Abstract
Background
Iodinated and gadolinium-based contrast media (ICM; GBCM) induce immediate hypersensitivity (IH) reactions. Differentiating allergic from non-allergic IH is crucial; allergy contraindicates the culprit agent for life. We studied frequency of allergic IH among ICM or GBCM reactors.
Methods
Patients were recruited in 31 hospitals between 2005 and 2009. Clinical symptoms, plasma histamine and tryptase concentrations and skin tests were recorded. Allergic IH was diagnosed by intradermal tests (IDT) with the culprit CM diluted 1:10, “potentially allergic” IH by positive IDT with pure CM, and non-allergic IH by negative IDT.
Findings
Among 245 skin-tested patients (ICM = 209; GBCM = 36), allergic IH to ICM was identified in 41 (19.6%) and to GBCM in 10 (27.8%). Skin cross-reactivity was observed in 11 patients with ICM (26.8%) and 5 with GBCM (50%). Allergy frequency increased with clinical severity and histamine and tryptase concentrations (p < 0.0001). Cardiovascular signs were strongly associated with allergy. Non-allergic IH was observed in 152 patients (62%) (ICM:134; GBCM:18). Severity grade was lower (p < 0.0001) and reaction delay longer (11.6 vs 5.6 min; p < 0.001). Potentially allergic IH was diagnosed in 42 patients (17.1%) (ICM:34; GBCM:8). The delay, severity grade, and mediator release were intermediate between the two other groups.
Interpretation
Allergic IH accounted for < 10% of cutaneous reactions, and > 50% of life-threatening ones. GBCM and ICM triggered comparable IH reactions in frequency and severity. Cross-reactivity was frequent, especially for GBCM. We propose considering skin testing with pure contrast agent, as it is more sensitive than the usual 1:10 dilution criteria.
Research in Context
Evidence Before This Study
Immediate hypersensitivity (IH) reactions to iodinated contrast media have been an everlasting problem for radiologists. Severe reactions are rare, happen within minutes, and are difficult to handle by an imaging team, which is often not trained or experienced in managing unexpected severe reactions. This leads to a poor prognosis when vasoactive drugs are not immediately used in patients experiencing anaphylactic shock or cardiac arrest. Many studies have attempted to decipher the underlying mechanisms involved in the hope of circumventing them. For decades, a true allergic mechanism was discounted by the community, who have advocated non-specific, so-called “anaphylactoïd” or “pseudo-allergic” reactions, and identified risk factors such as “previous reaction”, “asthma”, and “allergy to drugs”. Several pretreatment protocols have been tested, mainly based on antihistaminic drugs and corticosteroids. However, these do not prevent severe reactions and anaphylactic shocks, which are called “breakthrough reactions”.
Gadolinium chelates used as contrast agents for Magnetic Resonance Imaging were initially thought to be safe and to induce less adverse reactions than iodinated agents. This is probably true for mild reactions, since the osmotic load of a regular gadolinium chelate injection is 4 times lower than an iodinated contrast one. However, severe reactions and cardiovascular arrests have still been described with all the gadolinium chelates available on the market, leading to similar pretreatment strategies despite the lack of evidence supporting them.
Drug allergy is associated with increased tryptase and histamine concentrations in plasma during the first hours of the reaction, and is diagnosed by positive intradermal skin testing with diluted drug solutions. We searched NHL, Embase, and Medline databases with the terms “iodinated contrast media”, “gadolinium”, “allergy”, “hypersensitivity” and “skin tests” and found no study with a prospective design. Other authors have performed skin tests in patients with hypersensitivity reactions to iodinated contrast agents and found allergy in 13 to 65% of reactors. Most of these studies included retrospective cases tested years after the reaction, or lacked precise clinical history, name of culprit agent, or were mixed immediate and delayed reactions. Measurements of plasma histamine and tryptase were not performed. Only a few allergic reactions to gadolinium-based contrast agents have been described as clinical cases.
We conducted the first prospective study of IH reactions to iodinated or gadolinium-based agents. It needed to be multicenter, since the incidence of severe reactions is so low, in order to include a few hundred reactions over the term of the study. Based on an incidence of 0.1% moderate and severe reactions, we included 31 centers from across France that were able to provide allergy testing shortly after the reaction. We assumed that each center could perform at least 7000 injected examinations per year, meaning that 600,000 examinations could be obtained over a 3-year period, so that we could include 600 reactions.
However, after two years, the inclusion rate was lower than expected, and we decided to continue the study for a total of 4.5 years. Between 2005 and 2009, 319 patients presenting with IH reactions to iodinated or gadolinium-based contrast media were included. After appropriate medical treatment, blood sampling for histamine and tryptase measurements was performed, and 6 weeks later an appointment with an allergist for skin testing was organized. All 10 iodinated and 5 gadolinium agents on the French market were tested. An adjudication committee reviewed all the cases, based on clinical history and symptoms, and biochemical and skin test results. The committee classified the reactions as allergic when intradermal skin tests were positive to the culprit contrast solution diluted to the tenth (as recommended by the European Network for Drug Allergy), potentially allergic when skin tests were positive only with the pure solution, and non-allergic otherwise.
Added Value of This Study
To our knowledge, this is the first prospective multicenter study to explore IH reactions to iodinated and gadolinium-based contrast agents. Among 245 skin-tested patients, we identified 41 allergic reactions to iodinated agents, and 10 to gadolinium-based ones. The frequency of allergy increased with the severity of the reaction (9.5% in cutaneous reactions; 22.9% in moderate systemic ones; 52.9% in life-threatening ones, and 100% in cardiac arrest). Similarly, histamine and tryptase concentrations increased with the severity of the reaction, confirming the findings. Cardiovascular symptoms were highly linked to allergy.
The group called “Potentially Allergic” presented clinical symptoms and concentrations of histamine and tryptase intermediate between those of the Allergic and Non-Allergic groups, suggesting that some allergic patients are missed when using the recommended skin testing criteria.
Skin cross-reactivity with non-culprit contrast media diluted 1 in 10 was found for 31.4% of the allergic patients (with 1 to 4 other contrast agents), and in 62.7% with pure solutions (1 to 7 contrast agents).
Implications of the Available Evidence
This prospective study shows that allergy is responsible for 21% (and possibly more) IH reactions to contrast agents. Allergic patients are at high risk of recurrence if skin-test positive contrast media (culprit or non-culprit) are administered. Patients who have experienced life-threatening reactions and cardiovascular symptoms in particular should be managed with the highest care, as they are most probably allergic to one or more other contrast media.
A systematic follow-up of the patients experiencing IH reactions would vastly improve the safety of patients, by blood sampling rapidly after the onset of the reaction to measure histamine and tryptase, and then by sending the patient to an allergist with competence in drug allergy, in order to perform skin tests. The culprit agent should be contraindicated for life, together with the other agents inducing skin cross-reactivity. Since intradermal tests are positive only with the pure solution in some allergic patients, it seems also advisable to perform intradermal tests up to the pure solution in order to increase the sensitivity of diagnosis and detection of cross reactivity.
These results strongly support reorganization of radiology departments, with better identification of previous reactors, elimination of systematic premedication, availability of sampling kits with needles and vials on resuscitation trolleys, and identification of drug allergists to send reacting patients within the 6 weeks to 6 months following the reaction.
Alt-text: Unlabelled Box
1. Introduction
Among adverse events to contrast media (CM), immediate hypersensitivity (IH) reactions [1] raise the highest level of concern for radiologists and patients, since they may lead to severe anaphylactic shock within minutes after injection of CM, sometimes leading to death. The frequency of reactions to iodinated CM (ICM) was reduced with the use of non-ionic ICM in the 90s, but not the frequency of death [2], [3]. Numerous pretreatment protocols have been implemented, but their overall efficacy remains unclear [4]. The frequency of IH reactions to gadolinium-based CM (GBCM) is somewhat lower than with iodinated agents, but the severity can be as high, and deaths have been described with any agent available on the market [5]. Although rare (1/50,000 to 1/200,000), these events require early recognition and awareness of the radiological team.
IH reactions are defined by their onset, less than 1 h after administration of the agent, and by specific clinical signs involving four organs, alone or together: the skin and mucosa, the cardiovascular system, the respiratory and the digestive tracts [6], [7]. IH reactions have been considered for decades to be non-allergic, resulting from non-specific activation of basophils and other biochemical mechanisms, such as the effect of CM hyperosmolarity or complement activation [4]. Over the last 20 years, cumulative evidence has been published in the literature about the involvement of a true allergic mechanism in some IH reactions to contrast material for iodinated agents [6], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], and a few cases have been reported for GBCM [18], [19], [20].
It is important to differentiate allergic from non-allergic reactions [21], because allergy implies immune memory of the epitope, and recurrence (even at very low doses) with the culprit CM and potentially with other CM containing the same epitope (cross-reactivity).
Diagnosis of allergy relies on skin testing with the culprit drug [22]. False positive results may occur with concentrated drug solutions, and false negative results can lead to reaction recurrences. Mediator measurements in blood obtained during the reaction may be useful to ascertain the allergy diagnosis, but are not devoid of pitfalls: blood samples must be obtained within the first hour(s) of reaction because mediator half-life is short (15–20 min for histamine; 90–120 min for tryptase), and tryptase concentrations are rarely increased during mild reactions [9]. Recent reports evaluated skin testing in patients with IH reactions to ICM, with divergent results [23]. This may be because cases were often tested retrospectively, and mediator release was not studied.
The main goal of this study was to elucidate the mechanisms of IH reactions to contrast media and to evaluate the frequency of allergy to CM among them. For this purpose, we conducted a prospective multicenter study in France over the last decade. Due to the rarity of severe reactions, 31 centers were needed over a 5-year period, in order to gather enough data. A large cohort of reacting patients was comprehensively evaluated, using clinical symptoms, mediator evaluation, and skin testing. Secondary goals included the study of cross-reactivity with related CM and of clinical parameters associated with allergic IH.
2. Material and Methods
Patients experiencing ICM- or G-BCM-induced immediate hypersensitivity within 1 h after administration of ICM or GBCM, independently of the route of administration, were prospectively included in this multicenter CIRTACI study involving 31 academic centers between January 2005 and March 2009. Pregnant patients were excluded. The study was approved by the local ethics committee (CCPPRB Paris Broussais HEGP 2004-027) and funded by the French ministry of Health (PHRC 2003, EudraCT 2004-027A4). Written informed consent was obtained from each patient.
2.1. Clinical Symptoms
They were classified according to the Ring and Messmer [7] severity scale. Grade 1 is cutaneous and/or mucosal signs (generalized erythema, extended urticaria, angioedema). Grade 2 includes mild cardiovascular (tachycardia, hypotension) and/or mild respiratory signs (coughing, dyspnea), with or without cutaneous or gastrointestinal signs (severe nausea, vomiting or diarrhea). Grade 3 indicates cardiovascular collapse, possibly associated with bronchoconstriction. Grade 4 is cardiac arrest.
The reaction delay, CM name, management of reaction and clinical outcome, history of previous CM administration and previous reactions, history of allergy or asthma, pretreatment, and usual medications were recorded.
2.2. Plasma Tryptase and Histamine
Blood samples were collected as soon as the patient was clinically stable, then 2 h afterwards, and 24 h later or at skin testing to obtain basal values. Plasma histamine (RIA-histamine, Immunotech, Beckman Coulter, France) (N < 6 nmol/L) and mast cell tryptase (UniCAP Tryptase, Phadia, ThermoFisher, France) (N < 12.5 μg/L) were measured in a single laboratory [9], [24]. Tryptase concentrations during the reaction were compared with basal values if available. Tryptase concentrations were considered increased where the value during the reaction exceeded 1.2 times the basal value plus 2 μg/L, as recommended [25]. Where basal values were lacking, concentrations exceeding 12.5 μg/L were considered increased.
2.3. Skin Testing
The test was scheduled 6 weeks to 6 months after the reaction. Mini-vials of the ten available ICM (amidotrizoate; iobitridol; iodixanol; iohexol; iomeprol; iopamidol; iopromide; ioversol; ioxaglate; ioxithalamate) and five GBCM (gadobenate; gadodiamide; gadopentetate; gadoterate; gadoteridol) were kindly provided by the respective manufacturing companies (Bayer Healthcare, Bracco, GE Healthcare, Guerbet). Prick tests (PT) and intradermal tests (IDT) were performed with the culprit CM and the other related CM, diluted and undiluted, as previously described [12]. PT with a latex emulsion (Stallergenes, Antony, France) were performed. Photographs or drawings were recorded. PT was considered positive if, within 15–20 min of applying the drug solution on the forearm, a wheal equal to at least half the positive control and larger than the negative control appeared. IDT was considered positive if, within 20 min of injecting the drug solution on the back, a wheal (usually pruriginous) equal to at least the double of the injection bleb appeared, surrounded by a flare. CM cross-reactivity with related CM (either ICM or GBCM) was evaluated through PTs and IDTs.
2.4. Consensus Diagnosis and Classification Into 3 Groups
All the cases were reviewed by a consensus panel including 2 allergists, a biochemist, an anesthesiologist, and a radiologist. Patients without typical signs of IH were excluded. Patients were considered allergic (i.e. IgE-mediated allergy) to the culprit CM if the skin testing was positive (either positive prick test or positive IDT up to the 1/10 dilution) [22]. Patients were considered non allergic if all the skin tests were negative. Patients who had a positive IDT only for the pure solution of the culprit CM were classified as potentially allergic.
2.5. Statistical Analysis
Continuous variables were expressed as mean ± SD, and categorical variables as numbers and percentages. They were compared using Student's t- or chi-square tests. Mediator concentrations were log-transformed to obtain normally distributed variables and geometric means were calculated. Between-group comparisons were performed using ANOVA (SAS software version 9.2, SAS Inc., Cary, North Carolina). Cochran Armitage test was performed to search for a tendency between the three groups. Significance was assumed for p < 0.05. The diagnostic odds ratio (DOR) was calculated as the ratio of the symptom frequency for allergic reactions to that for non-allergic ones [26]. The DOR is significant when the lower limit of the 95% confidence interval exceeds 1.
2.6. Role of the Funding Source
The funders of the study (French Ministry of Health) had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Description of the Cohort
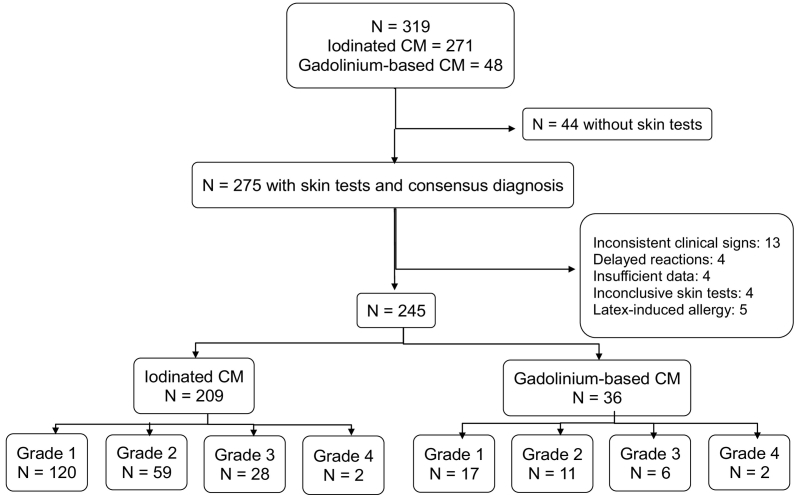
Over the five-year duration of this prospective study, the thirty-one centers enrolled 319 patients, and 275 presented for skin-testing (Fig. 1). Thirty were excluded (latex-induced allergy: 5; inconsistent clinical signs: 13, including 3 vasovagal reactions and 3 with aggravation of pre-existing respiratory symptoms; delayed reactions: 4; abnormal skin reactivity or insufficient data: 8). The final cohort consisted of 245 patients. Histamine concentrations were measured during the reaction in 224 patients and tryptase in 222. Basal values of tryptase were obtained in 192 (142 at skin testing and 50 at 24 h). Seven patients had moderately increased basal tryptase concentrations (range: 12.7–17.8 μg/L).
Fig. 1.
Flow chart and description of the study cohort. Severity grades were evaluated according to the Ring and Messmer scale [7]. CM: contrast material
3.2. Comparative Description of ICM and GBCM Reactions
Reactions occurred following ICM in 209 patients (85.3%) and following GBCM in 36 (14.7%) (Fig. 1). All the ICM and GBCM used in France were involved (Table 1). For reactions after ICM, the mean (S.D.; range) injected volume was 112 mL (49; 4–390). The route of administration of ICM was intravenous for 169 patients (80.9%), intra-arterial for 27 (12.9%) and other (intra-articular: 3; intra-cavity: 3; missing data: 7) for 13 (6.2%). The administered ICM were: iomeprol in 79 patients; iobitridol in 62; iodixanol in 15; iohexol in 16; ioxaglate in 16; iopamidol in 8; ioversol in 5; iopromide in 4; ioxithalamate in 2; and amidotrizoate in 2. For reactions after GBCM, the mean injected volume was 19.7 mL (13.7; 4–75). The route was intravenous. Gadoterate was administered in 10 patients; gadopentetate in 11; gadobenate in 8; gadoteridol in 5 and gadodiamide in 2.
Table 1.
Description of cohort and comparison of patients with allergic; potentially allergic, or non-allergic immediate hypersensitivity reactions to contrast material (CM).
| Total cohort | Allergic | Potentially allergic | Non-allergic | p-Value | |
|---|---|---|---|---|---|
| Number (%), otherwise stated | (N = 245) | (N = 51) | (N = 42) | (N = 152) | |
| Male | 119 (48.6%) | 28 (54.9%) | 22 (52.4%) | 69 (45.4%) | 0.43 |
| Age (y), mean ± SD | 48.8 ± 15.7 | 47.4 ± 16.2 | 49.9 ± 16.8 | 49.0 ± 15.3 | 0.73 |
| BMI (kg/m2), mean ± SD | 25.1 ± 4.6 | 25.3 ± 4.8 | 25.7 ± 4.9 | 24.9 ± 4.5 | 0.56 |
| History of allergy | 77 (31.4%) | 15 (29.4%) | 11 (26.2%) | 51 (33.6%) | 0.62 |
| History of asthma | 11 (4.5%) | 3 (5.9%) | 2 (4.8%) | 6 (3.9%) | 0.84 |
| Pretreatment | 23 (9.4%) | 3 (5.9%) | 7 (16.7%) | 13 (8.6%) | 0.18 |
| Usual medications | 154 (62.9%) | 28 (54.9%) | 24 (57.1%) | 102 (67.1%) | 0.21 |
| Previous CM administration | 165 (67.3%) | 31 (60.8%) | 28 (66.7%) | 106 (69.7%) | 0.50 |
| Previous reaction | 32 (13.1%) | 1 (2.0%) | 11 (26.2%) | 20 (13.2%) | 0.0026 |
| Delay between injection and reaction (min) mean ± SD | 9.7 ± 10.3 | 5.6 ± 4.7 | 7.3 ± 7.7 | 11.6 ± 11.6 | 0.001 |
| Contrast material | 0.272 | ||||
| Iodinated | 209 (85.3%) | 41 (80.4%) | 34 (81.0%) | 134 (88.2%) | |
| Amidotrizoate | 2 (0.8%) | 1(2.0%) | 0 | 1 (0.7%) | |
| Iobitridol | 62 (25.3%) | 10 (19.6%) | 8 (19.1%) | 44 (29.0%) | |
| Iodixanol | 15 (6.1%) | 3 (5.9%) | 1 (0.4%) | 11 (7.2%) | |
| Iohexol | 16 (6.5%) | 2 (3.9%) | 3 (7.1%) | 11 (7.2%) | |
| Iomeprol | 79 (32.2%) | 14 (27.5%) | 15 (37.7%) | 50 (32.9%) | |
| Iopamidol | 8 (3.3%) | 0 | 4 (9.5%) | 4 (2.6%) | |
| Iopromide | 4 (1.6%) | 1 (2.0%) | 1 (2.4%) | 2 (1.3%) | |
| Ioversol | 5 (2.0%) | 1 (2.0%) | 1 (2.4%) | 3 (2.0%) | |
| Ioxaglate | 16 (6.5%) | 9 (17.7%) | 0 | 7 (4.6%) | |
| Ioxithalamate | 2 (0.8%) | 0 | 1 (2.4%) | 1 (0.7%) | |
| Gadolinium-based | 36 (14.7%) | 10 (19.6%) | 8 (19.0%) | 18 (11.8%) | |
| Gadobenate | 8 (3.3%) | 2 (3.9%) | 1 (2.4%) | 5 (3.3%) | |
| Gadodiamide | 2 (0.8%) | 0 | 0 | 2 (1.3%) | |
| Gadopentetate | 11 (4.5%) | 3 (5.9%) | 4 (9.5%) | 4 (2.6%) | |
| Gadoterate | 10 (4.1%) | 3 (5.9%) | 2 (4.8%) | 5 (3.3%) | |
| Gadoteridol | 5 (2.0%) | 2 (3.9%) | 1 (2.4%) | 2 (1.3%) | |
| Severity grade | < 0.0001 | ||||
| Grade 1 | 137 (55.9%) | 13 (25.5%) | 24 (57.1%) | 100 (65.8%) | |
| Grade 2 | 70 (28.6%) | 16 (31.4%) | 10 (23.8%) | 44 (28.9%) | |
| Grade 3 | 34 (13.9%) | 18 (35.3%) | 8 (19.0%) | 8 (5.3%) | |
| Grade 4 | 4 (1.6%) | 4 (7.8%) | 0 | ||
| Increased tryptase concentrationsa | 66/222 (29.7%) | 31/41 (75.6%) | 17/38 (44.7%) | 18/143 (12.6%) | < 0.0001 |
| Increased histamine concentrationsa | 87/224 (38.8%) | 31/42 (73.8%) | 20/37 (54.1%) | 35/145 (24.1%) | < 0.0001 |
Number increased/number tested.
The reactions occurred within 15 min after administration of CM in 75% of patients. The mean delay between injection and reaction was shorter in grade 3–4 reactions (5.4 ± 4.7 min) than in grade 1–2 (10.5 ± 10.9 min) (p = 0.002), and was not different for ICM or GBCM reactions (p = 0.23). The severity grade of the reaction was 1 in 137 patients; 2 in 70; 3 in 34, and 4 in 4 (2 ICM and 2 GBCM) (Fig. 1), and was not different between ICM and GBCM reactions (p = 0.23). Cutaneous/mucous signs were present in 228 patients (93.1%), cardiovascular signs in 67 (27.3%), respiratory signs in 88 (35.9%) and digestive signs in 34 (13.9%). Digestive signs were not observed alone. Sign frequencies were not significantly different between ICM and GBCM (cutaneous signs: p = 0.98; cardiovascular: 0.50; respiratory: 0.87; digestive: 0.19). Sixty-seven patients had prolonged hospital surveillance. All the patients recovered.
3.3. Classification of Reactions
Skin tests were performed with the culprit CM in the 245 patients. Only 5 had positive PT (ICM: 3, GBCM: 2). Fifty had positive IDT to diluted solutions of CM (ICM: 41; GBCM: 9; at dilution 1:10 in 24; 1:100 in 20; 1:1000 in 6), and were classified allergic to CM, together with one patient with positive PT who did not undergo IDT. Among the 194 others, 42 patients had positive IDT to the pure CM solution, and were classified as potentially allergic. The remaining 152 patients had negative IDT and PT, and were diagnosed as non-allergic.
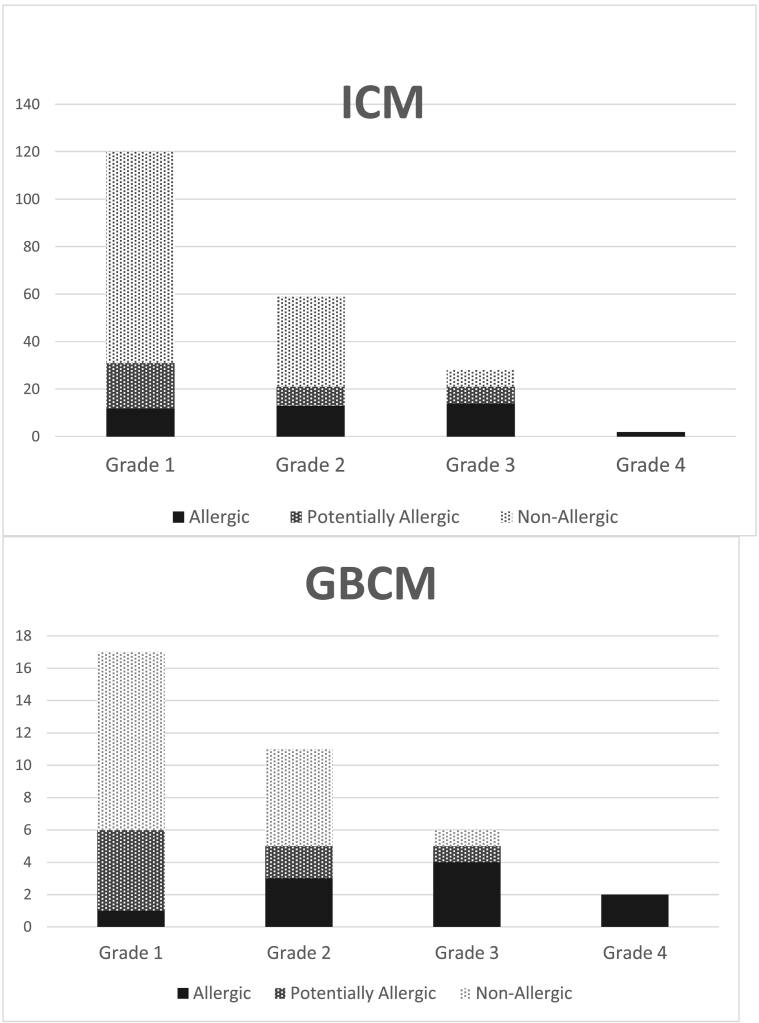
Allergy frequency was not significantly different between ICM (19.6%) and GBCM (27.8%) reactions (p = 0.37). Allergy frequency increased with the clinical severity of the reaction: among patients with grade 1 reactions, 9.5% were allergic; 22.9% among grade 2; 52.9% among grade 3, and 100% among grade 4 (p < 0.0001) (Fig. 2).
Fig. 2.
Number of cases of allergic (black bars) or non-allergic (light gray bars) immediate hypersensitivity according to the severity grade of the reaction. Potentially allergic group is represented in dark gray. ICM: iodinated contrast material; GBCM: gadolinium-based contrast material. The severity grade was determined according to the Ring and Messmer scale [7].
3.4. Patients with Allergic Reactions (Group Allergic)
Group Allergic consisted of 51 patients (28 male; ICM: 41; GBCM: 10) (Table 1). Eight out of the ten ICM and four out of the five GBCM used in France were involved. Fifteen (29.4%) patients described a previous history of allergy (rhinitis: 15; drug: 4; food: 3; other: 11) and 3 of asthma. Three patients (5.9%) reacted despite pretreatment (anxiolytics: 2; missing data: 1), and 20 (39.3%) had never previously received CM. Thirty-one patients had undergone previous contrast procedures: one had had a previous reaction (with the same CM) and 6 had previously received the culprit CM uneventfully. Cutaneous/mucous signs were present in 44 patients (86.3%), cardiovascular signs in 33 (64.7%), respiratory signs in 25 (49.0%), and digestive signs in 12 (23.5%) (Table 2). At least two categories of signs were present in 38 patients (74.5%) (Table 2; see also Supplementary Table 1, which describes the individual associations of signs and their association with allergic IH). Finally, 25.5% of allergic reactions were graded 1; 31.4% were graded 2; and 43.1% were graded 3 or 4 (life-threatening reactions) (Table 1).
Table 2.
Clinical signs (associated or not) reported for allergic and non-allergic reactions and their respective diagnostic odds ratios to predict an allergic hypersensitivity mechanism. Signs are linked to allergic hypersensitivity where the lower limit of the 95% confidence interval (95% CI) of the diagnostic odds ratio exceeds 1.
| Allergic |
Non-allergic |
Diagnostic odds ratio |
|
|---|---|---|---|
| N = 51 |
N = 152 |
[95% CI] for allergy to CM |
|
| Clinical signs | N (%) | N (%) | |
| Cutaneous-mucous signs | 44 (86.3%) | 143 (94.1%) | 0.35 [0.12, 1.02] |
| Digestive signs | 12 (23.5%) | 15 (9.9%) | 2.81 [1.22, 6.5] |
| Respiratory signs | 25 (49.0%) | 48 (31.6%) | 2.08 [1.09, 3.97] |
| Cardiovascular signs | 33 (64.7%) | 23 (15.1%) | 10.28 [4.98, 21.24] |
| Only 1 category of signs | 13 (25.5%) | 96 (63.2%) | 0.2 [0.1, 0.41] |
| 2 categories of signs | 16 (31.4%) | 39 (25.7%) | 1.32 [0.66, 2.64] |
| 3 or 4 categories of signs | 22 (43.1%) | 17 (11.2%) | 6.02 [2.85, 12.74] |
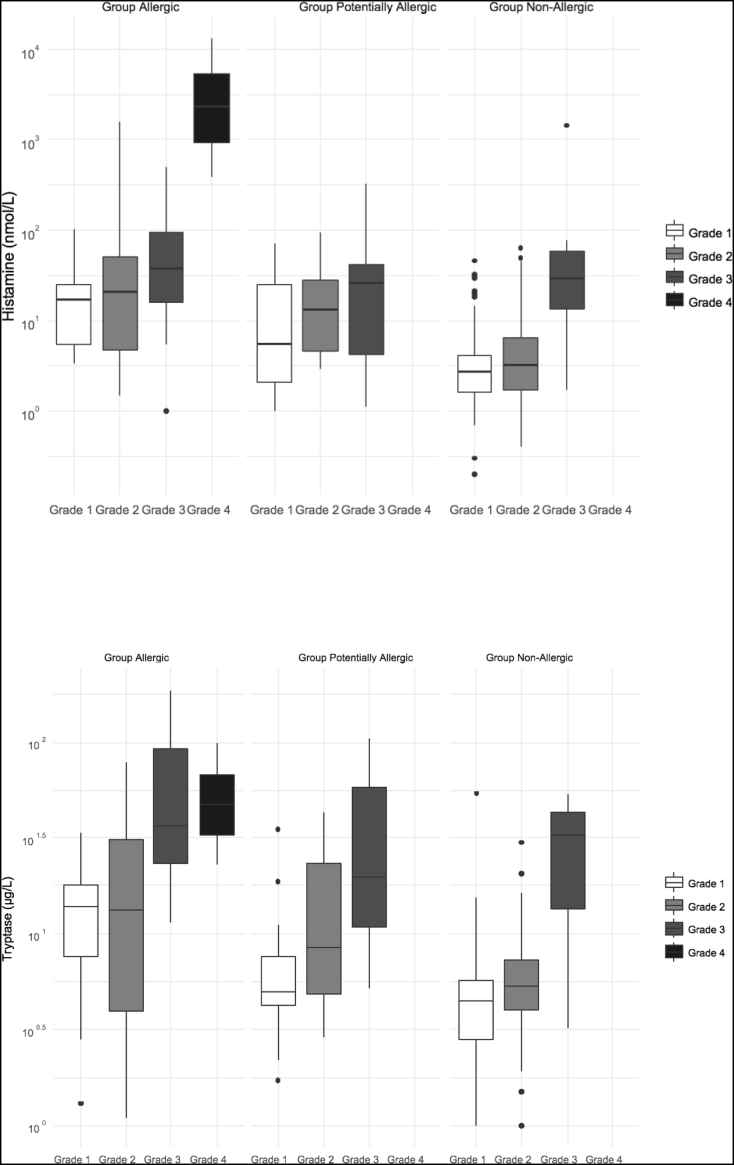
Tryptase concentrations were increased in 75.6% of allergic patients and plasma histamine in 73.8% (Table 1). Concentrations were not significantly different between ICM and GBCM reactions. Tryptase and histamine concentrations increased significantly with the severity grade of the reaction (p < 0.0001 for each) (Fig. 3; see also Supplementary Table 2 which displays the effect of the severity grade on mediator concentrations).
Fig. 3.
Plasma concentrations of histamine (upper panel) and tryptase (lower panel) within the first two hours after the immediate hypersensitivity reaction, according to the severity grade of Allergic, Potentially Allergic, and Non-allergic reactions (logarithmic scale). The severity grade was determined according to the Ring and Messmer scale [7].
Skin cross-reactivity with diluted solutions of the non-administered nine ICM or four GBCM respectively, was positive in 16 patients (31.4%): 11 (26.8%) to ICM (6 to one ICM; 1 to two; 2 to three, and 2 to four) and in 5 (50.0%) to GBCM (4 to one GBCM; and 1 to four), (see Supplementary Tables 3 and 4, which displays the number of cross-reacting CM per patient according to the mechanism of the reaction and its severity grade). The different cross-reacting ICM or GBCM related to the culprit CM appear in Supplementary Fig. 1. With pure solutions, IDT were positive in 32 patients (62.7%); 25 (61%) to ICM (9 to one ICM; 4 to two; 5 to three and 7 to four to seven ICM) and 7 (70.0%) to GBCM (2 to one GBCM; 2 to two; 2 to three and 1 to four). The frequency of cross-reactivity was not different between ICM and GBCM (p = 0.30).
3.5. Patients With Non-allergic Reactions (Group Non-Allergic)
Group Non-Allergic consisted of 152 patients (Table 1) (69 male; ICM: 134; GBCM: 18). A history of allergy was reported in 51 cases (33.6%) (rhinitis: 33; drug: 27; food: 14; other: 23) and asthma in 6. Thirteen patients reacted despite pretreatment (anti-H1: 1; corticosteroids: 3; both: 1; anxiolytics: 8). One hundred and six had undergone previous contrast procedures, 20 had previously reacted (4 with the culprit CM). There were no significant differences between Group Allergic and Group Non-Allergic for gender, age, BMI, history of allergy or asthma, name of administered CM, pretreatment, or previous CM administration (Table 1). Previous CM reactions were more frequent in Group Non-Allergic (p = 0.0026). The severity grade was lower in Group Non-Allergic than in Group Allergic (p < 0.0001) (Table 1) and the reaction delay was longer (11.6 versus 5.6 min; p < 0.001). Cutaneous/mucous signs were present in 143 patients (94.1%); respiratory signs in 48 (31.6%); cardiovascular signs in 23 (15.1%), and digestive signs in 15 (9.9%). In most patients, only one category of signs was present (63.2%) (Table 2 and Supplementary Table 1). Eighteen patients had increased tryptase concentrations (12.6%, 143 tested) and 35 had increased histamine (24.1%, 145 tested) (Table 1; Supplementary Table 2). Histamine and tryptase concentrations were significantly lower than in Group Allergic (p < 0.0001; Fig. 3).
Skin cross-reactivity was negative in all the patients with diluted CM, but positive in 13 (8.6%) with pure solutions, 8 (6.0%) to ICM (5 to one ICM; 1 to two; 1 to three and 1 to four) and 5 (27.8%) to GBCM (2 to one GBCM; 2 to two and 1 to three).
3.6. Clinical Signs Associated With Allergy or Non-allergy
According to diagnostic odds ratios, cardiovascular signs were highly associated with allergy (Table 2), especially when cutaneous or respiratory signs were also present (Suppl Table 1). Respiratory or digestive signs were less clearly associated with allergy and cutaneous signs were not associated. When three or four different organs were affected simultaneously, allergy was highly likely (Table 2). In contrast, non-allergic IH was likely when only one category of signs was present. Single cutaneous manifestations, especially isolated urticaria indicated a high probability of non-allergic reaction (Supplementary Table 1).
3.7. Patients With Potentially Allergic Reactions (Group Potentially Allergic)
Forty-two patients (22 men) had negative IDT with diluted solutions but positive IDT with pure solutions of CM, 34 with ICM and 8 with GBCM (Table 1). A history of allergy was described by 11 patients (26.2%) (rhinitis: 6; drug: 6; food: 5; latex: 1; other: 4) and asthma by 2 (4.8%). Seven (16.7%) had been pretreated (anti-H1: 1; anti-H1 and corticosteroids: 2; anxiolytics: 3; missing data: 1). Twenty-eight had previous CM administration and 11 had previous reactions (6 with the same CM), which was more frequent than in the other groups (p < 0.01). The time delay between CM injection and reaction was intermediate between the shortest (allergic reactions) and the largest ones (non-allergic) (p = 0.001). The severity grade of the reactions was also intermediate between the two other groups (p < 0.0001). Individual clinical signs are reported in Suppl Table 1. Tryptase concentrations were positive in 17 patients (44.7%, 38 tested), and histamine in 20 (54.1%, 37 tested). Mediator concentrations were intermediate between those observed in Groups Allergic and Non-Allergic (p = < 0.0001; Fig. 3). Skin cross-reactivity with diluted CM was positive in one patient (2.4%) to two ICM. With pure solutions, positive tests were obtained in 21 patients (50.0%), 14 (41.2%) to ICM (7 to one ICM; 5 to two and 2 to three) and 7 (87.5%) to GBCM (3 to one GBCM and 4 to two). The frequency of cross-reactivity to ICM was intermediate between those of the two other groups (p < 0.001).
4. Discussion
Immediate hypersensitivity reactions to CM are rare events but are potentially harmful and may lead to death. Clinical identification of such reactions and elucidation of their mechanisms (allergic or non-allergic) are important to allow safe future radiological procedures [14], [16]. Using recommended skin testing in 245 patients with IH reactions, we identified 51 patients with allergic reactions to the administered CM. Cardiovascular signs were significantly associated with allergic IH, confirming previous reports [27], [28] whereas urticaria and bronchospasm were associated with non-allergic IH, contrasting with another report [10]. Among the 51 CM allergic patients, 16 showed skin cross-reactivity to one or more other CM. All CM eliciting positive skin tests should be definitively avoided in future procedures. Cross-reactivity between CM explains the increased risk of recurrence of reaction when the responsible CM is the only contraindicated one [17].
Reactions to ICM have been a concern to radiologists for several decades [2], [3], [4] and, despite the use of non-ionic ICM, severe and fatal reactions still occur. GBCM were first considered as safe, but appear to also elicit IH reactions [5]. Our results reveal that, in terms of the likelihood of an allergy reaction and the severity of the reaction, ICM and GBCM are comparable. We identified 36 cases of reactions to GBCM, compared with 209 reactions to ICM. The ratio of GBCM/ICM reactions was 0.17, which is not dissimilar to the 0.27 market-share ratio in France during the time of the study (personal communication). We found no differences between ICM and GBCM reactions for delay, signs, severity grade of the reaction, or frequency of allergic mechanism. Thirty-nine percent of allergic reactions occurred in patients with no history of CM administration, which confirms other reports [27], [29] and raises the possibility of a sensitizing agent present in the patients' environment, like in ICM-contaminated drinking water [30]. Thus, a potential allergic IH reaction should be considered whenever CM is administered.
Other reports identified allergic IH in 5% to 80% of patients reacting to ICM [23], [28] compared with our finding of 19.6%. Several reasons may account for this variability, such as the type of clinical sign(s) being assessed and the severity of the reaction, or the delay between the reaction and skin tests to ascertain positivity [10], [29]. In our study, we recruited all the patients during the reaction, whereas previous studies recruited patients at skin testing. Thus, the present cohort is homogeneous, the culprit CM is known, and the clinical presentations are well-characterized allowing exclusion of adverse events unrelated to IH, contrary to studies which included retrospective cases or with unknown culprits [10], [11], [29]. Skin test positivity declines with the time elapsed after reaction, thus we performed skin tests within 6 months of reaction to enhance reliability. In the present study, grade 1 reactions (cutaneous/mucous reactions) were the most frequent (55.9% of patients), and appeared allergic in only 9.5% of cases. On the contrary, life-threatening reactions or cardiac arrest were rare (13.9% and 1.6%, respectively) and were diagnosed as allergic IH in 52.9% and 100% of cases, respectively. Thus, recruitment bias may account for the wide range of percentages of allergic IH, especially in patients recruited at skin testing, as patients with severe reactions are more prone to come for investigation than patients with minor ones. Concerning GBCM, we identified allergy in 27.8% of reactions, whereas other authors reported only a few clinical cases [18], [19]. This much higher number found in a prospective study supports the systematic investigation of hypersensitivity reactions to GBCM. We used mediator measurements during the first hours following the reaction as an alternative diagnostic tool, which is rarely used in CM reactions where the radiologists and intensive care doctors concentrate on emergency treatment. Increased tryptase concentrations (indicating mast cell activation) were found in 75.6% of allergic reactions and in 12.6% of non-allergic ones, and increased histamine (indicating mast cell and/or basophil activation) in respectively 73.8% and 24.1%. Concentrations correlated with severity of reactions, as already described in a smaller series [9]. Non-increased values found in some allergic patients could be explained by a low-severity grade of reactions or by a too large delay between reaction and blood withdrawing, compared with mediator half-life. Increased values in some non-allergic patients could be due to non-specific basophil/mast cell activation, which is a possible mechanism of non-allergic reactions [31].
Positive skin tests are considered as the gold standard to diagnose allergy. Concerning CM allergy, prick tests are insufficiently sensitive, as shown by this study and others [23]. IDT with diluted solutions of CM is recommended, and specificity has been calculated in 82 controls as 96.3% but sensitivity is unknown [29]. Concentrated drug solutions may be irritants and produce falsely positive IDT results. However, no positive IDT was reported in 106 controls with pure solutions of iopamidol, ioversol and iobitridol [14]. IDT with pure solutions never induced any systemic side effect in our study and others. In the present study, we identified a group of 42 patients with positive IDT with pure solutions of CM, who would have been classified as non-allergic using the recommended criteria. This group was considered as potentially allergic and the non-allergic group was reduced consequently. The potentially allergic group had more frequently previous reactions than the other groups, but the time delay between CM injection and reaction, severity of the reactions, and frequency of skin cross-reactivity were intermediate between those of the allergic and the non-allergic groups. Furthermore, increased tryptase concentrations were found in 44.7% of potentially allergic reactions and increased histamine in 54.1%, which is intermediate between the frequencies observed for allergic and non-allergic reactions. Put altogether, these findings suggest that the potentially allergic group contains a number of allergic patients, due to a lack of sensitivity of IDT performed with diluted solutions. Consequently, some allergic patients could be missed using the recommended 1:10 dilution IDT. Diluted solutions for IDT could also lead to cross-reactivity frequency being underestimated in allergic patients. In the present study, cross-reactivity was demonstrated in 31.4% of allergic patients with diluted CM solutions, but in 62.7% with pure solutions. This could explain the recurrence of reactions despite the use of an IDT-negative CM for re-administration [16], [23]. Alternatively, a continuum in IH reactions could be considered, with non-allergic reactions corresponding to unresponsiveness to IDT, possibly allergic ones with IDT response to high CM concentrations, and allergic ones responding to low CM concentrations, with the levels of mediators and severity of signs increasing progressively.
Potential pitfalls of this study include the non-exhaustiveness of inclusions in the centers and the lack of drug challenge to confirm the diagnosis. However, re-administration of the culprit CM is contraindicated in allergic patients and is unsafe even with low doses. Low doses of CM may elicit reactions in some patients with negative IDT, and low-dose negative challenge does not prevent reaction to full dose, although these reactions are generally mild [16], [17].
Our study has important consequences for daily radiological practice: Contrary to current opinion, GBCM are not safer than ICM regarding IH reactions, and patients should be managed identically. Patients experiencing their first injection of CM are usually considered as not at risk for allergic IH. However, the present data and data from others [15], [29] indicate that they may be sensitized to CM via some molecule in their environment and should thus be considered as potential reactors.
Patients who have experienced life-threatening reactions and cardiovascular symptoms should be managed with the highest care, as they are most probably allergic to one or more CM. Previous reactors are classically not skin-tested, and usually administered a different CM after pretreatment. Our data show that this approach might be valid in mild, non-allergic reactions (Grade 1 and 2) with low levels of histamine release whose effects can be prevented by antihistaminic drugs. However, it is not safe for the huge levels of histamine release measured for severe reactions, which cannot be challenged at the level of the receptors by antihistaminic drugs. This also explains the occurrence of breakthrough reactions in pretreated patients [32]. A safer approach is to elucidate the mechanism of the reaction through blood and skin testing, and to identify the culprit and cross-reactive agents, thus allowing selection of a safe CM for future opacifications of allergic patients.
Funding
French Ministry of Health (PHRC 2003, EudraCT 2004-027A4).
Acknowledgments/Funding
This study was funded by the French Ministry of Health (PHRC 2003, EudraCT 2004-027A4). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Mini-vials of the ten available ICM (amidotrizoate; iobitridol; iodixanol; iohexol; iomeprol; iopamidol; iopromide; ioversol; ioxaglate; ioxithalamate) and five GBCM (gadobenate; gadodiamide; gadopentetate; gadoterate; gadoteridol) were kindly provided by the respective manufacturing companies for skin testing (Bayer Healthcare, Bracco, GE Healthcare, Guerbet).
Author Contributions
All authors contributed equally to the following 4 criteria:
-
1.
Conception and design of the work and analysis, or interpretation of data for the work.
-
2.
Drafting of the work or revising it critically for important intellectual content.
-
3.
Final approval of the version to be published.
-
4.
Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
DL and LG were responsible for transport and measurements of histamine and tryptase samples.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2018.07.002.
Contributor Information
Olivier Clement, Email: olivier.clement@aphp.fr.
Martine Audebert, Email: audebert.martine@chu-amiens.fr.
Béatrice Benabes-Jezraoui, Email: jezbeatrice@gmail.com.
Sylvie Beot, Email: sbeot@gmx.fr.
Frédéric Berard, Email: frederic.berard@chu-lyon.fr.
Yves Berthezene, Email: yves.berthezene@chu-lyon.fr.
Philippe Bertrand, Email: bertrand@med.univ-tours.fr.
Jean-Luc Bourrain, Email: jl-bourrain@chu-montpellier.fr.
Bruno Boyer, Email: b.boyer@nancy.unicancer.fr.
Marie-France Carette, Email: marie-france.carette@aphp.fr.
Christine Caron-Poitreau, Email: cchristine3@numericable.fr.
Jean Pierre Cercueil, Email: jpcercueil@gmail.com.
Denis-André Charpin, Email: denis-andre.charpin@ap-hm.fr.
Evelyne Collet, Email: ecollet@chu-dijon.fr.
Arielle Crombe-Ternamian, Email: cab.radio.imev@hotmail.fr.
Eric Decoux, Email: eric.decoux@groupecrp.fr.
Pascal Demoly, Email: pascal.demoly@inserm.fr.
Claude Depriester, Email: claude.depriester@yahoo.com.
Alain Didier, Email: didier.a@chu-toulouse.fr.
Martine Drouet, Email: madrouet@chu-angers.fr.
Dominique Dupre-Goetchebeur, Email: domidupre@me.com.
Charles Dzviga, Email: charles.dzviga@wanadoo.fr.
Christine Fabre, Email: christine.fabre@chu-nimes.fr.
Gilbert Ferretti, Email: gferretti@chu-grenoble.fr.
Pascal Girardin, Email: pascal.girardin@wanadoo.fr.
Jacques Giron, Email: jajagila@wanadoo.fr.
Marion Gouitaa, Email: marion.gouitaa@mail.ap-hm.fr.
Nicolas Grenier, Email: nicolas.grenier@chu-bordeaux.fr.
Lydie Guenard Bilbault, Email: guenard-bilbault@orange.fr.
Stéphane Guez, Email: stephane.guez@chu-bordeaux.fr.
Nathalie Gunera-Saad, Email: nathalie.gunera@laposte.net.
Jean-François Heautot, Email: heautot@chu-rennes.fr.
Dominique Herbin, Email: herbin.dominique@wanadoo.fr.
Cyrille Hoarau, Email: hoarauc@med.univ-tours.f.
Claude Jacquot, Email: cldjacq@sfr.fr.
Christian Julien, Email: christjulien@wanadoo.fr.
Laurent Laborie, Email: laborie.laurent@wanadoo.fr.
Claude Lambert, Email: claude.lambert@chu-st-etienne.fr.
Xavier Leclerc, Email: xleclerc@chru-lille.fr.
Laurent Lemaitre, Email: llemaitre@chru-lille.fr.
Agnès Lillo-Le-Louet, Email: agnes.lillo-lelouet@aphp.fr.
Jean-Pierre Louvel, Email: jean-pierre.louvel@chu-rouen.fr.
Nathalie Louvier, Email: nlouvier@dijon.fnclcc.fr.
Nicolas Mennesson, Email: docteurmennesson@gmail.com.
Liliane Metge, Email: liliane.metge@chu-nimes.fr.
Yannick Meunier, Email: yannick.meunier@chu-rouen.fr.
Laurence Monnier-Cholley, Email: laurence.monnier-cholley@sat.aphp.fr.
Mariano Musacchio, Email: Mariano.musacchio@ch-colmar.fr.
Brigitte Nicolie, Email: brnicolie@chu-angers.fr.
Hélène Oesterle, Email: helene.oesterle@ch-colmar.fr.
Francine Paisant-Thouveny, Email: FrThouveny@chu-angers.fr.
Michel Panuel, Email: michel.panuel@mail.ap-hm.fr.
Frédérique Rety-Jacob, Email: frederique.rety-jacob@chu-lyon.fr.
Cécile Rochefort-Morel, Email: cecile.rochefort@chu-rennes.fr.
Catherine Roy, Email: catherine.roy@chru-strasbourg.fr.
Musa Sesay, Email: musa.sesay@chu-bordeaux.fr.
Catherine Sgro, Email: catherine.sgro@gmail.com.
Patrice Taourel, Email: p-taourel@chu-montpellier.fr.
Patrick Terrier, Email: terrier.patrick2@orange.fr.
Odile Theissen, Email: theissen-lavalo@ghrmsa.fr.
Francis Veillon, Email: francis.veillon@chru-strasbourg.fr.
Marie-Claude Vergnaud, Email: vergnaudmc@yahoo.fr.
Charles Veyret, Email: charles.veyret@chu-st-etienne.fr.
Denis Vincent, Email: denis.vincent@chu-nimes.fr.
Benoit Wallaert, Email: bwallaert@chru-lille.fr.
François Wessel, Email: fwessel@wanadoo.fr.
Marc Zins, Email: mzins@hpsj.fr.
Appendix A. Supplementary data
Supplementary material
References
- 1.Johansson S.G.O., Bieber T., Dahl R. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 2.Lasser E.C., Lyon S.G., Berry C.C. Reports on contrast media reactions: analysis of data from reports to the U.S. Food and Drug Administration. Radiology. 1997;203:605–610. doi: 10.1148/radiology.203.3.9169676. [DOI] [PubMed] [Google Scholar]
- 3.Katayama H., Yamaguchi K., Kozuka T., Takashima T., Seez P., Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175:621–628. doi: 10.1148/radiology.175.3.2343107. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman P.L., Seigle R.L. Reactions to radiocontrast material. Anaphylactoid events in radiology. Clin Rev Allergy Immunol. 1999;17:469–496. doi: 10.1007/BF02737651. [DOI] [PubMed] [Google Scholar]
- 5.Prince M.R., Zhang H., Zou Z., Staron R.B., Brill P.W. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol. 2011;196:W138–W143. doi: 10.2214/AJR.10.4885. [DOI] [PubMed] [Google Scholar]
- 6.Brockow K., Christiansen C., Kanny G. Management of hypersensitivity reactions to iodinated contrast media. Allergy. 2005;60:150–158. doi: 10.1111/j.1398-9995.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- 7.Ring J., Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;1:466–469. doi: 10.1016/s0140-6736(77)91953-5. [DOI] [PubMed] [Google Scholar]
- 8.Mita H., Tadokoro K., Akiyama K. Detection of IgE antibody to a radiocontrast medium. Allergy. 1998;53:1133–1140. doi: 10.1111/j.1398-9995.1998.tb03832.x. [DOI] [PubMed] [Google Scholar]
- 9.Laroche D., Aimone-Gastin I., Dubois F. Mechanisms of severe, immediate reactions to iodinated contrast material. Radiology. 1998;209:183–190. doi: 10.1148/radiology.209.1.9769830. [DOI] [PubMed] [Google Scholar]
- 10.Kvedariene V., Martins P., Rouanet L., Demoly P. Diagnosis of iodinated contrast media hypersensitivity: results of a 6-year period. Clin Exp Allergy. 2006;36:1072–1077. doi: 10.1111/j.1365-2222.2006.02532.x. [DOI] [PubMed] [Google Scholar]
- 11.Trcka J., Schmidt C., Seitz C.S., Bröcker E.-B., Gross G.E., Trautmann A. Anaphylaxis to iodinated contrast material: nonallergic hypersensitivity or IgE-mediated allergy? AJR Am J Roentgenol. 2008;190:666–670. doi: 10.2214/AJR.07.2872. [DOI] [PubMed] [Google Scholar]
- 12.Dewachter P., Laroche D., Mouton-Faivre C. Immediate reactions following iodinated contrast media injection: a study of 38 cases. Eur J Radiol. 2011;77:495–501. doi: 10.1016/j.ejrad.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Goksel O., Aydın O., Atasoy C. Hypersensitivity reactions to contrast media: prevalence, risk factors and the role of skin tests in diagnosis—a cross-sectional survey. Int Arch Allergy Immunol. 2011;155:297–305. doi: 10.1159/000320760. [DOI] [PubMed] [Google Scholar]
- 14.Prieto-García A., Tomás M., Pineda R. Skin test-positive immediate hypersensitivity reaction to iodinated contrast media: the role of controlled challenge testing. J Investig Allergol Clin Immunol. 2013;23:183–189. [PubMed] [Google Scholar]
- 15.Salas M., Gomez F., Fernandez T.D. Diagnosis of immediate hypersensitivity reactions to radiocontrast media. Allergy. 2013;68:1203–1206. doi: 10.1111/all.12214. [DOI] [PubMed] [Google Scholar]
- 16.Sesé L., Gaouar H., Autegarden J.-E. Immediate hypersensitivity to iodinated contrast media: diagnostic accuracy of skin tests and intravenous provocation test with low dose. Clin Exp Allergy. 2016;46:472–478. doi: 10.1111/cea.12703. [DOI] [PubMed] [Google Scholar]
- 17.Schrijvers R., Breynaert C., Ahmedali Y., Bourrain J.-L., Demoly P., Chiriac A.M. Skin testing for suspected iodinated contrast media hypersensitivity. J Allergy Clin Immunol Pract. 2018 Jul - Aug;6(4):1246–1254. doi: 10.1016/j.jaip.2017.10.040. (published online Jan 19) [DOI] [PubMed] [Google Scholar]
- 18.Hasdenteufel F., Luyasu S., Renaudin J.-M. Anaphylactic shock after first exposure to gadoterate meglumine: two case reports documented by positive allergy assessment. J Allergy Clin Immunol. 2008;121:527–528. doi: 10.1016/j.jaci.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Galera C., Pur Ozygit L., Cavigioli S., Bousquet P.J., Demoly P. Gadoteridol-induced anaphylaxis - not a class allergy. Allergy. 2010;65:132–134. doi: 10.1111/j.1398-9995.2009.02137.x. [DOI] [PubMed] [Google Scholar]
- 20.Fok J.S., Smith W.B. Hypersensitivity reactions to gadolinium-based contrast agents. Curr Opin Allergy Clin Immunol. 2017;17:241–246. doi: 10.1097/ACI.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 21.Böhm I., Schild H. Contrast-media-induced hypersensitivity or allergic/allergic-like reactions? Suggestion for a more appropriate use of the nomenclature. Eur J Clin Pharmacol. 2008;64:931–932. doi: 10.1007/s00228-008-0527-1. [author reply 933-934] [DOI] [PubMed] [Google Scholar]
- 22.Brockow K., Garvey L.H., Aberer W. Skin test concentrations for systemically administered drugs — an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013;68:702–712. doi: 10.1111/all.12142. [DOI] [PubMed] [Google Scholar]
- 23.Yoon S.H., Lee S.-Y., Kang H.-R. Skin tests in patients with hypersensitivity reaction to iodinated contrast media: a meta-analysis. Allergy. 2015;70:625–637. doi: 10.1111/all.12589. [DOI] [PubMed] [Google Scholar]
- 24.Fisher M.M., Baldo B.A. Mast cell tryptase in anaesthetic anaphylactoid reactions. Br J Anaesth. 1998;80:26–29. doi: 10.1093/bja/80.1.26. [DOI] [PubMed] [Google Scholar]
- 25.Valent P., Akin C., Arock M. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157:215–225. doi: 10.1159/000328760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glas A.S., Lijmer J.G., Prins M.H., Bonsel G.J., Bossuyt P.M.M. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–1135. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 27.Kim M.-H., Lee S.-Y., Lee S.-E. Anaphylaxis to iodinated contrast media: clinical characteristics related with development of anaphylactic shock. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morales-Cabeza C., Roa-Medellín D., Torrado I. Immediate reactions to iodinated contrast media. Ann Allergy Asthma Immunol. 2017;119:553–557. doi: 10.1016/j.anai.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Brockow K., Romano A., Aberer W. Skin testing in patients with hypersensitivity reactions to iodinated contrast media - a European multicenter study. Allergy. 2009;64:234–241. doi: 10.1111/j.1398-9995.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- 30.Böhm I. Iodinated X-ray contrast media in aquatic environment in general and in drinking water in particular: a possible source for the primary sensitization of patients. Chemosphere. 2018;194:28–29. doi: 10.1016/j.chemosphere.2017.11.154. [DOI] [PubMed] [Google Scholar]
- 31.McNeil B.D., Pundir P., Meeker S. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519:237–241. doi: 10.1038/nature14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim Y.S., Choi Y.H., Cho Y.J. Incidence of breakthrough reaction in patients with prior acute allergic-like reactions to iodinated contrast media according to the administration route. Korean J Radiol. 2018;19:352–357. doi: 10.3348/kjr.2018.19.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material