Abstract
Leishmania aethiopica is the main causative species for cutaneous leishmaniasis (CL) in Ethiopia. Despite its considerable burden, L. aethiopica has been one of the most neglected Leishmania species. In this review, published evidence on L. aethiopica history, geography, vector, reservoir, epidemiology, parasitology, and immunology is discussed and knowledge gaps are outlined. L. aethiopica endemic regions are limited to the highland areas, although nationwide studies on CL prevalence are lacking. Phlebotomus pedifer and P. longipes are the sandfly vectors and hyraxes are considered to be the main reservoir, but the role of other sandfly species and other potential reservoirs requires further investigation. Where and how transmission occurs exactly are also still unknown. Most CL patients in Ethiopia are children and young adults. Lesions are most commonly on the face, in contrast to CL caused by other Leishmania species which may more frequently affect other body parts. CL lesions caused by L. aethiopica seem atypical and more severe in their presentation as compared to other Leishmania species. Mucocutaneous leishmaniasis and diffuse cutaneous leishmaniasis are relatively common, and healing of lesions caused by L. aethiopica seems to take longer than that of other species. A thorough documentation of the natural evolution of L. aethiopica as well as in depth studies into the immunological and parasitological characteristics that underpin the atypical and severe clinical presentation are needed. Better understanding of CL caused by this parasite species will contribute to interventions related to transmission, prevention, and treatment.
Keywords: Cutaneous leishmaniasis, Ethiopia, Leishmania aethiopica
1. Introduction
Cutaneous leishmaniasis (CL) is a skin disease caused by the bite of sandflies infected with the Leishmania parasite [1]. New World CL in South and Central America is caused by a broad range of species. Old World CL is found across a wide geographical area, ranging from sub-Saharan Africa and the Mediterranean to the Middle East and Central and South Asia, where Leishmania major and L. tropica are the main causative pathogens [1]. Worldwide, 70–75% of the CL burden is carried by ten countries: Afghanistan, Iran, Syria, Algeria, North Sudan, Colombia, Brazil, Costa Rica, Peru and Ethiopia [2].
Leishmania aethiopica is the main causative species for CL in Ethiopia, although sporadically, cases of L. tropica and L. major have been reported. It is almost solely confined to Ethiopia, with an additional pocket in the highlands of Kenya [3]. CL caused by L. aethiopica has a number of interesting features, including a unique vector, reservoir, and a pleiotropic clinical presentation. Its yearly burden is estimated at around 20–50,000 cases per year in Ethiopia [2]. Despite the substantial amount of cases, L. aethiopica has been one of the most neglected Leishmania species. Nevertheless, a range of studies have been conducted over the past 50 years, providing some insights into this intriguing parasite. At the same time, huge knowledge gaps remain.
2. History
The first reported CL cases in Ethiopia dating back to the early 20th century were Italian soldiers who suffered from oriental sore [4], [5]. In the 1960's, severe cases of CL initially misdiagnosed as lepromatous leprosy were described in the areas surrounding Dessie (North-East Ethiopia) and Dembidollo (South-West Ethiopia) [6], [7], [8]. Those were later classified as diffuse cutaneous leishmaniasis (DCL). Soon it became apparent that CL is an endemic disease in Ethiopia [8], exemplified by the fact that it has a vernacular name in different regions and languages [9], [10], [11], [12]. Field studies were undertaken in areas near known CL foci in the late 1960's [9], often combining community surveys with studies aimed at identifying the vector and reservoir [11], [13]. A separate specific status was awarded to Ethiopian CL in 1973 by Bray, who described the parasite as Leishmania aethiopica, Phlebotomus longipes and P. pedifer as its sandfly vectors, hyraxes as reservoir hosts, and a geographical spread limited to the highlands of Ethiopia and the slopes of Mount Elgon, Kenya [3].
The next decades publications were few, limited to case reports on specific clinical presentations of CL seen in the Addis Ababa Leprosy hospital [14], [15], including the first mention of cases of mucocutaneous leishmaniasis (MCL) in Ethiopia [14]. Later, research centers treating and reporting on large groups of CL patients were established both in Addis Ababa (All Africa Leprosy Rehabilitation and Training center (ALERT) and Addis Ababa University) and in Mekele in the North (Italian Dermatological center (IDC)). In 2005, an outbreak of skin lesions was reported in Silti, 150 km south of Addis Ababa. The skin disease was called ‘recent lesion’ in the local language, and since no CL cases had been reported here before, this suggests that CL is spreading and establishing new endemic sites [16]. Different aspects of the studies mentioned are described in more detail below.
3. Geography
Ethiopia is located in the horn of Africa and has a high central plateau that ranges from 1200 to 3000 m. This plateau covers most of the country, with lowland areas mainly in the border regions. The climate is highly influenced by this altitude, as it is significantly cooler than other regions close to the equator.
It was estimated that almost 30 million people are at risk for CL in Ethiopia, affecting especially those living in the highlands, while CL does not occur in the lowland areas [3], [17]. A large Ethiopian mapping study showed that areas with steep slopes (> 7·5 degrees), high altitudes (between 2645 and 3563 m) and heavy rainfall were predictive for the occurrence of CL [17]. Areas in Ethiopia at high or very high risk are located along the mountain ridges spanning from the north to the south west, and along the mountain ridge ranging from the south to the north east. The two mountain ranges are separated by the Ethiopian rift valley, which has a lower altitude and risk of CL.
Traditionally, CL in Ethiopia is reported to occur between 1600 and 2650 m elevation [3], which is also confirmed by more recent studies (Table 1). However, it seems CL is spreading to new areas as shown by the CL outbreak in Silti where CL was not reported previously [16]. Reasons may be related to spread of the reservoir or vector into new areas, possibly associated with ecological changes such as deforestation.
Table 1.
Overview of publications on clinical studies on cutaneous leishmaniasis due to L. aethiopica.
| Ref | Location | Study type | Population | N | Age (years) | Sex distribution | Type and location of lesion | Diagnostic method | Species subtyping | Duration of lesion reported by patient | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Community-based | |||||||||||
| [9] Bryceson 1966 | Dembidollo area (SW Ethiopia) 1700 m | Survey | Villages where CL cases have previously been seen. Total surveyed not reported | 14 active lesions 12 scars |
Active lesions: Mean 25·1, median 23 Scars: mean 15·9 median 14·5 Total: mean 18·5 median 17 |
Active lesions: Male 5 (38·5%) Female 8 (61·5%) Scars: Male 8 (61·5%) Female 6 (38·5%) |
2 MCL (14·3%), one ‘chiclero ulcer’, 23 ‘oriental sore’ (9 active, 13 scars, 1 active &scar) 96·4% of lesions on the head/neck, 92·3% single lesion |
Clinical, Leishmania antigen test done on some, 7/14 ‘active’ cases smear positive 2 random biopsies done |
No | Means: scar: 20·7 months Active: 12·9 months |
Numbers table and text inconsistent Authors proposed natural evolution |
| [11] Lemma 1969 | Dessie area (NE Ethiopia), Wolega Province (W Ethiopia) Dembidollo area (SW Ethiopia), Shewa Province, Meta Abo (C Ethiopia), Rift valley lake area (S Ethiopia) All > 1800 m |
Survey | 2 areas chosen with known CL cases, one by chance observation, selection of the last area is not described. Mostly schoolchildren, also peasants, farmers and villagers. > 2000 surveyed | 57 active CL, 58 scars, (Prevalence active +/− 2·9%, total +/− 5·8%) |
For active CL: all age groups affected, mostly children and young adults | Except for one area, no male predominance in active CL/scars | Almost all oriental sore, a ‘few’ with MCL, no new DCL 97·6% of lesions on the head, 75–93% single lesion |
Parasite positivity was tested by Giemsa stained smears or culture. 22/61 tested were positive | No | 22/61 < 6 months, 21/61 6–12 months, 18 > 12 months | LST done in whole population surveyed, positivity ranging from 5·5 to 52% (52% in family of DCL patient); Numbers do not add up. Authors proposed natural evolution for CL |
| [10] Wilkins 1971 | Meta Abo (C Ethiopia) 2250–2500 m | Survey | All villagers of Meta Abo valley (estimated 95% included), a region home to CL cases. 1635 surveyed | 9 active cases, 52 past cases (Prevalence active 0.6%, total 3·7%) |
Cases: 3–70, mean 22·6, median 35 Mean whole population 27 |
Male 3 (33·3%) Female 6 (66·7%) |
9 LCL (100%), [8] 88·9% lesion on the head, [8] 88·9% single lesion | Clinical, 7 parasitologically confirmed by Giemsa Stained smears, culture or biopsy | No | NA | |
| [13] Ashford 1973 | Kutaber (C Ethiopia)2500 m, Ochollo (S Ethiopia)2100 m |
Survey | Villages with infected sandflies and hyraxes. Kutaber 357/370 compounds surveyed In Ochollo around 1/5 of compounds were visited 895 surveyed, areas were chosen by geographical features (steep slopes and flatter areas) |
Kutaber 28 cases (16 locally acquired of which 13 active) prevalence active 0·9% Ochollo: 96 active, 272 scars (prevalence 10·7% & 41·1% resp.) |
Kutaber: 8/13 between 6 and 15, none < 6, one elderly male Ochollo: 82·7% of active lesions < 16 years. 41·2% < 6 years, also < 1 year encountered |
NA | NA | Clinical, cases were checked by laboratory confirmation ‘as much as possible’ | No | NA | The Aleku focus is already described in Lemma et al. [11] |
| [12] Mengistu 1992 | Ochollo (S Ethiopia) >2000 m |
Repeated cross-sectional survey with 3 consecutive visits over 3 years | Residents of Ochollo district (> 95% surveyed). CL cases have been reported here in previous studies. 3022 surveyed. | 120 active cases, 1037 scars, Prevalence active 4·0%, total 38·3% |
80·0% of active infections in 0–10 years, 3 children 6 months, one woman > 60 | ‘both sexes equally affected’ | 2 DCL (1·7%), 2 MCL (1·7%), 116 LCL (active cases). 85·1% of lesions are above the neck, 67·6% had one lesion | Clinical, skin smears and culture for some active CL cases | L. aethiopica found in vector and in 6 patients by isoenzyme essay | For 50% of patients' duration 9·6 +/− 5·7 months, in 10% > 3 years | 65 (54%) of 120 schoolchildren positive for LST |
| [18] Sang 1993 | Mt Elgon (3 sites) (W Kenya) >1700 m |
Survey | Villages where CL had previously been reported, compared to a general population survey in the same area, not described in detail. 1979 and 18,525 surveyed respectively | 43 active cases. Prevalence 0·01–1·9% | No conclusions on age due to small sample size | NA | 3 DCL, rest LCL. 84·7% of lesions were on the head | Clinical, 19 parasitologically confirmed by Giemsa stained smear or culture | Two tested L. aethiopica by Isoenzyme assay | NA | Numbers in table and text are inconsistent |
| [16] Negera 2008 | Silti (C Ethiopia) | Survey | Simple random sampling of villagers in towns with previous reports of skin lesions. 1907 surveyed | 92 active cases (prevalence 4·8%) | Cases: Mean 17·9 − + 1.5SE range 2–70. Significantly more CL cases in 11–20 age group | Male 44 (47·8%) Female 48 (52·2%) |
14/73 MCL (19·2%), others presumably LCL. 68·5% had a lesion on the head, 46·7% had one lesion | Clinical, 73/92 confirmed by skin smear/culture/histopathology | Yes, all L. aethiopica by PCR | 69/92 > 6 months | Multivariate Risk Factor analysis: presence of adhatoda shrubs and presence of hyraxes near house significantly associated with CL |
| [20] Lemma 2009 | Addis Ababa (C Ethiopia) three localities in/around gorges of Bulbula-Akaki river (close to international airport) >2326 m |
Survey | Survey population not described. Total surveyed not reported | 35 cases (9 active, 26 healed). | Age group 0–9 and 10–19 years were the most affected. 4 of the 12 > 30 years were employed as night guards | Active lesions & scars combined: Male 17 (48·6%) Female 18 (51·4%) |
3 DCL (33·3%), 6 LCL (66·7%). 97% of scars were in the face | Clinical suspicion with parasitological confirmation for all by Giemsa stained smear or culture | L. aethiopica found in 3 hyraxes by PCR, not done on human samples | NA | |
| [32] Negera 2012 | Silti (C Ethiopia) | Prospective cohort identified by active and passive case finding | Survey population not described. Total surveyed not reported | 92 active cases from survey (31 from HC). | For all (including HC) Mean age 18·6 |
For all (including HC): Male 67 (54·5%) Female 56 (45·5%) |
Subtypes not described, 78·9% had a lesion on the head, 51·2% had one lesion | Clinical, 48/85 were culture positive, 44/54 positive for histopathology, 59/71 positive for PCR | All infections identified by PCR due to L. aethiopica | Of 54 described, 26% < 3 months, 48% 3–12 months, 26% > 12 months | Data from passive and active case finding is combined |
| [52] Buggsa 2014 | Ochollo (S Ethiopia) > 2000 m | Survey | Schoolchildren (all in 1 school) age 6–25 (523/600 participated) where previous CL studies had been done. 523 surveyed | 21 active lesions, 313 scars, and 8 active & scar Prevalence active 5·5%, total 65·4 |
For all: more prominent in 11–15 (49·7%), and 6–10 (41·8%), for active: unclear distribution | Active lesions & scars combined: Male 165 (48·2%) Female 177 (51·8%) |
1 MCL, 4 ‘recidivans’ (definition unclear), 4 MCL + LCL, rest (168) LCL 82·2% of scars and 84·9% of lesions on the face, 64·1% of scars and 41·4% of lesions singular |
Clinical, 4 active cases culture positive, 1 DAT positive, 1 smear positive | NA | NA | Numbers inconsistent for MCL and total prevalence |
| [21] Bsrat 2015 | Saesie Tsaeda-emba (N Ethiopia) > 2350 m | Survey | 6 randomly (multistage random sampling) selected peasant associations and a house to house survey 2011–2012. 2106 surveyed | 331 CL (141 active, 154 scar) Prevalence active 6·7%, total 14·0% |
Highest prevalence active lesion in age group 10–19 (12·8%, 64), followed by 0–9 (9·8%, 51). Central measure NA | Active lesions: Male 74 (52·5%) Female 67 (47·5%) Scars: Male 74 (48·1%) Female 80 (51·9%) |
Subtypes not described, 83·2% are at the head/neck, and 78·7% had one active lesion | Clinical, 10 culture confirmed, 30 smear confirmed | Yes, unmentioned number of PCR subtyping all L. aethiopica | NA | Univariate Risk factor analysis: age, study peasant organization, presence of cliff/gorge, walls with cracks and/or holes, presence of hyrax, animal burrow, animal dung and farm land near the residents' houses |
| Health center or hospital-based | |||||||||||
| [7] Poirier 1964 | Princess Zenebework Hospital, Addis Ababa (C Ethiopia) | Case series with passive case finding | Patients hospitalized in leprosy hospital in Addis Ababa, where later the diagnosis CL was made | 8 | Mean 21, Median 25, range 10–30 | Male 4 (50%) Female 4 (50%) |
3 lepromatous leprosy, 1 tuberculoid leprosy, 1 intermediate leprosy, 1 erythematous lupus, 1 tuberculoid lupus, 1 oriental sore | All parasitologically confirmed (microscopy or culture) | No | Mean(years) 10.4, median 6, range 2–20 years | |
| [8] Price 1965 | Princess Zenebework Hospital, Addis Ababa (C Ethiopia) | Case series with passive case finding | Patients falsely diagnosed as lepromatous leprosy | 22 | Range 4–52 (mainly 4–12) | ‘no sex predominance’ | 8 “lepromatoid type”, 2 “tuberculoid type”, 12 “intermediate type”. 10/21 (47·6%) lesions on head. | Histological, classification of CL in different categories over a spectrum, as done for leprosy | No | NA | Two of the 22 cases have been described in another paper (which is unclear) |
| [61] Bryceson 1969 | Princess Zenebework Hospital, Addis Ababa (C Ethiopia) | Case series with passive case finding | DCL patients admitted at Addis Ababa leprosarium | 33 | Range 8–40. Mean age at presentation is 20, all disease started before age 28 | Male 21 (63·6%) Female 12 (36·4%) |
33 DCL, 4 having mucosal involvement. Primary lesion: 14 on face (42·4%), 10 (42·4%) legs, 6 arms. 100% finally had lesions on the face |
Parasitological. In all leishmaniasis was confirmed by skin smears stained with Leishman's stain. NNN culture was done for a few cases | No | Range 1–20 years | The article itself has mapped the cases but not reported the location. Please see the article itself for the distribution of cases 16 of the 33 cases have been described elsewhere |
| [53] Lindtjorn 1981 | Sidamo Regional Hospital, Yirga Alem (SE Ethiopia) | Case series with passive case finding | Patients presenting with CL symptoms and parasitologically proven | 25 | 14 (56%) were < 20, 7 (28%) < 10, one (4%) 51-year-old | Male 14 (56%) Female 11 (44%) |
24 LCL (96%), one DCL (4%). 21/25 (84%) lesions on the face | Parasitological: 19 biopsied, 6 smear | No | Mean 7.1 months, range 1 month–3 years | |
| [33] Sarojini 1984 | ALERT, Addis Ababa (C Ethiopia) | Case series with passive case finding | All patients presenting to Alert | 104 | Range 4–70, 6·7% in 0–9 30·8% in 10–19 28·8% in 20–29 14·4% in 30–39 12·5% in 40–49 2·9% in 50–59 3·9% in 60 + |
Male 64 (61·5%) Female 40 (38·5%) |
98 LCL (94·2%), 6 DCL (5·8%). 120/124 (96·8%) of all lesions were on the head/neck. 73 (74·5%) had single lesions | Parasitological: By histology of smears or isolation of promastigotes from tissue obtained at biopsy or smear taking. Numbers not reported | All cultured samples had isoenzyme analysis done. All 20 were L. aethiopica | Range 1 month − 10 years. 83·7% had < 1 year, for DCL mean > 5 years | |
| [57] Padovese 2009 | IDC Mekele (N Ethiopia) | Case series with passive case finding | Patients presenting at IDC | 167 | High prevalence among adolescents. By category: 24·0% in 5–14 67·7% in 15–44 1·2% < 5 7·2% > 45 |
Male 126 (75·4%) Female 41 (24·6%) |
123 LCL (73·7%), 11 DCL (6·6%), 2 RCL (1·2%), 29 MCL (17·4%), 1 ML (only lips) (0·6%). 5 (5·6%) HIV +, 1 (0·6%) PKDL. 115 (68·9%) had a lesion on the head | Parasitological. Fine needle aspirates for skin smear microscopy and biopsy for histopathology | No | Range 12 weeks to 2 years | |
| [58] Morrone 2011 | IDC, Mekele, cases from all over Tigray (N Ethiopia) > 2000 m | Case series with passive case finding | Patients with clinical diagnosis of CL presenting to IDC during the study period | 471 | Mean 23·7. By category: 59·9% in 15–44 28% in < 14 |
Male 335 (71·1%) Female 136 (28·9%) |
405 LCL (86·0%), 52 MCL (11·0%), 11 DCL (2·3%), 3 PKDL (0·6%) 15 HIV positive (3·2%). Location and number of lesions not reported | All confirmed by either skin smear microscopy, FNAC or histopathology (skin smear microscopy and FNAC done on all, histopathology on FNAC negative) | No | NA | Recruitment from 2005 to 2008 which overlaps with Padovese et al. [57] who recruited from 2005 to 2007 |
| [65] Bekele 2014 | ALERT, 96 from Addis, Oromia-71(30·3) Amhara- 35 (15%) SNNP- 26 (11·1%) Tigray − 6 (2·6%) |
Case series with passive case finding | CL cases diagnosed at ALERT | 234 (14·2%) diagnosed from 1651 suspected | Mean 25, range 1–78. Age group 11–20 and 21–30 were most affected |
Male 133 (56·8%) Female 101 (43·2%) |
21 (9·0%) DCL, 24 (10·3%) MCL, 8 (3·4%) LCL, mostly not recorded (191). >48·3% of lesions on the face (26·1% on arms and legs). # of single lesions NA |
All Giemsa and/or histopathology confirmed (no numbers reported) | No | 15·0% < 3 months 18·4% 4–6 months 7·7% 7–9 months 16·7% 10–12 months 42·3% > 12 months |
The locations described are not detailed enough to map, so for this article only the cases in Addis are displayed on the map |
| [56] Tilahun 2014 | Ayder Referral Hospital, Mekele, (N Ethiopia) | Case series with passive case finding | CL cases diagnosed at Ayder Referral Hospital | 35 diagnosed from 486 patents visiting the dermatology OPD | By category 6 (17·1%) 1–15 24 (68·6%) 16–45 5 (14·3%) > 46 |
Male 26 (74·3%) Female 9 (25·7%) | 18 LCL(51·4%), 9 (25·7%) MCL, 8 (22·9%) DCL. 16(45·7%) had facial, 16 (45·7) had mucosal lesions, and 3 (8·6%) had lesions on extremities. |
11 (31·4%) were confirmed with skin slit microscopy, while 24 (68·6%) were negative for skin slit microsocpy | No | NA | The numbers and percentages indicated for overall prevalence and prevalence per type of disease are not consistent, therefore there is some doubt regarding the numbers reported here. |
| [59] Fikre 2017 | Leishmania Research and Treatment Center, Gondar (NW Ethiopia) | Case series with passive case finding | Cases of confirmed CL (one with strong clinical suspicion but negative smear) presenting to the LRTC | 154 | Median 23, IQR 16–38. By category: 6·5% < 10 20·1% 11–17 54·5% 18–44 18·8% > 45 |
Male 110 (71·4) Female 44 (28·6%) |
80 LCL (51·9%), 67 MCL (43·5%), 7 DCL (4·6%), 4 concomitant leprosy; 5 (3·2%) HIV +, 80·5% of lesions on head/neck, 61% had 1 lesion | Parasitological (99·3%), 0·7% (1 case) clinical diagnosis with negative aspirate | No | Median: 12 months (IQR 6–24), for MCL 12 (6–24), for DCL 13 (12–84) | |
| [106] Seife, 2018 | Boru Meda Hospital, dessie (NE Ethiopia) | Case series with passive case finding | Leishmaniasis patients presenting at Boru Meda dermatology department | 97 | By category < 15: 33 (35%) 16–45: 45 (45.4%) >45: 19 (19.6%) |
Male 62 (63.9%) Female 35 (36.1%) | 52 (53.6%) LCL, 28 (28.9%) MCL, 17 (17.5%) DCL. | 91 (93.8%) parasitologically confirmed 82 (84.5%) with skin slit smear, 9 (9.3%) with negative skin slit smear but positive FNAC, 6 (6.2%) negative for skin slit and FNAC (clinical diagnosis). | No | NA | Numbers for age are inconsistent, whether the numbers reported here are correct is not certain. |
The only area besides Ethiopia where L. aethiopica has been described in detail is the eastern slopes of Mount Elgon in Kenya [18]. This is a volcano on the border of Kenya and Uganda, and rises from the surrounding plateau which is at an altitude of 1700–2000 m. The area is characterized by a temperate climate with numerous caves and is dominated by steep escarpment cliffs on the southern slopes.
There is one report of a Sudanese patient with clinical visceral leishmaniasis (VL), who was found to have a lymph node aspirate positive for both L. donovani and L. aethiopica, even though clinically he did not appear to have symptoms of CL [19]. In-depth clinical information including a travel history is not reported. In addition, the article lacks a documented validation of the species identification method, and since both the clinical presentation, diagnostic method used, and the geographical location are remarkable, this report should be interpreted with caution.
To our knowledge, no CL has been reported on the Ugandan side of Mount Elgon, and CL due to L. aethiopica has not been reported in other areas of Kenya. Perhaps only steep cliffs in high altitude areas – like on the Kenyan side of mount Elgon- are conducive to L. aethiopica transmission. Nevertheless, CL could also remain underdiagnosed and the distribution of L aethiopica could be wider than currently thought, occurring in other highland areas across the rift valley region.
4. Vector and Reservoir
The first extensive sandfly studies were carried out in Ethiopia in the late 1960's, and identified P. longipes [11] and P. pedifer [13] as vectors for L. aethiopica. In Ethiopia, P. pedifer and P. longipes have only been found in high altitude areas [13], [20], [21], [22], [23], [24], granted that systematic Phlebotomine searches are lacking. A recent study collected sandflies from seven different locations in north-west Ethiopia. Presence of P. longipes by light trap collection was significantly and positively associated with altitude, and was found only in the two sites above 2000 m altitude, while P. pedifer was not found at all [25].
Although P. longipes and P. pedifer are not known to transmit other Leishmania species, they were found in a wide variety of places in Ethiopia including indoors [13], [20], [21], [22]. They seem to have a preference for places that are relatively cool, with high humidity, shady/dark, without smoke, and protected from wind [22]. Caves and cliffs are common places where sandflies were found [13], [23], [26]. One Ethiopian study noted that CL cases were found closer to gorges, which were often visited by children and young adults for recreation and fetching water during the daytime, as well as churchgoers spending many hours engaged in ceremonies [20]. Having a gorge close by [16], [21], the presence of cracks or holes in the wall [21], having an animal burrow, farm or dung near the home [21], and the presence of Adhatoda schimperiana shrubs [16] were significantly associated with CL. In Kenya, both P. longipes and P. pedifer have been found mainly in caves [23], [24], although infection with Leishmania was not demonstrated.
P. longipes is active throughout the night, although it was also found to bite in the day during warm, overcast weather [13]. P. longipes was capable of flying up to 240 m per night in search of a bloodmeal [22], potentially putting any person residing close to a resting site at risk of getting bitten. However, more detailed and systematic studies generating information on vector behavior including resting sites and feeding habits are needed.
A large study in the Awash valley in southern Ethiopia showed that other sandfly species could also carry Leishmania promastigotes associated with CL, with one L. aethiopica infection identified in P. sergenti [27] and L. tropica infections found in P. sergenti and P. saevus [27]. Several years later, the first Ethiopian CL case due to L. tropica was reported in the Awash valley [28]. Interestingly, this region is at an altitude of around 1000 m, which is much lower than where most CL cases as well as P. pedifer and P. longipes have been found. P. sergenti, which is well known for spreading L. tropica in many countries including Spain, has been reported from several regions in Ethiopia, ranging from 1230 to 1590 m altitude [29]. The role of P. sergenti in the spread of CL to lower altitude areas both by L. tropica and L. aethiopica needs more evaluation.
Transmission of CL due to L. aethiopica is considered to be zoonotic. Both the Procavia capensis and Heterohyrax brucei (rock hyraxes) - which mostly reside in rocky cliffs -, have been shown to carry Leishmania parasites in Ethiopia [13], [20] of which Lemma et al. were also able to show infection was due to L. aethiopica. An early epidemiological study found infected hyraxes in three locations across Ethiopia, of which up to 27% (6/22) was asymptomatically infected with Leishmania [13]. Presence of hyraxes close to the house was significantly associated with CL [16], [21]. In Kenya, Leishmania parasites have been isolated from cutaneous lesions in hyraxes [24]. Other animals may also serve as reservoir, like a giant rat in Kenya [24], a Kenyan goat [30], and an Ethiopian squirrel [31] were reported to carry L. aethiopica, although the latter was found in a region where no CL cases occur, and these reports were not substantiated in other studies. Since cows are commonly bitten by the identified Leishmania vectors [13], [26], they may also play a – yet uninvestigated - role as a reservoir.
When P. longipes was allowed to feed on lesions of CL patients in central Ethiopia (Kutaber), six out of 39 sandflies tested were carrying promastigotes seven to ten days later [13]. While humans are often considered as accidental hosts in a cycle of transmission between hyrax as the reservoir and P. longipes and P. pedifer as vectors, this suggests that transmission could also be anthroponotic. A 52% leishmanin skin test (LST) positivity rate in family members of a DCL patient also suggests there may be anthroponotic transmission [11], although similar geographical and behavioral risk factors may also explain the high LST positivity in these people.
Although the dogma is that humans are the accidental host in a cycle of transmission between hyraxes as the reservoir and P. longipes and P. pedifer as vectors, more research into potential secondary reservoirs, the role of other sandfly species, and a possible anthroponotic transmission is needed.
5. Parasitology
Epidemiological and clinical studies show that CL in Ethiopia is mainly caused by Leishmania aethiopica [12], [16], [18], [21], [32], [33], although routine species identification is rarely done. Nevertheless, cutaneous disease may also be inflicted by L. donovani, L. tropica, and L. major, which are also found in the country. A close genetic relationship between L. aethiopica and African strains of L. tropica exists [34].
Even though several tools have been developed to detect populations and intra-species differences, so far, a correlation between the vast array of clinical phenotypes and the different parasite populations could not be demonstrated. Methods that have been developed include multilocus enzyme electrophoresis [35], [36], restriction fragment length polymorphism (RFLP) analysis [37], microsatellites [38], and amplified fragment length polymorphism analysis (AFLP) [39]. The Leishmania RNA virus (LRV), which has been shown to be linked to disease severity [40] has been documented in L. aethiopica isolates collected between 1985 and 2011 as well, but a correlation with the clinical presentations could not be established [41]. Since intra-species identification has not been widely and systematically applied, the effect of the L. aethiopica genotype on disease remains unclear.
L. donovani, which causes VL and post kala-azar dermal leishmaniasis in Ethiopia, has also been isolated from cutaneous lesions in patients with HIV and concomitant VL [42], but not from immunocompetent patients. However, in Sri Lanka L. donovani is known to primarily cause CL [43]. Taken together it is relevant to systematically identify the species, especially in Ethiopian CL patients that live in VL endemic areas.
A hybrid between L. donovani and L. aethiopica has been found in a genome-wide screening using AFLP [39]. MHOM/ET/94/ABAUYE was isolated from a cutaneous lesion in Arba Minch (south-west Ethiopia), and was originally described as a variant of L. aethiopica [37], but AFLP revealed that about 30% of its genome is composed of L. donovani DNA. One of the methods used most in Ethiopia for species discrimination, namely RFLP analysis of the ribosomal ITS1 target [44], can discriminate the hybrid from actual L. aethiopica, given that results are analyzed with caution. Indeed, the RFLP pattern of both species is detectable, but the L. donovani fragment is less intense, and fragment sizes are similar [45]. Together with the fact that species identification is not part of clinical practice, the importance of such hybrids is currently unknown.
Two other dermotropic Leishmania species, L. tropica and L. major, have also been observed in Ethiopia. In the Awash region in the East, L. tropica was found in rodents [46], bats [47], and sand flies [27]. More importantly, L. tropica was found in a CL patient [28] in north-east Ethiopia. L. major was encountered in bats in the south-west and middle of the country [47], and in sand flies in the south [48]. To investigate their role in the epidemiology of CL, systematic species identification in patients is relevant.
Several methods are available to discriminate the four Leishmania species endemic in Ethiopia [45]. By far the most widely used in studies in the country is based upon the ITS1 ribosomal DNA target [16], [46], [47], [49], which is best described by Schonian et al. [44] Earlier studies relied on multilocus enzyme electrophoresis [12], [18], [33], [49]. Even though ITS1 can discriminate the species complexes from the Old world, when relying on RFLP, results should be interpreted with caution (as already pointed out above for the hybrid MHOM/ET/94/ABAUYE). Sequencing of the ITS1 locus would be an alternative, even though it can be technically challenging, making it less suited for routine practice. Also, PCR primers specific for L. aethiopica have been developed [50], [51], which could be implemented as a first line diagnostic tool to differentiate lesions caused by L. aethiopica from those caused by other species, but some of these were not validated properly for their use in Ethiopia.
In short, a more systematic screening of parasite identity in patients displaying the various forms of CL is needed to assess the impact of parasite identity on CL in Ethiopia. The species is only one factor, but equally important are intra-species differences such as the presence of LRV. Ideally, whole-genome sequencing on a large well-documented cohort from a nation-wide cross-sectional study would provide more insight into the role of the infecting parasite.
6. Epidemiology
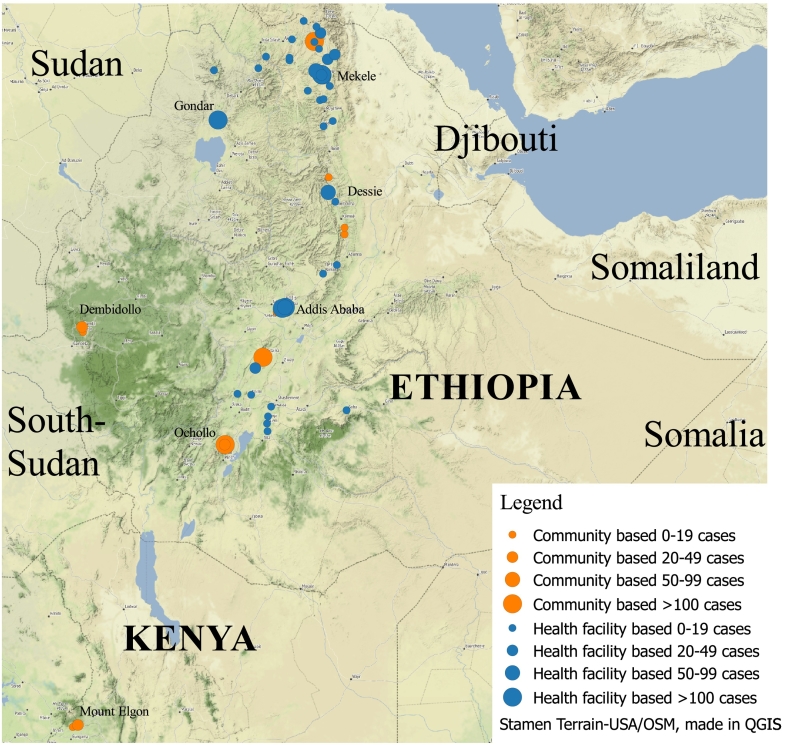
Table 1 provides an overview of twenty key papers providing relevant information on the epidemiology of CL. Fig. 1 gives an overview of clinical studies that have been published describing at least five cases of cutaneous leishmaniasis in Ethiopia or caused by L. aethiopica.
Fig. 1.
Map of published clinical cutaneous Leishmaniasis studies due to L. aethiopica. Orange indicates community-based studies, while blue dots indicate hospital-based studies. The size of the dot indicates the number of cases concerned. Studies reporting less than five patients are not reported here. For community-based studies the dots are on the (estimated) location of the site studied, while for hospital-based studies the origin of the case was reported where possible. For patients from hospital-based studies where the origin of patients was not described, the location of the hospital is indicated. An interactive version of the map can be accessed on: http://e.itg.be/saskia/. By clicking on the dots representing the studies, the first author, reference number, year of publication, name of location, type of study, case load, and name of the health facility (if applicable) can be viewed. Studies are described in detail in Table 1.
Prevalence of active CL ranges from 0·01 to 10·8% [13], [18], while prevalence including past CL (suspected scar lesions) ranged from 14·0 [21] to 65·4% [52]. The population investigated for the community studies was not random. They were often villagers in towns selected because of previous CL reports [9], [11], [16], [18], or towns where infected sandflies or hyraxes had been found [13]. The highest number of scars were reported in school children in Ochollo, which already had the highest active prevalence 40 years prior [13]. One study on the slopes of Mount Elgon in Kenya compared prevalence in villages with previous CL cases (0·55% and 1·86%) to a general survey in the area (0·01%) [18]. Unsurprisingly, prevalence of active lesions was much higher in villages known to be affected, although still relatively low when compared to Ethiopian sites. Studying prevalence of CL in sites with known cases could cause overreporting, but the one study with random selection of sampling towns (even though in a region known to be endemic for CL) still had a substantial prevalence of 6·7% [21].
The LST relies on eliciting delayed type hypersensitivity in those sensitized to Leishmania, by inoculation of Leishmania extracts into the skin. It can be used to assess endemicity. A few studies have used LST in Ethiopia. Positivity for LST ranged from 5·5% in school children in an area where no CL cases were found [11] (although cases were reported from here at later timepoints) [53] to 54% [11], [12], [54], [55] in school children in Ochollo which was already known to have a high number of CL cases. Thus, prevalence of CL in Ethiopia seems high in areas that have been studied, with suggested presence of hyperendemic pockets. However, nation-wide surveys are needed to provide a better understanding of the degree of endemicity of CL in Ethiopia.
The number of males and females affected in the community-based studies were equal, while the studies based in health centers showed a clear overrepresentation of males [56], [57], [58], [59], most likely explained by different health seeking behaviors for both sexes.
All age-groups are affected by CL, but prevalence seems to be highest in children and young adults [8], [9], [12], [13], [16], [20], [21], [53]. In Silti, where CL seems to have been introduced recently, prevalence was highest in the age group 11–20 and < 11 years with 7·7 and 4·1%, respectively, with the age group 11–20 years significantly overrepresented [16]. Interestingly, all three studies from the highly endemic area of Ochollo showed that most of the patients are younger than 16 years [12], [13], [52]. In fact, 90·6% of participants said their CL lesions had appeared before the age of five [52]. It is generally accepted that resolution of primary infection leads to long lasting immunity [60], which would explain why in highly endemic areas the age of those affected is lower than in less endemic regions since most adults will be immune. However, eight out of the 29 patients with active CL lesions had both a healed scar and an active lesion [52], and relapse of CL was not uncommon [59], indicating that resolution of a lesion is not fully protective against appearance of future lesions.
7. Clinical Features
There are three main forms of CL described in the literature on Ethiopia: Localized cutaneous leishmaniasis (LCL), MCL, and DCL. LCL is most commonly seen and usually starts with a singular lesion, which may ulcerate several months after appearance. However, many Ethiopian CL patients do not have a classic ulcer, but rather have crusty lesions with a patchy distribution, local edema, and color changes (Fig. 2). Mucosal involvement in Ethiopian CL patients is common, which generally causes lesions to be classified as MCL, in contrast to South American MCL which is characterized by hematogenous spread years after a primary CL lesion. Clinical observations show that in Ethiopia, mucosal lesions may present simultaneously with lesions on the skin [59], primarily on the skin with spread to the mucosa afterwards [57], or directly on the mucosa after which it may expand to the skin [59].
Fig. 2.
Common presentations of CL due to L. aethiopica are crusty lesions with a patchy distribution, local oedema and color changes with frequent mucosal involvement.
A subset of CL patients exhibits continued lesion growth which may last for decades and mostly manifests itself as DCL [6], [61]. DCL is a chronic and progressive condition affecting large areas of the skin, with multiple popular, nodular, or plaque lesions that often lack ulceration. It may resemble lepromatous leprosy [6], [7], [8].
Other clinical forms have also been described in Ethiopia. Large hypopigmented lesions that resemble borderline-tuberculoid leprosy [62], ‘chiclero ulcer’ [9], lymphedema and/or elephantiasis [8], [61], and leishmaniasis recidivans [52], [57], [63], which shows a chronic and relapsing course that may persist for years and can occur both in previously healthy individuals [63] and HIV-patients [64], have been reported in Ethiopia.
The percentage of DCL and MCL cases differs greatly per study site, as in some sites 43·5% of all cases were MCL [59], while other sites report percentages around 10% [57], [58], [65]. For DCL a percentage of 1·7 [12] to 33·3 [20] was reported. Possibly, due to the more mutilating clinical presentations, DCL and MCL are overrepresented at the clinical sites. Nevertheless, a community-based study investigating a new CL outbreak also showed a high MCL burden of 19·2% of all CL cases [16], and the highest percentage of DCL was actually reported from a community-based study [20].
CL lesions are commonly seen on body parts exposed to the vector. In Ethiopia it was observed that preferred sandfly biting sites were the face and the head as opposed to the arms and hands [11], which could explain why the majority of CL lesions is found in facial areas. Percentages of patients with primary facial lesions range from around 45 [8], [61] for studies describing DCL patients to up to 97·6 [11] in the overall CL population. Other body parts commonly affected are the arms and legs [61], [65].
Most study sites show that CL patients commonly have only one lesion [12], [21], [32], [33], [59], [61]. However, in Ochollo, which has repeatedly been studied as a hyperendemic CL focus, the average number of scars and active lesions were 1·5 and 1·7 respectively, with up to eight lesions (both active and scar) present at a time [52].
Data on natural evolution is limited, especially in recent studies. The few available publications indicate that LCL commonly starts healing after one year [9], [11], [12], [32], [33], [57]. Nevertheless, there is great diversity in duration of disease, as a subset of patients has a continued growth which may last for decades and mostly manifests itself as DCL [6], [7], [33], [61].
8. Immunology
The Leishmania parasite primarily survives and replicates as non-flagellated amastigotes in skin macrophages and dendritic cells (DC) [66]. Unlike other intracellular parasites (e.g. toxoplasma or Trypanosoma cruzi), Leishmania does not escape from the entry vacuole but survives and replicates within the digestive environment of the phagolysome. Neutrophils can also engulf the parasites, but appear to have an ambiguous role towards parasite control across different Leishmania spp. [60], and their role remains unknown for L. aethiopica. To counteract the evasion of innate immune mechanisms, the adaptive immune system is triggered to induce a strong Leishmania-specific response. In humans, control of Leishmania is therefore dependent upon generating CD4+ T helper (Th)-1 cells that produce interferon-γ (IFNγ), leading to macrophage activation and eventual killing of intracellular parasites through increased production of reactive oxygen species and to a lesser extent nitric oxide within the phagolysome.
CL caused by L. aethiopica is characterized by a wide clinical spectrum of disease influenced by both parasite diversity and host immune status. Different modes of macrophage killing discriminate the diverse clinical presentations, even though they share a single mechanism for resistance [60]. In general, MCL seems to be characterized by a low parasite load in an unmitigated inflammatory environment with strong delayed type hypersensitivity (DTH), cytotoxic, and Th1 responses. In contrast, DCL is defined by a high parasite load in the absence of a DTH and T-cell response, with high antibody and interleukin (IL)-10 levels.
The influence of parasite characteristics on clinical presentation was shown by limited studies from the 90s, that showed antigens derived from DCL isolates caused Peripheral Blood Mononuclear Cells from infected individuals to produce less IL-2 and IFNγ, and reduced proliferative capacity as compared to antigens derived from LCL isolates [67]. It was suggested that promastigotes from DCL patients preferentially stimulate immune suppressive activities resulting in anergy [67], which could relate to the observed unresponsiveness to standard drug treatment in DCL patients. In line with this hypothesis, high levels of the inhibitory molecule programmed death ligand 1 (PD-L1) in stimulated monocytes (65% vs 35% in negative controls) and severe reduced levels of IFNγ and granzyme B-producing cells were observed in an American DCL patient infected with L. amazoniensis [68]. In addition, high levels of functionally exhausted CD8+ T-cells were shown in four L. mexicana infected DCL patients, as compared to LCL patients [69].
Natural killer (NK) cells are able to provide initial protection against Leishmania parasites by production of initial IFNγ in draining lymph nodes, until a strong Th1 cell-mediated immunity is established. This was shown for L. aethiopica specifically by a set of studies in the 90s, in which non-exposed healthy Swedish individuals were able to respond to Leishmania antigen mainly by NK cell activity, compared to a dominant CD4+ T-cell response in patients with L. aethiopica infection [70], [71]. Increased NK proliferation upon re-stimulation was also seen in cured patients and negative endemic controls from Ethiopia, which was associated with enhanced CD4+ T-cell proliferation in some patients [72]. In animal models, direct contact points between Leishmania promastigotes and naïve human NK cells were found which were associated with cytotoxicity. They caused immediate destruction of the NK cells in a non-apoptotic way after initial exocytosis, leading to impaired NK cell activity during active CL [73].
CD8+ T-cells have not been characterized in depth for CL caused by L. aethiopica, although they have been reported to promote resistance and influence disease severity across Leishmania spp. [60] Both effects may be explained by production of primarily IFNγ or rather cytolytic activity, as increased cytolytic activity promotes a pathologic inflammatory response [74]. The protective role of Th17 cells in VL development [75] is also unexplored territory regarding L. aethiopica, although human studies show a detrimental role in L. braziliensis infection [76], [77]. In contrast to mice studies, the degree to which CD4+ Th2 or regulatory T-cells mediate susceptibility in human leishmaniasis is unclear.
Despite clear immunopathological differences between clinical presentations after L. aethiopica infection, current treatment options only target the parasite. Research efforts should focus on immunotherapeutic approaches that could reduce the severity of pathology. Likewise, the impact of HIV infection on CL should be studied as HIV is a considerable problem in Ethiopia and could significantly impact immunopathology and consequently skew clinical presentations [78].
A pool of short-lived effector CD4+ T-cells, capable of rapid response to a challenge, is maintained after control of primary infection, which is believed to protect against reinfection [60]. This argues for a non-sterile cure and the lack of knowledge on the generation of long-lived memory CD4+ T-cells is hampering the development of a successful vaccine and requires further research on the severe forms of CL caused by L. aethiopica [79].
9. Diagnosis
Different methods for diagnosing CL have been employed in Ethiopia. Clinical diagnosis was mainly used in community-based and older studies (see Table 1). Culture on different types of skin samples and Giemsa-stained microscopy on skin slit smears have commonly been used for decades (Table 1). Histopathology was used a lot historically, but as it requires relatively large and invasive biopsies it is now less commonly used while superficial sampling using skin slit scraping combined with microscopy has become the mainstay of diagnosis of CL. PCR has been introduced in Ethiopia in the last 10 years, first mainly for species identification [6], [79], but later also for diagnosis [32]. New promising tests such as the loop-mediated isothermal amplification (LAMP) assay and the CL detect Rapid Test [80], [81] have not yet been evaluated in Ethiopia.
Formal comparisons of the diagnostic tests used have not been made in Ethiopia. Although several studies use multiple tests to diagnose CL, most use different patient subgroups for different respective tests, and diagnostic tests are often performed on different kinds of samples, which may also impact the parasite yield, and thus the performance of the test. Only two studies compared different tests on the same group of patients [7], [16], but even here, it is not clear whether the two tests were done on the same sample type, and only 8 and 71 patients were tested respectively. Although care should be taken when interpreting the studies available, some preliminary conclusions can be drawn. Skin slit smears tested with microscopy seem to be more sensitive than culture [7], [21] (although one study shows opposite results [82]), while histology and PCR also detect a higher number of CL cases among CL suspected individuals [16], [32]. However, large scale studies evaluating the diagnostic techniques currently employed as well as new promising tests are direly needed. Ultimately, knowledge on the performance of the different diagnostic tests could result in development of a diagnostic algorithm, facilitating diagnosis at the different health care levels.
High copy number genes such as the ribosomal DNA array [44], [83], [84], kinetoplast minicircles [85], and the mini-exon [86] have been popular targets to demonstrate Leishmania parasites in cutaneous lesions using conventional or real-time PCR. However, application of such methods remains problematic in primary settings and are at best restricted to tertiary reference centers in Ethiopia. As point-of-care routine test, DNA extraction and PCR are too expensive, too complicated, and their turnaround time is too high. Isothermal amplification methods such as nucleic acid sequence based amplification [87] or LAMP [88], [89] alone do not solve these problems, as a simplified extraction method is also needed. Ideally, all-in-one systems combining DNA extraction and amplification should also become available for CL, similar to GeneXpert which is increasingly being used in point-of-care settings for diagnosis of tuberculosis [90].
10. Treatment
A wide range of treatments has been tried against CL in Ethiopia, reviewed in [91]. Most studies on treatment of L. aethiopica are outdated, involve only few patients, and are marked by poor methodology such as the lack of a control group. Since there have been no large clinical trials, the evidence-base remains very thin. For MCL, DCL, and more complicated forms of LCL, systemic pentavalent antimonials (most commonly Sodium Stibogluconate (SSG) and meglumine antimoniate) are widely used.
Evidence from observational studies using systemic SSG indicates an efficacy of 19·2 [59]–89·5% [32] across different patient groups. However, the first study was done retrospectively, and had very high loss to follow-up, while the second documented only 20 patients. Another prospective observational study reported an efficacy of 69% for systemic and intralesional antimonials treatments combined (efficacy for systemic treatment not reported separately) [57]. Additionally, there are a few case series; three Kenyan LCL patients [92] and one of two HIV/CL [64] patients were successfully treated with systemic SSG, while two patients with leishmaniasis recidivans (typically lesion recurrence within the edge of the scar after the initial lesion has healed) were cured with meglumine antimoniate [63]. A potential alternative systemic treatment is oral miltefosine, which is relatively safe and has successfully been used for other Leishmania species [93], [94]. Although in vitro studies indicate L. aethiopica is highly susceptible to miltefosine [95], clinical evidence is not yet available.
Three small clinical trials have been done so far. The only placebo-controlled trial was done on LCL patients using a Chinese medical ointment called Shiunko [96], which was not better than placebo, with cure rates of 20% (4/20) and 25% (5/20) respectively. Two small clinical trials were done over 20 years ago, using rifampicine with amithiozone versus pentamidine (LCL only) [97] and itraconazole versus placebo (10 LCL and 4 DCL) [98], of which only all six patients treated with pentamidine and two treated with placebo clinically improved. Historically, pentamidine has been used more frequently, as it was shown to be effective against complicated CL cases in several case reports [8], [14], [15], [33], but currently its use is rather limited due to safety concerns [33]. However, as pointed out by Naafs, pentamidine toxicity may only be linked to the pentamidine mesylate, while toxicity of pentamidine isethionate may be limited [99]. Additionally, differences in dosing of pentamidine might also explain differences in reported adverse events across studies [100].
There are several other treatments which have been used on a small scale. Paromomycin is recommended as one of the options in the Ethiopian national guidelines, but the only evidence available is from a small case series on DCL patients [101] in which two patients were successfully treated with paromomycin. However, both relapsed and were subsequently successfully treated with an extended course of paromomycin with systemic SSG. Small studies were done using metronidazole [102], ketoconazole [103], and Camolar [104], but they were not promising. AmBisome has also successfully been used for the treatment of one Ethiopian MCL patient, one Eritrean immunosuppressed man with LCL who now resided in Germany [105], and in combination with miltefosine for another patient resistant to several cycles of systemic SSG [59]. Several other combination treatments have also been tried. A small poorly documented descriptive study [106] from Eastern Ethiopia reported that 19/24 MCL and DCL patients were successfully treated with a combination of systemic SSG and allopurinol. 14 MCL patients were treated with a combination of systemic and intralesional SSG, of which 12 were cured, while six of seven DCL patients treated with a combination of systemic SSG, intralesional SSG and cryotherapy were treated successfully.
Cryotherapy and intralesional antimonials are most commonly used for uncomplicated LCL, although evidence is limited. Three studies have reported on efficacy of cryotherapy using liquid nitrogen. Cure was achieved in 80·5% of patients in southern Ethiopia [32], 60·8% in Addis Ababa [107], and 92·3% in Eastern Ethiopia [106] after treatment with cryotherapy, although treatment duration varied widely and is not always reported. The efficacy of intralesional SSG alone is not described, as one study only stated that the efficacy of systemic SSG and intralesional SSG taken together was 69% [57], while another reported that 25/26 patients treated with a combination of cryotherapy and intralesional SSG were cured [106].
11. Discussion
While a substantial number of studies have been performed over the decades, a clear knowledge gap on CL due to L. aethiopica remains (Table 1). Studies on epidemiology, parasite, reservoir, and vector are outdated and warrant contemporary systematic studies.
Where and when transmission takes place exactly are still unclear, as no nation-wide studies were done and information on Leishmania infected sandflies is scarce. Information on the national distribution of CL vectors is not properly established, since almost all sites studied were restricted to CL endemic areas. Whether sandflies are the limiting factor for transmission or whether other factors limit transmission when sandflies are abundant is unknown. More recent data on transmission as well as spread of CL vectors is vital for intervention strategies.
While most cases of CL in Ethiopia seem to be spread by P. longipes and P. pedifer sandflies and are caused by L. aethiopica, the role of other sandfly and Leishmania species in Ethiopian CL and the potential spread of CL to lower altitude areas needs to be better defined. While some sandfly species might not be directly implicated in human infections, it could well be that some (e.g. P. sergenti) maintain the cycle within the natural host.
Although hyraxes have been implicated as the reservoir, the role of humans and other animals such as cows as (incidental) hosts and potential reservoirs requires further investigation. If humans can transmit CL without another reservoir, it implicates that migration of people from endemic to non-endemic regions could lead to spread of CL into new habitats.
Why Ethiopia seems to have a relatively high burden of MCL and DCL is unclear, and the reason why a disproportional amount of CL lesions in Ethiopia are on the face is unknown. The atypical presentation of many Ethiopian CL patients and the large number of patients with primary mucosal involvement raises the need for a different classification approach.
The natural course of CL due to L. aethiopica is not properly established. The extent and duration of self-healing for L. aethiopica needs more investigation in order to decide who to treat and when to start. Treatment studies with a placebo -if deemed ethically appropriate- or observational studies in uncomplicated cases could provide insight in the natural evolution of CL due to L. aethiopica.
The different clinical presentations of CL due to L. aethiopica are characterized by clear immunopathological differences as well as parasite diversity. Whole-genome sequencing from a nationwide study could provide more insight into parasite related factors that may affect infectivity, pathogenicity, and virulence. Immunotherapeutic approaches may be used to boost the immune response and reduce the burden in MCL or DCL cases.
The most common way of diagnosing CL due to L. aethiopica uses skin slit samples with Giemsa-stained microscopy slides. However, large scale studies evaluating the diagnostic techniques currently employed as well as new promising tests are direly needed.
Various therapeutic options have been tried against CL in Ethiopia. However, most studies on treatment of L. aethiopica are outdated and defined by poor methodology, while large clinical trials are lacking. Therefore, the evidence-base is very limited. Clinical trials are sorely needed. For LCL these could compare cryotherapy, intralesional SSG, and thermotherapy, while topical treatment such as paromomycin crème should also be explored. Although systemic SSG currently remains the cornerstone of treatment for more complicated forms of CL, alternatives such as miltefosine and paromomycin require evaluation.
Search Strategy and Selection Criteria
References for this review were identified through searches in Pubmed and Google Scholar, through references from relevant articles, and by searching the authors’ own databases. Articles published in English, French and Italian were included. Search terms covered all sections of this review, and included but were not limited to “cutaneous leishmaniasis & Ethiopia” and “Leishmania aethiopica”
Conflicts of Interest
There are no conflicts of interest.
Funding
The authors received no specific funding for this work.
Author Contributions
Saskia van Henten: Conceptualization, data collection, visualization, writing, review and editing
Wim Adriaensen: Writing, review and editing
Helina Fikre: Data collection, visualization, review and editing
Hannah Akuffo: Writing, review and editing
Ermias Diro: Review and editing
Asrat Hailu: Review and editing
Gert Van der Auwera: Writing, review and editing
Johan van Griensven: Conceptualization, writing, review and editing
Declaration of Interest
The authors declare no competing interests.
Acknowledgements
We thank Carlos Kiyan for his assistance with GIS mapping, and Lieselotte Cnops for the enlightening discussions on diagnosis of cutaneous leishmaniasis. The authors received no specific funding for this work.
References
- 1.Reithinger R., Dujardin J.-C., Louzir H., Pirmez C., Alexander B., Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. Sep 2007;7(9):581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 2.Alvar J., Vélez I.D., Bern C. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. May 31 2012;7(5) doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray R.S., Ashford R.W., Bray M.A. The parasite causing cutaneous leishmaniasis in Ethiopia. Trans R Soc Trop Med Hyg. 1973;67(3):345–348. doi: 10.1016/0035-9203(73)90111-9. [DOI] [PubMed] [Google Scholar]
- 4.Poggi I. Oriental sore in Agamé (Abyssinia) Arch Ital Sci Med Trop Parassitol. 1937;18(3) [Google Scholar]
- 5.Monti G. Oriental sore in an Italian soldier. Arch Ital Sci Med Trop Parassitol. 1937;18(10) [Google Scholar]
- 6.Balzer R.J., Destombes P., Schaller K.F., Serie C. Leishmaniose cutanee pseudolepromateuse en Ethiopie. Bull Soc Pathol Exot. 1960;53:293–298. [PubMed] [Google Scholar]
- 7.Poirier A. Note preliminaire sur les leishmanioses en Ethiopie. Ann l'institut Pasteur d’ Ethiop. 1964;5:88–94. [Google Scholar]
- 8.Price E.W., Fitzherbert M. Cutaneous leishmaniasis in Ethiopia: a clinical study and review of the literature. Ethiop Med J. 1965;3:57–83. [Google Scholar]
- 9.Bryceson A., Nichol Thomas W. Cutaneous leishmaniasis in Wollega Province. Ethiop Med J. 1966;5:35–42. [Google Scholar]
- 10.Wilkins H.A. Studies on leishmaniasis in Ethiopia. VI:incidence rates of cutaneous leishmaniasis at Meta Abo. Ann Trop Med Parasitol. 1972;66(4):457–466. [PubMed] [Google Scholar]
- 11.Lemma A., Foster W.A., Gemetchu T., Preston P.M., Bryceson A., Minter D.M. Studies on leishmaniasis in Ethiopia. I. Preliminary investigations into the epidemiology of cutaneous leishmaniasis in the highlands. Ann Trop Med Parasitol. 1969 Dec;63(4):455–472. [PubMed] [Google Scholar]
- 12.Mengistu G., Laskay T., Gemetchu T. Cutaneous leishmaniasis in south-western Ethiopia: Ocholo revisited. Trans R Soc Trop Med Hyg. 1992;86(2):149–153. doi: 10.1016/0035-9203(92)90546-o. [DOI] [PubMed] [Google Scholar]
- 13.Ashford R.W., Bray M.A., Hutchinson M.P., Bray R.S. The epidemiology of cutaneous leishmaniasis in Ethiopia. Trans R Soc Trop Med Hyg. 1973;67(4):568–601. doi: 10.1016/0035-9203(73)90088-6. [DOI] [PubMed] [Google Scholar]
- 14.Barnetson R.S., Ridley D.S., Wheate H.W. A form of muco-cutaneous leishmaniasis in the Old World. Trans R Soc Trop Med Hyg. 1978;72(5):516–518. doi: 10.1016/0035-9203(78)90173-6. [DOI] [PubMed] [Google Scholar]
- 15.Barnetson R., Bryceson A.D.M. Cutaneous leishmaniasis and leprosy. Trans R Soc Trop Med Hyg. 1978;82(2):160–163. doi: 10.1016/0035-9203(78)90052-4. [DOI] [PubMed] [Google Scholar]
- 16.Negera E., Gadisa E., Yamuah L. Outbreak of cutaneous leishmaniasis in Silti woreda, Ethiopia: risk factor assessment and causative agent identification. Trans R Soc Trop Med Hyg. 2008;102(9):883–890. doi: 10.1016/j.trstmh.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Seid A., Gadisa E., Tsegaw T. Risk map for cutaneous leishmaniasis in Ethiopia based on environmental factors as revealed by geographical information systems and statistics. Geospat Health. 2014;8(2):377–387. doi: 10.4081/gh.2014.27. [DOI] [PubMed] [Google Scholar]
- 18.Sang D.K., Okelo G.B., Chance M.L. Cutaneous leishmaniasis due to Leishmania aethiopica, on Mount Elgon, Kenya. Ann Trop Med Parasitol. Aug 1993;87(4):349–357. doi: 10.1080/00034983.1993.11812778. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim M.E., Smyth A.J., Ali M.H., Barker D.C., Kharazmi A. The polymerase chain reaction can reveal the occurrence of naturally mixed infections with Leishmania parasites. Acta Trop. Sep 1 1994;57(4):327–332. doi: 10.1016/0001-706x(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 20.Lemma W., Erenso G., Gadisa E., Balkew M., Gebre-Michael T., Hailu A. A zoonotic focus of cutaneous leishmaniasis in Addis Ababa, Ethiopia. Parasit Vectors. 2009;2(1):60. doi: 10.1186/1756-3305-2-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bsrat A., Berhe N., Balkew M. Epidemiological study of cutaneous leishmaniasis in Saesie Tsaeda-emba district, eastern Tigray, northern Ethiopia. Parasit Vectors. 2015;8(1):149. doi: 10.1186/s13071-015-0758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster W.A. Studies on leishmaniasis in Ethiopia. III. Resting and breeding sites, flight behaviour, and seasonal abundance of Phlebotomus longipes (Diptera: Psychodidae) Ann Trop Med Parasitol. 1972;66(3):313–328. [PubMed] [Google Scholar]
- 23.Sang D.K., Chance M.L. Studies on the Phlebotomus fauna of Mount Elgon, Kenya. Ann Trop Med Parasitol. Jan 15 1993;87(5):509–515. doi: 10.1080/00034983.1993.11812803. [DOI] [PubMed] [Google Scholar]
- 24.Mutinga M.J. The animal reservoir of cutaneous leishmaniasis on Mount Elgon, Kenya. East Afr Med J. 1975;52(3) [PubMed] [Google Scholar]
- 25.Yared S., Gebresilassie A., Akililu E. Diversity and altitudinal distribution of phlebotomine sand flies (Diptera: Psychodidae) in visceral leishmaniasis endemic areas of northwest Ethiopia. Acta Trop. Dec 2017;176:1–10. doi: 10.1016/j.actatropica.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mutinga M.J. Phlebotomus longipes, a vector of cutaneous leishmaniasis in Kenya. Trans R Soc Trop Med Hyg. Jan 1 1971;65(1):106. doi: 10.1016/0035-9203(71)90200-8. [DOI] [PubMed] [Google Scholar]
- 27.Gebre-Michael T., Balkew M., Ali A., Ludovisi A., Gramiccia M. The isolation of Leishmania tropica and L. aethiopica from Phlebotomus (Paraphlebotomus) species (Diptera: Psychodidae) in the Awash Valley, northeastern Ethiopia. Trans R Soc Trop Med Hyg. Jan 1 2004;98(1):64–70. doi: 10.1016/s0035-9203(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 28.Hailu A., Di Muccio T., Abebe T., Hunegnaw M., Kager P.A., Gramiccia M. Isolation of Leishmania tropica from an Ethiopian cutaneous leishmaniasis patient. Trans R Soc Trop Med Hyg. 2006;100(1):53–58. doi: 10.1016/j.trstmh.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Ashford R.W. Sandflies (Diptera: Phlebotomidae) from Ethiopia: taxonomic and biological notes. J Med Entomol. Nov 25 1974;11(5):605–616. [PubMed] [Google Scholar]
- 30.Williams A.O., Mutinga J., Rodgers M. Leishmaniasis in a domestic goat in Kenya. Mol Cell Probes. Oct 1991;5(5):319–325. doi: 10.1016/s0890-8508(06)80002-2. [DOI] [PubMed] [Google Scholar]
- 31.Abebe A., Evans D.A., Gemetchu T. The isolation of Leishmania aethiopica from the ground squirrel Xerus rutilus. Trans R Soc Trop Med Hyg. Sep 1 1990;84(5):691. doi: 10.1016/0035-9203(90)90147-7. [DOI] [PubMed] [Google Scholar]
- 32.Negera E., Gadisa E., Hussein J. Treatment response of cutaneous leishmaniasis due to Leishmania aethiopica to cryotherapy and generic sodium stibogluconate from patients in Silti, Ethiopia. Trans R Soc Trop Med Hyg. 2012;106(8):496–503. doi: 10.1016/j.trstmh.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Sarojini P.A., Humber D.P., Yemane-Berhan T. Cutaneous leishmaniasis cases seen in two years at the All Africa Leprosy and Rehabilitation Training Centre Hospital. Ethiop Med J. 1984;22(1):7–11. [PubMed] [Google Scholar]
- 34.Krayter L., Schnur L.F., Schönian G. The genetic relationship between Leishmania aethiopica and Leishmania tropica revealed by comparing microsatellite profiles. PLoS One. Jul 21 2015;10(7) doi: 10.1371/journal.pone.0131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Blancq S.M., Belehu A., Peters W. Leishmania in the Old World: 3. The distribution of L. aethiopica zymodemes. Trans R Soc Trop Med Hyg. Jan 1 1986;80(3):360–365. doi: 10.1016/0035-9203(86)90318-4. [DOI] [PubMed] [Google Scholar]
- 36.Pratlong F., Dereure J., Ravel C. Geographical distribution and epidemiological features of Old World cutaneous leishmaniasis foci, based on the isoenzyme analysis of 1048 strains. Trop Med Int Health. Sep 1 2009;14(9):1071–1085. doi: 10.1111/j.1365-3156.2009.02336.x. [DOI] [PubMed] [Google Scholar]
- 37.Schönian G., Akuffo H., Lewin S. Genetic variability within the species Leishmania aethiopica does not correlate with clinical variations of cutaneous leishmaniasis. Mol Biochem Parasitol. Mar 5 2000;106(2):239–248. doi: 10.1016/s0166-6851(99)00216-9. [DOI] [PubMed] [Google Scholar]
- 38.Kebede N., Oghumu S., Worku A., Hailu A., Varikuti S., Satoskar A.R. Multilocus microsatellite signature and identification of specific molecular markers for Leishmania aethiopica. Parasit Vectors. Jun 4 2013;6(1):160. doi: 10.1186/1756-3305-6-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odiwuor S., De Doncker S., Maes I., Dujardin J.-C., Van der Auwera G. Natural Leishmania donovani/Leishmania aethiopica hybrids identified from Ethiopia. Infect Genet Evol. Dec 1 2011;11(8):2113–2118. doi: 10.1016/j.meegid.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 40.Ives A., Ronet C., Prevel F. Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science. Feb 11 2011;331(6018):775–778. doi: 10.1126/science.1199326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zangger H., Hailu A., Desponds C. Leishmania aethiopica field isolates bearing an endosymbiontic dsRNA virus induce pro-inflammatory cytokine response. PLoS Negl Trop Dis. Apr 24 2014;8(4) doi: 10.1371/journal.pntd.0002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gelanew T., Hurissa Z., Diro E. Case report: disseminated cutaneous leishmaniasis resembling post-kala-azar dermal leishmaniasis caused by Leishmania donovani in three patients co-infected with visceral leishmaniasis and human immunodeficiency virus/acquired immunodeficiency syndrome in. Am J Trop Med Hyg. 2011;84(6):906–912. doi: 10.4269/ajtmh.2011.11-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karunaweera N.D. Leishmania donovani causing cutaneous leishmaniasis in Sri Lanka: a wolf in sheep's clothing? Trends Parasitol. Oct 1 2009;25(10):458–463. doi: 10.1016/j.pt.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Schönian G., Nasereddin A., Dinse N. PCR diagnosis and characterization of leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. Sep 1 2003;47(1):349–358. doi: 10.1016/s0732-8893(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 45.Van der Auwera G., Dujardin J.-C. Species typing in dermal leishmaniasis. Clin Microbiol Rev. Apr 1 2015;28(2):265–294. doi: 10.1128/CMR.00104-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kassahun A., Sadlova J., Dvorak V. Detection of Leishmania donovani and L. tropica in Ethiopian wild rodents. Acta Trop. May 1 2015;145:39–44. doi: 10.1016/j.actatropica.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Kassahun A., Sadlova J., Benda P. Natural infection of bats with Leishmania in Ethiopia. Acta Trop. Oct 1 2015;150:166–170. doi: 10.1016/j.actatropica.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 48.Gebre-Michael T., Pratlong F., Lane R.P. Phlebotomus (Phlebotomus) duboscqi (Diptera: Phlebotominae), naturally infected with Leishmania major in southern Ethiopia. Trans R Soc Trop Med Hyg. Jan 1 1993;87(1) doi: 10.1016/0035-9203(93)90399-b. [10–1] [DOI] [PubMed] [Google Scholar]
- 49.Gadisa E., Genetu A., Kuru T. Leishmania (Kinetoplastida): species typing with isoenzyme and PCR–RFLP from cutaneous leishmaniasis patients in Ethiopia. Exp Parasitol. Apr 1 2007;115(4):339–343. doi: 10.1016/j.exppara.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 50.Kuru T., Janusz N., Gadisa E., Gedamu L., Aseffa A. Leishmania aethiopica: development of specific and sensitive PCR diagnostic test. Exp Parasitol. Aug 1 2011;128(4):391–395. doi: 10.1016/j.exppara.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Odiwuor S., Ageed Saad A., De Doncker S. Universal PCR assays for the differential detection of all Old World Leishmania species. Eur J Clin Microbiol Infect Dis. Feb 9 2011;30(2):209–218. doi: 10.1007/s10096-010-1071-3. [DOI] [PubMed] [Google Scholar]
- 52.Bugssa G. The current status of cutaneous leishmaniasis and the pattern of lesions in Ochollo primary school students, Ochollo, southwestern Ethiopia. Sci J Clin Med. 2014;3(6):111. [Google Scholar]
- 53.Lindtjorn B. Cutaneous leishmaniasis in the Sidamo highlands. Ethiop Med J. 1981;19(3):97–98. [PubMed] [Google Scholar]
- 54.Adam G.K., Ali K.M., Abdella Y.H. Trend in cumulative cases and mortality rate among visceral leishmaniasis patients in eastern Sudan: a 14-year registry, 2002–2015. Int J Infect Dis. Oct 2016;51:81–84. doi: 10.1016/j.ijid.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 55.Ali A. Leishmaniases survey in the Awash Valley: leishmanin skin test profile in the upper awash and surrounding areas. Ethiop Med J. Oct 1997;35(4):225–233. [PubMed] [Google Scholar]
- 56.Tilahun F., Alemu W., Magnitude Mulatu G. Associated factors of cutaneous leishmaniasis. Clin Med Res. 2014;3(6):189–199. [Google Scholar]
- 57.Padovese V., Terranova M., Toma L., Barnabas G.A., Morrone A. Cutaneous and mucocutaneous leishmaniasis in Tigray, northern Ethiopia: clinical aspects and therapeutic concerns. Trans R Soc Trop Med Hyg. 2009;103(7):707–711. doi: 10.1016/j.trstmh.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 58.Morrone A., Pitidis A., Pajno M.C. Epidemiological and geographical aspects of leishmaniasis in Tigray, northern Ethiopia: a retrospective analysis of medical records, 2005–2008. Trans R Soc Trop Med Hyg. 2011;105(5):273–280. doi: 10.1016/j.trstmh.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Fikre H., Mohammed R., Atinafu S., van Griensven J., Diro E. Clinical features and treatment response of cutaneous leishmaniasis in North-West Ethiopia. Trop Med Int Health. Oct 2017;22(10):1293–1301. doi: 10.1111/tmi.12928. [DOI] [PubMed] [Google Scholar]
- 60.Scott P., Novais F.O. Cutaneous leishmaniasis: immune responses in protection and pathogenesis. Nat Rev Immunol. Sep 18 2016;16(9):581–592. doi: 10.1038/nri.2016.72. [DOI] [PubMed] [Google Scholar]
- 61.Bryceson A.D.M. Diffuse cutaneous leishmaniasis in Ethiopia. I. the clinical and histological features of the disease. Trans R Soc Trop Med Hyg. 1969;63(6):708–737. doi: 10.1016/0035-9203(69)90116-3. [DOI] [PubMed] [Google Scholar]
- 62.Dassoni F., Abebe Z., Naafs B., Morrone A. Cutaneous and mucocutaneous leishmaniasis resembling borderline-tuberculoid leprosy: a new clinical presentation? Acta Derm Venereol. 2013;93(1):74–77. doi: 10.2340/00015555-1338. [DOI] [PubMed] [Google Scholar]
- 63.Dassoni F., Daba F., Naafs B., Morrone A. Leishmaniasis recidivans in Ethiopia: cutaneous and mucocutaneous features. J Infect Dev Ctries. 2017;11(1):106–110. doi: 10.3855/jidc.8516. [DOI] [PubMed] [Google Scholar]
- 64.Berhe N., Hailu A., Gemetchu T. Human immunodeficiency virus and recurrence of cutaneous leishmaniasis long after healed localized cutaneous leishmaniasis due to Leishmania aethiopica. Trans R Soc Trop Med Hyg. 1995;89(4):400–401. doi: 10.1016/0035-9203(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 65.Bekele S., Bekele Y., Lemma T. Recent trends of cutaneous leishmaniasis in Alert hospital, Addis Ababa. Ethiop Med J. 2014;(Suppl):37–41. [PubMed] [Google Scholar]
- 66.von Stebut E., Tenzer S. Cutaneous leishmaniasis: distinct functions of dendritic cells and macrophages in the interaction of the host immune system with Leishmania major. Int J Med Microbiol. Jan 6 2018;308(1):206–214. doi: 10.1016/j.ijmm.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 67.Akuffo H.O., Fehniger T.E., Britton S. Differential recognition of Leishmania aethiopica antigens by lymphocytes from patients with local and diffuse cutaneous leishmaniasis. Evidence for antigen-induced immune suppression. J Immunol. Oct 1 1988;141(7):2461–2466. [PubMed] [Google Scholar]
- 68.Barroso D.H., Falcão S.D.A.C., da Motta J. de O.C. PD-L1 may mediate T-cell exhaustion in a case of early diffuse leishmaniasis caused by Leishmania (L.) amazonensis. Front Immunol. May 11 2018;9:1021. doi: 10.3389/fimmu.2018.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hernández-Ruiz J., Salaiza-Suazo N., Carrada G. CD8 cells of patients with diffuse cutaneous leishmaniasis display functional exhaustion: the latter is reversed, in vitro, by TLR2 agonists. PLoS Negl Trop Dis. Nov 2 2010;4(11):e871. doi: 10.1371/journal.pntd.0000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akuffo H., Maasho K., Howe R. Natural and acquired resistance to Leishmania: cellular activation by Leishmania aethiopica of mononuclear cells from unexposed individuals is through the stimulation of natural killer (NK) cells. Clin Exp Immunol. 1993;94(3):516–521. doi: 10.1111/j.1365-2249.1993.tb08227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nylen S., Maasho K., Soderstrom K., Ilg T., Akuffo H. Live Leishmania promastigotes can directly activate primary human natural killer cells to produce interferon-gamma. Clin Exp Immunol. Mar 1 2003;131(3):457–467. doi: 10.1046/j.1365-2249.2003.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maasho K., Sanchez F., Schurr E., Hailu A., Akuffo H. Indications of the protective role of natural killer cells in human cutaneous leishmaniasis in an area of endemicity. Infect Immun. Jun 1 1998;66(6):2698–2704. doi: 10.1128/iai.66.6.2698-2704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lieke T., Nylen S., Eidsmo L., Schmetz C., Berg L., Akuffo H. The interplay between Leishmania promastigotes and human natural killer cells in vitro leads to direct lysis of Leishmania by NK cells and modulation of NK cell activity by Leishmania promastigotes. Parasitology. Dec 9 2011;138(14):1898–1909. doi: 10.1017/S0031182011001363. [DOI] [PubMed] [Google Scholar]
- 74.Novais F.O., Scott P. CD8 + T cells in cutaneous leishmaniasis: the good, the bad, and the ugly. Semin Immunopathol. May 24 2015;37(3):251–259. doi: 10.1007/s00281-015-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pitta M.G.R., Romano A., Cabantous S. IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani. J Clin Invest. Aug 13 2009;119(8):2379–2387. doi: 10.1172/JCI38813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boaventura V.S., Santos C.S., Cardoso C.R. Human mucosal leishmaniasis: neutrophils infiltrate areas of tissue damage that express high levels of Th17-related cytokines. Eur J Immunol. Oct 2010;40(10):2830–2836. doi: 10.1002/eji.200940115. [DOI] [PubMed] [Google Scholar]
- 77.Bacellar O., Faria D., Nascimento M. Interleukin 17 production among patients with American cutaneous leishmaniasis. J Infect Dis. Jul 1 2009;200(1):75–78. doi: 10.1086/599380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martínez D.Y., Verdonck K., Kaye P.M. Tegumentary leishmaniasis and coinfections other than HIV. PLoS Negl Trop Dis. Mar 1 2018;12(3) doi: 10.1371/journal.pntd.0006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Glennie N.D., Scott P. Memory T cells in cutaneous leishmaniasis. Cell Immunol. Nov 2016;309:50–54. doi: 10.1016/j.cellimm.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bennis I., Verdonck K., El Khalfaoui N. Accuracy of a rapid diagnostic test based on antigen detection for the diagnosis of cutaneous leishmaniasis in patients with suggestive skin lesions in Morocco. Am J Trop Med Hyg. Sep 2018;99(3):716–722. doi: 10.4269/ajtmh.18-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vink M.M.T., Nahzat S.M., Rahimi H. Evaluation of point-of-care tests for cutaneous leishmaniasis diagnosis in Kabul, Afghanistan. EBioMedicine. Nov 2018;37:453–460. doi: 10.1016/j.ebiom.2018.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mengistu G., Akuffo H., Fehniger T.E., Negese Y., Nilsen R. Comparison of parasitological and immunological methods in the diagnosis of leishmaniasis in Ethiopia. Trans R Soc Trop Med Hyg. Mar 1 1992;86(2):154–157. doi: 10.1016/0035-9203(92)90548-q. [DOI] [PubMed] [Google Scholar]
- 83.van Eys Guillaume J.J.M., Schoone G.J., NCM Kroon, Ebeling S.B. Sequence analysis of small subunit ribosomal RNA genes and its use for detection and identification of Leishmania parasites. Mol Biochem Parasitol. Mar 1 1992;51(1):133–142. doi: 10.1016/0166-6851(92)90208-2. [DOI] [PubMed] [Google Scholar]
- 84.Deborggraeve S., Laurent T., Espinosa D. A simplified and standardized polymerase chain reaction format for the diagnosis of leishmaniasis. J Infect Dis. Nov 15 2008;198(10):1565–1572. doi: 10.1086/592509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nicolas L., Prina E., Lang T., Milon G. Real-time PCR for detection and quantitation of leishmania in mouse tissues. J Clin Microbiol. May 1 2002;40(5):1666–1669. doi: 10.1128/JCM.40.5.1666-1669.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marfurt J., Niederwieser I., Makia N.D., Beck H.-P., Felger I. Diagnostic genotyping of Old and New World Leishmania species by PCR-RFLP. Diagn Microbiol Infect Dis. Jun 1 2003;46(2):115–124. doi: 10.1016/s0732-8893(03)00040-3. [DOI] [PubMed] [Google Scholar]
- 87.Mugasa C.M., Laurent T., Schoone G.J. Simplified molecular detection of Leishmania parasites in various clinical samples from patients with leishmaniasis. Parasit Vectors. Mar 2 2010;3(1):13. doi: 10.1186/1756-3305-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adams E.R., Schoone G., Versteeg I. Development and evaluation of a novel loop-mediated isothermal amplification assay for diagnosis of cutaneous and visceral Leishmaniasis. J Clin Microbiol. Apr 25 2018;56(7) doi: 10.1128/JCM.00386-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mukhtar M., Ali S.S., Boshara S.A. Sensitive and less invasive confirmatory diagnosis of visceral leishmaniasis in Sudan using loop-mediated isothermal amplification (LAMP) PLoS Negl Trop Dis. Feb 14 2018;12(2) doi: 10.1371/journal.pntd.0006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lawn S.D., Nicol M.P. Xpert ® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. Sep 30 2011;6(9):1067–1082. doi: 10.2217/fmb.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Griensven J., Gadisa E., Aseffa A., Hailu A., Beshah A.M., Diro E. Treatment of cutaneous leishmaniasis caused by Leishmania aethiopica: a systematic review. PLoS Negl Trop Dis. Mar 3 2016;10(3) doi: 10.1371/journal.pntd.0004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chulay J.D., Anzeze E.M., Koech D.K., Bryceson A.D. High-dose sodium stibogluconate treatment of cutaneous leishmaniasis in Kenya. Trans R Soc Trop Med Hyg. 1983;77(5):717–721. doi: 10.1016/0035-9203(83)90213-4. [DOI] [PubMed] [Google Scholar]
- 93.Castro M.D.M., Gomez M.A., Kip A.E. Pharmacokinetics of miltefosine in children and adults with cutaneous leishmaniasis. Antimicrob Agents Chemother. Mar 1 2017;61(3) doi: 10.1128/AAC.02198-16. [e02198-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mohebali M., Fotouhi A., Hooshmand B. Comparison of miltefosine and meglumine antimoniate for the treatment of zoonotic cutaneous leishmaniasis (ZCL) by a randomized clinical trial in Iran. Acta Trop. Jul 1 2007;103(1):33–40. doi: 10.1016/j.actatropica.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 95.Utaile M., Kassahun A., Abebe T., Hailu A. Susceptibility of clinical isolates of Leishmania aethiopica to miltefosine, paromomycin, amphotericin B and sodium stibogluconate using amastigote-macrophage in vitro model. Exp Parasitol. May 2013;134(1):68–75. doi: 10.1016/j.exppara.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 96.Na-Bangchang K., Ahmed O., Hussein J. Exploratory, phase II controlled trial of Shiunko ointment local application twice a day for 4 weeks in Ethiopian patients with localized cutaneous leishmaniasis. Evid Based Complement Alternat Med. Apr 19 2016;2016:1–8. doi: 10.1155/2016/5984709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van der Meulen J., Mock B., Fekete E., Sarojini P.A. Limited therapeutic action of rifampicin/isoniazid against Leishmania aethiopica. Lancet. 1981;2:197–198. doi: 10.1016/s0140-6736(81)90374-3. [DOI] [PubMed] [Google Scholar]
- 98.Akuffo H., Dietz M., Teklemariam S., Tadesse T., Amare G., Berhan T. The use of itraconazole in the treatment of leishmaniasis caused by Leishmania aethiopica. Trans R Soc Trop Med Hyg. 1990;84(4):532–534. doi: 10.1016/0035-9203(90)90028-d. [DOI] [PubMed] [Google Scholar]
- 99.Naafs B. Pentamidine-induced diabetes mellitus. Trans R Soc Trop Med Hyg. Jan 1 1985;79(1):141. doi: 10.1016/0035-9203(85)90267-6. [DOI] [PubMed] [Google Scholar]
- 100.Dorlo T.P.C., Kager P.A. Pentamidine dosage: a base/salt confusion. PLoS Negl Trop Dis. May 28 2008;2(5):e225. doi: 10.1371/journal.pntd.0000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Teklemariam S., Hiwot A.G., Frommel D., Miko T.L., Ganlov G., Bryceson A. Aminosidine and its combination with sodium stibogluconate in the treatment of diffuse cutaneous leishmaniasis caused by Leishmania aethiopica. Trans R Soc Trop Med Hyg. 1994;88(3):334–339. doi: 10.1016/0035-9203(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 102.Belehu A., Naafs B., Touw-Langendijk E. Failure of metronidazole treatment in Ethiopian mucocutaneous leishmaniasis. Br J Dermatol. 1978;99:421–422. doi: 10.1111/j.1365-2133.1978.tb06181.x. [DOI] [PubMed] [Google Scholar]
- 103.Viallet J., MacLean J.D., Robson H. Response to ketoconazole in two cases of longstanding cutaneous leishmaniasis. Am J Trop Med Hyg. 1986;35(3):491–495. doi: 10.4269/ajtmh.1986.35.491. [DOI] [PubMed] [Google Scholar]
- 104.Bryceson A., Foster W.A., Lemma A. Clinical trial of CI-501 (Camolar) against cutaneous leishmaniasis in Ethiopia. Trans R Soc Trop Med Hyg. 1969;63:152–153. doi: 10.1016/0035-9203(69)90111-4. [DOI] [PubMed] [Google Scholar]
- 105.Zanger P., Kötter I., Raible A., Gelanew T., Schönian G., Kremsner P.G. Case report: successful treatment of cutaneous leishmaniasis caused by Leishmania aethiopica with liposomal amphothericin B in an immunocompromised traveler returning from Eritrea. Am J Trop Med Hyg. 2011;84(5):692–694. doi: 10.4269/ajtmh.2011.10-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seife T., Benecha A.K., Zewdu F.T., Ayal A., Misganaw M. Treatment patterns and effectivness of anti-leishmaniasis agents for patients with cutaneous leishmaniasis at Boru Meda Hospital, South Wollo, North East Ethiopia, 2017/18. J Clin Exp Dermatol Res. 2018;09(03) [Google Scholar]
- 107.Leish-mapping team at AHRI in collaboration with WHO-Ethiopia . 2011. Proceedings of the International Consultative Meeting on Cutaneous Leishmaniasis in Ethiopia. Addis Ababa. [Google Scholar]