Abstract
Many small RNAs (sRNAs) regulate gene expression by base pairing to their target messenger RNAs (mRNAs) with the help of Hfq in Escherichia coli. The sRNA DsrA activates translation of the rpoS mRNA in an Hfq-dependent manner, but this activation ability was found to partially bypass Hfq when DsrA is overproduced. The precise mechanism by which DsrA bypasses Hfq is unknown. In this study, we constructed strains lacking all three rpoS-activating sRNAs (i.e., ArcZ, DsrA, and RprA) in hfq+ and Hfq− backgrounds, and then artificially regulated the cellular DsrA concentration in these strains by controlling its ectopic expression. We then examined how the expression level of rpoS was altered by a change in the concentration of DsrA. We found that the translation and stability of the rpoS mRNA are both enhanced by physiological concentrations of DsrA regardless of Hfq, but that depletion of Hfq causes a rapid degradation of DsrA and thereby decreases rpoS mRNA stability. These results suggest that the observed Hfq dependency of DsrA-mediated rpoS activation mainly results from the destabilization of DsrA in the absence of Hfq, and that DsrA itself contributes to the translational activation and stability of the rpoS mRNA in an Hfq-independent manner.
Keywords: DsrA, Escherichia coli, Hfq, rpoS, small RNAs
INTRODUCTION
There are about 100 small noncoding RNA (sRNA) in Escherichia coli. Many sRNAs are involved in fine tuning gene regulation for different growth environments, thereby helping the cell survive under various stress conditions (Bobrovskyy and Vanderpool, 2013; De Lay et al., 2013; Gottesman and Storz, 2011; Majdalani et al., 2005; Murina and Nikulin, 2015; Storz et al., 2011; Wassarman et al., 1999; Waters and Storz, 2009). Base pairing between an sRNA and a messenger RNA (mRNA) can regulate gene expression by changing the accessibility of the ribosome-binding site or altering the RNA-turnover rate (Majdalani et al., 2005; Santiago-Frangos et al., 2016). In most cases, sRNA-mediated regulation requires the presence of Hfq, a host protein that is required for Qβ bacteriophage replication (Vogel and Luisi, 2011). Hfq is an Sm-like protein that forms a homohexameric ring-like structure (Brennan and Link, 2007; Link et al., 2009; Sauter et al., 2003). A uridine-rich RNA sequence in an sRNA can bind to the proximal face (Lorenz et al., 2010; Panja et al., 2015; Sauer and Weichenrieder, 2011; Updegrove et al., 2016; Wang et al., 2011; Zhang et al., 2013) and outer rim (Panja et al., 2015; Sauer et al., 2012; Zhang et al., 2013) of Hfq, whereas an (ARN)n sequence motif of an mRNA can bind to its distal face (Małecka et al., 2015; Mikulecky et al., 2004; Schu et al., 2015; Updegrove et al., 2016). Hfq participates in sRNA-dependent translational regulation in various ways. First, Hfq can accelerate the base pairing between sRNAs and their mRNA targets (Hopkins et al., 2011; Panja and Woodson, 2012; Ross et al., 2013; Schu et al., 2015). While the binding of Hfq to sRNAs can prevent them from being degraded (Ikeda et al., 2011; Møller et al., 2002; Sledjeski et al., 2001; Vogel and Luisi, 2011), Hfq can also accelerate degradation of both sRNA and mRNA by recruiting the degradosome to sRNA–mRNA complexes (Andrade et al., 2012; Folichon et al., 2003; Ikeda et al., 2011). Moreover, Hfq reportedly plays more sophisticated roles in the sRNA-mediated translational regulation of the mRNAs for Spot42, SgrS, and RyhB (Bandyra et al., 2012; Desnoyers and Massé, 2012; Salvail et al., 2013).
DsrA, which is an 84-nucleotide Hfq-dependent RNA that can regulate multiple mRNAs (Lalaouna and Massé, 2016; Lalaouna et al., 2015; Lease et al., 1998; Sledjeski et al., 2001; Soper and Woodson, 2008), has been shown to activate the expression of the rpoS mRNA by an anti-antisense mechanism (Lease and Belfort, 2000; Majdalani et al., 1998; McCullen et al., 2010; Sledjeski et al., 1996). DsrA synthesis is increased at low temperatures, contributing to high levels of RpoS under these conditions (Hämmerle et al., 2013; Repoila and Gottesman, 2001; Sledjeski et al., 1996). The increases of both the dsrA promoter activity and the DsrA stability at low temperatures are responsible for the enhanced DsrA expression (Hämmerle et al., 2013; Repoila and Gottesman, 2001; Sledjeski et al., 1996). Therefore, it was thought that DsrA may be functional only under cold shock conditions. Nevertheless, DsrA can act on rpoS activation at 37°C (Mandin and Gottesman, 2010). Since DsrA is also induced by acid stress at 37°C (Bak et al., 2014), its activity is not limited to cold shock stress conditions. The rpoS mRNA usually forms a large stem–loop structure upstream of the start codon, which inhibits ribosome binding (Lease and Woodson, 2004; Soper et al., 2010; Wang et al., 2011). When DsrA binds to an upstream region in the 5′-UTR of rpoS, this stem–loop is disrupted, the ribosome binding site (RBS) is revealed, and translation of the rpoS mRNA is efficiently activated (Lease and Woodson, 2004). The DsrA-mediated activation of rpoS translation is Hfq-dependent at 30°C (Sledjeski et al., 2001) as well as at 25°C and 37°C (Supplementary Fig. S1). Hfq forms a stable ternary complex with DsrA and the rpoS mRNA, and this complexation increases the annealing rate of DsrA to the rpoS mRNA in vitro (Resch et al., 2008). However, overexpressed DsrA has also been shown to partially bypass the requirement of Hfq for rpoS activation (Soper et al., 2010; Večerek et al., 2010). In this respect, DsrA differs from two other rpoS-activating sRNAs, RprA and ArcZ, which stringently require Hfq for rpoS activation (McCullen et al., 2010). It has been proposed that the ability of overexpressed DsrA to partially bypass the requirement of Hfq for rpoS activation may be related to the ability of DsrA to tightly bind the 5′-UTR of the rpoS mRNA even in the absence of Hfq (Soper et al., 2010). However, the precise mechanism underlying the ability of overexpressed DsrA to bypass the requirement for Hfq remains unknown.
In the present work, we investigated the detailed mechanism underlying the requirement for Hfq in DsrA-mediated rpoS activation. For this purpose, we constructed strains lacking all three rpoS-activating sRNAs (i.e., ArcZ, DsrA, and RprA) in hfq+ and hfq− backgrounds, and controlled the cellular DsrA concentrations in these cells by ectopic expression. We then examined how the expression level of rpoS changed according to alterations in the concentration of DsrA. We found that the DsrA-mediated translational activation of rpoS occurred at similar levels in hfq− and hfq+ cells, but that DsrA and the rpoS mRNA both showed instability in hfq− cells. Our results suggest that the in vivo Hfq dependency of DsrA-mediated rpoS activation mainly results from the destabilization of DsrA in the absence of Hfq, but that DsrA itself contributes to the translational activation and stabilization of the rpoS mRNA in an Hfq-independent manner.
MATERIALS AND METHODS
Strains and plasmids
The bacterial strains and plasmids used in this study are listed in Table 1. Strain PM1409 carrying a chromosomal rpoS-lacZ translational fusion was gifted by Dr. S. Gottesman and referred to WT. The PM1409 Δhfq mutant was obtained by P1 transduction (Moore, 2011; Thomason et al., 2007) using the deletion strain, which was obtained from the E. coli Keio strain collection (Baba et al., 2006). PM1409Δ3 (a mutant strain having deletion of dsrA and rprA, and an arcZ promoter mutation) was obtained by P1 transduction using the relevant deletion strains (Bak et al., 2014). Briefly, kanamycin-marked mutations were transferred into the desired strain background using P1 transduction. The FRT-flanked kanamycin cassette introduced into the first dsrA deletion strain was removed using the Flp recombinase from pCP20 plasmid (Cherepanov and Wackernagel, 1995). The second rprA deletion was introduced by P1 transduction (Müller-Hill, 1985), and the kanamycin cassette was once again removed. To construct PM1409Δ3Δhfq, an additional hfq deletion was introduced. The arcZ promoter mutation was finally introduced by P1 transduction. PM1409ΔaΔr was constructed by the first rprA deletion and the second arcZ promoter mutation through P1 transduction. PM1409ΔaΔd and PM1409ΔdΔr were constructed by the first dsrA deletion and the second arcZ promoter mutation or rprA deletion. PM1409ΔaΔrΔhfq was constructed by the first rprA deletion, the second hfq deletion, and the final arcZ promoter mutation.
Table 1.
Strains and plasmids used in this study
| Name | Description | Source |
|---|---|---|
| Strains | ||
| PM1409 | Escherichia coli PM1205 lacI′::PBAD-rpoS-lacZ | (Soper et al., 2010) |
| PM1409Δhfq | PM1409 Δhfq::kanR | This study |
| PM1409Δ3 | PM1409 arcZ−::kanR ΔrprA ΔdsrA | This study |
| PM1409Δ3Δhfq | PM1409 arcZ−::kanR ΔrprA ΔdsrA Δhfq | This study |
| PM1409ΔaΔr | PM1409 arcZ−::kanR ΔrprA | This study |
| PM1409ΔaΔd | PM1409 arcZ−::kanR ΔdsrA | This study |
| PM1409ΔdΔr | PM1409 ΔdsrA::kanR ΔrprA | This study |
| PM1409ΔaΔrΔhfq | PM1409 arcZ−::kanR ΔdsrA Δhfq | This study |
| Plasmids | ||
| pHMB1 | A derivative of pHM1 (54), AmpR, IPTG-inducible transcription from immediately after the EcoRI site, modified rnpB terminator (GAUUU to GGAGU) next to the XbaI site. | (Bak et al., 2014) |
| pArcZ | pHMB1 derivative expressing ArcZ | (Bak et al., 2014) |
| pRprA | pHMB1 derivative expressing RprA | (Bak et al., 2014) |
| pDsrA | pHMB1 derivative expressing DsrA | (Bak et al., 2014) |
| pCP20 | FLP+, λ cI857+, λ PR Repts, AmpR, CmR, expression of site-specific Flp recombinase under control of a heat inducible promoter, temperature sensitive replication. | (Cherepanov and Wackernagel, 1995) |
LacZ activity assay
Three colonies for each strain were cultured in LB medium containing ampicillin (100 μg/ml) at 37°C or 25°C when necessary, and the overnight culture was diluted to 1:100 and cultured with the fresh medium. Arabinose (0.02%) and isopropyl β-D-1-thiogalactopyranoside (IPTG) were added at 2 h and 3.5 h, respectively, and the culture was incubated further for 0.5 h. LacZ activity was assayed as described previously (Zhang and Bremer, 1995). At least three independent measurements were performed for each strain.
RNA purification
Three colonies for each strain were cultured in LB medium containing ampicillin (100 μg/ml) at 37°C, and the overnight culture was diluted to 1:100 and cultured with the fresh medium. Arabinose (0.02%) and IPTG were added at 2 h and 3.5 h, respectively, and the culture was incubated further for 0.5 h. Total cellular RNAs were extracted using the acidic hot-phenol method, as described previously (Kim et al., 1996).
In vitro transcription
To prepare DsrA and LacZ200 (a transcript consisting of 200 nt of the lacZ mRNA), DNA templates were obtained via polymerase chain reaction (PCR) using appropriate primer pairs (Supplementary Table S1) and in-vitro transcription was carried out using T7 RNA polymerase (Promega, USA).
Northern blot analysis
For sRNA analysis, 0.5 to 20 μg of total RNAs were fractionated on a 7 M urea, 5% polyacrylamide gel, and electrotransferred onto a Hybond-XL membrane (Amersham Biosciences, UK), as previously described (Park et al., 2013). Known amounts of in vitro-transcribed DsrA were loaded along with RNA samples for quantification standards. For mRNA analysis, total RNAs (10 μg) were loaded on an agarose gel (1%, 1× MOPS) and transferred onto a Hybond-XL membrane through capillary diffusion (Streit et al., 2009). The membrane was hybridized with 32P-labeled DNA probes in PerfectHyb Plus Hybridization Buffer (Sigma-Aldrich, USA) according to the manufacturer’s instructions. Hybridization signals were analyzed using an Image Analyzer FLA7000 (Fuji, Japan). The utilized probes are listed in Supplementary Table S1.
Quantitative real-time PCR
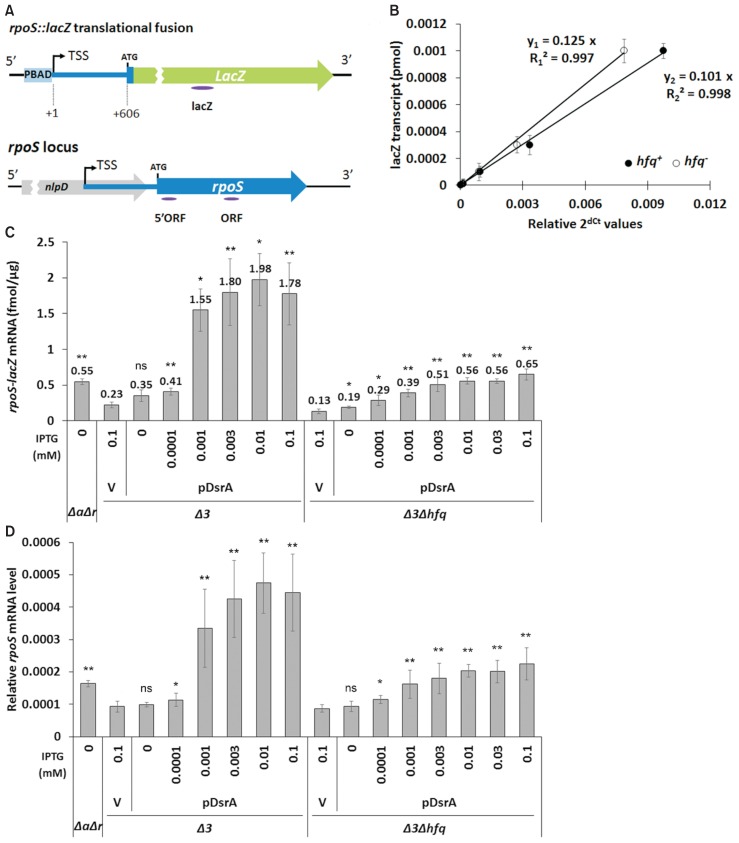
To measure the levels of transcripts, 5 μg of total RNA were DNase treated using a TURBO DNA-free Kit (Ambion, USA). Complementary DNAs (cDNAs) were synthesized from 0.5 μg of DNase-treated RNA using a SuPrimeScript RT-PCR premix (Genet Bio, Korea), cDNAs were amplified with SuPrimeScript qRT-PCR Premix (Genet Bio) using a Bioneer Exicycler 96 Real-Time Quantitative Thermal Block (Bioneer, Korea). Primer pairs specific to the lacZ ORF, rpoS ORF, rpoS 5′ORF, or rrsA mRNA were used for quantitative real-time reverse transcription-PCR (qRT-PCR). The used primers are listed in Supplementary Table S1. Cycle threshold (Ct) data were normalized to rrsA (16S rRNA gene) expression. To generate quantification standards of rpoS-lacZ mRNA, total cellular RNAs isolated from non-induced (without arabinose) PM1409Δ3 cells and PM1409Δ3Δhfq cells were mixed with known amounts of in vitro-transcribed LacZ200 and used for qRT-PCR, as described previously (Park et al., 2013). The abundance of rpoS-lacZ mRNA was estimated using the standard curves.
RNA stability assay
RNA stability was assessed as described previously (Kim et al., 1996). Briefly, three colonies for each strain were cultured in LB medium containing ampicillin (100 μg/ml) at 37°C, and the overnight culture was diluted to 1:100 and cultured with the fresh medium. Arabinose (0.02%) and IPTG were added at 2 h and 3.5 h, respectively, and the culture was incubated further for 0.5 h. For DsrA and rpoS transcription were halted by the addition of rifampicin (Milligan and Uhlenbeck, 1989) at a final concentration of 500 μg/ml. For rpoS-lacZ mRNA, the cultured cells were washed with LB medium lacking arabinose and then cultured for different time periods in LB medium containing ampicillin (100 μg/ml) and 0.1 mM IPTG. Total cellular RNAs were prepared and subjected to Northern blot analysis or qRT-PCR.
RESULTS
Activation of rpoS by ectopically expressed sRNAs
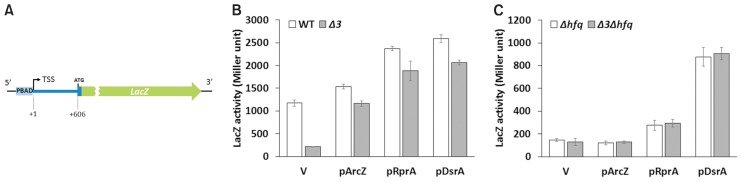
E. coli expresses three rpoS-activating sRNAs: ArcZ, DsrA, and RprA. It was previously shown that rpoS activation occurs in arcZ− rprA− cells but not in arcZ− rprA− hfq− cells, suggesting that the activation of rpoS by DsrA is Hfq-dependent (Majdalani et al., 1998; McCullen et al., 2010; Repoila and Gottesman, 2001; Sledjeski et al., 1996). However, it is not known whether this dependency on Hfq reflects an impact on DsrA stability, translational activation, or both due possible coincident effects of Hfq and DsrA on rpoS activation. To clarify the role of DsrA on rpoS activation, we first constructed arcZ− dsrA− rprA− strains in hfq+ and hfq− backgrounds carrying a rpoS-lacZ translational fusion; this generated PM1409Δ3 and PM1409Δ3Δhfq. RNA expression plasmids expressing each of the three sRNAs under IPTG induction were introduced into the generated strains, and the expression of the LacZ fusion was measured (Fig. 1). The lack of all three rpoS-activating sRNAs in hfq+ cells (PM1409Δ 3 cells) decreased the LacZ activity arising from the rpoS-lacZ translational fusion to less than 20% of the level in sRNA-expressing cells (PM1409 cells). Ectopic overexpression of any one of the sRNAs restored LacZ activity and even further stimulated rpoS-lacZ translation (Fig. 1B). In contrast, hfq− cells (PM1409Δhfq or PM1409Δ3Δhfq cells) exhibited sharply decreased LacZ activity regardless of sRNA gene knockout (Fig. 1C). Then we examined overexpression effects of three sRNAs on rpoS-lacZ translation in hfq− cells. Ectopic overexpression of ArcZ and RprA in these cells had relatively minor effects on rpoS-lacZ expression, regardless of sRNA gene knockout: about 2-fold decrease and increase by ArcZ and RprA, respectively. However, overexpression of DsrA in hfq− cells highly activated rpoS expression, increasing it by ~7-fold although it is approximately 50% of the level activated by DsrA overexpression in hfq+ cells (Fig. 1C).
Fig. 1. Stimulation of rpoS translation by DsrA overexpression in the absence of Hfq.
(A) The rpoS::lacZ chromosomal reporter fusion in strain PM1409. PBAD, the arabinose-inducible pBAD promoter; Position +1, the transcription start site (TSS) of rpoS; ATG, the translation start codon of rpoS. The sequence encoding the 5′-terminal 606 nt of the rpoS messenger RNA was fused to lacZ. (B) rpoS activation by overexpression of small RNAs (sRNAs) in PM1409 (WT) and PM1409Δ3 (Δ3) lacking all three rpoS-activating sRNAs. Cells containing sRNA-overexpressing plasmids, which were grown at 37°C, were induced with 0.02% arabinose and 0.1 mM IPTG, and LacZ activity was measured. Control vector (V), pHMB1. Plasmids pArcZ, pRprA, and pDsrA overexpress DsrA, RprA, and ArcZ, respectively. (C) rpoS activation by overexpression of sRNAs in PM1409Δhfq (Δhfq) and PM1409Δ3Δhfq (Δ3Δhfq). LacZ activity was measured as described in (B). WT, arcZ+ dsrA+ rprA+ hfq+; Δhfq, arcZ+ dsrA+ rprA+ hfq−; Δ3, arcZ− dsrA− rprA− hfq+; Δ3Δhfq, arcZ− dsrA− rprA− hfq−. At least three independent measurements were performed for each strain. Error bars represent SD.
Protection from degradation of DsrA by Hfq
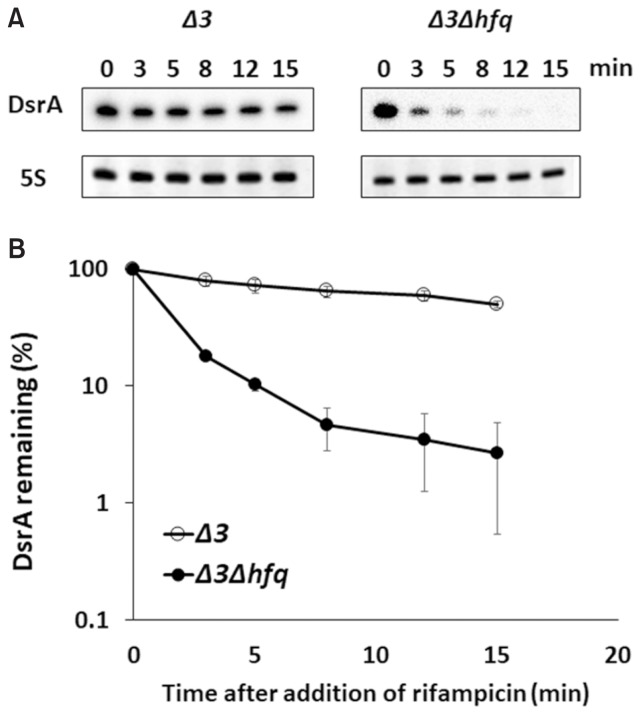
Ectopic expression of DsrA from pDsrA by induction with 0.1 mM IPTG was capable of stimulating rpoS translation in hfq− cells (PM1409Δhfq or PM1409Δ3Δhfq cells), but the expression level achieved in these cells was significantly lower than that obtained in hfq+ cells (PM1409 or PM1409Δ 3 cells) (Figs. 1B and 1C). The level of rpoS activation seen in hfq− cells was consistent with that described in the previous report showing that overexpression of DsrA could bypass the requirement of Hfq for rpoS activation (Soper et al., 2010). The observed weaker rpoS activation in hfq− cells might be in some part due to the low level of DsrA. Since endogenous DsrA was shown to be rapidly decayed in hfq− cells (Sledjeski et al., 2001), we speculated that overexpressed DsrA might be also rapidly degraded in hfq− cells. We found that the half-life of ectopically overexpressed DsrA was 1.5 min in hfq− cells (PM1409Δ3Δhfq cells), compared to 14 min in hfq+ cells (PM1409Δ3 cells) (Fig. 2). These data indicated that Hfq helps protect DsrA against degradation in vivo even when DsrA is overexpressed. This is contrast with the previous results that ectopically overexpressed DsrA had comparable stability to endogenous one (Sledjeski et al., 2001).
Fig. 2. Half-lives of DsrA in Δ3 and Δ3Δhfq cells.
(A) Total cellular RNA was prepared from 0.02% arabinose- and 0.1 mM IPTG-induced cells containing pDsrA, which were grown at 37°C, at the indicated times after rifampicin treatment. Cellular levels of DsrA were measured using Northern blot analysis. DsrA was probed with an anti-DsrA oligonucleotide and the 5S ribosomal RNA was detected as a loading control. Representative blots are shown. Δ 3, arcZ− dsrA− rprA− hfq+; Δ3Δhfq, arcZ− dsrA− rprA− hfq−.(B) The % RNA remaining against time are presented relative to that in cells before rifampicin treatment on a semi-log scale. Three Northern experiments were conducted and the mean DsrA concentrations ± SD were calculated.
Effects of Hfq on the activation of rpoS by different cellular levels of DsrA
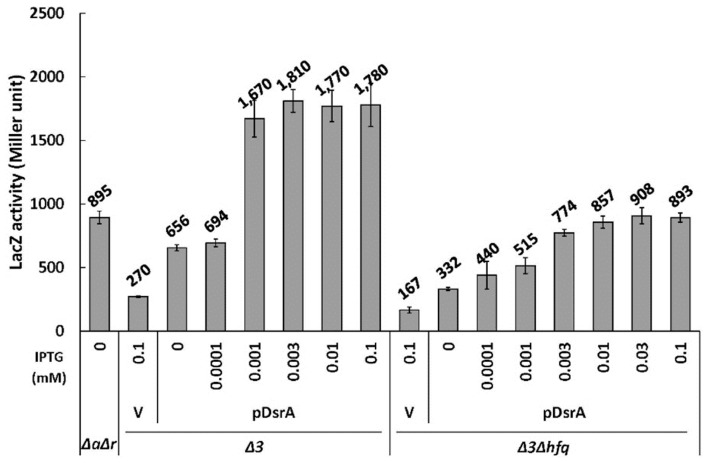
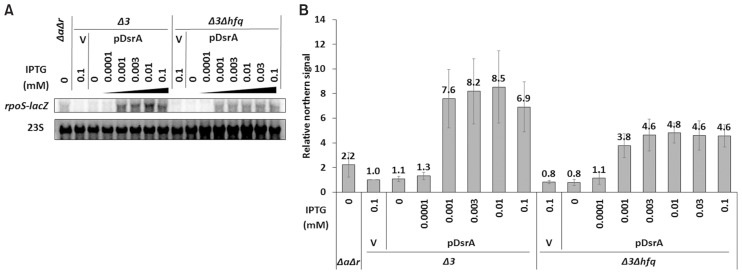
Next, we used different IPTG concentrations to change the cellular levels of ectopic DsrA expressed from pDsrA in PM1409Δ3 and PM1409Δ3Δhfq cells, which were referred to hfq+ (or Δ3) and hfq− (or Δ3Δhfq) cells in the subsequent studies, respectively, unless specified, and monitored the activation of rpoS. We found that pDsrA was a bit leaky so that it could produce a small amount of DsrA without the IPTG treatment. The steady-state concentration of DsrA increased with the concentration of IPTG in both hfq+ and hfq− cells, but the saturation level of DsrA was about 5-fold lower in hfq− cells. This might be due to the rapid decay of DsrA in hfq− cells. Interestingly we found that the level of DsrA was much lower in hfq+ cells exposed to no IPTG or to 0.0001 mM IPTG, compared to equivalently treated hfq− cells, whereas DsrA was highly accumulated in hfq+ cells exposed to 0.001 mM or higher IPTG concentrations. This may imply that Hfq uses some DsrA RNAs to bind other target mRNAs (e.g., mreB, hns, and/or rbsD), which could lead to the rapid decay of DsrA in the presence of Hfq (Lalaouna and Massé, 2016). The steady-state concentration of DsrA in hfq+ cells exposed to 0.001 mM IPTG was equivalent to that in hfq− cells exposed to 0.01 mM IPTG (Fig. 3). The LacZ activity in hfq+ cells exposed to 0.001 mM IPTG was 2-fold higher than that in hfq− cells exposed to 0.01 mM IPTG (Fig. 4). Moreover, Northern blot analysis (Fig. 5) and qRT-PCR (Fig. 6) revealed that the mRNA level of rpoS-lacZ was 2- to 3-fold higher in hfq+ cells than in hfq− cells at the above-listed IPTG concentrations. Since we also found that the endogenous level of DsrA in PM1409ΔaΔr (arcZ− dsrA+ rprA− hfq+) cells was comparable to the ectopic DsrA level resulting from basal expression in PM1409Δ3Δhfq (arcZ− dsrA− rprA− hfq−) cells without IPTG induction (Fig. 3), we compared the LacZ activity and the level of rpoS-lacZ mRNA between these two cells. Both the LacZ activity and the rpoS-lacZ mRNA level in PM1409ΔaΔr (hfq+) cells were about 2.5-fold higher than those in PM1409Δ3Δhfq (hfq−) cells (Figs. 4 and 6). Therefore, it is likely that the LacZ activities were correlated to the rpoS-lacZ mRNA levels regardless of the presence of Hfq, implying that the effects of Hfq on the translatability of rpoS-lacZ mRNA would be rather slight. Altogether, these data suggest that the higher-level activation of rpoS by DsrA in hfq+ cells is mainly due to the presence of higher rpoS mRNA levels.
Fig. 3. Cellular levels of DsrA in hfq+ and hfq− cells.
(A) Total cellular RNA was prepared from 0.02% arabinose- and IPTG-treated cells grown at 37°C, and subjected to Northern blot analysis as in Figure 2B. In vitro DsrA transcripts were used as standards for the quantitation of in vivo DsrA levels. Cells containing pDsrA were treated with IPTG at increasing concentrations from 0 to 0.1 mM. Representative blots are shown. The spliced image from the same Northern membrane was shown with the insertion of a dividing line between spliced lanes. (B) Standard curves for quantification of cellular DsrA. For the standard curve, the data with in vitro transcribed DsrA transcripts were used. Relative northern signals of DsrA of 0.0008 to 0.2 pmol were measured and graphs of relative northern signals vs. DsrA amounts were drawn. The standard curve equations for Northern membranes of PM1409Δ3 (Δ3) and PM1409Δ3Δhfq (Δ3Δhfq) cells and of PM1409ΔaΔr (ΔaΔr) cells from panel (A) were represented on the left and right graphs, respectively. R-squared means coefficient of determination. (C) The quantity of DsrA in a cell was estimated using the standard curve shown in (B). Three Northern experiments were conducted and the mean DsrA concentrations ± SD were calculated. Δ3, arcZ− dsrA− rprA− hfq+; Δ3Δhfq, arcZ− dsrA− rprA− hfq−; ΔaΔr, arcZ− dsrA+ rprA− hfq+; V, vector control.
Fig. 4. Up-regulation of rpoS translation by DsrA in hfq+ and hfq− cells.
Cells containing pDsrA, which were grown at 37°C, were induced with 0.02% arabinose and IPTG at increasing concentrations from 0 to 0.1 mM and LacZ activity was measured. The indicated values were calculated from at least three independent experiments. Error bars represent SD. Δ3, arcZ− dsrA− rprA− hfq+; Δ3Δhfq, arcZ− dsrA− rprA− hfq−; ΔaΔr, arcZ− dsrA+ rprA− hfq+; V, vector control.
Fig. 5. Northern analysis of effects of DsrA on rpoS-lacZ mRNA accumulation in Δ3 and Δ3Δhfq cells.
(A) Total cellular RNA was prepared from 0.02% arabinose- and IPTG-treated cells grown at 37°C, and subjected to Northern blot analysis. Cells containing pDsrA were treated with IPTG at increasing concentrations from 0 to 0.1 mM. The rpoS-lacZ mRNA was probed with an anti-lacZ oligonucleotide and the 23S ribosomal RNA was detected as a loading control. Representative blots are shown. (B) Northern signals were presented in a bar graph. Δ3, arcZ− dsrA− rprA− hfq+; Δ3Δhfq, arcZ− dsrA− rprA− hfq−; ΔaΔr, arcZ− dsrA+ rprA− hfq+; V, vector control. Three Northern experiments were conducted and the mean rpoS-lacZ concentrations ± SD were calculated.
Fig. 6. qRT-PCR analysis of effects of DsrA on rpoS mRNA accumulation in hfq+ and hfq− cells.
(A) Schematic diagrams of the rpoS::lacZ chromosomal reporter fusion and the rpoS gene structure. The PBAD promoter is indicated by PBAD, while the rpoS promoter is located within the nlpD gene. TSS, transcription start site; ATG, translation start codon. The locations of the qRT-PCR amplicons are indicated by ellipse below each diagram. (B) To generate the standard curve for quantitation of the rpoS-lacZ mRNA, total cellular RNA prepared from PM1409Δ3 and PM1409Δ3Δhfq cells grown at 37°C with no arabinose was mixed with known amounts of LacZ200, an in vitro transcript consisting of 200 nucleotides from lacZ mRNA, and also subjected to qRT-PCR using the lacZ amplicons. Cycle threshold (Ct) data of lacZ mRNA were normalized to rrsA expression. Graphs of relative Ct values vs. amounts of the lacZ transcript were drawn. The standard curve equations, y1 and y2, shown on the graph, represent equations for hfq+ and hfq− respectively. R-squared means coefficient of determination. (C) Total cellular RNA was prepared from arabinose-and IPTG-treated cells, which were grown at 37°C, and subjected to qRT-PCR. After normalization to rrsA expression the amount of rpoS-lacZ transcript per μg of total cellular RNA was estimated using the standard curve of (B). Values are means ± SD; n = 3; **P < 0.01, *P < 0.05; ns, non-significant (Student’s t-test, equal variance with the V/Δ3 value for hfq+ cells and with the V/Δ3Δhfq value for hfq− cells). (D) Levels of the rpoS transcript in PM1409ΔaΔr, PM1409Δ3, and PM1409Δ3Δhfq cells were determined by qRT-PCR, which was performed as described in (C) using the ORF amplicon. Δ3, arcZ− dsrA− rprA-−hfq+; Δ3Δhfq, arcZ− dsrA− rprA− hfq−; ΔaΔr, arcZ− dsrA+ rprA− hfq+; V, vector control.
The level of rpoS-lacZ in hfq− cells that lacked any DsrA expression was about 2-fold lower than that in hfq+ cells (Figs. 5 and 6C), suggesting that Hfq alone could protect the rpoS-lacZ mRNA from degradation or translation enhanced by Hfq could lead to a stabilization of rpoS-lacZ mRNA. We also examined how the ectopic expression of DsrA affected the endogenous rpoS mRNA level (Fig. 6D and Supplementary Fig. S2). Our results indicated that the endogenous rpoS mRNA level showed an increasing pattern similar to that of the rpoS-lacZ mRNA under DsrA overexpression, suggesting that the 5′ leader sequence of the rpoS mRNA is responsible for the ability of DsrA to increase the rpoS mRNA level. We also found that the level of endogenous rpoS mRNA increased with the level of DsrA, regardless of the presence of Hfq.
Effects of DsrA on the premature transcription termination of rpoS in the absence of Hfq
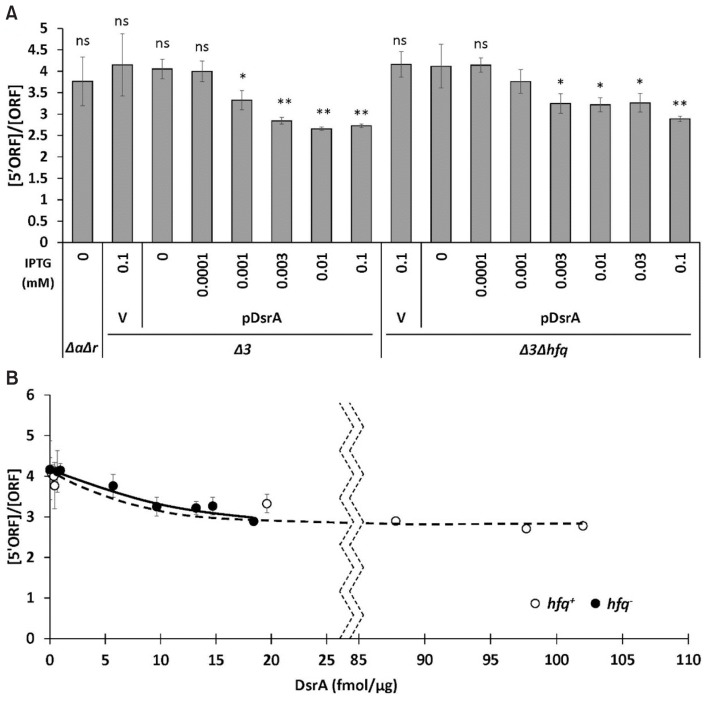
DsrA, ArcZ, and RprA have all been shown to suppress premature Rho-dependent transcription termination by binding the 5′ leader sequence of the rpoS mRNA (Sedlyarova et al., 2016), suggesting that the ability of DsrA to increase the rpoS mRNA level might result from an inhibition of Rho-dependent transcriptional termination. We thus examined the effect of Hfq on this DsrA-mediated antitermination. We selected two rpoS regions that had been amplified in previous studies (Sedlyarova et al., 2016), and used them as amplicons for qRT-PCR to assess the amounts of rpoS mRNA carrying the 5′ region and the internal region. The selected regions comprised the 5′ proximal sequence of +37 to +134 of the rpoS ORF (“5′ORF” amplicon) and the internal ORF sequence of +484 to +593 relative to the +1 translation start site (“ORF” amplicon) (Figs. 6A and 7). The final product ratio of the two amplicons was taken as representing the Rho-dependent termination efficiency. The [5′ORF]/[ORF] ratio was not significantly altered by the deletion of hfq in the absence of all three sRNAs, but DsrA overexpression decreased it by 20% in both hfq+ and hfq− cells. This suggests that DsrA-mediated antitermination occurs in the absence of Hfq and contributes to increasing the rpoS mRNA level. The antitermination effect first appeared at a low concentration of DsrA, but did not increase further as the concentration of DsrA increased (Fig. 7). Although future work may be warranted to examine why this effect does not increase with the concentration of DsrA, our present results suggest that DsrA-mediated antitermination seems to have only a minor contribution to increasing rpoS mRNA levels.
Fig. 7. Effects of DsrA on premature termination of rpoS transcription in hfq+ and hfq− cells.
(A) Levels of rpoS transcripts in PM1409Δ 3 and PM1409Δ3Δhfq cells grown at 37°C, were determined by performing qRT-PCR of the 5′ ORF amplicon, as described in Figure 6A. After cycle threshold (Ct) data were normalized to rrsA expression, the normalized values were divided by those of the rpoS ORF amplicon. An increase in the [5′ORF]/[ORF] ratio corresponds to an increase in the Rho-dependent termination efficiency, whereas a decrease in the [5′ORF]/[ORF] ratio corresponds to a decrease in the Rho-dependent termination efficiency. Values are means ± SD; n = 3; **P < 0.01, *P < 0.05; ns, non-significant (Student’s t-test, equal variance with V/Δ3Δhfq value). (B) The [5′ORF]/[ORF] ratio was plotted against the concentration of DsrA fmol/μg of total RNA. Δ3, arcZ− dsrA− rprA− hfq+; Δ3Δhfq, arcZ− dsrA− rprA− hfq−; ΔaΔr, arcZ− dsrA+ rprA− hfq+; V, vector control.
Effects of Hfq and DsrA on rpoS-lacZ decay
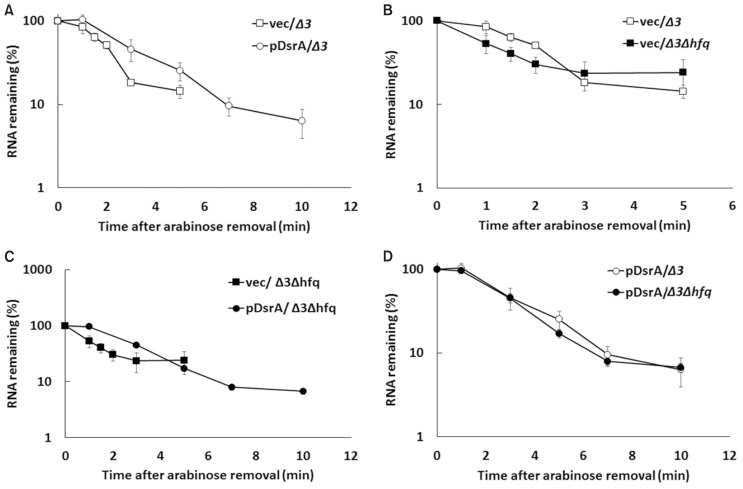
We also examined how Hfq and DsrA might increase the rpoS-lacZ mRNA level. To examine whether this effect reflected a simple increase in the half-life of the rpoS-lacZ mRNA, we determined the half-life of rpoS-lacZ mRNA produced under the control of pBAD by monitoring its disappearance after the removal of arabinose. Our results revealed that the half-life of the rpoS-lacZ mRNA was slightly increased in the presence of Hfq and also by overexpression of DsrA, regardless of Hfq (Fig. 8 and Table 2). Although the more common rifampicin chase-experiment could potentially mask the precise effects of DsrA because rifampicin might also inhibit the transcription of DsrA (Milligan and Uhlenbeck, 1989), we performed rifampicin chase experiments to see any effects of DsrA on stability of rpoS mRNA (Supplementary Fig. S3 and Supplementary Table S2). We found that DsrA also increased the half-live of rpoS mRNA. These results altogether suggest that the binding of DsrA to the rpoS mRNA inhibits the decay of the rpoS mRNA regardless of the presence of Hfq although Hfq may inhibit the decay of the rpoS mRNA. However, it should be noted that the inhibitory effect of DsrA or Hfq on the rpoS mRNA decay could be indirectly achieved through the increased translation in the presence of DsrA or the decreased translation in the absence of Hfq because the alteration of translation efficiency can affect mRNA stability.
Fig. 8. Effects of DsrA on the stability of the rpoS-lacZ mRNA in hfq+ and hfq− cells.
Total cellular RNA was prepared from 0.02% arabinose- and 0.1 mM IPTG-induced DsrA-expressing cells grown at 37°C, at the indicated times after arabinose was washed. Cellular levels of rpoS-lacZ mRNA were analyzed by qRT-PCR of PM1409Δ3 cells containing control vector and pDsrA (A), PM1409Δ3 and PM1409Δ3Δhfq cells containing control vector (B), PM1409Δ3Δhfq cells containing control vector and pDsrA (C), and PM1409Δ3 and PM1409Δ3Δhfq cells containing pDsrA (D). Cycle threshold (Ct) values were normalized to rrsA expression. The normalized values are used to calculate the fraction (%) of RNA remaining. The % RNA remaining was plotted on a semi-log scale as a function of time. Values are means ± SD; n = 3. Δ3, arcZ− dsrA− rprA− hfq+; Δ3Δhfq, arcZ− dsrA− rprA− hfq−; V, vector control.
Table 2.
Half-lives of the rpoS-lacZ mRNA
| Strain | Half-lives (min)a | |
|---|---|---|
|
| ||
| Vector | pDsrA | |
| hfq+ | 1.76 ± 0.08 | 2.64 ± 0.49 |
| hfq− | 1.22 ± 0.33 | 2.35 ± 0.22 |
Values are means ± SD for three independent experiments.
Half-lives were determined by linear regression analysis from the data presented in Figure 7. We assumed that the disappearance of rpoS-lacZ mRNA after arabinose washing followed a first-order decay.
Translational activation of rpoS by DsrA
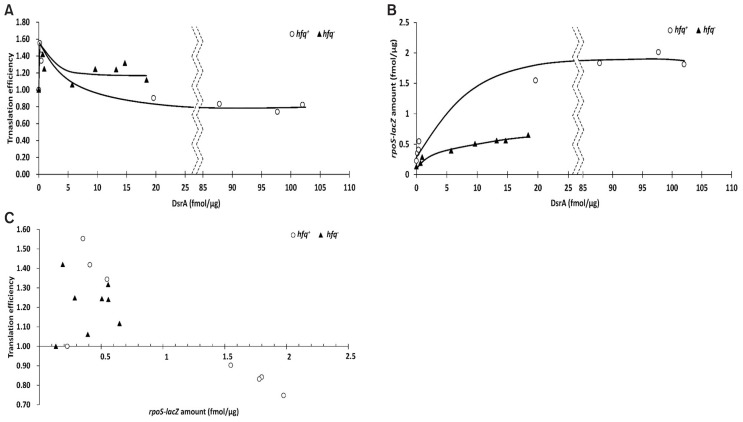
Finally, we examined whether DsrA activates the translation of rpoS in the absence of Hfq. To determine how DsrA affected the translation of LacZ from the rpoS-lacZ mRNA (Fig. 9), we defined translation efficiency as the ratio of LacZ activity to the amount of rpoS-lacZ mRNA. The relative translational efficiencies obtained in hfq+ and hfq− cells expressing various amounts of DsrA were calculated relative to that obtained in the absence of DsrA, which was given an arbitrary value of 1. Ectopic expression of DsrA increased the relative translation efficiency to about 1.5 regardless of Hfq unless the rpoS-lacZ mRNA was abundant (Fig. 9). Higher translation efficiencies were observed at very low concentrations of DsrA, but these increased efficiencies were reduced as the DsrA concentration increased. This contrasts with our observation that the rpoS-lacZ mRNA level increased with the DsrA level until a plateau was reached at 7-fold and 4-fold increases in rpoS-lacZ mRNA at DsrA concentration of about 20 fmol/μg of total RNA in hfq+ and hfq− cells, respectively (Fig. 9B). Therefore, it seems likely that a small amount of DsrA can activate translation of the rpoS mRNA, but that more DsrA is required to stabilize the rpoS mRNA. Translational activation of the rpoS-lacZ mRNA by DsrA was observed at up to rpoS-lacZ mRNA concentrations of 0.55 fmol/μg of total RNA in hfq+ cells and at up to 0.65 fmol/μg in hfq− cells, but was not observed at 1.5 fmol/μg in hfq+ cells (Figs. 6C and 9). Therefore, DsrA-mediated translational activation may not be effective at more than rpoS-lacZ mRNA concentration of 1.5 fmol/μg of total RNA. Endogenous DsrA activated the translation of the rpoS-lacZ mRNA with a relative translation efficiency of 1.34 at rpoS-lacZ mRNA concentration of 0.55 fmol/μg of total RNA.
Fig. 9. Effects of DsrA on the translational activation of rpoS in hfq+ and hfq− cells.
Translational efficiency was defined as the ratio of LacZ activity to the amount of rpoS-lacZ messenger RNA (mRNA) and calculated from the data presented in Figures 3, 4, and 6. Translational efficiencies (A) and rpoS-lacZ mRNA concentrations (B) in hfq+ and hfq− cells are plotted against the concentration of DsrA fmol/μg of total RNA. (C) Translational efficiencies in hfq+ and hfq− cells are plotted against the concentration of rpoS-lacZ mRNA fmol/μg of total RNA.
DISCUSSION
To determine the precise mechanism underlying the Hfq-independent DsrA-mediated regulation of rpoS translation at 37°C, we herein expressed ectopic DsrA in hfq+ and hfq− strains lacking all three rpoS-activating sRNAs (i.e., ArcZ, DsrA, and RprA). We then examined the translational regulation of rpoS mostly using an rpoS-lacZ translational fusion, as the translation of the 5′ leader sequence of the rpoS mRNA fused to lacZ can be taken as representing the regulatory characteristics of rpoS translation (McCullen et al., 2010; Peng et al., 2014; Resch et al., 2008; Soper et al., 2010). First, we found that ectopically expressed DsrA was very unstable in cells lacking Hfq. This is consistent with a previous report that the stability of endogenous DsrA is markedly decreased in the absence of Hfq at 30°C (Sledjeski et al., 2001). However, the previous authors reported that plasmid-expressed DsrA did not show a significant decrease of stability in the hfq− background (Sledjeski et al., 2001), which contrasts with our present findings. Although future work is needed to resolve this discrepancy, it is likely that ectopically expressed DsrA in our system mimics endogenous DsrA. Second, we found that the absence of Hfq was associated with a decrease in rpoS mRNA stability, which should contribute to the observed decrease in its translation. Binding of Hfq to rpoS mRNA may contribute to increasing rpoS mRNA stability because its 5′ leader sequence has Hfq-binding sites (Hämmerle et al., 2013; Lease and Woodson, 2004; Soper et al., 2010; Updegrove and Wartell, 2011). Alternatively, the reduction of rpoS translation by altered ribosome biogenesis in the absence of Hfq (Andrade et al., 2018) could also contribute to the decrease in rpoS mRNA stability because a lower abundance of translating ribosomes would mean that fewer mRNAs would be undergoing translation at a given moment, and more non-translating mRNAs would be vulnerable to RNases. In addition, since the Hfq binding to the 5′ leader sequence of rpoS mRNA can remodel the RBS structure of rpoS mRNA for efficient translation (Hämmerle et al., 2013), this binding can in turn enhance rpoS mRNA stability by increasing translation. Third, we showed that rpoS mRNA stability is enhanced by DsrA regardless of the presence of Hfq. The DsrA-mediated increase of rpoS mRNA stability resulted in accumulation of the rpoS mRNA. The DsrA-mediated accumulation of rpoS mRNA could be achieved through protection from RNase E degradation (McCullen et al., 2010) or the alternative RNase III processing (Resch et al., 2008). It is possible that the impact of DsrA on rpoS mRNA stability, to some extent, can result from the DsrA-mediated translation activation. However, the DsrA-mediated translation activation in both hfq+ and hfq− cells appears not to make a major contribution to rpoS mRNA stability because we showed here that the amount of rpoS mRNA was not correlated to translation efficiency but to the amount of DsrA in each strain. Rather, base-pairing between DsrA and rpoS mRNA to a large extent contributes to the stability of rpoS mRNA, leading to the increased levels of rpoS mRNA.
We found that the increased levels of rpoS mRNA by the same amount of DsrA was lower in hfq− cells than in hfq+ cells. The similar reduction of rpoS mRNA with its decreased half-life was also observed in the absence of DsrA, suggesting that Hfq affects rpoS mRNA stability regardless of the presence of DsrA.
Furthermore, we found that suppression of Rho-dependent transcription termination by DsrA can occur in the absence of Hfq, also resulting in rpoS activation. Finally, we found that the translational activation of the rpoS mRNA by DsrA is Hfq-independent. Although it has been reported that a ternary complex of DsrA-rpoS mRNA-Hfq forms well in vitro (Hämmerle et al., 2013; McCullen et al., 2010; Peng et al., 2014; Soper and Woodson, 2008; Updegrove and Wartell, 2011), the complex, even if formed in vivo, may not be required for translational activation. Instead, it may be related to the stabilization of the rpoS mRNA. Interestingly, translational activation of rpoS mRNA occurs in the presence of a small amount of DsrA, while stabilization of rpoS mRNA requires more DsrA, suggesting that DsrA may have the concentration-dependent dual actions. Another interesting finding of the present work is that translational activation was effective only at low concentrations of the rpoS mRNA. Although we do not yet know why translational activation by Hfq does not occur at high levels of the rpoS mRNA, we speculate that this activation could be coupled with ribosome loading. If an mRNA is relatively abundant, the ribosome-loading rate would be a rate-limiting step due to competition among available mRNAs.
Our results that DsrA itself can contribute to the translational activation and stabilization of the rpoS mRNA in an Hfq-independent manner in vivo may be contradictory to previous in vitro findings: Hfq interacts specifically with the 5′ leader sequence of rpoS mRNA to accelerate annealing of DsrA and rpoS mRNA (Soper and Woodson, 2008), and induces conformational changes of DsrA, potentially allowing for efficient base-pairing with rpoS mRNA (Večerek et al., 2008). The relatively high stability of DsrA-rpoS mRNA complex in the absence of Hfq (Soper et al., 2010) may allow DsrA to stimulate rpoS activation without Hfq in vivo even though Hfq is essential for activating the annealing process between DsrA and rpoS mRNA in vitro. In this regard, it is noteworthy that we cannot exclude additional roles of Hfq in DsrA-mediated rpoS activation through enhancement of rpoS mRNA stability or facilitation of ribosome loading on the mRNA in vivo.
A previous study (Hämmerle et al., 2013) reported that RpoS synthesis was sharply reduced at early exponential phase at 24°C in the absence of Hfq despite DsrA-rpoS mRNA duplex formation by overexpressed DsrA and that this sharp reduction is due to the lack of Hfq that is required to re-structure the RBS of the rpoS mRNA for efficient ribosome loading at low temperatures. However, data from other study (Soper et al., 2010) as well as ours (Supplementary Fig. S4) showed that rpoS activation by DsrA overexpression in the absence of Hfq (as assayed using rpoS-lacZ translational fusions) was almost half of that seen in the presence of Hfq at 25°C. Although the basis of the difference in levels of DsrA-mediated RpoS synthesis at low temperatures remains to be clarified, it seems likely that DsrA-rpoS mRNA base-pairing without Hfq still can contribute to a large extent (at least at specific growth phases) to rpoS activation at the low temperatures.
It was reported that RpoS synthesis is rather independent of Hfq and DsrA at 37°C because synthesis of RpoS in hfq− cells was found to be moderately reduced compared to that in hfq+ cells at the early exponential phase (Hämmerle et al., 2013). Nevertheless, since there was still a reduction of RpoS synthesis in hfq− cells at this specific growth phase, the reduction should be due to the absence of Hfq and the absence of rpoS activation by DsrA itself and possibly by other Hfq-dependent RpoS-activating sRNAs AcrZ and RprA. We found that the basal level of DsrA among three rpoS-activating sRNAs had the largest positive effects on the rpoS-lacZ translational fusion in hfq+ cells at the late exponential phase at 37°C (Supplementary Fig. S5) and similar results were also previously reported by Mandin and Gottesman (2010). Cells expressing only DsrA (ΔaΔr cells) synthesized LacZ from the rpoS-lacZ fusion 3-fold higher than Δ3 cells (Supplementary Fig. S5). When the ΔaΔr cells were shifted from 37°C to 25°C for 1.5 h, the rpoS-lacZ expression was slightly lowered at 25°C although a larger fold increase (about 4-fold) was observed in cells kept growing at 37°C (Supplementary Fig. S5D). Furthermore, DsrA is induced following acid challenge during the exponential phase at 37°C (Bak et al., 2014). Therefore, it is likely that DsrA-mediated rpoS activation plays an important role in RpoS synthesis at 37°C as well as at low temperatures.
While DsrA activates rpoS expression by binding to the 5′-UTR of its mRNA, it negatively regulates the hns mRNA by binding to the translation initiation region to inhibit translation. When DsrA represses hns and rbsD expression, Hfq is essential even if DsrA is overexpressed (Lalaouna et al., 2015; Lease and Belfort, 2000). This difference may reflect the presence of a repression mechanism in which the pairing of an sRNA with its mRNA targets most often results in degradation of those mRNAs. Since Hfq is believed to be involved in recruiting the RNA degradation machinery, it would be essential for the DsrA-mediated repressions of hns or rbsD. Alternatively, Hfq may play a critical role in facilitating DsrA-hns or rbsD mRNA interactions. In this regard, we note that while DsrA binds well to the rpoS mRNA in the absence of Hfq, the other two rpoS-activating sRNAs, ArcZ and RprA, which absolutely require Hfq for rpoS mRNA binding (McCullen et al., 2010).
To summarize, we herein dissected the coincident effects of Hfq and DsrA on rpoS activation to gain novel insights into the mechanisms underlying the DsrA-mediated translational activation of the rpoS mRNA. As shown in a proposed model (Fig. 10), we reveal that the translation and stability of the rpoS mRNA are enhanced by DsrA regardless of the presence of Hfq, although Hfq depletion causes a rapid degradation of DsrA and decreases the stability of the rpoS mRNA. This Hfq-independent DsrA-mediated rpoS activation occurs not only at the overexpression levels but also at the endogenous levels. These results suggest that the observed Hfq dependency of DsrA-mediated rpoS activation mainly results from the destabilization of DsrA in the absence of Hfq, but that DsrA itself can contribute to the translational activation and stability of the rpoS mRNA in an Hfq-independent manner. We further found that the proper concentrations of DsrA and rpoS mRNA can modulates the levels of the translational activation and of stability of rpoS mRNA. This work expands our understanding of the functions of sRNAs and their relationships with those of Hfq.
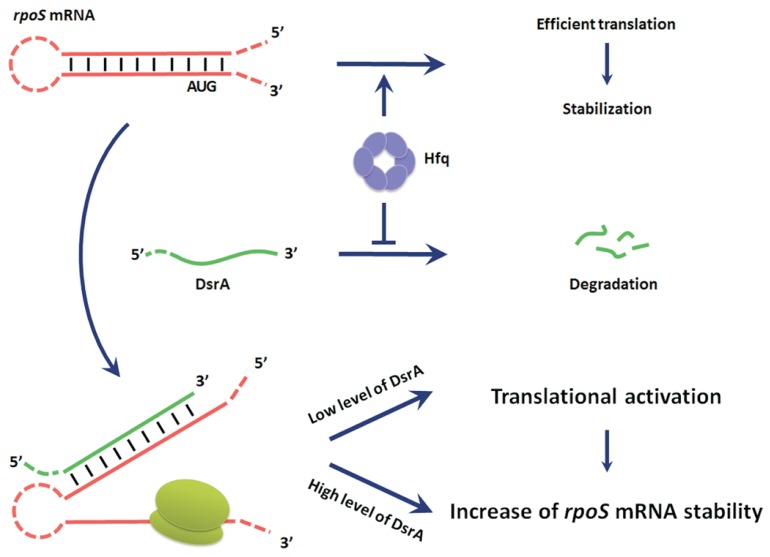
Fig. 10. A model for DsrA-mediated rpoS activation and the role of Hfq.
Hfq stabilizes rpoS messenger RNA (mRNA) and is required for efficient translation of rpoS mRNA, while it inhibits degradation DsrA. The efficient translation can cause an increase of the stability of rpoS mRNA. Binding of DsrA to the rpoS mRNA enhances the stabilization and translation of the rpoS mRNA. Translational activation of rpoS mRNA occurs in the presence of a small amount of DsrA, while stabilization of rpoS mRNA requires more DsrA. The translational activation can further contribute to stabilization of rpoS mRNA.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the National Resear ch Foundation of Korea (NRF) Grant by the Korean governme nt (MSIT) (2017R1A2B4010713; 2019R1H1A2039730) and the Intelligent Synthetic Biology Center of Global Frontier Proj ect funded by MSIT (2013M3A6A8073557). The authors wo uld like to thank NBRP-E. coli at NIG for providing E. coli stra ins containing the Keio knockout library and Dr. S. Gottesm an for providing strain PM1409. We also would like to thank Dr. D. Lalaouna for giving some information useful for rpoS mRNA northern blotting.
Footnotes
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Andrade J.M., Dos Santos R.F., Chelysheva I., Ignatova Z., Arraiano C.M. The RNA-binding protein Hfq is important for ribosome biogenesis and affects translation fidelity. EMBO J. 2018;37:e97631. doi: 10.15252/embj.201797631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade J.M., Pobre V., Matos A.M., Arraiano C.M. The crucial role of PNPase in the degradation of small RNAs that are not associated with Hfq. RNA. 2012;18:844–855. doi: 10.1261/rna.029413.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K.A., Tomita M., Wanner B.L., Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2 doi: 10.1038/msb4100050. 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak G., Han K., Kim D., Lee Y. Roles of rpoS-activating small RNAs in pathways leading to acid resistance of Escherichia coli. Microbiologyopen. 2014;3:15–28. doi: 10.1002/mbo3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyra K.J., Said N., Pfeiffer V., Górna M.W., Vogel J., Luisi B.F. The seed region of a small RNA drives the controlled destruction of the target mRNA by the endoribonuclease RNase E. Mol Cell. 2012;47:943–953. doi: 10.1016/j.molcel.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrovskyy M., Vanderpool C.K. Regulation of bacterial metabolism by small RNAs using diverse mechanisms. Annu Rev Genet. 2013;47:209–232. doi: 10.1146/annurev-genet-111212-133445. [DOI] [PubMed] [Google Scholar]
- Brennan R.G., Link T.M. Hfq structure, function and ligand binding. Curr Opin Microbiol. 2007;10:125–133. doi: 10.1016/j.mib.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Cherepanov P.P., Wackernagel W. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene. 1995;158:9–14. doi: 10.1016/0378-1119(95)00193-A. [DOI] [PubMed] [Google Scholar]
- De Lay N., Schu D.J., Gottesman S. Bacterial small RNA-based negative regulation: Hfq and its accomplices. J Biol Chem. 2013;288:7996–8003. doi: 10.1074/jbc.R112.441386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnoyers G., Massé E. Noncanonical repression of translation initiation through small RNA recruitment of the RNA chaperone Hfq. Genes Dev. 2012;26:726–739. doi: 10.1101/gad.182493.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folichon M., Arluison V., Pellegrini O., Huntzinger E., Régnier P., Hajnsdorf E. The poly(A) binding protein Hfq protects RNA from RNase E and exoribonucleolytic degradation. Nucleic Acids Res. 2003;31:7302–7310. doi: 10.1093/nar/gkg915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Storz G. Bacterial small RNA regulators: versatile roles and rapidly evolving variations. Cold Spring Harb Perspect Biol. 2011;3:a003798. doi: 10.1101/cshperspect.a003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerle H., Veečrek B., Resch A., Bläsi U. Duplex formation between the sRNA DsrA and rpoS mRNA is not sufficient for efficient RpoS synthesis at low temperature. RNA Biol. 2013;10:1834–1841. doi: 10.4161/rna.27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins J.F., Panja S., Woodson S.A. Rapid binding and release of Hfq from ternary complexes during RNA annealing. Nucleic Acids Res. 2011;39:5193–5202. doi: 10.1093/nar/gkr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Yagi M., Morita T., Aiba H. Hfq binding at RhlB-recognition region of RNase E is crucial for the rapid degradation of target mRNAs mediated by sRNAs in Escherichia coli. Mol Microbiol. 2011;79:419–432. doi: 10.1111/j.1365-2958.2010.07454.x. [DOI] [PubMed] [Google Scholar]
- Kim S., Kim H., Park I., Lee Y. Mutational analysis of RNA structures and sequences postulated to affect 3′ processing of M1 RNA, the RNA component of Escherichia coli RNase P. J Biol Chem. 1996;271:19330–19337. doi: 10.1074/jbc.271.32.19330. [DOI] [PubMed] [Google Scholar]
- Lalaouna D., Massé E. The spectrum of activity of the small RNA DsrA: not so narrow after all. Curr Genet. 2016;62:261–264. doi: 10.1007/s00294-015-0533-7. [DOI] [PubMed] [Google Scholar]
- Lalaouna D., Morissette A., Carrier M.C., Massé E. DsrA regulatory RNA represses both hns and rbsD mRNAs through distinct mechanisms in Escherichia coli. Mol Microbiol. 2015;98:357–369. doi: 10.1111/mmi.13129. [DOI] [PubMed] [Google Scholar]
- Lease R.A., Belfort M. Riboregulation by DsrA RNA: trans-actions for global economy. Mol Microbiol. 2000;38:667–672. doi: 10.1046/j.1365-2958.2000.02162.x. [DOI] [PubMed] [Google Scholar]
- Lease R.A., Cusick M.E., Belfort M. Riboregulation in Escherichia coli: DsrA RNA acts by RNA:RNA interactions at multiple loci. Proc Natl Acad Sci U S A. 1998;95:12456–12461. doi: 10.1073/pnas.95.21.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lease R.A., Woodson S.A. Cycling of the Sm-like protein Hfq on the DsrA small regulatory RNA. J Mol Biol. 2004;344:1211–1223. doi: 10.1016/j.jmb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Link T.M., Valentin-Hansen P., Brennan R.G. Structure of Escherichia coli Hfq bound to polyriboadenylate RNA. Proc Natl Acad Sci U S A. 2009;106:19292–19297. doi: 10.1073/pnas.0908744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz C., Gesell T., Zimmermann B., Schoeberl U., Bilusic I., Rajkowitsch L., Waldsich C., von Haeseler A., Schroeder R. Genomic SELEX for Hfq-binding RNAs identifies genomic aptamers predominantly in antisense transcripts. Nucleic Acids Res. 2010;38:3794–3808. doi: 10.1093/nar/gkq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalani N., Cunning C., Sledjeski D., Elliott T., Gottesman S. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc Natl Acad Sci U S A. 1998;95:12462–12467. doi: 10.1073/pnas.95.21.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalani N., Vanderpool C.K., Gottesman S. Bacterial small RNA regulators. Crit Rev Biochem Mol Biol. 2005;40:93–113. doi: 10.1080/10409230590918702. [DOI] [PubMed] [Google Scholar]
- Małecka E.M., Stróżecka J., Sobańska D., Olejniczak M. Structure of bacterial regulatory RNAs determines their performance in competition for the chaperone protein Hfq. Biochemistry. 2015;54:1157–1170. doi: 10.1021/bi500741d. [DOI] [PubMed] [Google Scholar]
- Mandin P., Gottesman S. Integrating anaerobic/aerobic sensing and the general stress response through the ArcZ small RNA. EMBO J. 2010;29:3094–3107. doi: 10.1038/emboj.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullen C.A., Benhammou J.N., Majdalani N., Gottesman S. Mechanism of positive regulation by DsrA and RprA small noncoding RNAs: pairing increases translation and protects rpoS mRNA from degradation. J Bacteriol. 2010;192:5559–5571. doi: 10.1128/JB.00464-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulecky P.J., Kaw M.K., Brescia C.C., Takach J.C., Sledjeski D.D., Feig A.L. Escherichia coli Hfq has distinct interaction surfaces for DsrA, rpoS and poly(A) RNAs. Nat Struct Mol Biol. 2004;11:1206–1214. doi: 10.1038/nsmb858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J.F., Uhlenbeck O.C. Synthesis of small RNAs using T7 RNA polymerase. Methods Enzymol. 1989;180:51–62. doi: 10.1016/0076-6879(89)80091-6. [DOI] [PubMed] [Google Scholar]
- M⊘ller T., Franch T., H⊘jrup P., Keene D.R., Bächinger H.P., Brennan R.G., Valentin-Hansen P. Hfq: a bacterial Sm-like protein that mediates RNA-RNA interaction. Mol Cell. 2002;9:23–30. doi: 10.1016/S1097-2765(01)00436-1. [DOI] [PubMed] [Google Scholar]
- Moore S.D. Assembling new Escherichia coli strains by transduction using phage P1. Methods Mol Biol. 2011;765:155–169. doi: 10.1007/978-1-61779-197-0_10. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B. Experiments with gene fusions. Trends Genet. 1985;1:61. doi: 10.1016/0168-9525(85)90025-3. [DOI] [Google Scholar]
- Murina V.N., Nikulin A.D. Bacterial small regulatory RNAs and Hfq protein. Biochemistry (Mosc) 2015;80:1647–1654. doi: 10.1134/S0006297915130027. [DOI] [PubMed] [Google Scholar]
- Panja S., Santiago-Frangos A., Schu D.J., Gottesman S., Woodson S.A. Acidic residues in the Hfq chaperone increase the selectivity of sRNA binding and annealing. J Mol Biol. 2015;427:3491–3500. doi: 10.1016/j.jmb.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panja S., Woodson S.A. Hfq proximity and orientation controls RNA annealing. Nucleic Acids Res. 2012;40:8690–8697. doi: 10.1093/nar/gks618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Bak G., Kim S.C., Lee Y. Exploring sRNA-mediated gene silencing mechanisms using artificial small RNAs derived from a natural RNA scaffold in Escherichia coli. Nucleic Acids Res. 2013;41:3787–3804. doi: 10.1093/nar/gkt061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Soper T.J., Woodson S.A. Positional effects of AAN motifs in rpoS regulation by sRNAs and Hfq. J Mol Biol. 2014;426:275–285. doi: 10.1016/j.jmb.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repoila F., Gottesman S. Signal transduction cascade for regulation of RpoS: temperature regulation of DsrA. J Bacteriol. 2001;183:4012–4023. doi: 10.1128/JB.183.13.4012-4023.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch A., Afonyushkin T., Lombo T.B., McDowall K.J., Bläsi U., Kaberdin V.R. Translational activation by the noncoding RNA DsrA involves alternative RNase III processing in the rpoS 5′-leader. RNA. 2008;14:454–459. doi: 10.1261/rna.603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J.A., Ellis M.J., Hossain S., Haniford D.B. Hfq restructures RNA-IN and RNA-OUT and facilitates antisense pairing in the Tn10/IS10 system. RNA. 2013;19:670–684. doi: 10.1261/rna.037747.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvail H., Caron M.P., Bélanger J., Massé E. Antagonistic functions between the RNA chaperone Hfq and an sRNA regulate sensitivity to the antibiotic colicin. EMBO J. 2013;32:2764–2778. doi: 10.1038/emboj.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Frangos A., Kavita K., Schu D.J., Gottesman S., Woodson S.A. C-terminal domain of the RNA chaperone Hfq drives sRNA competition and release of target RNA. Proc Natl Acad Sci U S A. 2016;113:E6089–E6096. doi: 10.1073/pnas.1613053113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer E., Schmidt S., Weichenrieder O. Small RNA binding to the lateral surface of Hfq hexamers and structural rearrangements upon mRNA target recognition. Proc Natl Acad Sci U S A. 2012;109:9396–9401. doi: 10.1073/pnas.1202521109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer E., Weichenrieder O. Structural basis for RNA 3′-end recognition by Hfq. Proc Natl Acad Sci U S A. 2011;108:13065–13070. doi: 10.1073/pnas.1103420108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter C., Basquin J., Suck D. Sm-like proteins in Eubacteria: the crystal structure of the Hfq protein from Escherichia coli. Nucleic Acids Res. 2003;31:4091–4098. doi: 10.1093/nar/gkg480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schu D.J., Zhang A., Gottesman S., Storz G. Alternative Hfq-sRNA interaction modes dictate alternative mRNA recognition. EMBO J. 2015;34:2557–2573. doi: 10.15252/embj.201591569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlyarova N., Shamovsky I., Bharati B.K., Epshtein V., Chen J., Gottesman S., Schroeder R., Nudler E. sRNA-mediated control of transcription termination in E. coli. Cell. 2016;167:111–121.e13. doi: 10.1016/j.cell.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledjeski D.D., Gupta A., Gottesman S. The small RNA, DsrA, is essential for the low temperature expression of RpoS during exponential growth in Escherichia coli. EMBO J. 1996;15:3993–4000. doi: 10.1002/j.1460-2075.1996.tb00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledjeski D.D., Whitman C., Zhang A. Hfq is necessary for regulation by the untranslated RNA DsrA. J Bacteriol. 2001;183:1997–2005. doi: 10.1128/JB.183.6.1997-2005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper T., Mandin P., Majdalani N., Gottesman S., Woodson S.A. Positive regulation by small RNAs and the role of Hfq. Proc Natl Acad Sci U S A. 2010;107:9602–9607. doi: 10.1073/pnas.1004435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper T.J., Woodson S.A. The rpoS mRNA leader recruits Hfq to facilitate annealing with DsrA sRNA. RNA. 2008;14:1907–1917. doi: 10.1261/rna.1110608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G., Vogel J., Wassarman K.M. Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell. 2011;43:880–891. doi: 10.1016/j.molcel.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit S., Michalski C.W., Erkan M., Kleeff J., Friess H. Northern blot analysis for detection and quantification of RNA in pancreatic cancer cells and tissues. Nat Protoc. 2009;4:37–43. doi: 10.1038/nprot.2008.216. [DOI] [PubMed] [Google Scholar]
- Thomason L.C., Costantino N., Court D.L. E. coli genome manipulation by P1 transduction. Curr Protoc Mol Biol. 2007;Chapter 1(Unit 1.17) doi: 10.1002/0471142727.mb0117s79. [DOI] [PubMed] [Google Scholar]
- Updegrove T.B., Wartell R.M. The influence of Escherichia coli Hfq mutations on RNA binding and sRNA • mRNA duplex formation in rpoS riboregulation. Biochim Biophys Acta. 2011;1809:532–540. doi: 10.1016/j.bbagrm.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Updegrove T.B., Zhang A., Storz G. Hfq: the flexible RNA matchmaker. Curr Opin Microbiol. 2016;30:133–138. doi: 10.1016/j.mib.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecerek B., Beich-Frandsen M., Resch A., Bläsi U. Translational activation of rpoS mRNA by the non-coding RNA DsrA and Hfq does not require ribosome binding. Nucleic Acids Res. 2010;38:1284–1293. doi: 10.1093/nar/gkp1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecerek B., Rajkowitsch L., Sonnleitner E., Schroeder R., Bläsi U. The C-terminal domain of Escherichia coli Hfq is required for regulation. Nucleic Acids Res. 2008;36:133–143. doi: 10.1093/nar/gkm985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J., Luisi B.F. Hfq and its constellation of RNA. Nat Rev Microbiol. 2011;9:578–589. doi: 10.1038/nrmicro2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wang L., Zou Y., Zhang J., Gong Q., Wu J., Shi Y. Cooperation of Escherichia coli Hfq hexamers in DsrA binding. Genes Dev. 2011;25:2106–2117. doi: 10.1101/gad.16746011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman K.M., Zhang A., Storz G. Small RNAs in Escherichia coli. Trends Microbiol. 1999;7:37–45. doi: 10.1016/S0966-842X(98)01379-1. [DOI] [PubMed] [Google Scholar]
- Waters L.S., Storz G. Regulatory RNAs in bacteria. Cell. 2009;136:615–628. doi: 10.1016/j.cell.2009.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A., Schu D.J., Tjaden B.C., Storz G., Gottesman S. Mutations in interaction surfaces differentially impact E. coli Hfq association with small RNAs and their mRNA targets. J Mol Biol. 2013;425:3678–3697. doi: 10.1016/j.jmb.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Bremer H. Control of the Escherichia coli rrnB P1 promoter strength by ppGpp. J Biol Chem. 1995;270:11181–11189. doi: 10.1074/jbc.270.19.11181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.