Abstract
Background
The possible association between β-adrenoceptor antagonists (β-blockers) and risk of COPD is controversial. The objective of the present study was to test whether β-blocker use is associated with susceptibility to the disease.
Methods
A total of 301,542 new users of β-blockers and 1,000,633 new users of any other antihypertensive drugs aged 30–90 years without any history of COPD hospitalizations were included in the present study and followed in the Danish National Patient Registry for incident admissions for COPD and COPD death between 1995 and 2015. Multiple adjusted cox regression models were used to examine the association between use of β-blockers and COPD hospitalization. Additionally, subgroup analyses based on underlying diseases at baseline or duration of treatment were performed.
Findings
People treated with β-blockers continuously for more than 6 months had a lower risk of COPD hospitalization during follow-up compared to people treated with any other antihypertensive drugs (adjusted hazard ratio [HRadjusted] 0·80, 95% CI 0·79–0·82). Risk of COPD hospitalization was lowered in the groups treated with β-blockers among patients with ischemic heart disease (0·72, 0·69–0·75), cardiac arrhythmias (0·76, 0·72–0·80), asthma (0·69, 0·61–0·79), hypertension (0·91, 0·86–0·96), and diseases of the pulmonary circulation (pulmonary embolism and cor pulmonale) (0·72, 0·59–0·87). All-cause mortality as well as risk of COPD death during follow-up was lower in the group treated with β-blockers compared to the group treated with any other antihypertensive drugs (0·56, 0·53–0·59).
Interpretation
Treatment with β-blockers seems to reduce risk of COPD hospitalization and mortality compared to treatment with any other antihypertensive drugs.
Funding
The Danish Council for Independent Research in Denmark (grant no. 4183-00569B), The Research Foundation of Health Science in Region Zealand (grant no. RSSF2017000661 and no. 15-000342), The Research Foundation of Medical Science (A.P. Møller Foundation, grant no. 16-68), The Research Foundation in memory of King Christian 10th (grant no. 142/2017), Aase & Ejnar Danielsen's Research Foundation (grant no. 10-001946), and Lundbeck Foundation (grant no. R252-2017-1690).
Keywords: β-Blockers, Obstructive pulmonary disease, Pharmacology, Epidemiology
Highlights
-
•
Users of β-blockers had an overall 19·7% lower risk of COPD hospitalization compared to users of any other antihypertensive drug during follow-up.
-
•
Users of β-blockers had an overall 44% lower risk of death from COPD compared to users of any other antihypertensive drug.
-
•
Results from this study advocates changes in the present hesitation of treatment with β-blockers in patients at risk of or with concomitant COPD.
Research in context
Evidence before this study
We searched PubMed for studies published before July 1st 2018 on the association between β-blockers and COPD with the search terms “β-blockers OR β-antagonists AND COPD OR obstructive pulmonary disease”, “β-blockers OR β-antagonists AND lung function” and “hypertension AND lung function”. We found no studies evaluating risk of COPD or COPD hospitalizations in individuals treated with β-blockers using an unselected material.
Added value of this study
The results show that treatment with β-blockers, regardless of their selectivity on bronchial tone, was associated with an overall lower risk of COPD hospitalization compared to users of any other antihypertensive drug during follow-up. Stratifying for age and gender did not change this association. Additionally, use of β-blockers was associated with a 44% lower risk of death from COPD compared to use of any other antihypertensive drug.
Implications of all available evidence
Results from this study advocates changes in the present hesitation of treatment of hypertension with β-blockers in patients at risk of or with concomitant COPD.
Alt-text: Unlabelled Box
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory health problem characterized by persistent respiratory symptoms, such as dyspnea, cough, and airflow limitations [1]. The airflow limitation is caused by parenchymal destruction and narrowing of the small airways leading to an increase in small-airway resistance [2], [3]. The respiratory symptoms can be ameliorated with β2-agonists; drugs that stimulate the β2-adrenergic receptors causing relaxation of smooth muscles in the airways and thereby bronchodilation [1].
The β-adrenergic receptors are situated om smooth muscle cells in many organs and are divided into 3 subtypes, β1, β2, and β3-adrenoceptors, according to their specific actions [4]. β1-adrenoceptors are found mainly in the heart where activation increases heart rate and force of contraction [4]. β2-adrenoceptors are primarily found in the airways where activation leads to muscle relaxation and bronchodilation [5]. β-adrenoceptor antagonists (β-blockers) are drugs that block the β-adrenergic receptors and are mainly used in treatment of hypertension, cardiac arrhythmia, myocardial infarction, and ischemic heart disease [4]. β-blockers are divided into β1-selective and non-selective β-blockers, respectively, and the primarily beneficial effects are achieved by blocking the β1-adrenoceptors in the heart and peripheral blood vessels whereby heart rate, force of contraction, and peripheral resistance are reduced [4], [6].
Even though β1-selective β-blockers are targeted specifically towards the β1-adrenoceptors, binding to the β2-adrenoceptor can occur, and a well-known side effect of β-blockers is bronchoconstriction [4], [6]. In line with this, studies have shown that β-blockers are associated with worsening of airway symptoms and reduced lung function in patients with COPD [7], [8], [9]. On the contrary, other studies have shown that treatment with β-blockers reduces risk of exacerbations as well as mortality following myocardial infarction in patients with COPD [10], [11], [12]. However, research on the association between β-blockers and COPD in individuals in the general population is to our knowledge limited. To test the hypothesis that long-term use of β-blockers is associated with COPD in the general population, we have followed users of β-blockers and users of any other antihypertensive drugs (calcium antagonists, ACE inhibitors, angiotensin II receptor antagonists, and diuretics) with no history of COPD hospitalizations and calculated their risk of COPD hospitalization according to treatment exposure.
2. Material and Methods
The present study was based on data registered in four nationwide Danish health registries between January 1995 and December 2015. The paper has been structured according to the STROBE guidelines (Supplementary Table S1).
2.1. Data Sources and Cohorts
2.1.1. The Danish National Prescription Registry
The Danish National Prescription Registry contains data on all prescription drugs sold in Denmark since 1995. The Registry is a sub-register from the Register of Medicinal Products Statistics established by law in 1994, of which researchers at authorized institutions can apply for access to data [13]. Tracking of individual prescription histories is made possible by a unique personal identification number (CPR-number) given to all Danish citizens at birth or upon immigration [14]. In this study, the CPR-numbers were anonymized and replaced by coded identification numbers. Drugs registered in the Prescription Registry are identified by the global Anatomical Therapeutic Chemical classification (ATC) codes: β-blockers, non-selective (C07AA01-C07AA03, C07AA05-C07AA07, C07AA16) and β-blockers, β1-selective (C07AB02-C07AB05, C07AB07, C07AB12). Other antihypertensive drugs: calcium antagonists (C08DA01, C08CA01-C08CA06, C08CA08-C08CA10, C08CA13, C08DB01), ACE inhibitors (C09AA01-C09AA07, C09AA09, C09AA10, C09AA13), angiotensin II receptor antagonists (C09CA01-C09CA04, C09CA06-C09CA08), and diuretics (C03AA01, C03AB01, C03BA11, C03CA01, C03CA02, C03CB02, C03DA01, C03DA04, C03EA01). Specification of antihypertensive drugs is given in the Supplementary Material (Table S2, Fig. S1, Fig. S2).
2.1.2. The Danish National Patient Registry
The Danish National Patient Registry includes discharge diagnoses from all Danish hospitals since 1978 and from outpatient clinics since 1995 [15]. Diseases are classified according to the International Classification of Diseases, 8th revision (ICD-8) through 1993 and 10th revision afterwards [15]. ICD-8 codes for COPD are 490, 491, and 492, and ICD-10 codes are J41, J410, J411, J418, J42, J429, J43, J430, J431, J431A, J432, J438, J439, J439A, J44, J440, J448, J448A, J448C, J449, J47, J479. For each hospitalization, diagnoses directly causing the hospitalization (action diagnoses, labeled with A in the registry) and diagnoses of comorbidities (labeled with B in the registry) are noted. We only included A-diagnoses in the analyses of the study. By that, it is possible to exclude patients hospitalized for other reasons than COPD. Specification of ICD-codes is given in the Supplementary Material (Table S3).
2.1.3. Danish Register of Causes of Death
Since 1871 data on all causes and numbers of deaths occurring in Denmark have been collected [16]. Causes of death have since 1948 been registered according to ICD-codes and the data collected in the Danish Register of Causes of Death [16]. On the death certificate, the diagnosis directly causing death is labeled as the “direct cause of death”, whereas comorbidities not directly causing death are labeled as “secondary diagnoses”. In the present study, we have defined death related to COPD with the above-mentioned ICD-8 and ICD-10 codes when COPD was registered as the direct cause of death, and calculated all-cause mortality and risk of death from COPD between the two treatment groups.
2.2. Study Design
We searched the Danish National Prescription Registry for users of either β-blockers or any other antihypertensive drugs from 1995 to 2015. Only people aged 30 to 90 years without any history of COPD hospitalizations from the Patient Registry were included. Follow-up (Index date) started on the day of the second prescription of either β-blockers or any other antihypertensive drugs within 12 months, as long-term pharmaceutically impaired β2-adrenergic receptor function was defined as > 6 months of treatment. For the analyses of risk of COPD hospitalization, follow-up ended at the date of first COPD admission, date of death, emigration or December 312,015, whichever came first. COPD admission refers to all three types of contacts registered in the Patient Registry; inpatient, outpatient, and emergency department contacts [15].
First hospital contact for COPD after Index date was classified as incident contact. For the control group, we selected users of any other antihypertensive drugs, as this group was considered the most appropriate control group regarding health and lifestyle.
2.3. Statistical Analyses
Hazard ratios (HR) for risk of COPD hospitalization and death from COPD in users of β-blockers were calculated using Cox regression models with adjustment for known confounders: age, sex, calendar year, comorbidities, income level, employment status, and marital status. In the analyses of risk of COPD hospitalization, death is censored.
Taking into account different indications for choice of treatment, we performed subgroup analyses based on different comorbidities at baseline. Subgroups that were specified in the statistical analysis plan (details provided in Supplementary Material) included diabetes mellitus, hypertension, ischemic heart disease, diseases of the pulmonary circulation (pulmonary embolism or cor pulmonale), cardiac arrhythmias (atrial fibrillation, tachycardia, or ventricular disorders), and asthma. In addition, we performed subgroup analyses stratified for age and sex as specified in the statistical analysis plan.
All analyses were performed using SAS statistical software package v. 9.4 for Windows. The study was approved by Statistics Denmark and The Danish Health Data Board (Reg. no.: 704586).
3. Results
3.1. Patient Characteristics
Novel users of either β-blockers or any other antihypertensive drugs without COPD at baseline aged 30–90 years between 1995 and 2015 were included in the current study. Baseline demographic statistics of the cohort are listed in Table 1. The sample population consisted of 301,542 users of β-blockers with a mean age of 58·1 years at baseline, and 1,000,633 users of other antihypertensive drugs with a mean age of 61·6 years at baseline. Among users of β-blockers, 151,420 (50·2%) were females, whereas 535,471 (53·5%) were females in the group of users of any other antihypertensive drugs. Among users of β-blockers, 34,058 (11·3%) were widows/widowers and 193,930 (64·3%) were married. 164,558 (16·4%) in the group of users of any other antihypertensive drugs were widows/widowers while 591,312 (59·1%) were married. Relative numbers of divorced and singles were equally distributed between the two groups of treatment. Relatively more people in the group of users of β-blockers had high incomes compared to users of any other antihypertensive drugs (54·7% vs. 48·6%) which was in line with the distribution of employed people in the two groups (47·1% vs. 39·7%). No differences in the relative numbers of unemployed or people receiving social support were observed between the two groups. Users of β-blockers were more likely to have had a history of ischemic heart disease (22·3% vs. 6·3%) and cardiac arrhythmia (15·2% vs. 3·8%). However, users of any other antihypertensive drugs were more likely to have diabetes mellitus type 1 or type 2 (5·5% vs. 2·6%).
Table 1.
Baseline characteristics of the cohort.
| Users of β-blockers | Users of any other AH drugs | |
|---|---|---|
| Number of participants | 301,542 | 1,000,633 |
| Mean age - years | 58·1 | 61·6 |
| Age – n. (%) | ||
| 30–39 | 28,255 (9·4) | 54,896 (5·5) |
| 40–49 | 56,645 (18·8) | 153,093 (15·3) |
| 50–59 | 77,840 (25·8) | 237,779 (23·8) |
| 60–69 | 72,240 (24·0) | 245,404 (24·5) |
| 70–79 | 48,974 (16·2) | 199,144 (19·9) |
| 80–89 | 17,588 (5·8) | 110,317 (11·0) |
| Sex – n. (%) | ||
| Female | 151,420 (50·2) | 535,471 (53·5) |
| Male | 150,122 (49·8) | 465,162 (46·5) |
| Charlson comorbidity index – n. (%) | ||
| None | 221,332 (73·4) | 723,912 (72·3) |
| 1–2 | 70,020 (23·2) | 229,269 (22·9) |
| 3 + | 10,190 (3·4) | 47,452 (4·7) |
| Comorbidities – n. (%) | ||
| Diabetes type 1/type 2 | 7852 (2·6) | 55,010 (5·5) |
| Hypertension | 29,393 (9·7) | 90,501 (9·0) |
| Ischemic heart disease | 67,180 (22·3) | 63,237 (6·3) |
| Diseases of the pulmonary circulation | 1452 (0·5) | 5267 (0·5) |
| Cardiac arrhythmia | 45,758 (15·2) | 38,068 (3·8) |
| Asthma | 1801 (0·6) | 9108 (0·9) |
| Income level – n. (%) | ||
| Lowest percentile | 67,664 (22·4) | 257,797 (25·8) |
| Low/mid percentile | 68,908 (22·9) | 256,527 (25·6) |
| Mid/high percentile | 79,776 (26·5) | 245,733 (24·6) |
| Highest percentile | 85,134 (28·2) | 240,383 (24·0) |
| Employment status – n. (%) | ||
| Employed | 142,128 (47·1) | 396,886 (39·7) |
| Unemployed | 18,111 (6·0) | 4503 (4·6) |
| On the social | 49,158 (16·3) | 155,232 (15·5) |
| Retired | 92,085 (30·5) | 401,819 (40·2) |
| Civil status – n. (%) | ||
| Widow/widower | 34,058 (11·3) | 164,558 (16·4) |
| Divorced | 37,398 (12·4) | 127,632 (12·8) |
| Married | 193,930 (64·3) | 591,312 (59·1) |
| Single | 36,096 (12·0) | 116,938 (11·7) |
AH = antihypertensive. n = number.
3.2. Risk of COPD Hospitalization
Among the 301,542 users of β-blockers, 17,813 incident COPD hospitalizations were registered between 1995 and 2015, corresponding to 2,743,220 person-years, and an incident rate of 649 cases per 100,000 person-years. In the same period, 71,129 incident cases of COPD hospitalizations were observed among the 1,000,633 users of any other antihypertensive drugs, corresponding to 7,737,523 person-years, and an incident rate of 919 cases per 100,000 person-years.
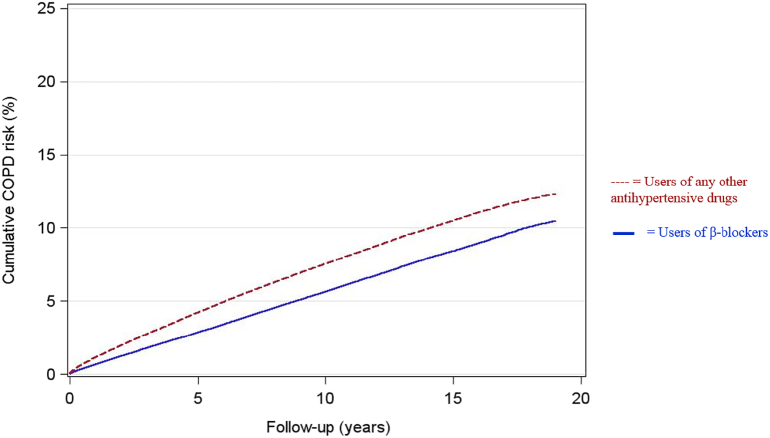
After adjustment for all covariates, users of β-blockers had an overall 19·7% lower risk of COPD hospitalization compared to users of any other antihypertensive drug during follow-up (adjusted hazard ratio [HRadjusted] 0·80, 95% CI 0·79–0·82). HR for COPD hospitalization in the first 5 years after treatment start was 0·75 (0·73–0·77), while exclusion of the first 5 years of follow-up resulted in a HR for COPD hospitalization of 0·85 (0·83–0·87) (Table 2). Stratifying for age and gender did not change the overall association between β-blockers and reduced risk of COPD hospitalization (Table 2). Furthermore, the association of β-blockers remained in those participants taking β-blockers continuously for 5 years (0·84, 0·82–0·87).
Table 2.
Adjusted hazard ratios for COPD hospitalization in users of β-blockers.
| Hazard ratio | 95% CI | |
|---|---|---|
| Overall risk | ||
| β-Blockers | 0·80 | 0·79–0·82 |
| β-Blockers (only first year of follow up) | 0·67 | 0·64–0·71 |
| β-Blockers (0–5 years of follow-up) | 0·75 | 0·73–0·77 |
| β-Blockers (excluding first year of follow-up) | 0·82 | 0·81–0·84 |
| β-Blockers (excluding first 5 years of follow-up) | 0·85 | 0·83–0·87 |
| Stratified by age (years) | ||
| 30–39 | 0·82 | 0·74–0·91 |
| 40–49 | 0·91 | 0·86–0·96 |
| 50–59 | 0·91 | 0·88–0·94 |
| 60–69 | 0·84 | 0·82–0·87 |
| 70–79 | 0·76 | 0·74–0·79 |
| 80–89 | 0·74 | 0·69–0·79 |
| Stratified by gender | ||
| Female | 0·84 | 0·82–0·86 |
| Male | 0·87 | 0·85–0·89 |
| Risk by selectivity on bronchial tone | ||
| β1-Selective β-blockers | 0·79 | 0·78–0·81 |
| Non-selective β-blockers | 0·84 | 0·82–0·87 |
| Stratified by underlying disease | ||
| Diabetes type 1/type 2 | ||
| Yes | 0·95 | 0·87–1·05 |
| No | 0·80 | 0·79–0·81 |
| Hypertension | ||
| Yes | 0·91 | 0·86–0·96 |
| No | 0·79 | 0·78–0·81 |
| Ischemic heart disease | ||
| Yes | 0·72 | 0·69–0·75 |
| No | 0·80 | 0·78–0·82 |
| Diseases of the pulmonary circulation | ||
| Yes | 0·72 | 0·59–0·87 |
| No | 0·80 | 0·79–0·82 |
| Cardiac arrhythmia | ||
| Yes | 0·76 | 0·72–0·80 |
| No | 0·79 | 0·78–0·81 |
| Asthma | ||
| Yes | 0·69 | 0·61–0·79 |
| No | 0·81 | 0·79–0·82 |
Cumulative risk of COPD hospitalizations in the 2 study groups are shown in Fig. 1.
Fig. 1.
Cumulative risk of COPD hospitalizations.
When analyzing the association between risk of COPD hospitalization and β-blockers with respect of their selectivity (or not) on bronchial tone, we did not find any significant difference between β1-selective and non-selective β-blockers. Users of β1-selective blockers had an overall 21% lower risk of COPD hospitalization (adjusted hazard ratio 0·79, 95% CI 0·78–0·81), whereas users of non-selective β-blockers had an overall 16% lower risk of COPD hospitalization (adjusted hazard ratio 0·84, 95% CI 0·82–0·87) compared to users of any other antihypertensive drug (Table 2).
3.3. Subgroup Analyses
As data on indication for the current treatment with either β-blockers or any other antihypertensive drug is not available in the registries, we performed subgroup analyses based on underlying diseases at baseline to avoid confounding by indication (Table 2). No major differences in risk of COPD hospitalization in users of β-blockers from the overall hazard ratio were seen between the groups of patients with ischemic heart disease (0·72, 0·69–0·75), cardiac arrhythmias (0·76, 0·72–0·80), asthma (0·69, 0·61–0·79), hypertension (0·91, 0·86–0·96), and diseases of the pulmonary circulation (pulmonary embolism and cor pulmonale) (0·72, 0·59–0·87). In the group of patients with diabetes mellitus type 1/type 2 (0·95, 0·87–1·05) no effect on COPD hospitalization was seen for β-blockers (Table 2).
Furthermore, propensity score adjustment by inverse probability weighting did not change the overall association between use of β-blockers and COPD. Similar results were found when dividing β-blockers into β1-selective blockers and non-selective blockers (Supplementary Table S4).
3.4. Mortality
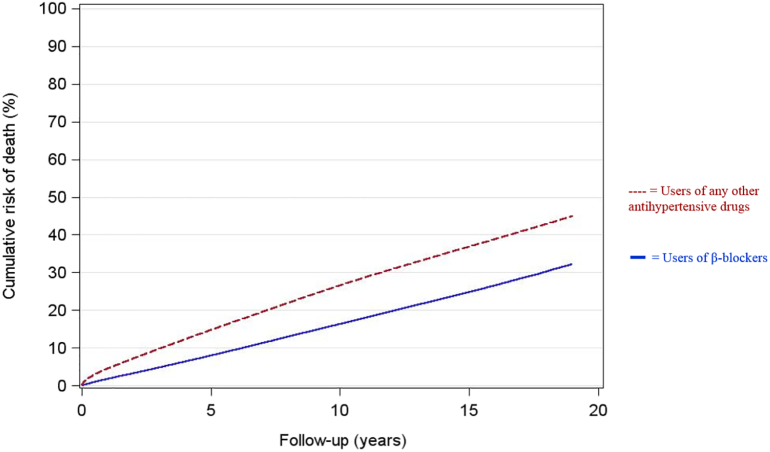
All-cause mortality was substantially lower in users of β-blockers during follow-up. Among the 301,542 users of β-blockers, 52,070 deaths were registered between 1995 and 2015, corresponding to 2,824,553 person-years, and an incident rate of 1843 deaths per 100,000 person-years. In the same period, 251,720 deaths were observed among the 1,000,633 users of any other antihypertensive drugs, corresponding to 8,043,900 person-years, and an incident rate of 3129 deaths per 100,000 person-years.
During follow-up, use of β-blockers was associated with a lower risk of death from COPD compared to use of any other antihypertensive drugs (0·56, 0·53–0·59). The association between β-blockers and reduced risk of COPD death seemed to be persistent when excluding the first 5 years of follow-up (0·72, 0·67–0·78) (Table 3).
Table 3.
Adjusted hazard ratios for COPD death in users of β-blockers.
| Hazard ratio | 95% CI | |
|---|---|---|
| Overall risk | ||
| β-Blockers | 0.56 | 0.53–0.59 |
| β-Blockers (only first year of follow up) | 0.60 | 0.57–0.63 |
| β-Blockers (0–5 years of follow-up) | 0.57 | 0.55–0.60 |
| β-Blockers (excluding first year of follow-up) | 0.58 | 0.55–0.61 |
| β-Blockers (excluding first 5 years of follow-up) | 0.72 | 0.67–0.78 |
Cumulative risk of all-cause mortality in the 2 study groups are shown in Fig. 2.
Fig. 2.
Cumulative risk of all-cause mortality.
4. Discussion
In this large Danish population-based study, we have evaluated the risk of COPD hospitalization in patients treated with β-blockers in the period 1995–2015. The study revealed that treatment with β-blockers, regardless of their selectivity on bronchial tone, was associated with reduced risk of COPD hospitalization, all-cause mortality, and death from COPD compared with other antihypertensive drugs.
This is in line with former studies showing that β-blockers reduce risk of exacerbations in patients with COPD. Du et al. performed in 2014 a meta-analysis including 15 observational cohort studies of β-blocker treatment in patients with COPD and revealed that treatment with β-blockers significantly decreased the risk of exacerbation of COPD [10]. Bhatt et al. performed in 2015 a prospective multicenter observational study including subjects with COPD GOLD stage 2 to 4 and found that β-blockers were associated with a significant reduction in COPD exacerbations regardless of severity of airflow obstruction [11]. The mechanisms behind this beneficial effect of β-blockers on risk of COPD exacerbations have not been clarified. However, it has been shown in a murine model that long-term pharmaceutically blocking of pulmonary β-adrenoceptors led to overexpression of β-receptors [17], and that this in turn could decrease bronchoconstriction [18]. Moreover, long-term administration of β-blockers has been shown to reduce airway inflammation and decrease mucus production in another murine asthma model [19].
Even though several studies have indicated a beneficial effect of β-blockers in patients with COPD, there is still a certain reluctance to prescribe β-blockers to patients with airway symptoms such as cough and shortness of breath, or if the patient has a history of cigarette smoking. The explanation behind the 19·7% overall lowered risk of COPD hospitalization found in this study could be that only the healthiest patients are prescribed β-blockers. To avoid for this “healthy user phenomenon” we have looked at the effect of β-blockers after excluding the first 5 years of follow-up. By this, we assume that we are only following patients free of airway symptoms at baseline, as patients already presenting airway symptoms at baseline are at higher risk of COPD hospitalization in the first 5 years. The adjusted HR after exclusion of the first 5 years of follow-up still showed the beneficial association between β-blockers and risk of COPD hospitalization, suggesting that the reduction seen with β-blockers is a class effect.
Another way of adjusting for the healthy user phenomenon is by stratifying for underlying diseases at baseline. In the groups of patients with ischemic heart disease and cardiac arrhythmia, risk of COPD hospitalization was decreased in users of β-blockers compared to users of any other antihypertensive drugs. This could be explained by the cardioprotective effect of β-blockers, as studies have shown that many COPD exacerbations may have cardiovascular causes [11]. The decreased risk for COPD hospitalization in patients with underlying asthma is surprising as many studies have shown significant reduction in lung function in asthmatics treated with β-blockers [20]. An explanation for this finding could be that some patients with known asthma in treatment with β-blockers when hospitalized with airway symptoms are misclassified as asthma hospitalization when they have COPD exacerbation.
Adjusted hazard ratio for death from COPD during follow-up in users of β-blockers was 0·56 (95% CI 0·53–0·59), suggesting that β-blockers are associated with reduced mortality regardless of underlying diseases. Exclusion of the first 5 years of follow-up did not change this association. This is in line with other studies evaluating risk of mortality in COPD patients treated with β-blockers following myocardial infarction, concluding that β-blockers' apparent benefits on exacerbations are not solely due to a “healthy” user phenomenon [11], [12]. Supporting this, the above mentioned meta-analysis by Du et al. found a 28% reduction in overall mortality in COPD patients treated with β-blockers [10]. However, the present study is to our knowledge the first study to compare risk of mortality in patients treated with either β-blockers versus any other antihypertensive drug.
Major strengths of the current study include 1) a very large unselected sample size, even in the subgroups, and 2) data are collected from completed databases with an almost 100% coverage of the Danish population. However, the study has some limitations and biases as well. A review of 1581 patients with COPD from the Danish Patient Registry showed a positive predictive value for COPD of 92% [21], however, studies have shown that a diagnosis based on clinical assessment or based on spirometry may be incorrect, as external obstruction of the small airways by pulmonary congestion, such as in unrecognized heart failure, also causes obstruction [22], [23], [24]. Importantly, the results from this study remained when stratifying the analysis for ischemic heart disease and cor pulmonale. As the study is based on register-based data, we cannot know for sure if the enrolled participants are actually taking the drugs. However, the Danish National Prescription Registry includes data on drugs handed out to the recipient in drugstores, with additional information on pack size and price. The findings in this study are therefore based on data on drugs sold to the patients and not only prescriptions made from the general practitioner. We therefore assume that individuals are actually in treatment with the drug if it is bought on a regular basis consistently with pack size, as suggested by Kildemoes and colleagues in 2011 [13]. Another potential limitation is the lack of diagnoses reported by general practitioners. In the present study, only patients hospitalized with COPD were included, which can tend to underestimate our risk estimate. For this reason, we supplemented the primary endpoint with all-cause mortality and COPD mortality specifically. Furthermore, we do not have data regarding the participants' smoking habits. Trying to take this into account, we have adjusted for socio-economic data, such as income and employment levels as well as marital status. Several studies have revealed that these socio-economic factors influence smoking status. Pennanen et al. showed that lower education, income, and living without a spouse are factors associated with an increased heaviness of smoking index [25]. Zhang et al. found that current smoking is associated with low socio-economic status and being never married, while former smoking is associated with secondary school education, middle-high income, and being widowed [26]. Thus, we do not think that smoking substantially influenced our study results. Finally, included patients are mainly whites of Danish descent, and the results may not automatically apply to other ethnic groups or to other countries with a significant different lifestyle.
To our knowledge, this is the first study to examine the association between β-blocker use and risk of COPD compared specifically with other antihypertensive drugs. This is important because other cardiovascular drugs (ACE inhibitors and angiotensin II receptor antagonists) also seem to have beneficial effects in COPD [27], [28].
In conclusion, the current study has revealed that long-term treatment with β-blockers was associated with reduced risk of COPD hospitalization, all-cause mortality, and death from COPD compared with treatment with other antihypertensive drugs, and thus β-blockers can be considered as safe, even in patients with COPD.
5. Outstanding questions
To further explore whether patients receiving β-blockers have reduced COPD hospitalizations and COPD deaths, a future randomized clinical trial is warranted.
The following are the supplementary data related to this article.
Supplementary material
STROBE Statement—Checklist of items that should be included in reports of cohort studies.
Antihypertensiva sold regularly in Denmark during the period from 1995 through 2015 by number of individuals who purchased the drug ≥ 2 times pr. year.
Specification of ICD codes for COPD.
Propensity scores adjusted by inverse probability weighting for COPD hospitalization in users of β-blockers.
Supplementary figures
Author Contributions
All authors conceived and designed the study. AON, BS, and MD collected the data. AON and LP did the statistical analyses. All authors contributed to data interpretation. AON wrote the original draft of the paper and all authors reviewed and edited drafts and approved the final version for submission. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Conflict of Interests
All authors declare no competing interests.
Acknowledgements/Funding
The project was financially supported by The Danish Council for Independent Research in Denmark (grant no. 4183-00569B), The Research Foundation of Health Science in Region Zealand (grant no. RSSF2017000661 and no. 15-000342), The Research Foundation of Medical Science (A.P. Møller Foundation) (grant no. 16-68), The Research Foundation in memory of King Christian 10th (grant no. 142/2017), Aase & Ejnar Danielsen's Research Foundation (grant no. 10-001946), and Lundbeck Foundation (grant no. R252-2017-1690). The funding sources are public or nonprofit organizations and support science in general. They had no influence in paper design, data collection, data analysis, interpretation, or writing of the paper.
Contributor Information
Lars Pedersen, Email: lap@clin.au.dk.
Birgitte Fischer Sode, Email: bfischer@dadlnet.dk.
Morten Dahl, Email: modah@regionsjaelland.dk.
References
- 1.From the “Global Strategy for the Diagnosis, Management and Prevention of COPD” Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018. http://goldcopd.org/ [cited 2018 Jun 24]. Available from.
- 2.McDonough J.E., Yuan R., Suzuki M. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogg J.C. A pathologist's view of airway obstruction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(5):5–7. doi: 10.1164/rccm.201206-1130ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rang H., Dale M., Ritter J., Moore P. 5th ed. Elsevier Churchill Livingstone; 2003. Pharmacology; p. 797s. Bd. [Google Scholar]
- 5.Nielsen A.O., Jensen C.S., Arredouani M.S., Dahl R., Dahl M. Variants of the ADRB2 Gene in COPD: Systematic Review and Meta-Analyses of Disease Risk and Treatment Response. COPD. 2017;14:451–460. doi: 10.1080/15412555.2017.1320370. [DOI] [PubMed] [Google Scholar]
- 6.Baker J.G., Wilcox R.G. β-Blockers, heart disease and COPD: current controversies and uncertainties. Thorax. 2017;72(3):271–276. doi: 10.1136/thoraxjnl-2016-208412. [DOI] [PubMed] [Google Scholar]
- 7.Lewis R.V., Lofthouse C. Adverse reactions with beta-adrenoceptor blocking drugs. An update. Drug Saf. 1993;9(4):272–279. doi: 10.2165/00002018-199309040-00005. [DOI] [PubMed] [Google Scholar]
- 8.Clague H.W., Ahmad D., Carruthers S.G. Influence of cardioselectivity and respiratory disease on pulmonary responsiveness to beta-blockade. Eur J Clin Pharmacol. 1984;27(5):517–523. doi: 10.1007/BF00556885. [DOI] [PubMed] [Google Scholar]
- 9.van der Woude H.J., Zaagsma J., Postma D.S., Winter T.H., van Hulst M., Aalbers R. Detrimental effects of β-blockers in copd*: a concern for nonselective β-blockers. Chest. 2005;127(3):818–824. doi: 10.1378/chest.127.3.818. [DOI] [PubMed] [Google Scholar]
- 10.Du Q., Sun Y., Ding N., Lu L., Chen Y. Beta-Blockers Reduced the Risk of Mortality and Exacerbation in Patients with COPD: A Meta-Analysis of Observational Studies. PLoS ONE. 2014;9(11):e113048. doi: 10.1371/journal.pone.0113048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatt S.P., Wells J.M., Kinney G.L. β-Blockers are associated with a reduction in COPD exacerbations. Thorax. 2016;71(1):8–14. doi: 10.1136/thoraxjnl-2015-207251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coiro S., Girerd N., Rossignol P. Association of beta-blocker treatment with mortality following myocardial infarction in patients with chronic obstructive pulmonary disease and heart failure or left ventricular dysfunction: a propensity matched-cohort analysis from the High-Risk Myocardial Infarction Database Initiative. Eur J Heart Fail. 2017;19(2):271–279. doi: 10.1002/ejhf.647. [DOI] [PubMed] [Google Scholar]
- 13.Kildemoes H.W., Sørensen H.T., Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen C.B. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 Suppl):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M., Schmidt S.A.J., Sandegaard J.L., Ehrenstein V., Pedersen L., Sørensen H.T. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health. 2011;39(7_suppl):26–29. doi: 10.1177/1403494811399958. [DOI] [PubMed] [Google Scholar]
- 17.Callaerts-Vegh Z., Evans K.L.J., Dudekula N. Effects of acute and chronic administration of β-adrenoceptor ligands on airway function in a murine model of asthma. Proc Natl Acad Sci U S A. 2004;101(14):4948–4953. doi: 10.1073/pnas.0400452101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGraw D.W., Forbes S.L., Mak J.C.W. Transgenic overexpression of β2-adrenergic receptors in airway epithelial cells decreases bronchoconstriction. Am J Physiol-Lung Cell Mol Physiol. 2000;279(2):L379–L389. doi: 10.1152/ajplung.2000.279.2.L379. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen L.P., Omoluabi O., Parra S. Chronic exposure to beta-blockers attenuates inflammation and mucin content in a murine asthma model. Am J Respir Cell Mol Biol. 2008;38(3):256–262. doi: 10.1165/rcmb.2007-0279RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morales D.R., Jackson C., Lipworth B.J., Donnan P.T., Guthrie B. Adverse respiratory effect of acute β-blocker exposure in asthma: a systematic review and meta-analysis of randomized controlled trials. Chest. 2014;145(4):779–786. doi: 10.1378/chest.13-1235. [DOI] [PubMed] [Google Scholar]
- 21.Thomsen R.W., Lange P., Hellquist B. Validity and underrecording of diagnosis of COPD in the Danish National Patient Registry. Respir Med. 2011;105(7):1063–1068. doi: 10.1016/j.rmed.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Güder G., Brenner S., Störk S., Hoes A., Rutten F.H. Chronic obstructive pulmonary disease in heart failure: accurate diagnosis and treatment. Eur J Heart Fail. 2014;16(12):1273–1282. doi: 10.1002/ejhf.183. [DOI] [PubMed] [Google Scholar]
- 23.Rutten F.H., Moons K.G.M., M-JM Cramer. Recognising heart failure in elderly patients with stable chronic obstructive pulmonary disease in primary care: cross sectional diagnostic study. BMJ. 2005;331(7529):1379. doi: 10.1136/bmj.38664.661181.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenner S., Güder G., Berliner D. Airway obstruction in systolic heart failure--COPD or congestion? Int J Cardiol. 2013;168(3):1910–1916. doi: 10.1016/j.ijcard.2012.12.083. [DOI] [PubMed] [Google Scholar]
- 25.Pennanen M., Broms U., Korhonen T. Smoking, nicotine dependence and nicotine intake by socio-economic status and marital status. Addict Behav. 2014;39(7):1145–1151. doi: 10.1016/j.addbeh.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Zhang D.M., Hu Z., Orton S. Socio-economic and psychosocial determinants of smoking and passive smoking in older adults. Biomed Environ Sci BES. 2013;26(6):453–467. doi: 10.3967/0895-3988.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Vasileiadis I.E., Goudis C.A., Giannakopoulou P.T., Liu T. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers: a promising medication for chronic obstructive pulmonary disease? COPD. 2018;15(2):148–156. doi: 10.1080/15412555.2018.1432034. [DOI] [PubMed] [Google Scholar]
- 28.Mancini G.B.J., Etminan M., Zhang B., Levesque L.E., FitzGerald J.M., Brophy J.M. Reduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006;47(12):2554–2560. doi: 10.1016/j.jacc.2006.04.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
STROBE Statement—Checklist of items that should be included in reports of cohort studies.
Antihypertensiva sold regularly in Denmark during the period from 1995 through 2015 by number of individuals who purchased the drug ≥ 2 times pr. year.
Specification of ICD codes for COPD.
Propensity scores adjusted by inverse probability weighting for COPD hospitalization in users of β-blockers.
Supplementary figures