Abstract
Background
Invasive Pneumococcal Disease (IPD) is a major public health concern. The effectiveness of 23-valent polysaccharide pneumococcal vaccine (PPV23) against IPD in older age-groups is not fully understood. We measured PPV23 effectiveness against IPD and interpreted changes in IPD incidence between 2000 and 2017.
Methods
Public Health England conducts enhanced national IPD surveillance in England and Wales. The indirect cohort method was used to estimate PPV23 effectiveness against IPD in individuals aged ≥ 65 years eligible for PPV23 vaccination during 2012–2016. IPD incidence in 2016/17 was compared to rates during 2000–2003, when neither PPV23 nor pneumococcal conjugate vaccines (PCVs) were routinely used in England and Wales.
Findings
PPV23 effectiveness, irrespective of time since vaccination, was 27% (95% CI, 17–35) after adjusting for age, co-morbidity and year of infection. Vaccine effectiveness reduced non-significantly (p = 0.13) with time since vaccination, from 41% (95% CI, 23–54) for those vaccinated within two years, to 34% (95% CI, 16–48) for those vaccinated 2–4 years previously, and 23% (95% CI, 12–32) for those vaccinated ≥ 5 years previously. Vaccine effectiveness did not vary significantly by age but was highest in previously healthy individuals (45%; 95%CI, 27–59). IPD incidence for PPV23 serotypes not included in the PCVs did not decrease after routine PPV23 use but increased significantly since PCV introduction in 2006.
Interpretation
PPV23 offers moderate short-term protection against IPD in older adults. PPV23 serotypes comprise an increasing proportion of IPD cases in older adults because of serotype replacement following routine PCV use in children.
Funding
European Union's Horizon 2020.
Keywords: Pneumococcal polysaccharide vaccine, PPV23, Effectiveness, Impact, Broome method, Trends
1. Introduction
Streptococcus pneumoniae is a major cause of morbidity and mortality worldwide, especially at the extremes of age and among those with underlying comorbidities. Almost 100 pneumococcal serotypes have been identified, based on their unique capsular polysaccharide structure. These serotypes each have different propensities for carriage and invasive disease, including meningitis, septicaemia and pneumonia, which can all be life-threatening. In order to prevent invasive pneumococcal disease (IPD), two different types of vaccines are currently available: the 23-valent pneumococcal polysaccharide vaccine (PPV23) and the pneumococcal conjugate vaccines (PCVs).
In the UK, prior to 2003, PPV23 had been available for more than 20 years and was recommended for individuals aged at least two years with specific underlying clinical conditions that significantly increased their risk of IPD [1]. Since 2003, a single dose of PPV23 was recommended for adults on their 65th birthday alongside a two-year catch-up (2003–2005) for all ≥ 65 year-olds, starting with ≥ 80 year-olds and moving to 75–79 and 65–74 year-olds by 2005. Currently, around 70% of ≥ 65 year-olds have received a dose of PPV23, including 19% already vaccinated as part of the at-risk programme by 65 years of age, 16% of new 65 year-olds and the remainder who receive the vaccine in subsequent years, usually before the age of 70 years [2].
In September 2006, the 7-valent PCV (PCV7) was introduced into the infant immunisation programme alongside a 12-month catch-up for all < 2 year-olds. This programme led to a rapid and sustained decline in PCV7-serotype IPD across all age groups through direct and indirect (herd) protection [3]. The overall decline in IPD was, however, offset by a small increase in IPD due to non-PCV7 serotypes across all age groups because of serotype replacement in carriage and disease. In April 2010, PCV7 was replaced with a 13-valent PCV (PCV13) without a catch-up, which led to further declines in both PCV13-type and overall IPD across all age groups although, again, with a small increase in replacement disease due to non-PCV13 serotypes [4].
Between 2003 and 2010, vaccine effectiveness (VE) of PPV23 using the Broome method [5], was estimated to be 48% within two years of vaccination, declining to 15% after five years [6]. Stratification by clinical risk group and age suggested declining VE with age and lower vaccine effectiveness in those with underlying co-morbidity, although these were not statistically significant. In previously healthy 65–74 year-olds, PPV23 VE was 65% (95% CI, 23–84) with 2 years of vaccination, 62% (95% CI, 21–82) from 2 to 5 years and 28% (95% CI, − 72 to 70) beyond 5 years.
That study had been informative in deliberations by the United Kingdom Joint Committee on Vaccines and Immunisation (JCVI) in 2013 and led to the decision to continue the elderly PPV23 programme. The programme was reviewed again in 2015 and the same decision was made, with a plan to re-assess PPV23 impact and VE because of the rapidly changing epidemiology of IPD due to large declines in PCV13-serotype IPD and replacement disease due to non-PCV13 serotypes as a result of the childhood PCV13 programme [7]. This was undertaken by Public Health England (PHE) in 2015, with additional resources from the Horizon 20-20 Integrated Monitoring of Vaccines Effectiveness (I-MOVE +) network in Europe [8]. In this study, we report the population impact and effectiveness of PPV23 on IPD among older adults in England and Wales over a 16-year period.
2. Methods
2.1. Vaccine Effectiveness Against IPD
PHE conducts enhanced national surveillance of IPD in England and Wales, as described previously [4]. Laboratory-confirmed cases with PPV23-type IPD (VT) and controls with non-PPV23 type IPD (NVT) are identified through a combination of clinical and laboratory reporting. National Health Service (NHS) hospital laboratories routinely submit invasive pneumococcal isolates to PHE for confirmation and serotyping, which is not performed locally. The laboratories also electronically report clinically significant infections to PHE through the Second Generation Surveillance System (SGSS, previously LabBase2). Reports without isolate submission are actively followed-up by PHE to ensure consistently high serotyping rates for all invasive isolates across England and Wales.
For this study, PHE asked general practitioners to complete a short questionnaire on the clinical and immunisation history for all confirmed IPD cases with known serotype among ≥ 65 years-olds, who were diagnosed between 1 January 2012 and 30 June 2016. IPD was defined as detection of S. pneumoniae (culture, PCR or antigen) from a normally sterile site. Final outcome (alive/dead) was ascertained by cross-checking all cases with the Patient Demographics Service (PDS; https://digital.nhs.uk/Demographics) in February 2017.
Based on the questionnaire returned by the GP underlying medical conditions were categorised into three risk-groups as follows: none, high-risk immunocompetent (chronic respiratory/heart/liver/renal disease; diabetes mellitus; cerebrospinal fluid leaks; cochlear implants) and immunocompromised (asplenia/splenic dysfunction; malignancy; or an immunosuppressive drug).
2.1.1. Statistical Methods for Effectiveness
Key variables (age, gender, period, clinical risk group, vaccination, ethnicity, clinical presentation, time registered at the GP, intensive care unit (ICU) admission, hospital stay ≥ 14 days, and death within 30 days) were compared between cases and controls using the chi-square test or Fisher's exact test as appropriate.
Vaccine effectiveness was calculated as 100% × (1 − the odds of vaccination in VT cases / odds of vaccination in NVT controls) using logistic regression to adjust for potential confounding factors of age (65–69, 70–74, 75–79, 80–84 and ≥ 85 years), clinical risk group category, gender, year of notification, and ethnicity. The influence of these factors, which may be related to both vaccination and IPD, was investigated by adding them sequentially in the order shown above to the model. For missing values on a factor such as clinical risk group, we used complete case analysis. This design method which is a variant on a case–control design in which non vaccine type cases serve as controls is known as the indirect cohort method or Broome method5 and was originally developed for assessment of PPV.
In the primary analysis for overall VE, vaccination was defined as a PPV23 dose at any time prior to IPD. This was then assessed by time since vaccination using a factor for vaccination with 4 levels (unvaccinated, vaccinated within < 2 years, 2–4 years, ≥ 5 years). To further investigate the long-term decline VE was also estimated using a factor for each individual year from vaccination to IPD (up to 16 years) and with a cubic spline and linear decline. Stratification was also performed by age (65–74, 75–84, ≥ 85 years) and clinical risk group (high risk immunocompetent, immunocompromised and none). Differences across these strata were tested using the likelihood ratio test or Wald's test. VE against individual serotypes was assessed by just including the controls and cases due to the individual serotype. Those vaccinated with unknown date were considered in the analysis as vaccinated ever but dropped from the analyses involving timing since vaccination.
Vaccine effectiveness against the different clinical manifestations (meningitis, septicaemia and pneumonia), case fatality rate, ICU admission and hospital admission for ≥ 14 days were also estimated.
Sensitivity analyses were as follows: i) VE by age at vaccination was considered rather than age at disease, ii) VE against vaccine-related serotypes (i.e. those of the same serogroup rather than the same serotype) by comparing these to the remaining controls, iii) only including records where the individual was registered at the GP practice for ≥ 2 or ≥ 5 years at the date of IPD (on the assumption that the patient's vaccination history was more likely to be accurate in those registered at the same practice for longer).
2.2. Vaccine Impact Against IPD
National IPD data were analysed from the 2000/01 to 2016/17 epidemiological years (from 01 July to 30 June the following year); the study population comprised those aged ≥ 65 years (split across 5-year age bands to ≥ 85 years) with IPD in England and Wales. Annual incidence was calculated by dividing the number of corrected cases by the population size for that age-group and year in England and Wales. Corrected case numbers were calculated with adjustments for missing age, serotype and underlying trends, and compared using an over-dispersed Poisson model as described previously [4], [9]. This method assumes that the serotype distribution in those with missing serotype is the same as in those with complete serotype. Also the age distribution in those with missing age is the same as the age distribution where the age is known. In this model, the inflation/deflation factors to correct for underlying trends in total IPD prior to PCV introduction (which were applied until 2009/10, after which it was assumed levels stabilised) were 3.82% for 65–69, 3.01% for 70–74, − 1.08% for 75–79, 0.25% for 80–84, − 0.21% for 80 +, and 0.99% for ≥ 65. So, for example, for 2004/05 (5 years before 2009/10), IPD was inflated by a factor of 1.03825 = 1.206. To assess impact to date, 2016/17 incidences were compared to 2000/01–2002/03 (pre-routine PPV23) in the Poisson model. To distinguish between PPV23 effects and PCV effects, serotypes were grouped as PCV7, PCV13 only (in PCV13 but not PCV7), PPV23 only (in PPV23 but not PCV13) and NVT (not in any vaccine).
2.3. Ethics Approval
PHE has legal permission, provided by Regulation 3 of The Health Service (Control of Patient Information) Regulations 2002, to process patient confidential information for national surveillance of communicable diseases (http://www.legislation.gov.uk/uksi/2002/1438/regulation/3/made). This includes PHE's responsibility to monitor the safety and effectiveness of vaccines.
2.4. Role of the Funding Source
Invasive pneumococcal disease surveillance is internally funded by Public Health England. The research is funded by a European Commission project I-MOVE + (Integrated Monitoring of Vaccines Effectiveness in Europe) that received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement #634446. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England.
3. Results
3.1. Vaccine Effectiveness Against IPD
3.1.1. Description of Cases and Controls
A total of 9847 IPD cases with serotyped isolates diagnosed in England and Wales in older adults aged ≥ 65 years during 4.5 years of surveillance (2012 to mid-2016) were followed-up and 6319 (64%) returned the questionnaire. A higher response rate was obtained for cases diagnosed during 2014–2016, when questionnaires were sent closer to the time of IPD. Vaccination history was missing in 74 leaving 6245 cases for analysis; of these, 4423 (71%) were due to PPV23 serotypes (cases) and 1822 (29%) were due to non-PPV23 serotypes (controls) (Table 1). Healthy adults represented 20% (1235/6173), high-risk immunocompetent individuals 52% (3194/6173) and immunocompromised individuals 28% (1744/6173) of cases and controls where clinical risk group was known. Within the high-risk immunocompetent group, the most commonly reported conditions were respiratory disease (52%), cardiovascular disease (50%), renal disease (32%) and diabetes (30%) with other conditions reported for ≤ 5% of individuals. Within the immunocompromised group, malignancy (84%) was most common. The number of cases and controls stratified by age, risk and time since vaccination are shown in Table 2.
Table 1.
Characteristics of cases and controls subject to age at disease, gender, period, underlying conditions, time since vaccination, 30 days mortality, ICU, hospital admission, ethnicity, clinical manifestations General Practice registrations, and age at vaccination.
| Variables | Level | Cases | Controls | Total | p-Value⁎ |
|---|---|---|---|---|---|
| All data | 4423 (71%) | 1822 (29%) | 6245 | ||
| Age Group at Disease | 65–74 | 1827 (76%) | 569 (24%) | 2396 | < 0.0001 |
| 75–84 | 1576 (69%) | 703 (31%) | 2279 | ||
| 85 + | 1020 (65%) | 550 (35%) | 1570 | ||
| Gender | Female | 2386 (73%) | 904 (27%) | 3290 | 0.008 |
| Male | 1990 (69%) | 897 (31%) | 2887 | ||
| Missing | 47 (69%) | 21 (31%) | 68 | ||
| Period | 2012 | 379 (77%) | 112 (23%) | 491 | < 0.0001 |
| 2013 | 623 (65%) | 333 (35%) | 956 | ||
| 2014 | 979 (68%) | 471 (32%) | 1450 | ||
| 2015 | 1457 (71%) | 602 (29%) | 2059 | ||
| 2016 | 985 (76%) | 304 (24%) | 1289 | ||
| Risk Group | High risk immunocompetent | 2275 (71%) | 919 (29%) | 3194 | < 0.0001 |
| Immunocompromised | 1164 (67%) | 580 (33%) | 1744 | ||
| None | 932 (75%) | 303 (25%) | 1235 | ||
| Missing | 52 (72%) | 20 (28%) | 72 | ||
| Time since vaccination | Unvaccinated | 1682 (76%) | 534 (24%) | 2216 | < 0.0001 |
| 0 to < 2 yrs | 194 (66%) | 101 (34%) | 295 | ||
| 2 to < 5 yrs | 260 (69%) | 117 (31%) | 377 | ||
| ≥ 5 yrs | 2096 (68%) | 972 (32%) | 3068 | ||
| Yes & unknown time | 191 (66%) | 98 (34%) | 289 | ||
| Died in 30 days | No | 3417 (71%) | 1365 (29%) | 4782 | 0.018 |
| Yes | 960 (68%) | 446 (32%) | 1406 | ||
| Missing | 46 (81%) | 11 (19%) | 57 | ||
| Intensive Care Unit Admission | No | 2247 (72%) | 887 (28%) | 3134 | < 0.0001 |
| Yes | 518 (79%) | 140 (21%) | 658 | ||
| Missing | 1658 (68%) | 795 (32%) | 2453 | ||
| Hospitalised for > 14 days | No | 2100 (74%) | 755 (26%) | 2855 | < 0.0001 |
| Yes | 847 (68%) | 392 (32%) | 1239 | ||
| Missing | 1476 (69%) | 675 (31%) | 2151 | ||
| Ethnicity | White | 3922 (71%) | 1576 (29%) | 466 | |
| Asian | 102 (69%) | 46 (31%) | 5498 | 0.004 | |
| Black | 34 (51%) | 33 (49%) | 148 | ||
| Other | 47 (71%) | 19 (29%) | 67 | ||
| Missing | 318 (68%) | 148 (32%) | 66 | ||
| Clinical Manifestations | Pneumonia | 3046 (73%) | 1118 (27%) | 4164 | < 0.0001 |
| Meningitis | 152 (63%) | 90 (37%) | 242 | ||
| Septicaemia/Bacteraemia | 279 (65%) | 150 (35%) | 429 | ||
| Others | 465 (66%) | 242 (34%) | 707 | ||
| Missing | 481 (68%) | 222 (32%) | 703 | ||
| General Practice Registration | < 2 yrs | 589 (66%) | 305 (34%) | 894 | 0.001 |
| 2 to < 5 yrs | 234 (75%) | 79 (25%) | 313 | ||
| ≥ 5 yrs | 3090 (72%) | 1220 (28%) | 4310 | ||
| Missing | 510 (70%) | 218 (30%) | 728 | ||
| Age Group at Vaccination | Unvaccinated | 1682 (76%) | 534 (24%) | 2216 | < 0.0001 |
| < 65 | 637 (74%) | 221 (26%) | 858 | ||
| 65–74 | 1315 (69%) | 597 (31%) | 1912 | ||
| 75 + | 598 (62%) | 372 (38%) | 970 | ||
| Yes & unknown time | 191 (66%) | 98 (34%) | 289 |
p-Value comparing case and control distribution for each factor by chi-squared test or Fisher's exact test.
Table 2.
Number of cases and controls by risk group, age group and time since vaccination as used in the vaccine effectiveness analyses.
| Time since vaccination | Risk group | Age |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 65–74 |
75–84 |
85 + |
All |
||||||
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | ||
| Unvaccinated | High risk immunocompetent | 355 | 75 | 233 | 87 | 191 | 86 | 779 | 248 |
| Immunocompromised | 193 | 83 | 97 | 47 | 65 | 32 | 355 | 162 | |
| None | 306 | 51 | 112 | 36 | 97 | 27 | 515 | 114 | |
| Missing | 17 | 4 | 5 | 3 | 11 | 3 | 33 | 10 | |
| All | 871 | 213 | 447 | 173 | 364 | 148 | 1682 | 534 | |
| 0 to < 2 years | High risk immunocompetent | 33 | 18 | 25 | 19 | 20 | 11 | 78 | 48 |
| Immunocompromised | 55 | 23 | 22 | 13 | 8 | 4 | 85 | 40 | |
| None | 22 | 8 | 5 | 1 | 4 | 3 | 31 | 12 | |
| Missing | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| All | 110 | 50 | 52 | 33 | 32 | 18 | 194 | 101 | |
| 2 to < 5 years | High risk immunocompetent | 86 | 23 | 27 | 13 | 18 | 9 | 131 | 45 |
| Immunocompromised | 63 | 34 | 17 | 11 | 6 | 1 | 86 | 46 | |
| None | 31 | 15 | 2 | 7 | 7 | 2 | 40 | 24 | |
| Missing | 1 | 0 | 1 | 0 | 1 | 2 | 3 | 2 | |
| All | 181 | 72 | 47 | 31 | 32 | 14 | 260 | 117 | |
| ≥ 5 years | High risk immunocompetent | 342 | 101 | 508 | 230 | 323 | 209 | 1173 | 540 |
| Immunocompromised | 192 | 70 | 282 | 150 | 112 | 66 | 586 | 286 | |
| None | 62 | 21 | 142 | 49 | 118 | 70 | 322 | 140 | |
| Missing | 3 | 2 | 8 | 2 | 4 | 2 | 15 | 6 | |
| All | 599 | 194 | 940 | 431 | 557 | 347 | 2096 | 972 | |
| Yes unknown time | High risk immunocompetent | 35 | 10 | 59 | 15 | 20 | 13 | 114 | 38 |
| Immunocompromised | 22 | 24 | 18 | 14 | 12 | 8 | 52 | 46 | |
| None | 8 | 6 | 13 | 5 | 3 | 2 | 24 | 13 | |
| Missing | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | |
| All | 66 | 40 | 90 | 35 | 35 | 23 | 191 | 98 | |
3.1.2. Vaccine Effectiveness Overall, by Age Group, Risk Group and Time Since Vaccination
Investigation of potential confounding factors showed that age-group, risk-group and year of infection changed the estimate by only a small amount (− 4%, − 2%, + 1%, respectively) while gender and ethnicity had no effect (< 1%). Therefore, adjusted estimates are presented with age-group, year of infection and risk-group adjustment. The overall crude VE estimate was 32% (95% CI, 24–40) and adjusted VE was 27% (95% CI, 17–35). The VE estimates by time since vaccination, age and risk group are summarised in Table 4; VE declined with time since vaccination, from 41% (95% CI, 23–54) for those vaccinated within two years to 34% (95% CI, 16–48) for those vaccinated within 2–4 years and 23% (95% CI, 12–32) for those vaccinated ≥ 5 years previously. Although the overall linear decline was not significant (p = 0.13), the longer term decline was best fitted with the spline model which indicates an initial drop from about 50% to a plateau after about 5 years at 20–25% (Fig. 1). The mean time since vaccination in this cohort was quite long at 9.55 years.
Table 4.
Overall adjusted vaccine effectiveness of PPV23 and estimates stratified by age at IPD, risk group and time since vaccination.
| Factor | Level | VE% (95% CI) | p-Value⁎ |
|---|---|---|---|
| All | – | 27 (17, 35) | |
| Age at IPD | 65–74 | 31 (16, 44) | 0.85 (0.22) |
| 75–84 | 17 (− 3, 32) | ||
| 85 + | 34 (17, 47) | ||
| Risk group | High risk immunocompetent | 25 (11, 37) | 0.050 |
| Immunocompromised | 13 (− 9, 30) | ||
| None | 45 (27, 59) | ||
| Time since vaccination | 0 to < 2 years | 41 (23 to 54) | 0.13 (0.23) |
| 2 to < 5 years | 34 (16 to 48) | ||
| ≥ 5 years | 23 (12 to 32) |
p-Value for difference in VE by strata and, in brackets the trend by age at IPD and time since vaccination.
Fig. 1.
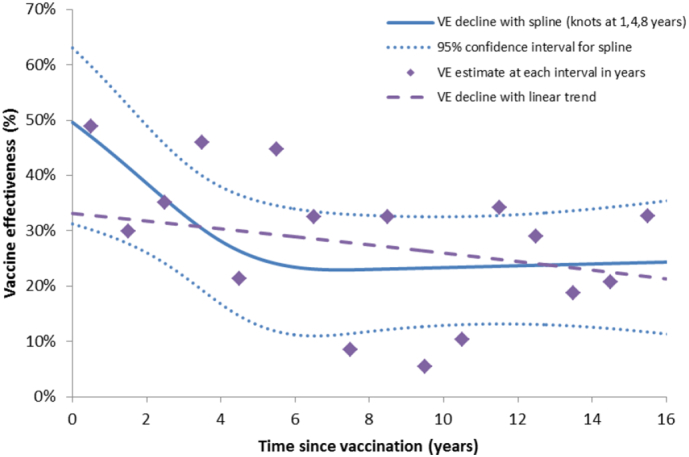
Vaccine effectiveness by time since vaccination using the spline model and a linear decline. Individual estimates for each year are also shown but are based on small numbers within each year.
When comparing VE between the three age-groups (Table 4), a higher VE was observed for 65–74 year-olds (31%; 95% CI, 16–44) and ≥ 85 years old (34%; 95% CI, 17–35), but lower among 75–84 year-olds (17%, 95% CI: − 3 to 32). This difference by age groups was not significant (p = 0.85) nor was a trend in VE by age significant (p = 0.22). Vaccine effectiveness by risk group achieved borderline significance (p = 0.05) and was highest in those without any risk factors (45%; 95% CI, 27–59) followed by the high-risk immunocompetent (25%; 95% CI, 11–37) and lowest in the immunocompromised (13%; 95% CI, − 9 to 30).
VE differed significantly (p = 0.005) by vaccine serotype (Supplementary Table 1) with no effectiveness seen for ST3 (2%; 95% CI, − 21 to 21) and high VE for 9 N (50%; 95% CI, 37–61). Although there was also low VE for ST 6B (− 10%; 95%CI, − 155 to 53) and 17F (2%, 95%CI, − 61 to 41) there was low precision for these serotypes. When ST3 was excluded from the analysis, there was no difference in VE across the remaining serotypes (p = 0.13). VE across age-time-risk strata varied from < 0 to 90%, but with wide confidence intervals (Table 3). For serotypes with sufficient numbers of cases, there was not a significant difference in waning by serotype. Within individual serotypes, too, there was limited power to assess waning. For the three major serotypes, VE (95% CI) at < 2 years, 2–4 years and ≥ 5 years for serotype 8 was 53% (27–70%), 40% (12–59%) and 18% (− 1–33%); for serotype 9 N, 69% (40–86%), 67% (41–82%) and 45% (29–58%); and for serotype 12F, 40% (− 1–65%), 37 (− 1–61%) and 24% (3–41%), respectively.
Table 3.
Adjusted vaccine effectiveness of PPV23 (% with 95% confidence interval) stratified by age at IPD, risk group and time since vaccination.
| Time since vaccination | Risk Group | Age at IPD |
||
|---|---|---|---|---|
| 65–74 |
75–84 |
85 + |
||
| VE% (95% CI) | VE% (95% CI) | VE% (95% CI) | ||
| 0 to < 2 years | High risk immunocompetent | 63 (30, 80) | 53 (9, 75) | 20 (− 75, 64) |
| Immunocompromised | 2 (− 72, 44) | 22 (− 70, 64) | − 10 (− 301, 70) | |
| None | 53 (− 12, 80) | − 65 (− 1434, 82) | 60 (− 92, 92) | |
| Any risk | 43 (17, 61) | 38 (1, 62) | 26 (− 36, 60) | |
| 2 to < 5 years | High risk immunocompetent | 21 (− 35, 54) | 20 (− 63, 61) | 3 (− 127, 58) |
| Immunocompromised | 20 (− 32, 51) | 30 (− 64, 70) | − 174 (− 2304, 69) | |
| None | 63 (26, 82) | 91 (51, 98) | 11 (− 375, 83) | |
| Any risk | 35 (11, 53) | 39 (0, 63) | 6 (− 81, 52) | |
| ≥ 5 years | High risk immunocompetent | 22 (− 11, 44) | 19 (− 9, 40) | 32 (7, 50) |
| Immunocompromised | − 8 (− 59, 27) | 12 (− 32, 41) | 14 (− 46, 49) | |
| None | 48 (2, 73) | 9 (− 52, 45) | 54 (22, 73) | |
| Any risk | 18 (− 3, 36) | 15 (− 6, 31) | 35 (18, 49) | |
The current PPV23 programme for ≥ 65 year-olds focuses on the healthy group who were not already eligible for the vaccine, based on risk status. In this age group (65–74 year-olds), the VE was 54%, 65% and 47% at < 2, 2–4 and ≥ 5 years since vaccination. VE against IPD cases that required intensive care admission was 34% (95% CI, 00–57) and 19% (95% CI, 07–31) against hospital admission for ≥ 14 days; VE was 25% (95% CI, − 38 to 59) against meningitis, 29% (95% CI, 17–40) against bacteraemic pneumonia and 28% (95% CI, − 12 to 53) against septicaemia, while VE against IPD death was 18% (95% CI, − 5 to 36).
3.1.3. Sensitivity Analysis
Table 5 shows the overall adjusted VE of PPV23 with vaccination status also defined by the age at which vaccination occurred and interval between vaccination and disease. The VE for vaccination among 65–74 year-olds (when most individuals received the vaccine) was 25% (95%CI, 13–35), irrespective of the interval between vaccination and disease, and 36% (95% CI, 17–50) for vaccination within the 5 years of IPD.
Table 5.
Adjusted vaccine effectiveness of PPV23 by age at vaccination according to timing of vaccination relative to IPD.
| Timing of vaccination relative to IPD within those vaccinated | Age at vaccination if vaccinated | VE% (95% CI) | p-Value⁎ |
|---|---|---|---|
| Any time before IPD | < 65 | 17 (− 01, 31) | 0.033 |
| 65–74 | 25 (13, 35) | ||
| 75 + | 35 (22, 46) | ||
| < 5 years before IPD | < 65 | 54 (24, 71) | 0.050 |
| 65–74 | 36 (17, 50) | ||
| 75 + | 30 (04, 48) | ||
| ≥ 5 years before IPD | < 65 | 12 (− 07, 28) | 0.027 |
| 65–74 | 20 (07, 32) | ||
| 75 + | 35 (20, 47) |
p-Value for difference in VE by age group.
VE against vaccine-related serotypes (Table 6) was 4% (95% CI, − 18 to 22), which justified including this group as part of the controls. Individuals who were registered at GP practices for ≥ 2 and ≥ 5 years, respectively, at the time of diagnosis were used as a proxy for certainty of their vaccination status. For the 4623 individuals registered for ≥ 2 years; the adjusted VE, for vaccination at any time, was 39% (95%CI, 29–48), and for the 4310 individuals registered for ≥ 5 years, the adjusted VE was 40% (95%CI, 30–49).
Table 6.
Adjusted vaccine effectiveness for vaccination at any time of PPV23 against vaccine related serotypes, IPD clinical manifestations, IPD severity, and against IPD when cases and controls are restricted to those registered at their GP for at least 2, 5 years.
| Any time and all ages | VE% (95% CI) |
|---|---|
| Vaccine related serotypes | 04 (− 18, 22) |
| Meningitis | 25 (− 38, 59) |
| Pneumonia | 29 (17, 40) |
| Septicaemia/Bacteraemia | 28 (− 12, 53) |
| ICU admission | 34 (00, 57) |
| Hospital stay > 14 days | 19 (07, 31) |
| Mortality ≤ 30 days | 18 (− 05, 36) |
| Registered at GP ≥ 2 years | 39 (29, 48) |
| Registered at GP ≥ 5 years | 40 (30, 50) |
3.2. Vaccine Impact Against IPD
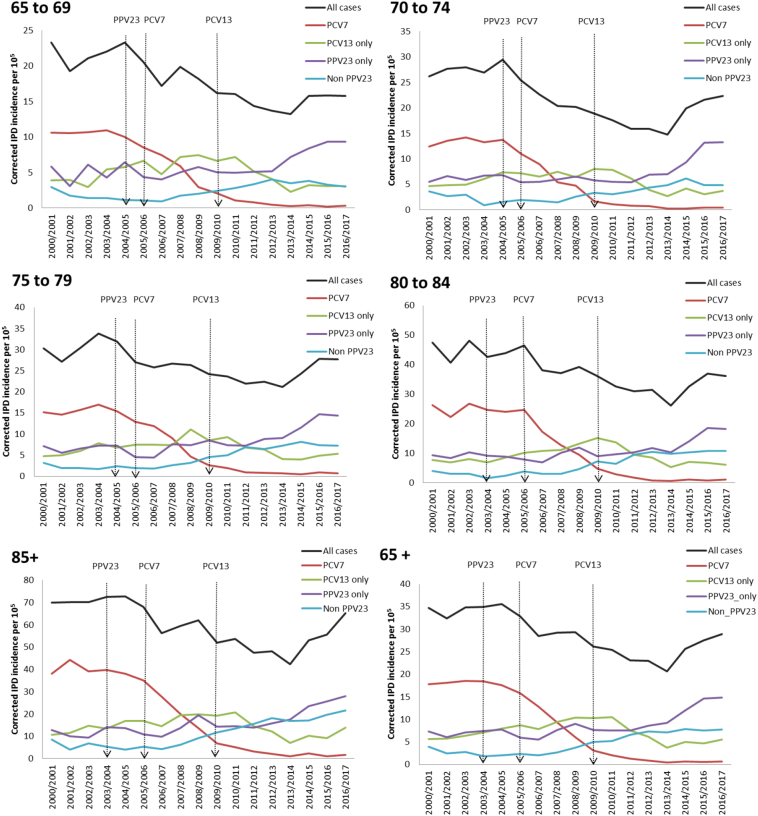
Between 2000/01 and 2016/17, there were 42,802 confirmed IPD cases in older adults, including 7600 PCV7-type IPD, 8675 cases due to the 6 additional PCV13 serotypes, 11,026 cases due to the 11 additional PPV23 serotypes, 6253 non-PPV23 IPD cases and 9248 cases where the serotype was not known. IPD incidence trends by age and serotype group are summarised in Fig. 2, and the incidence rate ratios in Table 7. In 2016/17, overall IPD incidence for ≥ 65 year-olds had dropped by 15% compared to the pre-PPV23 period. While IPD incidence increased sharply with age, the greatest proportional decline in overall IPD incidence was in 65 to 69 year-olds. During this period, there was a 96% decrease in PCV7-type IPD in all aged ≥ 65 years, 7% decrease in cases due to the additional PCV13 serotypes and a 117% increase in cases due to the additional PPV23 serotypes.
Fig. 2.
Corrected trends in the elderly IPD incidence in England and Wales from 2000/01 to 2016/17.
Table 7.
Incidence rate ratios for all IPD, PCV7, PCV13 only, PPV23 only and non-PPV23 in 2016/17 versus average 2000–03 baseline by age.
| Age | Serotype | 2000–03 corrected (raw) cases | 2000–03 incidence per 105 | 2016/17 corrected (raw) cases | 2016/17 incidence per 105 | IRR 2016/17: 2000–03 | 95% CI⁎ |
|---|---|---|---|---|---|---|---|
| 65–69 | All cases | 534 (351) | 21.20 | 397 (508) | 15.77 | 0.74 | 0.64–0.87 |
| PCV7 | 267 (89) | 10.60 | 8 (10) | 0.32 | 0.03 | 0.01–0.08 | |
| PCV13 only | 90 (30) | 3.58 | 78 (97) | 3.10 | 0.87 | 0.58–1.32 | |
| PPV23 only | 126 (42) | 4.99 | 235 (292) | 9.32 | 1.87 | 1.37–2.51 | |
| Non PPV23 | 51 (17) | 2.03 | 76 (95) | 3.03 | 1.49 | 0.93–2.50 | |
| 70–74 | All cases | 590 (434) | 27.29 | 483 (565) | 22.33 | 0.82 | 0.71–0.94 |
| PCV7 | 289 (107) | 13.39 | 10 (11) | 0.45 | 0.03 | 0.01–0.08 | |
| PCV13 only | 104 (39) | 4.84 | 81 (92) | 3.76 | 0.78 | 0.52–1.15 | |
| PPV23 only | 130 (48) | 5.99 | 288 (326) | 13.31 | 2.22 | 1.67–2.94 | |
| Non PPV23 | 66 (24) | 3.07 | 104 (118) | 4.82 | 1.57 | 1.03–2.41 | |
| 75–79 | All cases | 516 (545) | 29.30 | 489 (530) | 27.78 | 0.95 | 0.82–1.09 |
| PCV7 | 268 (137) | 15.20 | 13 (14) | 0.75 | 0.05 | 0.02–0.11 | |
| PCV13 only | 93 (48) | 5.28 | 94 (100) | 5.35 | 1.01 | 0.70–1.47 | |
| PPV23 only | 113 (58) | 6.45 | 254 (269) | 14.40 | 2.23 | 1.70–2.96 | |
| Non PPV23 | 42 (21) | 2.37 | 128 (136) | 7.28 | 3.07 | 2.01–4.75 | |
| 80–84 | All cases | 591 (513) | 45.43 | 471 (517) | 36.23 | 0.80 | 0.69–0.92 |
| PCV7 | 326 (130) | 25.09 | 14 (15) | 1.10 | 0.04 | 0.02–0.09 | |
| PCV13 only | 99 (39) | 7.59 | 80 (84) | 6.15 | 0.81 | 0.54–1.22 | |
| PPV23 only | 122 (49) | 9.38 | 236 (248) | 18.15 | 1.93 | 1.44–2.60 | |
| Non PPV23 | 44 (17) | 3.36 | 141 (148) | 10.83 | 3.22 | 2.05–5.12 | |
| ≥ 85 | All cases | 846 (700) | 70.06 | 786 (915) | 65.11 | 0.93 | 0.83–1.04 |
| PCV7 | 490 (194) | 40.56 | 21 (24) | 1.75 | 0.04 | 0.02–0.08 | |
| PCV13 only | 149 (59) | 12.32 | 167 (189) | 13.81 | 1.12 | 0.83–1.50 | |
| PPV23 only | 130 (51) | 10.73 | 338 (383) | 27.99 | 2.61 | 1.99–3.43 | |
| Non PPV23 | 78 (31) | 6.45 | 260 (295) | 21.56 | 3.34 | 2.38–4.67 | |
| ≥ 65 | All cases | 3043 (2543) | 34.01 | 2588 (3035) | 28.93 | 0.85 | 0.80–0.90 |
| PCV7 | 1624 (658) | 18.15 | 65 (74) | 0.73 | 0.04 | 0.03–0.06 | |
| PCV13 only | 530 (215) | 5.92 | 494 (562) | 5.52 | 0.93 | 0.79–1.10 | |
| PPV23 only | 614 (248) | 6.86 | 1334 (1518) | 14.91 | 2.17 | 1.92–2.47 | |
| Non PPV23 | 275 (111) | 3.08 | 696 (792) | 7.78 | 2.53 | 2.11–3.06 |
95% confidence interval inflated from a Poisson interval based on over-dispersion of 2.1 seen from modelling 2000–03 pre-PCV7 all invasive pneumococcal disease data.
During 2003–2006, post-PPV23 campaigns and prior to PCV introduction, Fig. 2 shows no clear evidence of an impact of these campaigns on IPD, with varying trends in PPV23 only, PCV13 and PCV7 serotypes (all of which are also in PPV23 except serotype 6A). Following PCV7 and PCV13 introduction in infants, herd effects led to large drops in IPD due to the serotypes in these two vaccines, as well as increases in serotype replacement disease, both among non-vaccine serotypes and, more recently, among the additional PPV23 serotypes. This means that, despite the reduction in IPD cases due to PPV23 serotypes also contained in PCV13, the additional PPV23 serotypes still form a large proportion of all IPD among ≥ 65 year-olds in 2016/17. The recent increases in replacement disease varied by age group, with overall IPD rates increasing particularly steeply in ≥ 85 year olds.
4. Discussion
In England and Wales, PPV23 was 27% effective against the vaccine serotypes causing IPD in older adults, irrespective of time since vaccination. This is a very important finding given the large changes in serotypes causing IPD in older adults since the introduction of the childhood pneumococcal conjugate vaccination programme more than a decade ago. PPV23 effectiveness declined from 41% for those vaccinated within two years to 34% for those vaccinated within 2–4 years and 23% for those vaccinated at least 5 years previously. Among ≥ 65 year-olds, vaccine effectiveness was higher in those who had no underlying medical conditions and in the younger age groups, although the associations with age were not significant. PPV23 provided no measurable protection against vaccine-related serotypes. In 2016/17, overall IPD incidence for ≥ 65 year-olds had dropped by 15% compared to the pre-PPV23 period, almost entirely because of reductions in IPD cases due to PCV7 and PCV13 serotypes that resulted from an indirect population impact of the childhood PCV7 and PCV13 programmes, respectively.
Our findings are similar to our previous analysis during 1998/99 to 2009/10, where we reported an overall VE of 24% which declined over time, from 48% within 2 years of vaccination to 15% after 5 years [6]. At that time, too, the only impact on IPD incidence among ≥ 65 year-olds was due to the childhood PCV7 immunisation programme, which was introduced in 2006. Our estimates are lower than a recent population-based case–control study of PPV23 VE against IPD among people over 60 years in Spain, which reported an adjusted VE of 72% (95% CI, 46–85) against overall IPD and 77% (95% CI, 40–92) against vaccine-type IPD [11]. In a recent meta-analysis, too, significant VE for PPV23 was reported against both IPD and pneumococcal pneumonia in the elderly; the pooled VE against IPD was 73% (95% CI, 10–92) in four clinical trials, 45% (95% CI, 15–65) in three cohort studies, and 59% (95% CI, 35–74) in three case–control studies [12]. It is likely that the higher vaccine effectiveness in some of the studies, especially clinical trials, may be due to a shorter follow-up periods compared to the longer observational studies, where waning immunity is likely to account for the lower overall effectiveness.
An important difference when compared to our previous analysis was the replacement of PCV7 with PCV13 in the childhood immunisation programme in April 2010, without any catch-up. This change resulted in a rapid and sustained decline in IPD due to the additional PCV13 serotypes, especially serotypes 7F and 19A, across all age groups through the direct and indirect protection offered by the programme. Consequently, overall IPD rates also declined during the first 4 years of the programme. Since 2013/14, however, this trend reversed, with acceleration in replacement disease due some of the non-PCV13 serotypes, especially those included in PPV23 [9]. In particular, serotypes 8, 9 N and 12F increased to such an extent – almost exclusively in adults and older adults – that they are currently responsible for 40% of all IPD in England and Wales [9]. It is, therefore, reassuring that PPV23 has a significant VE against these major replacing serotypes albeit for a limited duration. Currently, more than half the IPD cases in older adults are due to the additional PPV23 serotypes, making the health economics for this vaccine even more favorable, even with its limited individual-level effectiveness.
VE against serotypes that are common to both PPV23 and PCV13 show very wide confidence intervals because of relatively small numbers of cases. Notably, though, despite the successes of the childhood PCV13 programme, IPD cases due to serotype 3 have continued to increase, especially in adults and older adults, while cases due to serotype 19A are no longer declining and appear to be plateauing. The protection afforded by either PPV23 or PCV13 against serotype 3 IPD is likely to be very limited, although it is reassuring that PPV23 has a positive VE against serotype 19A IPD [9].
Despite individual-level PPV23 effectiveness in older adults, there was no clear impact at the population level of the routine use and catch-up campaigns in the target population during 2003–2006 (Fig. 2). This was assessed in more detail previously [6], with the conclusion that the expected impact based on incremental vaccine uptake and moderate vaccine effectiveness was small and, therefore, it was not surprising that no clear decreases were seen in IPD incidence. National surveillance does indicate recent increases in non-PCV13 IPD rates, especially among ≥ 85 year-olds, who had the highest disease incidence. As the population ages, these changes will lead to increasing numbers of IPD cases in the oldest populations. Given that VE remains positive in the older age groups, this does raise the question as to PPV23 might be more beneficial if given at an older age or if given at regular intervals within the ≥ 65 year age-group. This would require additional analyses [13], [14].
We have previously reported the strengths and limitations of this methodology [6]. A major strength remains the large numbers of confirmed IPD cases across all age groups identified through national surveillance over a period of almost two decades, which has allowed us to calculate VE with narrow confidence intervals, and stratify by age, risk group and time. The use of non-PPV23 type IPD cases as controls has the advantage of close matching in terms of underlying comorbidities, clinical characteristics, and other possible unmeasured confounders since the pneumococcal serotype in patients with IPD is only determined after the sample is submitted to the national reference laboratory. A limitation that affects interpretation of the lower VE in those with risk factors is that risk group status was reported at the time of IPD and not at the time of vaccination. It is likely that many will have developed risk factors after vaccination. Interpretation of the age effects and decline in VE by time since vaccination also requires caution due to high case fatality rates in the oldest age groups, meaning that those who survive may be those who also had higher and better vaccine responses. This is arguably not a bias but a feature of the population represented and would generate the decline to a plateau in VE by time since vaccination and the increased VE in the oldest age group.
One potential limitation is that we only had 64% questionnaire return rate from GPs and this response rate was lower in 2012 and 2013, when questionnaires were sent 2–3 years after the case occurred; also, questionnaires were less likely to be completed for fatal cases. However, it is very unlikely that there was any bias within the completed questionnaires in terms of vaccination status or the serotype responsible for IPD. Another limitation is that the GP may not know about PPV23 vaccination status if the vaccine was given outside their surgery or prior to the patient's enrolment to their surgery. This would misclassify some vaccinated patients as unvaccinated and slightly underestimate the VE. Another limitation is that some variables have a fairly high proportion of missing values. A further limitation of the study is that we only analysed invasive disease so VE estimates only apply to IPD and not to the larger population who experience non-invasive pneumococcal infections; although different studies have reported mixed results, there is some evidence that PPV23 protects against non-invasive pneumococcal infections, including non-bacteraemic pneumococcal pneumonia [10].
PPV23 continues to demonstrate moderate short-term effectiveness against IPD caused by the vaccine serotypes in older adults, but this was insufficiently high to have an impact on disease incidence at the population level following the campaigns in 2003–2005. Significant declines in IPD rates among older adults have been due to the indirect impact of PCV7 followed by PCV13 introduction into the childhood immunisation programme. The recent increase in IPD due to some of the additional PPV23 serotypes in adults, especially serotypes 8, 12F and 9N, for which significant VE were demonstrated with PPV23 is reassuring and could make the PPV23 programme for older adults even more cost-effective. Our findings should be generalisable to similar to other countries with established childhood PCV programmes and will help inform modelling and cost-effectiveness studies to assess whether changing the age at which PPV23 is offered and/or offering additional PPV23 doses to this vulnerable age group might help prevent more cases of IPD.
The following is the supplementary data related to this article.
Number of cases vaccinated and unvaccinated and adjusted vaccine effectiveness for vaccination at any time for the 4423 PPV23 ID cases by serotype.
Author Contributions
All authors were involved in the conception and design of the study and interpretation of the results. AD and NJA were responsible for the statistical analysis and wrote the first draft of the paper. RP was the project consultant and NJA was the project lead. SNL was the clinical lead for pneumococcal surveillance. NKF was the scientific lead for the national reference laboratory surveillance activities. All authors contributed to the data interpretation and read, commented on, and approved the final version of the report.
Declaration of Interests
SNL, CLS and NKF do contract research for vaccine manufacturers (including GlaxoSmithKline, Pfizer, and Sanofi Pasteur) on behalf of their employees, but receive no personal remuneration. The Immunisation, Hepatitis, and Blood Safety Department, where MER and SNL are employees, provides vaccine manufactures (including GlaxoSmithKline and Pfizer) with post-marketing surveillance reports on vaccine preventable diseases, including pneumococcal infections, which the companies are required to submit to the UK Licensing Authority in compliance with their Risk Management Strategy. A cost recovery charge is made for these reports. AD and NJA declare no competing interests.
Acknowledgements
We thank the staff involved with pneumococcal surveillance at Public Health England for the follow-up of invasive isolates and data collection for confirmed cases. We also thank the staff at hospital laboratories in England and Wales, who referred isolates for serotyping and provided additional information on request.
References
- 1.Department of Health . Chapter 25: pneumococcal. In: Salisbury D., Ramsay M., Noakes K., editors. Immunisation against infectious disease - ‘The Green Book’. 2006 updated ed. TSO (The Stationary Office); Norwich: 2013. pp. 295–313.https://www.gov.uk/government/publications/pneumococcal-the-green-book-chapter-25 [accessed Jan 10, 2018] [Google Scholar]
- 2.Public Health England Vaccine uptake guidance and the latest coverage data. 2017. https://www.gov.uk/government/publications/pneumococcal-polysaccharide-vaccine-ppv-vaccine-coverage-estimates
- 3.Miller E., Andrews N.J., Waight P.A., Slack M.P., George R.C. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–768. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
- 4.Waight P.A., Andrews N.J., Ladhani S.N., Sheppard C.L., Slack M.P., Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15:535–543. doi: 10.1016/S1473-3099(15)70044-7. [DOI] [PubMed] [Google Scholar]
- 5.Broome C.V., Facklam R.R., Fraser D.W. Pneumococcal disease after pneumococcal vaccination. An alternative method to estimate the efficacy of pneumococcal vaccine. N Engl J Med. 1980;303:549–552. doi: 10.1056/NEJM198009043031003. [DOI] [PubMed] [Google Scholar]
- 6.Andrews N.J., Waight P.A., George R.C., Slack M.P., Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012;30:6802–6808. doi: 10.1016/j.vaccine.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 7.JCVI Interim JCVI statement on adult pneumococcal vaccination in the UK. 2015. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/477966/JCVI_pnemococcal.pdf
- 8.Integrated Monitoring of Vaccines Effectiveness (I-MOVE+) in Europe Generic protocol for studies for vaccine effectiveness against Invasive Pneumococcal Disease and/or Pneumococcal Community Acquired Pneumonia using the indirect cohort method in the European Union/European Economic Area. 2015. http://www.i-moveplus.eu/wp3
- 9.Ladhani S.N., Collins S., Djennad A., Sheppard C.L., Borrow R., Fry N.K. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. 2018 doi: 10.1016/S1473-3099(18)30052-5. [published online Jan 25] [DOI] [PubMed] [Google Scholar]
- 10.Suzuki M., Dhoubhadel B.G., Ishifuji T., Yasunami M., Yaegashi M., Asoh N. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis. 2017;17:313–321. doi: 10.1016/S1473-3099(17)30049-X. [DOI] [PubMed] [Google Scholar]
- 11.Vila-Corcoles A., Ochoa-Gondar O., Guzman J.A. Effectiveness of the 23-valent polysaccharide pneumococcal vaccine against invasive pneumococcal disease in people 60 years or older. BMC Infect Dis. 2010;10:73. doi: 10.1186/1471-2334-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falkenhorst G., Remschmidt C., Harder T., Hummers-Pradier E., Wichmann O., Bogdan C. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine (PPV23) against pneumococcal disease in the elderly: systematic review and meta-analysis. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0169368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melegaro A., Edmunds W.J. The 23-valent pneumococcal polysaccharide vaccine. Part I. Efficacy of PPV in the elderly: a comparison of meta-analyses. Eur J Epidemiol. 2004;19:353–363. doi: 10.1023/b:ejep.0000024701.94769.98. [DOI] [PubMed] [Google Scholar]
- 14.Melegaro A., Edmunds W.J. The 23-valent pneumococcal polysaccharide vaccine. Part II. A cost-effectiveness analysis for invasive disease in the elderly in England and Wales. Eur J Epidemiol. 2004;19:365–375. doi: 10.1023/b:ejep.0000024752.48929.bd. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Number of cases vaccinated and unvaccinated and adjusted vaccine effectiveness for vaccination at any time for the 4423 PPV23 ID cases by serotype.