Abstract
Background
As there is limited data on the sustainability of desensitization of multifood-oral immunotherapy (multifood-OIT), we conducted a multisite multifood-OIT study to compare the efficacy of successful desensitization with sustained dosing vs discontinued dosing after multifood-OIT.
Methods
We enrolled 70 participants, aged 5–22 years with multiple food allergies confirmed by double-blind placebo-controlled food challenges (DBPCFCs). In the open-label phase of the study, all participants received omalizumab (weeks 1–16) and multi-OIT (2–5 allergens; weeks 8–30) and eligible participants (on maintenance dose of each allergen by weeks 28–29) were randomized 1:1:1 to 1 g, 300 mg, or 0 mg arms (blinded, weeks 30–36) and then tested by food challenge at week 36. Success was defined as passing 2 g food challenge to at least 2 foods in week 36.
Findings
Most participants were able to reach a dose of 2 g or higher of each of 2, 3, 4, and 5 food allergens (as applicable to the participant's food allergens in OIT) in week 36 food challenges. Using an intent-to-treat analysis, we did not find evidence that a 300 mg dose was effectively different than a 1 g dose in maintaining desensitization, and both together were more effective than OIT discontinuation (0 mg dose) (85% vs 55%, P = 0.03). Fifty-five percent of the intent-to-treat participants and 69% of per protocol participants randomized to the 0 mg arm showed no objective reactivity after 6 weeks of discontinuation. Cross-desensitization was found between cashew/pistachio and walnut/pecan when only one of the foods was part of OIT. No statistically significant safety differences were found between the three arms.
Interpretation
These results suggest that sustained desensitization after omalizumab-facilitated multi-OIT best occurs through continued maintenance OIT dosing of either 300 mg or 1 g of each food allergen as opposed to discontinuation of multi-OIT.
Funding
Sean N. Parker Center for Allergy and Asthma Research at Stanford University, Jeff and MacKenzie Bezos, NIAID AADCRC U19AI104209.
Trial Registration Number
ClinicalTrials.gov number, NCT02626611.
Keywords: Food allergy, Oral immunotherapy, Omalizumab, Sustained unresponsiveness, Food allergen
Research in context
Evidence before this study
There are currently no approved treatments for food allergy; however, oral immunotherapy (OIT) for food allergy has shown promise. Further, recent OIT protocols that include omalizumab have been shown to decrease treatment length and risk of severe reaction for patients with food allergies, particularly for those with multiple food allergies. In a prior single-site placebo-controlled clinical study, we demonstrated that omalizumab-facilitated multifood-OIT improved the efficacy and safety of desensitization at 36 weeks of OIT. However, there remains no information regarding sustainability of clinical reactivity after treatment cessation of multifood-OIT. Limited evidence suggests that patients may require continued consumption of allergens to sustain desensitization; however, whether continued consumption of 300 mg or 1000 mg of each food allergen per day is required by all patients to sustain desensitization is unclear. We searched CENTRAL, Ovid MEDLINE, Embase, the International Clinical Trials Registry Platform, ClinicalTrials.gov, and WHO International Clinical Trials Registry Platform using the search terms “food allergy,” “immunotherapy,” “omalizumab,” “desensitization,” “long-term,” and “sustained.”
Added value of this study
Our results indicate that desensitization with omalizumab-facilitated multifood OIT (up to 5 allergens) can be achieved in the majority of participants at multiple clinical sites within 30 weeks. Importantly, around half of multifood allergic individuals who discontinued therapy for 6 weeks were able to maintain their multifood desensitization. Further, it is suggested that sustained desensitization best occurs through continued maintenance OIT dosing, preferably 1 g or 300 mg per day of each food allergen.
Implications of all the available evidence
Our data demonstrate that omalizumab-facilitated multifood-OIT can be performed in multiple centers with a standardized protocol in a safe manner, with a high rate of completion. Evidence to date suggests that omalizumab-facilitated OIT allows for a rapid and safe dose escalation process. Our study shows sustained desensitization is possible, even after a period of withdrawal, and gives practical information for doses to maintain desensitization to multiple foods. Our trial's impact could improve compliance and quality of life for those participants on multifood OIT. In summary, withdrawal from therapy is possible in around half of patients after omalizumab-facilitated multifood OIT; however, the use of regular, daily therapy is optimal for sustained effect of multifood-OIT.
Alt-text: Unlabelled Box
1. Introduction
Among patients with food allergy, currently estimated in the United States to be approximately 5–8% of young children and about 4% of adults, about 40% of children are allergic to multiple foods [1]. Patients with multiple food (multifood) allergies face additional challenges (as compared to single food allergic patients) related to increased length of oral immunotherapy (OIT) treatment and risk of severe reaction following treatment with OIT. Limited evidence indicates that continued consumption of multifood allergens after OIT to multiple allergens simultaneously (multi-OIT) reduces recurrence of clinical reactivity [2]. We previously reported success of long-term OIT [3], [4]. However, the minimal daily dose required to sustain treatment effect in multi-OIT is unclear. Further, there is no evidence to date to indicate whether discontinuation of multi-OIT will be able to sustain a comparable reduction in recurrence of reactivity to each allergen as that of continued consumption of multi-OIT.
We therefore designed a multisite, double-blind randomized phase 2 study to determine dosing effects on maintenance of desensitization, including a discontinuation arm, in children and adults with multifood allergies. We used omalizumab (Xolair®; Genentech, South San Francisco, CA), an anti-IgE monoclonal antibody, that has been recently shown to facilitate OIT [5], [6], [7], [8], [9], [10], [11]. The primary endpoint was the proportion of participants with no objective allergic reactions on at least 2 food challenges to 2 g protein each of food allergens tested at 36 weeks.
Clinical cross-reactivity occurs in some foods, such as cashew and pistachio or walnut and pecan [12], [13], [14], [15], [16]. An exploratory endpoint was to evaluate whether desensitization with one allergen, such as cashew, can modulate reactivity to a related allergen, such as pistachio, that is not part of the OIT (i.e. cross-desensitization effects). If cross-desensitization is feasible, this has important clinical implications, as patients undergoing OIT to one food may benefit from protection to a related cross-reacting allergen.
2. Methods
2.1. Study Design
We performed a randomized, double-blind controlled phase 2 trial comparing the proportion of participants with sustained desensitization to 2 g protein to at least 2 food allergens following 6 weeks of continued vs discontinued OIT at 7 U.S. clinical sites from January 2016 to November 2016 (Appendix p2). The research protocol was approved by the relevant institutional review boards (IRB) or ethics committees under a Food and Drug Administration approved-Investigational New Drug.
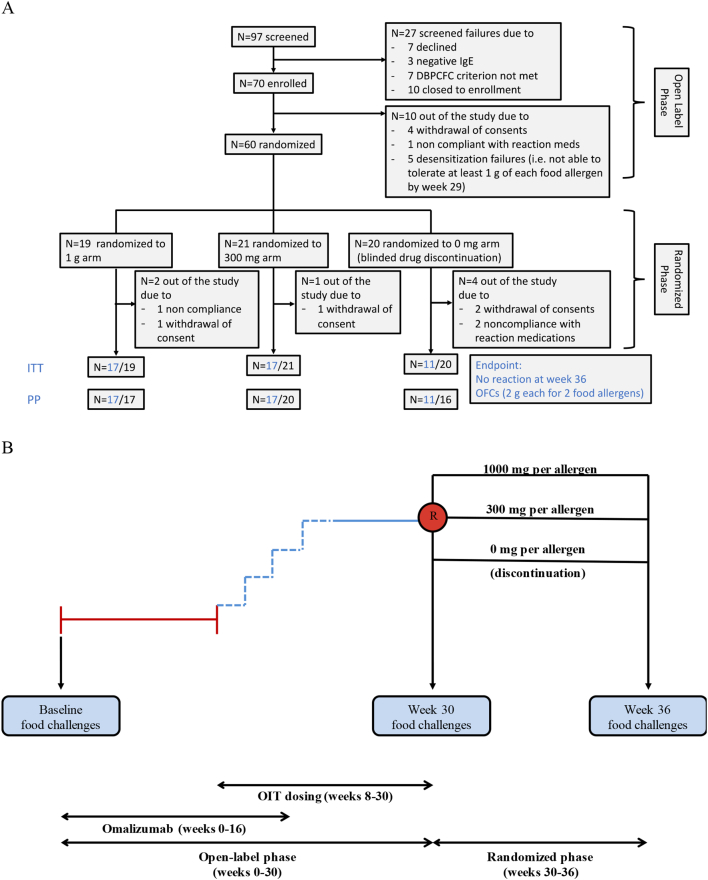
The 36-week study consisted of 2 phases, an open-label (weeks 0–30) and a randomized parallel-arm blinded phase (weeks 30–36) (Appendix p3). All participants in the open-label phase received omalizumab for the first 16 weeks. At week 8, participants underwent an initial rapid desensitization to a minimum of 2 and a maximum of 5 food allergens (depending on the number of foods each participant was allergic to). Participants then came to the sites every two weeks up through weeks 28–29 for investigator-supervised multi-OIT updosing to reach a maintenance dose of ≥ 1 g of each allergen. Participants that reached maintenance (≥ 1 g of each food allergen in the multi-OIT) by weeks 28–29 were randomized and eligible to receive week 30 food challenges. For the randomized phase, eligible participants were randomized into one of three blinded study arms: either of 2 active arms (1 g or 300 mg protein of each food allergen) or a 0 mg arm (a blinded OIT discontinuation arm consisting of an inactive placebo made up of oat flour) (Fig. 1).
Fig. 1.
Consort diagram (A) and study design (B).
At week 36, randomized, blinded participants underwent food challenge to evaluate the efficacy of the active treatment arms. The study was powered to detect a statistical significance (P < 0.05) in the achievement of the primary endpoint on the two treatment arms vs the discontinuation arm at week 36. Allergy skin tests and assessment of peanut-specific IgE and IgG4 were performed at designated time points. Participants who withdrew in the open-label phase or were otherwise not eligible for week 30 food challenge formed the non-randomized arm and were included in the safety analysis.
2.2. Participants
The study population eligibility criteria included individuals 4–55 years of age (actual enrollment: 5–22 years) with multiple food allergies proven by a clinical reaction in DBPCFCs (performed up to 12 months prior to enrolment) at ≤ 125 mg dose, equivalent to a cumulative total of 500 mg food protein. Additional inclusion criteria included a positive skin prick test (SPT) of ≥ 6 mm (wheal diameter, above the negative control) and/or a food-specific IgE concentration of > 4 kU/L for each food, as well as a total IgE of < 2000 kU/L. To be eligible to progress into the randomized phase of the study, participants must have reached a maintenance dose of at least 1 g per food by weeks 28–29. A full list of inclusion and exclusion criteria is shown in the protocol. All participants gave written informed consent.
2.3. Randomization and Masking
Eligible participants were randomly assigned 1:1:1 to 1 g, 300 mg, or 0 mg arms, using a computer and block randomization. Randomization was stratified by site and prepared by a blinded statistician. Enrollment was competitive among sites. Stanford's pharmacy randomized the participants and entered them into their respective site's randomization list sequentially, but only after they were confirmed to be eligible for week 30 food challenge. Upon completion of each participant's end-of-study visit and confirmation of data entry lock, study staff submitted a formal request for participant-specific unblinding to the Stanford Investigational Pharmacist.
2.4. Procedures
Food challenges were performed using standardized, validated, staged doses and were deemed positive if objective symptoms were diagnosed by trained personnel. Screening food challenges were all DBPCFCs and week 30 and week 36 food challenges were either OFCs or DBPCFCs since it was the screening DBPCFC which was important to rule out any placebo-related symptoms. Since OFC and DBPCFC have been shown to be comparable for immediate symptoms within the time frame of the food challenges we performed, we allowed for OFCs to be used instead of DBPCFC to improve participant scheduling [17]. For simplicity, we refer all week 30 and week 36 food challenges as OFCs. For a subset of those participants who had both cashew and pistachio or walnut and pecan food allergy, only cashew or walnut was included in the multifood OIT. Adverse events (AEs) and drug relatedness were evaluated by a trained physician. Dosing of omalizumab was given according to the manufacturer's instructions and the product insert. After the initial dose escalation day, the participants continued at-home self-administration of the combined allergens in their OIT at the maximum tolerated dose, returning every 2–4 weeks for an increase in their daily dose. When participants reached the maintenance dose, this dose was maintained daily until the food challenges at week 30.
2.5. Endpoints
The primary endpoint was the percent of participants who tolerated a food challenge (i.e. no objective reaction of grade 1 or more according to Bock's criteria [18]) a cumulative tolerated dose (CTD) of at least 2 g of each of at least 2 allergens at week 36.
Secondary endpoints included the percent of participants who passed food challenge at week 36 to a CTD of at least 2 g of each of their 3, 4, or 5 food allergens and, separately, the percent of participants who passed a food challenge at week 36 to a CTD of at least 4 g of each of 2 food allergens. Additional secondary outcomes included the concordance between changes in SPT wheal and changes in peanut specific-IgE, IgG4, and IgG4/IgE and clinical outcomes, the cross-desensitization effects of cashew with pistachio and walnut with pecan allergies by SPT and OFCs across arms, and the determination of ‘loss of protection’ of participants defined as non-tolerant after the 6 week withdrawal phase by calculating the food allergen CTD during a food challenge at week 30 compared to week 36.
Safety and AEs were collected as per Good Clinical Practice guidelines and documented at every visit to the clinical research unit. On-call numbers were provided to each participant and family for immediate reporting. In addition, diaries were obtained to obtain information on daily dosing and symptoms.
2.6. Outcomes
The primary outcome was comparing the primary endpoint between the combined active treatment arms versus the 0 mg arm. Secondary outcomes included all pairwise comparisons of the primary and major secondary endpoints.
2.7. Analysis Populations
Intent-to-treat (ITT): All participants who were randomized to one of the three blinded study arms. Per protocol (PP): All participants who underwent at least 2 food challenge to food allergens and one to placebo at week 36. Safety: All participants enrolled in the study.
2.8. Statistical Analysis
We designed our study including our analysis plan prior to participant enrollment. The primary efficacy analyses were conducted using the ITT analysis population. We first compared the 2 active treatment arms to the 0 mg discontinuation arm using a Fisher's exact test (R function fisher.test) [19]. Similarly, secondary analyses involved pairwise comparisons between each arm. Using an exact conditional test of independence in 2 × 2 × k tables (R function mantelhaen.test(…, exact = TRUE)) [20], we additionally compared the primary and main secondary endpoints between arms after adjusting for the number of food allergens in the participant's OIT. Similar methods were used to test whether the results of the primary endpoint differed across the k = 7 sites. Odds ratios (OR) and exact 95% binomial confidence intervals (CI) were reported. Unless otherwise specified, all efficacy analyses were conducted on the ITT population.
Safety was assessed on the safety analysis population, which included participants who were not randomized. The rates of any AEs were compared across the 1 g, 300 mg, and 0 mg discontinuation and non-randomized arms within each of the 3 study periods (weeks 8–16, 17–29 and 30–36) using a Kruskal-Wallis test.
Tests were two-sided and conducted at the 0.05 level of significance. No corrections were made for multiple comparisons as specified in the protocol. All analyses were conducted using R software v3.4.1 [19].
3. Results
3.1. Participants
Seventy participants were enrolled across 7 sites between January 2016 and November 2016 and underwent omalizumab-facilitated multifood-OIT as specified in the protocol. Prior to the randomized phase, 10 participants did not reach the week 30 food challenge (Fig. 1). The remaining 60 participants completed the omalizumab-assisted multi-OIT updosing, and were randomized into one of three blinded arms: 1 g (n = 19), 300 mg (n = 21), or 0 mg (n = 20) of each of their food allergens (Fig. 1).
Baseline characteristics for the ITT population by randomization arm were largely comparable; however, there was a higher proportion of males in the 1 g arm relative to the other two (n = 16, 84% vs n = 11, 52% and n = 10, 50%) (Table 1). The distribution of comorbidities such as asthma, atopic dermatitis and allergic rhinitis varied, where the 1 g arm had relatively higher rate of all conditions relative to the other two arms. Demographics between randomized and not randomized participants were mostly comparable (Table S1).
Table 1.
Demographic and clinical characteristics at baseline (randomized ITT).
| Baseline characteristic | Total (n = 60) | 1 g (n = 19) | 300 mg (n = 21) | Blinded discontinuation (n = 20) |
|---|---|---|---|---|
| Age in years, mean (SD) | 9.8 (3.4) | 9.1 (2.4) | 10.0 (4.0) | 10.3 (3.6) |
| Sex, n (%) | ||||
| Male | 37 (62%) | 16 (84%) | 11 (52%) | 10 (50%) |
| Female | 23 (38%) | 3 (16%) | 10 (48%) | 10 (50%) |
| Ethnicity, n (%) | ||||
| Hispanic | 4 (7%) | 1 (5%) | 1 (5%) | 2 (10%) |
| Non-Hispanic | 56 (93%) | 18 (95%) | 20 (95%) | 18 (90%) |
| History of comorbid conditions, n (%) | ||||
| Asthma | 42 (70%) | 18 (95%) | 13 (62%) | 11 (55%) |
| Atopic dermatitis | 49 (82%) | 17 (89%) | 16 (76%) | 16 (80%) |
| Allergic rhinitis | 50 (83%) | 18 (95%) | 15 (71%) | 17 (85%) |
| FEV1, mean (SD) | 97.1 (12.3) | 95.0 (9.7) | 99.0 (12.4) | 97.1 (14.4) |
| Number allergens in OIT, median (IQR) | 3 (2) | 3 (2) | 3 (3) | 3 (1) |
| Participants with…, n (%) | ||||
| 2 allergens in OIT | 18 (30%) | 5 (26%) | 9 (43%) | 4 (20%) |
| 3 allergens in OIT | 15 (25%) | 6 (32%) | 2 (10%) | 7 (35%) |
| 4 allergens in OIT | 13 (22%) | 3 (16%) | 4 (19%) | 6 (30%) |
| 5 allergens in OIT | 14 (23%) | 5 (26%) | 6 (29%) | 3 (15%) |
| Participants with…, n (%) | ||||
| Almond in OIT | 4 (7%) | 2 (11%) | 1 (5%) | 1 (5%) |
| Cashew in OIT | 34 (57%) | 10 (53%) | 13 (62%) | 11 (55%) |
| Egg in OIT | 17 (28%) | 5 (26%) | 5 (24%) | 7 (35%) |
| Hazelnut in OIT | 17 (28%) | 7 (37%) | 4 (19%) | 6 (30%) |
| Milk in OIT | 20 (33%) | 7 (37%) | 4 (19%) | 9 (45%) |
| Peanut in OIT | 51 (85%) | 17 (90%) | 20 (95%) | 14 (70%) |
| Pecan in OIT | 13 (22%) | 4 (21%) | 5 (24%) | 4 (20%) |
| Sesame in OIT | 8 (13%) | 1 (5%) | 4 (19%) | 3 (15%) |
| Shrimp in OIT | 3 (5%) | 0 (0%) | 2 (10%) | 1 (5%) |
| Soy in OIT | 1 (2%) | 0 (0%) | 1 (5%) | 0 (0%) |
| Walnut in OIT | 31 (52%) | 11 (58%) | 9 (43%) | 11 (55%) |
| Wheat in OIT | 4 (7%) | 1 (5%) | 2 (10%) | 1 (5%) |
| Median CTD across participant's OIT foods in baseline food challenge (mg), median (IQR) | 25 (45.6) | 25 (108.8) | 25 (45) | 13.8 (22.5) |
| CTD by food (mg), median (IQR) | ||||
| Almond | 90 (221.3) | 87.5 (87.5) | 375 (NA) | 5 (NA) |
| Cashew | 5 (22.5) | 5 (23.8) | 5 (5) | 5 (10) |
| Egg | 5 (25) | 25 (20) | 5 (25) | 0 (15) |
| Hazelnut | 25 (20) | 25 (37.5) | 15 (32.5) | 25 (18.8) |
| Milk | 25 (70) | 25 (112.5) | 150 (280) | 5 (20) |
| Peanut | 25 (70) | 25 (370) | 25 (70) | 15 (23.8) |
| Pecan | 5 (20) | 200 (356.3) | 5 (20) | 5 (6.3) |
| Sesame | 15 (21.3) | 25 (NA) | 25 (18.8) | 5 (2.5) |
| Shrimp | 75 (35) | NA | 40 (35) | 75 (NA) |
| Soy | 500 (NA) | NA | 500 (NA) | NA |
| Walnut | 25 (120) | 25 (122.5) | 25 (70) | 75 (110) |
| Wheat | 37.5 (150) | 0 (NA) | 225 (150) | 0 (NA) |
| Total IgE (kU/L), median (IQR) | 739.5 (776.2) | 993.7 (761.5) | 671 (802) | 870 (673.5) |
| Peanut specific IgE (kU/L), median (IQR) | 49.6 (193.1) | 32.9 (81.6, n = 12) | 72.5 (217.8, n = 16) | 84.4 (187.1, n = 10) |
| Peanut specific IgG4 (mg/L), median (IQR) | 0.9 (2.1) | 1.5 (2.1, n = 12) | 0.8 (2.3, n = 16) | 1.2 (1.5, n = 10) |
| Median SPT across participant's OIT foods (mm), median (IQR) | 10.8 (6.1) | 11.5 (6.5) | 10.3 (5) | 11.3 (7.9) |
| SPT (mm), median (IQR) | ||||
| Almond | 6.5 (2) | 7.8 (0.7) | 3.5 (NA) | 6.0 (NA) |
| Cashew | 11.8 (8.3) | 14.0 (9) | 11.5 (4.5) | 11.5 (9.8) |
| Egg | 10.5 (4.5) | 10.0 (1) | 12.0 (3) | 10.0 (6) |
| Hazelnut | 9.5 (15) | 13.5 (8.8) | 8.8 (11.9) | 6.5 (2.9) |
| Milk | 11.8 (9.5) | 11.0 (6.8) | 10.2 (2.4) | 17.5 (10) |
| Peanut | 13.0 (7) | 13.5 (7) | 10.5 (6.9) | 14.8 (7.4) |
| Pecan | 7.5 (4) | 6.8 (2.8) | 10.0 (2.5) | 5.5 (2.6) |
| Sesame | 14.0 (11.9) | 15.0 (NA) | 9.5 (9.4) | 17.0 (10.8) |
| Shrimp | 10.0 (2) | NA | 9.0 (2) | 9.0 (NA) |
| Soy | 10.0 (NA) | NA | 10.0 (NA) | NA |
| Walnut | 11.5 (9.8) | 13.0 (7) | 10.5 (11) | 9.0 (7) [n = 10, 1 NA] |
| Wheat | 9.0 (2.4) | 9.5 (NA) | 8.2 (3.3) | 8.5 (NA) |
OIT: oral immunotherapy; IQR: inner quartile range; NA: not applicable; FEV1: forced expiratory volume; CTD: cumulative tolerated dose; SPT: skin prick test wheal diameter.
Note: n for IgE and skin prick test data per allergen per group given only when different from the number listed under participants with the food in OIT.
3.2. Primary Endpoint
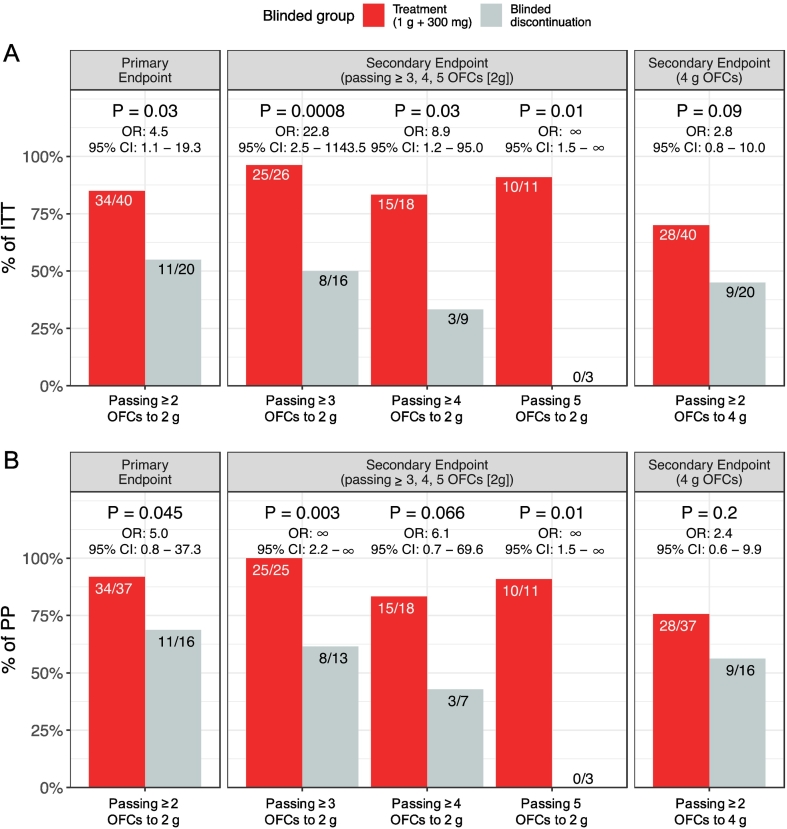
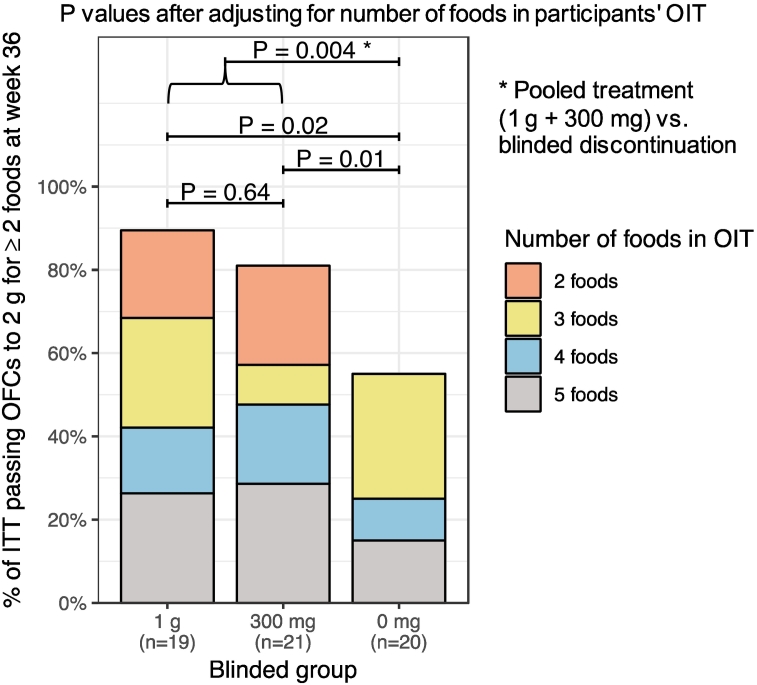
Since participants had a minimum of 2 food allergies each as per screening DBPCFC for enrollment, a minimum of 2 food allergens was tested in the primary endpoint for this study. The ITT participants who remained on active OIT throughout the OIT treatment period were more likely to reach the primary endpoint compared to the discontinuation arm (n = 34/40, 85% vs n = 11/20, 55%; OR: 4.5, 95% CI: 1.1–19.3, P = 0.03) (Table 2, Fig. 2a). This significant difference persisted when the number of food allergens (2, 3, 4 or 5) in the participant's OIT was taken into account (P = 0.004) (Table 2, Fig. 3), and when accounting for enrollment site (OR: 4.9, 95% CI: 1.2–24.1, P = 0.02) (Table S2).
Table 2.
Efficacy outcome for primary endpoint and major secondary endpoints.
| 1 g plus 300 mg (n = 40) Active doses |
Blinded discontinuation (n = 20) |
Odds ratio (95% CI) |
P-valuea | ||
|---|---|---|---|---|---|
| n/participants with that number foods in that group (%) | |||||
| Primary endpoint: Tolerated 2 g of ≥ 2 allergens | |||||
| Total | 34/40 (85%) | 11/20 (55%) | 4.5 (1.1, 19.3) | 0.03 | |
| # foods in OIT | 2 | 9/14 (64%) | 0/4 (0%) | 0.004 | |
| 3 | 7/8 (88%) | 6/7 (86%) | |||
| 4 | 7/7 (100%) | 2/6 (33%) | |||
| 5 | 11/11 (100%) | 3/3 (100%) | |||
| Secondary endpoints | |||||
| Tolerated 4 g of ≥ 2 allergens | |||||
| Total | 28/40 (70%) | 9/20 (45%) | 2.8 (0.8, 10.0) | 0.09 | |
| # foods in OIT | 2 | 5/14 (36%) | 0/4 (0%) | 0.02 | |
| 3 | 7/8 (88%) | 4/7 (57%) | |||
| 4 | 7/7 (100%) | 2/6 (33%) | |||
| 5 | 9/11 (82%) | 3/3 (100%) | |||
| Tolerated 2 g of ≥ 3 allergens | |||||
| Total | 25/26 (96%) | 8/16 (50%) | 22.8 (2.5, 1143.5) | 0.0008 | |
| # foods in OIT | 3 | 7/8 (88%) | 4/7 (57%) | 0.005 | |
| 4 | 7/7 (100%) | 2/6 (33%) | |||
| 5 | 11/11 (100%) | 2/3 (67%) | |||
| Tolerated 2 g of ≥ 4 allergens | |||||
| Total | 15/18 (83%) | 3/9 (33%) | 8.9 (1.2, 95.0) | 0.03 | |
| # foods in OIT | 4 | 4/7 (57%) | 1/6 (17%) | 0.06 | |
| 5 | 11/11 (100%) | 2/3 (67%) | |||
| Tolerated 2 g of 5 allergens | |||||
| 5 (Total) | 10/11 (91%) | 0/3 (0%) | ∞ (1.5, ∞) | 0.01 | |
Fisher's exact test or Exact conditional test of independence in 2 × 2 × k tables.
Fig. 2.
Percent of ITT and PP participants who passed endpoints.
(A) Percentage of ITT participants in the pooled treatment arm (1 g plus 300 mg) and discontinued arm (0 mg) who passed food challenge to 2 g to at least 2 foods (primary endpoint), as well as to at least 3, 4, or 5 foods or at least 2 food challenge to 4 g (secondary endpoints) at week 36. (B) Percentage of PP participants in the pooled treatment arm (1 g plus 300 mg) and discontinued arm (0 mg) who passed food challenge to 2 g to at least 2 foods (primary endpoint), as well as to at least 3, 4, or 5 foods or at least 2 food challenge to 4 g (secondary endpoints) at week 36.
Fig. 3.
Percentage of ITT participants per arm who tolerated 2 g in food challenge to at least 2 foods at week 36. The proportion of participants passing the primary endpoint and having the different numbers of foods (2–5) in their OIT is highlighted. Significance between the arms, including the pooled treatment arm, was assessed taking the number of foods in OIT for each participant into account.
The PP participants showed a difference between the combined treatment (1 g and 300 mg arms) and the 0 mg arm in achieving the primary endpoint (n = 34/37, 92% vs n = 11/16, 69%; OR: 5.0; 95% CI: 0.8–37.3; P = 0.045) (Fig. 1, Fig. 2b), but demonstrated no significant differences between the active treatment arms (1 g vs 300 mg) (n = 17/17, 100% vs n = 17/20, 85%, OR: 0; 95% CI: 0–2.8; P = 0.23). However, we acknowledge that the absence of a difference in these outcomes could simply be the result of limited power (small number of participants, limited period of follow up, and highly selective cohort) of the study to detect such changes.
3.3. Secondary Endpoints
ITT participants in the active treatment arms were significantly more likely to reach the primary endpoint compared to those in the 0 mg discontinuation arm when success was conditional on reaching 2 g to at least 3, 4 or 5 foods in week 36 food challenge (Table 2 and Fig. 2a, P = 0.0008, P = 0.03, and P = 0.01, respectively). These associations also persisted when accounting for the number of foods included in OIT (Table 2).
The percentage of participants in the combined active treatment arms that tolerated 2 g allergen protein to at least 2, 3, 4 or all 5 food allergen food challenge at week 36 were comparatively consistent across the number of allergens passed (between 83% and 96%) (Table 2, Fig. 2a). In contrast, in the 0 mg discontinuation arm the percent of ITT participants who tolerated 2 g allergen protein at week 36 OFCs decreased with increasing number of allergens to be passed; 55% (11/20; 2 allergens), 50% (8/16; 3 allergens), 33% (3/9; 4 allergens), and 0% (0/3; 5 allergens) (Table 2, Fig. 2a), although the low number of participants has to be taken into account when interpreting this.
The participants in the pooled active treatment arms were not more likely to successfully pass a food challenge to 4 g protein of at least 2 food allergens at week 36 compared to the 0 mg discontinuation arm (Table 2, and Fig. 2A; 70% vs 45%; OR: 2.8, 95% CI: 0.8–10.0, P = 0.09). However, when the number of food allergens (2, 3, 4 or 5) in the participant's OIT was taken into account, this was significantly different between the combined active treatment arms and the discontinuation arm (P = 0.02, Table 2).
The active 1 g and 300 mg arms were compared against each other and no significant differences were detected in the rate of tolerating 2 g allergen protein to at least 2 food challenges to 2 different food allergens at week 36 (Table 3, P = 0.66). The 1 g and 300 mg arms were independently tested against the 0 mg discontinuation arm (Tables S3A and S3B). The 1 g arm had a higher likelihood of successfully completing food challenge with 2 g protein of at least 2 food allergens at week 36 compared to the 0 mg discontinuation arm (Table S3A; OR: 6.6, 95% CI: 1.1–47.1, P = 0.03). Differences were also significant when the number of foods in each participant's OIT was taken into account (Table S3A, Fig. 3, P = 0.02). While there was no significant difference in study success between the 300 mg arm vs the 0 mg discontinuation arm (Table S3B, OR: 3.4, 95% CI: 0.7–18.9, P = 0.10), when the number of foods in the multi-OIT was taken into account, the difference in study success rates was significant (Table S3B, Fig. 3, P = 0.01).
Table 3.
Efficacy outcome for 1 g vs 300 mg.
| 1 g (n = 19) |
300 mg (n = 21) |
Odds ratio (95% CI) |
P-valuea | ||
|---|---|---|---|---|---|
| n/participants with that number foods in that group (%) | |||||
| Primary endpoint: Tolerated 2 g of ≥ 2 allergens | |||||
| Total | 17/19 (89%) | 17/21 (81%) | 2.0 (0.2, 24.5) | 0.66 | |
| # foods in OIT | 2 | 4/5 (80%) | 5/9 (56%) | 0.64 | |
| 3 | 5/6 (83%) | 2/2 (100%) | |||
| 4 | 3/3 (100%) | 4/4 (100%) | |||
| 5 | 5/5 (100%) | 6/6 (100%) | |||
| Secondary endpoints | |||||
| Tolerated 4 g of ≥ 2 allergens | |||||
| Total | 14/19 (74%) | 14/21 (67%) | 1.4 (0.3, 7.0) | 0.74 | |
| # foods in OIT | 2 | 2/5 (40%) | 3/9 (33%) | 1.00 | |
| 3 | 5/6 (83%) | 2/2 (100%) | |||
| 4 | 3/3 (100%) | 4/4 (100%) | |||
| 5 | 4/5 (80%) | 5/6 (83%) | |||
| Tolerated 2 g of ≥ 3 allergens | |||||
| Total | 13/14 (93%) | 12/12 (100%) | 0 (0, 45.5) | 1.00 | |
| # foods in OIT | 3 | 5/6 (83%) | 2/2 (100%) | 1.00 | |
| 4 | 3/3 (100%) | 4/4 (100%) | |||
| 5 | 5/5 (100%) | 6/6 (100%) | |||
| Tolerated 2 g of ≥ 4 allergens | |||||
| Total | 7/8 (88%) | 8/10 (80%) | 1.7 (0.1, 117.7) | 1.00 | |
| # foods in OIT | 4 | 2/3 (67%) | 2/4 (50%) | 1.00 | |
| 5 | 5/5 (100%) | 6/6 (100%) | |||
| Tolerated 2 g of 5 allergens | |||||
| 5 (Total) | 5/5 (100%) | 5/6 (83%) | ∞ (0.02, ∞) | 1.00 | |
Fisher's exact test or Exact conditional test of independence in 2 × 2 × k tables.
Presence of cross-desensitization to related allergens was tested for participants, when relevant. Participants receiving OIT to walnut and/or cashew were evaluated for cross-desensitization to pecan and/or pistachio, respectively, although these latter two food allergens were not included in OIT. Cross-desensitization challenges (performed as per the food challenge specified in the study protocol) were optional and walnut (in OIT) and pecan (not in OIT) was tested in 4 participants (1 each in the 1 g and 300 mg arm, and 2 in the 0 mg discontinuation arm). All 4 participants passed the food challenge to walnut as well as pecan at week 36. Interestingly one participant (0 mg arm) reacted to pecan in week 30 (CTD: 750 mg), but passed both the walnut and pecan food challenge at week 36. The median walnut SPT wheal across these 4 participants was 10.5 mm at baseline, 5.5 mm in week 30 and 5 mm in week 36. The median SPT wheal for pecan of these 4 participants was 10 mm at baseline, 4.8 mm in week 30 and 3.5 mm in week 36. Eight participants had cashew in their OIT, were also allergic to pistachio (baseline DBPCFC CTD < 500 mg, not in OIT), and underwent food challenge to cashew and pistachio at baseline and week 36. Three were randomized to the 1 g arm, 3 to the 300 mg arm, and 2 to the 0 mg discontinuation arm. All but one passed the 2 g food challenges to both cashew and pistachio at weeks 36. One participant from the 0 mg discontinuation arm passed the food challenge in week 30 but failed them to cashew as well as pistachio in week 36. The cashew median SPT wheal across these 8 participants was 16 mm at baseline, 10.5 mm in week 30 and 8 mm in week 36. The median SPT wheal for pistachio of these 8 participants was 15 mm at baseline, 8 mm in week 30 and 7 mm in week 36. Therefore, in summary, not all but most participants with either walnut or cashew in the multi food OIT were cross-desensitized to pecan (4/4 in week 36, 3/4 in week 30) or pistachio (7/8 in week 36, 8/8 in week 30), respectively.
3.3.1. Immunologic Parameters
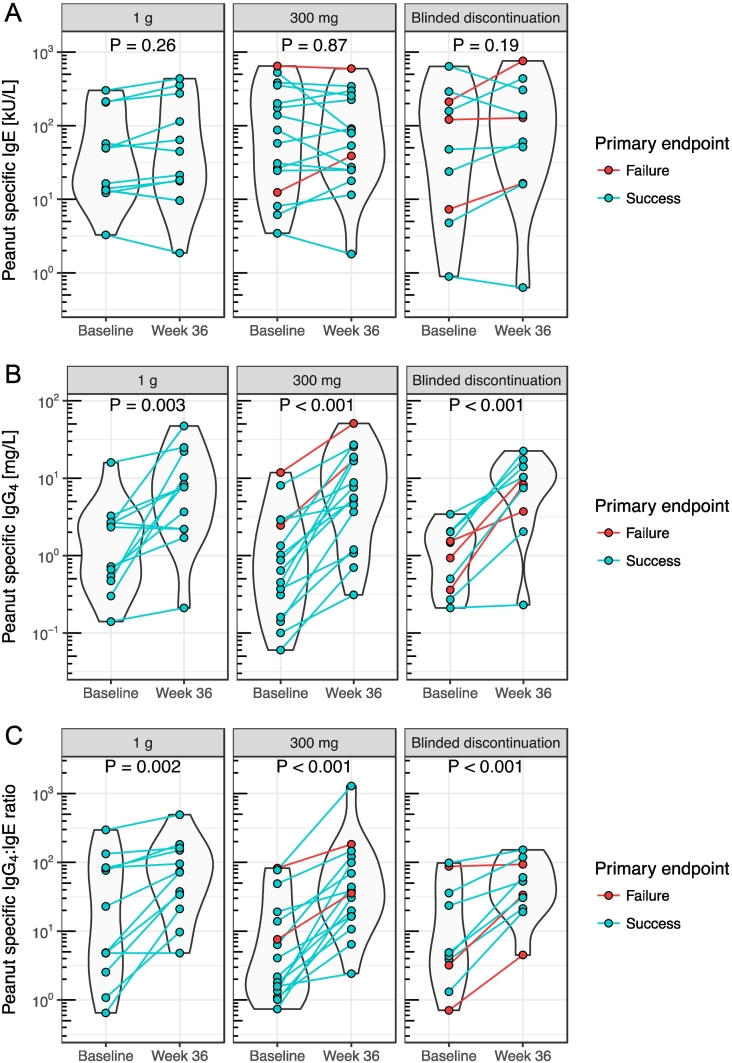
No significant difference in peanut-specific IgE at baseline was detected between participants that met or failed the primary endpoint containing peanut in their multi-OIT (Fig. S1A, P = 0.71). Peanut-specific IgE, IgG4, and IgG4/IgE levels at baseline and week 36 stratified by blinded study arm are shown in Fig. 4. In all three study arms, a significant increase in peanut-specific IgG4/IgE from baseline to week 36 can be seen (Fig. 4C). This was primarily driven by significant increases in IgG4 rather than changes in IgE (Fig. 4). When stratified by primary endpoint outcome, even the study failures showed a significant increase in peanut-specific IgG4/IgE from baseline to week 36 (Fig. S2C). Independent of the outcome of the food challenge at week 36, the SPT wheal did not show significant difference for any food, stratified by the three randomization groups between week 30 and week 36.
Fig. 4.
Peanut specific IgE, IgG4 and IgG4:IgE stratified by blinded study group.
Peanut specific IgE (A), IgG4 (B) and IgG4:IgE (C) levels of all ITT participant at baseline and week 36 stratified by blinded study group. (P values by F test in linear mixed effects model.)
3.4. Safety
Overall, omalizumab-facilitated multi-OIT was found to be safe with no clinically significant differences in AE rates between the 1 g, 300 mg, 0 mg discontinuation and non-randomized arms (Tables 4 and S4). Antihistamine and inhaler use were similar across arms (Table S5). Higher number of doses of injectable epinephrine occurred in the 1 g (4 doses of injectable epinephrine) vs discontinuation (1 dose of injectable epinephrine) vs 300 mg (no doses of injectable epinephrine) arms (Table S6). No cases of life-threatening anaphylaxis or eosinophilic esophagitis occurred during the study. None of the baseline characteristics were shown to be significantly associated with the percent of AE doses (Table S7).
Table 4.
Number and percentage of participants that experienced Adverse Events (AEs) by week range and randomization arm including those non-randomized.
| Study arm and period | Number of participants in phase and arm | Any AE |
Organ system |
Treated |
Grade AEb |
P-valuea |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GI |
Generalc |
Resp |
Skin |
Otherd |
1 |
2 |
3 |
|||||
| Number of participants with AEs (percentage of total) | ||||||||||||
| Weeks 8–16 (Omalizumab & OIT) | ||||||||||||
| 1 g | 19 | 11 (58%) | 10 (53%) | 0 | 2 (11%) | 3 (16%) | 1 (5%) | 5 (26%) | 10 (53%) | 6 (32%) | 0 | 0.75 |
| 300 mg | 21 | 16 (76%) | 16 (76%) | 4 (19%) | 3 (14%) | 7 (33%) | 2 (10%) | 11 (52%) | 16 (76%) | 10 (48%) | 0 | |
| Discontinuation | 20 | 15 (75%) | 14 (70%) | 1 (5%) | 4 (20%) | 6 (30%) | 3 (15%) | 7 (35%) | 14 (70%) | 8 (40%) | 0 | |
| Non-randomized | 10 | 9 (90%) | 9 (90%) | 1 (10%) | 5 (50%) | 2 (20%) | 1 (10%) | 8 (80%) | 8 (80%) | 8 (80%) | 0 | |
| Weeks 17–29 (OIT) | ||||||||||||
| 1 g | 19 | 13 (68%) | 10 (53%) | 2 (11%) | 6 (32%) | 6 (32%) | 2 (11%) | 9 (47%) | 12 (63%) | 8 (42%) | 0 | 0.90 |
| 300 mg | 21 | 18 (86%) | 16 (76%) | 7 (33%) | 6 (29%) | 6 (29%) | 4 (19%) | 14 (67%) | 17 (81%) | 13 (62%) | 1 (5%) | |
| Discontinuation | 20 | 19 (95%) | 18 (90%) | 3 (15%) | 8 (40%) | 5 (25%) | 2 (10%) | 10 (50%) | 17 (85%) | 11 (55%) | 0 | |
| Non-randomized | 9 | 9 (100%) | 9 (100%) | 0 | 4 (44%) | 3 (33%) | 2 (22%) | 7 (78%) | 9 (100%) | 7 (78%) | 0 | |
| Weeks 30–36 (Randomized withdrawal/tolerance) | ||||||||||||
| 1 g | 19 | 9 (47%) | 5 (26%) | 3 (16%) | 6 (32%) | 5 (26%) | 1 (5%) | 7 (37%) | 9 (47%) | 6 (32%) | 0 | 0.07 |
| 300 mg | 21 | 16 (76%) | 11 (52%) | 5 (24%) | 4 (19%) | 6 (29%) | 0 | 10 (48%) | 10 (48%) | 9 (43%) | 0 | |
| Discontinuation | 20 | 10 (50%) | 10 (50%) | 1 (5%) | 1 (5%) | 0 | 0 | 5 (25%) | 9 (45%) | 4 (20%) | 0 | |
| Non-randomized | 6 | 4 (67%) | 4 (67%) | 0 | 1 (17%) | 0 | 0 | 0 | 4 (67%) | 0 | 0 | |
GI: gastrointestinal; Resp: respiratory; OIT: oral immunotherapy.
Note: AEs during dosing days are included in this table. Allergic AEs are included.
Based on Kruskal-Wallis test comparing ‘Any AE’ between all four study arms.
CTCAE v.4.03 grade where 1, 2, 3 is grade 1, 2, and 3, respectively.
General indicates skin reactions at injection site.
Other indicates eye, nervous system, or vascular reactions.
After the end of the study, participants were offered OIT of their food allergens in outpatient clinics.
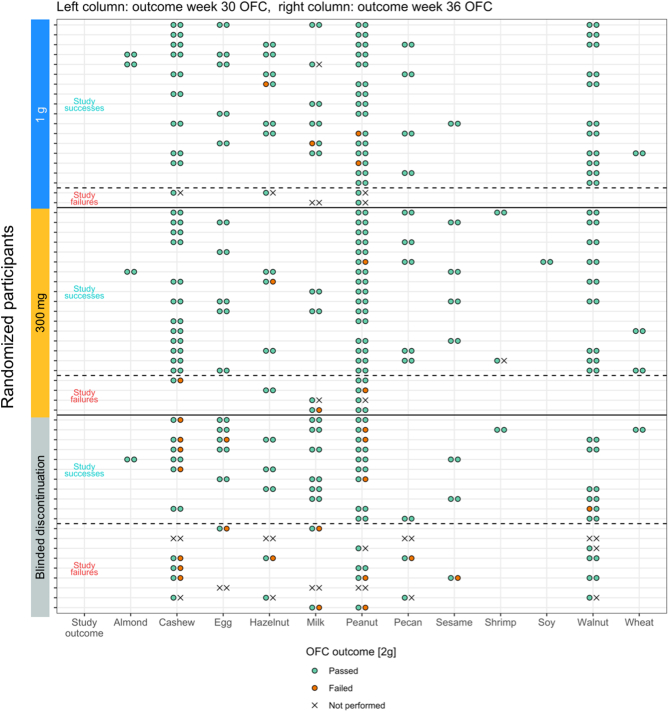
3.5. Additional Analyses
Figs. 5 and S3 show an overview of the foods in each participant's OIT regimen and the outcome of the week 36 food challenges. Even the nine participants who failed the primary endpoint in the 0 mg arm continued to maintain a higher dose threshold if week 36 food challenge were performed (median of median CTD per participant was 15 mg (IQR: 12.5 mg) at baseline and 750 mg (IQR: 375, n = 5) at week 36) after blinded discontinuation. In the 0 mg discontinuation arm, some foods had a higher OFC failure rate in week 36 than other foods. For example, of 9 participants that discontinued OIT with cashew (median CTD at baseline: 5 mg), 7 did not pass the food challenge at week 36, whereas of 8 participants that discontinued OIT with walnut (median CTD at baseline: 75 mg), 8 passed the food challenge at week 36 (Figs. 5 and S3). In more detail, of the 5 participants in the 0 mg discontinuation arm with cashew in their OIT who failed the primary endpoint, 3 underwent a food challenge at week 36 to cashew and all failed it. But even of the 6 participants with cashew OIT who passed the primary endpoint, 4 failed their week 36 cashew food challenge (each with a CTD of 750 mg) and only 2 passed it. All these participants passed the week 30 food challenges to cashew if performed (Fig. 5). Overall, across the three study arms all week 30 cashew food challenges were passed, showing successful desensitization for cashew. The food with the second highest rate of participants who failed to tolerate 2 g of allergen protein at week 36 food challenge within the 0 mg arm was peanut. Of the 9 participants that met the primary endpoint with peanut in their OIT, 3 failed their week 36 peanut food challenge (two with a CTD of 750 mg and one with 0 mg, Fig. S3).
Fig. 5.
Overview of food challenge outcomes at weeks 30 and 36.
Overview of food challenge outcomes to foods in each participant's multi-food OIT regimen at week 30 and week 36. Green indicates passed and orange failed challenges (to 2 g). “X” is shown if no food challenge was performed.
No significant difference in the medians of the baseline CTD for each of the foods included in that participant's multi-OIT for the primary endpoint outcome (passing at least 2 food challenge to 2 g) was detected (Fig. S4A, P = 0.16). Assessing the same differences in the 0 mg discontinuation arm showed no significant differences between the participants who failed or met the primary endpoint (Fig. S4B). In the same analysis of median SPT wheal for each of the foods included in that participant's OIT at baseline, no significant difference was detected between successes or failures (Fig. S5A, P = 0.63) or within the 0 mg discontinuation arm between participants meeting or failing the primary endpoint (Fig. S5B).
In post hoc analyses, no baseline characteristics were significantly associated with study success.
4. Discussion
This controlled, randomized, double-blind phase 2 study is the first multicenter phase 2 clinical trial evaluating various OIT maintenance doses, including discontinuation, after omalizumab-facilitated multi-allergen OIT. Consistent with a pilot study [11], our data here show success in safety and feasibility in desensitizing participants with up to 5 food allergens simultaneously in conjunction with a short course of omalizumab treatment. Based on eligibility criteria, the participants in this study were highly allergic with persistent food allergies. They had high frequencies of co-morbidities including asthma, allergic rhinitis and atopic dermatitis (Table 1). The median CTD across participant's OIT foods in baseline food challenges was only 25 mg, indicating, for example, that 1/10th of a peanut kernel (one peanut kernel is approximately 240 mg protein) could induce an allergic reaction in the typical peanut allergic participant enrolled in the study.
Our primary endpoint was achieved in 85% vs 55% of ITT population in the combined active treatment arms vs discontinuation arm. Several participants reached 2 g in food challenge to more than 2 allergens at week 36 (Figs. 5 and S3) without an objective allergic reaction. Even the participants labeled as study failures within the 0 mg discontinuation arm (9/20) continued to maintain a higher dose threshold after OIT discontinuation (Fig. 5). Importantly, no further omalizumab would likely have been detected in the circulation based on prior studies [21], [22], thus inferring other immune mediated mechanisms during multifood-OIT are likely present. Interestingly, the ability to discontinue with no objective allergic reaction upon rechallenge differed per food allergen (e.g. higher frequency for walnut compared to cashew). Thus, which individual food allergens may be discontinued vs continued in OIT needs further investigation. These differences may be due to immunological mechanisms specific to each food allergen's ability to desensitize and this is an area of active investigation. Furthermore, in the PP analysis, there was less of a difference between the active and discontinuation arms (92% vs 69%) which could indicate that successful discontinuation may be possible in certain participants. There was a higher rate of participants that discontinued in the 0 mg arm (4/20, 20%) compared to the combined treatment arms (3/40, 7.5%) during the randomized phase and this could have enriched the PP population for the participants that could pass the primary endpoint.
There was no evidence that continuing a daily multi-OIT 300 mg protein (about one nut kernel) dose for each allergen was effectively different than continuing a 1 g dose (about 5 peanut kernels or 1 oz. of milk) for each allergen in maintaining desensitization (passing ≥ 2 food challenge at 2 g protein for each of 2 food allergens in week 36); however, we acknowledge that the absence of a difference in these outcomes could simply be the result of limited power. The percentage of participants in the combined active treatment arms that passed at least 2 g protein of each of 2, 3, 4 or all 5 food allergen challenges at week 36 were comparatively consistent across the number of allergens passed. The ability to ingest 2 g of protein (equivalent to one tablespoon of peanut butter or 2 oz. cow's milk) for each of up to 5 food allergens is seen as a substantial improvement in the threshold to eat foods without reacting (compared to baseline where the threshold to allergic reaction occurred at mg levels of each food allergen tested).
We tested the ability to tolerate higher than 2 g of each protein in food challenges. The majority of participants in the pooled active treatment arms vs 0 mg discontinuation arm passed food challenge to at least 2 food allergens at 4 g protein each (70% vs 45%). We found that most participants could achieve cross-desensitization to homologous proteins not used in the multi-OIT. A few (n = 5) individuals reached 2 g of each of their food allergens (compared to 1 g of each of their food allergens) prior to week 29; however, this group showed no significant trends in efficacy or safety in either the primary or secondary endpoints. These results are based on a small sample size and further testing with larger sample sizes are needed.
AE rates were similar to single food allergen OIT studies and other multi-OIT studies [3], [4], [9], [11], [23], [24]. The data do not show statistically significant differences in safety and efficacy between the 300 mg and 1 g daily doses; however, the absence of a difference in these outcomes could simply be the result of limited power. There was an increased use of injectable epinephrine in the 1 g arm (Table S6). This is an important finding when considering improvements to compliance in long-term dosing for OIT. Lower allergen maintenance doses would likely be better tolerated and preferred by patients and thereby improve adherence. No statistically significant safety differences were found between the three arms; however, this should be interpreted in light of the limited power (small number of participants, limited period of follow up, and highly selective cohort) of the study to detect such changes.
Our study is the first to show, in participants with multiple food allergies, evidence that discontinuation is possible with omalizumab-facilitated multi-OIT. Note that discontinuation studies vary by the amount of time of withdrawal of OIT. Our clinical trial did not permit the ingestion of any food allergen during the entire course of the study. Previous studies have shown withdrawal was possible in single allergen desensitization (without omalizumab). A study by Burks et al. found 28% after 4–6 weeks blinded discontinuation of egg had no objective reaction upon rechallenge [25]. Discontinuation was possible in 50% of participants who stopped peanut consumption for 1 month [26]. Another study by Syed et al. of peanut OIT found 35% of participants successfully passed a rechallenge after 3 months of blinded discontinuation [27]. In a recent study by Wood et al. [8], there was no statistically-significance in successful discontinuation when milk OIT was given with omalizumab vs milk OIT with placebo; however, in this study, discontinuation success was not tested until 28 months after combined omalizumab-OIT dosing. Compared to the present study, dosing occurred with milk only without rapid updosing. In another study in which egg OIT was given for up to 48 months, successful discontinuation was achieved in 50% of participants in the absence of omalizumab by increasing the duration of OIT [28]. Our study is the first to test successful discontinuation in multi-OIT and the first to test successful discontinuation as early as 7 months after starting OIT. In summary, the result that 55% of the ITT had no objective reaction upon rechallenge after 6 weeks of discontinuation is comparable to other studies that have measured sustained desensitization rates; however these results need further testing with a larger sample size. Many participants prefer shorter duration of treatment (less than a year) compared to longer (i.e. most clinical trials to date are 2 + years) so there is potential for an omalizumab-facilitated therapy regimen to enhance effectiveness, compliance, and efficiency of therapy.
Even though several half-lives would have passed since the cessation of omalizumab in the protocol (20 weeks therefore 6 half-lives), it is possible that omalizumab was present in tissues and therefore had an effect on outcome. Another possible contribution to successful outcomes is the achievement of higher doses early on in the study (compared to other OIT studies [8], [29], [30], [31], [32], [33], [34]).
There were limitations to our study. The majority of participants were non-Hispanic (93% of ITT). There was no comparison to a multi-allergen OIT alone arm (i.e. without omalizumab-facilitation); further studies are needed to test this comparison. The sample size was relatively small but was similar in size to other OIT studies [8], [25], [26]. Further, as there were no corrections for multiple comparisons or adjustments made for imbalances in baseline characteristics across the treatment arms, interpretations of results should be made with caution. As with previous multi-OIT studies, regimens were customized to the participant's food allergies. As such, we were unable to identify which foods caused AEs; however, this was not a major concern of participants or investigators as all food allergens tested at week 36 food challenge showed decreased allergic reactions (Fig. 5). Some patients withdrew prior to week 36 or did not undergo more than 3 food challenges (including the placebo challenge) at week 36 which led to many food challenges not being completed (Fig. S3).
OIT is a promising approach for participants with food allergy. Our results show that multi-OIT can be performed in multiple centers with a standardized protocol in a safe manner, with a high rate of completion. Evidence to date suggests that omalizumab-facilitated OIT allows for a rapid and safe dose escalation process. This may help improve compliance and quality of life for those participants on OIT. Finally, 55% of individuals who discontinued OIT showed no objective allergic reaction upon rechallenge; however, the data show that regular, daily therapy is optimal for sustained effect of multifood-OIT. Further studies are needed to determine the optimal maintenance dose needed to sustain desensitization.
Contributors
Study was designed by SC, and KN. Study was performed by DK, AL, KO, SS, HS, AC, TW, DP, MM, RR, ML, SL, ER, AA, SC, JP, JS, JT, ST, JW, and KN. Data was collected by DK, AL, KO, SS, HS, AC, TW, DP, MM, RR, ML, SL, ER, AA, JP, JS, JT, ST, JW, and KN. Data was analyzed by SA, NP, and MD. Manuscript was written by SA, NP, DK, SJG, SC, and KN. Manuscript was critically reviewed by SS, HS, AC, TW, DP, MM, RR, SL, SJG, ER, AA, SC, JP, JS, JT, ST and JW.
Declaration of Interests
Dr. Kari Nadeau: NIAID CoFAR, NIAID Immune Tolerance Network, NHLBI Data and Safety Monitoring Board, NIEHS grant awardee, EPA grant awardee, FARE Center of Excellence Director, WAO Center of Excellence Director, site principal investigator (Aimmune Phase 3, DBV phase 3, AnaptysBio Phase 1, Astellas Phase 1). Dr. Sharon Chinthrajah receives grant support from CoFAR NIAID, Aimmune, DBV Technologies, Astellas, AnaptysBio, and is on the Regional Advisory Board for Aimmune. Dr. Julie Wang: NIAID CoFAR, Aimmune, DBV Technologies, UpToDate. Dr. Scott Sicherer: NIAID CoFAR, HAL-Allergy, UpToDate, Johns Hopkins University Press, Board of Directors of the American Academy of Allergy, Asthma and Immunology, and is associate editor of J Allergy Clin Immunol Pract. Dr. Hugh Sampson: NIAID and is the Chief Scientific Officer at DBV Technologies (60%) and a consultant for Allertein Therapeutics, LLC. Dr. Jonathan Spergel receives grant support from DBV Technologies, Aimmune and NIH, is a consultant with Regeneron and Shire, and is on the Scientific Advisory Board at DBV Technologies. Dr. Whitehorn is a consultant and principal investigator for DBV Technologies. Dr. Cianferoni receives funding from DBV Technologies. Dr. Stephen J. Galli receives grant support from NIAID, NIAMS, and the Tobacco-Related Disease Research Program (U. California). Dr. Assa'ad is a principal investigator for DBV Technologies, Aimmune Pharmaceuticals, Astellas, and the National Institute of Health. All other authors declare no conflicts of interest.
Acknowledgments
Acknowledgements
Funding sources include Sean N. Parker Center for Allergy and Asthma Research at Stanford University, Jeff and MacKenzie Bezos, NIAID AADCRC U19AI104209 (Galli, Nadeau, and Chinthrajah), Myra Reinhard Foundation, FARE Center of Excellence, EAT (End Allergies Together), Trip Advisor Foundation, CHOP-FARE, Children's Hospital of Philadelphia's Food Allergy Fund, The Sunshine Foundation, the Crown Family Philanthropies, and NCATS (NIH) Grant# UL1TR001422 (Pongracic). All medications and study drug (omalizumab) were purchased as commercially available products. We thank the nurses, dieticians, study coordinators, and patients and their families who participated in the study. We also thank Jennifer Bosworth, RN, Jennifer Howard, RN, Melissa Jain, RN, Michelle Catalano, LPN, Nashmia Qamar, DO, Rajesh Kumar, MD, Amanda Johnson, RD, Stefanie McCormack, BS, Christine Szychlinski, APN, CPNP, Jennifer Fishman, Jennifer Heimall, Lisa Clark, Jennifer Jennings, RN, Christina McDougall, Jane Robertson, Megan Lewis, Rushani Saltzman, Vanitha Sampath, PhD, Wendy Davidson, PhD, Marshall Plaut, MD, and Lisa Wheatley, MD, for reading and editing the manuscript.
The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the paper for publication. The funder did not fund any serological or cellular mechanistic studies. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data Sharing
All of the individual participant data collected during the trial (after de-identification), study protocol, statistical analysis plan, informed consent form, and clinical study report will be available immediately to anyone who wishes to access the data for any purpose. Data will be available indefinitely at Stanford University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2018.12.006.
Appendix A. Supplementary Data
Supplementary material 1
Supplementary material 2
References
- 1.Gupta R.S., Springston E.E., Warrier M.R. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 2.Lin C., Lee I.T., Sampath V. Combining anti-IgE with oral immunotherapy. Pediatr Allergy Immunol. 2017;28:619–627. doi: 10.1111/pai.12767. [DOI] [PubMed] [Google Scholar]
- 3.Andorf S., Manohar M., Dominguez T. Feasibility of sustained response through long-term dosing in food allergy immunotherapy. Allergy Asthma Clin Immunol. 2017;13:52. doi: 10.1186/s13223-017-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andorf S., Manohar M., Dominguez T. Observational long-term follow-up study of rapid food oral immunotherapy with omalizumab. Allergy Asthma Clin Immunol. 2017;13:51. doi: 10.1186/s13223-017-0223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung D.Y., Sampson H.A., Yunginger J.W. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348:986–993. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 6.Nadeau K.C., Schneider L.C., Hoyte L., Borras I., Umetsu D.T. Rapid oral desensitization in combination with omalizumab therapy in patients with cow's milk allergy. J Allergy Clin Immunol. 2011;127:1622–1624. doi: 10.1016/j.jaci.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider L.C., Rachid R., LeBovidge J., Blood E., Mittal M., Umetsu D.T. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol. 2013;132:1368–1374. doi: 10.1016/j.jaci.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood R.A., Kim J.S., Lindblad R. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow's milk allergy. J Allergy Clin Immunol. 2015;137:1103–1110.e1–11. doi: 10.1016/j.jaci.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begin P., Dominguez T., Wilson S.P. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using Omalizumab. Allergy Asthma Clin Immunol. 2014;10:7. doi: 10.1186/1710-1492-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacGinnitie A.J., Rachid R., Gragg H. Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol. 2016;139:873–881.e8. doi: 10.1016/j.jaci.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andorf S., Purington N., Block W.M. Anti-IgE treatment with oral immunotherapy in multifood allergic participants: a double-blind, randomised, controlled trial. Lancet Gastroenterol Hepatol. 2018;3:85–94. doi: 10.1016/S2468-1253(17)30392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andorf S., Borres M.P., Block W. Association of clinical reactivity with sensitization to allergen components in multifood-allergic children. J Allergy Clin Immunol Pract. 2017;5:1325–1334. doi: 10.1016/j.jaip.2017.01.016. [e4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goetz D.W., Whisman B.A., Goetz A.D. Cross-reactivity among edible nuts: double immunodiffusion, crossed immunoelectrophoresis, and human specific igE serologic surveys. Ann Allergy Asthma Immunol. 2005;95:45–52. doi: 10.1016/S1081-1206(10)61187-8. [DOI] [PubMed] [Google Scholar]
- 14.Uotila R., Kukkonen A.K., Pelkonen A.S., Makela M.J. Cross-sensitization profiles of edible nuts in a birch-endemic area. Allergy. 2016;71:514–521. doi: 10.1111/all.12826. [DOI] [PubMed] [Google Scholar]
- 15.Noorbakhsh R., Mortazavi S.A., Sankian M. Pistachio allergy-prevalence and in vitro cross-reactivity with other nuts. Allergol Int. 2011;60:425–432. doi: 10.2332/allergolint.10-OA-0222. [DOI] [PubMed] [Google Scholar]
- 16.Savvatianos S., Konstantinopoulos A.P., Borga A. Sensitization to cashew nut 2S albumin, Ana o 3, is highly predictive of cashew and pistachio allergy in Greek children. J Allergy Clin Immunol. 2015;136:192–194. doi: 10.1016/j.jaci.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Venter C., Pereira B., Voigt K. Comparison of open and double-blind placebo-controlled food challenges in diagnosis of food hypersensitivity amongst children. J Hum Nutr Diet. 2007;20:565–579. doi: 10.1111/j.1365-277X.2007.00828.x. [DOI] [PubMed] [Google Scholar]
- 18.Bock S.A., Sampson H.A., Atkins F.M. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: a manual. J Allergy Clin Immunol. 1988;82:986–997. doi: 10.1016/0091-6749(88)90135-2. [DOI] [PubMed] [Google Scholar]
- 19.R Core Team . R Foundation for Statistical Computing; 2017. R: a language and environment for statistical computing.https://www.R-project.org/ [Google Scholar]
- 20.Agresti A. Wiley; New York: 1990. Categorical data analysis. [Google Scholar]
- 21.Lawrence M.G., Woodfolk J.A., Schuyler A.J., Stillman L.C., Chapman M.D., Platts-Mills T.A. Half-life of IgE in serum and skin: consequences for anti-IgE therapy in patients with allergic disease. J Allergy Clin Immunol. 2017;139:422–428. doi: 10.1016/j.jaci.2016.04.056. [e4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubinsztajn R., Chazan R. Monoclonal antibodies for the management of severe asthma. Adv Exp Med Biol. 2016;935:35–42. doi: 10.1007/5584_2016_29. [DOI] [PubMed] [Google Scholar]
- 23.Virkud Y.V., Burks A.W., Steele P.H. Novel baseline predictors of adverse events during oral immunotherapy in children with peanut allergy. J Allergy Clin Immunol. 2017;139:882–888. doi: 10.1016/j.jaci.2016.07.030. [e5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Begin P., Winterroth L.C., Dominguez T. Safety and feasibility of oral immunotherapy to multiple allergens for food allergy. Allergy Asthma Clin Immunol. 2014;10:1. doi: 10.1186/1710-1492-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burks A.W., Jones S.M., Wood R.A. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–243. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vickery B.P., Scurlock A.M., Kulis M. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133:468–475. doi: 10.1016/j.jaci.2013.11.007. [e6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Syed A., Garcia M.A., Lyu S.C. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014;133:500–510. doi: 10.1016/j.jaci.2013.12.1037. [e11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones S.M., Burks A.W., Keet C. Long-term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapy. J Allergy Clin Immunol. 2016;137:1117–1127. doi: 10.1016/j.jaci.2015.12.1316. [e10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anvari S., Tran D., Nguyen A., Devaraj S., Davis C.M. Peanut oral immunotherapy dose variations do not result in allergic reactions. Pediatr Allergy Immunol. 2018;29:218–220. doi: 10.1111/pai.12837. [DOI] [PubMed] [Google Scholar]
- 30.Du Toit G., Roberts G., Sayre P.H. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Togias A., Cooper S.F., Acebal M.L. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Pediatr Nurs. 2017;32:91–98. doi: 10.1016/j.pedn.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Pajno G.B., Fernandez-Rivas M., Arasi S. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy. 2017 doi: 10.1111/all.13319. [DOI] [PubMed] [Google Scholar]
- 33.Bird J.A., Spergel J.M., Jones S.M. Efficacy and safety of AR101 in oral immunotherapy for peanut allergy: results of ARC001, a randomized, double-blind, placebo-controlled phase 2 clinical trial. J Allergy Clin Immunol Pract. 2017;6:476–485. doi: 10.1016/j.jaip.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Nagakura K.I., Sato S., Yanagida N. Oral immunotherapy in Japanese children with anaphylactic peanut allergy. Int Arch Allergy Immunol. 2018;175:181–188. doi: 10.1159/000486310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2