SUMMARY
BACKGROUND
Tuberculosis (TB) diagnosis in human immunodeficiency virus (HIV) positive persons is difficult, particularly in resource-limited settings. The relationship between TB culture status and mortality in HIV-positive persons treated for TB is unclear.
METHODS
We evaluated HIV-positive adults treated for TB at or after their first HIV clinic visit in Argentina, Brazil, Chile, Honduras, Mexico or Peru from 2000 to 2015. Anti-tuberculosis treatment included 2 months of isoniazid, rifampicin (RMP)/rifabutin (RBT), pyrazinamide ± ethambutol, followed by continuation phase treatment with isoniazid + RMP/RBT.
RESULTS
Of 759 TB-HIV patients, 238 (31%) were culture-negative, 228 (30%) had unknown culture status or did not undergo culture and 293 (39%) were culturepositive. The median CD4 at TB diagnosis was 96 (interquartile range 40–228); 636 (84%) received concurrent antiretroviral therapy (ART) and antituberculosis treatment. There were 123 (16%) deaths: 90/466 (19%) with TB culture-negative, unknown or not performed vs. 33/293 (11%) who were TB culturepositive (P=0.005). In Kaplan-Meier analysis, mortality in TB patients without culture-confirmed disease was higher (P = 0.002). In a Cox model adjusted for age, sex, CD4, ART timing, disease site and stratified by study site, mortality in persons without culture-confirmed TB was not significantly increased compared to those with culture-positive TB (hazard ratio 1.39, 95%CI 0.89–2.16, P = 0.15).
CONCLUSION
Most HIV-positive patients treated for TB did not have culture-confirmed TB, and mortality tended to be higher in patients without culture-confirmed disease, although the association was not statistically different after adjusting for other variables. Accurate TB diagnosis in HIV-positive persons is crucial.
Keywords: human immunodeficiency virus and TB, Latin America, culture-negative tuberculosis, anti-tuberculosis treatment
RÉSUMÉN
CONTEXTE
Le diagnostic de la tuberculose (TB) chez les personnes positives á l’infection par le virus de l’immunodéficience humaine (VIH) est difficile, particulièrement dans des contextes de ressources limitées. La relation entre statut de la culture de TB et mortalité parmi les personnes positives au VIH traitées pour TB n’est pas clair.
MÉTHODE
Nous avons évalué des adultes positifs au VIH traités pour TB lors de la premiére consultation, ou par la suite, dans un centre VIH en Argentine, au Brésil, au Chili, au Honduras, au Mexique ou au Pérou entre 2000 et 2015. Le traitement de la TB a inclus 2 mois d’isoniazide (INH), de rifampicine (RMP) ou rifabutine (RBT), de pyrazinamide ± éthambutol, suivis d’une phase de continuation par INH + RMP/RBT.
RÉSULTATS
Sur 759 patients TB-VIH, 238 (31%) ont eu une culture négative, 228 (30%) ont eu un résultat de culture inconnu/culture non réalisée et 293 (39%), une culture positive. Le taux de CD4 médian lors du diagnostic de TB a été de 96 (intervalle interquartile 40–228) et 636 patients (84%) ont reçu en parallèle un traitement antirétroviral (ART) et un traitement de TB. Il y a eu 123 (16%) décès : 90/466 (19%) à culture de TB négative, inconnue ou non réalisée contre 33/293 (11%) avec une TB à culture positive (P=0,005). En analyse de Kaplan-Meier, la mortalité des patients TB sans confirmation par la culture a été plus élevée (P = 0,002). Dans un modèle de Cox ajusté sur l’âge, le sexe, les CD4, le moment de mise en route de l’ART, le site de la maladie, et stratifié; par site d’étude, la mortalité des patients sans confirmation de TB par la culture n’a pas été significativement augmentée comparée à la TB à culture positive (risque relative 1,39; IC95% 0,89–2,16; P = 0,15).
CONCLUSION
La majorité; des patients positifs au VIH traités pour TB n’ont pas eu de confirmation par la culture et la mortalité a eu tendance à être plus élevée chez les patients sans confirmation de leur maladie par la culture, bien que l’association n’ait pas été statistiquement différente après ajustement sur d’autres variables. Un diagnostic exact de la TB parmi les personnes VIH positives est crucial.
RESUMEN
ANTECEDENTES
El diagnóstico de tuberculosis (TB) en personas que viven con virus de la innmunodeficiencia humana (VIH) es difícil, particularmente en países con recursos limitados. La relación entre el resultado del cultivo de TB y la mortalidad en personas con VIH no está clara.
MÉTODOS
Se evaluaron adultos con VIH tratados por TB en la primera visita o después de ésta, en Argentina, Brasil, Chile, Honduras, Mexico o Perú del 2000 al 2015. El tratamiento de TB incluyó 2 meses de isoniacida (INH), rifampicina (RMP) o rifabutina (RBT), pirazinamida ± etambutol, posteriormente la fase de mantenimiento con INH + RMP/RBT.
RESULTADOS
De los 759 pacientes con diagnóstico de TB-VIH, 238 (31%) presentaron cultivo negativo, 228 (30%) con cultivo desconocido/no realizado y 293 (39%) con cultivo positivo. La mediana de CD4 al diagnóstico de TB fue 96 (intervalo intercuartílico 40–228) y 636 (84%) recibieron tratamiento antirretroviral (ART) y tratamiento para TB. Se presentaron 123 (16%) muertes: 90/466 (19%) con cultivo negativo de TB, desconocido o no realizado comparado con 33/293 (11%) con cultivo positivo de TB (P = 0,005). En el análisis Kaplan-Meier, la mortalidad en pacientes con TB sin un cultivo confirmado fue mayor (P = 0,002). En un modelo de Cox ajustado para edad, sexo, CD4, tiempo con ART, sitio de la enfermedad y estratificado por el sitio del estudio, la mortalidad en personas sin un cultivo confirmado de TB no se incrementó) significativamente comparado con las personas con cultivo positivo de TB (riesgo relativo 1,39; 95%IC 0,89–8,16; P = 0,15).
CONCLUSIONES
La mayoría de los pacientes con VIH tratados por TB no tuvieron un cultivo realizado y en dichos pacientes, hubo una tendencia a una mayor mortalidad, aunque la asociación no fue estadísticamente significativa posterior al ajuste para otras variables. Por tanto, la precisión en el diagnóstico de TB en pacientes con VIH, es crucial.
THE GLOBAL BURDEN OF TUBERCULOSIS (TB) is enormous, with an estimated 10.4 million new TB cases and 1.7 million deaths in 2016.1,2 Human immunodeficiency virus (HIV) infection has contributed to the global TB burden—it is estimated that 10% of TB cases and 22% of TB deaths in 2016 were in persons with HIV.1 Globally, people living with HIV are 19 times more likely to develop TB than those without HIV.1
Prompt, accurate diagnosis of TB and initiation of appropriate treatment is important to interrupt Mycobacterium tuberculosis transmission and reduce TB mortality;1–3 however, diagnosing TB among those who are HIV-positive is difficult and often only based on clinical symptoms in resource-limited settings. Diagnosis of TB in HIV-positive persons can be limited by atypical or minimal clinical and chest X-ray presentations.4–7 The gold standard diagnostic test for TB is mycobacterial culture. Although specific, the sensitivity of culture can be low; in settings where culture is routinely performed, approximately 20% of TB cases are culture-negative.4 Rates of culture-negative TB are particularly high in resource-limited settings, due in part, to limited infrastructure for culture-based diagnostic tests. Despite World Health Organization (WHO) policy recommending liquid-based culture since 2007,8 its availability in resource-poor settings has been limited. Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) is a genotypic, non-culture-based diagnostic test that is sensitive and specific for M. tuberculosis,8 but has limited access. In an international cohort of persons with HIV treated for TB, only 4% were tested using Xpert.9
In the present study conducted in HIV-positive individuals in Latin America treated for TB, we compared mortality risk among those with vs. those without culture-confirmed TB. We hypothesised that those without culture-confirmed TB were at higher mortality risk, in part because many received treatment for the wrong disease. Acid-fast bacilli (AFB) smear-negative TB is associated with increased mortality in HIV-positive persons,10–12 likely due to delays in TB diagnosis. However, there are few data on mortality risk in TB patients without culture-confirmed TB.
METHODS
Study population
The Caribbean, Central and South America network for HIV epidemiology (CCASAnet) cohort has been described elsewhere.13 The collaboration was established in 2006 as part of the International epidemiology Databases to Evaluate AIDS (IeDEA). The following sites contributed data to the study: Hospital Juan A Fernández and Centro Médico Huésped, Buenos Aires, Argentina (HF-CMH-Argentina); Institute Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz, Rio de Janeiro, RJ, Brazil (FC-Brazil); Fundación Arriarán, Santiago, Chile (FA-Chile); Instituto Hondureño de Seguridad Social and Hospital Escuela Universitario, Tegucigalpa, Honduras (IHSS/HE-Honduras); El Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico (INCMNSZ-Mexico); and Instituto de Medicina Tropical Alexander von Humboldt, Lima, Peru (IMTAvH-Peru). The requirement for individual patient informed consent was waived locally by the review board at each participating site, and by the CCASAnet Data Coordinating Center at Vanderbilt University Medical Center, Nashville, TN, USA.
All HIV-positive adults treated for TB at or after entry into the CCASAnet cohort between 2000 and 2015 were included. Those who received a standard 2-month initiation phase of isoniazid (INH), rifampicin (RMP) or rifabutin (RBT), pyrazinamide ± ethambutol followed by continuation phase comprising INH + RMP or RBT where included. Participants with an unknown TB treatment initiation date or site of TB (pulmonary vs. extra-pulmonary), no followup after TB diagnosis, resistance to INH and/or RMP, were excluded.
Most sites used directly observed treatment (DOT) according to local guidelines. Community-based DOT was used in Honduras, Chile, Peru and Mexico, but not in Brazil. The mycobacterial detection method most commonly used was the smear test (Ziehl-Neelsen) and culture; the medium most frequently used was Löwenstein-Jensen agar, followed by liquid media (BACTEC™ MGIT™ [Mycobacterial Growth Indicator Tube]; BD, Sparks, MD, USA). The Xpert test was available at some sites after 2014. Diagnostic tests were performed according to physician choice and test availability.
Study definitions
Date of TB diagnosis was considered the date of TB treatment initiation. Baseline was defined as the first TB diagnosis on, or after, the participant’s CCASAnet enrolment date. Baseline CD4 was defined based on the count obtained closest to the date of TB diagnosis, within 180 days before and 30 days after baseline. Site of TB disease was characterised as either pulmonary only or any extra-pulmonary disease. Antiretroviral therapy (ART) was defined as any regimen containing ⩾3 antiretroviral agents. Participants were characterised as never on ART, ART concurrent with anti-tuberculosis treatment and ART before or after anti-tuberculosis treatment. When a study participant met more than one category, concurrent ART and anti-tuberculosis treatment took precedence. In addition, ART use was characterised as not on ART at TB diagnosis vs. on ART at TB diagnosis. Missing ART treatment end dates were replaced with the participant’s last day of follow-up. If the date of TB treatment completion was missing, it was assumed to be 180 days after date of TB diagnosis, unless they died before then, in which case the last date alive or date of death was used.
Statistical analysis
The primary outcome was all-cause mortality after TB diagnosis. A sensitivity analysis was performed for all-cause mortality only during the first year after TB diagnosis. The primary exposure variable was TB culture status, positive vs. not positive (combination of culture-negative, unknown or not performed). This permitted a comparison of mortality among those treated for TB with vs. those without a positive TB culture. A sensitivity analysis was performed to compare culture-positive vs. culture-negative patients.
Descriptive statistics were reported as median and interquartile ranges (IQRs) or percentage and frequency, as appropriate. The survival probability after TB diagnosis was estimated using Kaplan-Meier survival curves; the log-rank test was used for comparisons. Cox proportional hazards models was used to investigate the association between culture status and time from TB diagnosis to death after adjusting for covariates a priori considered potential confounders: age, sex, baseline CD4, site of TB (pulmonary only vs. any extra-pulmonary disease) and timing of ART relative to anti-tuberculosis treatment. A sensitivity analysis was also performed based on year of TB diagnosis as a potential confounder to account for changes in care over time. All analyses were stratified by study site; a separate baseline hazard was assumed for each site.14 Missing covariates were multiply imputed using 20 replications; both complete case analyses and analyses using multiply imputed data are presented. The linearity assumption between time to death and continuous predictor variables were relaxed by restricting cubic splines; however, this also means that a single hazard ratio does not fully describe the variable’s relationship to time to death. Several hazard ratios were therefore reported using a common reference to provide a more complete view of the relationship between variables and time to death over a range of variable values. Patients lost to follow-up (defined as no visit within 12 months) were censored at their last visit date.
RESULTS
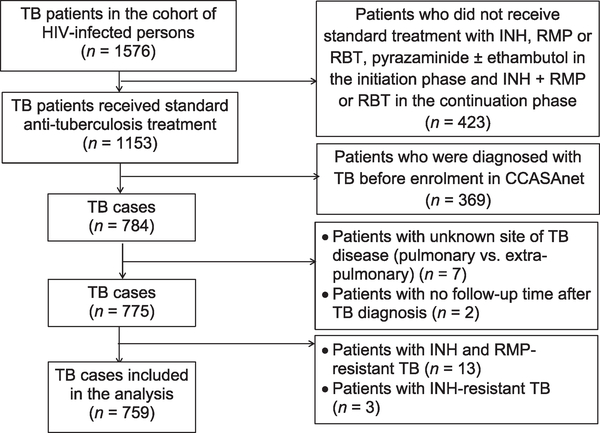
Of the 19 526 persons living with HIV who were enrolled between 2000 and 2015 at the CCASAnet study sites participating in this analysis, 1576 were diagnosed with TB. Of these, 1153 received standard anti-tuberculosis treatment; 369 were excluded because their TB episode was before enrolment into CCASAnet. Of the 784, the site of TB disease was unknown in seven, and two did not have any followup after TB diagnosis. Of the remaining 775, 13 were resistant to INH or RMP and three were INH-resistant. A final total of 759 subjects undergoing standard anti-tuberculosis treatment were included in this analysis (Figure 1). The mycobacterial detection method most frequently used was Löwenstein-Jensen agar, followed by liquid media (MGIT) and Xpert (after 2014). Amplification tests or lipoarabinomannan (LAM) were not performed in this cohort.
Figure 1.
HIV-infected TB patients included in the analysis. TB = tuberculosis; HIV = human immunodeficiency virus; INH = isoniazid; RMP = rifampicin; RBT = rifabutin; CCASAnet = Caribbean, Central and South America network for HIV epidemiology.
Of the 759 study participants, 238 (31%) were culture-negative, 228 (30%) had unknown culture status/did not undergo culture and 293 (39%) were culture-positive. Baseline characteristics are given in Table 1. The median age was 35 years; 77% were male; 47% had pulmonary TB only; the median CD4 count at TB diagnosis was 96 cells/mm3 (interquartile range [IQR] 40–228), and 636 (84%) received concomitant ART and anti-tuberculosis treatment. Of the 759 study participants, 463 (61%) were smear-negative or had unknown smear status/did not undergo smear testing and 296 (39%) were smearpositive. The median follow-up was 3.79 years (IQR 1.66–6.36). There were 113 patients (14.9%) lost to follow-up; 7.4% in the first year after TB diagnosis.
Table 1.
Baseline characteristics of study population (n = 759)
| Total (n = 759) | Culture-negative (n = 238) | Culture status unknown/ culture not performed (n = 228) | Culture-positive (n = 293) | |
|---|---|---|---|---|
| Characteristic | n (%) | n (%) | n (%) | n (%) |
| Age, years, median [IQR] | 35 [30–43] | 36 [29–43] | 36 [30–43] | 35 [30–43] |
| Male sex | 581 (77) | 194 (82) | 160 (70) | 227 (77) |
| Study site | ||||
| Argentina | 61 (8) | 13 (5) | 9 (4) | 39 (13) |
| Brazil | 237 (31) | 48 (20) | 53 (23) | 136 (46) |
| Chile | 44 (6) | 19 (8) | 16 (7) | 9 (3) |
| Honduras | 26 (3) | 11 (5) | 11 (5) | 4 (1) |
| Mexico | 27 (4) | 10 (4) | 0 (0) | 17 (6) |
| Peru | 364 (48) | 137 (58) | 139 (61) | 88 (30) |
| Site of TB disease | ||||
| Pulmonary only | 356 (47) | 117 (49) | 98 (43) | 141 (48) |
| Any extra-pulmonary | 403 (53) | 121 (51) | 130 (57) | 152 (52) |
| CD4 at TB diagnosis, median [IQR] | 96 [40–228] | 87 [37–223] | 100 [41–222] | 97 [45–241] |
| Missing CD4 at TB diagnosis | 149 (20) | 57 (24) | 36 (16) | 56 (19) |
| AFB smear status | ||||
| Negative | 347 (46) | 173 (73) | 70 (31) | 104 (35) |
| Unknown/not performed | 116 (15) | 7 (3) | 97 (43) | 12 (4) |
| Positive | 296 (39) | 58 (24) | 61 (27) | 177 (60) |
| TB diagnosis relative to ART | ||||
| Never on ART | 38 (5) | 3 (1) | 20 (9) | 15(5) |
| ART concurrent with anti-tuberculosis treatment | 636 (84) | 190 (80) | 198 (87) | 248 (85) |
| ART before or after anti-tuberculosis treatment | 85 (11) | 45 (19) | 10 (4) | 30 (10) |
| TB diagnosis relative to ART | ||||
| Not on ART at TB diagnosis | 570 (75) | 173 (73) | 159 (70) | 238 (81) |
| On ART at TB diagnosis | 189 (25) | 65 (27) | 69 (30) | 55 (19) |
IQR = interquartile range; TB = tuberculosis; AFB = acid-fast bacilli; ART =antiretroviral therapy.
Persons who underwent culture testing (whether positive or negative) were more likely to be male and have received ART than individuals who did not undergo culture testing. There was no statistically significant difference between the groups in terms of age, CD4 count at TB diagnosis and site of TB disease (pulmonary vs. extra-pulmonary) (data not shown).
Mortality
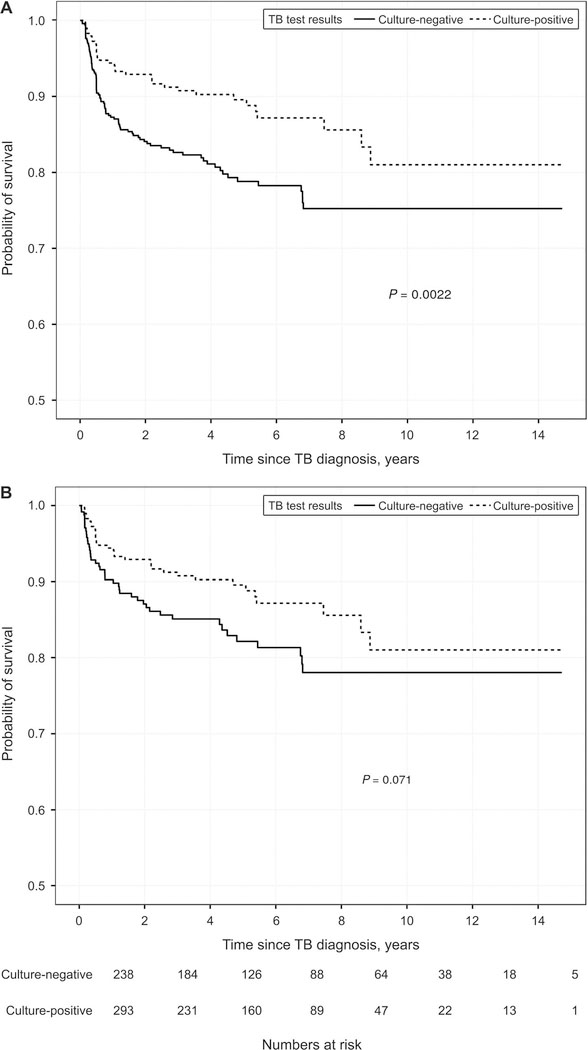
Of the 759 study participants, 123 (16%) died: 42/ 238 (18%) were TB culture-negative, 38/167 (23%) had unknown culture status, 10/61 (16%) did not undergo culture and 33/293 (11%) were culturepositive. After combining all participants without a positive culture, 90/466 (19%) died vs. 33/293 (11%) participants who were TB culture-positive (P = 0.005; χ2 test). In Kaplan-Meier analysis, risk of death in TB patients without culture-confirmed disease was higher than in persons with culture-positive TB (P = 0.002; log-rank test; Figure 2A). In a multivariable Cox model stratified by study site and adjusted for age, sex, CD4 count, timing of ART initiation relative to anti-tuberculosis treatment, and site of TB disease, the risk of death in persons without culture-confirmed TB was higher than those with culture-positive TB; however, the difference was not statistically significant (hazard ratio [HR] 1.39, 95% confidence interval [CI] 0.89–2.16; P = 0.15; Table 2A; model with multiple imputation for missing data).
Figure 2.
Kaplan-Meier curves of time to death according to culture status among HIV-infected TB patients. A) Culture-positive vs. all others (negative, unknown, not performed); B) culture-positive vs. culture-negative. TB = tuberculosis; HIV = human immunodeficiency virus.
Table 2A.
Factors associated with death among HIV-infected TB patients: Cox proportional hazards models among persons with culture-positive TB vs. all others (culture-negative, culture unknown and culture not performed)*
| Covariate | Univariate |
Multivariable complete case |
Multivariable imputed |
|||
|---|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Culture test results | 0.22 | 0.79 | 0.15 | |||
| Positive (reference) | 1.00 | 1.00 | 1.00 | |||
| All others | 1.30 (0.85–1.99) | 1.06 (0.67–1.69) | 1.39 (0.89–2.16) | |||
| CD4 at TB diagnosis | <0.001 | <0.001 | <0.001 | |||
| 50 | 3.14 (1.81–5.45) | 3.96 (2.20–7.13) | 3.71 (2.12–6.50) | |||
| 100 | 2.29 (1.35–3.89) | 2.74 (1.56–4.84) | 2.70 (1.57–4.66) | |||
| 200 | 1.46 (0.93–2.28) | 1.61 (1.00–2.59) | 1.65 (1.04–2.64) | |||
| 350 (reference) | 1.00 | 1.00 | 1.00 | |||
| 500 | 0.74 (0.43–1.30) | 0.68 (0.38–1.22) | 0.65 (0.36–1.18) | |||
| Timing of TB relative to ART | <0.001 | <0.001 | <0.001 | |||
| Never on ART (reference) | 1.00 | 1.00 | 1.00 | |||
| ART concurrent with anti-tuberculosis treatment | 0.17 (0.10–0.28) | 0.11 (0.06–0.19) | 0.14 (0.08–0.24) | |||
| ART before or after anti-tuberculosis treatment | 0.1 1 (0.05–0.23) | 0.16 (0.07–0.41) | 0.14 (0.06–0.32) | |||
| Age at TB diagnosis, years | 0.43 | 0.03 | 0.06 | |||
| 25 | 1.03 (0.68–1.56) | 1.24 (0.79–1.95) | 1.12 (0.73–1.71) | |||
| 30 | 1.00 (0.84–1.19) | 1.07 (0.89–1.29) | 1.03 (0.86–1.23) | |||
| 35 (reference) | 1.00 | 1.00 | 1.00 | |||
| 40 | 1.05 (0.96–1.15) | 1.08 (0.98–1.19) | 1.08 (0.98–1.19) | |||
| 50 | 1.26 (0.89–1.78) | 1.69 (1.15–2.48) | 1.57 (1.08–2.27) | |||
| Sex | 0.08 | 0.10 | 0.21 | |||
| Female (reference) | 1.00 | 1.00 | 1.00 | |||
| Male | 0.70 (0.47–1.05) | 0.69 (0.44–1.08) | 0.76 (0.50–1.16) | |||
| Site of TB | 0.36 | 0.40 | 0.20 | |||
| Pulmonary only (reference) | 1.00 | 1.00 | 1.00 | |||
| Any extra-pulmonary | 1.18 (0.83–1.70) | 1.19 (0.79–1.79) | 1.28 (0.88–1.86) | |||
Multiple imputation used for missing data. In accordance with the standard convention, univariate analysis was not performed with imputed data. All analyses were stratified by study site.
HIV = human immunodeficiency virus; TB = tuberculosis; HR = hazard ratio; CI = confidence interval.
Results were similar when the timing of ART initiation relative to anti-tuberculosis treatment was dichotomised (on ART vs. not on ART at TB diagnosis), and all of the other variables above were included in the Cox model: risk of death among those without culture-confirmed disease was HR 1.24 (95%CI 0.81–1.92; P = 0.32). After controlling for year of TB diagnosis, HR for no culture-confirmed disease was 1.39 (95%CI 0.89–2.16, P = 0.15). Results were also similar when follow-up was limited to the first year after TB diagnosis: HR for no culture-confirmed disease was 1.39 (95%CI 0.76–2.51, P = 0.28). When the comparison was limited to persons with culture-negative vs. those with culture-positive TB, an increasing trend towards mortality was observed; however, this was not statistically significant (P = 0.07; Kaplan-Meier log-rank test, Figure 2B; HR 1.37, 95%CI 0.84–2.22, P = 0.20, Table 2B, Cox model with multiple imputation for missing data).
Table 2B.
Factors associated with death among HIV-infected TB patient: Cox proportional hazards models among persons with culture-positive vs. culture-negative TB*
| Covariate | Univariate |
Multivariable complete case |
Multivariable imputed |
|||
|---|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Culture test results | 0.84 | 0.63 | 0.20 | |||
| Positive (reference) | 1.00 | 1.00 | 1.00 | |||
| Negative | 1.05 (0.64–1.72) | 1.15 (0.66–2.00) | 1.37 (0.84–2.22) | |||
| CD4 at TB diagnosis | <0.001 | <0.001 | <0.001 | |||
| 50 | 3.14 (1.81–5.45) | 4.34 (2.02–9.33) | 3.77 (2.02–7.02) | |||
| 100 | 2.29 (1.35–3.89) | 3.15 (1.52–6.53) | 2.72 (1.48–4.98) | |||
| 200 | 1.46 (0.93–2.28) | 1.85 (1.01–3.40) | 1.65 (0.99–2.74) | |||
| 350 (ref) | 1.00 | 1.00 | 1.00 | |||
| 500 | 0.74 (0.43–1.30) | 0.57 (0.27–1.20) | 0.65 (0.35–1.23) | |||
| Timing of TB relative to ART | <0.001 | <0.001 | <0.001 | |||
| Never on ART (reference) | 1.00 | 1.00 | 1.00 | |||
| ART concurrent with anti-tuberculosis treatment | 0.17 (0.10–0.28) | 0.07 (0.03–0.16) | 0.13 (0.07–0.23) | |||
| ART before or after anti-tuberculosis treatment | 0.11 (0.05–0.23) | 0.09 (0.03–0.27) | 0.12 (0.05–0.30) | |||
| Age at TB diagnosis, years | 0.43 | 0.24 | 0.05 | |||
| 25 | 1.03 (0.68–1.56) | 1.28 (0.74–2.21) | 1.10 (0.71–1.69) | |||
| 30 | 1 (0.84–1.19) | 1.09 (0.86–1.37) | 1.02 (0.85–1.22) | |||
| 35 (reference) | 1.00 | 1.00 | 1.00 | |||
| 40 | 1.05 (0.96–1.15) | 1.05 (0.92–1.20) | 1.09 (0.99–1.20) | |||
| 50 | 1.26 (0.89–1.78) | 1.52 (0.91–2.53) | 1.59\ (1.10–2.30) | |||
| Sex | 0.08 | 0.41 | 0.22 | |||
| Female (reference) | 1.00 | 1.00 | 1.00 | |||
| Male | 0.70 (0.47–1.05) | 0.77 (0.42–1.42) | 0.76 (0.50–1.17) | |||
| Site of TB | 0.36 | 0.35 | 0.17 | |||
| Pulmonary only (reference) | 1.00 | 1.00 | 1.00 | |||
| Any extra-pulmonary | 1.18 (0.83–1.70) | 1.28 (0.76–2.16) | 1.30 (0.89–1.89) | |||
Multiple imputation used for missing data. In accordance with the standard convention, univariate analysis was not performed with imputed data. All analyses were stratified by study site.
HIV = human immunodeficiency virus; TB = tuberculosis; HR = hazard ratio; CI = confidence interval; ART =antiretroviral therapy.
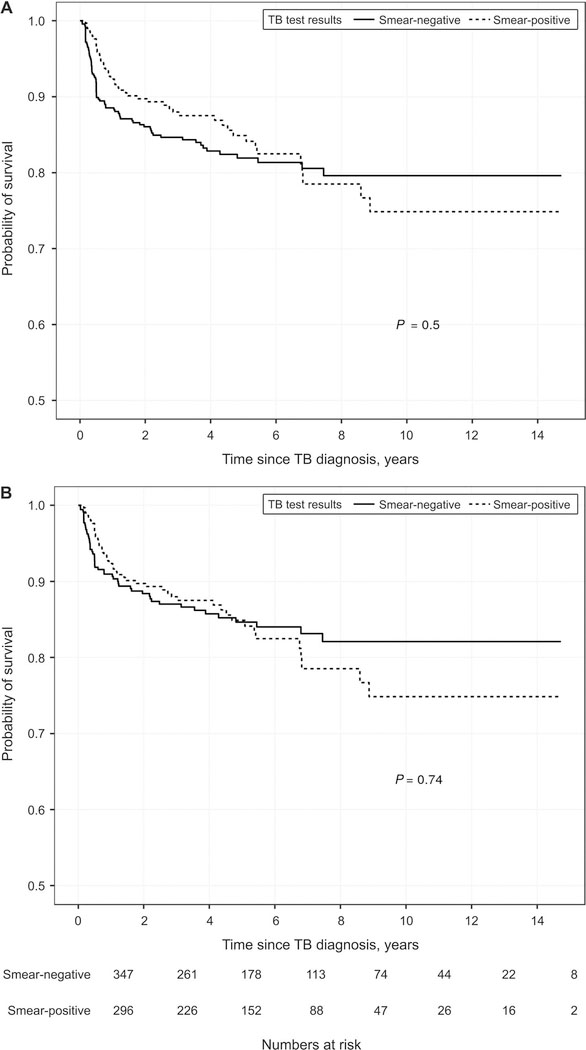
Among the 759 study participants, the proportion who died was 16% (77/463) smear-negative, smear-unknown or smear not performed vs. 16% (46/296) smear-positive (P = 0.77; χ2 test). The Kaplan-Meier curves of time to death by smear status are shown in Figure 3 (all others vs. smear-positive; P = 0.50, logrank test; smear-negative vs. smear-positive; P = 0.74, log-rank test).
Figure 3.
Kaplan-Meier curves of time to death according to smear status among TB patients. A) Smear-positive vs. all others (negative, unknown, not performed); B) smear-positive vs. smear-negative. TB = tuberculosis.
DISCUSSION
In this large multicentre cohort study of persons with HIV treated for TB at six sites in Latin America, most patients did not have culture-confirmed TB. The risk of death tended to be higher in patients without culture-confirmed TB than those with culture-confirmed disease; however, after adjusting for other variables CIs included the null of no difference. Both key findings—that most patients treated for TB may not have TB, and that such patients have at least as high a risk of death as those with culture-confirmed disease—highlight the importance of improving our ability to diagnose TB and distinguishing it from other diseases in HIV-positive persons.
Our findings may be due to several factors. First, there can be difficulties in diagnosing TB, particularly in persons with advanced HIV (median CD4 count in our study was 96 cells/mm3). In addition, culture-negative TB is more common in persons with disseminated disease, which is more prevalent in advanced HIV disease.15 There was heterogeneity in the TB diagnostic tests used, and the study population was assessed for TB only if symptoms were present; however, as persons with advanced HIV can have TB without signs or symptoms,16–18 culture would not be performed.
The prevalence of bacteriologically confirmed TB in low-income countries has been approximately 30% according to previous reports.19 Most culture-negative cases are also smear-negative, which has been associated with unfavourable outcomes.12,20,21 However, in advanced HIV, where the risk of smear-negative TB is increased, a high proportion have culture-confirmed TB when culture is performed. Conversely, the proportion of persons who are culture-negative but smear-positive is low, at 3–9%.19 Smear-negative TB was not associated with an increased mortality risk than smear-positive TB in this cohort, as in previous reports from the United States.22 The reason for the lack of increased mortality risk when comparing smear-positive to smear-negative TB in some settings may be due to the availability of several TB diagnostic tests, and therefore not due to delays in TB diagnosis. In addition, in our study, patients included in the analysis were those who received anti-tuberculosis treatment; in other studies, anti-tuberculosis treatment may have required a positive diagnostic test. Finally, TB was the most prevalent opportunistic infection reported in our cohort,23 which may indicate greater awareness and prompt empiric anti-tuberculosis treatment.
It is, of course, possible that patients with culture-negative TB had other causes of disease, and may have died from these causes.24,25 In some settings, histoplasmosis often has a similar clinical presentation and such patients are therefore culture-negative for TB,26 which could indicate that many patients were treated for the wrong disease. Again, it is imperative to improve TB diagnosis, so that patients can be accurately diagnosed. Finally, as seen before, high CD4 cell counts at TB diagnosis and concomitant ART use were strongly predictive of improved survival.25,27
The study had several limitations. First, not all study participants had data on mycobacterial culture. There were some differences between persons who did vs. those who did not have cultures performed, although these two groups were similar according to age, CD4 and TB disease site. In a sensitivity analysis excluding patients who did not undergo culture testing, results were similar, with those patients who were not culture-positive having a higher risk of mortality. Our decision to compare those with and without TB culture confirmation as the primary analysis was intended to reflect ground realities in resource-limited settings, where it is quite common to treat without microbiological confirmation. As microbiological confirmation is included in national guidelines and WHO recommendations, lack of compliance with these could suggest patients are receiving low-standard care, which could be associated with poorer outcomes. However, it is worth noting that every site except for the Mexican site (which had only 27 cases) had a substantial proportion of patients with missing or unknown culture status.
Other study limitations are due to a lack of information on potential confounding variables and other issues that arise from using retrospective observational data. We did not have data on drug resistance in culture-negative TB cases, which could have contributed to the increased risk of death after anti-tuberculosis treatment. We did not have any data on adherence. Although most sites perform DOT, its implementation varies across sites and could potentially confound relationships seen in this study. Information on cause of death was not available. We presume that part of the increased risk of death among persons without culture-confirmed TB was due to treatment of the incorrect disease. However, additional data would confirm this hypothesis. A non-trivial proportion of our patients were lost to follow-up and rates of death were likely different among lost patients. We used multiple imputation to address missing covariate information, but it is possible that data were not missing at random even after conditioning on several relevant covariates. Our study spanned 16 years, and although results were similar when controlling for year of TB diagnosis, care of TB-HIV changed over this period and may have impacted study findings. A high proportion of patients were excluded because they did not receive standard anti-tuberculosis treatment. We did not have data on the reasons for non-standard treatment, but it could have been due, in part, to drug-drug interactions with ART. Finally, although our study comprised a large number of patients from six sites throughout Latin American, CIs for our adjusted analyses were wide, suggesting an insufficient number of events to make strong conclusions.
With the above limitations noted, we conclude that in our cohort, most HIV-positive persons treated for TB did not have culture-confirmed disease. Time to death in persons treated for TB without culture-confirmed disease tended to be shorter than in culture-positive TB patients, although this association was not statistically significant after adjusting for other factors. These results suggest that other diseases may be inappropriately treated as TB and underscores the importance of prompt, accurate TB diagnosis in HIV-positive persons. Additional studies in resource-limited settings, particularly studies that include autopsies, are important to better understand why mortality is particularly high for culture-negative, HIV-positive persons treated for TB.
Acknowledgements
The authors thank O Sued for his critical review of the manuscript; and all patients, care givers and data managers involved in the Caribbean, Central and South America network for HIV epidemiology (CCASAnet) cohort.
This work was supported by the National Institutes of Health-funded CCASAnet, a member cohort of the International Epidemiologic Databases to Evaluate AIDS (leDEA) (U01AI069923). This award is funded by the following institutes: Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Cancer Institute (NCI), National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Mental Health (NIMH), and the Office of the Director, National Institutes Of Health (OD), Bethesda, MD, USA (NIH U01 AI069923, K24 AI065298).
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report, 2017. WHO/HTM/TB/2017.23. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 2.Zenner D, Abubakar I, Conti S. Impact of TB on the survival of people living with HIV infection in England, Wales and Northern Ireland. Thorax 2015; 70: 566–573. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global tuberculosis report, 2016. WHO/HTM/TB/2016.13. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 4.Dorman S Advances in the diagnosis of tuberculosis: current status and future prospects. Int J Tuberc Lung Dis 2015; 19: 504–516. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Reported tuberculosis in the United States, 2015. Atlanta, GA, USA: US Department of Health and Human Services, 2016. https://www.cdc.gov/tb/statistics/reports/2015/default.htm-Table7. Accessed October 2018. [Google Scholar]
- 6.Perlman DC, El Sadr WM, Nelson ET, et al. Variation of chest radiographic patterns in pulmonary tuberculosis by degree of human immunodeficiency virus-related immunosuppression. Clin Infect Dis 1997; 25: 242–246. [DOI] [PubMed] [Google Scholar]
- 7.Pepper T, Joseph P, Mwenya C, et al. Normal chest radiography in pulmonary tuberculosis: implications for obtaining respiratory specimen cultures. Int J Tuberc Lung Dis 2008; 12: 397–403. [PubMed] [Google Scholar]
- 8.World Health Organization. Policy statement: liquid media for culture and DST. Geneva, Switzerland: WHO, 2007. [Google Scholar]
- 9.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010; 363: 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clouse K, Blevins M, Lindegren ML, et al. Low implementation of Xpert MTB/RIF among HIV/TB co-infected adults in the International epidemiologic Databases to Evaluate AIDS (IeDEA) program. PLOS ONE 2017; 12: e0171384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawn SD, Wood R. Tuberculosis in antiretroviral treatment services in resource-limited settings: addressing the challenges of screening and diagnosis. J Infect Dis 2011; 204 (Suppl 4): S1159–S1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton NT, Forson A, Lurie MN, Kudzawu S, Kwarteng E, Kwara A. Factors associated with mortality and default among patients with tuberculosis attending a teaching hospital clinic in Accra, Ghana. Trans R Soc Trop Med Hyg 2011; 105: 675–682. [DOI] [PubMed] [Google Scholar]
- 13.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet 2007; 369: 2042–2049. [DOI] [PubMed] [Google Scholar]
- 14.Giganti MJ, Luz PM, Caro-Vega Y, et al. A comparison of seven Cox regression-based models to account for heterogeneity across multiple HIV treatment cohorts in Latin America and the Caribbean. AIDS Res Hum Retroviruses 2015; 31: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassett IV, Wang B, Chetty S, et al. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis 2010; 51: 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shepherd BE, Rebeiro PF. Brief report: assessing and interpreting the association between continuous covariates and outcomes in observational studies of HIV using splines. J Acquir Immune Defic Syndr 2017; 74: e60–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawn SD, Kranzer K, Edwards DJ, McNally M, Bekker LG, Wood R. Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS 2010; 24: 1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belay M, Bjune G, Abebe F. Prevalence of tuberculosis, HIV, and TB-HIV co-infection among pulmonary tuberculosis suspects in a predominantly pastoralist area, northeast Ethiopia. Glob Health Action 2015; 8: 27949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGowan CC, Cahn P, Gotuzzo E, et al. Cohort Profile: Caribbean, Central and South America Network for HIV research (CCASAnet) collaboration within the International Epidemiologic Databases to Evaluate AIDS (IeDEA) programme. Int J Epidemiol 2007; 36: 969–976. [DOI] [PubMed] [Google Scholar]
- 20.Madico G, Mpeirwe M, White L et al. Detection and quantification of Mycobacterium tuberculosis in the sputum of culture-negative HIV-infected pulmonary tuberculosis suspects: a proof-of-concept study. PLOS ONE 2016; 11: e0158371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawn SD, Ayles H, Egwaga S, et al. Potential utility of empirical tuberculosis treatment for HIV-infected patients with advanced immunodeficiency in high TB-HIV burden settings. Int J Tuberc Lung Dis 2011; 15: 287–295. [PubMed] [Google Scholar]
- 22.Nakiyingi L, Ssengooba W, Nakanjako D, et al. Predictors and outcomes of mycobacteremia among HIV-infected smear-negative presumptive tuberculosis patients in Uganda. BMC Infect Dis 2015; 15: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavanaugh JS, Shah NS, Cain KP, Winston CA. Survival among patients with HIV infection and smear-negative pulmonary tuberculosis—United States, 1993–2006. PLOS ONE 2012; 7: e47855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crabtree-Ramírez B, Caro-Vega Y, Shepherd BE, et al. Time to HAART initiation after diagnosis and treatment of opportunistic infections in patients with AIDS in Latin America. PLOS ONE 2016; 11: e0153921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantipong P, Murakami K, Moolphate S, Aung MN, Yamada N. Causes of mortality among tuberculosis and HIV co-infected patients in Chiang Rai, Northern Thailand. HIV AIDS 2012; 4: 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adenis A, Nacher M, Hanf M, et al. Tuberculosis and histoplasmosis among human immunodeficiency virus-infected patients: a comparative study. Am J Trop Med Hyg 2014; 90: 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cain KP, Anekthananon T, Burapat C, et al. Causes of death in HIV-infected persons who have tuberculosis, Thailand. Emerg Infect Dis 2009; 15: 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]