Abstract
Background
Studies increasingly suggest that the efficacy of certain dual antiretroviral therapy (ART) combinations is equal to triple ART. Increasing concerns among HIV-positive patients and physicians in Switzerland include ART cost and long-term ART safety and toxicity, i.e. taking only as many ART agents as necessary. The aims of this retrospective analysis are to report on the de-escalation of our entire clinic population of eligible patients with well-controlled HIV-infection to dolutegravir-containing dual ART.
Methods
Starting in March 2015, we systematically considered the de-escalation of eligible patients to either dolutegravir/emtricitabine or dolutegravir/lamivudine, by discontinuing tenofovir disoproxil fumarate or abacavir. We report on the virological efficacy, tolerability and patient satisfaction ≥ 48 weeks after de-escalation.
Findings
Of 106 HIV-positive patients followed in our clinic, 70 patients were de-escalated. Three returned to triple ART (insomnia after dolutegravir start, n = 2; new wish for single tablet regimen, n = 1). All de-escalated patients and all who continued triple ART had suppressed HIV viremia at last follow-up and were satisfied with their ART regimen, except for one patient who had virological failure after ART discontinuation in the setting of major depression. The most common reasons to not de-escalate included hepatitis B co-infection (n = 6), physician's concern about ART adherence (n = 6), patient reluctance to switch from a single tablet to a 2-tablet regimen (n = 7), patient satisfied with current ART (n = 5) and others (n = 12).
Interpretation
ART de-escalation to dolutegravir/FTC or dolutegravir/3TC is possible in the majority of patients virologically suppressed on triple ART, and may effectively address patient and physician concerns about long-term safety and cost of ART.
Keywords: HIV infection, Dual antiretroviral therapy, Dolutegravir, De-escalation, Induction and maintenance treatment
Research in context
Evidence before this study
Four randomized trials conducted in patients with well-controlled HIV infection document that de-escalating from triple antiretroviral therapy (ART) including a protease inhibitor plus two nucleoside reverse transcriptase inhibitors (NRTI) to dual ART with only one NRTI is as effective as continuing triple ART. Dolutegravir was approved for clinical use in Europe in 2014 and rapidly emerged as a well-tolerated and highly potent ART agent with a high barrier to HIV resistance. Several case series in ART-naïve patients and in well-controlled patients de-escalating to dual ART based on dolutegravir plus either emtricitabine or lamivudine showed high rates of virological success.
Added value of this study
In March 2015, we systematically started de-escalating HIV-positive patients followed in our Swiss University-affiliated clinic to dual therapy containing dolutegravir plus either emtricitabine or lamivudine. Considering contraindications to de-escalation (most notably, chronic hepatitis B infection and limited likelihood of ART adherence), we de-escalated 70 of our 103 patients. Of the 70 de-escalated patients, 4 returned to triple ART and 66 have successfully continued dual ART for at least 48 weeks.
Implications of all the available evidence
Together with the recent data from the randomized SWORD-1 and -2 studies, our data suggest that dual dolutegravir-based ART is similarly effective as standard triple ART for maintenance therapy in patients virologically suppressed on triple ART. Dual therapy may effectively address patient and physician concerns about cost and about potential long-term adverse effects of ART. The recently published GEMINI-1 and -2 trials now establish that dual dolutegravir-based ART is as effective as triple ART also in ART-naïve patients. Dolutegravir monotherapy may still emerge as a valid HIV treatment option in selected patients in the future.
Alt-text: Unlabelled Box
1. Introduction
Current antiretroviral therapy (ART) guidelines recommend combination treatment using 3 drugs. However, concerns about long-term ART safety have emerged, including the potential for renal dysfunction [1] and osteoporosis [2] (for tenofovir disoproxil fumarate [TDF] and certain protease inhibitors [PI]), and for cardiovascular events (with abacavir [ABC] and certain PIs) [3]. Several randomized studies now document that dual therapy including a PI therapy plus one non-nucleoside reverse transcriptase inhibitor (NRTI) is similarly effective to triple PI-based ART combined with 2 NRTIs [4], [5], [6], [7]. Case series in naïve patients and in virologically suppressed patients de-escalating to dual ART using dolutegravir plus either emtricitabine (FTC) or lamivudine (3TC) have also shown high rates of virological success [8], [9], [10], [11], [12], [13]. In some countries, such dual therapy now is frequently and successfully prescribed for cost reasons.
On this background, it was our best assessment that dual treatment using dolutegravir plus either FTC or 3TC represents an appropriate option to diminish long-term safety concerns and medication cost for patients with well-controlled HIV infection. Dolutegravir monotherapy was not considered because the virological failure rate appeared unacceptably high [14]. Here we report on the systematic de-escalating of an entire population of eligible patients in a Swiss university-affiliated HIV clinic from virologically suppressive triple ART to dual therapy with either dolutegravir/FTC or dolutegravir/3TC.
2. Methods
As of 1.3.2015, 2 infectious diseases specialists (SAW and PET) followed 106 HIV-positive patients in an HIV out-patient clinic. All patients were enrolled in the Swiss HIV Cohort Study (SHCS; www.shcs.ch) and provided written informed consent for anonymized collection and analysis of their clinical and laboratory data. All patients on stable suppressive triple ART (HIV RNA < 50 copies/mL for ≥ 12 months) were considered for de-escalation to dual therapy with dolutegravir/FTC or dolutegravir/3TC, similar to our systematic switching away from protease inhibitors based on the best available assessment of the literature many years ago.
Ineligibility for de-escalation was assessed clinically by the attending HIV physician and included patients with pre-existing NRTI- or integrase inhibitor-associated mutations or hepatitis B co-infection, in whom TDF was not discontinued, and physician concern about ART adherence. Patients were extensively informed about the potential merits and limitations of dual therapy. Infectious diseases specialists in Switzerland are able to prescribe antiretroviral therapy agents approved by the federal authorities and in combinations they select, without requiring any written informed consent. For example, our center has been systematically avoiding protease inhibitors for many years (note in Table 1 that patients were typically de-escalated from an NNRTI- or integrase inhibitor-based triple ART regimen). Dolutegravir monotherapy was not considered because the virological failure rate appeared too high, and we agree with concerns expressed at meetings and in the literature [17], [18] that dolutegravir monotherapy should be given only in the setting of a research protocol and with the written informed consent of the patient.
Table 1.
Baseline characteristicsa.
| All (n = 103) | Continued triple ART (n = 33) | De-escalated to dual ART (n = 70) | |
|---|---|---|---|
| Age, years (median, IQR) | 52 (43–56) | 51 (40–56) | 52 (44–56) |
| Male (n, %) | 66 (64%) | 22 (67%) | 44 (63%) |
| Duration since HIV diagnosis, years (median, IQR) | 12 (8–19) | 11 (6–21) | 12 (8–17) |
| Mode of HIV transmission | MSM (37) | MSM [13] | MSM [24] |
| Heterosexual (54) | Heterosexual [17] | Heterosexual (37) | |
| IDU [11] | IDU [3] | IDU [8] | |
| unknown [1] | unknown [1] | ||
| Most frequent ART regimens before de-escalation | TDF/FTC/EFV [23] | TDF/FTC/EFV [6] | TDF/FTC/EFV [17] |
| TDF/FTC/RLP [17] | TDF/FTC/RLP [8] | TDF/FTC/RLP [9] | |
| ABC/3TC/DTG [11] | ABC/3TC/DTG [2] | ABC/3TC/DTG [9] | |
| TDF/FTC/DTG [10] | TDF/FTC/DTG [2] | TDF/FTC/DTG [8] | |
| TDF/FTC/EVG/COBI [5] | TDF/FTC/EVG/COBI [2] | TDF/FTC/EVG/COBI [3] | |
| TDF/3TC/DTG [4] | TDF/3TC/DTG [1] | TDF/3TC/DTG [3] | |
| Patients with nadir CD4 < 200 cells/mL (n, %) | 50 (49%) | 13 (39%) | 37 (53%) |
| eGFR (mean)b | 92 mL/min | 92 mL/min | 93 mL/min |
Note. 3TC, lamivudine; ABC, abacavir: cobi, cobicistat; DTG, dolutegravir; EVG, elvitegravir; EFV, efavirenz; RLP, rilpivirine; TDF, tenofovir disoproxil fumarate.
At time of de-escalation or at last visit in continued triple ART group, respectively.
Last measurement before de-escalation in this group, and at last visit in the continued triple ART group, respectively.
The outcomes of interest in this case series were the number of patients who could or could not be de-escalated, the patient/physician reasons for this, the number of satisfied patients in each group, assessed by personal questioning, and the prevalence of HIV RNA < 50 copies/mL, measured ≥ 48 weeks after de-escalation or as of 31.08.2018 in patients continuing triple ART, respectively. This paper represents a retrospective, secondary analysis of routinely collected data. Standard HIV care was provided to all patients, including routine clinical and laboratory evaluation 2–3, 8–12, and 24 weeks after de-escalation, and at least twice yearly thereafter. We follow this same schedule in all patients in whom we change ART, i.e. no additional visits were scheduled because of de-escalation, but patients in whom ART was not changed were offered follow-up visits at the usual frequency, i.e. every 6 months.
Organ morbidity at baseline included decline in estimated glomerular filtration rate (eGFR) to < 60 mL/min in those with previously normal eGFR [19], low trauma fracture [20], osteoporosis by DXA, and ALT elevation ≥ 1.5 × normal. In any patient, ≥ 1 organ morbidities could be present. Concern about future renal or bone morbidity included patients who had confirmed > 10% eGFR drop without drop to eGFR < 60 mL/min, and DXA-confirmed osteopenia. Some patients were enrolled in the SHCS core project metabolism and aging and underwent DXA and coronary CT angiography every 2 years; however, no additional imaging or laboratory tests were scheduled because of ART de-escalation.
3. Results
Of 106 HIV-positive patients, 3 were elite controllers without ART, i.e. 103 patients were eligible to be considered for de-escalation. Their clinical characteristics are summarized in Table 1. Organ morbidity was present in 25 (24.2%) patients (n = 9 renal, n = 20 osteoporosis, n = 3 liver), and concern about future morbidity in 24 (23.3%) patients (n = 8 renal, n = 19 osteopenia, n = 2 liver). All patients have been followed for ≥ 48 weeks after de-escalation or during continued triple ART, with no missing follow-up visits or laboratory values.
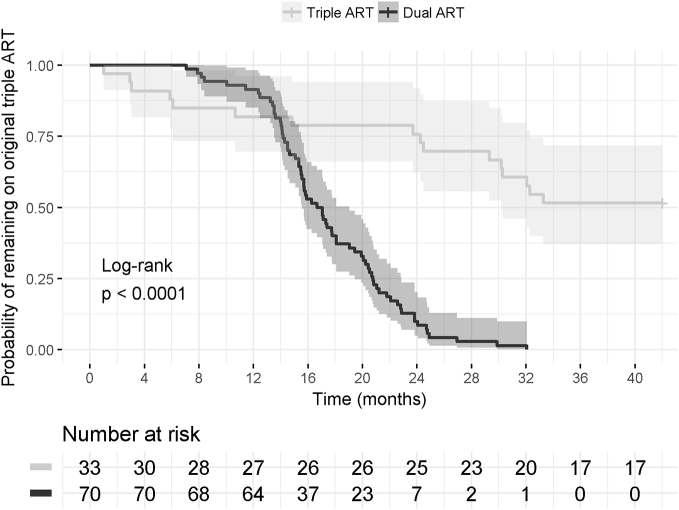
As shown in Fig. 1, over the course of about 2 years, seventy of the 103 (67.9%) patients were de-escalated (n = 61 to DTG/FTC, n = 9 to DTG/3TC). Reasons for not de-escalating included patient-related reasons (n = 15) and physician-related reasons (n = 18). Patient-related reasons included reluctance to switch from a single tablet to a 2-tablet regimen (n = 7), patient satisfied with current ART (n = 5), anxiety about de-escalation (n = 1), patient unwilling to attend 2 additional clinic appointments after de-escalation (n = 1), and patient's HIV-positive partner unwilling to de-escalate (n = 1). Physician reasons for not de-escalating included NRTI resistance mutations (n = 2), hepatitis B co-infection (n = 6), concern about ART adherence (n = 6), concern about future need to modify epilepsy medication (n = 1), headache during previous DTG exposure (n = 2), and patient soon moving to another country (n = 1).
Fig. 1.
Proportions of patients that were switched to dual therapy or that remained on original triple ART regimen after 01.03.2015.
Kaplan Meier Plot for probability of remaining on the ART regimen in use on 01.03.2015 over 183 weeks of follow-up in the dual and triple ART groups with 95% confidence intervals (log-rank test p < 0.0001). The graph illustrates patients who were de-escalated to dolutegravir-containing dual therapy (n = 70) and patients who continued triple ART (n = 33; of which n = 16 switched to another triple ART combination, and n = 17 continued the original triple ART regimen).
One patient elected to de-escalate ART but has been followed < 48 weeks. In the de-escalation group, 66 of 70 (94.3%) patients were satisfied and expressed the wish to continue the new ART regimen; all these patients remain on the initially selected dual ART at last follow-up, ≥ 48 weeks after de-escalation. Four patients returned to triple ART because of insomnia after starting DTG within the first 2 weeks (n = 2), or because of a newly expressed wish to return to single tablet ART after 2 months of DTG-FTC (n = 1). One patient had virological failure (1.62 log HIV RNA copies/mL), 9 months after de-escalation, who admitted to discontinuing DTG/FTC in the setting of severe depression. No resistance-associated mutations were identified and 4 weeks into re-starting DTG/FTC, HIV RNA decreased to 1615 copies/mL. However, because of continued concerns about adherence, this patient was then switched back to triple therapy (TAF/FTC/DTG) and has suppressed HIV RNA at last follow-up.
As shown in Fig. 1, 33 patients were not de-escalated to dual therapy. Of these, 17 patients remain on the original triple ART regimen at last follow-up. Sixteen patients were switched to other triple ART regimens within approximately 3 years, because of the availability of tenofovir alafenamide, in order to reduce the number of pills, or for other reasons. The new ART regimens included TAF/FTC/EVG/COBI (n = 5), TAF/FTC/DTG (n = 4), ABC/3TC/DTG (n = 3), or another triple regimen (n = 3). All 33 patients were satisfied with their ART choice, and all had suppressed HIV RNA at last follow-up except for 2 patients (one patient with a blip who had suppressed viremia at the last 2 routine follow-up visits, and one patient with limited ART adherence who had detectable viremia at the last 4 visits and was then lost to follow-up).
4. Discussion
Here we report on the systematic de-escalation to dolutegravir-based dual ART of the majority of a Swiss clinic population of patients with well-controlled HIV infection while on triple ART. Existing organ morbidity or concerns about future morbidity was recorded in almost half of the patients, providing a rationale for considering de-escalation to a regimen that avoids TDF, boosted PIs, or ABC for bone, renal, or cardiovascular health reasons. After careful consideration of contra-indications, patient preferences, and likelihood of ART adherence, about 2/3 of our patients were de-escalated. Only few de-escalated patients subsequently returned to triple ART, and none because of insufficient virological efficacy but for tolerability reasons or personal preference.
While emphasis continues to be placed on triple ART in current guidelines, some HIV clinicians have begun sparing one NRTI in patients with suppressed HIV [21], for two main reasons; first, evidence for the virological efficacy of dual therapy using 3TC or FTC plus either a PI [4], [5] or dolutegravir [8], [9], [10], [11], [12], [13] is now convincing, suggesting that TDF, tenofovir alafenamide (TAF), or ABC as “third” agent may be unnecessary, because they add to long-term toxicity and cost concerns in well-controlled patients. Second, compared to triple ART using TDF, the alternative of switching to TAF has only limited bone and renal benefits, a slightly unfavorable LDL-cholesterol effect, and the cost of TAF is high compared to generic TDF. This suggests that systematic switching of all patients from TDF to TAF is neither ideal nor always necessary. The single pill, fixed-dose combination TAF/FTC/bictegravir may now provide an additional effective alternative ART regimen for patients in whom the third agent cannot be stopped.
Our best assessment today is that de-escalation from suppressive triple ART to dolutegravir/3TC or dolutegravir/FTC is safe, effective, and carries only a very small resistance risk [8], [9], [10], [11], [12], [13], suggesting that previously expressed concerns about the merits, cost, and safety of ART simplification to dual therapy should be re-assessed [17], [18]. Intriguingly, the manufacturer of dolutegravir is now actively pursuing the dual therapy strategy, consistent with perceived efficacy and commercial viability of this approach [13], [22]. While the virological failure rate with dolutegravir monotherapy may be unacceptably high [14], dolutegravir monotherapy may still emerge as a valid de-escalation option in selected patients in the future.
In our patients, we preferred FTC over 3TC due to longer half life and potential association with protection from end-stage liver disease [15]. However, randomized studies now suggest similar virological efficacy and thus the interchangeability of 3TC and FTC [16]. Additional arguments favoring the selection of 3TC rather than FTC today include reduced cost compared to FTC due to availability of generic 3TC, and in the future may include convenience (i.e. availability of fixed-dose combination 3TC-dolutegravir).
Our data is limited by the number of patients de-escalated and the follow-up time attained during dual ART, and does not apply to the selection of initial ART. Longer observation periods are required to document the durability of the virological success and any measurable clinical benefit as regards e.g. renal function or bone mineral density. In addition, regular follow-up in the setting of the successfully ongoing Swiss HIV Cohort Study, with high rates of viral suppression and modern ART regimens, may indicate a clinic population with a higher future likelihood of successful dual therapy than in other settings. Most of our patients had suppressed viremia for many years, and were continuously treated (some for > 10 years) by the same attending physician, suggesting high levels of ART adherence, strong physician-patient relationships, and high levels of reciprocal trust. For these reasons, our favorable de-escalation experience should only cautiously be extrapolated to other populations.
Interestingly, an increasing pill number was considered by few patients as reason against de-escalation. On the contrary, the vast majority of patients were convinced that minimization of pill number was less important than prescription of not more than the necessary number of ART agents with the least potential for long-term toxicity. We suggest that limitation of ART agents prescribed to the minimally necessary number represents an appropriate goal for modern HIV care. A main topic of conversation with our patients now is the management of existing or avoidance of future “metabolic” complications, including dyslipidemia, cardiovascular, renal, and bone events. In addition, ART cost is regularly perceived as excessive by our patients. This may surprise given that ART is covered by Swiss insurance carriers and our clinic is located in a resource-rich country. In our clinic, many patients try to pursue a healthy lifestyle, seek medical information from multiple sources including the internet, perhaps increasingly reflecting phenomena that have been interpreted in the setting of trends towards “postmodern medicine” [23] and “healthism” [24], including (possibly increasing) skepticism towards the pharmaceutical industry.
The effectiveness of dual, dolutegravir-based ART was recently recorded in a systematic review and meta-analysis [25] and is receiving increasing attention at scientific meetings [21]. Dolutegravir-based dual therapy is being prescribed to an increasing even though still limited number of patients by Swiss HIV clinicians. In January 2018, 142 of 9458 (1.5%) ART-treated SHCS participants were taking dual, dolutegravir-based ART (increasing from 0.5% of participants in 2015 to 1.3% in 2016 and 1.6% in 2017). Fourty-eight of 196 (24.5%) SHCS-affiliated physicians were treating > 1 patient with dual, dolutegravir-based ART outside of clinical trials in 2017 (Alexandra Scherrer, SHCS data center, University of Zurich, personal communication). Dolutegravir-3TC dual ART was recently confirmed to be non-inferior vs. dolutegravir-TDF-FTC in randomized trials in ART-naive patients (GEMINI-1 and -2, ClinicalTrials.gov NCT02831673 and NCT02831764) [26]. We await with interest the results of an ongoing clinical trial of de-escalation to dolutegravir-3TC dual ART in patients with well-controlled HIV infection in Switzerland (simpl’HIV, ClinicalTrials.gov NCT03160105).
Funding
No funding was obtained in the preparation of this report.
Author Contributions
PET and SAW extracted the data from the electronic medical records. NDD and CS analyzed the data and wrote the first draft of the manuscript. SG generated the Kaplan Meier curve. All authors provided insights and critically reviewed the manuscript, and approved the submitted version.
Acknowledgments/Funding/Conflicts of interest
The authors have received no financial support for the clinical management of their patients or for the preparation of this report. Our institution has received unrestricted research grants and advisory fees from ViiV and Gilead – no fees went to any of the authors personally. The funders had no role in paper design, data collection, data analysis, interpretation, writing of the paper.
References
- 1.Kooij K.W., Vogt L., Wit F.W.N.M. Higher prevalence and faster progression of chronic kidney disease in human immunodeficiency virus-infected middle-aged individuals compared with human immunodeficiency virus-uninfected controls. J Infect Dis. 2017;216:622–631. doi: 10.1093/infdis/jix202. [DOI] [PubMed] [Google Scholar]
- 2.Bedimo R., Maalouf N.M., Zhang S., Drechsler H., Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012;26:825–831. doi: 10.1097/QAD.0b013e32835192ae. [DOI] [PubMed] [Google Scholar]
- 3.Sabin C.A., Reiss P., Ryom L. Is there continued evidence for an association between abacavir usage and myocardial infarction risk in individuals with HIV? A cohort collaboration. BMC Med. 2016;14:61. doi: 10.1186/s12916-016-0588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahn P., Andrade-Villanueva J., Arribas J.R. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis. 2014;14:572–580. doi: 10.1016/S1473-3099(14)70736-4. [DOI] [PubMed] [Google Scholar]
- 5.Arribas J.R., Girard P.-M., Landman R. Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15:785–792. doi: 10.1016/S1473-3099(15)00096-1. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Molina J.A., Rubio R., Rivero A. Dual treatment with atazanavir-ritonavir plus lamivudine versus triple treatment with atazanavir-ritonavir plus two nucleos(t)ides in virologically stable patients with HIV-1 (SALT): 48 week results from a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15:775–784. doi: 10.1016/S1473-3099(15)00097-3. [DOI] [PubMed] [Google Scholar]
- 7.Lombardi F., Belmonti S., Quiros-Roldan E. Evolution of blood-associated HIV-1 DNA levels after 48 weeks of switching to atazanavir/ritonavir + lamivudine dual therapy versus continuing triple therapy in the randomized AtLaS-M trial. J Antimicrob Chemother. 2017;72:2055–2059. doi: 10.1093/jac/dkx068. [DOI] [PubMed] [Google Scholar]
- 8.Gubavu C., Prazuck T., Niang M. Dolutegravir-based monotherapy or dual therapy maintains a high proportion of viral suppression even in highly experienced HIV-1-infected patients. J Antimicrob Chemother. 2016;71:1046–1050. doi: 10.1093/jac/dkv430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maggiolo F., Gulminetti R., Pagnucco L. Lamivudine/dolutegravir dual therapy in HIV-infected, virologically suppressed patients. BMC Infect Dis. 2017;17:215. doi: 10.1186/s12879-017-2311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joly V., Burdet C., Landman R. Abstract 458, Conference on Retroviruses and Opportunistic Infections, Seattle WA, 13–16 February 2017. 2017. Promising results of lamivudine + dolutegravir maintenance therapy in ANRS 167 Lamidol trial.http://www.croiconference.org/sessions/promising-results-dolutegravir-lamivudine-maintenance-anrs-167-lamidol-trial (accessed April 27, 2018) [Google Scholar]
- 11.Achhra A.C., Mwasakifwa G., Amin J., Boyd M.A. Efficacy and safety of contemporary dual-drug antiretroviral regimens as first-line treatment or as a simplification strategy: a systematic review and meta-analysis. Lancet HIV. 2016;3:e351–e360. doi: 10.1016/S2352-3018(16)30015-7. [DOI] [PubMed] [Google Scholar]
- 12.Cahn P., Rolon M.J., Figueroa M.I., Gun A., Patterson P., Sued O. Dolutegravir-lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study. J Int AIDS Soc. 2017;20:21678. doi: 10.7448/IAS.20.01.21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taiwo B.O., Zheng L., Stefanescu A. ACTG A5353: a pilot study of dolutegravir plus lamivudine for initial treatment of human immunodeficiency virus-1 (HIV-1)-infected participants with HIV-1 RNA < 500000 Copies/mL. Clin Infect Dis. 2018;66:1689–1697. doi: 10.1093/cid/cix1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katlama C., Soulié C., Caby F. Dolutegravir as monotherapy in HIV-1-infected individuals with suppressed HIV viraemia. J Antimicrob Chemother. 2016;71:2646–2650. doi: 10.1093/jac/dkw186. [DOI] [PubMed] [Google Scholar]
- 15.Ryom L., Lundgren J.D., De Wit S. Use of antiretroviral therapy and risk of end-stage liver disease and hepatocellular carcinoma in HIV-positive persons. AIDS. 2016;30:1731–1743. doi: 10.1097/QAD.0000000000001018. [DOI] [PubMed] [Google Scholar]
- 16.Ford N., Hill A., Vitoria M., Mills E.J. Editorial commentary: comparative efficacy of lamivudine and emtricitabine: comparing the results of randomized trials and cohorts. Clin Infect Dis. 2015;60:154–156. doi: 10.1093/cid/ciu767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr A., Hoy J., Pozniak A. The ethics of switch/simplify in antiretroviral trials: non-inferior or just inferior? PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallant J., Sugarman J. Dolutegravir monotherapy: when should clinical practice be clinical research? Antivir Ther (Lond) 2017;22:93–95. doi: 10.3851/IMP3113. [DOI] [PubMed] [Google Scholar]
- 19.Mocroft A., Lundgren J.D., Ross M. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet HIV. 2016;3:e23–e32. doi: 10.1016/S2352-3018(15)00211-8. [DOI] [PubMed] [Google Scholar]
- 20.Junier T., Rotger M., Biver E. Contribution of genetic background and clinical risk factors to low-trauma fractures in human immunodeficiency virus (HIV)-positive persons: the Swiss HIV cohort study. Open Forum Infectious Diseases. 2016;3(ofw101) doi: 10.1093/ofid/ofw101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HIV treatment bulletin Dolutegravir-based dual therapy as switch option in multiple studies. 2017. http://i-base.info/htb/32843 published online Nov 28. (accessed April 27, 2018)
- 22.ViiV Healthcare ViiV healthcare submits regulatory applications for the first HIV maintance regimen comprising only two medicines. 2017. https://www.viivhealthcare.com/media/press-releases/2017/june/viiv-healthcare-submits-regulatory-applications-for-the-first-hiv-maintenance-regimen-comprising-only-two-medicines.aspx published online June 1. (accessed April 27, 2018)
- 23.Gray J.A. Postmodern medicine. Lancet. 1999;354:1550–1553. doi: 10.1016/s0140-6736(98)08482-7. [DOI] [PubMed] [Google Scholar]
- 24.Greenhalgh T., Wessely S. ‘Health for me’: a sociocultural analysis of healthism in the middle classes. Br Med Bull. 2004;69:197–213. doi: 10.1093/bmb/ldh013. [DOI] [PubMed] [Google Scholar]
- 25.Buzzi M., Wandeler G., Anderegg N. Abstract PS1/2, 16th European AIDS Conference, Milan, Italy, 25–27 October. 2017. Dolutegravir-based simplified maintenance therapy in HIV-infected patients. A systematic review and meta-analysis.http://www.professionalabstracts.com/eacs2017/programme-eacs2017.pdf (accessed April 27, 2018) [Google Scholar]
- 26.Cahn P., Sierra Madero J., Arribas J.R. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet HIV. 2018 doi: 10.1016/S0140-6736(18)32462-0. [DOI] [PubMed] [Google Scholar]