Abstract
BACKGROUND
Approximately 20% of low-grade gliomas (LGG) display visible protoporphyrin fluorescence during surgery after 5-aminolevulinic acid (5-ALA) administration.
OBJECTIVE
To determine if fluorescence represents a prognostic marker in LGG.
METHODS
Seventy-four consecutive patients with LGG (World Health Organization 2016) were operated on with 5-ALA. Fluorescent tissue was specifically biopsied. Tumor size, age, Karnofsky index, contrast-enhancement, fluorescence, and molecular factors (IDH1/IDH2-mutations, Ki67/MIB1 Index, 1p19q codeletions, ATRX, EGFR, p53 expression, and O6-methylguanine DNA methyltransferase promotor methylation), were related to progression-free survival (PFS), malignant transformation-free survival (MTFS) and overall survival (OS).
RESULTS
Sixteen of seventy-four LGGs (21.6%) fluoresced. Fluorescence was partially related to weak enhancement on magnetic resonance imaging and increased (positron emission tomography)PET-FET uptake, but not to Karnofsky Performance Score, tumor size, or age. Regarding molecular markers, only EGFR expression differed marginally (fluorescing vs nonfluorescing: 19% vs 5%; P = .057). Median follow-up was 46.4 mo (95% confidence interval [CI]: 41.8-51.1). PFS, MTFS, and OS were shorter in fluorescing tumors (PFS: median 9.8 mo, 95% CI: 1.00-27.7 vs 45.8, 31.9-59.7, MTFS: 43.0 [27.5-58.5] vs 64.6 [57.7-71.5], median not reached, P = .015; OS: 51.6, [34.8-68.3] vs [68.2, 62.7-73.8], P = .002). IDH mutations significantly predicted PFS, MTFS, and OS. In multivariate analysis IDH status and fluorescence both independently predicted MTFS and OS. PFS was not independently predicted by fluorescence.
CONCLUSION
This is the first report investigating the role of ALA-induced fluorescence in histologically confirmed LGG. Fluorescence appeared to be a marker for inherent malignant transformation and OS, independently of known prognostic markers. Fluorescence in LGG might be taken into account when deciding on adjuvant therapies.
Keywords: Diffuse astrocytoma, 5-ALA, Fuorescence-guided resection, Malignization transformation-free survival (MTFS), PET, Low-grade glioma
ABBREVIATIONS
- 5-ALA
-aminolevulinic acid
- CI
confidence interval
- DNA
deoxyribonucleic acid
- LGG
low-grade gliomas
- EoR
extent of resection
- MGMT
O6-methylguanine DNA methyltransferase
- FET-PET
fluoroethyl-L-tyrosine positron emission tomography
- KPS
Karnofsky performance score
- MLPA
multiplex ligation-dependent probe amplification
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- MTFS
malignant transformation-free survival
- OS
overall survival
- PFS
progression-free survival
Five-aminolevulinic acid (5-ALA) induces the accumulation of fluorescing porphyrins in malignant glioma tissue and has recently been approved by the US Food and Drug Administration for fluorescence-guided resection of these tumors. Porphyrins can be visualized with specially adapted surgical microscopes during surgery.1-13. In gliomas harboring anaplastic foci, regions with a higher proliferation rate can be identified by the accumulation of fluorescence, which can be specifically interrogated pathologically.7,13,14 Spectrographically detectable porphyrin fluorescence has been related to malignancy in gliomas.15
In confirmed low grade diffuse gliomas (LGG, WHO grade II), on the other hand, visible fluorescence can be detected in only about 20% of patients.7,13,14,16 In an earlier study LGG with fluorescence were found not to differ histologically from LGG without fluorescence.14 The reasons for the nonuniform accumulation in LGG are not understood. Simply assuming porphyrin accumulation to be a hallmark of malignancy may be erroneous, as benign tumors such as meningeomas,15,17-20 or grade II ependymomas have been found to accumulate fluorescence.21-24 We therefore questioned whether patients with LGG with visible fluorescence have a prognosis different to that of patients without fluorescence. We interrogated our database regarding LGGs operated on using 5-ALA to determine prognosis, and related prognosis to known clinical and molecular factors.
METHODS
Consecutive patients entered into a prospective data base formed the basis of this study. This retrospective analysis was approved by the ethical committee of the University of Münster (reference: 2014-560-f-N). Due its retrospective nature, no informed consent was available or necessary. However, informed consent had been obtained from all patients regarding the use of their tissues for scientific purposes prior to their entry into the data base.
We identified 74 consecutive patients operated on using 5-ALA between October 2010 and January 2016 with a final diagnosis of WHO 2016 grade II glioma, for whom follow-up data greater than 3 mo was available.
Surgery
We have adapted a generous policy toward using 5-ALA in patients with assumed LGG since a number of these tumors will ultimately turn out to harbor grade III pathologies, especially if there is any weak or indistinct contrast-enhancement. It has previously been demonstrated that ALA induced fluorescence can be used to detect anaplastic foci in tumors that appear as low grade lesion on the magnetic resonance imaging (MRI).13,14,16,25
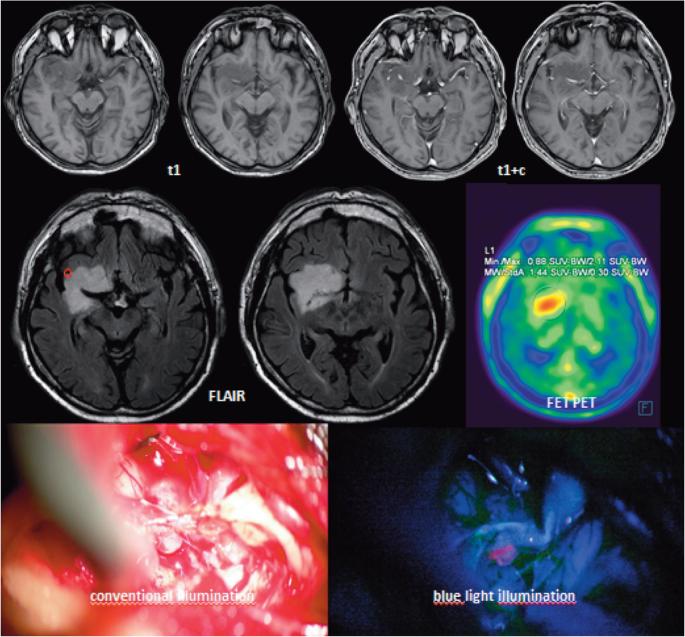
Patients received 5-ALA (Gliolan®, medac, Wedel, Germany) at a dose of 20 mg/kg dissolved in 50 mL of tap water 3 to 4 hr prior to induction of anaesthesia.11 During surgery surgeons frequently toggled between white light and the fluorescence mode of the microscope (OPMI Pentero, Carl Zeiss Meditech, Oberkochen, Germany, equipped with the BLUE400 fluorescence option) for detecting fluorescence. Neuronavigation was used to correlate areas with weak enhancement on MRI or regions with increased uptake ratios, as derived from O-(2-[18F]-fluoroethyl)-L-tyrosine positron emission tomography (FET-PET). Neuronavigation was used as early as possible in order to minimize brainshift. Fluorescence, if present, was either found in a diffuse fashion throughout the tumor, or focally (Figure 1) and specifically biopsied. Fluorescence was confirmed visibly after extraction of tissue before securing samples in preprepared vials for processing.
FIGURE 1.
Example for MRI of a LGG glioma of the right insula and perisylvian structures (top row: t1 + /− GD, middle row: FLAIR images and representative FET-PET image). Bottom row left: intraoperative white light image of insula viewed from top with partially resected tumor, right: blue white image. Note greenish fluorescence of insular M3 branch of middle cerebral artery. The circle on the FLAIR image marks the region of focal fluorescence encountered during surgery.
Surgery was only performed by 1 of 3 surgeons (W.S., C.E., J.W.).
Preoperative Imaging
Magnetic resonance (MR) imaging and FET-PET imaging were performed as described in detail previously.14,26
Neuropathology
Tumor tissues were graded in accordance with 2016 WHO criteria.27 Neuropathologists were blinded as to whether tumors had fluoresced during surgery. The Ki-67/MIB-1 proliferation index, ATRX expression, EGRFR expression and the IDH1 (R132H) mutation status were determined using immunohistochemistry.28 All tumors showing negative staining for mutated IDH1 (R132H) protein were sequenced for non-R132H-IDH1 and IDH2 hotspot mutations.29 In tumors with possible oligodendroglial differentiation, 1p/19q co-deletions were determined by multiplex ligation-dependent probe amplification (MLPA) using probes (SALSA MLPA P088 Oligodendroglioma 1p-19q probemix) and protocols provided by the manufacturer (MRC Holland, Amsterdam, The Netherlands) in all tumors, in which an oligodendroglial differentiation was suspected histopathologically.
O6-methylguanine DNA methyltransferase (MGMT) promoter methylation status was determined by methylation-specific polymerase chain reaction of bisulfite converted deoxyribonucleic acid (DNA) (EZ DNA Methylation-Gold Kit; Zymo Research, Orange, California) as previously described.30
Clinical Data
We recorded age, gender, Karnofsky Performance Score (KPS), extent of resection (EoR) based on the MRI fluid-attenuated inversion-recovery image, and presence of weak contrast-enhancement on preoperative MRI. Treatment with radiotherapy or chemotherapy after initial surgery (adjuvant therapy) was documented.
Treatment decisions regarding adjuvant therapies were in all cases based on evaluations in our multidisciplinary tumor board. In the face of progression or malignant degeneration patients were treated according to accepted guidelines.31
Patients were followed for overall survival (OS), progression-free survival (PFS), or malignant transformation-free survival (MTFS). Progression was defined according to RANO criteria by an independent neuroradiologist and was based on imaging with or without histological corroboration.32 PFS consequently incorporated increases in size, malignant degeneration, or death. Malignant degeneration was only assumed when histology, obtained during surgery or from stereotactic biopsy, proved high-grade glioma pathology. Tissue was collected in all patients in whom malignant degeneration was assumed by resection or stereotactic biopsy.
The present cohort partially coincides with patients analyzed for an earlier study without follow-up, but included only patients from that cohort in whom follow-up data of >3 mo were available and that were not lost to follow-up.
Statistical Methods
Commercially available software (Statistical Package for Social Sciences, version 23.0; IBM Inc, Armonk, New York) was used for all statistical analyses. The Person Chi2 square statistic was calculated for categorial variables. Difference in average values for independent groups were test using analysis of variance for testing the significance of differences in mean values between independent groups. For testing univariate differences in outcome data, the Kaplan–Meier estimator was employed with the log rank test statistic for assessing significance for categorical variables and cox regressions for continuous variables. Cox regressions with stepwise forward inclusion were used for testing the effects of multiple variables in time to event analyses. Multinomial logisitic regression was employed for testing possible relationships between independent variables and fluorescence as outcome. We assumed a 2-sided error probability level P of less than .05 to indicate significance.
RESULTS
Patient and Imaging Characteristics
A typical case example is given in Figure 1, which summarizes MR, PET, and intraoperative findings in a patient with an IDH1 mutated diffuse astrocytoma. MRI indicated weak, spotty enhancement. Fluorescence was found in a focal distribution. Pathologically, no differences were detected between fluorescing and nonfluorescing tissue. The patient harbored an IDH1 mutated diffuse astrocytoma WHO (2016) grade II. Both a sample from fluoresceing as well as a sample from nonfluorescing tumor tissue had a MIB-Index of approximately 1%.
Table 1 summarizes the clinical characteristics of patients stratified by the presence or lack of fluorescence. Among 74 patients fluorescence was observed in 16 (21.6%) cases. No significant differences were noted between patients with fluorescing and nonfluorescing tumors regarding age, tumors size, gender, and EoR. Table 2 summarizes the results of our molecular analyses stratified by fluorescence findings. 1p19q co-deletions/IDH1 mutated tumors were observed in 12 patients (16.2%), indicating oligodendroglioma according to the 2016 WHO classification. Of the remaining 62 diffuse astrocytomas, IDH1 mutations were observed in 46 (74.2%) cases. No IDH2 mutations were observed. Median MIB-Index was 4.3% in the pooled cohorts, which did not differ between fluorescing and nonfluorescing tumor samples. No significant differences were found regarding p53, ATRX expression or MGMT promotor methylation. Interestingly, EGFR expression was three times more common in fluorescing LGG than in nonfluorescing LGG, but not significantly so (19 vs 5%; P = .057).
TABLE 1.
Clinical Characteristics
| ALA | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 74) | No fluorescence (n = 58) | Fluorescence (n = 16) | |||||||||||||||
| Variable | Avg | SD | M | n | n/N | Avg | SD | M | n | n/N | Avg | SD | M | n | n/N | P a | |
| Age | 42.8 | 13.4 | 41.0 | 42.8 | 13.1 | 41.0 | 43.0 | 14.7 | 41.1 | .956 | |||||||
| Gender | male | 42 | 56.8% | 35 | 60.3% | 7 | 43.8% | .236 | |||||||||
| female | 32 | 43.2% | 23 | 39.7% | 9 | 56.3% | |||||||||||
| KPS | 80 | 4 | 5.4% | 3 | 5.2% | 1 | 6.3% | .030 | |||||||||
| 90 | 12 | 16.2% | 6 | 10.3% | 6 | 37.5% | |||||||||||
| 100 | 58 | 78.4% | 49 | 84.5% | 9 | 56.3% | |||||||||||
| Volume | ccm | 11.4 | 20.1 | 6.00 | 13.0 | 22.5 | 6.00 | 5.79 | 3.16 | 6.0 | .207 | ||||||
| Enhancement | no | 56 | 75.7% | 49 | 84.5% | 7 | 43.8% | .001 | |||||||||
| (weak, indistinct) | yes | 18 | 24.3% | 9 | 15.5% | 9 | 56.3% | ||||||||||
| FETmaxb | 2.45 | 1.29 | 2.35 | 2.24 | 1.29 | 2 | 3.21 | 0.952 | 3 | .009 | |||||||
| EoR | >90% | 24 | 32.4% | 21 | 36.2% | 3 | 18.8% | .187 | |||||||||
| <90% | 50 | 67.6% | 37 | 63.8% | 13 | 81.3% | |||||||||||
| Volume | ccm | 11.4 | 20.1 | 6.00 | 13.0 | 22.5 | 6.00 | 5.79 | 3.16 | 6.0 | .207 | ||||||
| Adj. | none | 39 | 52.7% | 32 | 55.2% | 7 | 43.8% | .335 | |||||||||
| Cytotoxic | chemo | 9 | 12.2% | 8 | 13.8% | 1 | 6.3% | ||||||||||
| Therapy | RT | 24 | 32.4% | 16 | 27.6% | 8 | 50.0% | ||||||||||
| missing | 2 | 2.7% | 2 | 3.4% | 0 | 0.0% | |||||||||||
aChi2 for categories and ANOVA for continuous data; avg, average; SD, standard deviation; M, median; EoR, extent of resection; KPS, Karnofsky Performance Score; ccm, cubic centimeters; FETmax, maxium standard uptake ratio; RT, radiotherapy; chemo, chemotherapy; bn = 70 patients; non-fluorescing tumors: 55, fluorescing tumors 15.
TABLE 2.
Molecular Data
| ALA | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 74) | No fluorescence (n = 58) | Fluorescence (n = 16) | |||||||||||||||
| Avg | SD | M | n | n/N | Avg | SD | M | n | n/N | Avg | SD | M | n | n/N | P a | ||
| MIB-index % | 4.3 | 4.0 | 3.0 | 4.0 | 3.3 | 3.0 | 5.3 | 5.9 | 4.0 | .242 | |||||||
| WHO 2016 oligo | 12 | 16.2% | 9 | 15.5% | 3 | 18.7% | .859 | ||||||||||
| DA | IDH wildtype | 16 | 21.6% | 12 | 20.7% | 4 | 25.0% | ||||||||||
| IDH mutated | 46 | 62.1% | 37 | 63.8.% | 9 | 56.3% | |||||||||||
| EGFR expression | yes | 6 | 8.1% | 3 | 5.2% | 3 | 18.8% | .057 | |||||||||
| no | 53 | 71.6% | 44 | 75.9% | 9 | 56.2% | |||||||||||
| missing | 15 | 20.3% | 11 | 18.9% | 4 | 0.25% | |||||||||||
| P53 mutation | yes | 22 | 29.7% | 16 | 27.6% | 6 | 37.5% | .294 | |||||||||
| no | 43 | 58.1% | 36 | 62.1% | 7 | 43.7% | |||||||||||
| missing | 9 | 12.2% | 6 | 10.3% | 3 | 18.8% | |||||||||||
| ATRX loss | yes | 20 | 27.0% | 18 | 31.0% | 2 | 12.5% | .100 | |||||||||
| no | 38 | 51.4% | 27 | 46.6% | 11 | 68.8% | |||||||||||
| missing | 16 | 21.6% | 13 | 22.4% | 3 | 18.7% | |||||||||||
| MGMT meth. | yes | 40 | 54.1% | 33 | 56.9% | 7 | 43.8% | .813 | |||||||||
| no | 20 | 27.0% | 16 | 27.6% | 4 | 25.0% | |||||||||||
| missing | 14 | 18.9% | 9 | 15.5% | 5 | 31.2% | |||||||||||
aChi2 for categories and ANOVA for continuous data; avg, average; SD, standard deviation, M, median; DA, diffuse astrocytoma; oligo, oligodendroglioma (1p19q co-deleted); EGFR,epidermanl growth factor receptor; MGMT meth, meythlation of the MGMT promotor gene.
We did observe differences in the distribution of KPS, with more patients in the group with tumor fluorescence having a KPS of 90 than in the group of patients without fluorescence.
Overall, in 18 (24.3%) tumors, weak, indistinct, or spotty enhancement was observed on the MRI. No strong enhancement was observed. Patients with tumors showing fluorescence more frequently had MR images with such weak contrast-enhancement (9/16 patients, 56.3% vs 9/49, 15.5%, P = .001).
Patients with intraoperative fluorescence had a slightly increased PET maximal standardized uptake value (SUV) ratio of 3.21 ± .952 (SD) compared to patients without fluorescence 2.24 ± 1.29 (P = .009).
No differences regarding adjuvant therapies after initial surgery (radiotherapy or chemotherapy) were observed.
Multivariate Analysis of Factors Predicting Fluorescence
We considered contrast-enhancement, tumor volume, 1p19q co-deletions, IDH1, MIB1 Index, and the FET-PET to possibly influence the accumulation of fluorescence. In multinomial logistic regression analysis only FET SUV maximum and preoperative contrast-enhancement predicted fluorescence independently (Table 3).
TABLE 3.
Factors Affecting Fluorescence
| Factor/covariate | HR | 95% CI | P |
|---|---|---|---|
| Volumea | 1.09 | 0.894-1.335 | .388 |
| Max. SUV ratio PETa | 2.103 | 1.04-4.26 | .039 |
| 1p19q | 2.88 | 0.402-20.6 | .293 |
| IDH1 mutation | 2.74 | 0.562-13.3 | .213 |
| MIB index | 0.903 | 0.785-1.040 | .157 |
| Enhancement | 8.31 | 1.83-37.7 | .006 |
aContinuous.
Univariate Outcome Analyses
Median follow-up was 46.4 mo (95% CI: 41.8-51.1).
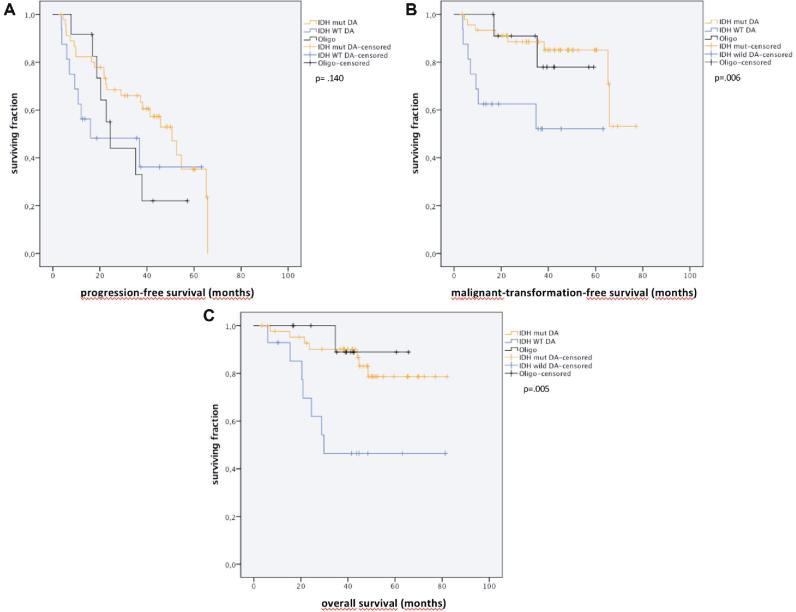
Kaplan–Meier (univariate) analysis showed WHO grade II subtypes (IDH mutated diffuse astrocytomas, IDH wildtype diffuse astrocytomas, IDH mutant/1p19q codeleted oligodendrogliomas) to predict PFS, MTFS, and OS to varying extents (Figure 2A-2C).
FIGURE 2.
Kaplan–Meier curves for A, PFS, B, malignant transformation-free survival, MTFS, and C, OS, stratified by WHO 2016 grade II pathology (Oligo: oligodendrglioma; IDHmut DA: IDH1 mutated diffuse astrocytoma; and IDH WT DA: IDH1 wildtype diffuse astrocytoma).
Patients with IDH1 wild type diffuse astrocytomas tumors tended to have a shorter PFS compared to IDH 1 mutated tumors (median, 95% CI: 16.0, 0,0-47.2 vs 50.7, 38.0-63.3, P = .099). Regarding MTFS and OS, median survivals were not reached in either group. MTFS was shorter in IDH 1 wild type tumors than in tumors with IDH1 mutations (median not reached; average, 95% CI: 39.0, 25.6-52.4; 64.6 vs 57.3-71.9 mo, P = .003). Similarly, average OS was shorter for patients with IDH1 wildtype tumors (49.0, 32.3-65.6 mo) compared to IDH1 mutated tumors (71.5, 64.4-78.6 mo, P = .004).
Prognosis in 1p19q codeleted/IDH mutant oligodendrogliomas was similar to IDH mutated diffuse astrocytomas (median PFS: 24.4, 19.8-29.0, average MTFS: 62.3, 55.9-68.7; average OS: 52.2, 43.5-60.9 mo).
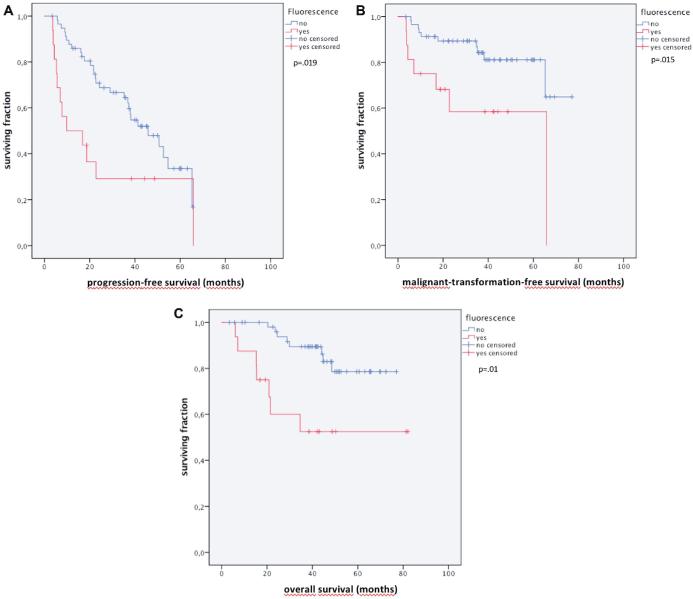
Importantly, fluorescence was prognostic for PFS, MTFS and OS. PFS in fluorescing was shorter in fluorescing vs. nonfluorescing tumors (9.8, 0.00-27.7 vs 45.8, 31.9-59.7 mo; P = .019), as was MTFS (43.0, 27.5-58.5 vs 64.6, 57.7-71.5 mo, median not reached, P = .015) and OS (51.6, 34.8-68.3 vs 68.2, 62.7-73.8 mo, P = .002; Figure 3A-3C).
FIGURE 3.
Kaplan–Meier curves for A, PFS, B, MTFS, and C, OS, stratified by the presence of tumor fluorescence.
On the other hand, no significant influence of contrast-enhancement, maximum PET SUV ratios or EoR on MTFS was found in univariate analysis. Average MTFS was 37.0 (39.7-43.4) mo in enhancing tumors and 60.3 (52.7-67.9) mo in nonenhancing tumors (P = .635). The hazard ratio for maximum PET SUV ratios for predicting MTFS, tested as a continuous variable, was 1.20 (P = .312). EoR had no significant influence on PFS (hazard ratio [HR]: .548, P = .065) OS (OS: HR .713, P = .817) or MTFS (HR = .636, P = .363). The low hazard ratios for resection indicated underpowering for this analysis.
Multivariate Outcome Analysis
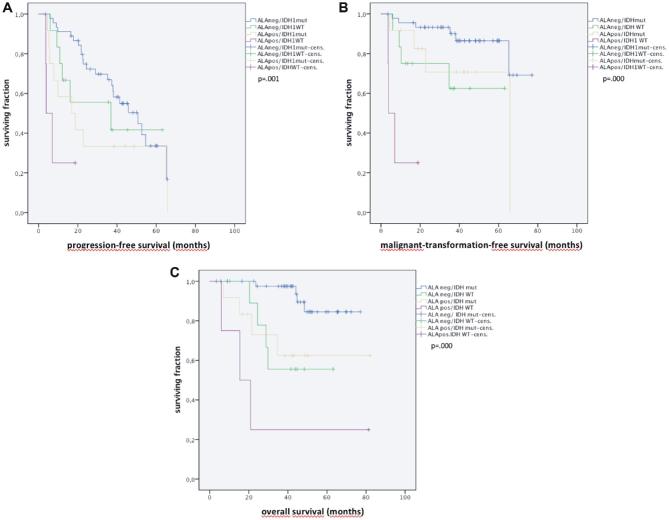
For multivariate cox regression analysis of OS and MTFS we considered four factors, which were significant on univariate survival analysis, that is age, fluorescence, IDH1 satus, and weak contrast-enhancement on MRI (Table 4). We found fluorescence, IDH1 status and age to be prognostic for OS, and only IDH1 status and fluorescence to be prognostic for MTFS. Malignant transformation was highly predictive for survival (HR 5.70, P = .001). Figure 4 illustrates the independent effects of fluorescence and IDH1 mutation status.
TABLE 4.
Cox Regression Analysis of Factors Predicting Outcomes in Univariate Analysis.
| PFS | 0.000b | MDFS | 0.003b | OS | 0.000b | |
|---|---|---|---|---|---|---|
| Factor/covariate | HR | P | HR | P | HR | P |
| Age | 1.01 | .294 | 1.03 | .135 | 1.06 | .011 |
| Enhancement | 3.09 | .012 | 1.34 | .673 | 3.07 | .062 |
| IDH1 wildtype | 5.30 | .000 | 3.17 | .043 | 5.007 | .006 |
| Visible fluorescence | 1.45 | .312 | 4.44 | .010 | 3.60 | .026 |
| Max. SUV ratio PETa | 1.41 | .009 | – | – | – | – |
amaximal standardized uptake ratio on FET-PET; univariate only significant for PFS.
bomnibus test for analysis.
FIGURE 4.
Kaplan–Meier curves for A, PFS, B, MTFS, and C, OS, stratified by IDH1 mutation (mutated: IDHmut, wildtype: IDHWT) and presence of fluorescence (fluorescing: ALApos, nonfluorescing: ALAneg) demonstrating the independent influence of both factors on outcome.
DISCUSSION
Demographic and Molecular Factors
ALA-induced tumor fluorescence has been linked to malignancy in presumed LGG.10,14,16 Previously however, looking exclusively at a population of histologically proven LGG patients, we observed a subpopulation of LGG with macroscopic fluorescence.14 Importantly, in this series histology was based on samples taken from fluorescing tissue, and corroborated by samples from nonfluorescing tumor, if available. According to institutional policy, we took great care to specifically collect samples from any suspicious regions based on enhancement, PET uptake or fluorescence to ensure tissue to be representative for the true tumor dignity and to rule out sampling errors in possibly anaplastic gliomas. All other extracted tissue was also given to the pathologist for histological analysis.
Fluorescence was not predicted by factors previously associated with worse prognosis, ie, higher MIB Index, IDH1 wildtype pathology or lack of 1p19q co-deletions.33,34 These molecular factors now play an integral part in the 2016 modification of the WHO classification of brain tumors.27 We now address the question of whether patients with fluorescing LGG differ prognostically from patients without fluorescence. We found no differences in the MIB index or light morphological features between fluorescing and nonfluorescing samples. However, we found a (marginally significant) difference in the expression of EGFR. This molecule has been associated malignancy and angiogenesis in gliomas.35
We observed a shorter time to malignant deterioration and a shorter survival in patients with fluorescing LGG, identifying visible fluorescence in LGGs as a possible intraoperative, independent biological marker for incipient unfavorable outcome.
Given the discussions regarding that patients with LGG are to be considered high risk,36 the observation of such fluorescence supports the recommendation of adjuvant cytotoxic therapies and shorter surveillance intervals especially in IDH wildtype diffuse LGG with fluorescence. This worse prognosis was independent of 1p19q-codeletion status, age, tumor size, or IDH1 mutations.
Stratified by fluorescence, our cohorts were found to be very similar. Young age and generally favorable KPS (90 or 100) are characteristic for LGG patients, as were the low median MIB values and the high fraction of patients with IDH1 mutated tumors. We found no IDH2 mutations, which are much rarer than IDH1 mutations. KPS, although somewhat different in our cohorts (but with minor exceptions either 90 or 100), did not influence outcomes. EoR was similar in both groups. We did not find a significant influence of EoR on outcome, as defined by PFS, MDFS, or OS. The respective hazard ratios of .548, .636, and .71, indicted that our cohorts were most likely underpowered for corroborating existing assumptions on the influence of EoR on outcome in LGG.37,38
Due to the similarities in prognostic variables between groups, adjuvant therapies were very comparable, consisting of observation in over 50% of cases, and radiotherapy or chemotherapy in the remaining cases, as expected. The observation of fluorescence did not lead to different adjuvant therapies in this series, which might have confounded our results.
In sum, therefore, we regard fluorescence to be an early indicator of malignant transformation, possibly related to EGFR expression and incipient angiogenic changes, which can not necessarily be determined by enhancement, higher uptake of amino acids, conventional light microscopy, or immunohistochemistry.
Mechanisms of Fluorescence Accumulation in LGG
Three mechanisms might be considered regarding fluorescence accumulation in a subgroup of low-grade glioma. Firstly, it has been suggested that IDH1 mutations might negatively influence porphyrin accumulation after in vitro exposure of tumor cells to 5-ALA39 because energy metabolism is impaired in IDH1 mutant cells. However, we found no relationship between visible fluorescence and IDH1 mutation status. Our observation does not necessarily contradict the in vitro data, since ALA does not cross the intact blood brain barrier and entry of ALA into the brain is a prerequisite of ALA metabolism by tumor cells.40
Secondly, fluorescence accumulation may be the result of very early angiogenic changes, a hallmark of malignancy. We observed a relationship between fluorescence, weak contrast-enhancement on the MRI and FET-PET, respectively, as summarized in Table 3. The common denominator may well be initial changes in vascular permeability, intravascular volume or vessel density within tumors. ALA for itself does not cross the blood-brain barrier,40 whereas FET uptake appears to depend on a blood-brain barrier bound L-type amino acid transporter.41 ALA derived porphyrins are observed if FET uptake in tumors exceeds a certain level,14,25 suggesting permeability changes to play a role for increased accumulation of both amino acids in tumors with early angiogenic changes. The threshold of FET uptake for predicting fluorescence in our cohort was 1.85. The values for 1.37 were observed by Stockhammer et al25 in their cohort, which also contained a number of high-grade gliomas.
Thirdly and finally, porphyrin accumulation in response to ALA has been linked to proliferation, cell cycle, and abnormal heme metabolism in tumors.42-44 However, cellular mechanisms of accelerated porphyrin synthesis can only become operational when ALA crosses a mildly dysfunctional blood-barrier, even if permeability increases do not have to be great to allow passage of ALA, which has a molecular weight of 131 Daltons, again supporting the permeability hypothesis.
Overall, the underlying mechanisms for FET and ALA uptake and ALA metabolism in LGG require further study.
Outcome Analyses
In multivariate analysis, PFS was not independently predicted by ALA induced fluorescence, whereas MTFS and OS were, indicating a role of fluorescence for heralding malignant deterioration and ultimately death but not simple low grade tumor growth. On the other hand, FET-PET was not prognostic for MTFS. We did however observe only a limited number of events, with 17 of 74 patients (23.0%) experiencing malignant deterioration. In total,15 of 74 patients (20.2%) died during the observation period. The relatively small number of events limits our conclusions on FET-PET. Possibly a longer follow-up will further clarify the role of FET-PET maxima for OS and MTFS.
FET accumulation has been associated with worse prognosis in LGG.45,46 and may better be assessed by dynamic PET imaging45,47 that was not performed in this study. Maximal tumor to brain ratios of FET have been shown to correlate with outcome with and without IDH1/2 mutations, and depend mostly on WHO grade.48 In our cohort we only studied patients with LGG and not HGG.
Limitations
Overall, we acknowledge the comparably small number of patients as a limitation of this study. Also, we did not objectively determine porphyrin fluorescence using spectrometry12,15,49 that might be considered another limitation. Spectrometry allows quantitative estimations of tissue Protoporphyrin IX (PPIX) concentrations rather than relying on than visual identification of fluorescent regions alone. Low concentrations of PPIX in tumor not visible to the eye cannot be ruled out even in completely non-conspicious LGG without visible fluorescence as previously demonstrated by Sanai et al, 2011.50 However, in routine use, fluorescence-guided resections rely on optical visualization. Thus, the conclusions from our present observations will still be of value for the practicing neurosurgeon, and fluorescence quantification would not add further value regarding the prognosis and treatment of LGG.
We acknowledge the retrospective nature of our study to limit our conclusions, as well as the relatively short follow up of 46 mo in the context of LGG. Also, we did not gather dynamic information on FET-PET, which might have better reflected the true role of FET-PET in predicting porphyrin accumulation or outcome.
CONCLUSION
Together, our cohort of LGG patients with visible fluorescence during surgery had a worse prognosis than those without visible fluorescence, independently of known molecular factors. This observation, which should be prospectively validated, may justify more aggressive adjuvant therapies in this distinct subgroup. Porphyrin accumulation requires a certain level of blood-brain barrier permeability increase and appears to herald malignant degeneration prior to changes becoming evident on histology.
Disclosures
Dr Stummer has received consultant and speaker's fees from medac and speakers fees from Zeiss, NXDC, Leica. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Notes
A part of the data was presented orally at the, 67th Annual Meeting of the German Society of Neurosurgery (DGNC), in Frankfurt am Main, Germany, June 12-15, 2016.
Neurosurgery Speaks! Audio abstracts available for this article at www.neurosurgery-online.com.
COMMENTS
In this study, the authors present data using 5-ALA fluorescence during resection of low-grade gliomas and report a correlation between visible fluorescence and both overall survival and progression to a more malignant phenotype. The role of 5-ALA fluorescence has been well-documented in the resection of high-grade gliomas. However, its role in low-grade gliomas remains to be fully elucidated. Approximately 16 (or 20%) low-grade gliomas in this study displayed fluorescence. Fluorescence was partially related to weak enhancement on MRI and increased uptake in PET. In this cohort with a median follow-up period of 46.6 months, low-grade gliomas with visible fluorescence had shorter overall survival (51.6 months vs 68.2 months) and had a shorter time to malignant transformation (43 months vs 64 months). Statistical analysis revealed fluorescence to be an independent predictor of both overall survival and malignant transformation-free survival. This is an interesting finding with potential clinical implications especially in cases where the aggressiveness of subsequent treatment may be nuanced. Overall, this study augments the body of literature on the role of 5-ALA fluorescence in low-grade gliomas.
Emanuela Binello
Boston, Massachusetts
The current article is timely as it will add to the growing use of fluorescence to guide glioma surgery, and suggests that (ALA-positive, ALA + ) fluorescence can be viewed as an independent biomarker for prognosis in histologically confirmed low grade gliomas (LGGs). This interesting, well-done, and provocative study will expand the value of fluorescence-guided surgery in the surgeon's armamentarium. The work, however, raises a few questions:
1) In the ALA + tumors, were these really LGGs? Or were these anaplastic foci, representing early malignant transformation, not yet detectable by histology, but detectably by pathophysiology?1
2) It is known that contrast enhancement is the functional byproduct of angiogenesis, with the formation of permeable, new vessels and breakdown of the blood-brain barrier.2 Likewise, there is an “angiogenic switch” in the stepwise transformation of LGG to secondary glioblastomas.3 The authors found no relationship to known prognostic molecular factors (MIB, 1p19q codeletions, IDH1/2 mutations), but it would be of interest if the ALA + tumors showed increased markers of angiogenesis: microvascular density, VEGF165, CD31, CD45, Tie2, etc.
3) The authors found no correlation with IDH-1 protein (R132) or proliferative markers (Ki67/MID1), but other determinants of malignancy, eg, TERT promoter, IL-6, EGFR, p53,4-6 or the TCGA atlas, were not interrogated. Is it possible that there is a molecular or genomic biomarker specifically linked to ALA-fluorescence?
The current study shows that ALA-positivity may be a tool to help the surgeon exploit that opportunity and potentially eradicate a pre-malignant or an “in situ” malignancy within a larger zone of LGG, moving closer to the ideal of a ‘preventive surgical neurooncology’.1,7-8 The current study will also stimulate the development of other fluorescent modalities, such as indocyanine green (ICG), which is highly sensitive, 9 and could potentially detect premalignant foci within a larger LGG. Taken together, the data reported here will strengthen the argument for aggressive, complete surgical removal of LGGs whenever possible.10 The silent evolution of LGGs to high-grade gliomas provides a window of opportunity to detect these tumors earlier7 that the neurosurgeon can utilize to improve the outlook for patients with low-grade glioma.
Steven Brem
Philadelphia, Pennsylvania
REFERENCES
- Aldave G, Tejada S, Pay E et al.. Prognostic value of residual fluorescent tissue in glioblastoma patients after gross total resection in 5-aminolevulinic Acid-guided surgery. Neurosurgery. 2013;72(6):915-921. [DOI] [PubMed] [Google Scholar]
- Díez Valle R, Tejada Solis S, Idoate Gastearena MA, García de Eulate R, Domínguez Echávarri P, Aristu Mendiroz J. Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: volumetric analysis of extent of resection in single-center experience. J Neurooncol. 2011;102(1):105-113. [DOI] [PubMed] [Google Scholar]
- Floeth FW, Pauleit D, Wittsack HJ et al.. Multimodal metabolic imaging of cerebral gliomas: positron emission tomography with [18F]fluoroethyl-L-tyrosine and magnetic resonance spectroscopy. J Neurosurg. 2005;102(2):318-327. [DOI] [PubMed] [Google Scholar]
- Lau D, Hervey-Jumper SL, Chang S et al.. A prospective phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J Neurosurgery. 2016;124(5):1300-1309. [DOI] [PubMed] [Google Scholar]
- Morshed RA, Han SJ, Lau D, Berger MS: Wavelength-specific lighted suction instrument for 5-aminolevulinic acid fluorescence-guided resection of deep-seated malignant glioma: technical note. J Neurosurg. 2018;128(5):1448-1453. [DOI] [PubMed] [Google Scholar]
- Nabavi A, Thurm H, Zountsas B et al.. 5-ALA recurrent glioma study group. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: a phase ii study. Neurosurgery. 2009;65(6):1070-1077. [DOI] [PubMed] [Google Scholar]
- Nishikawa R. Fluorescence illuminates the way… Neuro Oncol. 2011;13(8):805-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DW, Valdés PA, Harris BT et al.. Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between δ-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article. J Neurosurg 2011;114(3):595-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schucht P, Beck J, Abu-Isa J et al.. Gross total resection rates in contemporary glioblastoma surgery: results of an institutional protocol combining 5-aminolevulinic acid intraoperative fluorescence imaging and brain mapping. Neurosurgery. 2012;71(5):927-935. [DOI] [PubMed] [Google Scholar]
- Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme utilizing 5-ALA-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000;93(6):1003-1013. [DOI] [PubMed] [Google Scholar]
- Stummer W, Pichlmeier U, Meinel T et al.. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392-401. [DOI] [PubMed] [Google Scholar]
- Stummer W, Stocker S, Wagner S et al.. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery. 1998;42(3):518-526. [DOI] [PubMed] [Google Scholar]
- Widhalm G, Kiesel B, Woehrer A et al.. 5-aminolevulinic acid induced fluorescence is a powerful intraoperative marker for precise histopathological grading of gliomas with non-significant contrast-enhancement. PLoS One. 2013;8(10):e7698, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber M, Wölfer J, Ewelt C et al.. The value of 5-ALA in low-grade gliomas and High-grade gliomas lacking glioblastoma imaging features: An analysis based on fluorescence, MRI, 18F-FET PET, and tumor molecular factors. Neurosurgery. 2016;78(3):401-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés PA, Kim A, Leblond F et al.. Combined fluorescence and reflectance spectroscopy for in vivo quantification of cancer biomarkers in low- and high-grade glioma surgery. J Biomed Opt. 2011;16(11):116007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewelt C, Floeth FW, Felsberg J et al.. Finding the anaplastic focus in diffuse gliomas: the value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence. Clin Neurol Neurosurg. 2011;113(7):541-547. [DOI] [PubMed] [Google Scholar]
- Coluccia D, Fandino J, Fujioka M et al.. Intraoperative 5-aminolevulinic-acid-induced fluorescence in meningiomas. Acta Neurochir (Wien). 2010;152(10):1711-1719. [DOI] [PubMed] [Google Scholar]
- Cornelius JF, Slotty PJ, Kamp MA, Schneiderhan TM, Steiger HJ, El-Khatib M. Impact of 5-aminolevulinic acid fluorescence-guided surgery on the extent of resection of meningiomas–With special regard to high-grade tumors. Photodiagnosis Photodyn Ther. 2014;11(4):481-490. [DOI] [PubMed] [Google Scholar]
- Della Puppa A, Rustemi O, Gioffrè G et al.. Predictive value of intraoperative 5-aminolevulinic acid–induced fluorescence for detecting bone invasion in meningioma surgery. J Neurosurg. 2014;120(4):840-845. [DOI] [PubMed] [Google Scholar]
- Motekallemi A, Jeltema H-R, Metzemaekers J, van Dam G, Crane L, Groen R. The current status of 5-ALA fluorescence-guided resection of intracranial meningiomas—a critical review. Neurosurgery. 2015;38(4):619-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García B, Artero C, Zamorano M, Tejero G. Fluorescence-guided resection with 5- aminolevulinic acid of subependymomas of the fourth ventricle: report of 2 cases: technical case report. Neurosurgery. 2015;11(Suppl 2):E364-E371. [DOI] [PubMed] [Google Scholar]
- Inoue T, Endo T, Nagamatsu K, Watanabe M, Tominaga T. 5-aminolevulinic acid fluorescence-guided resection of intramedullary ependymoma: report of 9 cases. Neurosurgery. 2013;72(2 Suppl Operative):159-168. [DOI] [PubMed] [Google Scholar]
- Millesi M, Kiesel B, Woehrer A et al.. Analysis of 5-aminolevulinic acid-induced fluorescence in 55 different spinal tumors. J Neurosurg. 2014;36(2):E11. [DOI] [PubMed] [Google Scholar]
- Stummer W, Rodrigues F, Schucht P et al.. Predicting the “usefulness” of 5-ALA-derived tumor fluorescence for fluorescence-guided resections in pediatric brain tumors: a European survey. Acta Neurochir. 2014;156(12):2315-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockhammer F, Misch M, Horn P, Koch A, Fonyuy N, Plotkin M. Association of F18-fluoro-ethyl-tyrosin uptake and 5-aminolevulinic acid-induced fluorescence in gliomas. Acta Neurochir. 2009;151(11):1377-1383. [DOI] [PubMed] [Google Scholar]
- Stummer W, Reulen HJ, Meinel T et al.. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564-576. [DOI] [PubMed] [Google Scholar]
- Louis DN, Perry A, Reifenberger G et al.. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803-820. [DOI] [PubMed] [Google Scholar]
- Capper D, Zentgraf H, Balss J, Hartmann C, von Deimling A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118(5):599-601. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Meyer J, Balss J et al.. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118(4):469-474. [DOI] [PubMed] [Google Scholar]
- Felsberg J, Rapp M, Loeser S et al.. Prognostic significance of molecular markers and extent of resection in primary glioblastoma patients. Clin Cancer Res. 2009;15(21):6683-6693. [DOI] [PubMed] [Google Scholar]
- Soffietti R, Baumert BG, Bello L et al.. European Federation of Neurological Societies: Guidelines on management of low-grade gliomas: report of an EFNS-EANO task force. Eur J Neurol. 2010;17(9):1124-1133. [DOI] [PubMed] [Google Scholar]
- van den Bent MJ, Wefel JS, Schiff D et al.. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583-593. [DOI] [PubMed] [Google Scholar]
- Eckel-Passow JE, Lachance DH, Molinaro Annette M et al.. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller M, Weber RG, Willscher E et al.. Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015;129(5):679-693. [DOI] [PubMed] [Google Scholar]
- Keller S, Schmidt M. EGFR and EGFRvIII promote angiogenesis and cell invasion in glioblastoma: combination therapies for an effective treatment. Int J Mol Sci. 2017;18(6):1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelle L, Fontaine D, Mandonnet E et al.. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. J Neurosurg. 2013;118(6):1157-1168. [DOI] [PubMed] [Google Scholar]
- Delgado-López PD, Corrales-García EM, Martino J, Lastra-Aras E, Diffuse low-grade glioma: a review on the new molecular classification, natural history and current management strategies. Clin Transl Oncol. 2017;19(8):931-944. [DOI] [PubMed] [Google Scholar]
- Hervey-Jumper SL, Berger MS. Role of surgical resection in low- and high-grade gliomas. Curr Treat Options Neurol. 2014;16(4):284. [DOI] [PubMed] [Google Scholar]
- Kim JE, Cho HR, Xu WJ et al.. Mechanism for enhanced 5-aminolevulinic acid fluorescence in isocitrate dehydrogenase 1 mutant malignant gliomas. Oncotarget. 2015;6(24):20266-20277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis SR, Novotny A, Xiang J et al.. Transport of 5-aminolevulinic acid between blood and brain. Brain Res. 2003;959(2):226-234. [DOI] [PubMed] [Google Scholar]
- Langen KJ, Hamacher K, Weckesser M et al.. O-(2-[18F]fluoroethyl)-l-tyrosine: uptake mechanisms and clinical applications. Nucl Med Biol. 2006;33(3):287-294. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Paredes S, Batlle AM. Tumour-localizing properties of porphyrins. In 412 vivo studies using free and liposome encapsulated aminolevulinic acid. Comp 413 Biochem Physiology. 1992;102(2):433-436. [DOI] [PubMed] [Google Scholar]
- Idoate MA, Diez Valle R, Echeveste J, Tejada S. Pathological characterization of the glioblastoma border as shown during surgery using 5-aminolevulinic acid-induced fluorescence. Neuropathology. 2011;31(6):575-582. [DOI] [PubMed] [Google Scholar]
- Yang X, Palasuberniam P, Kraus D, Chen B. Aminolevulinic acid-based tumor detection and therapy: molecular mechanisms and strategies for enhancement. Int J Mol Sci. 2015;16(10):25865-25880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen NL, Suchorska B, Wenter V et al.. Dynamic 18F-FET PET in newly diagnosed astrocytic low-grade glioma identifies high-risk patients. J Nuclear Medicine. 2014;55(2):198-203. [DOI] [PubMed] [Google Scholar]
- Floeth FW, Pauleit D, Sabel M et al.. Prognostic value of O-(2-18F- fluoroethyl)-L-tyrosine PET and MRI in low-grade glioma. J Nucl Med. 2007;48(4):519-527. [DOI] [PubMed] [Google Scholar]
- Pöpperl G, Kreth FW, Mehrkens JH et al.. FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur J Nucl Med Mol Imaging. 2007;34(12):1933-1942. [DOI] [PubMed] [Google Scholar]
- Rapp M, Heinzel A, Galldiks N et al.. Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J Nucl Med. 2013;54(2):229-235. [DOI] [PubMed] [Google Scholar]
- Valdés PA, Bekelis K, Harris BT et al.. 5-Aminolevulinic acidinduced protoporphyrin IX fluorescence in meningioma: qualitative and quantitative measurements in vivo. Neurosurgery. 2014;10(Suppl 1):74-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Snyder LA, Honea N et al.. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J Neurosurg. 2011;115(4):740-748. [DOI] [PubMed] [Google Scholar]
References
- Al-Tamimi YZ, Palin MS, Patankar T, et al Low-grade glioma with foci of early transformation does not necessarily require adjuvant therapy after radical surgical resection. World Neurosurg. 2018;110:e346-e354. [DOI] [PubMed] [Google Scholar]
- Zagzag D, Goldenberg M, Brem S. Angiogenesis and blood-brain barrier breakdown modulate CT contrast enhancement: an experimental study in a rabbit brain tumor model. AJR Amer J Roentgenol. 1989;153(1):141-1146. [DOI] [PubMed] [Google Scholar]
- Huang Y, Rajappa P, Hu W, et al A proangiogenic signaling axis in myeloid cells promotes malignant progression of glioma. J Clin Invest. 2017;127(5):1826-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel-Passow JE, Lachance DH, Molinaro AM, et al Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola D, Balducci L, Chen DT, et al Senescence-associated-gene signature identifies genes linked to age, prognosis, and progression of human gliomas. J Geriatr Oncol. 2014;5(4):389-399. [DOI] [PubMed] [Google Scholar]
- Abdullah KG, Ramayya A, Thawani JP, et al Factors associated with increased survival after surgical resection of glioblastoma in octogenarians. PLoS One. 2015;10(5):e0127202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallud J, Capelle L, Taillandier L, Badoual M, Duffau H, Mandonnet E. The silent phase of diffuse low-grade gliomas. Is it when we missed the action? Acta Neurochir (Wien). 2013;155(12):2237-2242. [DOI] [PubMed] [Google Scholar]
- Duffau H. The rationale to perform early resection in incidental diffuse low-grade glioma: toward a “preventive surgical neurooncology”. World Neurosurg. 2013:80(5):e115-e117. [DOI] [PubMed] [Google Scholar]
- Lee JY, Thawani JP, Pierce J, et al Intraoperative near-infrared optical imaging can localize gadolinium-enhancing gliomas during surgery. Neurosurgery. 2016;79(6):856-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakola AS, Myrmel KS, Kloster R, et al Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308(18):1881-1888. [DOI] [PubMed] [Google Scholar]