiratory and Sleep Services, Southern Adelaide Local Health Network (RDM, SM); Respiratory Department, Hospital Universitari Arnau de Vilanova-Santa María, Lleida (FB); The Second Affiliated Hospital of Soochow University, Soochow (RC); Department of Cardiology, Fuwai Hospital, Beijing (ZL); Instituto do Coracao (Incor) and Hospital Universitario, Sao Paulo (GLF); The First Affiliated Hospital of Guangzhou Medical University, State Key Laboratory of Respiratory Disease, Guangzhou (YL); Neurology Department, All India Institute of Medical Sciences, Delhi (MT); Flinders Centre for Epidemiology and Biostatistics, Flinders University, Adelaide (RW); Department of Preventive Medicine and Public Health, Fukuoka University, Fukuoka (HA); Respiratory Department, Peking University Medical College, Beijing (YX); The First Affiliated Hospital of Nanjing Medical University, Nanjing (ZX).
References
- 1.Lee C.H., Khoo S.M., Tai B.C. Obstructive sleep apnea in patients admitted for acute myocardial infarction: prevalence, predictors, and effect on microvascular perfusion. Chest. 2009;135:1488–1495. doi: 10.1378/chest.08-2336. [DOI] [PubMed] [Google Scholar]
- 2.Lee C.H., Sethi R., Li R. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation. 2016;133:2008–2017. doi: 10.1161/CIRCULATIONAHA.115.019392. [DOI] [PubMed] [Google Scholar]
- 3.Somers V.K., White D.P., Amin R. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing; in collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 4.McEvoy R.D., Antic N.A., Heeley E. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 5.Engleman H.M., Martin S.E., Kingshott R.N., Mackay T.W., Deary I.J., Douglas N.J. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax. 1998;53:341–345. doi: 10.1136/thx.53.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peker Y., Glantz H., Eulenburg C., Wegscheider K., Herlitz J., Thunstrom E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194:613–620. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 7.Barbe F., Duran-Cantolla J., Sanchez-de-la-Torre M. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–2168. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 8.Yu J., Zhou Z., McEvoy R.D. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318:156–166. doi: 10.1001/jama.2017.7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saaresranta T., Hedner J., Bonsignore M.R. Clinical phenotypes and comorbidity in European sleep apnoea patients. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards B.A., Wellman A., Sands S.A. Obstructive sleep apnea in older adults is a distinctly different physiological phenotype. Sleep. 2014;37:1227–1236. doi: 10.5665/sleep.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee R.W., Sutherland K., Chan A.S. Relationship between surface facial dimensions and upper airway structures in obstructive sleep apnea. Sleep. 2010;33:1249–1254. doi: 10.1093/sleep/33.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckert D.J., White D.P., Jordan A.S., Malhotra A., Wellman A. Defining phenotypic causes of obstructive sleep apnea: identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zinchuk A., Jeon S., Koo B. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax. 2018;73:472–480. doi: 10.1136/thoraxjnl-2017-210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vavougios G.D., George D.G., Pastaka C., Zarogiannis S.G., Gourgoulianis K.I. Phenotypes of comorbidity in OSAS patients: combining categorical principal component analysis with cluster analysis. J Sleep Res. 2016;25:31–38. doi: 10.1111/jsr.12344. [DOI] [PubMed] [Google Scholar]
- 15.Ye L., Pien G.W., Ratcliffe S.J. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J. 2014;44:1600–1607. doi: 10.1183/09031936.00032314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagnadoux F., Le Vaillant M., Paris A. Relationship between OSA clinical phenotypes and CPAP treatment outcomes. Chest. 2016;149:288–290. doi: 10.1016/j.chest.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Kao D., Lewsey J., Anand I. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail. 2015;17(9):925–935. doi: 10.1002/ejhf.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins L.M., Lanza S.T. John Wiley & Sons Inc; Hoboken, NJ: 2010. Latent Class and latent transition analysis: with applications in the social, behavioral, and health sciences. [Google Scholar]
- 19.Clogg C.C. Latent class models: recent developments and prospects for the future. In: Arminger G., Clgg C.C., Sobel M.E., editors. Handbook of statistical modeling for the social and behavioral sciences. Plenum Press; New York, NY: 1995. pp. 311–359. [Google Scholar]
- 20.Ueshima H., Sekikawa A., Miura K. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118:2702–2709. doi: 10.1161/CIRCULATIONAHA.108.790048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaggi H.K., Concato J., Kernan W.N., Lichtman J.H., Brass L.M., Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 22.Shahar E., Whitney C.W., Redline S. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 23.Wilson P.W., D'Agostino R.B., Levy D., Belanger A.M., Silbershatz H., Kannel W.B. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 24.Campos-Rodriguez F., Martinez-Garcia M.A., Reyes-Nunez N., Caballero-Martinez I., Catalan-Serra P., Almeida-Gonzalez C.V. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. Am J Respir Crit Care Med. 2014;189:1544–1550. doi: 10.1164/rccm.201311-2012OC. [DOI] [PubMed] [Google Scholar]
- 25.Marin J.M., Carrizo S.J., Vicente E., Agusti A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Ceron E., Fernandez-Navarro I., Garcia-Rio F. Effects of continuous positive airway pressure treatment on glucose metabolism in patients with obstructive sleep apnea. Sleep Med Rev. 2016;25:121–130. doi: 10.1016/j.smrv.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Ceron E., Barquiel B., Bezos A.M. Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. A randomized clinical trial. Am J Respir Crit Care Med. 2016;194:476–485. doi: 10.1164/rccm.201510-1942OC. [DOI] [PubMed] [Google Scholar]
- 28.Joosten S.A., Hamza K., Sands S., Turton A., Berger P., Hamilton G. Phenotypes of patients with mild to moderate obstructive sleep apnoea as confirmed by cluster analysis. Respirology. 2012;17:99–107. doi: 10.1111/j.1440-1843.2011.02037.x. [DOI] [PubMed] [Google Scholar]
- 29.Palma J.A., Iriarte J., Fernandez S., Valencia M., Alegre M., Artieda J. Characterizing the phenotypes of obstructive sleep apnea: clinical, sleep, and autonomic features of obstructive sleep apnea with and without hypoxia. Clin Neurophysiol. 2014;125:1783–1791. doi: 10.1016/j.clinph.2014.01.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient flowchart.
Supplementary material