Abstract
Exposure to air pollution and other environmental inhalation hazards, such as occupational exposures to dusts and fumes, aeroallergens, and tobacco smoke, is a significant cause of chronic lung inflammation leading to respiratory disease. It is now recognized that resolution of inflammation is an active process controlled by a novel family of small lipid mediators termed “specialized pro-resolving mediators” or SPMs, derived mainly from dietary omega-3 polyunsaturated fatty acids. Chronic inflammation results from an imbalance between pro-inflammatory and pro-resolution pathways. Research is ongoing to develop SPMs, and the pro-resolution pathway more generally, as a novel therapeutic approach to diseases characterized by chronic inflammation. Here, we will review evidence that the resolution pathway is dysregulated in chronic lung inflammatory diseases, and that SPMs and related molecules have exciting therapeutic potential to reverse or prevent chronic lung inflammation, with a focus on lung inflammation due to inhalation of environmental hazards including urban particulate matter, organic dusts and tobacco smoke.
Keywords: pro-resolving mediators, SPMs, air pollution, chronic inflammation, COPD, cigarette smoking
1. Introduction
The adult human lung has an approximate surface area for gas exchange of 100m2, about the size of half a standard tennis court, and, resting, inhales and exhales over 10,000 liters of air per day (more with exertion). As such, the lung is often the first point of contact and first line of defense for pathogens, particulate matter, dusts and fumes, smoke, and other inhaled environmental toxicants. Tobacco smoking is probably the most widespread intentional inhalation environmental exposure. However, exposure to second hand smoke, wood and biomass smoke, agricultural dust, traffic-related and other forms of air pollution, and other industrial inhalation hazards, also contribute to significant chronic lung inflammation and lung disease (Utell, et al., 1990). The host inflammatory response evolved to deal with insults such as these, and inflammation is generally a beneficial response. Yet, inflammation must be tightly regulated, as chronic unresolved inflammation causes or contributes to many significant pathologies of its own (Serhan, 2010). It was long assumed that resolution of inflammation was a passive process that occurred when the original stimulus was eliminated, resulting in down-regulation of pro-inflammatory mediators. However, recent research has uncovered a novel family of specialized pro-resolving lipid mediators (SPMs), mainly derived from arachidonic acid and dietary omega-3 polyunsaturated fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) (Chiang, et al., 2017; Duvall, et al., 2016; Serhan, et al., 2015). These mediators are critical to self-limited inflammatory responses and there is considerable evidence that chronic inflammation is marked by decreased levels of these mediators. There is also excitement that these molecules represent a new therapeutic opportunity for the many diseases which include chronic inflammation as a pathogenic feature (Serhan, et al., 2008). Here, we review emerging evidence that SPMs may represent novel therapies for lung diseases that result from acute and chronic exposures to environmental hazards including tobacco smoking, second hand smoke exposure, air pollution, industrial pollution, and other inhalation hazards.
2. Air pollution, cigarette smoke and other environmental lung insults
This review will examine chronic lung inflammation and inflammatory diseases resulting from environmental lung insults, and the potential for SPMs and other modifiers of the pro-resolution circuitry to moderate and treat lung disease.
Environmental lung insults range from the common (e.g. urban air pollution), to the uncommon (e.g. mine and factory dust, swine dust from commercial feedlot operations) to cigarettes and other forms of tobacco smoking, and come from a variety of sources. Air pollution and other environmental hazards are well-documented to cause acute and chronic lung and cardiopulmonary disease (Sydbom, et al., 2001; Thurston, et al., 2017). Typical urban air pollution is composed of partially combusted hydrocarbons from vehicle exhaust, gases such as ozone and nitrogen and sulfur oxides, particulates derived from sand, soil and other local terrestrial sources, and biological materials such as agricultural dust, bacteria and viruses, and aeroallergens such as pollen, mold and house dust mite antigens (Tunno, et al., 2018; Zeb, et al., 2018). Generally, the particulates of most concern, and that are subject to air pollution regulation, are known as PM2.5, particulate matter <2.5 microns in diameter. This is the size threshold to be deposited in the alveoli. Although each inhalation hazard has its own unique chemistry, they can have overlapping constituents and thus, activate pro-inflammatory programming via similar mechanisms.
Black carbon (soot) from combustion sources has a large and highly active surface area and can adsorb materials including metals and hydrocarbons (Long, et al., 2013). Metals, particularly copper and iron, can promote inflammation through generation of reactive oxygen species (Guastadisegni, et al., 2010; Harrington, et al., 2015; Michael, et al., 2013). Polyaromatic hydrocarbons such as naphthalene and anthracene are found in diesel exhaust and other combustion particulates and promote inflammation through upregulation of the aryl hydrocarbon receptor (Awji, et al., 2015; den Hartigh, et al., 2010; Martey, et al., 2005). These inflammatory mechanisms are common to combustion source air pollution including diesel exhaust particulates, urban particulate matter, wood smoke, and biomass smoke. Combustion sources also generate sulfur and nitrogen oxides, which are pro-inflammatory on their own, and which can generate ozone in the presence of sunlight, all of which are potent stimuli of lung inflammation via reactive oxygen species (Muller, et al., 2012; Zielinska, et al., 2010).
There are a number of biological sources that contribute to environmental lung injury and that also interact with combustion particles. Agricultural dusts, such as from commercial poultry and swine feeding operations, are strongly pro-inflammatory as well as allergenic. House dust mite and cockroach allergens act in a similar manner. Biological dusts can contain LPS and other microbial pathogen associated molecular patterns (PAMPs), which can cause inflammation via multiple innate immune pathways (Alexis, et al., 2006; Poole, et al., 2012; Wunschel, et al., 2016). Agricultural particulate matter also upregulates production of pro-inflammatory eicosanoids including prostaglandins, thromboxanes and leukotrienes by airway epithelial cells (Malireddy, et al., 2013). Additionally, these biological insults can interact with combustion products such as urban particulate matter, diesel exhaust, and cigarette smoke, to potentiate the effects of both insults. For example, low dose exposure to diesel exhaust or cigarette smoke can increase the risk of allergic sensitization by aeroallergens (Rumold, et al., 2001; Santos, et al., 2014; Sydbom, et al., 2001).
Inhalation of air pollution can cause both acute and chronic diseases, including hypersensitivity pneumonitis, acute respiratory distress syndrome, bronchitis, bronchiolitis, and emphysema. This is seen as the result of an imbalance between inflammatory and resolution pathways due to repeated or continued pro-inflammatory stimulation (Balode, et al., 2012; Bozinovski, et al., 2013; Dudek, et al., 2016). Although it is not our intention to discuss the immunological basis of asthma in this review, we will discuss the contribution of environmental hazards such as diesel exhaust and aeroallergens to asthma, and how SPMs might be used to treat asthma related to environmental hazards. There are many excellent reviews on the general subject of asthma and SPMs (Duvall, et al., 2017; Levy, et al., 2014; Miyata, et al., 2015; Robb, et al., 2016).
Air pollution also contributes to systemic inflammatory diseases including obesity and cardiovascular disease (Brook, et al., 2010; Moore, et al., 2016; Thurston, et al., 2017). This is now thought to be caused in part by inhalation of ultrafine particulates (UFP); particulate matter less than 0.1 microns in diameter. UFP have an active surface area that is orders of magnitude larger than the surface area of an equal mass of PM2.5, and generation of UFP is currently unregulated. UFP can cross the alveolar epithelium and endothelium and enter the bloodstream and have effects on distant organs (Utell, et al., 2000). UFP increased levels of pro-inflammatory eicosanoids including leukotrienes, prostaglandins and 8-isoprotane relative to anti-inflammatory lipid mediators in both mice and rat alveolar epithelial cells (Beck-Speier, et al., 2012).
An important aspect of air pollution and other environmental hazards is its pervasiveness, as people are exposed whenever they go outdoors (Samet, et al., 1991). Workers in occupational exposure settings may receive personal protective equipment, but compliance may be haphazard, and non-workers in homes and businesses downwind are still exposed. Government has attempted to limit urban PM2.5 by placing emissions controls on pollution sources such as vehicles, powerplant, and even residential lawn mowers and charcoal grills, but urban dwellers are still at the mercy of weather conditions that can cause PM2.5 levels vary dramatically from day to day and season to season (Samet, et al., 1991).
Cigarettes and other forms of tobacco smoking are probably the most common intentional inhalation insult. Tobacco smoke shares similarities with other combustion-sourced pollutants, including carbon particulates, partially combusted hydrocarbons, LPS and other biological products, and gasses such as ammonia (Roemer, et al., 2004). Tobacco smoke is well-understood to be a significant cause of diseases including lung cancer and chronic obstructive pulmonary disease (COPD; emphysema, chronic bronchitis and small airway remodeling). In many cases, the severity of COPD continues to worsen, even after smoking cessation, and smoking-related lung inflammation can persist for 10 years or more. As discussed below, this represents, at least in part, a failure of the resolution pathway to self-limit the inflammation caused by smoking, once smoking has ceased.
Tobacco smoking also has direct consequences for non-smokers who are exposed to environmental tobacco smoke (ETS), often referred to as secondhand smoke. ETS is a combination of exhaled mainstream smoke, side stream smoke that burns off the tip of the cigarette between puffs, and smoke particulates that settle on furnishings and remain persistent in the environment. Exposure to ETS is associated with increased risk of respiratory symptoms exposed groups such as flight attendants, and is also associated with increased allergic sensitization and susceptibility to respiratory infection in children who live in a home with a smoker (Beatty, et al., 2011; Eisner, et al., 2005; Gergen, et al., 1998; Goodwin, 2007; Lang, et al., 2013). Tobacco smoking is also a significant added risk factor for disease in people who have occupational exposures to other environmental insults (Khelifi, et al., 2014). This suggests that failure to resolve inflammation due to smoke exposure leaves the lungs in a vulnerable state that makes them more susceptible to second insults such as pathogens and aeroallergens.
New therapies for chronic lung inflammatory diseases are urgently needed. Corticosteroids are sometimes used, but chronic use is associated with significant adverse effects; moreover, some asthma and COPD patients become steroid-resistant (Barnes, 2013; Raissy, et al., 2013). Importantly, corticosteroids do not modify the underlying cause of chronic inflammation, namely an imbalance between pro-inflammatory and pro-resolving pathways that leads to pathologic chronic inflammation. Here, we discuss a newly identified family of endogenous lipid mediators that activate and regulate resolution of inflammation, which are responsible for the typical self-limiting nature of inflammation, and whose disruption is now recognized as a key contributor to chronic inflammation. We also discuss in vitro and in vivo evidence that these mediators, and the pro-resolution pathway more generally, are an important emerging target for therapeutic intervention.
3. Specialized Pro-Resolving Mediators (SPMs)
“Specialized pro-resolving lipid mediators” (SPMs) describes a family of small lipid molecules derived from endogenous or dietary long chain fatty acids via enzymatic and non-enzymatic reactions. The lipoxins were first reported in 1984 by Serhan and colleagues (Samuelsson, et al., 1985; Serhan, et al., 1984), with identification of additional DHA and EPA-derived SPMs beginning in 2000 (Serhan, et al., 2000). Four main classes of compounds have been extensively studied to date; lipoxins, resolvins, protectins and maresins (Schwab, et al., 2006; Serhan, et al., 2008) (Figure 1). Most of the SPMs are generated from the dietary omega-3 polyunsaturated fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), although the lipoxins are generated from arachidonic acid, and DHA can be produced in limited quantities in vivo through the action of elongation of very long chain fatty acids protein 2 (ELOVL2) (Chiurchiu, et al., 2016). Recently, it was discovered that precursors of MaR1, PD1 and RvD1 can form conjugates with small peptides derived from glutathione resulting in MCTRs (maresin conjugates in tissue repair), PCTRs (protectin conjugates in tissue repair) and RCTRs (resolvin conjugates in tissue repair), which have independent biological activity in the repair of tissue injury (Serhan, 2017). Resolvins and lipoxins can be degraded by the enzyme eicosanoid oxidoreductase (EOR, also known as 15-hydroxyprostaglandin dehydrogenase or 15-PGDH) into 8-oxo- and 17-oxo-RvD1, which have significantly reduced biological effect (Clish, et al., 2000; Sun, et al., 2007); while the production of lipoxins can be inhibited by epoxide hydrolases (Flitter, et al., 2017; Ono, et al., 2014; Yang, et al., 2015). It is plausible that similar enzymes exist to degrade other SPMs, as the resolution pathway is known to be as tightly controlled by feedback mechanisms as inflammatory pathways. SPMs act through a family of G-protein coupled receptors that includes the lipoxin A4 receptor ALX, also known as formyl peptide receptor 2 or FPR2; the D resolvin receptors (DRV)1 and 2, also known as GPR32 and GPR18 (Chiang, et al., 2015); and the E resolvin receptors (ERV)1 (ChemR23) and BLT1 (Duvall, et al., 2016). Receptors for other SPMs remain to be identified, and the downstream signaling events are also not well understood at this time (reviewed in (Chiang, et al., 2017)). Thus, the status of this pro-resolving signaling network depends on the presence of precursors, synthetic and degrading enzymes, and appropriate GPCRs on target cells.
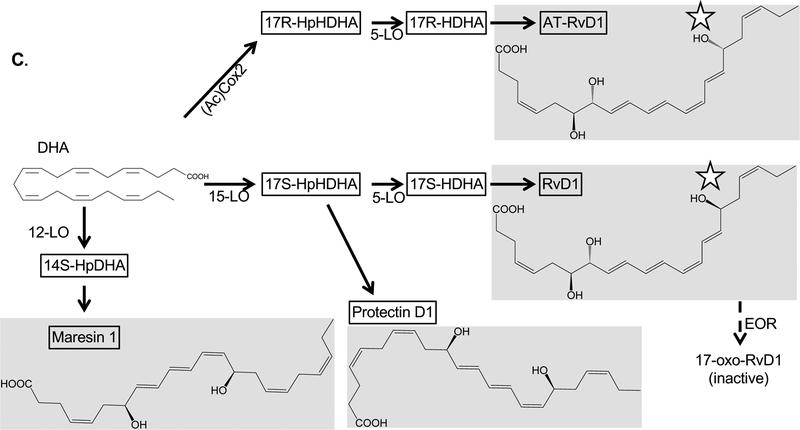
Figure 1.
Diagram of the synthetic pathways for the major classes of SPMs, with selected enzymatic processing steps, final products and degradation pathways. Stars indicate sites at which the aspirin-triggered epimers differ from the standard compound. Abbreviations: (Ac)Cox2, acetylated Cox2; CYP, Cytochrome P450 enzymes; EOR; epoxide oxoreductase; HDHA, hydroxydocosahexaenoic acid; HEPE, hydroxyeicosapentaenoic acid; HETE, hydroxyeicosatetraenoic acid; sHE, soluble epoxide hydrolase. Other abbreviations as defined in the main text.
Also of note is the effect of aspirin on SPM synthesis (Serhan, et al., 2008). Aspirin works in part by acetylating COX2, and acetylation changes the stereochemistry of some SPM synthesis reactions. For example, DHA is converted into 7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid (RvD1) by the action of 5-lipoxygenase (5-LO) and 15-lipoxygenase (15-LO). However, acetylated COX2 converts DHA into 7S,8R,17R-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid (abbreviated 17R-RvD1, aspirin triggered RvD1 or AT-RvD1) (Sun, et al., 2007). A similar reaction with arachidonic acid as the parent compound results in AT-LXA4 rather than LXA4 (Chiang, et al., 1998). AT-LXA4 can also be formed through cytochrome P450 enzymes that can generate 15R-HETE, the precursor to AT-LXA4, from arachidonic acid (thus, the description of these epimers as “aspirin-triggered” can be a misnomer)(Claria, et al., 1995; Keeney, et al., 1998)). These reactions are significant because the aspirin-triggered forms of the compounds have similar pro-resolving biological activity but are highly resistant to endogenous degradation mechanisms (Sun, et al., 2007). This presents a natural example of a modification that increases the compounds’ pharmaceutical utility while maintaining activity, and a number of animal model studies discussed below use AT-RvD1 rather than RvD1 for this reason.
SPMs exhibit a variety of anti-inflammatory and pro-resolving properties on numerous cell types. SPMs inhibit production of pro-inflammatory cytokines, downregulate neutrophil activation and migration, and increase non-phlogistic (non-inflammatory) activation of macrophage engulfment and removal of apoptotic inflammatory cells and debris (efferocytosis) (Basil, et al., 2016). SPMs also inhibit antigen presentation by dendritic cells, promote primary and memory B cell IgG and IgM responses while downregulating allergic IgE production and pro-inflammatory cytokines, and promote differentiation of Tregs while inhibiting Th1, Th2 and Th17 function (Duffney, et al., 2018). Importantly, SPMs are pro-resolution without being classically immunosuppressive, and may avoid the side-effects of chronic immune suppression of long-term therapy with corticosteroids and NSAIDs. In fact, by blocking the activity of COX2, NSAIDs have been shown to inhibit production of SPMs as well as inhibiting pro-inflammatory prostaglandins, resulting in delayed resolution of inflammation (Chan, et al., 2010; Willoughby, et al., 2000).
Key concepts in understanding the function of SPMs include lipid mediator class-switching and transcellular biosynthesis. Mediator class-switching describes the phenomenon in which the same cell type switches from producing pro-inflammatory mediators early in inflammation, to producing pro-resolving mediators during the resolution phase, by altering the expression of key synthetic enzymes to favor SPMs rather than inflammatory mediators. This was first described in a mouse model of air pouch inflammation, in which neutrophils switched from producing leukotrienes to lipoxins by downregulating 5-LO and upregulating 15-LO (Levy, et al., 2001), and has subsequently been demonstrated in human neutrophils, lung fibroblasts, and other cells (Lacy, et al., 2016). Transcellular biosynthesis is the observation that some cells only produce SPM precursors but lack the enzymes needed to produce the final SPM; while other cell types can produce the final SPM if provided the precursor (Capra, et al., 2015; Serhan, et al., 2000). Thus, under normal conditions, SPM biosynthesis is tightly controlled, both spatially and temporally.
Although not classified as SPMs, the epoxyeicosatrienoic acids (EETs) and hydroxyeicosatetraenoic acids (HETEs) are of interest, as they are produced by similar mechanism, and function as indirect precursors of some SPMs in transcellular biosynthesis. EETs are synthesized from omega-6 fatty acids including arachidonic acid via cytochrome P450 epoxygenases, while HETEs are generated by lipoxygenases (LOs) (reviewed in (Capra, et al., 2015; Lopez-Vicario, et al., 2016; Zeldin, 2001)). HETEs and EETs can serve as precursors of SPMs, and also have potent actions of their own, including regulating inflammatory signaling (Node, et al., 1999; Spector, et al., 2004; Thomson, et al., 2012). As resolvins can be degraded by endogenously produced EOR, EETs can be degraded by endogenous soluble epoxide hydrolases (sEHs), which can promote inflammation by limiting the production of SPMs through reduction in levels of precursors (Liu, et al., 2011; Schmelzer, et al., 2005; Yang, et al., 2015).
SPMs are of significant interest as potential pharmaceuticals (Lee, 2012). Since they are produced endogenously, they are expected to have an excellent safety profile, and the long carbon backbone is amenable to derivatization to increase potency and half-life, reduce local degradation, or to modify its receptor selectivity and mode of action (Serhan, et al., 2008). Several clinical trials of dietary supplementation with omega-3 polyunsaturated fatty acids generally, or purified DHA and EPA specifically, have been completed or are underway, albeit with mixed results, as discussed below. Trials of resolvins for psoriasis (NCT03483311) and chronic pain (NCT03095157) are planned or underway, and a trial of an RvE1 derivative for inflammatory dry eye disease has recently completed (NCT00799552). An LXA4 precursor showed efficacy in a clinical trial in infantile eczema (Wu, et al., 2013). The GPCRs through which SPMs signal are also targets for novel small molecule antagonists that would not necessarily need to be derived from SPMs themselves. As the longest-recognized SPM receptor, ALX/FPR2 is farthest along this developmental pathway (Bozinovski, et al., 2013; Corminboeuf, et al., 2015), while the fact that many SPMs receptors have not yet been identified is an obvious hurdle still to be overcome. Beyond treatment with SPMs themselves, other steps in the pro-resolution pathway offer potential therapeutic targets. For example, lipoxygenase activators may promote SPM production, while selected lipoxygenase inhibitors could enhance production of selected SPMs by blocking production of less desirable SPMs that compete for the same precursor pool. EOR and other enzymes that degrade SPMs are also potential targets for small molecule inhibitors, which would increase the half-life of endogenously produced SPMs (Pillarisetti, et al., 2012). Soluble epoxide hydrolase inhibitors (sEHIs) have shown promise in pre-clinical animal models of lung inflammation (Liu, et al., 2011; Podolin, et al., 2013; Schmelzer, et al., 2005; Yang, et al., 2015). Pro-resolving circuits can also be targeted with therapeutic micro-RNAs (Fredman, et al., 2012; Recchiuti, et al., 2011; Recchiuti, et al., 2012). The effectors of the pro-resolution pathway (SPMs, receptors, key enzymes) represent a fertile area for pharmaceutical development for chronic inflammatory lung diseases that have escaped their endogenous self-limited purpose.
4. SPMs and Air Pollution: Evidence from human studies
Epidemiological and human studies suggest that lipid mediators are markers of exposure to inhaled toxicants and cardiopulmonary diseases. Dysregulated lipid levels are also associated with exposure-related inflammation and diseases. However, studies of dietary supplementation with pro-resolving lipid mediators and precursors, as a preventative or treatment strategy, have revealed mixed results to date.
Increased levels of inflammatory lipid mediators are associated with the inhalation of air pollution. Oxylipins that promote airway inflammation, including 12,13-DiHOME, 9-HODE, 5-HETE, and 13-HODE, are increased in the plasma and bronchoalveolar lavage (BAL) fluid of adults up to 24 hours after an acute exposure to biodiesel exhaust or subway air (Gouveia-Figueira, et al., 2017; Gouveia-Figueira, et al., 2018; Lundstrom, et al., 2011). 8-isoprostane, an indicator of oxidative stress that is derived from arachidonic acid, is increased in people exposed to air pollutants. 8-isoprostane is also elevated in the exhaled breath condensate of children exposed to black carbon particulate matter and in the urine of individuals exposed to wood smoke from cooking fires (Allen, et al., 2011; Barregard, et al., 2006; Commodore, et al., 2013; Patel, et al., 2013). Similarly, another marker of lipid peroxidation, urinary 8‐epi‐prostaglandin F2α, is increased in individuals after short-term exposure to fine particulate matter (W. Li, et al., 2016). Even prenatal exposures to particulate matter have been correlated with increased levels of inflammatory lipoxygenase metabolites, such as 5-HETE, 9-HODE, and 15-HETE, in neonatal cord blood (Martens, et al., 2017). Increased inflammatory lipid levels in people are associated with the inhalation of pollutants and inflammatory diseases related to air pollution.
Asthma, an inflammatory allergic airway disease, is associated with increased concentrations of 8-isoprostane and decreased levels of pro-resolving LXA4 in blood, sputum, and bronchoalveolar lavage fluid (Duvall, et al., 2016; Ono, et al., 2014). This disease is associated with increased bronchoalveolar lavage levels of prostaglandin (PG)D2, which causes bronchoconstriction (Fajt, et al., 2013). Inflammatory cysteinyl leukotrienes derived from arachidonic acid are also elevated while protectin D1 (PD1) is reduced in the exhaled breath condensate of asthmatics (Duvall, et al., 2016; Levy, Kohli, et al., 2007; Linares Segovia, et al., 2014). Similarly, increased arachidonic acid levels and decreased DHA was found in the nasal scrapings of people with asthma (Freedman, et al., 2004). Inflammatory lipid mediators are correlated with airway inflammation.
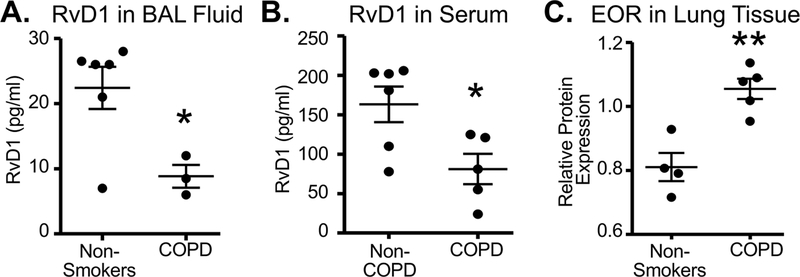
As tobacco smoking in various forms is the most common form of intentional exposure to “air pollution,” there is considerable interest in the effects of smoking on pro-inflammatory and pro-resolving lipids. For example, RvD1 is decreased, while 17-HDHA, 14-HDHA, 12-HETE, and 15-HETE are increased, in the BAL of individuals with COPD (Croasdell, et al., 2015). Similarly, patients with COPD exhibit increased leukotrienes and decreased LXA4 in BAL and sputum, suggesting an imbalance between pro-inflammatory and pro-resolving pathways (Balode, et al., 2012; Duvall, et al., 2016). Serum amyloid A is a pro-inflammatory mediator that binds to the ALX/FPR2 receptor and opposes the pro-resolving effects of LXA4 which acts through the same receptor. Serum amyloid A was detected in the serum and BAL fluid of COPD patients, and was dramatically increased relative to LXA4 during acute exacerbations of COPD (Bozinovski, et al., 2012), consistent with the hypothesis that smoking results in an imbalance between inflammation and resolution. Smokers with COPD also have higher serum levels of monounsaturated fatty acids and lower levels of omega 3 fatty acids compared to nonsmokers (Titz, et al., 2016). Increased PGE2 is also present in the sputum and exhaled breath condensate and increased serum amyloid A, which can bind to and block ALX receptors, is increased in the BAL of COPD patients (Duvall, et al., 2016; Zaslona, et al., 2015). Our own work with lung lipid mediators in cigarette smoking is summarized in Figure 2. We found that RvD1 is decreased in BAL and serum from COPD patients relative to healthy non-smokers (Croasdell, et al., 2015). Intriguingly, expression of eicosanoid oxidoreductase (EOR), was increased in lung tissue of COPD patients (Hsiao, et al., 2015), suggesting a possible explanation for diminished RvD1 levels (Figure 2C). Although we are not directly considering respiratory infections in this review, it is worth noting that individuals with cystic fibrosis exhibit reduced lung levels of omega 3 fatty acids and lipoxins (Eickmeier, et al., 2017; Karp, et al., 2004), and that Pseudomonas aeruginosa, a key pathogen in cystic fibrosis, expresses an epoxide hydrolase as a virulence factor. This enzyme hydrolyzes anti-inflammatory EETs including the lipoxin precursor 14,15-EET, inhibiting the production of pro-resolving lipoxins (Flitter, et al., 2017). Thus, induction of SPM-destroying enzymes as a pro-inflammatory response has been adopted as an evolutionary strategy by at least one significant pathogen. Taken together, our results along with others show that inhalation of cigarette smoke, diesel exhaust, and other inhalation insults, both acutely and chronically, can depress normal endogenous resolution signals in the lungs.
Figure 2. RvD1 is decreased in COPD patients.
Panels A and B: RvD1 was measured in bronchoalveolar lavage fluid (BAL) and serum from non-smokers without COPD and from COPD patients by EIA as previously reported (Croasdell, et al., 2015). Panel C: Levels of eicosanoid oxidoreductase (EOR), an enzyme that degrades and inactivates RvD1, were measured in lung tissue from non-smoking donors or COPD patients, as previously reported (Hsiao, et al., 2015).
Similarly, a Western high fat diet with arachidonic acid is associated with an increased risk for cardiovascular disease. Higher circulating levels of DHA and EPA in individuals are inversely correlated with cardiovascular disease (Superko, et al., 2014). Higher serum concentrations of arachidonic acid and reduced levels of RvD1 are also detected in symptomatic patients with carotid disease (Bazan, et al., 2017). People with CVD also have increased circulating levels of 8-isoprostane.
Evidence of the efficacy of supplementation with omega-3 polyunsaturated fatty acids is mixed. A recent controlled trial of fish oil supplementation found a significant reduction in wheeze, asthma, and respiratory infections in 3 year old children whose mothers received fish oil during the third trimester (Bisgaard, et al., 2016), while similar trials showed that prenatal omega-3 supplementation increased the levels of DHA and reduced allergic responses in infants (D’Vaz, et al., 2012; Miyake, et al., 2007). However, two other trials found no difference in allergic sensitization in children after pre-natal fish oil supplementation at 6 years of follow up (Best, et al., 2016; Best, et al., 2018). A systematic review of diet and air pollution found that a Mediterranean diet high in omega-3 fatty acids was associated with reduced airway disease in smokers and people exposed to air pollution, but there was insufficient data that omega-3 fatty acid supplementation was protective against the effects of exposure to air pollution other than tobacco smoke (Whyand, et al., 2018).
There have been a number of observational studies and clinical trials examining omega-3 fatty acid supplementation in individuals with COPD, also with mixed results. A recent placebo-controlled feasibility trial discovered challenges in recruitment participant retention, and adherence to the supplement protocol for studies of omega-3 supplementation in COPD patients (Fulton, et al., 2017). A longitudinal and a cross-sectional retrospective study, which determined DHA and EPA intake through patient-reported dietary information, found no association between omega-3 fatty acid consumption and COPD (McKeever, et al., 2008; Tabak, et al., 1998). Similarly, a prospective cohort study also found no association between diets reported to have higher amounts of omega-3 fatty acid foods and COPD mortality (Walda, et al., 2002). However, intake of omega-3 fatty acids by participant recall was inversely correlated with COPD in a prospective case-control study and a cross-sectional study (Ahmadi, et al., 2012; de Batlle, et al., 2012). Increased dietary omega-3 fatty acids were also associated with a decreased risk for COPD in the Nurses’ Health Study and lowered serum levels of inflammatory markers. In addition to dietary information, greater blood levels of EPA but not DHA were associated with inflammatory markers and COPD in prospective studies (De Castro, et al., 2007; Novgorodtseva, et al., 2013). Recent systematic meta-analyses report inconclusive evidence to support the hypothesis that omega-3 fatty acid supplementation reduces the risk of COPD (Atlantis, et al., 2016; Fulton, et al., 2015).
There is currently an ancillary study of a clinical trial in progress examining the effects of omega-3 fatty acid supplementation on COPD outcomes and exacerbations (Gold, et al., 2016). One of primary aims of this clinical trial (NCT01169259) and the OMEGA-SPM-DOSE study (NCT02719665) are to determine if omega-3 supplementation affects cardiovascular disease. This is an important research question since, similar to COPD, mixed results have been found with omega fatty acid intake and cardiovascular disease outcomes. Meta-analyses and studies have reported that omega-3 fatty acid supplementation did not reduce the risk for cardiovascular disease events or markers of oxidative stress and inflammation (Tenenbaum, et al., 2018). Yet, a recent meta-analysis of randomized controlled trials found that omega-3 fatty acids reduced inflammatory cytokines and improved vascular dysfunction in patients with coronary artery disease (Tenenbaum, et al., 2018). It’s thought that these variable results may be due in part, to differences in the dose of omega-3 fatty acid supplementation (Tenenbaum, et al., 2018). Additionally, a recent study found that higher serum levels of DHA and EPA were correlated with lower fibrinogen levels in patients exposed to ambient air pollution (Croft, et al., 2018). Similarly, omega-3 fatty acid supplementation was found to be protective against particulate matter-related cardiac function abnormalities (Peter, et al., 2015). There are variable data about the effects of omega-3 fatty acids and pro-resolving lipid mediators on cardiovascular disease.
Human studies show that inflammatory and pro-resolving lipid mediators could be potential biomarkers of exposure to air pollutants, as well as contribute to toxicant-related diseases. However, more clinical studies are needed to understand the effects of supplementing pro-resolving lipid mediators on toxicant-associated inflammatory lung diseases and respiratory infections. However, recent experimental studies provide strong evidence that pro-resolving lipid mediators may be effective therapies against pollutant induced-lung inflammation.
5. In vitro investigations in lung and airway cells.
Many important insights into the use of SPMs are potential therapies for environmental exposure driven lung disease have been obtained from in vitro studies of lung cells exposed to inflammatory stimuli (Table 1). Here, we consider epithelial cells as first point of contact with inhaled insults, macrophages as a key line of defense which act to engulf and remove inhaled particulate matter, and fibroblasts and other mesenchymal cells.
Table 1:
In vitro studies of SPMs in lung cells suggesting therapeutic potential
| Lipid/Lipid modifier | Targets | Mechanism | Cell types reported | Reference |
| EPA, DHA | Increased production of pro-resolution lipid mediators; increases recellularization of lung scaffolds | MSCs, lung fibroblasts, epithelial cells and monocytes | (Abreu, et al., 2018; Nordgren, et al., 2014; Nordgren, Heires, et al., 2018) | |
| RvD1 and AT-RvD1 | GPR32, ALX/FPR2 | Reduce inflammatory cytokines, increase macrophage phagocytosis and M2 phenotype, reduce NF-κB, STAT6, STAT1, TAK1, TBK signaling | Macrophages, Lung fibroblasts, bronchial epithelial cells, small airway epithelial cells | (Croasdell, et al., 2015; de Oliveira, et al., 2017; Hsiao, et al., 2013; Hsiao, et al., 2014) |
| RvD2 | GPR32, ALX/FPR2 | Reduce inflammatory cytokines, increase phagocytosis in macrophages exposed to cigarette smoke extract | Macrophages | (Croasdell, et al., 2015) |
| MaR1 | Serum Response element signaling | Reduction of IL-6 and IL-8, lowered neutrophil accumulation, IL-6 and TNFα levels in BAL | Bronchial epithelial cells | (Nordgren, et al., 2013) |
| Protectin | Increased differentiation of macrophages, increased efferocytosis/phagocytosis | Monocytes, macrophages | (Pistorius, et al., 2018) | |
| Soluble epoxide hydrolase inhibitors (sEHIs) | Soluble epoxide hydrolase | Reduced breakdown of pro-resolution lipid mediators | Ex vivo analysis of human and rodent blood, serum, sputum and BAL cells | (Ono, et al., 2014; Podolin, et al., 2013). |
Abbreviations:
AT-RvD1, aspirin-triggered resolvin D1
BAL, bronchoalveolar lavage
DHA, docosahexaenoic acid
EPA, eicosapentaenoic acid
MaR1, maresin 1
MSC, mesenchymal stem cells or mesenchymal stromal cells
RvD1, resolvin D1
RvD2, resolvin D2
sEHI, soluble epoxide hydrolase inhibitor
Epithelial Cells
Lung airway epithelial cells are critical to maintaining airway homeostasis and are often the first responders to exposures (Muller, et al., 2012). Several studies have revealed that diesel exhaust particles (DEP) alter lipid mediator production in epithelial cells. A549 cells, a transformed alveolar epithelial cell line, was exposed to DEP, and COX-2 and PGE2 production were measured (Ahn, et al., 2008). DEP lead to a dramatic increase in both COX-2 expression and production of its downstream metabolite, PGE2. In a more recent study, immortalized human bronchial epithelial cells were exposed to DEP for either acute or long-term periods (Rynning, et al., 2018). Short term exposure to DEP induced production of both PGE2 and PGF2α. Interestingly, the epithelial cells exposed to long term DEP showed a more fibroblast like morphology, expressed mesenchymal markers such as vimentin and released increased levels of PGE2 and PGF2α even after DEP was removed, though levels of LXA4 and HETE metabolites remained unchanged. This suggests DEP is able to transform these epithelial cells into a more pro-inflammatory cell type that may increase susceptibility and immunomodulation to promote allergic asthma and COPD (Rynning, et al., 2018). Mechanistically, wood smoke enhanced cigarette smoke-induced inflammation in human airway epithelial cells by blocking LXA4 production (Awji, et al., 2015), while RvD1 inhibited IL-8 production and NF-κB activation in human bronchial epithelial cells (Dong, et al., 2014).
House dust mite allergen includes both bacterial LPS and allergenic dust mite proteins, the combination of insults provokes strong inflammatory responses from airway epithelial cells and can cause airway inflammation and asthmatic symptoms. Both RvD1 and AT-RvD1 significantly reduced inflammatory cytokine production in bronchial epithelial cells with associated reductions in NF-κB, STAT6 and STAT1 signaling (de Oliveira, et al., 2017). This study also confirmed that the effects of RvD1 were dependent upon the known RvD1 receptor, FPR2/ALX, as had previously been reported for LXA4 (Bonnans, et al., 2006; Bozinovski, et al., 2012).
Airborne agricultural particular matter (PM) from poultry dust can also increase respiratory tract inflammation, common in agricultural workers. Respiratory epithelial cells exposed to agricultural PM produced increased levels of prostaglandins, thromboxane A2 and leukotrienes B4 and C4 (Malireddy, et al., 2013). Testing therapeutic uses of SPMs, several studies show that adding SPMs to cell cultures limits inflammatory responses from agricultural organic dust exposure in bronchial epithelial cells. MaR1 dose dependently reduced IL-6 and IL-8 production in bronchial epithelial cells exposed to swine dust (Nordgren, et al., 2013). Mechanistically, MaR1 appears to act by limiting protein kinase C (PKC) and Serum Response Element (SRE) transcriptional activity while not inhibiting other inflammatory signaling pathways such as NF-κB and AP-1 signaling. Another study by the same group showed that epidermal growth factor receptor (EGFR) signaling is disrupted in both human bronchiolar epithelial cells and in mice exposed to agricultural dust extract, while DHA supplementation increased EGFR signaling in both in vitro and in vivo models following dust exposure (Nordgren, Heires, et al., 2018). EGFR signaling is an important regulator of repair pathways in airway epithelial cells after injury. While DHA appears to increase production of the EGFR ligand, amphiregulin to increase signaling, it not clear how the omega-3 fatty acid signals in this context. These studies show that respiratory tract epithelial cells can respond to environmental insults through alterations in lipid mediator production.
Macrophages
Macrophages are key cells that coordinate the response to exposure related lung disease. Macrophages, derived from monocytes, aid in clearance of apoptotic cells, cell debris and foreign bodies. Air pollutants including cigarette smoke and diesel exhaust particulates promote production of inflammatory mediators including pro-inflammatory lipids like leukotrienes and prostaglandins via the COX2/arachidonic acid pathway. Rat alveolar macrophages exposed to DEP showed increased expression of COX2 and a concomitant increase in PGE2 production (Bhavaraju, et al., 2014). LPS, a powerful inflammatory stimulant and inducer of COX-2 on its own, could be further pushed to induce COX-2 expression in human monocytes with the presence of DEP (Hofer, et al., 2004). These studies went further to show that DEP could further enhance other pathways that induce COX-2 in human monocytes including activation with the Toll-like receptor 2 (TLR2) ligand, Pam3Cys. Additional studies have shown a prominent COX-2 response in macrophages when they are cultured with airborne particulate matter. RAW 264.7 mouse macrophages were exposed to air pollution particulate matter which caused large increases in oxidative stress and PGE2 production (Schneider, et al., 2005). Addition of glutathione, a powerful antioxidant was able to prevent the particulate matter’s ability to increase PGE2 levels. Agglomerates of ultrafine particles that contain carbon and titanium dioxide also induce changes in lipid mediators in alveolar macrophages (Beck-Speier, et al., 2001). Canine macrophages exposed to ultrafine particles produced high levels of PGE2 and LTB4. Alveolar macrophages are also a major source of the pro-inflammatory leukotriene, LTB4. LTB4 is elevated in asthmatics and recent work suggests that phthalate exposure is linked to LTB4 levels. A recent study showed that primary rat alveolar macrophages exposed to mono(2-ethylhexyl) phthalate produced elevated levels of LTB4 through phthalate induced ROS formation and the 5-LO pathway(Rakkestad, et al., 2010). Macrophages are a key effector cell and can respond robustly to a variety of environmental and pharmacological exposures.
Several studies show the importance of SPMs in this pathway. For instance, a recent study reported that the protectin metabolic pathway regulates human monocyte to macrophage differentiation and macrophage function (Pistorius, et al., 2018). The authors showed that inhibition of protectin synthesis in human and mouse monocytes led to a loss of macrophage differentiation and a dramatic reduction in efferocytosis and bacterial phagocytosis. Thus, a deficiency in protectin or protectin precursors leads to a loss of functional macrophages and likely prevents resolution of inflammation. Another recent study also showed macrophages can also produce maresins such as 13S,14S-epoxy-maresin (13,14-eMar) and MaR1, derived from DHA (Dalli, et al., 2013). 13,14-eMaR was shown to be a pro-resolution SPM by blocking LTA4 hydrolase and by promoting conversion of pro-inflammatory M1 macrophages to the pro-resolving M2 phenotype. In addition to protectins and maresins, RvD1 and RvD2 have been shown by our group to play an important role in pro-resolving macrophage function. RvD1 and RvD2 attenuated spontaneous release of pro-inflammatory cytokines including IL-8 and TNFα by BAL macrophages from COPD patients, while increasing their capacity to phagocytose labeled bacteria (Croasdell, et al., 2015). RvD1 and RvD2 had the same effect on monocyote derived macrophages exposed to cigarette smoke extract in vitro, as well as reducing the carbonylation of cytoplasmic proteins, a marker of oxidative stress. The resolvins acted by dampening NF-κB activation in a receptor-mediated mechanism (Croasdell, et al., 2015; Hsiao, et al., 2013). There appears to be a fine balance between production of lipid mediators based on the protective/resolving COX and pro-inflammatory LO pathways (namely PGE2 and LTB4, respectively). When pro-inflammatory LO pathways overtake the other side, serious lung disease can result.
Mesenchymal cells, fibroblasts and other lung cells
Mesenchymal stem/stromal cells (MSCs) are another key player in lung repair and resolution processes. A recent study on human lung MSCs shows that agricultural waste products disrupt lipid mediator signaling (Nordgren, Bailey, et al., 2018). Agricultural organic dust is an important exposure that triggers airway inflammation and it has been implicated in the increase in lung disease observed in agricultural workers. In the study, MSCs were stimulated with dust extract obtained from swine containment facilities. Exposed MSCs produced more inflammatory mediators such as TNFα, IL-6 and IL-8 as well as increased production of resolving molecules such as RvD1. These studies suggest lung resident MSCs are a source of pro-resolution molecules that are important in response to environmental exposures (Nordgren, Bailey, et al., 2018). Although this report did not identify specific functional mediators, a previous study in an LPS-induced acute lung injury model reported that the protective effect of MSCs was conveyed by LXA4 (Fang, et al., 2015). In studies by our group, primary human lung fibroblasts, small airway epithelial cells and blood monocytes were exposed to cigarette smoke extract, which increased pro-inflammatory responses including IL-6 and IL-8. Remarkably, RvD1 attenuated this proinflammatory signaling in all three cell types (Croasdell, et al., 2015; Hsiao, et al., 2013). Interestingly we also found that lung fibroblasts treated with cigarette smoke extract produced both pro-inflammatory and pro-resolving mediators in a temporally regulated manner (Lacy, et al., 2016). At day 1–2 after treatment with cigarette smoke extract, lung fibroblasts expressed increased COX2 and produced pro-inflammatory mediators including IL-6 and PGE2; however, at later time points, COX2 expression and PGE2 production waned and was replaced by production of anti-inflammatory prostaglandins including ligands for peroxisome proliferator-activated receptor γ (PPARγ). This is consistent with other studies showing that cells can produce both pro-inflammatory and pro-resolving mediators in a temporally regulated manner; responding to the inflammatory insult at first but with the capacity to limit their own inflammatory responses to prevent them from becoming pathogenic.
Few studies have examined the response of primary neutrophils to air pollution in vitro. One recent study showed neutrophils are readily activated by DEP exposure (Matsuzaki, et al., 2006). DEP induced IL-8, LTB4 and MMP-9 production in neutrophils demonstrating that activated neutrophils contribute to DEP-mediated chronic inflammatory lung diseases. There is ample evidence in other inflammatory models that neutrophils contribute to both the pro-inflammatory and pro-resolving environment, and that they can both produce and respond to SPMs to enhance resolution of inflammation (reviewed in (Jones, et al., 2016)). And, as discussed below, there is strong evidence for neutrophil involvement in therapeutic studies of SPMs in animal models of exposure to smoke and other inhalation insults.
6. Insights from animal models including therapeutic trials
Promising in vitro results combined with in vivo studies of pro-inflammatory and pro-resolving responses in lung inflammatory lung disease models suggest that SPMs have important therapeutic potential in chronic lung disease.
The majority of studies of SPMs in animal models of lung disease to date involve mouse models of allergic airways disease or asthma, usually initiated by model allergens such as ovalbumin (OVA) or house dust mite allergen. These studies have been comprehensively reviewed elsewhere (Barnig, et al., 2018; Duvall, et al., 2016; Miyata, et al., 2015). Briefly, there is strong evidence that SPM production is attenuated in allergic airways disease, including reductions in maresins, lipoxins, protectins and resolvins, consistent with reports from human studies (Bilal, et al., 2011; Kolmert, et al., 2018; Krishnamoorthy, et al., 2015; Pyrillou, et al., 2018). Different models show distinct SPM profiles, although whether this is due to differences in the antigen response or detection method, remains unclear.
A few studies to date have looked at the involvement of SPMs in asthma as exacerbated by different forms of air pollution. Ambient ultrafine particulate matter (UFP) is a well-documented risk factor for both cardiovascular and respiratory diseases. UFP inhalation induces oxidative stress in the lung that may contribute to allergen-induced lung inflammation (Beck-Speier, et al., 2001). UFP exposure in OVA-allergen challenged mice significantly increased production of both inflammatory (LTB4, 8-isoprostane) and anti-inflammatory/pro-resolving lipid mediators (LXA4, 15-HETE and PGE2) in the lung (Beck-Speier, et al., 2012). While both pro inflammatory and pro-resolution molecules were induced, UFP tips the balance towards inflammatory signals creating a more robust allergen response to OVA. Thus, an imbalance of lipid mediator signaling may be a root cause of the increased susceptibility of asthma patients to particulate matter. Another recent study investigated whether or not airborne UFP exposure alters fatty acid metabolism and lipid mediator production in the gut (R. Li, et al., 2015). Mice lacking the LDL receptor were exposed to UFP or filtered air for 10 weeks in a chronic exposure model. The authors first measured lipid mediators present in the small intestine. Several lipid mediators including: 15-HETE, 12-HETE, 5-HETE and PGD2 were upregulated in response to UFP. This result suggests that UFP, like PM2.5 impacts inflammatory signaling through altering lipid mediator production.
Wood smoke is another combustion-derived source of ambient air pollution, and chronic exposure is associated with bronchitis, respiratory infection, and lung cancer. In mice, wood smoke exposure induces neutrophilic inflammation similar to cigarette smoke, including significant decreases in LXA4 (Awji, et al., 2015). Interestingly, wood smoke also synergized with cigarette smoke to cause greater inflammation and suppression of LXA4 than either insult alone. LXA4 is also implicated in a guinea pig model of chronic bronchitis following cigarette smoke exposure. Cigarette smoke exposure alone significantly suppressed LXA4 in BAL fluid. Treatment with naringin, a flavanone extracted from citrus fruits, reversed the pro-inflammatory effects of smoke exposure, including inflammation, goblet cell hyperplasia and TNFα production, and also restored normal levels of LXA4 (Luo, et al., 2012). Overall, the evidence supports the conclusion that inhalation of combustion-derived air pollution disrupts the balance of inflammatory and resolution pathways in a profoundly pro-inflammatory way.
Many SPMs and SPM precursors that may aid in resolving lung disease have been investigated in preclinical animal models, using both dietary supplementation with DHA and EPA, or with direct therapeutic administration of specific purified SPMs. In rodent models of allergic airways inflammation or asthma, therapeutic efficacy has been demonstrated for LXA4, LXB4, MaR1, RvE1, RvD1, PD1 and fish oil dietary supplementation (Duvall, et al., 2016; Flesher, et al., 2014; Ishizuka, et al., 2008; Karra, et al., 2015; Krishnamoorthy, et al., 2015; Levy, Lukacs, et al., 2007; Navarro-Xavier, et al., 2016; Rogerio, et al., 2012). Pre-treatment of MSCs with EPA enhanced their ability to resolve lung inflammation after sensitization and challenge with house dust mite allergen (Abreu, et al., 2018). The importance of LXA4 was further illustrated by a study using an inhibitor of leukotriene A4 hydrolase (LTA4H), the enzyme responsible for production of the proinflammatory LTB4. The LTA4H inhibitor attenuated allergic airway hyperresponsiveness in mice, which was accompanied by dramatically increased levels of LXA4, possibly due to shunting of precursors to the lipoxin pathway (Rao, et al., 2010).
Studies examining the efficacy of SPM in animal models of lung disease due to air pollution and other inhaled insults are summarized in Table 2. To date there are few published studies examining the efficacy of SPMs in asthma models exacerbated by air pollution. One such study investigated the effects of urban particulate matter (PM), which is hypothesized to have contributed to the rapid increase in the number of people with asthma. In the OVA model of allergic asthma in mice, co-exposure to urban PM2.5 significantly increased lung inflammation, BAL cell counts, and the Th2 cytokines IL-5, IL-13 and IL-33. Treatment with LXA4 significantly attenuated the effect of urban PM by reducing BAL cell infiltrates, tissue inflammation, Th2 cytokines, and dampening expression of the key Th2 transcription factors RORα and GATA3 (Lu, et al., 2018).
Table 2:
Therapeutic uses of SPMs in animal models of lung disease influenced by air pollution and smoking exposures
| Lipid/Lipid Modifier | In vivo model | Result | Reference |
|---|---|---|---|
| EPA | Allergic airways inflammation to house dust mite antigen | MSCs pre-treated with EPA suppressed inflammation and produced elevated levels of SPMs | (Abreu, et al., 2018) |
| DHA | Instillation of agricultural dust | Dietary DHA reduced BAL neutrophil influx and pro-inflammatory cytokines | (Nordgren, et al., 2014) |
| DHA | Instillation of agricultural dust | Mice on a high-DHA diet were protected from inflammation and exhibited increased levels of the repair protein amphiregulin | (Nordgren, Heires, et al., 2018) |
| Omega-3 PUFA | RCT of omega-3 PUFA supplementation in horses with chronic respiratory disease | Supplementation increased plasma levels of DHA and significantly reduced clinical symptoms compared to a low-dust diet alone | (Nogradi, et al., 2015) |
| MaR1 | Instillation of agricultural dust | Pretreatment with MaR1 by i.p. injection attenuated tissue inflammation, BAL neutrophil influx, and pro-inflammatory cytokine production | (Nordgren, et al., 2015) |
| RvD1 | Acute cigarette smoke exposure | RvD1 given by inhalation reduced cigarette smoke-induced inflammation, and accelerated resolution of inflammation when given after smoking cessation | (Hsiao, et al., 2013) |
| AT-RvD1 | Chronic cigarette smoke exposure | AT-RvD1 given by inhalation or i.v. injection inhibited emphysematous changes in lung architecture, and reduced lung inflammation, oxidative stress and apoptosis | (Hsiao, et al., 2015) |
| RvD1 | Chronic cigarette smoke exposure | RvD1 inhibited structural emphysema and inflammation when given concurrently with smoke exposure, and promoted lung tissue regeneration when given after smoking cessation | (Kim, et al., 2016) |
| AT-RvD1 | Chronic cigarette smoke exposure | AT-RvD1 promoted tissue repair by upregulating the Nrf2/Keap1 pathway | (Posso, et al., 2018) |
| LXA4 | Urban particulate matter exacerbation of allergic airway inflammation (asthma) | LXA4 inhibited tissue inflammation, BAL eosinophils, Th2 cytokines, and expression of Th2 transcription factors | (Lu, et al., 2018) |
| Soluble epoxide hydrolase inhibitor (sEHI) t-TUCB | Mouse OVA model of allergic airway inflammation | t-TUCB increased tissue levels of anti-inflammatory EETs and reduced inflammation, eosinophils and Th2 cytokines | (Yang, et al., 2015). |
| sEHI GSK2256294A | Acute cigarette smoke exposure | GSK2256294A inhibited BAL neutrophilia and lung tissue CXCL1 | (Podolin, et al., 2013). |
Abbreviations:
AT-RvD1, aspirin-triggered resolvin D1
BAL, bronchoalveolar lavage
DHA, docosahexaenoic acid
EET, Epoxyeicosatrienoic acids
EPA, eicosapentaenoic acid
LXA4, lipoxin A4
MaR1, maresin 1
MSC, mesenchymal stem cells or mesenchymal stromal cells
PUFA, polyunsaturated fatty acids
RCT, randomized controlled trial
RvD1, resolvin D1
RvD2, resolvin D2
sEHI, soluble epoxide hydrolase inhibitor
t-TUCB, trans-4-{4-[3-(4-Trifluoromethoxyphenyl)ureido]cyclohexyloxy}benzoic acid
As mentioned above, agricultural waste dust extract can promote inflammatory signaling in cell culture systems. Animal studies in which mice are exposed to dust extract by intranasal challenge also show high levels of airway inflammation and neutrophil accumulation. Dietary supplementation with DHA reduced neutrophilic infiltration, pro-inflammatory cytokines, and inflammation, in mice challenged intranasally with dust from a commercial swine operation (Nordgren, et al., 2014). The investigators then tried purified MaR1, one of the downstream products of DHA metabolism, and which had previously reduced inflammatory cytokine production in bronchial epithelial cells exposed to agricultural dust (Nordgren, et al., 2013). MaR1 lowered BAL neutrophils, IL-6 and TNFα levels in mice exposed both acutely and chronically to agricultural dust, but did not reduce histological inflammation (Nordgren, et al., 2015). This suggested that MaR1 may only target some of the mechanisms of swine dust induced inflammation and that other DHA metabolites might also play important roles. While both lipid mediators proved useful, a difference in administration may prove advantageous for DHA, which was successfully delivered orally while MaR1 was given intraperitoneally. Interestingly, as opposed to many other studies on SPM mechanisms of action, MaR1 did not block NF-κB signaling, rather, it attenuated serum response element signaling in bronchial epithelial cells exposed to dust extract (Nordgren, et al., 2013). It is also possible these mediators influence EGFR signaling to promote successful resolution. Whether this mechanism is responsible for the in vivo effects of DHA and MaR1 are currently unknown. Dietary supplementation with omega-3 polyunsaturated fatty acids containing DHA was also effective at reducing symptoms of chronic respiratory disease in horses on a low-dust diet (Nogradi, et al., 2015).
Smoking is the most well-known risk factor for development of emphysema and COPD. Cigarette smoke exposure in animal models causes acute inflammation with neutrophilia and increased pro-inflammatory cytokines. Evidence that smoking promotes an imbalance between pro-inflammatory and pro-resolving pathways that is centered around ALX/FPR2 (the LXA4 and RvD1 receptor) was recently reviewed (Bozinovski, et al., 2013). Thus, several recent studies have examined RvD1 in mouse models of cigarette smoke exposure. AT-RvD1 given concurrently with cigarette smoke exposure for 3 days significantly blunted acute inflammation, neutrophil recruitment, and pro-inflammatory signaling (Hsiao, et al., 2013). Importantly, AT-RvD1 given after the final smoke exposure accelerated the resolution of inflammation (Hsiao, et al., 2013). Chronic exposure to cigarette smoke in rodent models leads to emphysematous changes including airspace enlargement, cellular inflammation, neutrophil activation, and oxidative stress. Our group showed that RvD1 prevented airspace enlargement and reduced macrophage and neutrophil infiltration in mice exposed chronically to cigarette smoke (Hsiao, et al., 2013). AT-RvD1 reduced production of pro-inflammatory cytokines IL-6 and Cxcl1, inhibited expression of COX2 and ICAM1, and induced the anti-inflammatory cytokine, IL-10. RvD1 also promoted differentiation of alternatively activated (M2) macrophages and neutrophil efferocytosis while reducing lung cell apoptosis. Subsequently, Posso et al. demonstrated that AT-RvD1 enhanced resolution and repair in mice when given after smoking cessation. AT-RvD1 reduced oxidative stress by upregulating the Nrf2 pathway, increased IL-10 expression, and promoted restoration of lung elastin fibers (Posso, et al., 2018). In a third study, RvD1 given for 6 weeks after smoking cessation (regeneration model) had the same beneficial effect as RvD1 given during the final 6 weeks of smoke exposure (prevention model) (Kim, et al., 2016).
In addition to testing SPMs as direct treatments, research is progressing to target other key enzymes in the SPM pathways. As discussed above, soluble epoxide hydrolases (sEHs) sEH promote inflammation by degrading lipid mediators and precursors derived from arachidonic acid, EPA and DHA, and soluble epoxide hydrolase inhibitors (sEHIs) have been proposed as treatments for inflammatory diseases by inhibiting degradation of anti-inflammatory EETs. A recent study tested an sEHI in the mouse OVA model of allergic airways inflammation (Yang, et al., 2015). Inhibition of sEH reduced BAL eosinophil numbers as well as the Th2 cytokines IL-4, IL-5, eotaxin and RANTES (Yang, et al., 2015). Another study tested the sEHI, GSK2256294A in a mouse model of cigarette smoke induced lung disease (Podolin, et al., 2013). GSK2256294A attenuated cigarette smoke induced leukocyte infiltration and BAL chemokine levels by inhibiting inflammatory signaling and promoting resolution signaling. Interestingly, the antimicrobial agent triclocarban is being increasingly used in personal care products, triclocarban is a potent inhibitor of sEH and was recently tested in an LPS challenge mouse model (Liu, et al., 2011). Again, the model showed that inhibition of sEH dramatically reduced LPS-induced inflammatory cytokine and chemokine production.
Although the currently available studies of interventions in animal models of air pollution related lung disease focus mainly on asthma and cigarette smoking, the commonality of disease processes suggest that these targets should also prove beneficial in other chronic lung diseases caused by exposure to diesel exhaust, wood smoke, industrial pollution, and other inhaled insults where continued chronic stimulation overwhelms the normal self-limiting nature of inflammation, leading to chronic inflammation and disease pathology.
7. Conclusion and future directions.
Inflammation is normally a beneficial and self-limited process. There is now considerable evidence that chronic inflammation represents a failure to resolve inflammation, and this failure is linked to dysregulation of SPMs and the pro-resolution pathways. Chronic lung inflammation may be especially difficult to resolve, since exposures to urban air pollution, diesel and traffic exhaust, and secondhand smoke, are often difficult to avoid, resulting in repeated insults. In COPD patients, the disease can persist and even worsen after smoking cessation, because the inflammatory cascade is self-perpetuating, with lung inflammatory cells producing reactive oxygen species and other pro-inflammatory signals that cause tissue damage and recruit further inflammatory cells. Although corticosteroids can be useful in treating acute inflammation and disease flare-ups, chronic use carries significant risks and side effects and does not address the underlying imbalance.
While dietary supplementation with fish oils and other sources of omega-3 may have some general benefits, their benefits in specific human chronic inflammatory diseases are unclear, although some trials are still ongoing. Therapeutic use of SPMs shows promise in cell culture and animal models of exposure to various categories of inhalation insult. The resolution program, broadly viewed, is a target-rich environment; potential therapeutics include SPMs and SPM derivatives, other synthetic small molecule agonists of SPM receptors, inhibitors of SPM-degrading enzymes, and modifiers of the SPM synthesis pathways. If therapies based on small inhibitor RNAs move out of the laboratory and into the clinic, the resolution program presents a rich array of potential targets.
Acknowledgements:
The authors thank Steven J. Pollock for assistance in assembling the figures.
Funding: This work was funded in part by The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. grant number HT9404-13-1-0030, NIH grant R01HL120908, R01HL133761, the Parkes Family Endowment and the C. Jane Davis and C. Robert Davis Professorship. The funders had no role in the preparation of this review article.
Abbreviations
- AT-RvD1
aspirin-triggered resolvin D1
- BAL
bronchoalveolar lavage
- COPD
Chronic Obstructive Pulmonary Disease
- COX2
cyclooxygenase 2
- DEP
diesel exhaust particles
- DHA
docosahexaenoic acid
- DiHOME
dihydroxyoctadeca(mono)enoic acid
- EET
epoxyeicosatrienoic acid
- EOR
eicosanoid oxidoreductase
- EPA
eicosapentaenoic acid
- FPR2
formyl peptide receptor 2
- GPCR
G-protein coupled receptor
- HDHA
hydroxydocosahexaenoic acid
- HDM
house dust mite allergen
- HETE
hydroxyeicosatetraenoic acid
- HODE
hydroxyoctadecadienoic acid
- LO
lipoxygenase
- LPS
lipopolysaccharide
- LTA4
leukotriene A4
- LTB4
leukotriene B4
- LXA4
lipoxin A4
- MaR1
maresin 1
- MSC
mesenchymal stem cell
- NSAID
nonsteroidal anti-inflammatory drug
- OVA
ovalbumin
- PD1
protectin D1
- PG
prostaglandin
- PKC
protein kinase C
- PM
particulate matter
- RvD1
resolvin D1
- RvD2
resolvin D2
- RvE1
resolvin E1
- sEH
soluble epoxide hydrolase
- sEHI
soluble epoxide hydrolase inhibitor
- SPM
specialized pro-resolving lipid mediator
- SRE
Serum Response Element
- UFP
ultrafine particulate matter
Footnotes
Conflicts of interest: The authors declare no conflicts of interest pertaining to this work.
References
- Abreu SC, Lopes-Pacheco M, da Silva AL, Xisto DG, de Oliveira TB, Kitoko JZ, de Castro LL, Amorim NR, Martins V, Silva LHA, Goncalves-de-Albuquerque CF, de Castro Faria-Neto HC, Olsen PC, Weiss DJ, Morales MM, Diaz BL, & Rocco PRM (2018). Eicosapentaenoic Acid Enhances the Effects of Mesenchymal Stromal Cell Therapy in Experimental Allergic Asthma. Front Immunol, 9, 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi A, Haghighat N, Hakimrabet M, & Tolide-ie H (2012). Nutritional evaluation in chronic obstructive pulmonary disease patients. Pak J Biol Sci, 15, 501–505. [DOI] [PubMed] [Google Scholar]
- Ahn EK, Yoon HK, Jee BK, Ko HJ, Lee KH, Kim HJ, & Lim Y (2008). COX-2 expression and inflammatory effects by diesel exhaust particles in vitro and in vivo. Toxicol Lett, 176, 178–187. [DOI] [PubMed] [Google Scholar]
- Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, Devlin RB, & Becker S (2006). Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Allergy Clin Immunol, 117, 1396–1403. [DOI] [PubMed] [Google Scholar]
- Allen RW, Carlsten C, Karlen B, Leckie S, van Eeden S, Vedal S, Wong I, & Brauer M (2011). An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am J Respir Crit Care Med, 183, 1222–1230. [DOI] [PubMed] [Google Scholar]
- Atlantis E, & Cochrane B (2016). The association of dietary intake and supplementation of specific polyunsaturated fatty acids with inflammation and functional capacity in chronic obstructive pulmonary disease: a systematic review. Int J Evid Based Healthc, 14, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awji EG, Chand H, Bruse S, Smith KR, Colby JK, Mebratu Y, Levy BD, & Tesfaigzi Y (2015). Wood smoke enhances cigarette smoke-induced inflammation by inducing the aryl hydrocarbon receptor repressor in airway epithelial cells. Am J Respir Cell Mol Biol, 52, 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balode L, Strazda G, Jurka N, Kopeika U, Kislina A, Bukovskis M, Beinare M, Gardjusina V, & Taivans I (2012). Lipoxygenase-derived arachidonic acid metabolites in chronic obstructive pulmonary disease. Medicina (Kaunas), 48, 292–298. [PubMed] [Google Scholar]
- Barnes PJ (2013). Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol, 131, 636–645. [DOI] [PubMed] [Google Scholar]
- Barnig C, Frossard N, & Levy BD (2018). Towards targeting resolution pathways of airway inflammation in asthma. Pharmacol Ther, 186, 98–113. [DOI] [PubMed] [Google Scholar]
- Barregard L, Sallsten G, Gustafson P, Andersson L, Johansson L, Basu S, & Stigendal L (2006). Experimental exposure to wood-smoke particles in healthy humans: effects on markers of inflammation, coagulation, and lipid peroxidation. Inhal Toxicol, 18, 845–853. [DOI] [PubMed] [Google Scholar]
- Basil MC, & Levy BD (2016). Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol, 16, 51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan HA, Lu Y, Jun B, Fang Z, Woods TC, & Hong S (2017). Circulating inflammation-resolving lipid mediators RvD1 and DHA are decreased in patients with acutely symptomatic carotid disease. Prostaglandins Leukot Essent Fatty Acids, 125, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty AL, Haight TJ, & Redberg RF (2011). Associations between respiratory illnesses and secondhand smoke exposure in flight attendants: A cross-sectional analysis of the Flight Attendant Medical Research Institute Survey. Environ Health, 10, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck-Speier I, Dayal N, Karg E, Maier KL, Roth C, Ziesenis A, & Heyder J (2001). Agglomerates of ultrafine particles of elemental carbon and TiO2 induce generation of lipid mediators in alveolar macrophages. Environ Health Perspect, 109 Suppl 4, 613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck-Speier I, Karg E, Behrendt H, Stoeger T, & Alessandrini F (2012). Ultrafine particles affect the balance of endogenous pro- and anti-inflammatory lipid mediators in the lung: in-vitro and in-vivo studies. Part Fibre Toxicol, 9, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best KP, Sullivan T, Palmer D, Gold M, Kennedy DJ, Martin J, & Makrides M (2016). Prenatal Fish Oil Supplementation and Allergy: 6-Year Follow-up of a Randomized Controlled Trial. Pediatrics, 137. [DOI] [PubMed] [Google Scholar]
- Best KP, Sullivan TR, Palmer DJ, Gold M, Martin J, Kennedy D, & Makrides M (2018). Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood - a longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ J, 11, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavaraju L, Shannahan J, William A, McCormick R, McGee J, Kodavanti U, & Madden M (2014). Diesel and biodiesel exhaust particle effects on rat alveolar macrophages with in vitro exposure. Chemosphere, 104, 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilal S, Haworth O, Wu L, Weylandt KH, Levy BD, & Kang JX (2011). Fat-1 transgenic mice with elevated omega-3 fatty acids are protected from allergic airway responses. Biochim Biophys Acta, 1812, 1164–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadottir E, Schoos AM, Wolsk HM, Pedersen TM, Vinding RK, Thorsteinsdottir S, Folsgaard NV, Fink NR, Thorsen J, Pedersen AG, Waage J, Rasmussen MA, Stark KD, Olsen SF, & Bonnelykke K (2016). Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N Engl J Med, 375, 2530–2539. [DOI] [PubMed] [Google Scholar]
- Bonnans C, Fukunaga K, Levy MA, & Levy BD (2006). Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. Am J Pathol, 168, 1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozinovski S, Anthony D, Anderson GP, Irving LB, Levy BD, & Vlahos R (2013). Treating neutrophilic inflammation in COPD by targeting ALX/FPR2 resolution pathways. Pharmacol Ther, 140, 280–289. [DOI] [PubMed] [Google Scholar]
- Bozinovski S, Uddin M, Vlahos R, Thompson M, McQualter JL, Merritt AS, Wark PA, Hutchinson A, Irving LB, Levy BD, & Anderson GP (2012). Serum amyloid A opposes lipoxin A(4) to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A, 109, 935–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr., Whitsel L, Kaufman JD, American Heart Association Council on, E., Prevention, C. o. t. K. i. C. D., Council on Nutrition, P. A., & Metabolism. (2010). Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation, 121, 2331–2378. [DOI] [PubMed] [Google Scholar]
- Capra V, Rovati GE, Mangano P, Buccellati C, Murphy RC, & Sala A (2015). Transcellular biosynthesis of eicosanoid lipid mediators. Biochim Biophys Acta, 1851, 377–382. [DOI] [PubMed] [Google Scholar]
- Chan MM, & Moore AR (2010). Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J Immunol, 184, 6418–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Dalli J, Colas RA, & Serhan CN (2015). Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med, 212, 1203–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, & Serhan CN (2017). Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med, 58, 114–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Takano T, Clish CB, Petasis NA, Tai HH, & Serhan CN (1998). Aspirin-triggered 15-epi-lipoxin A4 (ATL) generation by human leukocytes and murine peritonitis exudates: development of a specific 15-epi-LXA4 ELISA. J Pharmacol Exp Ther, 287, 779–790. [PubMed] [Google Scholar]
- Chiurchiu V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, & Serhan CN (2016). Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med, 8, 353ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claria J, & Serhan CN (1995). Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A, 92, 9475–9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clish CB, Levy BD, Chiang N, Tai HH, & Serhan CN (2000). Oxidoreductases in lipoxin A4 metabolic inactivation: a novel role for 15-onoprostaglandin 13-reductase/leukotriene B4 12-hydroxydehydrogenase in inflammation. J Biol Chem, 275, 25372–25380. [DOI] [PubMed] [Google Scholar]
- Commodore AA, Zhang JJ, Chang Y, Hartinger SM, Lanata CF, Mausezahl D, Gil AI, Hall DB, Aguilar-Villalobos M, Vena JE, Wang JS, & Naeher LP (2013). Concentrations of urinary 8-hydroxy-2’-deoxyguanosine and 8-isoprostane in women exposed to woodsmoke in a cookstove intervention study in San Marcos, Peru. Environ Int, 60, 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corminboeuf O, & Leroy X (2015). FPR2/ALXR agonists and the resolution of inflammation. J Med Chem, 58, 537–559. [DOI] [PubMed] [Google Scholar]
- Croasdell A, Thatcher TH, Kottmann RM, Colas RA, Dalli J, Serhan CN, Sime PJ, & Phipps RP (2015). Resolvins attenuate inflammation and promote resolution in cigarette smoke-exposed human macrophages. Am J Physiol Lung Cell Mol Physiol, 309, L888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft D, Block R, Cameron SJ, Evans K, Lowenstein CJ, Ling F, Zareba W, Hopke PK, Utell MJ, Thurston SW, Thevenet-Morrison K, & Rich DQ (2018). Do elevated blood levels of omega-3 fatty acids modify effects of particulate air pollutants on fibrinogen? Air Qual Atmos Health, 11, 791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Vaz N, Meldrum SJ, Dunstan JA, Lee-Pullen TF, Metcalfe J, Holt BJ, Serralha M, Tulic MK, Mori TA, & Prescott SL (2012). Fish oil supplementation in early infancy modulates developing infant immune responses. Clin Exp Allergy, 42, 1206–1216. [DOI] [PubMed] [Google Scholar]
- Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggstrom JZ, Petasis NA, & Serhan CN (2013). The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. Faseb j, 27, 2573–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Batlle J, Sauleda J, Balcells E, Gomez FP, Mendez M, Rodriguez E, Barreiro E, Ferrer JJ, Romieu I, Gea J, Anto JM, Garcia-Aymerich J, & Group P.-C. S. (2012). Association between Omega3 and Omega6 fatty acid intakes and serum inflammatory markers in COPD. J Nutr Biochem, 23, 817–821. [DOI] [PubMed] [Google Scholar]
- De Castro J, Hernandez-Hernandez A, Rodriguez MC, Sardina JL, Llanillo M, & Sanchez-Yague J (2007). Comparison of changes in erythrocyte and platelet phospholipid and fatty acid composition and protein oxidation in chronic obstructive pulmonary disease and asthma. Platelets, 18, 43–51. [DOI] [PubMed] [Google Scholar]
- de Oliveira JR, da Silva PR, & Rogerio AP (2017). AT-RvD1 modulates the activation of bronchial epithelial cells induced by lipopolysaccharide and Dermatophagoides pteronyssinus. Eur J Pharmacol, 805, 46–50. [DOI] [PubMed] [Google Scholar]
- den Hartigh LJ, Lame MW, Ham W, Kleeman MJ, Tablin F, & Wilson DW (2010). Endotoxin and polycyclic aromatic hydrocarbons in ambient fine particulate matter from Fresno, California initiate human monocyte inflammatory responses mediated by reactive oxygen species. Toxicol In Vitro, 24, 1993–2002. [DOI] [PubMed] [Google Scholar]
- Dong J, Zhang M, Liao Z, Wu W, Wang T, Chen L, Yang T, Guo L, Xu D, & Wen F (2014). Resolvin-D1 inhibits interleukin-8 and hydrogen peroxide production induced by cigarette smoke extract in 16HBE cells via attenuating NF-kappaB activation. Chin Med J (Engl), 127, 511–517. [PubMed] [Google Scholar]
- Dudek SE, Nitzsche K, Ludwig S, & Ehrhardt C (2016). Influenza A viruses suppress cyclooxygenase-2 expression by affecting its mRNA stability. Sci Rep, 6, 27275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffney PF, Falsetta ML, Rackow AR, Thatcher TH, Phipps RP, & Sime PJ (2018). Key roles for lipid mediators in the adaptive immune response. J Clin Invest, 128, 2724–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall MG, Bruggemann TR, & Levy BD (2017). Bronchoprotective mechanisms for specialized pro-resolving mediators in the resolution of lung inflammation. Mol Aspects Med, 58, 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall MG, & Levy BD (2016). DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur J Pharmacol, 785, 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickmeier O, Fussbroich D, Mueller K, Serve F, Smaczny C, Zielen S, & Schubert R (2017). Pro-resolving lipid mediator Resolvin D1 serves as a marker of lung disease in cystic fibrosis. PLoS One, 12, e0171249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner MD, Balmes J, Katz PP, Trupin L, Yelin EH, & Blanc PD (2005). Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health, 4, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, & Wenzel SE (2013). Prostaglandin D(2) pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol, 131, 1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Abbott J, Cheng L, Colby JK, Lee JW, Levy BD, & Matthay MA (2015). Human Mesenchymal Stem (Stromal) Cells Promote the Resolution of Acute Lung Injury in Part through Lipoxin A4. J Immunol, 195, 875–881. [DOI] [PubMed] [Google Scholar]
- Flesher RP, Herbert C, & Kumar RK (2014). Resolvin E1 promotes resolution of inflammation in a mouse model of an acute exacerbation of allergic asthma. Clin Sci (Lond), 126, 805–814. [DOI] [PubMed] [Google Scholar]
- Flitter BA, Hvorecny KL, Ono E, Eddens T, Yang J, Kwak DH, Bahl CD, Hampton TH, Morisseau C, Hammock BD, Liu X, Lee JS, Kolls JK, Levy BD, Madden DR, & Bomberger JM (2017). Pseudomonas aeruginosa sabotages the generation of host proresolving lipid mediators. Proc Natl Acad Sci U S A, 114, 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman G, Li Y, Dalli J, Chiang N, & Serhan CN (2012). Self-limited versus delayed resolution of acute inflammation: temporal regulation of pro-resolving mediators and microRNA. Sci Rep, 2, 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, Weed DA, Gelrud A, Regan MM, Laposata M, Alvarez JG, & O’Sullivan BP (2004). Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med, 350, 560–569. [DOI] [PubMed] [Google Scholar]
- Fulton AS, Coates AM, Williams MT, Howe PRC, Garg ML, Wood LG, Frith P, & Hill AM (2017). Fish oil supplementation in chronic obstructive pulmonary disease: feasibility of conducting a randomised controlled trial. Pilot Feasibility Stud, 3, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton AS, Hill AM, Williams MT, Howe PR, & Coates AM (2015). Paucity of evidence for a relationship between long-chain omega-3 fatty acid intake and chronic obstructive pulmonary disease: a systematic review. Nutr Rev, 73, 612–623. [DOI] [PubMed] [Google Scholar]
- Gergen PJ, Fowler JA, Maurer KR, Davis WW, & Overpeck MD (1998). The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988 to 1994. Pediatrics, 101, E8. [DOI] [PubMed] [Google Scholar]
- Gold DR, Litonjua AA, Carey VJ, Manson JE, Buring JE, Lee IM, Gordon D, Walter J, Friedenberg G, Hankinson JL, Copeland T, & Luttmann-Gibson H (2016). Lung VITAL: Rationale, design, and baseline characteristics of an ancillary study evaluating the effects of vitamin D and/or marine omega-3 fatty acid supplements on acute exacerbations of chronic respiratory disease, asthma control, pneumonia and lung function in adults. Contemp Clin Trials, 47, 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD (2007). Environmental tobacco smoke and the epidemic of asthma in children: the role of cigarette use. Ann Allergy Asthma Immunol, 98, 447–454. [DOI] [PubMed] [Google Scholar]
- Gouveia-Figueira S, Karimpour M, Bosson JA, Blomberg A, Unosson J, Pourazar J, Sandstrom T, Behndig AF, & Nording ML (2017). Mass spectrometry profiling of oxylipins, endocannabinoids, and N-acylethanolamines in human lung lavage fluids reveals responsiveness of prostaglandin E2 and associated lipid metabolites to biodiesel exhaust exposure. Anal Bioanal Chem, 409, 2967–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia-Figueira S, Karimpour M, Bosson JA, Blomberg A, Unosson J, Sehlstedt M, Pourazar J, Sandstrom T, Behndig AF, & Nording ML (2018). Mass spectrometry profiling reveals altered plasma levels of monohydroxy fatty acids and related lipids in healthy humans after controlled exposure to biodiesel exhaust. Anal Chim Acta, 1018, 62–69. [DOI] [PubMed] [Google Scholar]
- Guastadisegni C, Kelly FJ, Cassee FR, Gerlofs-Nijland ME, Janssen NA, Pozzi R, Brunekreef B, Sandstrom T, & Mudway I (2010). Determinants of the proinflammatory action of ambient particulate matter in immortalized murine macrophages. Environ Health Perspect, 118, 1728–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AD, Smirnov A, Tsirka SE, & Schoonen MA (2015). Metal-sulfide mineral ores, Fenton chemistry and disease--particle induced inflammatory stress response in lung cells. Int J Hyg Environ Health, 218, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer TP, Bitterle E, Beck-Speier I, Maier KL, Frankenberger M, Heyder J, & Ziegler-Heitbrock L (2004). Diesel exhaust particles increase LPS-stimulated COX-2 expression and PGE2 production in human monocytes. J Leukoc Biol, 75, 856–864. [DOI] [PubMed] [Google Scholar]
- Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, Olsen KC, Pollock SJ, Serhan CN, Phipps RP, & Sime PJ (2013). A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS One, 8, e58258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao HM, Thatcher TH, Colas RA, Serhan CN, Phipps RP, & Sime PJ (2015). Resolvin D1 Reduces Emphysema and Chronic Inflammation. Am J Pathol, 185, 3189–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao HM, Thatcher TH, Levy EP, Fulton RA, Owens KM, Phipps RP, & Sime PJ (2014). Resolvin D1 attenuates polyinosinic-polycytidylic acid-induced inflammatory signaling in human airway epithelial cells via TAK1. J Immunol, 193, 4980–4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka T, Hisada T, Aoki H, & Mori M (2008). Resolvin E1: a novel lipid mediator in the resolution of allergic airway inflammation. Expert Rev Clin Immunol, 4, 669–672. [DOI] [PubMed] [Google Scholar]
- Jones HR, Robb CT, Perretti M, & Rossi AG (2016). The role of neutrophils in inflammation resolution. Semin Immunol, 28, 137–145. [DOI] [PubMed] [Google Scholar]
- Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, Yang R, Uddin J, Guggino WB, Atabani SF, Belkaid Y, Xu Y, Whitsett JA, Accurso FJ, Wills-Karp M, & Petasis NA (2004). Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol, 5, 388–392. [DOI] [PubMed] [Google Scholar]
- Karra L, Haworth O, Priluck R, Levy BD, & Levi-Schaffer F (2015). Lipoxin B(4) promotes the resolution of allergic inflammation in the upper and lower airways of mice. Mucosal Immunol, 8, 852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney DS, Skinner C, Travers JB, Capdevila JH, Nanney LB, King LE Jr., & Waterman MR (1998). Differentiating keratinocytes express a novel cytochrome P450 enzyme, CYP2B19, having arachidonate monooxygenase activity. J Biol Chem, 273, 32071–32079. [DOI] [PubMed] [Google Scholar]
- Khelifi M, Zarrouk A, Nury T, Hamed H, Saguem S, Salah RB, Riedinger JM, & Lizard G (2014). Cytokine and eicosanoid profiles of phosphate mine workers. J Toxicol Sci, 39, 465–474. [DOI] [PubMed] [Google Scholar]
- Kim KH, Park TS, Kim YS, Lee JS, Oh YM, Lee SD, & Lee SW (2016). Resolvin D1 prevents smoking-induced emphysema and promotes lung tissue regeneration. Int J Chron Obstruct Pulmon Dis, 11, 1119–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmert J, Pineiro-Hermida S, Hamberg M, Gregory JA, Lopez IP, Fauland A, Wheelock CE, Dahlen SE, Pichel JG, & Adner M (2018). Prominent release of lipoxygenase generated mediators in a murine house dust mite-induced asthma model. Prostaglandins Other Lipid Mediat, 137, 20–29. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy N, Burkett PR, Dalli J, Abdulnour RE, Colas R, Ramon S, Phipps RP, Petasis NA, Kuchroo VK, Serhan CN, & Levy BD (2015). Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J Immunol, 194, 863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy SH, Woeller CF, Thatcher TH, Maddipati KR, Honn KV, Sime PJ, & Phipps RP (2016). Human lung fibroblasts produce proresolving peroxisome proliferator-activated receptor-gamma ligands in a cyclooxygenase-2-dependent manner. Am J Physiol Lung Cell Mol Physiol, 311, L855–L867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang JE, Dozor AJ, Holbrook JT, Mougey E, Krishnan S, Sweeten S, Wise RA, Teague WG, Wei CY, Shade D, Lima JJ, & American Lung Association-Asthma Clinical Research, C. (2013). Biologic mechanisms of environmental tobacco smoke in children with poorly controlled asthma: results from a multicenter clinical trial. J Allergy Clin Immunol Pract, 1, 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH (2012). Resolvins as new fascinating drug candidates for inflammatory diseases. Arch Pharm Res, 35, 3–7. [DOI] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, & Serhan CN (2001). Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol, 2, 612–619. [DOI] [PubMed] [Google Scholar]
- Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, & Serhan CN (2007). Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol, 178, 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Lukacs NW, Berlin AA, Schmidt B, Guilford WJ, Serhan CN, & Parkinson JF (2007). Lipoxin A4 stable analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism. Faseb j, 21, 3877–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, & Serhan CN (2014). Resolution of acute inflammation in the lung. Annu Rev Physiol, 76, 467–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Navab K, Hough G, Daher N, Zhang M, Mittelstein D, Lee K, Pakbin P, Saffari A, Bhetraratana M, Sulaiman D, Beebe T, Wu L, Jen N, Wine E, Tseng CH, Araujo JA, Fogelman A, Sioutas C, Navab M, & Hsiai TK (2015). Effect of exposure to atmospheric ultrafine particles on production of free fatty acids and lipid metabolites in the mouse small intestine. Environ Health Perspect, 123, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wilker EH, Dorans KS, Rice MB, Schwartz J, Coull BA, Koutrakis P, Gold DR, Keaney JF Jr., Lin H, Vasan RS, Benjamin EJ, & Mittleman MA (2016). Short-Term Exposure to Air Pollution and Biomarkers of Oxidative Stress: The Framingham Heart Study. J Am Heart Assoc, 5, e002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares Segovia B, Cortes Sandoval G, Amador Licona N, Guizar Mendoza JM, Nunez Lemus E, Rocha Amador DO, Ramirez Gomez XS, & Monroy Torres R (2014). Parameters of lung inflammation in asthmatic as compared to healthy children in a contaminated city. BMC Pulm Med, 14, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Qiu H, Morisseau C, Hwang SH, Tsai HJ, Ulu A, Chiamvimonvat N, & Hammock BD (2011). Inhibition of soluble epoxide hydrolase contributes to the anti-inflammatory effect of antimicrobial triclocarban in a murine model. Toxicol Appl Pharmacol, 255, 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CM, Nascarella MA, & Valberg PA (2013). Carbon black vs. black carbon and other airborne materials containing elemental carbon: physical and chemical distinctions. Environ Pollut, 181, 271–286. [DOI] [PubMed] [Google Scholar]
- Lopez-Vicario C, Rius B, Alcaraz-Quiles J, Garcia-Alonso V, Lopategi A, Titos E, & Claria J (2016). Pro-resolving mediators produced from EPA and DHA: Overview of the pathways involved and their mechanisms in metabolic syndrome and related liver diseases. Eur J Pharmacol, 785, 133–143. [DOI] [PubMed] [Google Scholar]
- Lu X, Fu H, Han F, Fang Y, Xu J, Zhang L, & Du Q (2018). Lipoxin A4 regulates PM2.5-induced severe allergic asthma in mice via the Th1/Th2 balance of group 2 innate lymphoid cells. J Thorac Dis, 10, 1449–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom SL, Levanen B, Nording M, Klepczynska-Nystrom A, Skold M, Haeggstrom JZ, Grunewald J, Svartengren M, Hammock BD, Larsson BM, Eklund A, Wheelock AM, & Wheelock CE (2011). Asthmatics exhibit altered oxylipin profiles compared to healthy individuals after subway air exposure. PLoS One, 6, e23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo YL, Zhang CC, Li PB, Nie YC, Wu H, Shen JG, & Su WW (2012). Naringin attenuates enhanced cough, airway hyperresponsiveness and airway inflammation in a guinea pig model of chronic bronchitis induced by cigarette smoke. Int Immunopharmacol, 13, 301–307. [DOI] [PubMed] [Google Scholar]
- Malireddy S, Lawson C, Steinhour E, Hart J, Kotha SR, Patel RB, Zhao L, Wilkins JR, Marsh CB, Magalang UJ, Romberger D, Wewers MD, & Parinandi NL (2013). Airborne agricultural particulate matter induces inflammatory cytokine secretion by respiratory epithelial cells: mechanisms of regulation by eicosanoid lipid signal mediators. Indian J Biochem Biophys, 50, 387–401. [PubMed] [Google Scholar]
- Martens DS, Gouveia S, Madhloum N, Janssen BG, Plusquin M, Vanpoucke C, Lefebvre W, Forsberg B, Nording M, & Nawrot TS (2017). Neonatal Cord Blood Oxylipins and Exposure to Particulate Matter in the Early-Life Environment: An ENVIRONAGE Birth Cohort Study. Environ Health Perspect, 125, 691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martey CA, Baglole CJ, Gasiewicz TA, Sime PJ, & Phipps RP (2005). The aryl hydrocarbon receptor is a regulator of cigarette smoke induction of the cyclooxygenase and prostaglandin pathways in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol, 289, L391–399. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T, Amakawa K, Yamaguchi K, Ishizaka A, Terashima T, Matsumaru A, & Morishita T (2006). Effects of diesel exhaust particles on human neutrophil activation. Exp Lung Res, 32, 427–439. [DOI] [PubMed] [Google Scholar]
- McKeever TM, Lewis SA, Cassano PA, Ocke M, Burney P, Britton J, & Smit HA (2008). The relation between dietary intake of individual fatty acids, FEV1 and respiratory disease in Dutch adults. Thorax, 63, 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael S, Montag M, & Dott W (2013). Pro-inflammatory effects and oxidative stress in lung macrophages and epithelial cells induced by ambient particulate matter. Environ Pollut, 183, 19–29. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Sasaki S, Tanaka K, Ohya Y, Miyamoto S, Matsunaga I, Yoshida T, Hirota Y, Oda H, Osaka M, & Child Health Study G. (2007). Fish and fat intake and prevalence of allergic rhinitis in Japanese females: the Osaka Maternal and Child Health Study. J Am Coll Nutr, 26, 279–287. [DOI] [PubMed] [Google Scholar]
- Miyata J, & Arita M (2015). Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int, 64, 27–34. [DOI] [PubMed] [Google Scholar]
- Moore BF, Clark ML, Bachand A, Reynolds SJ, Nelson TL, & Peel JL (2016). Interactions Between Diet and Exposure to Secondhand Smoke on Metabolic Syndrome Among Children: NHANES 2007–2010. J Clin Endocrinol Metab, 101, 52–58. [DOI] [PubMed] [Google Scholar]
- Muller L, & Jaspers I (2012). Epithelial cells, the “switchboard” of respiratory immune defense responses: effects of air pollutants. Swiss Med Wkly, 142, w13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Xavier RA, de Barros KV, de Andrade IS, Palomino Z, Casarini DE, & Flor Silveira VL (2016). Protective effect of soybean oil- or fish oil-rich diets on allergic airway inflammation. J Inflamm Res, 9, 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, & Liao JK (1999). Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science, 285, 1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogradi N, Couetil LL, Messick J, Stochelski MA, & Burgess JR (2015). Omega-3 fatty acid supplementation provides an additional benefit to a low-dust diet in the management of horses with chronic lower airway inflammatory disease. J Vet Intern Med, 29, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgren TM, Bailey KL, Heires AJ, Katafiasz D, & Romberger DJ (2018). Effects of Agricultural Organic Dusts on Human Lung-Resident Mesenchymal Stem (Stromal) Cell Function. Toxicol Sci, 162, 635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgren TM, Bauer CD, Heires AJ, Poole JA, Wyatt TA, West WW, & Romberger DJ (2015). Maresin-1 reduces airway inflammation associated with acute and repetitive exposures to organic dust. Transl Res, 166, 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgren TM, Friemel TD, Heires AJ, Poole JA, Wyatt TA, & Romberger DJ (2014). The omega-3 fatty acid docosahexaenoic acid attenuates organic dust-induced airway inflammation. Nutrients, 6, 5434–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgren TM, Heires AJ, Bailey KL, Katafiasz DM, Toews ML, Wichman CS, & Romberger DJ (2018). Docosahexaenoic acid enhances amphiregulin-mediated bronchial epithelial cell repair processes following organic dust exposure. Am J Physiol Lung Cell Mol Physiol, 314, L421–L431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgren TM, Heires AJ, Wyatt TA, Poole JA, LeVan TD, Cerutis DR, & Romberger DJ (2013). Maresin-1 reduces the pro-inflammatory response of bronchial epithelial cells to organic dust. Respir Res, 14, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novgorodtseva TP, Denisenko YK, Zhukova NV, Antonyuk MV, Knyshova VV, & Gvozdenko TA (2013). Modification of the fatty acid composition of the erythrocyte membrane in patients with chronic respiratory diseases. Lipids Health Dis, 12, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono E, Dutile S, Kazani S, Wechsler ME, Yang J, Hammock BD, Douda DN, Tabet Y, Khaddaj-Mallat R, Sirois M, Sirois C, Rizcallah E, Rousseau E, Martin R, Sutherland ER, Castro M, Jarjour NN, Israel E, Levy BD, National Heart L., & Blood Institute’s Asthma Clinical Research, N. (2014). Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. Am J Respir Crit Care Med, 190, 886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MM, Chillrud SN, Deepti KC, Ross JM, & Kinney PL (2013). Traffic-related air pollutants and exhaled markers of airway inflammation and oxidative stress in New York City adolescents. Environ Res, 121, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter S, Holguin F, Wood LG, Clougherty JE, Raederstorff D, Antal M, Weber P, & Eggersdorfer M (2015). Nutritional Solutions to Reduce Risks of Negative Health Impacts of Air Pollution. Nutrients, 7, 10398–10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillarisetti S, & Khanna I (2012). Targeting soluble epoxide hydrolase for inflammation and pain - an overview of pharmacology and the inhibitors. Inflamm Allergy Drug Targets, 11, 143–158. [DOI] [PubMed] [Google Scholar]
- Pistorius K, Souza PR, De Matteis R, Austin-Williams S, Primdahl KG, Vik A, Mazzacuva F, Colas RA, Marques RM, Hansen TV, & Dalli J (2018). PDn-3 DPA Pathway Regulates Human Monocyte Differentiation and Macrophage Function. Cell Chem Biol, 25, 749–760 e749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolin PL, Bolognese BJ, Foley JF, Long E 3rd, Peck B, Umbrecht S, Zhang X, Zhu P, Schwartz B, Xie W, Quinn C, Qi H, Sweitzer S, Chen S, Galop M, Ding Y, Belyanskaya SL, Israel DI, Morgan BA, Behm DJ, Marino JP Jr., Kurali E, Barnette MS, Mayer RJ, Booth-Genthe CL, & Callahan JF (2013). In vitro and in vivo characterization of a novel soluble epoxide hydrolase inhibitor. Prostaglandins Other Lipid Mediat, 104–105, 25–31. [DOI] [PubMed] [Google Scholar]
- Poole JA, & Romberger DJ (2012). Immunological and inflammatory responses to organic dust in agriculture. Curr Opin Allergy Clin Immunol, 12, 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posso SV, Quesnot N, Moraes JA, Brito-Gitirana L, Kennedy-Feitosa E, Barroso MV, Porto LC, Lanzetti M, & Valenca SS (2018). AT-RVD1 repairs mouse lung after cigarette smoke-induced emphysema via downregulation of oxidative stress by NRF2/KEAP1 pathway. Int Immunopharmacol, 56, 330–338. [DOI] [PubMed] [Google Scholar]
- Pyrillou K, Chairakaki AD, Tamvakopoulos C, & Andreakos E (2018). Dexamethasone induces omega3-derived immunoresolvents driving resolution of allergic airway inflammation. J Allergy Clin Immunol, 142, 691–695 e694. [DOI] [PubMed] [Google Scholar]
- Raissy HH, Kelly HW, Harkins M, & Szefler SJ (2013). Inhaled corticosteroids in lung diseases. Am J Respir Crit Care Med, 187, 798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakkestad KE, Holme JA, Paulsen RE, Schwarze PE, & Becher R (2010). Mono(2-ethylhexyl) phthalate induces both pro- and anti-inflammatory responses in rat alveolar macrophages through crosstalk between p38, the lipoxygenase pathway and PPARalpha. Inhal Toxicol, 22, 140–150. [DOI] [PubMed] [Google Scholar]
- Rao NL, Riley JP, Banie H, Xue X, Sun B, Crawford S, Lundeen KA, Yu F, Karlsson L, Fourie AM, & Dunford PJ (2010). Leukotriene A(4) hydrolase inhibition attenuates allergic airway inflammation and hyperresponsiveness. Am J Respir Crit Care Med, 181, 899–907. [DOI] [PubMed] [Google Scholar]
- Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N, & Serhan CN (2011). MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. Faseb j, 25, 544–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchiuti A, & Serhan CN (2012). Pro-Resolving Lipid Mediators (SPMs) and Their Actions in Regulating miRNA in Novel Resolution Circuits in Inflammation. Front Immunol, 3, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb CT, Regan KH, Dorward DA, & Rossi AG (2016). Key mechanisms governing resolution of lung inflammation. Semin Immunopathol, 38, 425–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer E, Stabbert R, Rustemeier K, Veltel DJ, Meisgen TJ, Reininghaus W, Carchman RA, Gaworski CL, & Podraza KF (2004). Chemical composition, cytotoxicity and mutagenicity of smoke from US commercial and reference cigarettes smoked under two sets of machine smoking conditions. Toxicology, 195, 31–52. [DOI] [PubMed] [Google Scholar]
- Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, & Levy BD (2012). Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J Immunol, 189, 1983–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumold R, Jyrala M, & Diaz-Sanchez D (2001). Secondhand smoke induces allergic sensitization in mice. J Immunol, 167, 4765–4770. [DOI] [PubMed] [Google Scholar]
- Rynning I, Neca J, Vrbova K, Libalova H, Rossner P Jr., Holme JA, Gutzkow KB, Afanou AKJ, Arnoldussen YJ, Hruba E, Skare O, Haugen A, Topinka J, Machala M, & Mollerup S (2018). In Vitro Transformation of Human Bronchial Epithelial Cells by Diesel Exhaust Particles: Gene Expression Profiling and Early Toxic Responses. Toxicol Sci, 166, 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM, & Utell MJ (1991). The environment and the lung. Changing perspectives. JAMA, 266, 670–675. [PubMed] [Google Scholar]
- Samuelsson B, Hammarstrom S, Hamberg M, & Serhan CN (1985). Structural determination of leukotrienes and lipoxins. Adv Prostaglandin Thromboxane Leukot Res, 14, 45–71. [PubMed] [Google Scholar]
- Santos KT, Florenzano J, Rodrigues L, Favaro RR, Ventura FF, Ribeiro MG, Teixeira SA, Ferreira HH, Brain SD, Damazo AS, Zorn TM, Camara NO, Muscara MN, Peron JP, & Costa SK (2014). Early postnatal, but not late, exposure to chemical ambient pollutant 1,2-naphthoquinone increases susceptibility to pulmonary allergic inflammation at adulthood. Arch Toxicol, 88, 1589–1605. [DOI] [PubMed] [Google Scholar]
- Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, & Hammock BD (2005). Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci U S A, 102, 9772–9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JC, Card GL, Pfau JC, & Holian A (2005). Air pollution particulate SRM 1648 causes oxidative stress in RAW 264.7 macrophages leading to production of prostaglandin E2, a potential Th2 mediator. Inhal Toxicol, 17, 871–877. [DOI] [PubMed] [Google Scholar]
- Schwab JM, & Serhan CN (2006). Lipoxins and new lipid mediators in the resolution of inflammation. Curr Opin Pharmacol, 6, 414–420. [DOI] [PubMed] [Google Scholar]
- Serhan CN (2010). Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol, 177, 1576–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN (2017). Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. Faseb j, 31, 1273–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, & Chiang N (2008). Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol, 153 Suppl 1, S200–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, & Dalli J (2015). The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol, 27, 200–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, & Gronert K (2000). Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med, 192, 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hamberg M, & Samuelsson B (1984). Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci U S A, 81, 5335–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AA, Fang X, Snyder GD, & Weintraub NL (2004). Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res, 43, 55–90. [DOI] [PubMed] [Google Scholar]
- Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, & Serhan CN (2007). Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem, 282, 9323–9334. [DOI] [PubMed] [Google Scholar]
- Superko HR, Superko AR, Lundberg GP, Margolis B, Garrett BC, Nasir K, & Agatston AS (2014). Omega-3 Fatty Acid Blood Levels Clinical Significance Update. Curr Cardiovasc Risk Rep, 8, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydbom A, Blomberg A, Parnia S, Stenfors N, Sandstrom T, & Dahlen SE (2001). Health effects of diesel exhaust emissions. European Respiratory Journal, 17, 733–746. [DOI] [PubMed] [Google Scholar]
- Tabak C, Feskens EJ, Heederik D, Kromhout D, Menotti A, & Blackburn HW (1998). Fruit and fish consumption: a possible explanation for population differences in COPD mortality (The Seven Countries Study). Eur J Clin Nutr, 52, 819–825. [DOI] [PubMed] [Google Scholar]
- Tenenbaum A, & Fisman EZ (2018). Omega-3 polyunsaturated fatty acids supplementation in patients with diabetes and cardiovascular disease risk: does dose really matter? Cardiovasc Diabetol, 17, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson SJ, Askari A, & Bishop-Bailey D (2012). Anti-inflammatory effects of epoxyeicosatrienoic acids. Int J Vasc Med, 2012, 605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston GD, Kipen H, Annesi-Maesano I, Balmes J, Brook RD, Cromar K, De Matteis S, Forastiere F, Forsberg B, Frampton MW, Grigg J, Heederik D, Kelly FJ, Kuenzli N, Laumbach R, Peters A, Rajagopalan ST, Rich D, Ritz B, Samet JM, Sandstrom T, Sigsgaard T, Sunyer J, & Brunekreef B (2017). A joint ERS/ATS policy statement: what constitutes an adverse health effect of air pollution? An analytical framework. Eur Respir J, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titz B, Luettich K, Leroy P, Boue S, Vuillaume G, Vihervaara T, Ekroos K, Martin F, Peitsch MC, & Hoeng J (2016). Alterations in Serum Polyunsaturated Fatty Acids and Eicosanoids in Patients with Mild to Moderate Chronic Obstructive Pulmonary Disease (COPD). Int J Mol Sci, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunno BJ, Tripathy S, Kinnee E, Michanowicz DR, Shmool JL, Cambal L, Chubb L, Roper C, & Clougherty JE (2018). Fine-Scale Source Apportionment Including Diesel-Related Elemental and Organic Constituents of PM2.5 across Downtown Pittsburgh. Int J Environ Res Public Health, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utell MJ, & Frampton MW (2000). Acute health effects of ambient air pollution: the ultrafine particle hypothesis. J Aerosol Med, 13, 355–359. [DOI] [PubMed] [Google Scholar]
- Utell MJ, & Samet JM (1990). Environmentally mediated disorders of the respiratory tract. Med Clin North Am, 74, 291–306. [DOI] [PubMed] [Google Scholar]
- Walda IC, Tabak C, Smit HA, Rasanen L, Fidanza F, Menotti A, Nissinen A, Feskens EJ, & Kromhout D (2002). Diet and 20-year chronic obstructive pulmonary disease mortality in middle-aged men from three European countries. Eur J Clin Nutr, 56, 638–643. [DOI] [PubMed] [Google Scholar]
- Whyand T, Hurst JR, Beckles M, & Caplin ME (2018). Pollution and respiratory disease: can diet or supplements help? A review. Respir Res, 19, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby DA, Moore AR, Colville-Nash PR, & Gilroy D (2000). Resolution of inflammation. Int J Immunopharmacol, 22, 1131–1135. [DOI] [PubMed] [Google Scholar]
- Wu SH, Chen XQ, Liu B, Wu HJ, & Dong L (2013). Efficacy and safety of 15(R/S)-methyl-lipoxin A(4) in topical treatment of infantile eczema. Br J Dermatol, 168, 172–178. [DOI] [PubMed] [Google Scholar]
- Wunschel J, & Poole JA (2016). Occupational agriculture organic dust exposure and its relationship to asthma and airway inflammation in adults. J Asthma, 53, 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Bratt J, Franzi L, Liu JY, Zhang G, Zeki AA, Vogel CF, Williams K, Dong H, Lin Y, Hwang SH, Kenyon NJ, & Hammock BD (2015). Soluble epoxide hydrolase inhibitor attenuates inflammation and airway hyperresponsiveness in mice. Am J Respir Cell Mol Biol, 52, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaslona Z, & Peters-Golden M (2015). Prostanoids in Asthma and COPD: Actions, Dysregulation, and Therapeutic Opportunities. Chest, 148, 1300–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeb B, Alam K, Sorooshian A, Blaschke T, Ahmad I, & Shahid I (2018). On the Morphology and Composition of Particulate Matter in an Urban Environment. Aerosol Air Qual Res, 18, 1431–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldin DC (2001). Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem, 276, 36059–36062. [DOI] [PubMed] [Google Scholar]
- Zielinska B, Samy S, McDonald JD, Seagrave J, & Committee H. E. I. H. R. (2010). Atmospheric transformation of diesel emissions. Res Rep Health Eff Inst, 5–60. [PubMed] [Google Scholar]