ABSTRACT
Background:
Manuka honey has attracted the attention of the scientific community for its antimicrobial and antioxidant properties. The active compounds of manuka honey to which its myeloperoxidase activity inhibition is owed are methyl syringate (MSYR) and leptosin (a novel glycoside of MSYR). The non-peroxide antibacterial activity is attributed to glyoxal, 3-deoxyglucosulose, and methylglyoxal. These properties make it an inexpensive and effective topical treatment in wound management. This study has focused on the evaluation of the effect of manuka honey and acacia honey on wound healing in nondiabetic and streptozotocin-induced diabetic rats.
Materials and Methods:
This study was conducted on a total of 42 rats (six rats in each group) and respective drug/substance was topically applied once daily on the excision wound for 21 days. Induction of diabetes was carried out in rats in groups IV, V, VI, and VII only. Measurement of wound contraction was carried out on days 3, 6, 9, 12, 15, 18, and 21 after operation. Time taken for the complete epithelization was recorded along with a histopathological examination of the healed wound bed.
Results:
Topical application of manuka honey achieved ≥80% wound contraction on day 9 after operation in both the nondiabetic and diabetic group. Complete epithelization was achieved 2 days earlier than the normal epithelization time in the manuka group. Histopathological examination showed well-formed keratinized squamous epithelium with normal collagen tissue surrounding hair follicles.
Conclusion:
This study provides good outcome with respect to wound healing (especially in diabetic condition) when manuka honey was compared to acacia honey and standard treatment.
KEYWORDS: Acacia honey, diabetes, manuka honey, wound management
INTRODUCTION
Wound healing is a complex and continual process that has three phases: inflammation, proliferative phase, and tissue remodeling. Basically, wound healing is the result of interactions among cytokines, growth factors, blood and cellular elements, and the extracellular matrix. The cytokines promote healing by various pathways, such as stimulating the production of components of the basement membrane, preventing dehydration, and increasing inflammation and formation of granulation tissue.[1,2] At the cellular level, monocytes infiltrate the wound site and become activated macrophages that release growth factors, such as platelet-derived growth factor (PDGF) and vascular endothelial growth factor, which initiate the formation of granulation tissue. Macrophages play a key role in inflammation and repair; likewise, macrophage depletion is associated with defective wound healing, and transfusion of macrophages will accelerate wound healing in nonhealing wounds.[3,4] Platelets facilitate hemostatic plug formation by secreting PDGF that further attracts and activates macrophages and fibroblasts. After the injury, reepithelialization process of the wounds starts quickly. Within 1–2 days, epidermal cells begin to proliferate at the wound margin. New granulation tissue appears and new capillaries proliferate through the new stroma on day 4. Later fibroblasts begin to synthesize the extracellular matrix.[3,4,5]
There has been a renaissance in recent times regarding the use of honey for the treatment of wounds, burns, and skin ulcers. In the past decade, there have been many reports of case studies, experiments using animal models, and randomized controlled clinical trials that provide a large body of very convincing evidence for its effectiveness, and biomedical research that explains how honey produces such good results.[6] Contaminated wounds, especially large wounds (e.g., major degloving injuries and burns), and conditions, such as necrotizing fasciitis acquired through infections with Pseudomonas species, Escherichia coli, or Streptococci, can be difficult and expensive to treat using conventional methods. Honey has been shown to be effective against the growth of bacteria, and its use enhances wound healing. Thus, it is an inexpensive topical treatment that is extremely effective in wound management and its use makes management of large, open wounds financially feasible.[7,8]
Honey is a viscous, supersaturated sugar solution derived from nectar gathered and modified by the honeybee, Apis mellifera. Honey contains approximately 30% glucose, 40% fructose, 5% sucrose, and 20% water.[9] Other components of honey are proteins, vitamins of the B complex, minerals, and antioxidants such as flavonoids, ascorbic acid, catalase, and selenium. Organic acids make up 0.57% of honey and are responsible for its acidity. The main enzymes in honey are invertase, amylase, and glucose oxidase. The specific percentages of all these different components may vary depending on the plant origin, the geographical location, the season in which the honey was collected, the treatment of honey since its harvesting, and its age.[10] The general effects on wound healing observed on using honey are as follows: (1) Great wound contraction, (2) promotion of granulation tissue formation, (3) promotion of epithelialization, (4) stimulation of tissue growth and synthesis of collagen, (5) stimulation of formation of new blood vessels in the bed of wounds, (6) reduction of postoperative adhesion, (7) reduction of edema, (8) reduction of inflammation, (9) deodorization of wounds, (10) promotion of moist wound healing, (11) facilitation of debridement, and (12) reduction of pain.[11] One of the most important strategies to keep the process of healing ongoing is to sterilize damaged tissue from any microbial infection. Honey has many effects, such as antibacterial, antioxidant, antitumor, anti-inflammatory, and various metabolic effects. The accelerative effect of honey in the wound healing process is related to its physical properties of hygroscopicity, hypertonicity, lower pH, and complex chemical composition.[12]
Within monofloral honey (bees forage predominantly on one type of plant and honey is named according to the source), manuka honey has attracted the attention of the scientific community for its antimicrobial and antioxidant properties. Manuka honey is derived from Leptospermum scoparium (manuka tree) of the Myrtaceae family, which is a small tree or shrub found in eastern Australia and New Zealand. Manuka honey contains various flavonoids such as pinobanksin, pinocembrin, and chrysin, and certain other compounds in minor concentration such as luteolin, quercetin, 8-methoxykaempferol, isorhamnetin, kaempferol, and galangin. The constituents of phenolic acids and volatile norisoprenoids are 4-hydroxybenzoic acid, dehydrovomifoliol, benzoic acid yields, kojic acid, 2-methoxyphenyllactic acid, and methyl syringate (MSYR). The active compounds of manuka honey to which its myeloperoxidase activity inhibition is owed are MSYR and leptosin (a novel glycoside of MSYR). The non-peroxide antibacterial activity is attributed to glyoxal, 3-deoxyglucosulose, and methylglyoxal (MGO).[13] Manuka honey also possesses antimicrobial activity against Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus species, Serratia marcescens, Listeria monocytogenes, and S. epidermidis.[13,14] Similarly, acacia honey is a widely and commercially available honey, and earlier conducted studies have shown its superiority over sulfadiazine in causing subsidence of acute inflammatory changes, better control of infections, and quicker wound healing.[14]
Therefore, this study was taken up for the evaluation of the effect of manuka honey and acacia honey on wound healing in nondiabetic and streptozotocin-induced diabetic rats.
MATERIALS AND METHODS
Description of procedure
Animals
Healthy, adult inbred albino rats of Wistar strain of male sex only, weighing 180–200g were used in the experimental study. They were housed in the animal house of the institute. The animals were housed individually in the polypropylene cages with sterile paddy husk bedding. The animals were provided free access to water and standard food ad libitum and were housed under controlled conditions of temperature (23°C ± 2°C), humidity (50% ± 5%), and 10–14h of light and dark cycles. However, rats were fasted for 24h and water was withdrawn 1h before administration of drugs. A total of 42 rats (seven groups of six rats each) were used for carrying out the experiment. The committee for the purpose of control and supervision of experiments on animals guidelines for animal maintenance was followed throughout the experiment. The study was carried out after obtaining clearance from the institutional animal ethics committee.
Materials
-
•
Manuka honey (Wedderspoon, Raw Manuka Honey, KFactor 16; Wedderspoon Organic New Zealand, Rangiora, New Zealand)
-
•
Acacia honey (Safa Honey, Bengaluru, India)
-
•
Sodium alginate (HiMedia Laboratories, Mumbai, India)
-
•
Animals: Inbred albino rats of Wistar strain (male sex) weighing 180–200 g
-
•
Drugs: Xylazine hydrochloride (6mg/kg), ketamine hydrochloride (85mg/kg), and 10% antiseptic povidone-iodine solution
-
•
Nalgene metabolic cage (Ancare Corp., Bellmore, NY), a feeding needle
-
•
Surgical instruments: Scalpel, forceps, needle holder, scissors, chromic catgut (4-0), and silk thread
Induction of diabetes mellitus
Diabetes mellitus was chemically induced in the rats using streptozotocin. Briefly, after a 12-h fasting, rats were given a single intraperitoneal injection of streptozotocin (65mg/kg) freshly prepared in 0.1-M sodium citrate buffer (pH 4.5). After 8 days of streptozotocin injection, blood glucose measurement was performed on the tail vein blood using a glucometer. Rats whose fasting blood glucose levels exceeded 250mg/dL were considered diabetic. Water intake and weight were monitored throughout the study, and to confirm the diabetic state, fasting blood glucose measurement was again carried out on the day of euthanasia.[15]
Creation of excision wound
The rats were anesthetized using an intramuscular injection of 6mg/kg of xylazine hydrochloride and 85mg/kg of ketamine hydrochloride. Thereafter, the back of the rats was shaved. The shaved area was sterilized using an antiseptic preparation of 10% povidone-iodine solution. An excision wound was created by cutting away 500mm2 (circular area) full thickness of a predetermined area on the depilated back of the rat. The day on which wound was created was considered as day 0. The wound area was measured immediately by placing a transparent tracing paper over the wound and tracing it out. The wounded animals were housed separately in different cages.[16,17]
Drug treatment
The study was conducted on a total of 42 rats (six rats in each group) and the respective drug/substance was topically applied once daily on the excision wound for 21 days. Induction of diabetes was carried out in rats in groups IV, V, VI, and VII only. Excision wound was created in the rats in all groups. Group I served as control and 2% w/w sodium alginate gel was topically applied on the wound. Group II received topical application of manuka honey (10% or 15% concentration added to 2% w/w sodium alginate gel). Group III received topical application of acacia honey (10% or 15% concentration added to 2% w/w sodium alginate gel). Group IV diabetic rats with excision wound were also treated topically with 2% w/w sodium alginate gel. Group V diabetic rats with excision wound were also treated topically with manuka honey (10% or 15% concentration added to 2% w/w sodium alginate gel). Group VI diabetic rats with excision wound were treated with acacia honey topically (10% or 15% concentration added to 2% w/w sodium alginate gel). Group VII diabetic rats with excision wound received standard treatment with silver sulfadiazine cream.[18,19] The activity study groups have been summarized in Table 1.
Table 1.
Activity study groups
| Groups | Protocol | No. of rats |
|---|---|---|
| Group I (control) | 2% w/w sodium alginate gel topically for 21 days | 6 |
| Group II | 10% or 15% concentration of manuka honey added to 2% w/w sodium alginate gel topically for 21 days | 6 |
| Group III | 10% or 15% concentration of acacia honey added to 2% w/w sodium alginate gel topically for 21 days | 6 |
| Group IV (diabetic control) | 2% w/w sodium alginate gel topically for 21 days | 6 |
| Group V (diabetic) | 10% or 15% concentration of manuka honey added to 2% w/w sodium alginate gel topically for 21 days | 6 |
| Group VI (diabetic) | 10% or 15% concentration of acacia honey added to 2% w/w sodium alginate gel topically for 21 days | 6 |
| Group VII (standard) (diabetic) | Silver sulfadiazine cream topically for 21 days | 6 |
| Total | 42 |
Evaluation parameters for wound healing
Evaluation of excision wound was carried out on days 3, 6, 9, 12, 15, 18, and 21 after operation.
Measurement of wound contraction
The excision wound margin was traced after wound creation by using transparent paper and the respective area was measured using a graph paper. Wound contraction was measured at every 2 days’ interval, until complete wound healing, and expressed in percentage of the healed wound area.[20] The evaluated surface area was then used to calculate the percentage of wound contraction, taking the initial size of wound 500mm2 as 100%, by using the following formula:[21]

Epithelialization period
The epithelialization period was evaluated by noting the number of days required for the eschar to fall off from the wound surface exclusive of leaving a raw wound behind.[21]
Histopathological studies
Wound tissue specimens (wound bed) from control, test, and standard groups were taken after complete healing of excision wound, and after usual processing, 6-mm thick sections were cut and stained with hematoxylin and eosin. Sections were qualitatively assessed under the light microscope and observed with respect to the fibroblast proliferation, collagen formation, angiogenesis, and epithelialization.[21]
Statistical analysis
The results were analyzed for statistical significance using one-way analysis of variance (ANOVA), followed by post hoc (Tukey’s) test, using the Statistical Package for the Social Sciences software (SPSS, IBM, New York), version 16.0. The P <0.05 was considered to be statistically significant.
RESULTS
Excision wound model in nondiabetic group
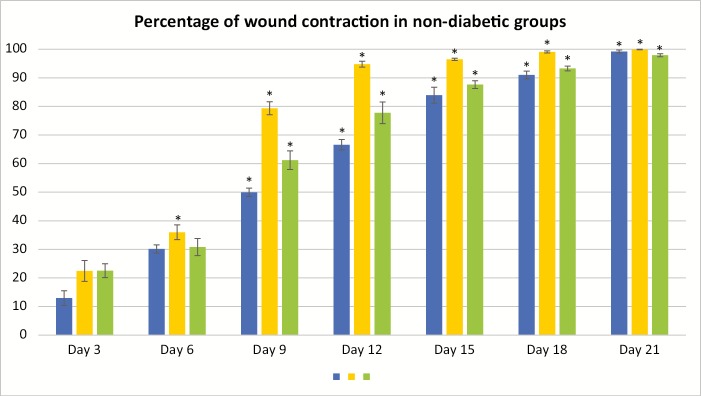
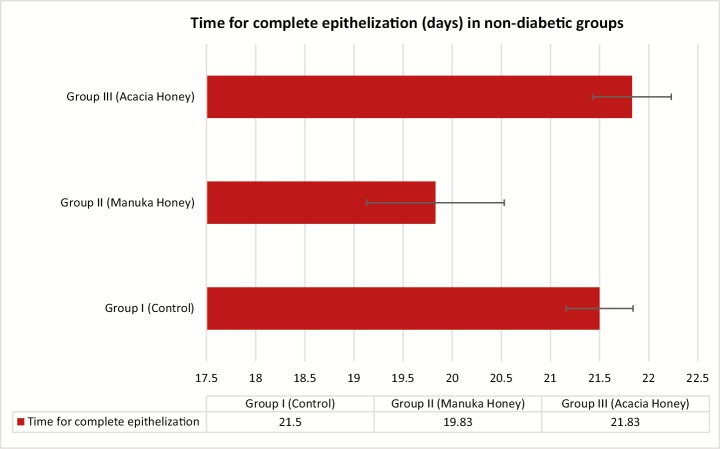
The percentage of wound contraction in group I (control) on day 3, 6, 9, 12, 15, 18, and 21 was 12.97% ± 2.57%, 30.13% ± 1.45%, 49.97% ± 1.47%, 66.60% ± 1.84%, 83.93% ± 2.80%, 91.00% ± 1.36%, and 99.20% ± 0.52%, respectively. The percentage of wound contraction in group II (manuka honey) on day 3, 6, 9, 12, 15, 18, and 21 was 22.47% ± 3.64%, 35.97% ± 2.58%, 79.37% ± 2.26%, 94.77% ± 1.03%, 96.47% ± 0.38%, 99.07% ± 0.36%, and 99.90% ± 0.10%, respectively, and the P value was significant (P <0.001) from day 6 onward. The percentage of wound contraction in group III (acacia honey) on day 3, 6, 9, 12, 15, 18, and 21 was 22.53% ± 2.42%, 30.80% ± 3.16%, 61.20% ± 3.26%, 77.77% ± 3.79%, 87.63% ± 1.34%, 93.27% ± 0.83%, and 97.90% ± 0.52%, respectively, and the P value was significant (P <0.001) from day 9 onward. The time for the complete epithelization in groups I, II, and III was 21.50±0.34, 19.83±0.70, and 21.83±0.40 days, respectively. The epithelization was completed 2 days prior in the manuka honey group as compared to that in the acacia honey and control group [Figures 1 and 2]. The histopathological examination of the wound bed after complete healing, that is, after day 21 showed well-formed keratinized epithelium and normal collagen tissue. The histopathological examination of the wound tissue specimen of the nondiabetic control (group I) [Figures 3 and 4] showed poorly formed keratinized squamous epithelium with loosely arranged collagen fibers in the underlying dermis [Figure 5].
Figure 1.
Wound contraction in the nondiabetic rats. *P <0.001. All the results are expressed as mean ± standard error mean (SEM). The differences between the experimental groups were compared by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test, using SPSS software, version 16.0. A P value <0.05 was considered as statistically significant
Figure 2.
Time for complete epithelization (days) in the nondiabetic rats
Figure 3.
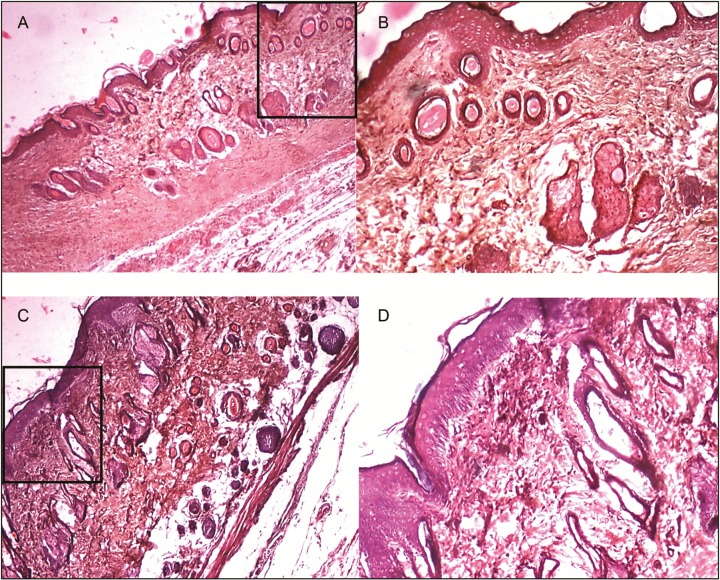
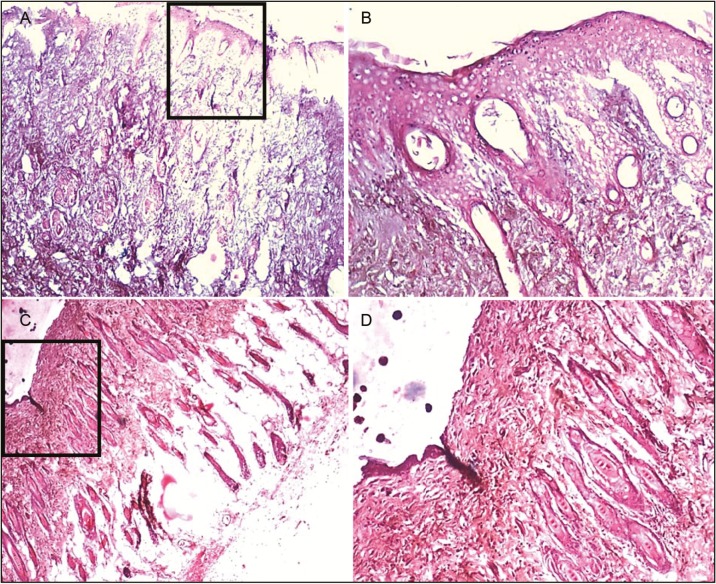
Histopathological slides for the acacia honey in diabetic and nondiabetic group. Figure (A) and (B) represents wound tissue specimen (wound bed) after complete healing of excision wound, stained with hematoxylin and eosin in the diabetic group (group VI: acacia honey) as viewed under light microscope 4× and 10×. The sections showed well-formed keratinized squamous epithelium. The underlying dermis showed area of loosely arranged collagen fibers surrounding hair follicles and rest of the area of the dermis showed normal collagen tissue. Figure (C) and (D) represents wound tissue specimen of nondiabetic group (group III: acacia honey) as viewed under light microscope 4× and 10×; sections revealed keratinized squamous epithelium and the underlying dermis showed pilosebaceous units and less amount of loosely arranged collagen fibers
Figure 4.
Histopathological slides for the manuka honey in diabetic and nondiabetic group. Figure (A) and (B) represents wound tissue specimen (wound bed) after complete healing of excision wound, stained with hematoxylin and eosin in the diabetic group (group V: manuka honey) as viewed under light microscope 4× and 10×. The sections showed well-formed keratinized squamous epithelium. The underlying dermis showed pilosebaceous units and small patches of loosely arranged collagen fibers, and rest of the area of the dermis showed normal collagen tissue. Figure (C) and (D) represents wound tissue specimen of nondiabetic group (group II: manuka honey) as viewed under light microscope 4× and 10×; the sections revealed keratinized squamous epithelium and the underlying dermis showed area of loosely arranged collagen fibers surrounding hair follicles
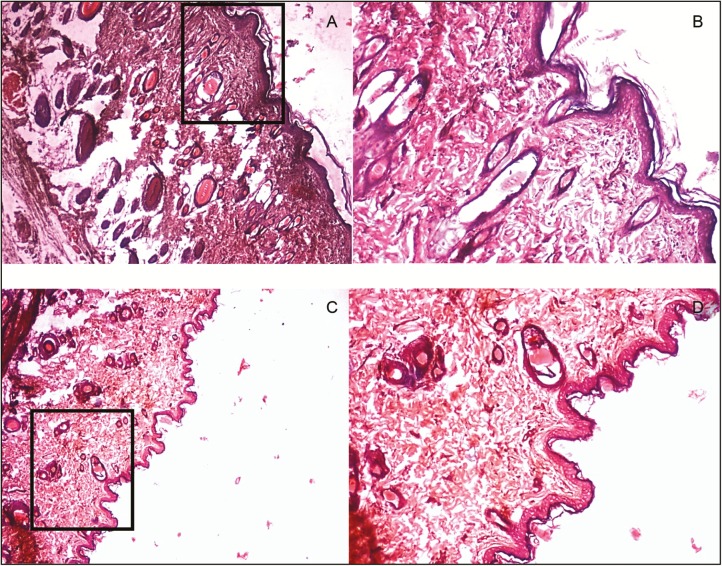
Figure 5.
Histopathological slides for the nondiabetic and diabetic control groups. Figure (A) and (B) represents wound tissue specimen (wound bed) after complete healing of excision wound, stained with hematoxylin and eosin in the nondiabetic group (group I) as viewed under light microscope 4× and 10×. The sections showed poorly formed keratinized squamous epithelium. The underlying dermis showed loosely arranged collagen fibers. Figure (C) and (D) represents wound tissue specimen after complete healing of excision wound in diabetic group (group IV) as viewed under light microscope 4× and 10×; sections showed ill-formed keratinized epithelium and poorly formed collagen tissue
Excision wound model in diabetic group
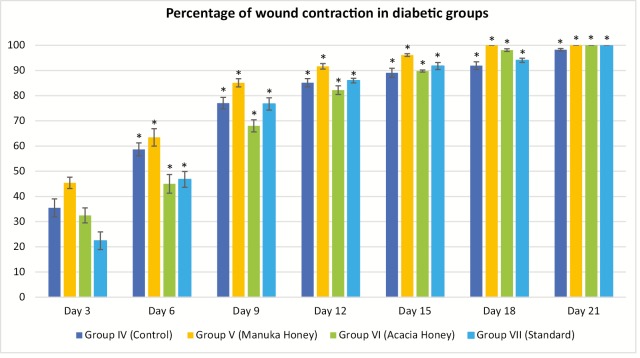
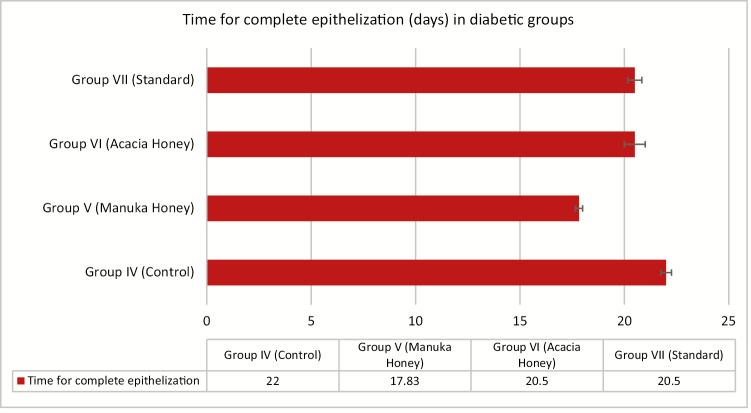
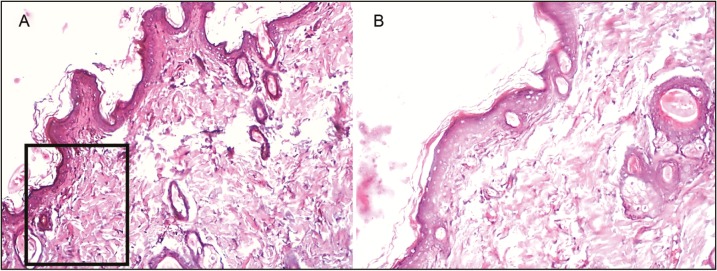
The percentage of wound contraction in group IV (control) on day 3, 6, 9, 12, 15, 18, and 21 was 35.47% ± 3.55%, 58.67% ± 2.58%, 77.03% ± 2.29%, 85.13% ± 1.64%, 89.07% ± 1.80%, 91.90% ± 1.55%, and 98.23% ± 0.49%, respectively. The percentage of wound contraction in group V (manuka honey) on day 3, 6, 9, 12, 15, 18, and 21 was 45.37% ± 2.24%, 63.43% ± 3.45%, 85.10% ± 1.64%, 91.63% ± 1.11%, 96.13% ± 0.55%, 100.00% ± 0.00%, and 100.00% ± 0.00%, respectively, and the P value was significant (P <0.001) from day 3 onward. In group V, 85% and 100% wound contraction was achieved at day 9 and 18, respectively. The percentage of wound contraction in group VI (acacia honey) on day 3, 6, 9, 12, 15, 18, and 21 was 32.47% ± 2.97%, 45.00% ± 3.72%, 68.03% ± 2.40%, 82.20% ± 1.70%, 89.77% ± 0.42%, 98.07% ± 0.54%, and 100.00% ± 0.00%, respectively. In group VI, 82% and 100% wound contraction occurred on day 12 and 21, respectively. In group VII (standard treatment), 86% and 100% wound contraction was achieved at day 12 and 21, respectively. The time for the complete epithelization in groups IV, V, VI, and VII was 22.00±0.26, 17.83±0.17, 20.50±0.50, and 20.50±0.34 days, respectively. The epithelization was completed 4 days prior than the normal healing time (21 days) in the manuka honey group versus 1 day earlier in the acacia honey group [Figures 6 and 7]. The histopathological examination of the wound tissue specimen in group VI (acacia honey) showed keratinized squamous epithelium with loosely arranged collagen fibers in the underlying dermis along with the zone of normal collagen tissue, whereas group V (manuka honey) showed well-formed keratinized squamous epithelium with areas of normal collagen in the underlying dermis along with few patches of loosely arranged collagen fibers surrounding hair follicles [Figures 3 and 4]. The histopathological examination of the wound bed after complete healing in the diabetic control (group IV) showed ill-formed keratinized epithelium and poorly formed collagen tissue [Figure 5]. The diabetic standard (silver sulfadiazine) group VII on histopathological examination revealed well-formed keratinized squamous epithelium, and the underlying dermis showed loosely arranged collagen fibers [Figure 8].
Figure 6.
Wound contraction in diabetic rats. *P <0.001. All the results are expressed as mean ± standard error mean (SEM). The differences between the experimental groups were compared by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test, using SPSS software, version 16.0. A P-value <0.05 was considered as statistically significant
Figure 7.
Time for complete epithelization (days) in the diabetic group
Figure 8.
Histopathological slides for the diabetic standard group. Figure (A) and (B) represents wound tissue specimen (wound bed) after complete healing of excision wound, stained with hematoxylin and eosin in the diabetic group (group VII: standard silver sulfadiazine) as viewed under light microscope 4× and 10×. The sections showed well-formed keratinized squamous epithelium and the underlying dermis showed loosely arranged collagen fibers
The histopathological examination of the wound specimen is best measured by the quantitative method, which is based on the absolute number of cells and tissue area. Therefore, a quantitative scoring system is highly specific and standardized, which is difficult to score as it requires to objectify the exact interval between two values. Hence, it is appropriate to use semiquantitative scoring systems based on the selected parameters of wound healing that includes the amount of granulation tissue, inflammatory infiltrate, collagen fiber orientation, the pattern of collagen, and the amount of early and late collagen.[22] The healing score based on the aforementioned six histological parameters given by Sultana et al.[23] grades the healing status as good (16–19), fair (12–15), and poor (8–11).[24] Moreover, in contrast to the quantitative method, the semiquantitative scoring system can evaluate keratinization, which is one of the important factors in the process of wound healing.[22] Henceforth, in this study, we have calculated the wound healing using semiquantitative scoring system by Sultana et al.[23] On the basis of this wound healing scoring system, the healing status was found as poor, fair, and good for groups I and IV (control); groups III, VI (acacia honey), and VII (standard); and groups II and V (manuka honey), respectively.
DISCUSSION
Various published reports regarding the use of honey for the wound management in animal models as well as clinical cases are available.[25,26] The fresh wounds treated with topical honey have shown to accelerate wound contraction along with increased granulation tissue.[27] All types of honey are not same; every individual honey has particular characteristics unique to that honey, which imparts it with antimicrobial, antioxidant, anti-inflammatory, and wound healing properties.[28,29] The use of acacia honey has been previously shown in animal experiments,[14] though no previous study is available to compare the effects of manuka honey versus acacia honey in wound healing in nondiabetic and diabetic rats.
The active manuka honey is nowadays being used as a functional food owing to its holistic properties. Manuka honey has been tried to heal surgical and accidental wounds, particularly in horses, and has shown good outcome.[30,31] Some published case reports mention the successful application of manuka honey in nonhealing wounds and ulcers where the conventional antibiotics have failed.[32,33] Manuka honey has also shown efficient antimicrobial activity against a wide range of bacteria and even against MRSA, methicillin-resistant S. epidermidis, vancomycin-resistant Enterococcus, and so on.[34] Diabetes is considered as one of the major factors for delayed wound healing, primarily because of decrease in the concentration of collagen and granulation tissue formation in addition to increased protease and collagenase.[35] Clinical cases in favor of manuka honey dressings being effective in neuropathic diabetic foot ulcers are available.[36] This study compares two different types of honey, that is, acacia honey versus manuka honey with respect to wound contraction and rate of epithelization achieved in the excision wound model.
In the nondiabetic group, group II treated with topical manuka honey showed 80% wound contraction on day 9 versus 61% in group III (acacia honey), whereas on day 18, there was nearly complete wound contraction (99%) in group II, which was 8% more in comparison to that in control group and 3 days earlier than the normal wound contraction rate. The complete epithelization also occurred a day prior in group II when compared to that in groups I and II. The histopathological examination showed the formation of well-arranged and loosely arranged collagen in dermis, normal hair follicles, and keratinized squamous epithelium in the epidermis in groups II and III. The enhanced healing as observed in group II can be attributed to the suitable healing environment provided by manuka honey–alginate hydrogel. It is noted that honey increases its weight by 150% when topically applied and alginate hydrogel maintains the moist environment around the wound; the wet environment allows quick absorption of exudates during the various phases of wound healing, cell migration, cell differentiation, angiogenesis, matrix formation, promotion of granulation tissue, rapid epithelization, and enhanced healing. In addition, honey possesses intrinsic wound healing properties because of hydrogen peroxide production, antioxidant, antibacterial, and hydroscopic properties.[37,38]
In the diabetic group, group V (manuka honey) showed 85% reduction in wound size on day 9 versus 68% and 77% wound contraction in groups VI and VII, respectively. At day 15, 96% wound contraction was observed in group V compared to 92% in group VII (standard). Complete epithelization was achieved in 18 days in group V, whereas it was 20.5 days in groups VI and VII. The histopathological examination revealed a well-formed keratinized epithelium with normally arranged collagen tissue in the dermis with intact hair follicles in group V. It has been suggested that manuka honey contains MGO-derived advanced glycosylated end (AGE) products (argpyrimidine), which cause activation of nuclear factor kappa B (NF-κB), leading to apoptosis in the epithelial cells.[39] Moreover, manuka honey exerts osmotic action, which induces outflow of lymph, promotes extra oxygenation, protects fibroblasts, and enriches nutrient supply to the surface of the wound.[40] In addition, its antimicrobial activity is enhanced due to inhibition of tissue catalases, which are responsible for metabolizing hydrogen peroxide.[41] The presence of a unique factor, that is, phytochemical factor in manuka honey imparts antibacterial action. This phytochemical factor is rated as the Unique Manuka Factor (UMF) number, greater the UMF number more will be its antibacterial property.[8] This study showed good positive outcomes in diabetic excision model in group V when compared to that in control and standard treatment group.
On the contrary, the active antibacterial component of manuka honey, that is, MGO, has been reported to react with lysine, arginine, and cysteine residues of the structural proteins, including collagen, and further leads to the formation of AGE products, which can cause disruption of extracellular matrix remodeling, impaired microcirculation and immune response, promotion of fibrosis in chronic tissue infection, and induction of endothelial cell dysfunction.[42] The aforementioned reasons contribute to the detrimental effect of MGO in diabetic wound healing.[42] The results of this study have shown expedition of healing in diabetic wound model and do not support the aforementioned findings. Although manuka honey has shown superiority in the diabetic wound healing, large-scale studies are needed in the future to elucidate the effect of manuka honey in the healing of chronic wounds. The implicated mechanism states that the elevated protease levels lead to degradation of various growth factors, cytokines, and extracellular matrix component, henceforth increased deposition of nonviable tissue. Proteases have optimal activity at alkaline pH; manuka honey lowers the pH and hence activity of proteases is lowered.[43,44] Another mechanism is that manuka honey inactivates the plasminogen activator inhibitor, thereby converting plasminogen to plasmin, which digests fibrin and decreases the amount of nonviable tissue.[44,45] Further studies are needed to explore the underlying mechanism of manuka honey for enhanced diabetic wound healing.
CONCLUSION
Honey can be used as biological wound dressings based on its various physical, chemical, and biological properties. To achieve proper wound healing, honey needs to be present at the wound interface at all the times to allow effective anti-inflammatory, antibacterial, and immunostimulatory action. Our study has shown excellent wound healing properties with both the types of honey (acacia and manuka), though it was better with the manuka honey. Plenty of case reports supports the evidence of using manuka honey in wound healing, especially in chronic and nonhealing leg ulcers. This study provides evidence in favor of manuka honey with respect to enhanced wound healing, especially in the diabetic condition where wound healing is delayed. Therefore, medicated creams and wound dressings containing manuka factor and honey (especially manuka honey) can be used to provide effective and faster wound healing.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
This study was supported by the Department of Pharmacology, Kasturba Medical College, Manipal, Manipal Academy of Higher Education (MAHE), Mangaluru, Karnataka, India, with regard to the supply of instruments and animals needed for the conduct of the study.
REFERENCES
- 1.Heldin C-H,, Westermark B, Chapter 7: Role of platelet-derived growth factor in vivo. In: Richard C, editor. The molecular and cellular biology of wound repair. 1st ed. New York: Springer US; 1988. pp. 249–73. [Google Scholar]
- 2.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 3.Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- 4.AL-Waili N. Peritoneal macrophages transfusion in the treatment of chronic postoperative wound infections. J Pak Med Assoc. 1989;39:310–2. [PubMed] [Google Scholar]
- 5.Clark RA, Nielsen LD, Welch MP, McPherson JM. Collagen matrices attenuate the collagen-synthetic response of cultured fibroblasts to TGF-beta. J Cell Sci. 1995;108:1251–61. doi: 10.1242/jcs.108.3.1251. [DOI] [PubMed] [Google Scholar]
- 6.Molan PC. Potential of honey in the treatment of wounds and burns. Am J Clin Dermatol. 2001;2:13–9. doi: 10.2165/00128071-200102010-00003. [DOI] [PubMed] [Google Scholar]
- 7.Mathews KA, Binning AG. Wound management using honey. Compend Con Edu. 2002;24:53–9. [Google Scholar]
- 8.Molan PC. The evidence supporting the use of honey as a wound dressing. Int J Low Extrem Wounds. 2006;5:40–54. doi: 10.1177/1534734605286014. [DOI] [PubMed] [Google Scholar]
- 9.Sato T, Miyata G. The nutraceutical benefit, part iii: honey. Nutrition. 2000;16:468–9. doi: 10.1016/s0899-9007(00)00271-9. [DOI] [PubMed] [Google Scholar]
- 10.Vandamme L, Heyneman A, Hoeksema H, Verbelen J, Monstrey S. Honey in modern wound care: a systematic review. Burns. 2013;39:1514–25. doi: 10.1016/j.burns.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Al-Waili N, Salom K, Al-Ghamdi AA. Honey for wound healing, ulcers, and burns; data supporting its use in clinical practice. ScientificWorldJournal. 2011;11:766–87. doi: 10.1100/tsw.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo SK, Huttenlocher A. Innate immunity: wounds burst H2O2 signals to leukocytes. Curr Biol. 2009;19:R553–5. doi: 10.1016/j.cub.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Suarez JM, Gasparrini M, Forbes-Hernández TY, Mazzoni L, Giampieri F. The composition and biological activity of honey: a focus on manuka honey. Foods. 2014;3:420–32. doi: 10.3390/foods3030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iftikhar F, Arshad M, Rasheed F, Amraiz D, Anwar P, Gulfraz M. Effects of acacia honey on wound healing in various rat models. Phytother Res. 2010;24:583–6. doi: 10.1002/ptr.2990. [DOI] [PubMed] [Google Scholar]
- 15.Mendes JJ, Leandro CI, Bonaparte DP, Pinto AL. A rat model of diabetic wound infection for the evaluation of topical antimicrobial therapies. Comp Med. 2012;62:37–48. [PMC free article] [PubMed] [Google Scholar]
- 16.Shanbhag TV, Sharma C, Adiga S, Bairy LK, Shenoy S, Shenoy G. Wound healing activity of alcoholic extract of Kaempferia galanga in Wistar rats. Indian J Physiol Pharmacol. 2006;50:384–90. [PubMed] [Google Scholar]
- 17.Prasad SK, Kumar R, Patel DK, Hemalatha S. Wound healing activity of Withania coagulans in streptozotocin-induced diabetic rats. Pharm Biol. 2010;48:1397–404. doi: 10.3109/13880209.2010.486837. [DOI] [PubMed] [Google Scholar]
- 18.Kaur LP. Topical gel: a recent approach for novel drug delivery. Asian J Biomed Pharmaceut Sci. 2013;3:1. [Google Scholar]
- 19.Tasleem S, Naqvi SB, Khan SA, Hashmi K. Efficacy of newly formulated ointment containing 20% active antimicrobial honey in treatment of burn wound infections. J Ayub Med Coll Abbottabad. 2013;25:145–8. [PubMed] [Google Scholar]
- 20.Sadaf F, Saleem R, Ahmed M, Ahmad SI, Navaid-ul-Zafar Healing potential of cream containing extract of Sphaeranthus indicus on dermal wounds in guinea pigs. J Ethnopharmacol. 2006;107:161–3. doi: 10.1016/j.jep.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Pawar RS, Chaurasiya PK, Rajak H, Singour PK, Toppo FA, Jain A. Wound healing activity of Sida cordifolia Linn. in rats. Indian J Pharmacol. 2013;45:474–8. doi: 10.4103/0253-7613.117759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta A, Kumar P. Assessment of the histological state of the healing wound. Plast Aesthet Res. 2015;2:239–42. [Google Scholar]
- 23.Sultana J, Molla MR, Kamal M, Shahidullah M, Begum F, Bashar MA. Histological differences in wound healing in maxillofacial region in patients with or without risk factors. Bangladesh J Pathol. 2009;24:3–8. [Google Scholar]
- 24.Lemo N, Marignac G, Reyes-Gomez E, Lilin T, Crosaz O, Ehrenfest DM. Cutaneous reepithelialization and wound contraction after skin biopsies in rabbits: a mathematical model for healing and remodelling index. Vet Arh. 2010;80:637–52. [Google Scholar]
- 25.Suguna I, Chandrakasan G, Ramamoorthy U, Joseph KT. Influence of honey on biochemical and biophysical parameters of wounds in rats. J Clin Biochem Nutr. 1993;14:91–9. [Google Scholar]
- 26.Molan PC, Betts JA. Clinical usage of honey as a wound dressing: an update. J Wound Care. 2004;13:353–6. doi: 10.12968/jowc.2004.13.9.26708. [DOI] [PubMed] [Google Scholar]
- 27.Osuagwu FC, Oladejo OW, Imosemi IO, Aiku A, Ekpos OE, Salami AA, et al. Enhanced wound contraction in fresh wounds dressed with honey in Wistar rats (Rattus novergicus) West Afr J Med. 2004;23:114–8. doi: 10.4314/wajm.v23i2.28100. [DOI] [PubMed] [Google Scholar]
- 28.Dunford C, Cooper R, Molan P, White R. The use of honey in wound management. Nurs Stand. 2000;15:63–8. doi: 10.7748/ns2000.11.15.11.63.c2952. [DOI] [PubMed] [Google Scholar]
- 29.Bergman A, Yanai J, Weiss J, Bell D, David MP. Acceleration of wound healing by topical application of honey. An animal model. Am J Surg. 1983;145:374–6. doi: 10.1016/0002-9610(83)90204-0. [DOI] [PubMed] [Google Scholar]
- 30.Bischofberger AS, Dart CM, Horadagoda N, Perkins NR, Jeffcott LB, Little CB, et al. Effect of manuka honey gel on the transforming growth factor β1 and β3 concentrations, bacterial counts and histomorphology of contaminated full-thickness in wounds in equine distal limbs. Aust Vet J. 2016;94:27–34. doi: 10.1111/avj.12405. [DOI] [PubMed] [Google Scholar]
- 31.Dart A, Bischofberger A, Dart C, Jeffcott L. A review of research into second intention equine wound healing using manuka honey: current recommendations and future applications. Equine Vet Educ. 2015;27:658–64. [Google Scholar]
- 32.Regulski M. A novel wound care dressing for chronic leg ulcerations. Podiatry Manag. 2008;27:235–46. [Google Scholar]
- 33.Smith T, Legel K, Hanft JR. Topical Leptospermum honey (Medihoney) in recalcitrant venous leg wounds: a preliminary case series. Adv Skin Wound Care. 2009;22:68–71. doi: 10.1097/01.ASW.0000345283.05532.9a. [DOI] [PubMed] [Google Scholar]
- 34.Carter DA, Blair SE, Cokcetin NN, Bouzo D, Brooks P, Schothauer R, et al. Therapeutic manuka honey: no longer so alternative. Front Microbiol. 2016;7:569. doi: 10.3389/fmicb.2016.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komesu MC, Tanga MB, Buttros KR, Nakao C. Effects of acute diabetes on rat cutaneous wound healing. Pathophysiology. 2004;11:63–7. doi: 10.1016/j.pathophys.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Kamaratos AV, Tzirogiannis KN, Iraklianou SA, Panoutsopoulos GI, Kanellos IE, Melidonis AI. Manuka honey-impregnated dressings in the treatment of neuropathic diabetic foot ulcers. Int Wound J. 2014;11:259–63. doi: 10.1111/j.1742-481X.2012.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giusto G, Vercelli C, Comino F, Caramello V, Tursi M, Gandini M. A new, easy-to-make pectin-honey hydrogel enhances wound healing in rats. BMC Complement Altern Med. 2017;17:266. doi: 10.1186/s12906-017-1769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–26. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Visavadia BG, Honeysett J, Danford MH. Manuka honey dressing: an effective treatment for chronic wound infections. Br J Oral Maxillofac Surg. 2008;46:55–6. doi: 10.1016/j.bjoms.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Molan P, Rhodes T. Honey: a biologic wound dressing. Wounds. 2015;27:141–51. [PubMed] [Google Scholar]
- 41.Alvarez-Suarez JM, Giampieri F, Cordero M, Gasparrini M, Forbes-Hernández TY, Mazzoni L, et al. Activation of AMPK/Nrf2 signalling by Manuka honey protects human dermal fibroblasts against oxidative damage by improving antioxidant response and mitochondrial function promoting wound healing. J Funct Foods. 2016;25:38–49. [Google Scholar]
- 42.Majtan J. Methylglyoxal—a potential risk factor of manuka honey in healing of diabetic ulcers. Evid Based Complement Alternat Med. 2011;2011:295494. doi: 10.1093/ecam/neq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996;4:321–5. doi: 10.1046/j.1524-475X.1996.40307.x. [DOI] [PubMed] [Google Scholar]
- 44.Gethin GT, Cowman S, Conroy RM. The impact of manuka honey dressings on the surface pH of chronic wounds. Int Wound J. 2008;5:185–94. doi: 10.1111/j.1742-481X.2007.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Labban L. Honey as a promising treatment for diabetic foot ulcers (DFU) J Med Soc. 2014;28:64–8. [Google Scholar]