Abstract
Type II diabetes (T2D) has been identified as a major risk factor for the development of Alzheimer’s disease (AD). Interestingly, both AD and T2D have similar characteristics including amyloid peptide aggregation, decreased metabolism, and increased oxidative stress and inflammation. Despite their prevalence, therapies for these diseases are limited. To date, most therapies for AD have targeted amyloid-β or tau. Unfortunately, most of these clinical trials have been largely unsuccessful, creating a crucial need for novel therapies. A number of studies have shown that metabolic hormone therapies are effective at ameliorating high blood glucose levels in diabetics as well as improving cognitive function in AD and mild cognitive impairment patients. Pramlintide, a synthetic analogue of the pancreatic hormone amylin, has been developed and used for years now as a treatment for both type I diabetes and T2D due to the loss of β-islet cells responsible for producing amylin. Importantly, recent data demonstrates its potential therapeutic role for AD as well. This review aims at addressing parallels between T2D and AD at a pathological and functional level, focusing on amylin signaling as a key, overlapping mediator in both diseases. The potential therapeutic use of this hormone to treat AD will also be explored from a mechanistic viewpoint.
Keywords: Alzheimer’s disease, amylin, cognition, pramlintide, type II diabetes
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by the accumulation of two peptides, amyloid-β (Aβ) and hyperphosphorylated tau. The accumulation of these pathological peptides is thought to give rise to progressive functional deficits that range from short-term and spatial memory loss in the early stages of the disease and progressing to a complete loss of cognitive function, daily living functional skills, and severe psychiatric events. Though the incidence and fatality rate of many other diseases is decreasing, the incidence of AD and other dementias are on the rise, increasing approximately 71% from 2000 to 2013 [1]. As such, AD and other dementias are a surmounting problem throughout the world with an estimated 5.4 million Americans suffering from AD as of 2016 [2]. Furthermore, approximately 15 million family members and unpaid caregivers of AD patients invested approximately 18.1 billion hours of care toward AD patients and those afflicted with other dementias, a time commitment estimated to be worth $221 billion [1]. This figure does not include the actual cost paid by family members, Medicare, or Medicaid, which amounted to an estimated $236 billion in 2016 [1]. As such, AD presents a substantial financial burden to the medical community as well as the general population and is only predicted to increase in the coming years.
While there are a vast number of environmental and genetic factors that contribute to the development of AD, a large body of work has demonstrated that metabolic disease, particularly type II diabetes (T2D), is strongly associated with AD [3–6]. T2D is a metabolic disease characterized by chronic hyperglycemia that leads to hyperinsulinemia and hyperamylinemia [7]. Similar to AD, the prevalence of T2D has steadily risen over time, with an estimated 250 million cases of T2D worldwide as of 2010 [8]. Like AD, this number is expected to increase substantially over time. One in ten people suffer from T2D and approximately 70% of patients with T2D report cognitive decline and eventually develop AD [3–6]. Furthermore, individuals that have suffered from T2D for five or more years have an increased likelihood of develop AD compared to those diagnosed with the disease for less than five years [9]. As such, it is likely that the staggering number of T2D cases will further contribute to the incidence of AD. Though these two diseases are among the most prevalent in the U.S., present a substantial financial burden, and are both within the top ten leading causes of death, therapies for both diseases remain limited [10].
Currently, there are six drugs on the market approved by the Food and Drug Administration for the treatment of AD and other dementias, most of which are cholinesterase inhibitors that have varying efficacy depending on the patient [11, 12]. Incredibly, five of the current six approved drugs for the treatment of AD were developed prior to 2003, demonstrating our lack of ingenuity over the past 15 years. Since then, a staggering amount of drug trials have failed worldwide, demonstrating our crucial need for the development of new and innovative therapies for the treatment of AD [13]. Most of these trials have been aimed to reduce or eliminate Aβ pathology (70 of 146 compounds) and have been largely unsuccessful. A large number of neuroprotective compounds (63 of 146) have also entered various stages of clinical trials with little success and an even smaller number of therapies (13 of 146) targeting tau pathologies. As of 2012, the Food and Drug Administration approved a meager 0.4% of drugs that have entered clinical trials, marking AD therapeutics some of the least successful throughout clinical trials.
Unfortunately, AD is a multifactoral disease, receiving influence from both genetic and environmental factors [1]. For this reason, it seems unlikely that any one drug will be an absolute solution in treating AD. To this end, there is a vital need for novel theories to the innate etiology of AD as well as alternative approaches to treating the disease. As such, furthering our mechanistic understanding of environmental and disease related risk factors should lead to breakthroughs in therapeutics capable of treating or delaying the onset of disease. Given the well-established relationship between AD, T2D, and amyloid-related pathologies, this review will evaluate the use of T2D therapies in AD prevention and treatment. This review will seek to highlight the use of the pancreatic hormone amylin, currently used to treat diabetes, as a treatment for AD and discuss a potential mechanism through which we hypothesize recombinant non-aggregating amylin yields neuronal and cognitive benefits in AD.
ALZHEIMER’S DISEASE AS A MULTIFACTORAL DISEASE
AD is a complicated, multifactoral disease, with both environment and genetics contributing to the risk for the development of AD [14–19]. A number of genes have been associated with the development of AD, including mutations in APP and presenilin [20], trisomy 21 (Down Syndrome) [21–23], and the presence of the APOE ε4 allele [24–26]. Given that these genetic mutations and diseases lead to aberrant Aβ processing and deposition, the study of Aβ pathology has dominated the field for more than 40 years. However, while there is a clear association between these above-mentioned genes and early onset AD, the role of Aβ is less clear in sporadic AD. To date, we have failed to connect any single gene to most late onset AD cases, suggesting that late onset AD is a result of a combination of additive or synergistic pathological factors involving genetics, environment, and lifestyle [27].
The body of evidence suggesting that environmental and lifestyle factors contribute to AD is ever expanding. Important to this review, nutrition and metabolism-based lifestyle diseases such obesity and T2D contribute to the development of AD pathology [1, 16–19, 28, 29]. While conflicting evidence exists with regard to the relationship between obesity and AD, T2D remains one of the strongest correlates to the development of AD [3, 6]. Importantly, there are a number of similarities between AD and T2D: 1) both diseases are characterized by an over-all decrease in metabolic activity [30–34], 2) AD and T2D give rise to oxidative stress (OS), inflammation [16], and aberrant ion flux and signaling [35–39], and 3) both diseases have a pathological component that is directly related to an amyloids [40–42].
REDUCTIONS IN METABOLIC ACTIVITY IN T2D AND AD
T2D is a metabolic disease characterized by chronic hyperglycemia that leads to hyperinsulinemia and hyperamylinemia [7]. Chronic hyperinsulinemia and hyperamylinemia result in hormone resistance that eventually leads to decreased insulin and amylin production as well as β-islet cell loss [43, 44]. Insulin resistance results in poor glucose utilization and an overall decrease in systemic metabolism. Importantly, decreased cerebral glucose utilization is one of the first signs of cognitive impairment during aging. Extensive research has shown that there is impaired insulin transport across the blood-brain barrier (BBB) and reduced CNS insulin [45], decreased insulin receptor expression, decreased insulin-like growth factor expression, and an overall decrease in brain metabolism in both mild cognitive impairment (MCI) and AD patients [30–34]. Chronic hyperinsulinemia reduces surface insulin receptor (IR) expression in the BBB and thus reduces insulin transport into the brain [46, 47]. Decreased IR signaling results in a deficit in insulin receptor substrates, which has a negative impact on the PI3K and MAPK pathways, both of which are involved in synaptic plasticity and cellular survival responses [4, 48]. Reduced IR signaling within the brain also reduces neuronal glucose uptake, affecting mitochondrial metabolism and reactive oxygen species (ROS)/reactive nitrogen species (RNS) production [49, 50].
OXIDATIVE STRESS, INFLAMMATION, ABERRANT ION FLUX, AND SIGNALING IN AD AND T2D
ROS are naturally produced during cellular metabolism but can be significantly increased by environment/lifestyle and disease events such as T2D. The human brain is particularly susceptible to OS given its high requirement for oxygen, high levels of cellular respiration, high concentration of iron, ascorbate, unsaturated fatty acids, and high glucose metabolism [51–55]. The brain’s ability to combat OS is diminished during aging and is further exacerbated by lifestyle and disease [52–54]. Unchecked OS damage causes damage to proteins, lipids, and nucleic acids, leading to inflammation [54]. Importantly, mitochondria, the main source of ROS/RNS, are particularly vulnerable if ROS are not effectively quenched [56]. A number of mitochondria aberrations have been identified in the aging brain and AD, including changes in the expression of a number of mitochondrial enzymes, more specifically, pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and cytochrome c oxidase, all of which are involved in oxidative phosphorylation [57, 58]. Furthermore, decreased mitochondrial membrane potential, impaired protein transport to the mitochondria, increased ROS production, and increased mitochondrial fragmentation [59, 60] have also been noted in aging and AD, demonstrating a clear role of mitochondrial aberrations in AD.
Hormone resistance and glucose intolerance also contribute to mitochondrial dysfunction [61, 62]. While glucose stimulation allows for crucial physiological hyperpolarization of the mitochondrial membrane, glucose intolerance, and insulin resistance disrupt the mitochondrial membrane potential by increasing uncoupling, decreasing ATP production, and facilitating ROS production [63–66].
Redox-active iron mediated OS within the brain is also closely associated with the formation of AD related neuritic plaques and neurofibrillary tangles [67]. These, in turn, have been linked to increased OS [68–70], neuroinflammation derived from oxidative insults and pathology, decreases in long-term potentiation (LTP), the molecular mechanism for learning and memory, [37, 38], and neuronal loss [15].
Sufficient oxidative damage gives rise to inflammation and is a well-established component of AD. Inflammation has a highly dynamic role in AD and can arise from OS [71], Aβ, or neurofibrillary tangle production, and even precede overt hallmark AD pathology [72]. As such, Aβ production and plaque formation cause an inflammatory reaction that is mediated by activated astrocytes and microglia. Microglial and astrocytic activation leads to the secretion of a number of pro-inflammatory cytokines, including interleukin-1β (IL-1β), IL-6, IL-18, tumor necrosis factor-α, and NFκB [72–74]. The secretion of these pro-inflammatory cytokines results in further astrogliosis, microglial activation, ROS, and amyloid production, ultimately contributing further to AD pathology. More recently, genome-wide association studies have identified a number of microglial genes are associated with AD, particularly PLCG2, ABI3, CR1, CD33, and TREM2 [75–77] suggesting that neuroinflammatory events may be both driven by oxidative damage and pathology but also potentially trigger these pathological events.
OS is also a clear component of T2D. T2D is commonly preceded by a long period of prediabetes, lasting approximately seven to ten years. During this period, hyperglycemia gives rise to OS within β-cells of the pancreas and is thought to precede β-cell loss [78]. Pre-diabetic OS leads to impairments in homeostatic repair [79], mitophagy, and mitochondrial function [80]. Mitochondrial dysfunction further propagates the diabetic phenotype, giving rise to increased insulin resistance. Furthermore, increased fat mass and decreased glucose metabolism in T2D necessitate increased utilization of β-oxidation. High levels of β-oxidation in T2D leads to increased ROS production and uncoupling as well as decreased ATP production [81]. While OS is a prominent component of T2D disease progression, the role of inflammation is less clear. Evidence suggests that several inflammatory cytokines, including IL-1, IL-6, and c-reactive protein may play a role in the progression of T2D [82–84]. Thus, a causal link between T2D and AD may be, at least partially, related to the ability of T2D to drive pathological events (OS and inflammation) known to directly trigger AD.
RELEVANCE OF AMYLOID PATHOLOGY IN T2D AND AD
An amyloid, by definition, is an extracellular proteinaceous aggregate exhibiting β-sheet structure. Amyloids also possess unique structural properties that allow other homologous proteins as well as structurally similar proteins to aggregate with one another. As discussed previously, the overproduction of Aβ and subsequent aggregation is thought to be one of the main pathological events in AD and its pathology the hallmark of this disease [85]. Interestingly, the aggregation of another amyloid, amylin, is closely associated with pancreatic β-cell death and thus is intimately linked to T2D [40–42]. Importantly, there is mounting evidence demonstrating that amylin and Aβ play a role in the disease progression of T2D and AD. Thus, understanding the role of amylin pathogenesis and how these two amyloids interact may yield important advances for both diseases.
As previously mentioned, there is an intimate relationship between Aβ and metabolic function, particularly through the action of insulin. Interestingly, the chief enzyme responsible for degrading insulin, insulin degrading enzyme (IDE), is also capable of metabolizing a number of other amyloid forming proteins, including calcitonin, glucagon, and Aβ [86, 87]. In the periphery, IDE is largely expressed in the liver and kidneys but is also present within the brain. Importantly, IDE expression is closely associated with the APOE ε4 allele and is reduced approximately 50% in those possessing this allele. Reductions in IDE expression give rise to the accumulation of Aβ, which is associated with a number of toxic functions. Insulin signaling is suggested to reduce the production of Aβ by increasing cleavage of AβPP by a-secretases and reducing cleavage by β-secretases [88]. Furthermore, extracellular Aβ within the brain results in internalization of the IR and contributes to CNS insulin resistance [89, 90]. Activation of the insulin signaling cascade is also crucial to the inactivation of glycogen synthase kinase-3β, which is intimately associated with tau phosphorylation [91]. Thus, impaired insulin signaling results in a failure to properly activate Akt, which is thus unable to inhibit glycogen synthase kinase-3β activity, resulting in the hyperphosphorylation of tau. As such, insulin signaling is crucial to physiological cerebral function and the prevention of neurodegenerative pathological features. Furthermore, production and signaling of the pancreatic hormone amylin is also deregulated in both T2D and AD.
Amylin, also known as islet amyloid polypeptide, is a 37-amino acid (Table 1) metabolic hormone produced by β-islet cells of the pancreas that is co-secreted with insulin and helps to regulate glucose homeostasis throughout the body by modulating glucagon production and gastrointestinal emptying [92, 93]. On a macro level, amylin helps to reduce glucose absorption within the gastrointestinal tract while simultaneously decreasing glucose release from the liver, which effectively lowers the systemic insulin demand [93, 94].
Table 1.
There are a number of compounds known to interact with the amylin receptor. The sequences of human amylin, pramlintide, and rat amylin are very similar, but differ slightly, allowing for differences in secondary structure properties
| Compound | Amino Acid Sequence |
|---|---|
| Human Amylin | KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY |
| Rat Amylin | KCNTATCATQRLANFLVRSSNNLGPVLPPTNVGSNTY |
| Pramlintide (Symlin) | KCNTATCATQRLANFLVHSSNNFGPILPPTNVGSNTY |
| AC187 | VLGKLSQELHKLQTYPRTNTGSNTY |
| AC253 | LGRLSQELHRLQTYPRTNTGSNTY |
The sequence of the two most common synthetic amylin receptor antagonists are also provided. Though AC187 and AC253 are extremely similar to one another, they differ significantly from endogenous amylins.
Amylin signals through the amylin receptor (AMYR) which is a heterodimeric receptor composed of the calcitonin receptor and a receptor-activity modifying protein (RAMP) 1, 2 or 3 [95]. While amylin can bind to calcitonin receptor itself, the addition of RAMP 1–3 vastly increases the binding affinity. Though amylin was originally studied as a glucose homeostatic agent in the periphery, it was later discovered that amylin readily crosses the BBB [96–98], leading to further inquiry as to receptor localization throughout the brain. While the AMYR was originally characterized in brain regions associated with glucose homeostasis and satiety, it was later discovered that the AMYR was expression in regions related to cognition, most importantly, the hippocampus and cortex [93, 99].
Though the signaling capabilities of the various AMYR have not been fully characterized in the CNS, peripherally, amylin modulates downstream targets of the insulin regulatory pathway such as STAT3, AMPK, and Akt [1, 100]. Amylin also modulates ERK and MAPK signaling cascades in various tissues; within the hippocampus, these cascades are known to modulate synaptic plasticity [1, 100, 101]. Currently, evidence suggests that AMYR3 is the main amylin receptor that functions in the brain and is the primary target of Aβ and pramilintide, discussed later in this review [102]. Given the presence of the AMYR in areas associated with cognition and amylin’s signaling capabilities in other tissues, it is likely that amylin signaling is involved in normal CNS functioning.
In the pathological state of T2D, hyperglycemia and β-cell dysfunction/death arise from hyperinsulinemia, hyperamylinemia, and subsequent hormone resistance [103]. More than 95% of T2D patients accumulate amylin derived amyloid fibrils around β-islet cells of the pancreas [40–42]. Importantly, a number of studies have also linked amylin to AD. The amino acid sequences of amylin and Aβ are similar but not identical. Because of their structural similarities, Aβ and human amlyin form similar β-sheet structures [39]. Interestingly, an in vitro study of human amylin demonstrated that amylin begins to fibrillize in as little time as 24 hours [76]. Though the half-life of amylin is more in the scope of minutes, chronic, high production of amylin easily facilitates fibrillization [93, 104]. Furthermore, amylin-derived amyloid deposits within the pancreas are associated with unregulated Ca2+ flux, which disrupts β-cell functionality, gives rise to OS, and eventually leads to cytotoxicity and cell loss [35, 36, 39].
Importantly, several studies have shown that amylin is also capable of accumulating within the brain [29, 105, 106]. Evaluation of human brains has shown that amylin oligomers and plaques are found in the cortex of T2D and AD patients. AD patients were found to have plaques with various compositions: Aβ only plaques, amylin only plaques, and the occasional mixed plaque (both amylin and Aβ) [105, 106]. Many of these plaques were also seen in the parenchyma and vasculature of both AD and T2D brains and were accompanied by markers of OS as well as inflammation [106]. Supporting this evidence in humans, rodent models expressing human amylin also show amylin plaques within the brain and amylin deposits were associated with increased 4-HNE, a marker for lipid peroxidation, as well as the pro-inflammatory cytokine IL-1β [29, 106].
Because amylin aggregates within the pancreas of virtually all T2D patients, causing cellular dysfunction and is also BBB permeable, it was originally thought that amylin might have similar properties and effects within the brain. As such, a number of studies were carried out to evaluate amylin’s electrophysiological signaling capabilities within the brain. AMYR3 has continually been shown to be the major signaling receptor within the brain [37, 38, 102, 107, 108]. Furthermore, Aβ and human amylin are capable of signaling through AMYR3 to modulate several signaling cascades, including PKA, MAPK, Akt, and cFos [102]. At lower concentrations, amylin and Aβ modulate these signaling cascades, but at higher concentrations both human amylin and Aβ have been shown to disrupt LTP. Additionally, pretreatment with the AMYR antagonist AC253 or PRAM attenuates these Aβ and human amylin induced deficits in LTP providing strong evidence that these two peptides are affecting circuitry within the brain.
Taken together, these data suggest that both Aβ and amylin have physiological properties important for brain circuitry. However, the aggregation of human amylin in T2D patients and evidence of the ability of Aβ to signal through amylin receptors also suggests a potential common mode of pathological action.
THEORY OF PATHOLOGY: LOSS OF NATIVE FUNCTION VERSUS AGGREGATION
There is currently a debate within the field with regard to the role of amylin in the disease initiation and progression of AD. While there is strong evidence that amylin fibrils within the pancreas of T2D patients are physiologically disruptive and eventually toxic, evidence for the role of amylin in AD remains controversial. Because amylin is over produced in T2D and is capable of crossing the BBB and aggregating, it is postulated that amylin is detrimental to the system and that blocking its toxic function is beneficial [29, 36, 105, 106].
On the other hand, there is also a body of evidence that suggests that there is a loss of native amylin signaling in the diabetic and AD brain. While aggregation may be problematic, it is the actual loss of native amylin signaling that is the root cause of dysfunction in the T2D and AD brains. As such, this theory of a loss of native function calls for a replacement of this lost signaling with either functional human amylin or a non-aggregating amylin analogue. Pramlintide (PRAM) is a synthetic analogue of the pancreatic hormone amylin. The sequence of PRAM differs by only three amino acids from that of human amylin but these key amino acids changes prevent PRAM from aggregating, while also maintaining its signaling capabilities. Evidence of the loss of native function is demonstrated by the fact that PRAM is currently used as an adjunct treatment for diabetics to improve metabolic health [109, 110]. PRAM has also been studied in animal models of AD, demonstrating a number of therapeutic effects in reducing hall-mark AD pathology while simultaneously improving cognition [111–114].
PRAM is approved by the Food and Drug Administration as a treatment for diabetes [115]. When administered in conjunction with insulin, PRAM improves diabetic hbA1C, a measure of long-term blood glucose levels, most likely by decreasing post-prandial glucose levels [94, 109, 110, 116–118]. Over the course of either 16 or 52 weeks obese participants lost an average of 5 lbs and obese/diabetic participants lost an average of 5.5 lbs [109, 110]. Clinical data also suggests that PRAM reduces serum markers of OS in diabetic patients [119]; PRAM treated patients had significantly reduced serum excursions of nitrotyrosine and decreased oxidized low-density lipoproteins, indicating that PRAM exhibits some anti-oxidant properties.
To date, no human studies that have utilized PRAM as a treatment or preventative measure for AD. However, a growing body of in vivo rodent work from our laboratory and others demonstrate that amylin replacement may be a viable preventative treatment for AD. Relatedly, plasma amylin levels were reduced in both AD and MCI patients compared to cognitively intact controls [111]. In a separate study, plasma amylin levels were also surveyed in diabetics versus nondiabetics and plasma amylin levels positively correlated with cognitive improvements in diabetics compared to nondiabetics [111, 120]. Interestingly, plasma amylin levels are also positively correlated with better cognitive function in the elderly in the absence of diabetes. Together, these studies suggest that low plasma amylin levels are associated with late-stage T2D, MCI, and AD and cognitive impairment. As such, it is important to consider the temporal course of pathological events in both T2D and AD. While prediabetes and early T2D are characterized by hyperamylinemia that contributes to the initial amylin-derived amyloid deposition, there is a stark reversal to hypophysiological amylin levels in late stage T2D and aging. Thus, regardless of an early or late disease state, there is a reduction in the availability of functional amylin in T2D and thus an overall loss of native amylin function that gives rise to cognitive impairment and AD-related pathology. As such, administration of functional amylin should improve function and reduce pathology.
AMYLIN AND PRAMLINTIDE MODULATION OF NEURONAL PLASTICITY
Human amylin, Aβ, and PRAM are all capable of interacting with the AMYR3 in the hippocampus and cortex to modulate LTP [37, 38]. Both human amylin and Aβ cause decreases in LTP in AD transgenic mouse hippocampal slice cultures. Pretreatment with PRAM, however, is able to block this decrease. Conversely, Gingell et al. demonstrated that along with having varying affinities for each AMYR human amylin, rat amylin, and PRAM were all capable of increasing cAMP in two separate in vitro models while Aβ1–42 was unable to induce any such change [121]. While it is unclear why human amylin, Aβ, and PRAM modulate LTP and cAMP differently, it is likely that aggregation plays a role. It is also possible that PRAM mediates its protective effects against human amylin and Aβ by removing these substances from the system, as suggested in Zhu et al., though this mechanism would not be present in vitro [113].
In addition to blocking the toxic effects of human amylin and Aβ on LTP, PRAM increases the expression proteins associated with synaptic plasticity and cognition. PRAM treated SAMP8 mice showed increased levels of the synaptic marker synapsin I as well as an improved recognition index in the Novel Object Recognition Task [111]. Human amylin was also shown to modulate Akt and ERK signaling as well as increase the expression of cFos [102]. Taken together, these data suggest that amylin signaling is capable of modulating markers of synaptic plasticity [102, 111], signaling cascades associated with synaptic plasticity [100, 102], and LTP [37, 38] which suggests that amylin has an innate role in healthy cognition.
AMYLIN AND PRAMLINTIDE MODULATION OF AD PATHOLOGY
PRAM and human amylin treatment have been shown to consistently reduce Aβ plaque burden in a number of rodent models of AD [113, 114]. 5XFAD mice treated chronically with intraperitoneal (IP) amylin or PRAM showed a marked reduction in dense-cored plaque burden, Aβ plaque size, and soluble Aβ1–42 throughout the brain [113]. Interestingly, Aβ1–42 levels were increased in the cerebrospinal fluid compared to the brain, suggesting that amylin treatment may assist in the transport of soluble Aβ out of the brain as an excretory measure. This work was further expanded as 5XFAD mice were treated with vehicle, amylin, or amylin + AC253 for 10 weeks via IP injection [114]. Importantly, amylin + AC253 treated mice showed significantly increased plaque burden compared to amylin treated controls, demonstrating a direct therapeutic effect of amylin on plaque burden. All reductions in amyloid pathology were accompanied by increased cognitive function [113, 114].
Evidence from multiple sources has also shown that amylin treatment reduces neuroinflammation and OS [111, 112]. SAMP8 mice treated chronically with peripheral PRAM showed reduced levels of HO-1, a marker of OS, in the hippocampus compared to controls [111]. PRAM treated SAMP8 mice also showed a decrease in cyclooxygenase-2 expression in the hippocampus compared to untreated controls. Similarly, 5XFAD mice treated chronically with IP rodent amylin showed decreased microglial activation in the cortex via CD68 and Iba-1 expression [112]. In vitro data from this study suggests that the effects on CD68 expression are mediated through AMYR3, as knockdown of RAMP3 diminished amylin’s effects in BV-2 cells, a microglial model. Wang et al. [112] also report changes in the expression of several mitochondrial genes between AD mice and controls. Interestingly, amylin treatment almost completely returned this gene expression to levels seen in control mice.
While the majority of research utilizing amylin or PRAM as a treatment for AD has focused on Aβ pathology and neuroinflammation, recent evidence suggests that amylin treatment is also capable of modulating tau phosphorylation. New evidence now suggests that peripheral treatment with human amylin reduces tau pathology in the 3xTg mouse model of AD [114]. 10-week, daily IP injection of amylin was capable of reducing tau phosphorylation via the CDK-5 and p35/25 pathway. Interestingly, modulation of the CDK-5 pathway with amylin treatment was also seen in Alder et al. [111], though Adler et al. [111] reported an increase in total CDK-5 expression with no change in p35/25 whereas Zhu et al. [114] reported a change in the expression of p35/25 and no change in CDK-5 expression. It is, however, important to note that these studies were conducted in different models of aging/AD. Data from these separate labs do seem to consistently suggest the involvement of CDK-5 pathway with amylin/PRAM treatment. Though research that utilizes amylin/PRAM as a treatment for AD is limited, the major studies appear to have similar results, even across rodent lines.
DISCUSSION
There is evidence supporting a role of amylin in the disease progression of T2D and AD but also evidence that amylin and its analogue PRAM are therapeutic in the treatment of T2D and AD. Epidemiological studies demonstrating a positive relationship between plasma amylin and cognition coupled with in vivo rodent studies demonstrating the therapeutic effects of both amylin and PRAM on cognition, Aβ pathology, OS, and inflammation in AD transgenic models all come together to support the idea that there is a loss of native amylin function in both T2D and AD. While there is also clear evidence that amylin and Aβ aggregate in the brain and the pancreas [29, 36, 105, 106], that the resulting amyloids cause cellular distress ranging from aberrant ion flux [36], impaired mitochondrial function, OS, and inflammation [106] we assert that these pathological phenomena arise due to the temporal progression of T2D as outlined in Lutz et al. [122]. As serum amylin levels rise during the early stages of diabetes, amylin is easily able to cross the BBB and aggregate as serum concentrations increase. Synonymously, high extracellular concentrations of amylin within the pancreas readily aggregate. Importantly, though amylin levels may appear to be high at these points and should coincide with improvements in cognition based on Adler et al. and Qiu et al., there may be paradoxical effects wherein amylin forms fibrils and fails to perform its native function [111, 123, 124].
The electrophysiological data regarding amylin, Aβ, and PRAM becomes difficult to interpret in the light of epidemiological data and in vivo data. Several studies clearly demonstrate that low dose (50 nM) human amylin or Aβ reduces the slope of EPSPs in hippocampal slice cultures in slices of AD transgenic models while PRAM also demonstrated no direct benefit on its own [38]. Kimura et al. hypothesized that the benefits of PRAM were related to the ability of this compound to block the effects of human amylin and Aβ in a similar fashion as the receptor antagonists AC187 and AC253. While this is a possibility, the fact that AC253 administration blocked the benefits of amylin and PRAM in vivo [114] suggests this may not be the case. As such, there seems to be a discrepancy between in vivo and ex vivo paradigms, suggesting that an intact in vivo system may be necessary to recapitulate the therapeutic effects of amylin and PRAM. Importantly, Gingell et al. [121] demonstrated conflicted evidence to Fu et al. [102], demonstrating that Aβ was not capable of activating the CALCR or any AMYR. Zhu et al. [114] also showed that use of the AMYR antagonist AC253 blocked the therapeutic effects of human amylin and most importantly did not pose any benefit to animals when administered alone.
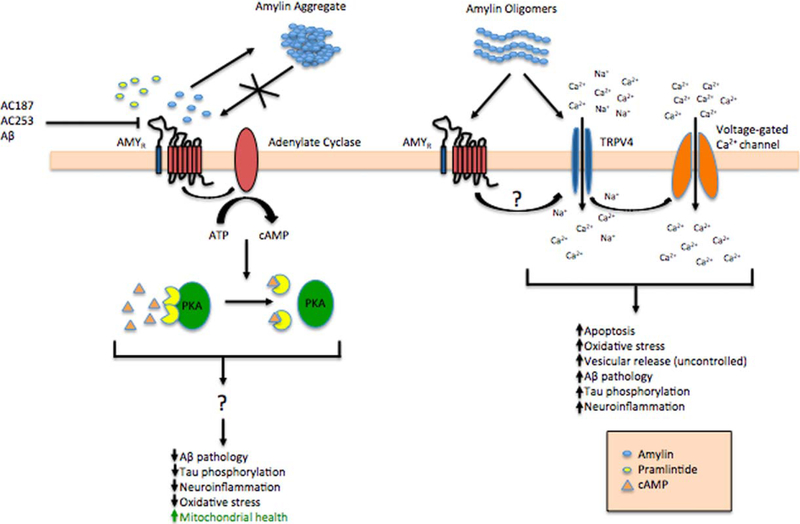
Interestingly, a novel study suggests that low doses of human amylin elicit small Ca2+ influx in a small number of neurons. As the concentration is increased the number of responsive neurons increases as well as the magnitude of the Ca2+ influx [76]. Importantly, low dose human amylin signals mostly through its native receptor. At higher doses (>1 µM), however, a greater number of neurons are activated and may also utilize a second receptor type, the TRPV4 receptor. This finding could help to demonstrate how higher concentrations of amylin and Aβ are able to induce cellular dysfunction. It is possible that the TRPV4 receptor mediates the toxic effects of amyloids through aberrant Ca2+ influx (Fig. 1). Future studies that take electrophysiological readings from animals that were chronically treated with human amylin or PRAM could prove to be extremely informative in determining how human amylin and PRAM affect LTP in vivo.
Fig. 1.
Soluble amylin and pramlintide are capable of activating the endogenous AMYR which is responsible for recruiting adenylate cyclase. Activation of adenylate cyclase results in the production of cAMP and the release and activation of PKA. This canonical amylin signaling pathway is what is hypothesized to give rise to the beneficial effects seen by amylin signaling. However, at higher concentrations amylin forms oligomers and eventually aggregate plaques, effectively removing signaling capable amylin. Amylin plaques are unable to signal through the endogenous AMYR but amylin oligomers are thought to interact with the AMYR and/or the TRPV4 receptor to initially depolarize the membrane through the influx of Na+ and Ca2+. This initial depolarization allows voltage-gated Ca2+ to reach threshold and results in further Ca2+ influx. Uncontrolled Ca2+ influx is severely detrimental and also occurs in β-cells of the pancreas and is attributed to late stage β-cell loss in T2D.
It is important to note that although human amylin has shown promise in rodent studies, it may not be a translatable therapy in humans. Historically, AD research has shown that interventions must be commenced prior to the development of pathology. As such, amylin or PRAM treatment for diabetics would theoretically need to be started early in the T2D disease course to prevent the accumulation of AD pathology. While replacing amylin signaling with human amylin in the rodent system poses benefits, adding human amylin to a system where aggregation may already be an issue will only exacerbate amylin derived plaque deposition in the pancreas and brain. To this end, PRAM holds a higher therapeutic potential in humans than simply replacing human amylin in T2D. This idea, however, does not detract from the fact that improving amylin signaling in vivo shows promise in slowing the progression of AD.
To date, in vivo studies utilizing human amylin and PRAM show promise as a preventative measure in the treatment of AD. It has yet to be determined, however, if amylin interventions induce their benefits by improving metabolic tone or through direct CNS intervention. It is also unclear whether amylin is capable of improving brain health in rodent models of diabetes and obesity or in rodent models of AD exposed to high fat diets. Additional studies are needed to determine how robust and potent amylin is at preventing T2D and AD related pathology. Furthermore, additional studies are needed to verify the mechanistic nature of amylin and PRAM utilizing CNS administered amylin receptor inhibitors as well as in vivo knockdown of AMYR. These aspects together with an in depth study of divergent signaling mechanisms between Aβ, amylin, and PRAM and receptor subtype signaling are needed to further elucidate the mechanisms underlying the benefits of PRAM and develop more optimally targeted therapies.
Footnotes
DISCLOSURE STATEMENT
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0433r1).
REFERENCES
- [1].Alzheimer’s Association (2016) 2016 Alzheimer’s disease facts and figures. Alzheimers Dement 12, 459–509. [DOI] [PubMed] [Google Scholar]
- [2].Hebert LE, Weuve J, Scherr PA, Evans DA (2013) Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80, 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ott A, Stolk R, Van Harskamp F, Pols H, Hofman A, Breteler M (1999) Diabetes mellitus and the risk of dementia The Rotterdam Study. Neurology 53, 1937–1937. [DOI] [PubMed] [Google Scholar]
- [4].Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB (2011) Diabetes mellitus and Alzheimer’s disease: Shared pathology and treatment? Br J Clin Pharmacol 71, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA (2014) Dementia and cognitive decline in type 2 diabetes and prediabetic stages: Towards targeted interventions. Lancet Diabetes Endocrinol 2, 246–255. [DOI] [PubMed] [Google Scholar]
- [6].Dash SK (2013) Cognitive impairment and diabetes. Recent Pat Endocr Metab Immune Drug Discov 7, 155–165. [DOI] [PubMed] [Google Scholar]
- [7].DeFronzo RA, Bonadonna RC, Ferrannini E (1992) Pathogenesis of NIDDM: A balanced overview. Diabetes Care 15, 318–368. [DOI] [PubMed] [Google Scholar]
- [8].Ginter E, Simko V (2012) Global prevalence and future of diabetes mellitus. Adv Exp Med Biol 771, 35–41. [DOI] [PubMed] [Google Scholar]
- [9].Leibson CL, Rocca WA, Hanson V, Cha R, Kokmen E, O’brien P, Palumbo P (1997) Risk of dementia among persons with diabetes mellitus: A population-based cohort study. Am J Epidemiol 145, 301–308. [DOI] [PubMed] [Google Scholar]
- [10].Murphy SL, Kochanek KD, Xu J, Heron M (2015) Deaths: Final data for 2012. Natl Vital Stat Rep 63, 1–117. [PubMed] [Google Scholar]
- [11].Hyde C, Peters J, Bond M, Rogers G, Hoyle M, Anderson R, Jeffreys M, Davis S, Thokala P, Moxham T (2012) Evolution of the evidence on the effectiveness and cost-effectiveness of acetylcholinesterase inhibitors and memantine for Alzheimer’s disease: Systematic review and economic model. Age Ageing 42, 14–20. [DOI] [PubMed] [Google Scholar]
- [12].Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, Burns A, Dening T, Findlay D, Holmes C (2012) Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 366, 893–903. [DOI] [PubMed] [Google Scholar]
- [13].Cummings JL, Morstorf T, Zhong K (2014) Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimers Res Ther 6, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Evans JL, Goldfine ID, Maddux BA, Grodsky GM (2003) Are oxidative stress–activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes 52, 1–8. [DOI] [PubMed] [Google Scholar]
- [15].LaFerla FM, Oddo S (2005) Alzheimer’s disease: Aβ, tau and synaptic dysfunction. Trends Mol Med 11, 170–176. [DOI] [PubMed] [Google Scholar]
- [16].Butterfield DA, Di Domenico F, Barone E (2014) Elevated risk of type 2 diabetes for development of Alzheimer disease: A key role for oxidative stress in brain. Biochim Biophys Acta 1842, 1693–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Whitmer R, Gustafson D, Barrett-Connor E, Haan M, Gunderson E, Yaffe K (2008) Central obesity and increased risk of dementia more than three decades later. Neurology 71, 1057–1064. [DOI] [PubMed] [Google Scholar]
- [18].Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Yaffe K (2005) Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. BMJ 330, 1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Whitmer RA, Gunderson EP, Quesenberry CP, Zhou J, Yaffe K (2007) Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res 4, 103–109. [DOI] [PubMed] [Google Scholar]
- [20].Goldman JS, Hahn SE, Catania JW, Larusse-Eckert S, But-son MB, Rumbaugh M, Strecker MN, Roberts JS, Burke W, Mayeux R (2011) Genetic counseling and testing for Alzheimer disease: Joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet Med 13, 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lott IT, Dierssen M (2010) Cognitive deficits and associated neurological complications in individuals with Down’s syndrome. Lancet Neurol 9, 623–633. [DOI] [PubMed] [Google Scholar]
- [22].McCarron M, Gill M, McCallion P, Begley C (2005) Health co-morbidities in ageing persons with Down syndrome and Alzheimer’s dementia. J Intellect Disabil Res 49, 560–566. [DOI] [PubMed] [Google Scholar]
- [23].Coppus A, Evenhuis H, Verberne GJ, Visser F, Van Gool P, Eikelenboom P, Van Duijin C (2006) Dementia and mortality in persons with Down’s syndrome. J Intellect Disabil Res 50, 768–777. [DOI] [PubMed] [Google Scholar]
- [24].Saunders AM, Strittmatter WJ, Schmechel D, George- Hyslop PS, Pericak-Vance M, Joo S, Rosi B, Gusella J, Crapper-MacLachlan D, Alberts M (1993) Association of apolipoprotein E allele 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 43, 1467–1467. [DOI] [PubMed] [Google Scholar]
- [25].Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, Van Duijn CM (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta-analysis. JAMA 278, 1349–1356. [PubMed] [Google Scholar]
- [26].Loy CT, Schofield PR, Turner AM, Kwok JB (2014) Genetics of dementia. Lancet 383, 828–840. [DOI] [PubMed] [Google Scholar]
- [27].Zhu X, Raina AK, Perry G, Smith MA (2004) Alzheimer’s disease: The two-hit hypothesis. Lancet Neurol 3, 219–226. [DOI] [PubMed] [Google Scholar]
- [28].Förstl H, Kurz A (1999) Clinical features of Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 249, 288–290. [DOI] [PubMed] [Google Scholar]
- [29].Oskarsson ME, Paulsson JF, Schultz SW, Ingelsson M, Westermark P, Westermark GT (2015) In vivo seeding and cross-seeding of localized amyloidosis: A molecular link between type 2 diabetes and Alzheimer disease. Am J Pathol 185, 834–846. [DOI] [PubMed] [Google Scholar]
- [30].Hoyer S (1998) Is sporadic Alzheimer disease the brain type of non-insulin dependent diabetes mellitus? A challenging hypothesis. J Neural Transm 105, 415–422. [DOI] [PubMed] [Google Scholar]
- [31].Hoyer S, Nitsch R, Oesterreich K (1991) Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer type: A cross-sectional comparison against advanced late-onset and incipient early-onset cases. J Neural Transm Park Dis Dement Sect 3, 1–14. [DOI] [PubMed] [Google Scholar]
- [32].Plum L, Schubert M, Brüning JC (2005) The role of insulin receptor signaling in the brain. Trends Endocrinol Metab 16, 59–65. [DOI] [PubMed] [Google Scholar]
- [33].Steen E, Terry BM, J Rivera E, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J Alzheimers Dis 7, 63–80. [DOI] [PubMed] [Google Scholar]
- [34].Talbot K, Wang H-Y, Kazi H, Han L-Y, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122, 1316–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Anguiano M, Nowak RJ, Lansbury PT (2002) Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry 41, 11338–11343. [DOI] [PubMed] [Google Scholar]
- [36].Kawahara M, Kuroda Y, Arispe N, Rojas E (2000) Alzheimer’s β-amyloid, human islet amylin, and prion protein fragment evoke intracellular free calcium elevations by a common mechanism in a hypothalamic GnRH neuronal cell line. J Biol Chem 275, 14077–14083. [DOI] [PubMed] [Google Scholar]
- [37].Kimura R, MacTavish D, Yang J, Westaway D, Jhamandas JH (2012) Beta amyloid-induced depression of hippocampal long-term potentiation is mediated through the amylin receptor. J Neurosci 32, 17401–17406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kimura R, MacTavish D, Yang J, Westaway D, Jhamandas JH (2017) Pramlintide antagonizes beta amyloid (Aβ)-and human amylin-induced depression of hippocampal longterm potentiation. Mol Neurobiol 54, 748–754. [DOI] [PubMed] [Google Scholar]
- [39].Lim Y-A, Ittner LM, Lim YL, Götz J (2008) Human but not rat amylin shares neurotoxic properties with Aβ42 in long-term hippocampal and cortical cultures. FEBS Lett 582, 2188–2194. [DOI] [PubMed] [Google Scholar]
- [40].Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846. [DOI] [PubMed] [Google Scholar]
- [41].Johnson K, O’Brien T, Jordan K, Westermark P (1989) Impaired glucose tolerance is associated with increased islet amyloid polypeptide (IAPP) immunoreactivity in pancreatic beta cells. Am J Pathol 135, 245. [PMC free article] [PubMed] [Google Scholar]
- [42].Johnson KH, O’Brien TD, Betsholtz C, Westermark P (1989) Islet amyloid, islet-amyloid polypeptide, and diabetes mellitus. N Engl J Med 321, 513–518. [DOI] [PubMed] [Google Scholar]
- [43].Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Pettiti M, Natali A, Mari A, DeFronzo R (2003) Predominant role of reduced beta-cell sensitivity to glucose over insulin resistance in impaired glucose tolerance. Diabetologia 46, 1211–1219. [DOI] [PubMed] [Google Scholar]
- [44].Group UPDS (1995) UK Prospective Diabetes Study 16: Overview of 6 years’ therapy of type II diabetes: A progressive disease. Diabetes 44, 1249–1258. [PubMed] [Google Scholar]
- [45].Gil-Bea FJ, Solas M, Solomon A, Mugueta C, Winblad B, Kivipelto M, Ramirez MJ, Cedazo-Mínguez A (2010) Insulin levels are decreased in the cerebrospinal fluid of women with prodomal Alzheimer’s disease. J Alzheimers Dis 22, 405–413. [DOI] [PubMed] [Google Scholar]
- [46].Schwartz MW, Figlewicz DF, Kahn SE, Baskin DG, Greenwood M, Porte D Jr (1990) Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides 11, 467–472. [DOI] [PubMed] [Google Scholar]
- [47].Wallum B, Taborsky G Jr, Porte D Jr, Figlewicz D, Jacobson L, Beard J, Ward W, Dorsa D (1987) Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab 64, 190–194. [DOI] [PubMed] [Google Scholar]
- [48].Horwood JM, Dufour F, Laroche S, Davis S (2006) Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci 23, 3375–3384. [DOI] [PubMed] [Google Scholar]
- [49].Neumann KF, Rojo L, Navarrete LP, Farías G, Reyes P, Maccioni RB (2008) Insulin resistance and Alzheimer’s disease: Molecular links & clinical implications. Curr Alzheimer Res 5, 438–447. [DOI] [PubMed] [Google Scholar]
- [50].de la Monte SM, Longato L, Tong M, DeNucci S, Wands JR (2009) The liver-brain axis of alcohol-mediated neurodegeneration: Role of toxic lipids. Int J Environ Res Public Health 6, 2055–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med 23, 134–147. [DOI] [PubMed] [Google Scholar]
- [52].Floyd RA (1999) Antioxidants, oxidative stress, and degenerative neurological disorders. Exp Biol Med 222, 236–245. [DOI] [PubMed] [Google Scholar]
- [53].Floyd RA (1996) Protective action of nitrone-based free radical traps against oxidative damage to the central nervous system. Adv Pharmacol 38, 361–378. [DOI] [PubMed] [Google Scholar]
- [54].Floyd RA, Carney JM (1992) Free radical damage to protein and DNA: Mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann Neurol 32, S22–S27. [DOI] [PubMed] [Google Scholar]
- [55].Yu BP (1994) Cellular defenses against damage from reactive oxygen species. Physiol Rev 74, 139–162. [DOI] [PubMed] [Google Scholar]
- [56].Dunn JD, Alvarez LA, Zhang X, Soldati T (2015) Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol 6, 472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Reddy PH (2007) Mitochondrial dysfunction in aging and Alzheimer’s disease: Strategies to protect neurons. Antioxid Redox Signal 9, 1647–1658. [DOI] [PubMed] [Google Scholar]
- [58].Reddy PH, Beal MF (2008) Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med 14, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sirk D, Zhu Z, Wadia JS, Shulyakova N, Phan N, Fong J, Mills LR (2007) Chronic exposure to sub-lethal betaamyloid (Aβ) inhibits the import of nuclear-encoded proteins to mitochondria in differentiated PC12 cells. J Neurochem 103, 1989–2003. [DOI] [PubMed] [Google Scholar]
- [60].Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Gräber S, Kovacs I, Lee WD, Waggoner J, Cui J (2006) Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J 25, 3900–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Anello M, Lupi R, Spampinato D, Piro S, Masini M, Boggi U, Del Prato S, Rabuazzo A, Purrello F, Marchetti P (2005) Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia 48, 282–289. [DOI] [PubMed] [Google Scholar]
- [62].Jheng H-F, Tsai P-J, Guo S-M, Kuo L-H, Chang C-S, Su I-J, Chang C-R, Tsai Y-S (2012) Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol 32, 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Engel MF, Khemtémourian L, Kleijer CC, Meeldijk HJ, Jacobs J, Verkleij AJ, de Kruijff B, Killian JA, Höppener JW (2008) Membrane damage by human islet amyloid polypeptide through fibril growth at the membrane. Proc Natl Acad SciUSA 105, 6033–6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Inestrosa N, Alvarez A, Godoy J, Reyes A, De Ferrari G (2000) Acetylcholinesterase–amyloid-β-peptide interaction and Wnt signaling involvement in Aβ neurotoxicity. Acta Neurol Scand 102, 53–59. [DOI] [PubMed] [Google Scholar]
- [65].Schubert D, Behl C, Lesley R, Brack A, Dargusch R, Sagara Y, Kimura H (1995) Amyloid peptides are toxic via a common oxidative mechanism. Proc Natl Acad Sci USA 92, 1989–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zraika S, Hull R, Udayasankar J, Aston-Mourney K, Subramanian S, Kisilevsky R, Szarek W, Kahn S (2009) Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia 52, 626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Smith MA, Harris PL, Sayre LM, Perry G (1997) Iron accumulation in Alzheimer disease is a source of redoxgenerated free radicals. Proc Natl Acad Sci U S A 94, 9866–9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Albers DS, Beal MF (2000) Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J Neural Transm Suppl 59, 133–54. [DOI] [PubMed] [Google Scholar]
- [69].Huang WJ, Zhang X, Chen WW (2016) Role of oxidative stress in Alzheimer’s disease. Biomed Rep 4, 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Giraldo E, Lloret A, Fuchsberger T, Viña J (2014) Aβ and tau toxicities in Alzheimer’s are linked via oxidative stress-induced p38 activation: Protective role of vitamin E. Redox Biol 2, 873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Verdile G, Keane KN, Cruzat VF, Medic S, Sabale M, Rowles J, Wijesekara N, Martins RN, Fraser PE, Newsholme P (2015) Inflammation and oxidative stress: The molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediators Inflamm 2015, 105828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Heneka MT, O’Banion MK, Terwel D, Kummer MP (2010) Neuroinflammatory processes in Alzheimer’s disease. J. Neural Transm 117, 919–947. [DOI] [PubMed] [Google Scholar]
- [73].Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T (2009) Inflammation in Alzheimer’s disease: Amyloid-β oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol 87, 181–194. [DOI] [PubMed] [Google Scholar]
- [74].Salminen A, Ojala J, Suuronen T, Kaarniranta K, Kauppinen A (2008) Amyloid-β oligomers set fire to inflammasomes and induce Alzheimer’s pathology. J Cell Mol Med 12, 2255–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Sims R, Van Der Lee SJ, Naj AC, Bellenguez C, Badarinarayan N, Jakobsdottir J, Kunkle BW, Boland A, Raybould R, Bis JC (2017) Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat Genet 49, 1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zhang N, Yang S, Wang C, Zhang J, Huo L, Cheng Y, Wang C, Jia Z, Ren L, Kang L (2017) Multiple target of hAmylin on rat primary hippocampal neurons. Neuropharmacology 113, 241–251. [DOI] [PubMed] [Google Scholar]
- [77].Miller JA, Woltjer RL, Goodenbour JM, Horvath S, Geschwind DH (2013) Genes and pathways underlying regional and cell type changes in Alzheimer’s disease. Genome Med 5, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bhadada SK, Rastogi A, Agarwal A, Kochhar R, Kochhar R, Bhansali A (2017) Comparative study of clinical features of patients with celiac disease & those with concurrent celiac disease & type 1 diabetes mellitus. Indian J Med Res 145, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Las G, Shirihai O (2010) The role of autophagy in β-cell lipotoxicity and type 2 diabetes. Diabetes Obes Metab 12, 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI (2003) Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science 300, 1140–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bugger H, Abel ED (2010) Mitochondria in the diabetic heart. Cardiovasc Res 88, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wang X, Bao W, Liu J, OuYang Y-Y, Wang D, Rong S, Xiao X, Shan Z-L, Zhang Y, Yao P (2013) Inflammatory markers and risk of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 36, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z (2010) Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol 11, 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Odegaard AO, Jacobs DR, Sanchez OA, Goff DC, Reiner AP, Gross MD (2016) Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc Diabetol 15, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Selkoe DJ, Schenk D (2003) Alzheimer’s disease: Molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol 43, 545–584. [DOI] [PubMed] [Google Scholar]
- [86].Bennett RG, Duckworth WC, Hamel FG (2000) Degradation of amylin by insulin-degrading enzyme. J Biol Chem 275, 36621–36625. [DOI] [PubMed] [Google Scholar]
- [87].Kurochkin IV (2001) Insulin-degrading enzyme: Embarking on amyloid destruction. Trends Biochem Sci 26, 421–425. [DOI] [PubMed] [Google Scholar]
- [88].Wang X, Yu S, Gao S-J, Hu J-P, Wang Y, Liu H-X (2014) Insulin inhibits Abeta production through modulation of APP processing in a cellular model of Alzheimer’s disease. Neuroendocrinol Lett 35, 224–229. [PubMed] [Google Scholar]
- [89].Zhao W-Q, Townsend M (2009) Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochim Biophys Acta 1792, 482–496. [DOI] [PubMed] [Google Scholar]
- [90].Zhao W-Q, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL (2008) Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J 22, 246–260. [DOI] [PubMed] [Google Scholar]
- [91].Watson GS, Craft S (2003) The role of insulin resistance in the pathogenesis of Alzheimer’s disease. CNS Drugs 17, 27–45. [DOI] [PubMed] [Google Scholar]
- [92].Gedulin BR, Rink TJ, Young AA (1997) Dose-response for glucagonostatic effect of amylin in rats. Metabolism 46, 67–70. [DOI] [PubMed] [Google Scholar]
- [93].Young A (2005) Inhibition of gastric emptying. Adv Pharmacol 52, 99–121. [DOI] [PubMed] [Google Scholar]
- [94].Ratner R, Dickey R, Fineman M, Maggs D, Shen L, Strobel S, Weyer C, Kolterman O (2004) Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in Type 1 diabetes mellitus: A 1-year, randomized controlled trial. Diabet Med 21, 1204–1212. [DOI] [PubMed] [Google Scholar]
- [95].Gebre-Medhin S, Mulder H, Zhang Y, Sundler F, Betsholtz C (1998) Reduced nociceptive behavior in islet amyloid polypeptide (amylin) knockout mice. Mol Brain Res 63, 180–183. [DOI] [PubMed] [Google Scholar]
- [96].Banks WA, Jaspan JB, Huang W, Kastin AJ (1997) Transport of insulin across the blood-brain barrier: Saturability at euglycemic doses of insulin. Peptides 18, 1423–1429. [DOI] [PubMed] [Google Scholar]
- [97].Banks WA, Jaspan JB, Kastin AJ (1997) Selective, physiological transport of insulin across the blood-brain barrier: Novel demonstration by species-specific radioimmunoassays. Peptides 18, 1257–1262. [DOI] [PubMed] [Google Scholar]
- [98].Olsson M, Herrington MK, Reidelberger RD, Permert J, Arnelo U (2007) Comparison of the effects of chronic central administration and chronic peripheral administration of islet amyloid polypeptide on food intake and meal pattern in the rat. Peptides 28, 1416–1423. [DOI] [PubMed] [Google Scholar]
- [99].Nakamoto H, Soeda Y, Takami S, Minami M, Satoh M (2000) Localization of calcitonin receptor mRNA in the mouse brain: Coexistence with serotonin transporter mRNA. Mol Brain Res 76, 93–102. [DOI] [PubMed] [Google Scholar]
- [100].Larson EB, Kukull WA, Katzman RL (1992) Cognitive impairment: Dementia and Alzheimer’s disease. Annu Rev Public Health 13, 431–449. [DOI] [PubMed] [Google Scholar]
- [101].Potes CS, Boyle CN, Wookey PJ, Riediger T, Lutz TA (2012) Involvement of the extracellular signal-regulated kinase 1/2 signaling pathway in amylin9s eating inhibitory effect. Am J Physiol Regul Integr Comp Physiol 302, R340–R351. [DOI] [PubMed] [Google Scholar]
- [102].Fu W, Ruangkittisakul A, MacTavish D, Shi JY, Ballanyi K, Jhamandas JH (2012) Amyloid β (Aβ) peptide directly activates amylin-3 receptor subtype by triggering multiple intracellular signaling pathways. J Biol Chem 287, 18820–18830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guénette S (2003) Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad SciUSA 100, 4162–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Colburn WA, Gottlieb AB, Koda J, Kolterman OG (1996) Pharmacokinetics and pharmacodynamics of AC137 (25, 28, 29 tripro-amylin, human) after intravenous bolus and infusion doses in patients with insulin-dependent diabetes. J Clin Pharmacol 36, 13–24. [DOI] [PubMed] [Google Scholar]
- [105].Jackson K, Barisone GA, Diaz E, Jin Lw, DeCarli C, Despa F (2013) Amylin deposition in the brain: A second amyloid in Alzheimer disease? Ann Neurol 74, 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Verma N, Ly H, Liu M, Chen J, Zhu H, Chow M, Hersh LB, Despa F (2016) Intraneuronal amylin deposition, peroxidative membrane injury and increased IL-1β synthesis in brains of Alzheimer’s disease patients with type-2 diabetes and in diabetic HIP rats. J Alzheimers Dis 53, 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Fu W, Patel A, Jhamandas JH (2013) Amylin receptor: A common pathophysiological target in Alzheimer’s disease and diabetes mellitus. Front Aging Neurosci 5, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Jhamandas JH, Li Z, Westaway D, Yang J, Jassar S, MacTavish D (2011) Actions of β-amyloid protein on human neurons are expressed through the amylin receptor. Am J Pathol 178, 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Singh-Franco D, Perez A, Harrington C (2011) The effect of pramlintide acetate on glycemic control and weight in patients with type 2 diabetes mellitus and in obese patients without diabetes: A systematic review and meta-analysis. Diabetes Obes Metab 13, 169–180. [DOI] [PubMed] [Google Scholar]
- [110].Hollander PA, Levy P, Fineman MS, Maggs DG, Shen LZ, Strobel SA, Weyer C, Kolterman OG (2003) Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes. Diabetes Care 26, 784–790. [DOI] [PubMed] [Google Scholar]
- [111].Adler BL, Yarchoan M, Hwang HM, Louneva N, Blair JA, Palm R, Smith MA, Lee H-g, Arnold SE, Casadesus G (2014) Neuroprotective effects of the amylin analogue pramlintide on Alzheimer’s disease pathogenesis and cognition. Neurobiol Aging 35, 793–801. [DOI] [PubMed] [Google Scholar]
- [112].Wang E, Zhu H, Wang X, Gower AC, Wallack M, Blusztajn JK, Kowall N, Qiu WQ (2017) Amylin treatment reduces neuroinflammation and ameliorates abnormal patterns of gene expression in the cerebral cortex of an Alzheimer’s disease mouse model. J Alzheimers Dis 56, 47–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zhu H, Wang X, Wallack M, Li H, Carreras I, Dedeoglu A, Hur JY, Zheng H, Li H, Fine R (2015) Intraperitoneal injection of the pancreatic peptide amylin potently reduces behavioral impairment and brain amyloid pathology in murine models of Alzheimer’s disease. Mol Psychiatry 20, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zhu H, Xue X, Wang E, Wallack M, Na H, Hooker JM, Kowall N, Tao Q, Stein TD, Wolozin B (2017) Amylin receptor ligands reduce the pathological cascade of Alzheimer’s disease. Neuropharmacology 119, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Aronoff SL (2017) Rationale for treatment options for mealtime glucose control in patients with type 2 diabetes. Postgrad Med 129, 231–241. [DOI] [PubMed] [Google Scholar]
- [116].Edelman S, Garg S, Frias J, Maggs D, Wang Y, Zhang B, Strobel S, Lutz K, Kolterman O (2006) A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care 29, 2189–2195. [DOI] [PubMed] [Google Scholar]
- [117].Riddle M, Frias J, Zhang B, Maier H, Brown C, Lutz K, Kolterman O (2007) Pramlintide improved glycemic control and reduced weight in patients with type 2 diabetes using basal insulin. Diabetes Care 30, 2794–2799. [DOI] [PubMed] [Google Scholar]
- [118].Wysham C, Lush C, Zhang B, Maier H, Wilhelm K (2008) Effect of pramlintide as an adjunct to basal insulin on markers of cardiovascular risk in patients with type 2 diabetes. Curr Med Res Opin 24, 79–85. [DOI] [PubMed] [Google Scholar]
- [119].Singh-Franco D, Robles G, Gazze D (2007) Pramlintide acetate injection for the treatment of type 1 and type 2 diabetes mellitus. Clin Ther 29, 535–562. [DOI] [PubMed] [Google Scholar]
- [120].Qiu WQ, Zhu H (2014) Amylin and its analogs: A friend or foe for the treatment of Alzheimer’s disease? Front Aging Neurosci 6, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Gingell JJ, Burns ER, Hay DL (2014) Activity of pramlintide, rat and human amylin but not Aβ1–42 at human amylin receptors. Endocrinology 155, 21–26. [DOI] [PubMed] [Google Scholar]
- [122].Lutz TA, Meyer U (2015) Amylin at the interface between metabolic and neurodegenerative disorders. Front Neurosci 9, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Qiu WQ, Au R, Zhu H, Wallack M, Liebson E, Li H, Rosenzweig J, Mwamburi M, Stern RA (2014) Positive association between plasma amylin and cognition in a homebound elderly population. J Alzheimers Dis 42, 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Qiu WQ, Wallack M, Dean M, Liebson E, Mwamburi M, Zhu H (2014) Association between amylin and amyloid-β peptides in plasma in the context of apolipoprotein E4 allele. PLoS One 9, e88063. [DOI] [PMC free article] [PubMed] [Google Scholar]