Abstract
Background
Despite growing waiting lists for renal transplants, hesitations persist with regard to the use of deceased after cardiac death (DCD) renal grafts. We evaluated the outcomes of DCD donations in The Netherlands, the country with the highest proportion of DCD procedures (42.9%) to test whether these hesitations are justified.
Methods
This study included all procedures with grafts donated after brain death (DBD) (n = 3611) and cardiac death (n = 2711) performed between 2000 and 2017. Transplant outcomes were compared by Kaplan Meier and Cox regression analysis, and factors associated with short (within 90 days of transplantation) and long-term graft loss evaluated in multi-variable analyses.
Findings
Despite higher incidences of early graft loss (+ 50%) and delayed graft function (+ 250%) in DCD grafts, 10-year graft and recipient survival were similar for the two graft types (Combined 10-year graft survival: 73.9% (95% CI: 72.5–75.2), combined recipient survival: 64.5% (95 CI: 63.0–66.0%)). Long-term outcome equivalence was explained by a reduced impact of delayed graft function on DCD graft survival (RR: 0.69 (95% CI: 0.55–0.87), p < 0.001). Mid and long-term graft function (eGFR), and the impact of incident delayed graft function on eGFR were similar for DBD and DCD grafts.
Interpretation
Mid and long term outcomes for DCD grafts are equivalent to DBD kidneys. Poorer short term outcomes are offset by a lesser impact of delayed graft function on DCD graft survival. This nation-wide evaluation does not justify the reluctance to use of DCD renal grafts. A strong focus on short-term outcome neglects the superior recovery potential of DCD grafts.
Keywords: Kidney transplantation, Donation after cardiac death, Donation after brain death, Outcome, Graft survival, Delayed graft function
Highlights
-
•
In the Netherlands, there is a frequent and liberal use of renal grafts donated after cardiac death.
-
•
Grafts donated after cardiac death present with high incidences of early graft loss and delayed graft function
-
•
Yet, grafts donated after cardiac and brain death show equivalent 10 year transplantation outcomes
-
•
Long-term outcome equivalence relates to a reduced impact of delayed graft function on graft survival of grafts donated after cardiac death.
Research in context
Evidence before this study
While some studies suggest good outcomes for kidney grafts donated after cardiac death (DCD), liberal use of these grafts is still considered controversial. The Netherlands has a longstanding tradition with DCD kidney grafts, and currently DCD procedures account for 50% of all deceased donor procedures. Using national transplantation registry data we compared the short and long term outcomes for kidney grafts donated after brain and cardiac death of all transplants performed between 2000 and 2017.
Added value of this study
Although this study confirms a higher incidence of short term graft loss and delayed graft function in DCD grafts. It shows equivalent 10-year graft survival and recipient survival.
Implications of all the available evidence
This study shows similar long-term outcomes for grafts donated after brain and cardiac death. Poorer short-term outcomes for DCD grafts do not translate in worse long-term outcomes. Results dismiss a reticent attitude towards DCD grafts in an era of pressing donor shortages.
Alt-text: Unlabelled Box
1. Introduction
Kidney transplantation profoundly improves quality of life and longevity of end-stage renal disease patients and remains the only curative option for patients with end-stage renal disease [1]. In an era of growing waiting lists for renal transplants, and unacceptably high waiting list-associated mortality, pressing donor shortages have led to an increased use of so called “extended criteria grafts” and kidney grafts obtained from donors deceased following cardiac death (DCD) [2]. Transplantation procedures with DCD grafts are associated with increased incidences of primary non-function/early graft loss and delayed graft function. The latter phenomenon is considered to negatively influence graft function and long-term graft survival [3], [4], [5], [6]. As a result, the use of DCD grafts remains controversial [7], with many countries refraining from using these grafts [8], [9], [10], [11].
Remarkably, some small cohort studies [12], [13], [14], [15], [16], and follow up data from the UK transplant registry do not support these reservations towards the use of DCD grafts [17]. In fact, the UK registry data indicate equal 5-year kidney graft survival rates for DCD grafts and grafts from donors donated after brain-death (DBD). However, the low proportion DCD grafts and concern with regard to differences in graft risk profiles; in particular the reported increased susceptibility of DCD grafts for (prolonged) cold-ischemia [17], raises questions on the generalizability of the UK registry data.
In the light of the emerging discussion regarding a more liberal use of DCD grafts, we considered an independent and adequately powered evaluation of outcomes of DCD renal graft procedures relevant. This analysis focuses on The Netherlands, which has a long and relative liberal tradition with regard to the use of DCD grafts [18]. In fact, an evaluation for 2013 showed that the Netherlands is the country with the highest proportion of DCD procedures [19]. In fact, DCD procedures currently account for 50% of all deceased donor procedures performed nationwide.
This national registry-based study evaluates the outcomes of all 2711 DCD transplantations performed between 2000 and 2017 in The Netherlands.
2. Methods
2.1. Study Population
The Netherlands Organ Transplant Registry (NOTR) is a mandatory nationwide registry of kidney transplant recipients from all eight kidney transplant centres in the Netherlands. The NOTR registry is managed by the Dutch Transplant Foundation and includes recipient and donor characteristics, and a variety of outcome parameters (Table 1). In the first year after transplantation, registry follow-up is at month 3, thereafter on a yearly basis. Quality checks are performed by on-site polls, business rules in application and cross checks with the national dialysis registry. We retrieved data on recipient and donor characteristics, and transplantation outcomes for all procedures performed between January 1st 2000 and January 1st 2017. There were no missings for type of donor, graft survival or recipient survival. With respect to follow-up time for graft survival, 2.5% was missing and for recipient survival no missing for follow-up time. Missing percentages were higher for some of the clinical parameters. If appropriate the percentage missings for these parameters are indicated in Table 1.
Table 1.
| DBD |
DCD |
||
|---|---|---|---|
| N = 3611 (57.1%) | N = 2711 (42.9%) | ||
| Sex donor (male) | 1934 (47.6%) | 1682 (58.4%) | |
| Age donor (yr) | 49.8 ± 15.2 | 49.4 ± 15.1 | |
| Body-mass index donor | 25.2 ± 4.3 | 25.3 ± 4.6 | |
| Last creatinine donor (μmol/l) | 77.4 ± 33.1 | 70.4 ± 26.0 | |
| eGFR (MDRD) donor (ml/min) | 90.0 ± 37.6 | 101.5 ± 39.2 | |
| Cause of death donor (%) | |||
| Trauma | 751 (20.8) | 832 (30.7) | |
| Stroke | 2153 (59.6) | 1060 (39.1) | |
| Cardiac arrest | 161 (4.5) | 470 (17.3) | |
| Other | 546 (15.1) | 349 (12.9) | |
| Hypertension donor (%) | |||
| No | 2210 (61.2) | 2040 (75.2) | |
| Yes | 946 (26.2) | 529 (19.5) | |
| Unknown | 455 (12.6) | 142 (5.2) | |
| Smoking donor (%) | |||
| No | 1625 (45.0) | 1282 (47.3) | |
| Yes | 1675 (46.4) | 1271 (46.9) | |
| Unknown | 311 (8.6) | 158 (5.8) | |
| Cold ischemia time (hrs) | 17.0 [13.2–22.0] | 16.1 [12.8–20.1] | |
| Cold ischemia time distribution (%) |
|||
| < 12 h | 633 (17.5) | 485 (17.9) | |
| 12–18 h | 1266 (35.1) | 1091 (40.2) | |
| 18–24 h | 969 (26.8) | 707 (26.1) | |
| > 24 h | 524 (14.5) | 249 (9.2) | |
| Unknown | 219 (6.1) | 179 (6.6) | |
| Machine perfused | 158 | 155 | |
| Graft anastomosis time (min) | 33 [26–41] | 32 [26–40] | |
| KDRI | 1.29 [1.04–1.62] | 1.38 [1.12–1.71] | |
| Sex recipient (male) | 2083 (57.7%) | 1692 (62.4%) | |
| Age recipient (years) | 51.9 ± 14.6 | 53.7 ± 13.3 | |
| BMI recipient (kg/m2) | 25.3 ± 4.4 | 25.9 ± 4.4 | |
| No of previous transplants (%) | |||
| 0 | 2705 (81.4) | 2216 (87.1) | |
| 1 | 479 (14.4) | 260 (10.7) | |
| 2 | 111 (3.3) | 43 (1.8) | |
| 3 | 22 (0.7) | 8 (0.3) | |
| 4 | 6 (0.2) | 2 (0.1) | |
| Mismatches (%) | |||
| HLA-Dr | 0 | 1509 (41.9) | 869 (32.3) |
| 1 | 1815 (5.4) | 1612 (59.9) | |
| 2 | 276 (7.7) | 209 (7.8) | |
| HLA-A | 0 | 1409 (39.1) | 815 (30.2) |
| 1 | 2005 (49.5) | 1583 (55.2) | |
| 2 | 587 (14.5) | 425 (14.8) | |
| HLA-B | 0 | 955 (26.5) | 445 (16.5) |
| 1 | 1810 (50.3) | 1616 (59.8) | |
| 2 | 836 (23.2) | 642 (23.8) | |
| Panel reactive antibodies > 5% (%) | 570 (15.8) | 252 (9.3) | |
| Induction therapy | |||
| Anti-IL2r | 1542 (42.7) | 1239 (45.6) | |
| ATG | 119 (3.3) | 81 (3.0) | |
| Initial immune suppression | |||
| Ciclosporin A | 964 (26.7) | 539 (19.9) | |
| Tacrolimus | 2705 (71.0) | 2245 (78.5) | |
| Sirolimus | 193 (5.4) | 108 (4.0) | |
| Mycophenolate | 3317 (91.9) | 2537 (93.6) | |
| Corticosteroids | 3513 (97.3) | 2641 (97.4) | |
Plus-minus values are means ± SD. Values between square brackets represent median and [interquartile range].
Starting 2016 all grafts were machine perfused.
Grafts were preserved via arterial cold perfusion, generally using University of Wisconsin (UW) or Histidine-tryptophan-ketoglutarate (HTK) preservation solution. Almost all organs were retrieved and preserved by means of static cold storage. However, as of 2016 most kidney grafts were preserved using hypothermic machine perfusion. Organs were allocated according to the Eurotransplant guidelines.
DCD kidneys were all from controlled circulatory death donors (Maastricht category 3 (controlled DCD: awaiting cardiac arrest after withdrawal of life-supporting treatments in the ICU) [20]. We excluded grafts from uncontrolled circulatory death donors (n = 136), as well as graft recipients younger than 12 years of age (n = 118), and combined organ recipients (n = 366). This resulted in 3611 DBD and 2711 DCD grafts available for final analysis.
The first warm ischemic period in the DCD donors was defined as the time between cardiac arrest and start of cold perfusion. The time after withdrawal of support with RR ≪ 50 mm Hg to cardiac arrest is not available. Cold ischemia time was defined as time from the start of cold perfusion in the donor to start of the actual implantation in the recipient. The anastomosis time was defined as the time from organ removal from cold storage to graft reperfusion in the recipient. Delayed graft function was defined by the need of dialysis because of initial non-function in the first week(s) after kidney transplantation that was followed by functional recovery. Early graft loss was defined as graft loss within 90 days of transplantation.
2.2. Study End Points
Post transplantation outcome was classified in the following categories: primary function, early graft loss (within 90 days of transplantation) or late graft loss (> 90 days of transplantation). The kidney donor risk index (KDRI) was calculated using the coefficients provided [18] and eGFR estimated by the Modification of Diet in Renal Disease (MDRD) formula. Patients who experienced graft failure, but did not die during follow-up were censored at the end of follow-up. For these analyses of -recipient overall survival-, only death was an event in the analyses.
2.3. Data Statement
This study is based on data made available by the Netherlands Organ Transplant Registry (NOTR). Access to the data set is handled by the registry (info@transplantatiestichting.nl).
2.4. Statistical Analyses
Differences in donor, recipient, transplantation procedure-related factors, and graft function between DBD and DCD donors were assessed using Fischer exact tests, unpaired t-tests or Mann–Whitney rank tests for categorical, normally-distributed and non-parametric data, respectively. The continuous variables that were entered in the logistic regression models were visually inspected for skewness. For the Kaplan Meier curves, the proportional hazard assumption was tested on the basis of Schoenfeld residuals after fitting the model and there was no evidence that the assumption was violated with a p-value of 0.88 for the graft survival and p = 0.28 for the recipient survival.
Kaplan Meier survival curves were generated for DBD versus DCD, and in combination with or without delayed graft function. Graft survival time was defined from date of transplantation to date of graft failure. Survival time was truncated at 10 years. Differences in graft and recipient survival were studied by Cox Proportional Hazard analyses. For the logistic regression analyses, variables were selected based on clinical relevance (a priori) based on previous pilot research in a hospital based dataset. All factors were studied in univariable analyses; thereafter, variables with a p-value of p < 0.1 were entered in the multivariable analyses. For the graft and recipient survival, two multivariable models were built: one adjusting for age and sex and a second full model including all baseline variables which were either different between the donor types or deemed clinically relevant.
Missing data were entered in the model as a category unknown and results are represented in the tables in case for categorical factors. In the continuous factors, missing values were excluded for analyses. There were no missings for the primary parameters (type of donor, graft survival or recipient survival). With respect to follow-up time for graft survival, 2.5% was missing and for recipient survival no missings for follow-up time. Missing information on secondary parameters is provided in Table 1.
Results are represented as Hazard Ratio (HR) for the survival analysis or Odds Ratio (OR) for the logistic regression with corresponding 95% Confidence Intervals (CI). Analyses were performed using STATA/SE version 12.0 (StataCorp, Texas, USA) and SPSS 22.0 (IBM, Amsterdam, The Netherlands).
3. Results
The NOTR registry, for the 2000–2017 interval, includes data for 3611 DBD (57.1%) and 2711 DCD (42.9%) kidney transplantations. The 17-year interval is associated with changes in medical treatment and decision making such as progressive use of older donors and DCD grafts, and changes in immune suppression therapy (e.g. induction therapy) which may positively and negatively affect transplant outcomes. This aspect is included as “calendar months since transplantation” in the multivariable analysis for early graft loss and DGF. Aspects included in this factor and their correlation with the number of months-passed since transplantation procedure are summarized in Supplemental Table 1.
DBD and DCD donors differed with regard to sex-distribution, eGFR, hypertension and smoking histories, and cause-of-death (Table 1). DCD donors had superior pre-donation creatinine clearance, a lower prevalence of hypertension, but immunological matching was less optimal than for DBD grafts (Table 1). Simple univariate correlations between the various factors and outcomes are provided in Supplemental Table 2. The slightly superior characteristics of DCD donors are reflected in their modest lower median KDRI's after exclusion of the DCD component from the equation (i.e.: 1.29 for the DCD group and 1.39 for the DBD group (p < 1.10−25)). Cold ischemia and anastomosis times were marginally shorter in the DCD donor group (Table 1).
The registry data indicate a 50% higher incidence of early graft loss, and an almost 150% higher incidence of delayed graft function in DCD grafts (Table 2). Multivariable analysis showed that only donor and procedure-related factors associated with early graft loss (Supplemental Table 1), whereas incident delayed graft function associated with donor, procedure and recipient-related factors (Supplemental Table 2). Donor and recipient female sex associated with reduced incidences of delayed graft function. The progressive association between the transplant date (calendar months since transplantation), and early graft loss and delayed graft function indicates lower incidences for the more recent procedures.
Table 2.
Transplant outcomes⁎.
| DBD | DCD | p-Value | |
|---|---|---|---|
| Early graft loss (< day 90) | |||
| Primary non function | 284 (7.9%) | 279 (10.3%) | < 0.0001 |
| Related to acute rejection | 17 (0.5%) | 12 (0.4%) | 0.87 |
| Delayed graft function | |||
| No | 2409 (66.7%) | 879 (32.4%) | < 0.0001 |
| Yes | 628 (17.4%) | 1141 (42.1%) | |
| Unknown | 574 (15.9%) | 691 (25.5%) | |
| Late graft loss (> day 90) | 732 (20.3%) | 533 (19.7%) | < 0.568 |
| 3 months eGFR [iqr] | |||
| − DGF | 48.1 [37.8–60.9] | 48.9 [37.1–58.8] | < 0.004 |
| + DGF | 39.6 [28.3–51.2] | 38.9 [29.2–51.2] | 0.13 |
| Year 1 eGFR [iqr] | |||
| − DGF | 49.5 [38.6–62.5] | 49.4 [38.3–63.0] | 0.61 |
| + DGF | 42.1 [30.9–54.8] | 43.6 [31.4–54.9] | 0.58 |
| Year 5 eGFR [iqr] | |||
| − DGF | 50.5 [37.6–66.1] | 50.7 [36.5–64.4] | 0.88 |
| + DGF | 44.8 [32.6–61.6] | 43.0 [31.4–58.6] | 0.15 |
Values represent mean (sd) or median [interquartile range IQR].
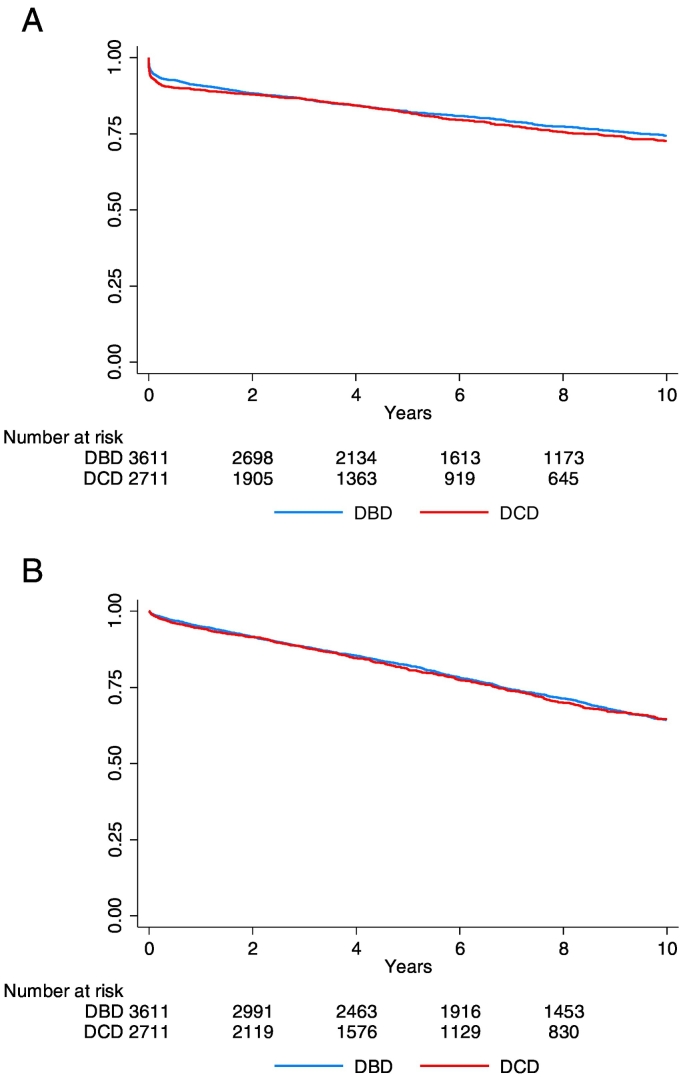
Notably, higher incidences of early graft loss and delayed graft function in the DCD grafts did not impact long-term graft or recipient survival. In fact, 10-year graft survival (Fig. 1A and Table 3) and 10-year recipient survival (Fig. 1B and Supplemental Table 3) were similar for DBD and DCD grafts (Fig. 1A: HR for graft loss (DBD reference): 1.07 (95% CI: 0.96–1.20); p = 0.22; Fig. 2: adjusted-for-age-HR for recipient death (DBD reference): 1.02 (95% CI: 0.92–1.12); p = 0.73). Considering the highly significant differences in donor characteristics (Table 1), an additional Cox proportional hazard analyses for the two survival outcomes (graft survival (Table 3) and recipient survival (Supplemental Table 3) was performed for two models; one model adjusting for age and sex, and one full model. The model adjusted for age and sex indicated an HR of respectively 1.12 (95% CI: 1.00–1.26; p = 0.05) and 0.98 (95% CI: 0.89–1.08; p = 0.72) for graft survival (Table 3) and recipient survival (Supplemental Table 3). HRs for the fully adjusted models were: 1.08 (95% CI: 0.95–1.24; p = 0.24) for graft survival, and 1.03 (95% CI: 0.92–1.16; p = 0.55) for recipient survival.
Fig. 1.
A. Recipient death censored 10-year graft survival of DBD (blue) and DCD (red) grafts transplanted in the Netherlands; HR (DBD reference): 1.07 (95% CI: 0.96–1.20); p = 0.22. Schoenfeld residuals the proportional hazard assumption after fitting the model: p: 0.88.
B. 10-year recipient survival for recipients of a DBD (blue) or DCD (red) graft; HR (DBD reference): 1.03 (0.93–1.14); p = 0.56.
Table 3.
Cox proportional hazard analyses for Graft Failure.
| Unadjusted HR donor type | DBD DCD |
Reference 1.07 (0.96–1.20) |
0.22 | ||
| Factor | HR (95% CI) | P-value | HR (95% CI) | p-value | |
| Model 1: adjusted age and sex | Model 2: fully adjusted | ||||
| Donor type | DBD | Reference | Reference | ||
| DCD | 1.12 (1.00–1.26) | 0.05 | 1.08 (0.95–1.24) | 0.24 | |
| Sex donor | Male | Reference | Reference | ||
| Female | 1.00 (0.89–1.12) | 0.97 | 1.01 (0.89–1.15) | 0.85 | |
| Age donor | Continuous | 1.02 (1.02–1.03) | < 0.001 | 1.01 (1.00–1.01) | < 0.001 |
| Sex recipient | Male | Reference | Reference | ||
| Female | 1.08 (0.96–1.21) | 0.19 | 1.08 (0.96–1.23) | 0.20 | |
| Age recipient | Continuous | 0.99 (0.98–0.99) | < 0.001 | 0.99 (0.98–0.99) | < 0.001 |
| BMI donor | Continuous | 0.99 (0.98–1.01) | 0.52 | ||
| Creatinine donor | Continuous | 1.00 (0.99–1.00) | 0.06 | ||
| Cause of death donor | Trauma | Reference | |||
| Stroke | 1.13 (0.95–1.34) | 0.18 | |||
| Cardiac arrest | 0.96 (0.74–1.24) | 0.77 | |||
| Other | 1.08 (0.85–1.35) | 0.51 | |||
| Hypertension donor | No | Reference | |||
| Yes | 1.14 (0.98–1.33) | 0.06 | |||
| Unknown | 1.11 (0.87–1.41) | 0.38 | |||
| Smoking donor | No | Reference | |||
| Yes | 1.10 (0.97–1.25) | 0.14 | |||
| Unknown | 0.94 (0.73–1.21) | 0.66 | |||
| Cold ischemia time | Continuous | 1.02 (1.02–1.03) | < 0.001 | ||
| Graft anastomosis time | Continuous | 1.00 (1.00–1.01) | 0.03 | ||
| Early graft loss | No | Reference | |||
| Yes | 154.7 (124–192) | < 0.001 | |||
| BMI recipient | Continuous | 1.01 (0.99–1.03) | 0.05 | ||
| Mismatches HLA-Dr | 0 | Reference | |||
| 1 | 1.20 (1.05–1.38) | 0.007 | |||
| 2 | 1.24 (0.94–1.64) | 0.12 | |||
| Mismatches HLA-A | 0 | Reference | |||
| 1 | 0.98 (0.85–1.13) | 0.84 | |||
| 2 | 1.15 (0.94–1.42) | 0.17 | |||
| Mismatches HLA-B | 0 | Reference | |||
| 1 | 0.85 (0.73–1.02) | 0.09 | |||
| 2 | 0.99 (0.81–1.21) | 0.95 | |||
| Panel reactive antibodies | Continuous | 1.01 (1.00–1.01) | < 0.001 | ||
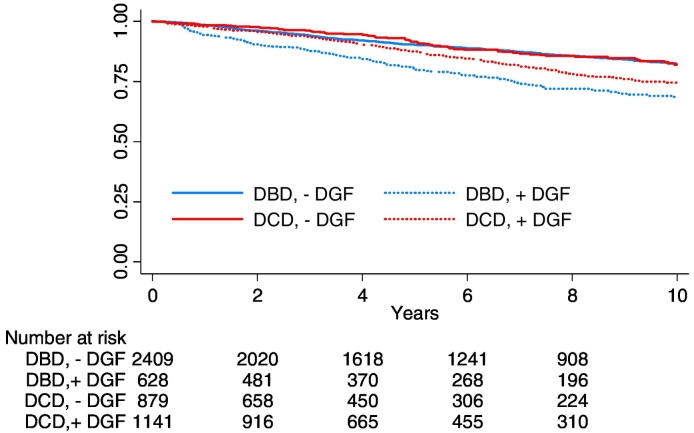
Fig. 2.
Differential impacts of delayed graft function on recipient death censored graft survival of DBD and DCD grafts (grafts with primary non function are excluded). HR for graft loss after delayed graft function in DCD grafts (DBD reference): 0.69 (95% CI: 0.55–0.87); P = 0.001.
In the light of expressed concerns with regard to a disproportionate impact of longer ischemia times on DCD grafts outcomes [17], we specifically addressed the impact of prolonged (> 24 h) cold ischemia time on outcome. In fact, prolonged cold ischemia time disproportionally impacted early graft survival (viz. graft loss within 90-days of transplantation) in the DCD group (early graft losses: 17.1 vs. 10.7% in the DCD and DBD group respectively, P = 0.007). However, the similar conditional graft survival for grafts surviving the first 90 days (HR for graft loss (DBD is reference) 1.15 (0.82–1.62); p = 0.41) indicates that the disproportionate effect reflects impaired graft recovery.
Comparable 10-year graft survival for DBD and DCD grafts in the context of a more than doubled incidence of delayed graft function in DCD grafts implies differential impacts of delayed graft function on graft outcome in the two donor types. A discordant effect is supported by the differential impacts of incident delayed graft function on graft survival when censored for early graft loss (and recipient death) (Fig. 2: HR for graft loss after delayed graft function 2.11 (95% CI: 1.73–2.57) for DBD grafts vs. 1.46 (95% CI: 1.22–1.75) for the DCD grafts, P = 0.001) (graft survival for DBD and DCD grafts without delayed graft function is reference)) and discordant associations between incident delayed graft function and graft loss in DBD and DCD grafts (Supplemental Tables 4–7).
The impact of delayed graft function on graft function (estimated clearance) on the other hand was similar for both donor types, with equal 1- and 5-year eGFRs in DBD and DCD grafts without delayed graft function, and approximately 12% lower eGFRs in grafts that sustained delayed graft function (Table 2).
4. Discussion
Results of this nation-wide evaluation of data from a society with an almost equal allocation of DCD and DBD renal grafts show similar mid- and long-term survival outcomes for DCD and DBD grafts.
Although some experts call for a more liberal use of DCD donor grafts in the light of the high waiting list associated mortality (in fact, for the US alone almost 5000 patients die annually while waiting for kidney transplant) [21], high incidences of delayed graft function and early graft loss in DCD grafts remain a major source of concern that prevents a more liberal use of this type of grafts [8]. As result, the use of kidney grafts donated after cardiac death as a highly significant source of donor organs remains a matter of great controversy [2]. In most societies including the US, the number of DCD procedures performed has stabilized at approximately 10–20% of the total of deceased donor procedures performed [22].
A less reticent attitude towards DCD grafts is supported by preliminary reports from small observational studies [12], [13], [14], [15], [16], and in particular by data from the UK registry [17], [19]. Although all reports indicate similar outcomes for DCD and DBD grafts, small sized studies are sensitive to publication bias [23], and small proportions of DCD procedures (less than 10% of the procedures) raise concerns on a potential selection bias with respect to superior donor characteristics of DCD grafts accepted for transplantation. This concern has been partially eliminated by updated evaluation for the UK that incorporated the increased use of DCD grafts in recent years [19], but follow up time still remains limited, and there is a considerable gap in recipient age between recipients of DBD and DCD grafts pointing to cautiousness with respect to the use of DCD grafts in younger patients. As result use of DCD grafts remains a matter of on-going debate, and the reticent towards use of DCD grafts persists. In order to help the transplantation communities and health authorities, that are currently discussing whether or not to adopt a more liberal attitude to the use DCD donor grafts in kidney transplantation, we performed a nation-wide evaluation of outcomes after DCD donation for the Netherlands.
With an almost equal share of DBD and DCD grafts, and comparable donor and recipient characteristics, the situation in the Netherlands is uniquely positioned to evaluate the outcomes for DBD and DCD procedures. This setting not only allows for the evaluation of a large number of DCD procedures, but it also limits selection biases that may result from a preferential use of DCD grafts with superior donor characteristics (i.e. young donor age; short ischemia time). The liberal attitude towards DCD grafts in Dutch transplantation centres is reflected in the high proportion of DCD grafts (41% for the 17 year observation period evaluated in this study, 50% for the year 2016), and comparable donor characteristics for DBD and DCD grafts. The Dutch policy with respect to use of DCD (and extended criteria) grafts presumably explains the relatively high incidence of early graft loss (overall incidence for the 2000–2017 interval in the Netherlands 6% vs. 2.8% for the UK [19]). Noticeably, this did not impact overall outcomes with 10-year graft survival rates for the Netherlands (73.9%) being similar to those reported for the UK (74.4%) [19]) and slightly better to those of Caucasian American recipients 71% [24].
Although the cohort data for the Netherlands confirm the higher incidences of early graft loss and delayed graft function in DCD grafts, long-term graft and recipient survival for DBD and DCD grafts were similar. We consider it unlikely that this equivalence relates to differences in donor- or recipient characteristics as the observed differences were minor, and as positive associations (e.g. higher percentage of male donors in the DCD group) are balanced by negative factors (higher recipient age in the DCD group). Outcome equivalence despite an almost two-and-a-half fold increase in the incidence of delayed graft function was explained by differential impacts of delayed graft function on graft survival in DCD than DBD grafts. As such a focus on short-term outcomes ignores the superior recovery characteristics of DCD grafts.
Dutch registry data confirm a higher susceptibility of DCD grafts for prolonged cold-ischemia as also reported for the UK-cohort [18]. Remarkably, detailed exploration of this phenomenon showed that this negative impact is limited to a higher risk for early graft loss: prolonged cold ischemia times did not disproportionately impact early graft loss-censored-graft-survival and rejection episodes. Implying the decisions to accept these grafts should be primarily based on the consequences of early graft loss to the recipient.
Long-term survival and functional equivalence, and comparable rates of rejection-related graft losses for DCD and DBD grafts imply that under the current immunosuppressive regimens immunological aspects are not a main point of concern with regard to the use of DCD grafts. The higher incidence of delayed graft function and more profound impact of prolonged cold ischemia time on early DCD graft survival, and the pronounced negative effect of delayed graft function on DBD-graft survival rather points to differences in graft resilience. In this context, DCD grafts appear to sustain a more profound ischemic insult, which is compensated by a superior functional recovery potential. The latter is supported by the graft recovery analyses (eGFR) showing equal function for DCD and DBD grafts during prolonged follow-up. One could speculate that the differences in resilience relate to a negative impact of donor brain death on DBD grafts [25], [26], and/or activation of tissue protective responses such as ischemic preconditioning [27], and activation of the innate repair receptor [28] during the initial warm ischemia episode following cardiac death in DCD grafts. A further, and non-exclusive explanation is that incident delayed graft function in DBD grafts marks a poorer graft quality.
Multivariable analyses stressed the impact of the first warm ischemic period on incident early graft loss and delayed graft function [29]. In fact, for incident delayed graft function the impact of 1 min of warm ischemia equalled the impact of 1 h cold ischemia. As such attempts to further minimize the first warm ischemic period could improve short term outcomes of DCD grafts. Longer anastomosis times primarily associate with early graft loss. Yet, it cannot be excluded that this association (partially) reflects technical difficulties during the transplant procedure. The analysis did not indicate cold-ischemia time as a negative factor for graft survival [17], this phenomenon may reflect the notion that the negative impact of cold ischemia times in mainly limited to ischemia times over 24 h [17], and the progressive awareness on avoiding long cold ischemia times in the Netherlands (Supplemental Table 2). As result the number of grafts with cold ischemia times exceeding 24 h in the current study is low.
Irrespective of the donor type, multivariable analysis indicated lower incident delayed graft function for grafts of female donors. Sex-associated differences in graft survival were not observed for mid and long term outcome (not shown). Conclusions from a recent experimental and data base study confirm this observation, and suggest that superior short term outcomes in females relate to an estrogen-mediated mechanism [30].
Observed beneficial associations between donor height and short term outcome may relate to a mass-effect with a higher number of functional units in kidneys from taller donors [31].
Limitations: this is a registry based study. As such all limitations accompanying registry databases such as missing data and registration errors apply. The limited number of early graft losses interferes with a more detailed evaluation of associated factors. Moreover, exploration of potential associations is limited to the factors represented in the data base, as such information on potentially relevant factors might be missing. Although we adjusted for clinically relevant factors, this might have led to residual confounding. Third, clinical practices and guidelines may have changed over time. Although we introduced the “calendar months since transplantation” as factor in the regression analysis for DGF and early graft loss, associations may have changed over time.
In conclusion, this detailed report covering 17 years' experience with DCD procedures and that includes more than 2700 DCD procedures shows that under the prevailing Dutch protocols mid- and long-term outcomes after DBD and DCD kidney graft transplantation are similar. These conclusions, and equal 10-year graft survival rates for The Netherland, UK and USA do not justify the reluctance to the use of DCD renal grafts. A strong focus on short-term outcomes neglects the superior recovery potential of DCD grafts.
The increased incidences of early graft loss following longer ischemia times (over 24 h) call for stricter guidelines with respect to the logistics of DCD procedures.
Outstanding Questions
In the light of donor shortages, there needs to be more attention for the apparent excellent recovery potential and adequate long-term survival of renal grafts donated after cardiac death. The reluctance to the use of these grafts should be questioned.
Conflicts of Interest
We declare no competing interests.
Acknowledgments
Acknowledgements
We greatly acknowledge the Dutch Transplant Foundation (Nederlandse Transplantatie Stichting) for providing the data, especially Cynthia Konijn-Janssen and Dilesh Kishoendajal.
Funding
This study did not receive external funding.
Author Contributions
Study conception and design: AFS, JHNL. Data collection: LGMW, DKdeV, APJdeV, FJB, JvdW, ADvZ, MHLC, LHH, MCB, ASN, SPB, and IPA. Data analysis: EB and JHNL. Drafting of the figures: EB. Data Interpretation: AFS, JHNL and EB. Writing of the manuscript: AFS, LGMW and JHNL. Critical revision of the manuscript: DKdeV, APJdeV, FJB, JvdW, ADvZ, MHLC, LHH, MCB, ASN, SPB, IPA, and EB.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2018.09.007.
Appendix A. Supplementary data
Supplementary tables
References
- 1.Abecassis M., Bartlett S.T., Collins A.J. Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin J Am Soc Nephrol. 2008;3:471–480. doi: 10.2215/CJN.05021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Childress J.F. Organ donor research: overcoming challenges, increasing opportunities. JAMA. 2017;318:2177–2178. doi: 10.1001/jama.2017.16442. [DOI] [PubMed] [Google Scholar]
- 3.Sharif A., Borrows R. Delayed graft function after kidney transplantation: the clinical perspective. Am J Kidney Dis. 2013;62:150–158. doi: 10.1053/j.ajkd.2012.11.050. [DOI] [PubMed] [Google Scholar]
- 4.de Sandes-Freitas T.V., Felipe C.R., Aguiar W.F., Cristelli M.P., Tedesco-Silva H., Medina-Pestana J.O. Prolonged delayed graft function is associated with inferior patient and kidney allograft survivals. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yarlagadda S.G., Coca S.G., Formica R.N., Jr., Poggio E.D., Parikh C.R. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24:1039–1047. doi: 10.1093/ndt/gfn667. [DOI] [PubMed] [Google Scholar]
- 6.Schröppel B., Legendre C. Delayed kidney graft function: from mechanism to translation. Kidney Int. 2014;86:251–258. doi: 10.1038/ki.2014.18. [DOI] [PubMed] [Google Scholar]
- 7.Formica R.N., Jr. Opportunities to increase availability of deceased donor kidneys. CJASN. 2017;12:871–873. doi: 10.2215/CJN.04490417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mallon D.H., Summers D.M., Bradley J.A., Pettigrew G.J. Defining delayed graft function after renal transplantation: simplest is best. Transplantation. 2013;96:885–889. doi: 10.1097/TP.0b013e3182a19348. [DOI] [PubMed] [Google Scholar]
- 9.Cavaille-Coll M., Bala S., Velidedeoglu E. Summary of FDA workshop on ischemia reperfusion injury in kidney transplantation. Am J Transplant. 2013;13:1134–1148. doi: 10.1111/ajt.12210. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez-Gil B., Haase-Kromwijk B., Van Leiden H. Current situation of donation after circulatory death in European countries. Transpl Int. 2011;24:676–686. doi: 10.1111/j.1432-2277.2011.01257.x. [DOI] [PubMed] [Google Scholar]
- 11.Wind J., Faut M., van Smaalen T.C., van Heurn E.L. Variability in protocols on donation after circulatory death in Europe. Crit Care. 2013;17:R217. doi: 10.1186/cc13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagaraja P., Roberts G.W., Stephens M., Horvath S., Fialova J., Chavez R. Influence of delayed graft function and acute rejection on outcomes after kidney transplantation from donors after cardiac death. Transplantation. 2012;94:1218–1223. doi: 10.1097/TP.0b013e3182708e30. [DOI] [PubMed] [Google Scholar]
- 13.Singh R.P., Farney A.C., Rogers J. Kidney transplantation from donation after cardiac death donors: lack of impact of delayed graft function on post-transplant outcomes. Clin Transpl. 2011;25:255–264. doi: 10.1111/j.1399-0012.2010.01241.x. [DOI] [PubMed] [Google Scholar]
- 14.Kusaka M., Kubota Y., Sasaki H. Combined predictive value of the expanded donor criteria for long-term graft survival of kidneys from donors after cardiac death: a single-center experience over three decades. Int J Urol. 2016;23:319–324. doi: 10.1111/iju.13045. [DOI] [PubMed] [Google Scholar]
- 15.Weber M., Dindo D., Demartines N., Ambuhl P.M., Clavien P.A. Kidney transplantation from donors without a heartbeat. N Engl J Med. 2002;347:248–255. doi: 10.1056/NEJMoa020274. [DOI] [PubMed] [Google Scholar]
- 16.Gentil M.A., Castro P., Ballesteros L. Survival of kidney allograft of donors after circulatory death is similar to donors after brain death: experience in a regional program. Transplant Proc. 2015;47:2572–2574. doi: 10.1016/j.transproceed.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 17.Summers D.M., Johnson R.J., Allen J. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet. 2010;376:1303–1311. doi: 10.1016/S0140-6736(10)60827-6. [DOI] [PubMed] [Google Scholar]
- 18.Moers C., Leuvenink H.G., Ploeg R.J. Donation after cardiac death: evaluation of revisiting an important donor source. Nephrol Dial Transplant. 2010;25:666–673. doi: 10.1093/ndt/gfp717. [DOI] [PubMed] [Google Scholar]
- 19.Summers D.M., Watson C.J., Pettigrew G.J. Kidney donation after circulatory death (DCD): state of the art. Kidney Int. 2015;88:241–249. doi: 10.1038/ki.2015.88. [DOI] [PubMed] [Google Scholar]
- 20.Kootstra G. Statement on non-heart-beating donor programs. Transplant Proc. 1995;27:2965. [PubMed] [Google Scholar]
- 21.http://optn.transplant.hrsa.gov
- 22.Hart A., Smith J.M., Skeans M.A. OPTN/SRTR 2016 annual data report: kidney. Am J Transplant. 2018;18(Suppl. 1):18–113. doi: 10.1111/ajt.14557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Easterbrook P.J., Berlin J.A., Gopalan R., Matthews D.R. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 24.Gondos A., Döhler B., Brenner H., Opelz G. Kidney graft survival in Europe and the United States: strikingly different long-term outcomes. Transplantation. 2013;95:267–274. doi: 10.1097/TP.0b013e3182708ea8. [DOI] [PubMed] [Google Scholar]
- 25.Bos E.M., Leuvenink H.G., van Goor H., Ploeg R.J. Kidney grafts from brain dead donors: inferior quality or opportunity for improvement? Kidney Int. 2007;72:797–805. doi: 10.1038/sj.ki.5002400. [DOI] [PubMed] [Google Scholar]
- 26.de Vries D.K., Lindeman J.H., Ringers J., Reinders M.E., Rabelink T.J., Schaapherder A.F. Donor brain death predisposes human kidney grafts to a proinflammatory reaction after transplantation. Am J Transplant. 2011;11:1064–1070. doi: 10.1111/j.1600-6143.2011.03466.x. [DOI] [PubMed] [Google Scholar]
- 27.de la Barca JM Chao, Bakhta O., Kalakech H. Metabolic signature of remote ischemic preconditioning involving a cocktail of amino acids and biogenic amines. JAHA. 2016;5 doi: 10.1161/JAHA.116.003891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brines M., Cerami A. The receptor that tames the innate immune response. Mol Med. 2012;18:486–496. doi: 10.2119/molmed.2011.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gill J., Rose C., Lesage J., Joffres Y., Gill J., O'Connor K. Use and outcomes of kidneys from donation after circulatory death donors in the United States. J Am Soc Nephrol. 2017;28:3647–3657. doi: 10.1681/ASN.2017030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aufhauser D.D., Jr., Wang Z., Murken D.R. Improved renal ischemia tolerance in females influences kidney transplantation outcomes. J Clin Invest. 2016;126:1968–1977. doi: 10.1172/JCI84712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz G.J. Height: the missing link in estimating glomerular filtration rate in children and adolescents. Nephrol Dial Transplant. 2014;29:944–947. doi: 10.1093/ndt/gft530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables