Abstract
The prevalence, host range and geographical bounds of chronic wasting disease (CWD), the prion disease of cervids, are expanding. Horizontal transmission likely contributes the majority of new CWD cases, but the mechanism by which prions are transmitted among CWD-affected cervids remains unclear. To address the extent to which prion amplification in peripheral tissues contributes to contagious transmission, we assessed the prion levels in central nervous and lymphoreticular system tissues in white-tailed deer (Odocoileus virginianus), red deer (Cervus elaphus elaphus) and elk (Cervus canadensis). Using real-time quaking-induced conversion, cervid prion cell assay and transgenic mouse bioassay, we found that the retropharyngeal lymph nodes of red deer, white-tailed deer and elk contained similar prion titres to brain from the same individuals. We propose that marked lymphotropism is essential for the horizontal transmission of prion diseases and postulate that shed CWD prions are produced in the periphery.
Keywords: prion, chronic wasting disease, transmissible spongiform encephalopathy, lymphoid tissue, cervid prion cell assay, RT-QuIC
Transmissible spongiform encephalopathies (TSEs) are fatal, incurable, infectious disorders of mammals that are caused by prions. Zoonotic transmission of bovine spongiform encephalopathy (BSE) caused variant Creutzfeldt–Jakob disease (vCJD) in young adults and teenagers [1]. vCJD not only illustrates the ability of TSEs to occur as unpredictable epidemics, but also raises concerns about the risk from additional animal prion diseases, including sheep scrapie and cervid chronic wasting disease (CWD). TSE prion replication results from corruption of the host-encoded cellular prion protein (PrPC) by its infective counterpart (PrPSc) through conformational templating [2]. The contagious transmission of CWD in free-ranging and captive animals is unique among prion diseases. Prevalence can reach 13 % in certain wild populations [3], while >80 % of captive deer housed in paddocks that previously contained CWD-infected animals ultimately developed disease [4]. By contrast, bovine spongiform encephalopathy incidence in dairy cattle herds was less than 3 % [5]. While the facile spread of CWD in wild populations is likely a result of both direct animal-to-animal contact and exposure to contaminated environments, the biological underpinnings of this process are not completely understood, and it is not entirely clear why horizontal transmissibility varies among TSEs.
Early, widespread and progressive accumulation of PrPSc in the lymphoid system is a feature of CWD in deer, and PrPSc or prion infectivity has been detected in many additional peripheral tissues [6–18]. While similar patterns of early lymphoid prion deposition and eventual widespread prion deposition are well established in sheep scrapie [19–24], PrPSc is restricted almost entirely to nervous tissue in BSE-affected cattle [25–28].
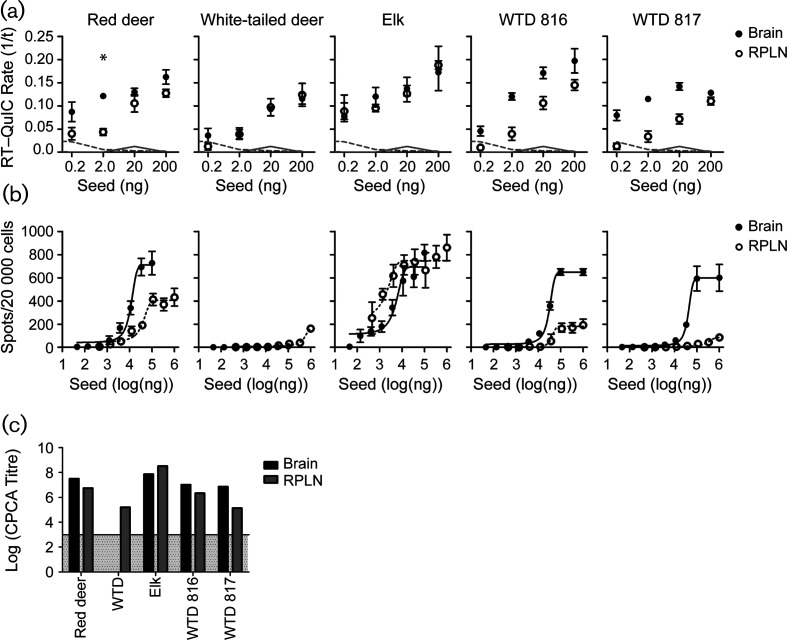
Despite significant involvement of the lymphoreticular system, studies of CWD infectivity have focused primarily on prions in the central nervous system (CNS). We therefore compared the properties of prions in retropharyngeal lymph nodes with prions in the CNS of the same individual for three cervid subspecies: red deer (Cervus elaphus elaphus) inoculated with CWD-affected brain homogenate, allowed to progress until terminal disease [16]; naturally infected elk (Cervus canadensis); and naturally or experimentallyinfected white-tailed deer (WTD) (Odocoileus virginianus) [29]. We used complementary approaches, including real-time quaking-induced conversion (RT-QuIC) [30], cervid prion cell assay (CPCA) [31] and bioassay in CWD-susceptible transgenic (Tg) mice [32]. In RT-QuIC with truncated Syrian hamster recombinant PrP (the standard RT-QuIC substrate [33]), the rate of amyloid formation (QuIC rate) is the inverse of the lag phase observed between the addition of a prion-containing seed and the detection of amyloid by thioflavin-T fluorescence. The rate of amyloid formation for prions from the retropharyngeal lymph node (RPLN) and the CNS were indistinguishable in natural CWD infections of WTD and elk (Fig. 1). Because RT-QuIC relies on the seeded conversion of recombinant PrP to an amyloid form, and the correlation of seeding activity to infectivity is uncertain, we also estimated the infectivity of each tissue using the CPCA. Accordingly, we infected transgenically modified RK13 rabbit kidney epithelial cells expressing deer or elk PrPC with WTD or elk prions of matching genotype. We calculated titres as previously described; briefly, the titre is the log of the number of spots (which is estimated to be the number of infected cells) per gram of tissue [31, 34, 35]. RK13 cells that do not express PrPC served as negative controls. The titres of CWD prions from RPLN and brain of elk and red deer were similar (elk RPLN: 8.5; elk brain: 7.9; red deer RPLN: 6.7; red deer brain: 7.5), but the titres were lower in the RPLN compared to the CNS for experimentally infected WTD 816 and 817 (816 RPLN: 6.3; 816 brain: 7.0; 817 RPLN: 5.1; 817 brain: 6.9) (Fig. 1). Interestingly, preparations from naturally infected WTD brain failed to infect susceptible RK13 cells (Fig. 1b–c), although we detected proteinase K (PK)-resistant PrPSc by Western blot (data not shown) and amyloid seeding activity (Fig. 1a) in RT-QuIC. In contrast, samples from experimentally infected WTD (#816, 817) produced robust infections of deer PrPC expressing cells. Therefore, prions were present in the naturally infected WTD brain, and the cells expressing deer PrPC were competent for infection. Previous studies have suggested that CPCA may be sensitive to CWD prion strain properties, since other infectious CWD isolates do not efficiently infect cells in the CPCA [31]. The same CWD isolates (from naturally and experimentally infected WTD) had similar seeding activity in RT-QuIC; it seems that their variability was only detectable in CPCA. Due to the sensitivity of CPCA to CWD strain properties, we hypothesize that naturally infected deer brain contained a different strain than the other WTD samples.
Fig. 1.
Prion loads in RPLN and CNS from CWD-infected cervids are comparable when assessed by RT-QuIC and CPCA. (a) RPLN and brain samples from red deer, WTD or elk were tested in RT-QuIC to determine the seeding activity of each sample (represented by the RT-QuIC rate, which is the inverse of the lag phase). Filled circles, brain; open circles, RPLN. Error bars, SEM at each dilution. *, significant differences between RPLN and brain samples (P<0.05, two-sided Mann–Whitney test). Dotted grey lines, mean spontaneous conversion rate for a negative brain sample; solid grey lines, mean spontaneous conversion rate for a negative RPLN sample. (b) CPCA of RPLN or brain from red deer, WTD or elk using RK13 cells expressing deer PrPC (WTD samples), or elk PrPC (elk and red deer samples). Infected cells appear as immunopositive spots on elispot plates following treatment with proteinase K. (c) Titres indicate the log spots g−1 tissue calculated in the linear range of curves, with a limit of detection of 103 (indicated by the grey region).
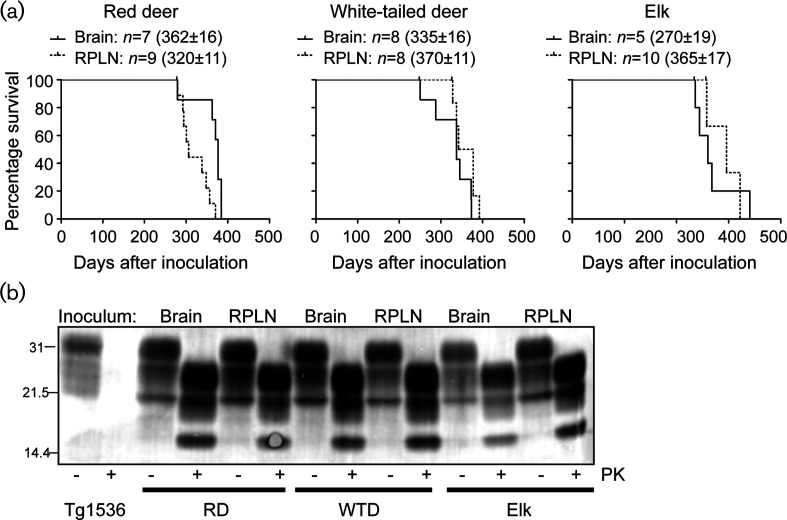
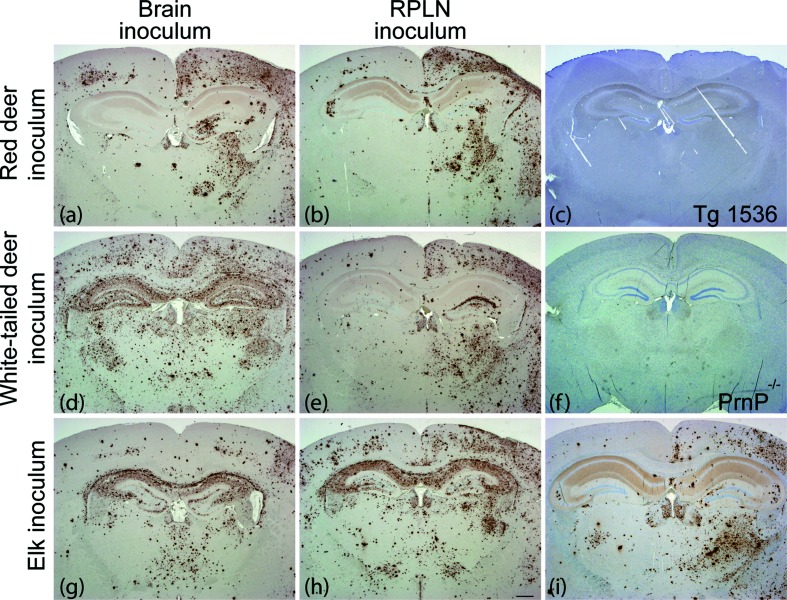
To assess the infectivity of prions from the lymph nodes and CNS of the various CWD-infected cervid subspecies, we intracerebrally inoculated Tg(DeerPrP) 1536 mice (as previously described, [32]). All of the inoculated animals in all six study groups displayed a constellation of neurological signs consistent with CWD, with mean incubation times ranging from 270 to 370 days (Fig. 2a). Mice inoculated with brain extracts from naturally infected WTD had shorter mean incubation times than mice inoculated with RPLN from the same animal (P<0.05, Mantel–Cox) (Fig. 2a). While CWD prions from the CNS of naturally infected elk produced disease with a shorter mean incubation time than prions from RPLN (P<0.005, Mantel–Cox) (Fig. 2a), Tg mice inoculated with red deer brain extracts had a longer mean incubation time than Tg mice inoculated with red deer RPLN (P<0.005, Mantel–Cox) (Fig. 2a). Immunoblots of brain extracts showing the presence of PrPSc in the brains of diseased Tg(DeerPrP) 1536 mice confirmed that mice had succumbed to prion infection in all cases (Fig. 2b). We analysed coronal hippocampal sections with immunohistochemistry using formic acid treatment, antigen retrieval and antibody D18 for standard neuropathological assessment of CWD strains in Tg(Deer) 1536 mice [36]. Mice inoculated with brain or RPLN from red deer had similar PrPSc deposition patterns: florid plaques were present primarily in the inoculated hemisphere and the hippocampi were spared, which is reminiscent of type II CWD [37] (Fig. 3a, b). Mice inoculated with WTD RPLN had a similar deposition pattern to red deer-inoculated mice, with florid plaques in the inoculated (right) hemisphere, which is characteristic of type II CWD (Fig. 3e). However, mice inoculated with WTD brain had florid plaques in both hemispheres and hippocampal PrPSc accumulation, which is reminiscent of type I CWD (Fig. 3d).
Fig. 2.
Prions from the CNS and lymphoreticular system of CWD-infected deer and elk are transmissible to CWD-susceptible transgenic mice. (a) Tg(DeerPrP) 1536 mice were intracerebrally inoculated with 1 % homogenates of brain and RPLN tissues from CWD-infected cervids. Kaplan–Meier survival curves are shown for each infected cohort. Mean incubation times (±SEM), where n indicates the number of infected and diseased animals, are also shown. Asterisks indicate statistical significance between the mean incubation periods of matched samples (*, P<0.05; **, P<0.005, Mantel–Cox). (b). All of the diseased Tg(DeerPrP) 1536 mouse brains were analysed by immunoblotting to confirm prion disease. Samples from two representative mice in each group are shown. The inoculum tissues are listed above the blots, and the inoculum species and PK treatments are listed below the blots.
Fig. 3.
The PrPSc deposition patterns in mice varied with inoculum source. We prepared coronal sections through the hippocampus of Tg(DeerPrP) 1536 mice inoculated with: (a) red deer brain, (b) red deer RPLN, (c) nothing (uninoculated), (d) white-tailed deer brain, (e) white-tailed deer RPLN, (g) elk brain, (h) elk RPLN, 320 days p.i., (i) elk RPLN, 400 days p.i. We used prion knockout mice (PrnP−/−) as a negative control for IHC (f). We stained paraffin-embedded sections with anti-PrP antibody 18 days after formic acid treatment and antigen retrieval. Scale bar (h), 500 µm. All mice were inoculated in the right hemisphere.
Mice inoculated with elk RPLN had an apparently bimodal distribution of incubation times (Fig. 2a). Mice with shorter incubation periods (320 days p.i.) had PrPSc in both hemispheres and the hippocampus (type I) (Fig. 3h), as did all mice inoculated with elk brain (Fig. 3g). Interestingly, mice inoculated with elk RPLN with longer incubation periods (400 days p.i.) displayed asymmetric deposition patterns (type II) (Fig. 3j). The variability in PrPSc deposition patterns between animals inoculated with brain versus lymphoid tissue and within the group inoculated with elk RPLN suggests the presence of multiple CWD strains in a single individual.
Understanding the biological properties of prions that are resident in non-CNS tissues of animals affected by CWD is important for several reasons. First, it offers clues about the rapid and contagious spread of CWD [38, 39]. While the pathogenesis of both CWD and sheep scrapie are characterized by prion replication in the lymphoid system [6, 7, 40], BSE is restricted almost exclusively to the nervous system, with a few reports of PrPSc detection in the distal ileum and the tonsil [25–27]. However, when cattle were intracerebrally inoculated with scrapie or CWD, there was essentially no PrPSc detected outside the CNS [41, 42]. In contrast, when sheep were inoculated with BSE, prions were detectable in the lymphoid system as well as the CNS [43, 44]. Taken together, these results suggest a significant host influence on prion lymphotropism, with deer and sheep tending to permit lymphotropism, while cattle do not. Additionally, the lymphoid system and CNS have different tolerances for cross-species prion transmission [45]. When Tg mice were inoculated with prion strains to which they are typically resistant, PrPSc propagation and prion infectivity were detectable in their spleens, despite an absence of prion replication in the brain [45]. CWD strains seem to have variable lymphotropic behaviour, which supports the hypothesis that the CNS and lymphoid system provide different environments for prion replication [37]. We propose that lymphotropism is an essential component of horizontally transmissible prion diseases, which are characterized by substantial titres of infectious prions outside the brain, particularly in the lymphoid system. Full characterization of the properties of peripheral CWD prions versus CNS prions and an assessment of their zoonotic potential remains to be determined. Our data suggest that (1) peripheral tissues accumulate high titres of infectious CWD prions and (2) different CWD strains may be harboured by peripheral tissues versus central nervous tissues.
Funding information
This work was supported by National Institutes of Health grants R01 NS040334 and P01 AI077774 to G. T. and grant F30OD021442 to K. A. D.
Acknowledgements
We thank Clare Hoover for useful discussions.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
Animals were collected in accordance with protocols of the Canadian Council on Animal Care or the Colorado State University Institutional Animal Care and Use Committee.
Footnotes
Abbreviations: BSE, bovine spongiform encephalopathy; CNS, central nervous system; CPCA, cervid prion cell assay; CWD, chronic wasting disease; PK, proteinase K; PrPC, normal prion protein; PrPSc, abnormal prion protein; RPLN, retropharyngeal lymph node; RT-QuIC, real-time quaking-induced conversion; Tg, transgenic; TSE, transmissible spongiform encephalopathy; vCJD, variant Creutzfeldt–Jakob disease; WTD, white-tailed deer.
References
- 1.Will RG, Ironside JW, Zeidler M, Estibeiro K, Cousens SN, et al. A new variant of Creutzfeldt-Jakob disease in the UK. The Lancet. 1996;347:921–925. doi: 10.1016/S0140-6736(96)91412-9. [DOI] [PubMed] [Google Scholar]
- 2.Cohen FE, Prusiner SB. Pathologic conformations of prion proteins. Annu Rev Biochem. 1998;67:793–819. doi: 10.1146/annurev.biochem.67.1.793. [DOI] [PubMed] [Google Scholar]
- 3.Monello RJ, Powers JG, Hobbs NT, Spraker TR, O'Rourke KI, et al. Efficacy of antemortem rectal biopsies to diagnose and estimate prevalence of chronic wasting disease in free-ranging cow elk (Cervus elaphus nelsoni) J Wildl Dis. 2013;49:270–278. doi: 10.7589/2011-12-362. [DOI] [PubMed] [Google Scholar]
- 4.Miller MW, Williams ES, Hobbs NT, Wolfe LL. Environmental sources of prion transmission in mule deer. Emerg Infect Dis. 2004;10:1003–1006. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collee JG, Bradley R. BSE: a decade on–Part 2. Lancet. 1997;349:715–721. doi: 10.1016/S0140-6736(96)08496-6. [DOI] [PubMed] [Google Scholar]
- 6.Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O'Rourke KI, et al. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus) J Gen Virol. 1999;80:2757–2764. doi: 10.1099/0022-1317-80-10-2757. [DOI] [PubMed] [Google Scholar]
- 7.Hoover CE, Davenport KA, Henderson DM, Denkers ND, Mathiason CK, et al. Pathways of prion spread during early chronic wasting disease in deer. J Virol. 2017;91:e00077-17. doi: 10.1128/JVI.00077-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spraker TR, Zink RR, Cummings BA, Sigurdson CJ, Miller MW, et al. Distribution of protease-resistant prion protein and spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus) with chronic wasting disease. Vet Pathol. 2002;39:546–556. doi: 10.1354/vp.39-5-546. [DOI] [PubMed] [Google Scholar]
- 9.Fox KA, Jewell JE, Williams ES, Miller MW. Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus) J Gen Virol. 2006;87:3451–3461. doi: 10.1099/vir.0.81999-0. [DOI] [PubMed] [Google Scholar]
- 10.Miller MW, Williams ES. Detection of PrPCWD in mule deer by immunohistochemistry of lymphoid tissues. Vet Rec. 2002;151:610–612. doi: 10.1136/vr.151.20.610. [DOI] [PubMed] [Google Scholar]
- 11.Race BL, Meade-White KD, Ward A, Jewell J, Miller MW, et al. Levels of abnormal prion protein in deer and elk with chronic wasting disease. Emerg Infect Dis. 2007;13:824–829. doi: 10.3201/eid1306.070186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angers RC, Browning SR, Seward TS, Sigurdson CJ, Miller MW, et al. Prions in skeletal muscles of deer with chronic wasting disease. Science. 2006;311:1117. doi: 10.1126/science.1122864. [DOI] [PubMed] [Google Scholar]
- 13.Race B, Meade-White K, Oldstone MB, Race R, Chesebro B. Detection of prion infectivity in fat tissues of scrapie-infected mice. PLoS Pathog. 2008;4:e1000232. doi: 10.1371/journal.ppat.1000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sigurdson CJ, Spraker TR, Miller MW, Oesch B, Hoover EA. PrPCWD in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J Gen Virol. 2001;82:2327–2334. doi: 10.1099/0022-1317-82-10-2327. [DOI] [PubMed] [Google Scholar]
- 15.Jewell JE, Brown J, Kreeger T, Williams ES. Prion protein in cardiac muscle of elk (Cervus elaphus nelsoni) and white-tailed deer (Odocoileus virginianus) infected with chronic wasting disease. J Gen Virol. 2006;87:3443–3450. doi: 10.1099/vir.0.81777-0. [DOI] [PubMed] [Google Scholar]
- 16.Balachandran A, Harrington NP, Algire J, Soutyrine A, Spraker TR, et al. Experimental oral transmission of chronic wasting disease to red deer (Cervus elaphus elaphus): early detection and late stage distribution of protease-resistant prion protein. Can Vet J. 2010;51:169–178. [PMC free article] [PubMed] [Google Scholar]
- 17.Haley NJ, Mathiason CK, Carver S, Zabel M, Telling GC, et al. Detection of chronic wasting disease prions in salivary, urinary, and intestinal tissues of deer: potential mechanisms of prion shedding and transmission. J Virol. 2011;85:6309–6318. doi: 10.1128/JVI.00425-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davenport KA, Hoover CE, Bian J, Telling GC, Mathiason CK, et al. PrPC expression and prion seeding activity in the alimentary tract and lymphoid tissue of deer. PLoS One. 2017;12:e0183927. doi: 10.1371/journal.pone.0183927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andréoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, et al. Early accumulation of PrPSc in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J Gen Virol. 2000;81:3115–3126. doi: 10.1099/0022-1317-81-12-3115. [DOI] [PubMed] [Google Scholar]
- 20.van Keulen LJ, Schreuder BE, Vromans ME, Langeveld JP, Smits MA. Scrapie-associated prion protein in the gastrointestinal tract of sheep with natural scrapie. J Comp Pathol. 1999;121:55–63. doi: 10.1053/jcpa.1998.0300. [DOI] [PubMed] [Google Scholar]
- 21.Jeffrey M, Begara-McGorum I, Clark S, Martin S, Clark J, et al. Occurrence and distribution of infection-specific PrP in tissues of clinical scrapie cases and cull sheep from scrapie-affected farms in Shetland. J Comp Pathol. 2002;127:264–273. doi: 10.1053/jcpa.2002.0592. [DOI] [PubMed] [Google Scholar]
- 22.Heggebø R, Press CM, Gunnes G, González L, Jeffrey M. Distribution and accumulation of PrP in gut-associated and peripheral lymphoid tissue of scrapie-affected Suffolk sheep. J Gen Virol. 2002;83:479–489. doi: 10.1099/0022-1317-83-2-479. [DOI] [PubMed] [Google Scholar]
- 23.Jeffrey M, Martin S, Thomson JR, Dingwall WS, Begara-McGorum I, et al. Onset and distribution of tissue PrP accumulation in scrapie-affected suffolk sheep as demonstrated by sequential necropsies and tonsillar biopsies. J Comp Pathol. 2001;125:48–57. doi: 10.1053/jcpa.2001.0476. [DOI] [PubMed] [Google Scholar]
- 24.Race R, Jenny A, Sutton D. Scrapie infectivity and proteinase K—resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis. J Infect Dis. 1998;178:949–953. doi: 10.1086/515669. [DOI] [PubMed] [Google Scholar]
- 25.Balkema-Buschmann A, Eiden M, Hoffmann C, Kaatz M, Ziegler U, et al. BSE infectivity in the absence of detectable PrPSc accumulation in the tongue and nasal mucosa of terminally diseased cattle. J Gen Virol. 2011;92:467–476. doi: 10.1099/vir.0.025387-0. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann C, Eiden M, Kaatz M, Keller M, Ziegler U, et al. BSE infectivity in jejunum, ileum and ileocaecal junction of incubating cattle. Vet Res. 2011;42:21. doi: 10.1186/1297-9716-42-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balkema-Buschmann A, Fast C, Kaatz M, Eiden M, Ziegler U, et al. Pathogenesis of classical and atypical BSE in cattle. Prev Vet Med. 2011;102:112–117. doi: 10.1016/j.prevetmed.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Wells GA, Spiropoulos J, Hawkins SA, Ryder SJ. Pathogenesis of experimental bovine spongiform encephalopathy: preclinical infectivity in tonsil and observations on the distribution of lingual tonsil in slaughtered cattle. Vet Rec. 2005;156:401–407. doi: 10.1136/vr.156.13.401. [DOI] [PubMed] [Google Scholar]
- 29.Denkers ND, Hayes-Klug J, Anderson KR, Seelig DM, Haley NJ, et al. Aerosol transmission of chronic wasting disease in white-tailed deer. J Virol. 2013;87:1890–1892. doi: 10.1128/JVI.02852-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atarashi R, Wilham JM, Christensen L, Hughson AG, Moore RA, et al. Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nat Methods. 2008;5:211–212. doi: 10.1038/nmeth0308-211. [DOI] [PubMed] [Google Scholar]
- 31.Bian J, Napier D, Khaychuck V, Angers R, Graham C, et al. Cell-based quantification of chronic wasting disease prions. J Virol. 2010;84:8322–8326. doi: 10.1128/JVI.00633-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Browning SR, Mason GL, Seward T, Green M, Eliason GA, et al. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J Virol. 2004;78:13345–13350. doi: 10.1128/JVI.78.23.13345-13350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilham JM, Orrú CD, Bessen RA, Atarashi R, Sano K, et al. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog. 2010;6:e1001217. doi: 10.1371/journal.ppat.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bian J, Kang HE, Telling GC. Quinacrine promotes replication and conformational mutation of chronic wasting disease prions. Proc Natl Acad Sci USA. 2014;111:6028–6033. doi: 10.1073/pnas.1322377111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bian J, Khaychuk V, Angers RC, Fernández-Borges N, Vidal E, et al. Prion replication without host adaptation during interspecies transmissions. Proc Natl Acad Sci USA. 2017;114:1141–1146. doi: 10.1073/pnas.1611891114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williamson RA, Peretz D, Pinilla C, Ball H, Bastidas RB, et al. Mapping the prion protein using recombinant antibodies. J Virol. 1998;72:9413–9418. doi: 10.1128/jvi.72.11.9413-9418.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Angers RC, Kang HE, Napier D, Browning S, Seward T, et al. Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science. 2010;328:1154–1158. doi: 10.1126/science.1187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams ES, Young S. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis. 1980;16:89–98. doi: 10.7589/0090-3558-16.1.89. [DOI] [PubMed] [Google Scholar]
- 39.Benestad SL, Mitchell G, Simmons M, Ytrehus B, Vikøren T. First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Vet Res. 2016;47:1–7. doi: 10.1186/s13567-016-0375-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Keulen LJ, Vromans ME, van Zijderveld FG. Early and late pathogenesis of natural scrapie infection in sheep. APMIS. 2002;110:23–32. doi: 10.1034/j.1600-0463.2002.100104.x. [DOI] [PubMed] [Google Scholar]
- 41.Cutlip RC, Miller JM, Race RE, Jenny AL, Katz JB, et al. Intracerebral transmission of scrapie to cattle. J Infect Dis. 1994;169:814–820. doi: 10.1093/infdis/169.4.814. [DOI] [PubMed] [Google Scholar]
- 42.Haley NJ, Siepker C, Greenlee JJ, Richt JA. Limited amplification of chronic wasting disease prions in the peripheral tissues of intracerebrally inoculated cattle. J Gen Virol. 2016;97:1720–1724. doi: 10.1099/jgv.0.000438. [DOI] [PubMed] [Google Scholar]
- 43.Foster JD, Bruce M, McConnell I, Chree A, Fraser H. Detection of BSE infectivity in brain and spleen of experimentally infected sheep. Vet Rec. 1996;138:546–548. doi: 10.1136/vr.138.22.546. [DOI] [PubMed] [Google Scholar]
- 44.Foster JD, Parnham D, Chong A, Goldmann W, Hunter N. Clinical signs, histopathology and genetics of experimental transmission of BSE and natural scrapie to sheep and goats. Vet Rec. 2001;148:165–171. doi: 10.1136/vr.148.6.165. [DOI] [PubMed] [Google Scholar]
- 45.Béringue V, Herzog L, Jaumain E, Reine F, Sibille P, et al. Facilitated cross-species transmission of prions in extraneural tissue. Science. 2012;335:472–475. doi: 10.1126/science.1215659. [DOI] [PubMed] [Google Scholar]